Abstract
Bovine NK cells have recently been characterized and the present study describes the interaction between NK cells, antigen-presenting cells, and secreted mycobacterial proteins. Gamma interferon (IFN-γ) production by NK cells was seen in approximately 30% of noninfected calves in response to the Mycobacterium tuberculosis complex-specific protein ESAT-6, MPP14 from Mycobacterium avium subsp. paratuberculosis, and purified protein derivative (PPD) from M. tuberculosis. In contrast, no response was induced by MPB70, which is another M. tuberculosis complex-specific secreted antigen. The production of IFN-γ by NK cells in whole blood in response to ESAT-6 and MPP14 was demonstrated using intracellular staining together with surface labeling for the NK cell-specific receptor, NKp46, or CD3. Furthermore, the depletion of NK cells from peripheral blood mononuclear cells completely abolished the IFN-γ production. The response was mediated through stimulation of adherent cells and was largely independent of contact between adherent cells and the NK cells. Neutralization of interleukin-12 only partly inhibited IFN-γ production, showing that other cytokines were also involved. The demonstration of NK cell-mediated IFN-γ production in young cattle provides an explanation for the nonspecific IFN-γ response frequently encountered in young cattle when using the IFN-γ test in diagnosis of mycobacterial infections.
Mycobacterial infections are widespread in ruminants and considered of major significance. The most important of these is bovine tuberculosis caused by Mycobacterium bovis, which has large economic implications and is a threat to human health in many parts of the developing world. Another widespread mycobacterial pathogen is Mycobacterium avium subsp. paratuberculosis. This bacterium causes paratuberculosis in ruminants and has been linked to Crohn's disease in humans (5). Although conclusive evidence for the role of M. avium subsp. paratuberculosis in Crohn's disease is lacking, the increased public focus on food-borne pathogens has given a renewed interest in this bacterium.
Both M. avium subsp. paratuberculosis and M. bovis are intracellular bacteria that survive and replicate inside host cells and they elicit immune responses with similar characteristics. The outcome of the infection is dependent on a complex interplay between the invading bacteria and the immune responses of the host. Protective immunity is dependent on activation of the cellular immune response with the production of the Th1 cytokine gamma interferon (IFN-γ), which is essential for resistance to mycobacteria (8, 16). The steering of the immune response onto a Th1 pathway is dependent on cytokines produced by the innate immune system. A key cytokine in this context is interleukin-12 (IL-12), which is essential for the development of a protective Th1 response. The importance of this cytokine has been demonstrated in humans, where disruption of IL-12 or IL-12 receptors gives an increased susceptibility to mycobacterial infections (1, 8, 10, 11). IL-12 also acts in synergy with IL-18 and several studies have demonstrated that monocytes/macrophages infected with mycobacteria secreted IL-12 and IL-18 (15, 17). There may however be a distinct difference in the ability of the antigen-presenting cells to induce IL-12 and IL-18 production (18). Hope et al. have shown that bovine dendritic cells infected with virulent M. bovis and M. bovis BCG produced IL-12, tumor necrosis factor alpha, and little IL-10, whereas macrophages produced tumor necrosis factor alpha, IL-10, and little IL-12 (22).
IL-12 and IL-18 also have a synergistic effect on IFN-γ production from NK cells, which are large granular lymphocytes belonging to the innate immune system (17). Several studies have shown that NK cells respond to mycobacterial infections but they are not believed to be essential for protection (24). Nevertheless, NK cells are likely to play a role in immune modulation and initiation of a Th1 pathway. The findings of Vankayalapati et al. supported a role for NK cells as a link between the innate and adapted immune response when they demonstrated that human NK cells regulated CD8+ T-cell effector function in response to Mycobacterium tuberculosis (42). A direct effect of mycobacteria on NK cells was also recently described by Esin et al. (14). They demonstrated that NK cells responded to extracellular BCG with proliferation, IFN-γ production, and cytotoxicity. Similar results has been reported in the bovine model where NK-like cells from naïve calves produced IFN-γ in response to M. bovis BCG-infected dendritic cells (21).
The ability of NK cells to produce large amounts of IFN-γ may also be important for diagnostic testing. IFN-γ is secreted at an early stage of mycobacterial infections and a commercial IFN-γ test using purified protein derivative (PPD) is available for bovine tuberculosis (37, 45). The rationale for measuring IFN-γ production as a diagnostic parameter is that previously sensitized T cells, through the T-cell receptor/major histocompatibility complex, will recognize antigens specific for one particular infection. Several groups have thus, for the last decade, successfully focused on the use of more specific antigens such as ESAT-6, CFP10, and MPB70 to increase the specificity of the IFN-γ test (4, 36, 43). However, it is well recognized that the test cannot be used in young cattle due to a large proportion of nonspecific reactors, which display high IFN-γ responses but are not infected (6, 23, 27).
We believed that the IFN-γ responses encountered in these young calves were not due to sensitization to other mycobacteria but rather caused by other mechanisms. This was supported by our previous study demonstrating that innate IFN-γ production occurred in young cattle after stimulation with MPP14, a secreted protein detected in the M. avium complex (33, 34). We hypothesized that NK cells were responsible for the observed IFN-γ production. Further characterization of the mechanisms and cells involved in the innate IFN-γ production is now feasible due to the recent characterization of bovine NK cells and the availability of a monoclonal antibody raised against the NK receptor NKp46 (38, 39). The aim of the present study was to investigate the interaction between NK cells, antigen-presenting cells, and secreted mycobacterial antigens, and we identified NK cells as the prime IFN-γ-producing cell in response to MPP14, ESAT-6, and PPD in uninfected animals.
