Abstract
The 42-kDa fragment of the merozoite surface protein 1 (MSP-142) is a leading candidate for the development of a vaccine to control malaria. We previously reported a method for the production of Plasmodium vivax MSP-142 (PvMSP-142) as a soluble protein (S. Dutta, L. W. Ware, A. Barbosa, C. F. Ockenhouse, and D. E. Lanar, Infect. Immun. 69:5464-5470, 2001). We report here a process to manufacture the same PvMSP-142 protein but as an insoluble inclusion body-derived protein which was then refolded in vitro. We compared the immunogenicity and protective efficacy of the soluble and refolded forms of PvMSP-142 protein by using a heterologous but closely related P. cynomolgi-rhesus monkey challenge model. As comparative controls we also expressed, purified, and immunized rhesus with the soluble and refolded forms of the P. cynomolgi MSP-142 (PcMSP-142) proteins. All proteins induced equally high-titer, cross-reacting antibodies. Upon challenge with P. cynomolgi, none of the MSP-142-vaccinated groups demonstrated sterile protection or a delay in the prepatent period. However, following an initial rise in parasitemia, all MSP-1-vaccinated animals had significantly lower parasite burdens as indicated by lower cumulative parasitemia, lower peak parasitemia, lower secondary peak parasitemia, and lower average daily parasitemia compared to the adjuvant control group (P < 0.05). Except the soluble PcMSP-142 group, monkeys in all other groups had fewer numbers of days with parasitemia of >10,000 parasites mm−3. Interestingly, there was no significant difference in the level of partial protection observed in the homologous and heterologous groups in this challenge model. The soluble and refolded forms of PcMSP-142 and PvMSP-142 proteins also appeared to have a similar partially protective effect.
Progress towards a vaccine against Plasmodium falciparum malaria is advancing rapidly, with several candidate antigens being tested for safety and efficacy in humans (4); comparatively, however, the development of a vaccine against P. vivax malaria has lagged behind. Unlike P. falciparum, where sporozoite challenge studies using the chloroquine-sensitive 3D7 strain are available, the relapsing nature of P. vivax hepatic stages and the lack of an in vitro culture system precludes any sporozoite challenge studies of P. vivax vaccine candidates in humans. Therefore, as more recombinant vaccine products become available, there will be an increasing need to compare and down-select vaccine candidate antigens in preclinical studies using animal models of P. vivax infection.
P. cynomolgi, which infects rhesus macaques in southeast Asia, is a closely related species to P. vivax; human transmission of P. cynomolgi has also been reported (25). The two parasites share a similar clinical course of infection (26), a reticulocyte-specific invasion (17), the presence of Schuffner's dots on infected erythrocytes (2), and a dormant liver hypnozoite stage that is responsible for a relapsing blood stage infection (23). P. cynomolgi and P. vivax have similar genomic GC content, and rRNA analysis confirms their close taxonomic relatedness (43). High homology of prime vaccine candidates such as the apical membrane antigen 1, 97% (13); circumsporozoite protein, 90% (16); erythrocyte binding protein, 76% (32); and the 42-kDa fragment of the merozoite surface protein 1 (MSP-1), 75% (31); have been reported. Although it is believed that the P. cynomolgi-rhesus model can serve as a model for testing P. vivax antigens, there is only one previous report where this model was used to test the efficacy of a recombinant P. vivax vaccine (22).
The merozoite surface protein 1 (MSP-1), found on the surface of Plasmodium merozoites, has been a prime vaccine candidate for many years (10). Following its synthesis as a 200-kDa precursor, the MSP-1 molecule undergoes step-wise proteolytic processing resulting in a glycosyl-phosphatidylinositol-anchored 42-kDa protein (MSP-142) on the surface of free merozoites (7). This 42-kDa intermediate undergoes secondary processing at the time of invasion, releasing a 33-kDa soluble polypeptide (MSP-133) and leaving behind on the invading merozoite a glycosyl-phosphatidylinositol-anchored 19-kDa form (MSP-119) (7). Depending on the species, MSP-119 contains 10 or 11 cysteine residues that form five disulfide bonds. Immunization with recombinant MSP-142 and MSP-119 raises antibodies that inhibit invasion of merozoites in vitro (9) and protects the immunized animals against live parasite challenge (29).