MATERIALS AND METHODS
Antigens.
A 14-kDa secreted antigen (MPP14) was purified from M. avium subsp. paratuberculosis as described previously (33). Purified protein derivative (PPD) from M. tuberculosis was obtained from the National Veterinary Institute, Oslo, Norway. Recombinant ESAT-6 produced in Escherichia coli and Lactococcus lactis, respectively, were kindly provided by John Pollock, Veterinary Science Division, Stormont, Belfast, and Peter Andersen, Statens Serum Institute, Copenhagen. The first batch was used for the initial screening of IFN-γ responses in calves, while the latter batch was used throughout the rest of the study. The two ESAT-6 batches were tested in parallel on several samples and showed a similar response in all animals. Native MPB70 was purified as described (31) and was provided from Morten Harboe, National Hospital, Oslo. MPP14, ESAT-6, and MPB70 were used at a concentration of 2 μg/ml and PPD was used at a concentration at 10 μg/ml.
Animals.
Clinically healthy Norwegian Red dairy cattle in areas free from paratuberculosis and bovine tuberculosis were used in the present study.
IFN-γ production in whole blood.
Heparinized whole blood was dispensed into 24-well tissue culture trays (1 ml/well) and stimulated with purified MPP14, ESAT-6, MPB70, and PPD and incubated at 37°C in 5% CO2 in air for 24 h. One well served as an unstimulated control. The plasma was removed and assayed for IFN-γ using the bovine IFN-γ enzyme-linked immunosorbent assay (ELISA) (Bovine IFN-γ EASIA, Biosource, Nivelles, Belgium) according to the manufacturer's instructions. Results were calculated as corrected optical density (OD): [(mean of antigen-stimulated wells − mean of control wells)/(test positive control − test negative control)]. A corrected OD above 0.3 was considered positive. Polymyxin B (Sigma Chemical Co., St. Louis, MO), at a concentration of 50 μg/ml, was added in parallel on some occasions to test for lipopolysaccharide contamination in antigen preparations.
Depletion of NK cells.
Peripheral blood mononuclear cells (PBMC) were isolated from EDTA blood by density gradient centrifugation (1,150 × g, 20 min) on Lymphoprep (Nycomed Pharma, Oslo, Norway). NK cells were removed using the MACS system with an LD column (Miltenyi Biotec Ltd., Bisley, United Kingdom). In short, PBMC were incubated with monoclonal antibodies against NKp46 (AKS1) (38) followed by anti mouse immunoglobulin G-coupled magnetic particles (Miltenyi Biotec). PBMC and the NK-depleted PBMC (4 × 105 cells/well) were cultivated in RPMI 1640 containing 10% fetal calf serum with l-glutamine and gentamicin. The efficacy of the NK cell depletion was analyzed on a FACScalibur flow cytometer (Becton Dickinson, NJ), equipped with Cell-Quest software. The cells were stimulated with MPP14, ESAT-6, PPD, or medium alone in 96-well flat-bottomed microculture plates and incubated at 37°C in 5% CO2 in air for 24 h. The supernatant was assayed for IFN-γ (Biosource) and a standard curve was made using purified bovine IFN-γ, kindly provided by Stephen Jones, Pfizer, Melbourne.
Isolation and stimulation of adherent cells.
PBMC were purified as described above and diluted in RPMI 1640 (3 × 106 cells/ml). Adherent cells were isolated from PBMC in 24-well culture plates (1 ml/well) by incubation for 1 h at 37°C. Nonadherent cells were carefully removed by rinsing at least three times with RPMI. The adherent cells were incubated for 24 h with MPP14, ESAT-6, MPB70, PPD, or medium alone in 500 μl RPMI 1640 (5% fetal calf serum, l-glutamine, and gentamicin). The supernatant was filtered through 0.2-μm filters (Millipore) to remove remaining cells and frozen until used for stimulation of NK cell cultures. Polymyxin B (50 μg/ml) was added in parallels to test for lipopolysaccharide contamination in antigen preparations.
Intracellular staining for IFN-γ.
We incubated 5 ml heparinized whole blood with PPD, MPP14, ESAT-6, or recombinant bovine IL-2 (rbIL-2) (100 units/ml) and rbIL-12 (5 units/ml) (20) for 24 h. Nonstimulated control cultures and positive control cultures stimulated with 50 μg/ml phorbol 12-myristate 13-acetate (Sigma Chemical Co., St. Louis, MO) and 1 μg/ml calcium ionophore A23187 (Sigma) were included. After 6 h of incubation, 10 μg/ml brefeldin A (Sigma) was added and samples were incubated for another 12 h. PBMC were separated on Lymphoprep as described above. Cells were washed twice in Hanks' balanced salt solution (GibcoBRL, Paisley, United Kingdom), and 106 cells were added to the wells of 96-well staining trays (Falcon, Becton Dickinson). Between the following steps the cells were washed twice in phosphate-buffered saline plus 1% bovine serum albumin plus 0.05% sodium azide.
The cells were labeled for surface markers at previous determined optimal final concentrations; NKp46 (AKS1) or CD3 (MM1A, VMRD, Pullman, Wash). Cell suspensions were incubated with monoclonal antibodies for 30 min on ice followed by incubation with fluorescein isothiocyanate-conjugated secondary antibodies (10 μg/ml, Southern Biotechnology Associates Inc, Birmingham, Ala) for 30 min on ice. The cells were then fixed and permeabilized by incubating for 20 min on ice in 125 μl Cytofix/Cytoperm solution (Pharmingen, San Diego, CA), washed twice with 200 μl Perm/Wash solution (Pharmingen), which was also used for antibody diluent and washing through the rest of the procedure.
The cells were incubated for 30 min on ice with an in-house monoclonal antibody (immunoglobulin G2a clone 6.19) raised against affinity-purified bovine IFN-γ, washed twice, and incubated with phycoerythrin-conjugated secondary antibody (5 μg/ml, Southern), followed by another wash and resuspension in 400 μl fluorescence-activated cell sorting buffer (Becton Dickinson). The samples were analyzed on a FACScalibur flow cytometer (Becton Dickinson), equipped with Cell-Quest software; 100,000 lymphocytes were read using forward and side scatter gating. Gates for identifying NKp46- and CD3-positive cells were set using cells stained with secondary antibodies only.
NK cell cultures and stimulations.
rbIL-2 activated NK cell cultures were established as previously described (38) Briefly, monoclonal antibody against NKp46 (AKS1) was incubated with PBMC isolated on Lymphoprep, and NKp46-positive cells were selected by incubation with anti mouse immunoglobulin G-coated Dynabeads (Dynal, Oslo, Norway) and magnetic separation. Positively selected cells were cultured in RPMI 1640 supplemented with 60 μg/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, nonessential amino acids, 50 μM 2-mercaptoethanol (all Invitrogen, Carlsbad, Ca), 10% fetal calf serum, and 100 biological units/ml rbIL-2 (medium). Proliferating NK cells were used after 7 to 14 days of culture.
NK cells were washed in medium without rbIL-2, diluted to a concentration of 2 × 10 6 cells/ml and added to wells of 96-well cell culture trays containing different stimulators. The final cell concentration was 106 cells/ml and stimulations were set up in triplicates. The supernatants from antigen stimulated adherent cells were used at a final dilution of 1/10. rbIL-12 was used at various concentrations as indicated and anti-IL-12 (clone cc301) (20) was used at a concentration of 10 μg/ml in neutralizing experiments.
Statistics.
The differences in responses to the various antigens were evaluated using the nonparametric Wilcoxon-Mann-Whitney test. P < 0.05 was considered significant.
RESULTS
IFN-γ production in response to secreted mycobacterial proteins in young cattle.
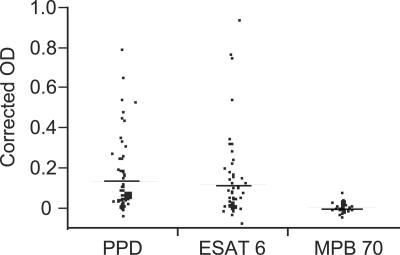
Young cattle in an area free from bovine tuberculosis and paratuberculosis were tested for IFN-γ production in response to ESAT-6, MPB70, and PPD. Clinically healthy cattle (n = 54) under the age of 18 months were tested four times each within a period of 6 months using a whole-blood assay. Altogether 31% of the animals had an IFN-γ response to ESAT-6 at one or more occasions, while 37% responded to PPD. In contrast, no IFN-γ response was detected in any sample stimulated with MPB70. There was also a significant difference in the average response to ESAT-6 and PPD compared to MPB70 (Fig. 1). To exclude the influence of possible lipopolysaccharide contamination, polymyxin B was added to the samples at some occasions. No inhibition of IFN-γ production was observed.
FIG. 1.
Extent of IFN-γ production in an M. bovis-free area. Young cattle between 2 and 18 months of age (n = 54) from three different herds were tested four times for IFN-γ production after stimulation of whole blood with PPD, ESAT-6, and MPB70 for 24 h. The mean response in each animal is shown as dots, while the means for each antigen stimulation are shown as lines. Corrected OD is expressed as [(mean of antigen-stimulated wells − mean control wells)/(test positive control − test negative control)].
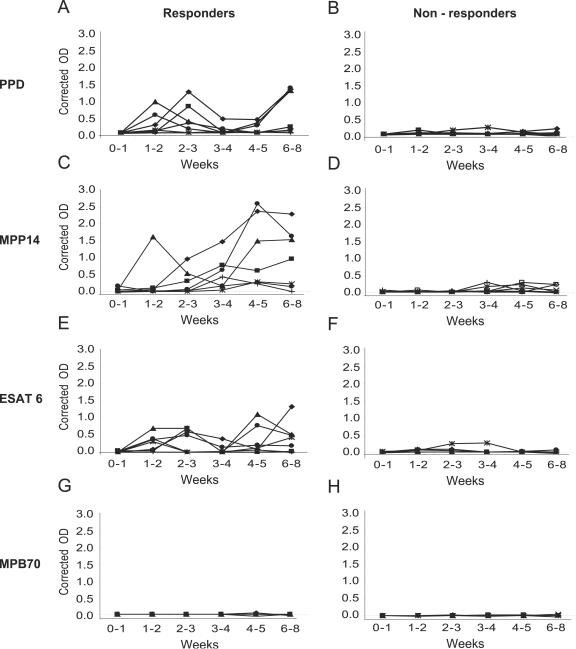
The IFN-γ production in response to secreted mycobacterial proteins was also followed in 14 calves from the first week of life and up to the age of approximately 2 months. IFN-γ was detected after stimulation with ESAT-6, MPP14, and PPD from the second week after birth, while no response was seen after stimulation with MPB70 (Fig. 2). Distinct differences between the animals were present. Seven animals had no responses to any of the antigens, while seven animals reacted (corrected OD > 0.3) to ESAT-6, MPP14, and PPD antigens at several occasions (Fig. 2). The animals that responded to ESAT-6 usually also responded to MPP14 and PPD.
FIG. 2.
IFN-γ responses in 14 young calves during the first 2 months of life. Whole blood was stimulated for 24 h with MPP14, ESAT-6, and MPB70 at a concentration of 2 μg/ml and PPD at a concentration of 10 μg/ml. The responders (n = 7) are animals with an IFN-γ response (corrected OD > 0.3) at at least one time point against any of the purified antigens. Nonresponders (n = 7) never had a corrected OD of >0.3 against any of the antigens. Stimulations were made with (A and B) PPD, (C and D) MPP14, (E and F) ESAT-6, or (G and H) MPB70.
NK cells produce IFN-γ in response to mycobacterial antigens.
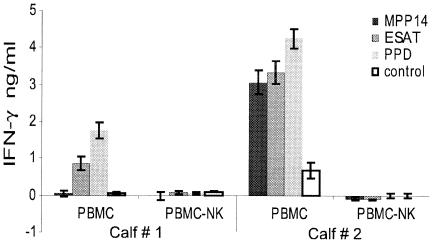
To test the hypothesis that NK cells were responsible for the IFN-γ production, PBMC from responding animals were depleted of NK cells using a monoclonal antibody directed against NKp46, followed by secondary antibodies coupled to magnetic beads. The removal of NK cells was monitored using flow cytometry and routinely more than 95% of the NK cells were removed. PBMC and PBMC depleted of NK cells were stimulated with MPP14, ESAT-6, and PPD, and the supernatant was assayed for IFN-γ production after 24 h of stimulation. The samples were stimulated in triplicate, and the assay was repeated three times using PBMC from six different animals, and two representative examples are shown in (Fig. 3). While the amount of IFN-γ produced varied from animal to animal, the IFN-γ responses were always eliminated after depletion of NK cells. This demonstrates that the NK cells produced IFN-γ, or alternatively, were necessary for IFN-γ production from other cell types.
FIG. 3.
IFN-γ production in PBMC and PBMC depleted of NK cells stimulated with mycobacterial antigens. The samples were stimulated with MPP14 (2 μg/ml), ESAT-6 (2 μg/ml), or PPD (10 μg/ml) for 24 h and the supernatant was assayed for IFN-γ production. The results are the means of cells stimulated in triplicates ± standard error of the mean. The experiment was repeated three times using PBMC from six different animals.
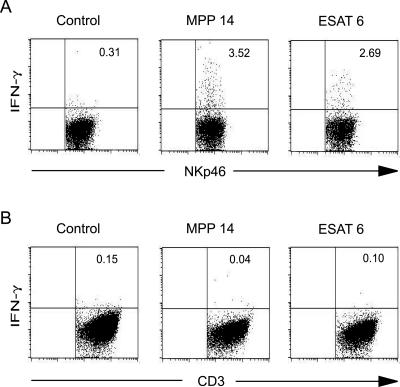
To confirm that the cells producing IFN-γ were indeed NK cells, whole blood from responder animals were incubated with ESAT-6 or MPP14 or left unstimulated. Intracellular staining for IFN-γ together with labeling for surface markers and analysis by flow cytometry identified NK cells as the main IFN-γ-producing cell type while little IFN-γ was produced by CD3+ cells (Fig. 4). The experiment was repeated six times, and between 1 and 4% of the NK cells produced IFN-γ after stimulation with ESAT-6 or MPP14. The relatively small number of IFN-γ-producing NK cells is in accordance with results from human studies where only a minor population of NK cells produced large amounts of IFN-γ (7). In comparison, less than 0.3% of CD3+ cells produced IFN-γ after stimulation with these antigens. CD3+ cells were however the major IFN-γ producers after stimulation with phorbol myristate acetate and calcium ionophore (data not shown). We also found a large individual variation in the number of IFN-γ-producing NK cells in nonstimulated samples ranging from 0.1 to 3.0% while no spontaneous IFN-γ production in CD3+ cells was detected. The two different methods, depletion of NK cells from PBMC and intracellular staining, conclusively established NK cells as a source of IFN-γ after stimulation with ESAT-6 and MPP14.
FIG. 4.
Flow cytometry plots verifying IFN-γ production by NK cells. Whole blood was stimulated with MPP14 or ESAT-6 or left unstimulated for 6 h. Brefeldin A was added to stop protein export and the samples were incubated for another 12 h. PBMC was subsequently purified by density gradient centrifugation. The cells were labeled with monoclonal antibodies against A) the NK cell marker NKp46 and B) CD3, followed by permeabilization and labeling for intracellular IFN-γ; 100,000 lymphocytes were analyzed and gated for NKp46-positive and CD3+ cells, respectively. Numbers in the upper right quadrant indicate the percentages of NK cells or CD3+ cells producing IFN-γ.
Antigen-induced IFN-γ production in NK cell cultures was dependent on adherent cells.
In an attempt to define the mechanism of IFN-γ production by NK cells, rbIL-2 activated NK cell cultures were stimulated with MPP14 and ESAT-6. No IFN-γ was detected, indicating that the antigens alone were unable to stimulate NK cells directly (data not shown). We thus hypothesized that the IFN-γ production by NK cells was mediated through stimulation of antigen-presenting cells. Adherent cells from four animals were incubated with MPP14, ESAT-6, MPB70, PPD, and medium alone, and the supernatants were assayed for the ability to induce IFN-γ production in nonautologous NK cell cultures. The assay was repeated four times, and the NK cell cultures were stimulated in triplicate. The results are given as the mean increase in IFN-γ production in response to the various antigens compared to medium alone.
There was a relatively large variation in IFN-γ production between NK cell cultures from different animals. However, in all the animals, supernatants from the MPP14-stimulated adherent cells induced significantly higher IFN-γ production compared to MPB70, while three out of four animals had a significantly higher response to ESAT-6 compared to MPB70 (Table 1). These results indicated that MPP14 and ESAT-6 induced cytokine production in adherent cells that subsequently induced IFN-γ production by NK cells. Since activated macrophages are able to secrete IFN-γ, the supernatants from stimulated adherent cells were assayed for the presence of this cytokine, and no IFN-γ was detected. Furthermore, lipopolysaccharide is a potent stimulator of antigen-presenting cells, and to exclude the influence of possible lipopolysaccharide contamination, polymyxin B was added to some of the samples. Minimal inhibition of IFN-γ production was observed.
TABLE 1.
Increase in IFN-γ production in NK cell cultures after stimulation with supernatant from antigen-stimulated adherent cells compared to medium alonea
| Calf no. | Mean IFN-γ production (ng/ml) ± SEM
|
|||
|---|---|---|---|---|
| MPP14 | ESAT-6 | PPD | MPB70 | |
| 1 | 1.3 ± 0.1* | 2.8 ± 0.7* | 4.6 ± 0.8 | 0.4 ± 0.3 |
| 2 | 2.5 ± 0.6* | 1.9 ± 0.4* | 1.8 ± 0.5 | 0.0 ± 0.5 |
| 3 | 5.0 ± 0.5* | 4.5 ± 0.5 | 0.8 ± 0.4 | 2.8 ± 0.5 |
| 4 | 4.8 ± 1.1* | 3.0 ± 0.8* | 3.8 ± 1.1* | 0.6 ± 0.2 |
*, P = 0.05, Wilcoxon rank test, compared to MPB70.
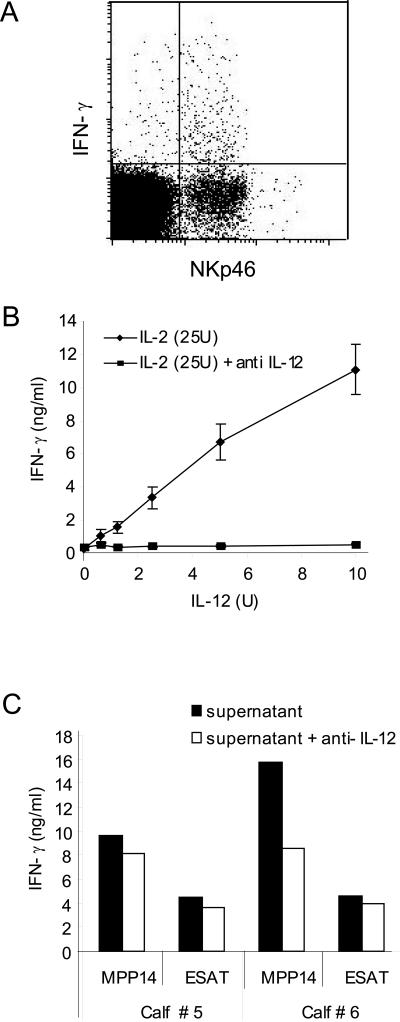
A likely mechanism for the IFN-γ production in NK cells in antigen-stimulated whole blood is through cytokines from stimulated monocytes. To see whether bovine NK cells in blood can produce IFN-γ in response to cytokines, heparinized whole-blood samples were stimulated with rbIL-2 and rbIL-12. IFN-γ production was subsequently measured using intracellular staining and flow cytometry. An average of 10.9% of the NK cells produced IFN-γ, with an individual variation ranging from 4.9 to 20.1% (n = 6) (Fig. 5A). In contrast, the average number of CD3+ cells producing IFN-γ was 1.3%. This showed that a combination of rbIL-2 and rbIL-12 induced IFN-γ production in NK cells in whole-blood (Fig. 5A). Likewise, NK cells in cultures stimulated with rbIL-2 and rbIL-12 produced IFN-γ (Fig. 5B), while low levels (1 to 2 ng/ml) of IFN-γ were detected when rbIL-2 (25 to 200U) or rbIL-12 (2.5 to 20 U) was used alone.
FIG. 5.
Mechanisms for IFN-γ production by NK cells. A) Intracellular flow cytometry staining for IFN-γ and surface markers in IL-2 (100 U) and IL-12 (5 U)-stimulated whole blood (100,000 gated lymphocytes); 4.9% of the NKp46-positive cells produced IFN-γ. B) IFN-γ production in IL-2-activated NK cell cultures after stimulation with IL-2 and different levels of IL-12 with or without neutralization by anti-IL-12. C) Production of IFN-γ by NK cells stimulated with supernatant from antigen-stimulated adherent cells. Supernatants from antigen-stimulated adherent cells were diluted 1:10 and incubated with or without anti-IL-12 for 1 hour before addition of NK cells. The amount of IFN-γ was measured by ELISA after 24 h of incubation.
Neutralization of IFN-γ production in NK cells was achieved by adding anti-IL-12 antibodies (Fig. 5B). We subsequently assessed whether IL-12 from antigen-stimulated adherent cells was responsible for the induced IFN-γ production by NK cells. The presence of IL-12 in the supernatants was estimated in an IL-12 assay as described previously (20) and small amounts of IL-12 (<1 U/ml) were detected. Anti-IL-12 was added to NK cell cultures together with supernatant from antigen-stimulated adherent cells to see if a possible effect of IL-12 could be neutralized. The addition of anti-IL-12 antibodies gave a reduction of IFN-γ production varying from 15% to 47%, showing that IL-12 was partly responsible for the observed IFN-γ production from NK cells (Fig. 5C). However, other cytokines were also likely to be involved.
DISCUSSION
The present study investigated the interaction between NK cells, adherent cells, and secreted mycobacterial antigens. We found that the mycobacterial antigens ESAT-6 from the M. tuberculosis complex and MPP14 from the M. avium complex induced IFN-γ production from NK cells. The response was dependent on adherent cells and likely mediated through cytokine production.
We have previously demonstrated innate IFN-γ production in healthy calves in response to MPP14 (34). The recent characterization of bovine NK cells and the generation of a monoclonal antibody raised against the bovine NK cell marker NKp46 (38, 39) facilitated a more detailed study of this innate IFN-γ production. ESAT-6, MPP14, and MPB70 are secreted antigens available to the immune system at an early stage of the infection, and several such proteins can induce strong IFN-γ production from sensitized T cells (2, 25). In the present study we have shown that ESAT-6 and MPP14 were able to induce IFN-γ production from NK cells.
NK cells are important in immune regulation and play a role in directing the immune system towards a Th1 pathway in other species (40, 42), however, the significance of NK cells in cattle remains to be established. ESAT-6 and MPP14 may be of importance in the development of a protective Th1 response and are thus good candidates to be evaluated in new vaccines against M. bovis and M. avium subsp. paratuberculosis. The ability of ESAT-6 to initiate a Th1 response has been shown previously in the mouse model, where ESAT-6 delivered as a fusion protein and as a DNA vaccine induced IFN-γ production to an unrelated antigen (29, 30). Mycobacteria are likely to harbor antigens that induce both beneficial and detrimental immune responses. A detailed examination of the effect of various mycobacterial antigens on the innate and the adaptive immune system will facilitate a rational strategy for the design of new vaccines.
The mechanisms for the antigen-induced IFN-γ production from NK cells were not fully explained. However, a definite requirement for antigen-stimulated adherent cells was clearly detected. Most likely, the observed IFN-γ production from NK cells was a result of cytokines secreted from the adherent cells. Despite the low levels of IL-12 present in the samples, neutralization of IL-12 partly inhibited the observed IFN-γ production from NK cells. It is thus likely that other cytokines were involved, and that these cytokines acted in synergy with IL-12. A likely candidate is IL-18. This cytokine is produced by monocytes and macrophages and has a variety of biological functions, including induction of type 1 cytokines and enhancement of NK cell functions (12). The production of IFN-γ from NK cells in response to IL-18 produced by Mycobacterium leprae-stimulated human monocytes was demonstrated by Garcia et al. (17).
The lack of antibodies to the bovine Toll-like receptors (TLRs) and other receptors hindered detailed investigation of the mechanisms for the interaction of ESAT-6 and MPP14 with adherent cells. The interaction of mycobacterial components with innate immune receptors such as TLR-2 and TLR-4 have been studied in murine and human models. Lipoarabinomannan and its precursor lipomannan from the mycobacterial cell wall can interact with TLR and induce production of proinflammatory cytokines (9, 28, 41). Furthermore, lipoproteins from M. tuberculosis induced IL-12 production mediated via TLR (3). In these proteins the lipid moiety was believed to be essential for activation. Activation through TLR by protein ligands has not been extensively investigated. However, Ohashi et al. showed that the human heat shock protein 60 induced tumor necrosis factor alpha and NO2− production through TLR-4 (32). We have here demonstrated that secreted mycobacterial protein antigens also can interact with the innate immune system.
There was a remarkable individual difference in the NK cell mediated IFN-γ responses. The explanation for these individual variations is unclear. Young calves will encounter numerous antigens for the first time and they are likely to have a highly activated immune system. The combination of viral, bacterial, and parasitic agents the naïve animal is exposed to, together with inherent predisposition, may steer the general immune response onto a Th1 or Th2 pathway. This was recently demonstrated by Elias et al., who showed that Schistosoma mansoni infection induced a general Th2 response, and that the S. mansoni-infected mice were less able to elicit a Th1 response than the controls (13). The infected mice had a reduced protective effect of BCG vaccination with a lower IFN-γ production.
A general activation of the immune system towards a Th1 or Th2 pathway in the different calves may explain the individual variations in IFN-γ production from NK cells. This may also be reflected in variations in spontaneous cytokines secretion in peripheral blood as detected in the present study, where nonstimulated NK cells showed large variations in IFN-γ secretion. Such variations have been observed by Walker et al. in patients and healthy humans in Malawi, where the frequency of mycobacterial and parasitic infections was high (44). They also showed that spontaneous cytokine production influenced the induced production. Donors who had spontaneous IFN-γ production by NK cells also showed increased production after stimulation with phorbol myristate acetate and calcium ionophore compared to donors without spontaneous IFN-γ production. This is in accordance with the findings in the present and our previous study, where animals that had innate IFN-γ production in response to MPP14 also had low-level spontaneous IFN-γ production (34).
The IFN-γ test has been used in several countries to diagnose early stages of bovine tuberculosis and the use of specific proteins such as ESAT-6 has clearly increased the specificity of this test (4, 35). However, it is widely recognized that the test cannot be used in young animals due to nonspecific IFN-γ production (6, 27). Our results demonstrated that NK cells are a source of innate IFN-γ production, and this provides an explanation for the problems encountered in the young calves. This is also in agreement with the finding that young calves had the highest proportion of NK cells in peripheral blood (26).
In a broader screening of IFN-γ production in young cattle, we found that approximately one third of the animals under the age of 18 months produced IFN-γ in response to ESAT-6 and that calves responding to ESAT-6 also usually responded to MPP14. ESAT-6 is present in the M tuberculosis complex and has also been detected in M. kansasii and M. flavescens (19), while MPP14 was detected by Western blotting in the M. avium complex and M. scrofulaceum (33). It is unlikely that as many as one-third of the animals were sensitized to mycobacteria harboring both MPP14 and ESAT-6. Consequently, it is likely that NK cells are responsible for the majority of the nonspecific IFN-γ production observed in young cattle. Methods that inhibit IFN-γ production from NK cells would therefore lead to an improvement of the IFN-γ test for detection of bovine mycobacterial diseases.
In conclusion, we have demonstrated that IFN-γ production from NK cells occurred in response to adherent cells stimulated with the secreted proteins ESAT-6 and MPP14. This will have consequences for the interpretation and use of the IFN-γ test for diagnosis of mycobacterial infections in young cattle.
Acknowledgments
We are grateful to Morten Harboe, National Hospital, Oslo; Peter Andersen, Statens Serum Institute, Copenhagen; and John Pollock, Veterinary Science Division, Belfast, for providing the MPB70 and ESAT-6 antigens. We also thank Inger Austrheim Heffernan and Ingvild Berg for excellent technical assistance and Annette Kampen and Elisabeth Kommisrud for organizing blood sampling.
This work was partly funded by GENO, the Norwegian Meat Research Center, and the Norwegian Research Council.
Editor: J. D. Clements
REFERENCES
- 1.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, P. 1994. The T-cell response to secreted antigens of Mycobacterium tuberculosis. Immunobiology 191:537-547. [DOI] [PubMed] [Google Scholar]
- 3.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 4.Buddle, B. M., A. R. McCarthy, T. J. Ryan, J. M. Pollock, H. M. Vordermeier, R. G. Hewinson, P. Andersen, and G. W. de Lisle. 2003. Use of mycobacterial peptides and recombinant proteins for the diagnosis of bovine tuberculosis in skin test-positive cattle. Vet. Rec. 153:615-620. [DOI] [PubMed] [Google Scholar]
- 5.Chiodini, R. J., H. J. Van Kruiningen, R. S. Merkal, W. R. Jr. Thayer, and J. A. Coutu. 1984. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. J. Clin. Microbiol. 20:966-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, M. T., and B. Y. Zhao. 1994. Comparison of the commercial serum antibody ELISA, gamma-interferon test kit, and radiometric fecal culture for early diagnosis of paratuberculosis in experimentally infected Holstein calves, p. 67-76. In R. J. Chiodini, M. T. Collins, and E. O. Bassey (ed.), Proceedings of the Fourth International Colloquium on Paratuberculosis.
- 7.Cooper, M. A., T. A. Fehniger, and M. A. Caligiuri. 2001. The biology of human natural killer-cell subsets. Trends Immunol. 22:633-640.11698225 [Google Scholar]
- 8.Cunningham, J. A., J. D. Kellner, P. J. Bridge, C. L. Trevenen, D. R. Mcleod, and H. D. Davies. 2000. Disseminated bacille Calmette-Guerin infection in an infant with a novel deletion in the interferon-gamma receptor gene. Int. J. Tuberc. Lung Dis. 4:791-794. [PubMed] [Google Scholar]
- 9.Dao, D. N., L. Kremer, Y. Guerardel, A. Molano, W. R. Jacobs, Jr., S. A. Porcelli, and V. Briken. 2004. Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect. Immun. 72:2067-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong, R., F. Altare, I. A. Haagen, D. G. Elferink, T. Boer, P. J. Breda Vriesman, P. J. Kabel, J. M. Draaisma, J. T. van Dissel, F. P. Kroon, J. L. Casanova, and T. H. Ottenhoff. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435-1438. [DOI] [PubMed] [Google Scholar]
- 11.de Jong, R., A. A. Janson, W. R. Faber, B. Naafs, and T. H. Ottenhoff. 1997. IL-2 and IL-12 act in synergy to overcome antigen-specific T-cell unresponsiveness in mycobacterial disease. J. Immunol. 159:786-793. [PubMed] [Google Scholar]
- 12.Dinarello, C. A. 1999. Interleukin-18. Methods 19:121-132. [DOI] [PubMed] [Google Scholar]
- 13.Elias, D., H. Akuffo, A. Pawlowski, M. Haile, T. Schon, and S. Britton. 2005. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine 23:1326-1334. [DOI] [PubMed] [Google Scholar]
- 14.Esin, S., G. Batoni, M. Pardini, F. Favilli, D. Bottai, G. Maisetta, W. Florio, R. Vanacore, H. Wigzell, and M. Campa. 2004. Functional characterization of human natural killer cells responding to Mycobacterium bovis bacille Calmette-Guerin. Immunology 112:143-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinberg, J., C. Fieschi, R. Doffinger, M. Feinberg, T. Leclerc, S. Boisson-Dupuis, C. Picard, J. Bustamante, A. Chapgier, O. Filipe-Santos, C. L. Ku, L. de Beaucoudrey, J. Reichenbach, G. Antoni, R. Balde, A. Alcais, and J. L. Casanova. 2004. Bacillus Calmette Guerin triggers the IL-12/IFN-gamma axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur. J. Immunol. 34:3276-3284. [DOI] [PubMed] [Google Scholar]
- 16.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia, V. E., K. Uyemura, P. A. Sieling, M. T. Ochoa, C. T. Morita, H. Okamura, M. Kurimoto, T. H. Rea, and R. L. Modlin. 1999. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J. Immunol. 162:6114-6121. [PubMed] [Google Scholar]
- 18.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T-cell response. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 19.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hope, J. C., L. S. Kwong, G. Entrican, S. Wattegedera, H. M. Vordermeier, P. Sopp, and C. J. Howard. 2002. Development of detection methods for ruminant interleukin (IL)-12. J. Immunol. Methods 266:117-126. [DOI] [PubMed] [Google Scholar]
- 21.Hope, J. C., P. Sopp, and C. J. Howard. 2002. NK-like CD8(+) cells in immunologically naive neonatal calves that respond to dendritic cells infected with. Mycobacterium bovis BCG. J. Leukoc. Biol. 71:184-194. [PubMed] [Google Scholar]
- 22.Hope, J. C., M. L. Thom, P. A. McCormick, and C. J. Howard. 2004. Interaction of antigen presenting cells with mycobacteria. Vet. Immunol. Immunopathol. 100:187-195. [DOI] [PubMed] [Google Scholar]
- 23.Jungersen, G., A. Huda, J. J. Hansen, and P. Lind. 2002. Interpretation of the gamma interferon test for diagnosis of subclinical paratuberculosis in cattle. Clin. Diagn. Lab. Immunol. 9:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junqueira-Kipnis, A. P., A. Kipnis, A. Jamieson, M. G. Juarrero, A. Diefenbach, D. H. Raulet, J. Turner, and I. M. Orme. 2003. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J. Immunol. 171:6039-6045. [DOI] [PubMed] [Google Scholar]
- 25.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulberg, S., P. Boysen, and A. K. Storset. 2004. Reference values for relative numbers of natural killer cells in cattle blood. Dev. Comp. Immunol. 28:941-948. [DOI] [PubMed] [Google Scholar]
- 27.McDonald, W. L., S. E. Ridge, A. F. Hope, and R. J. Condron. 1999. Evaluation of diagnostic tests for Johne's disease in young cattle. Aust. Vet. J. 77:113-119. [DOI] [PubMed] [Google Scholar]
- 28.Means, T. K., S. Wang, E. Lien, A. Yoshimura, D. T. Golenbock, and M. J. Fenton. 1999. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163:3920-3927. [PubMed] [Google Scholar]
- 29.Menon, S. A., M. J. Wannemuehler, G. G. Mahairas, and F. C. Minion. 2002. Mycobacterial ESAT-6 protein enhances mouse IFN-gamma responses to Mycoplasma hyopneumoniae P71 protein. J. Interferon Cytokine Res. 22:807-813. [DOI] [PubMed] [Google Scholar]
- 30.Minion, F. C., S. A. Menon, G. G. Mahairas, and M. J. Wannemuehler. 2003. Enhanced murine antigen-specific gamma interferon and immunoglobulin G2a responses by using mycobacterial ESAT-6 sequences in DNA vaccines. Infect. Immun. 71:2239-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagai, S., J. Matsumoto, and T. Nagasuga. 1981. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect. Immun. 31:1152-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 164:558-561. [DOI] [PubMed] [Google Scholar]
- 33.Olsen, I., L. J. Reitan, and H. G. Wiker. 2000. Distinct differences in repertoires of low-molecular-mass secreted antigens of Mycobacterium avium complex and Mycobacterium tuberculosis. J. Clin. Microbiol. 38:4453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen, I., and A. K. Storset. 2001. Innate IFN-gamma production in cattle in response to MPP14, a secreted protein from Mycobacterium avium subsp. paratuberculosis. Scand. J. Immunol. 54:306-313. [DOI] [PubMed] [Google Scholar]
- 35.Pollock, J. M., and P. Andersen. 1997. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J. Infect. Dis. 175:1251-1254. [DOI] [PubMed] [Google Scholar]
- 36.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 37.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1990. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust. Vet. J. 67:134-137. [DOI] [PubMed] [Google Scholar]
- 38.Storset, A. K., S. Kulberg, I. Berg, P. Boysen, J. C. Hope, and E. Dissen. 2004. NKp46 defines a subset of bovine leukocytes with natural killer cell characteristics. Eur. J. Immunol. 34:669-676. [DOI] [PubMed] [Google Scholar]
- 39.Storset, A. K., I. O. Slettedal, J. L. Williams, A. Law, and E. Dissen. 2003. Natural killer cell receptors in cattle: a bovine killer cell, immunoglobulin-like receptor multigene family contains members with divergent signaling motifs. Eur. J. Immunol. 33:980-990. [DOI] [PubMed] [Google Scholar]
- 40.Trinchieri, G. 1995. Natural killer cells wear different hats: effector cells of innate resistance and regulatory cells of adaptive immunity and of hematopoiesis. Semin. Immunol. 7:83-88. [DOI] [PubMed] [Google Scholar]
- 41.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vankayalapati, R., P. Klucar, B. Wizel, S. E. Weis, B. Samten, H. Safi, H. Shams, and P. F. Barnes. 2004. NK cells regulate CD8+ T-cell effector function in response to an intracellular pathogen. J. Immunol. 172:130-137. [DOI] [PubMed] [Google Scholar]
- 43.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker, D., J. Jason, K. Wallace, J. Slaughter, V. Whatley, A. Han, O. C. Nwanyanwu, P. N. Kazembe, H. Dobbie, L. Archibald, and W. R. Jarvis. 2002. Spontaneous cytokine production and its effect on induced production. Clin. Diagn. Lab. Immunol. 9:1049-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood, P. R., L. A. Corner, and P. Plackett. 1990. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res. Vet. Sci. 49:46-49. [PubMed] [Google Scholar]