We have previously reported a process for the production of correctly folded human vaccine-grade P. vivax MSP-142 protein in the bacterial expression host Escherichia coli Origami(DE3) (14). This bacterial strain has mutations in both the thioredoxin reductase (trxB) and glutathione reductase (gor) genes, which greatly enhances the disulfide bond formation of recombinant proteins expressed in its cytoplasm, hence resulting in a soluble product (6). However, the transposon-mediated genetic modifications of E. coli carried both the tetracycline and the kanamycin resistance genes as selectable markers and therefore made the cells incompatible with the production of human use vaccines that relied on plasmids carrying the Tetr or the Kanr genes. The use of a compatible expression host E. coli BL21(DE3) resulted in the production of an MSP-142 protein that was insoluble and located in the inclusion body fraction of the cells. In this report we have shown that soluble MSP-142 protein can be obtained from the BL21(DE3) cells by in vitro refolding of the inclusion body-derived protein under controlled redox conditions. Both the soluble and refolded P. vivax MSP-142 products appeared to be structurally similar based on biophysical analysis and reactivity with conformational epitope-specific monoclonal antibodies.
It was our goal, therefore, to seek evidence of the immunological equivalence of these proteins in a parasite challenge model. Towards that end we report here the results of a vaccination study comparing the immunogenicity and efficacy of the soluble and refolded P. vivax MSP-142 products in the P. cynomolgi-rhesus model. We have also produced equivalent homologous constructs of P. cynomolgi MSP-142 protein both in their soluble and refolded forms, and these two proteins were also used as positive control immunogens in this study.
MATERIALS AND METHODS
Gene cloning.
P. cynomolgi-infected monkey blood was spotted on an IsoCode Stix (Schleicher and Schuell, Keene, NH), and the genomic DNA was extracted in water by heating the paper spot at 95°C for 30 min. This extract was used as a template to PCR amplify the P. cynomolgi subsp. bastianelli MSP-142 gene (PcMSP-142) using a forward primer 5′CATGCCATGGCAGACCAAGAACAACGGGA3′ and reverse primer 5′AATAGTTTAGCGGCCGCTTAGCTACAGAAAAC3′. The PCR product was directly cloned into pTOPO 2.1 cloning vector (Invitrogen) and sequenced. The sequence has been deposited in GenBank (AY869723). The gene was excised from the pTOPO 2.1 plasmid and cloned into the NcoI-NotI sites of the expression vector pETAT(NK2) (3) in frame with a 6× His tag at the N terminal. The cloning and expression of an equivalent construct carrying the PvMSP-142 gene has been previously reported (14).
Expression and purification of soluble PcMSP-142 and PvMSP-142 proteins.
Recombinant expression plasmids carrying the PcMSP-142 or PvMSP-142 gene were transformed into E. coli Origami(DE3) strain cells (Novagen, San Diego, CA). Expression was carried out in a 10-liter fermentor (New Brunswick Scientific, New Brunswick, NJ) as described previously (14). Protein expression was induced with a final 0.1 mM concentration of isopropyl-β-d-thiogalactopyranoside at 25°C for 2 h, and cells were harvested by centrifugation. A two-step purification protocol comprising a nickel-nitrilotriacetic acid (Ni-NTA) column followed by a Q-Sepharose column was used to purify both soluble PvMSP-142 and soluble PcMSP-142 proteins essentially as described previously (14). The purified proteins were dialyzed against phosphate-buffered saline (PBS) prior to use.
Expression and purification of refolded PcMSP-142 and PvMSP-142 proteins.
The recombinant plasmids carrying the PcMSP-142 or PvMSP-142 gene were transformed into E. coli BL-21(DE3) strain cells (Novagen, San Diego, CA). The fermentation and induction conditions were identical to those described above for soluble protein expression. To allow upscaling of the procedure and evaluation of different lots of material, the amount of all buffers and resins were indexed to a starting cell weight (scw). Cell paste from both PcMSP-142 and PvMSP-142 fermentations were suspended (10 ml g−1 scw) in resuspension buffer (RB; 15 mM sodium phosphate, 450 mM NaCl, pH 7.4). Cells were lysed by microfluidization, and the insoluble inclusion bodies were harvested by centrifugation (12,000 × g, 45 min, 4°C). The pellet was suspended in cold RB (10 ml g−1 scw) using a manual glass tissue homogenizer, and the suspension was centrifuged as before. The washed pellet was resuspended in solubilization buffer (SB; 7 M urea in RB) (6.6 ml g−1 scw). The pellet was suspended by homogenization, incubated for 20 min on a shaking platform at room temperature (RT), and centrifuged (12,000 × g, 45 min, 10°C). A nickel-nitrilotriacetic acid (Ni-NTA) agarose (QIAGEN, Valencia, CA) column (0.6 ml gm−1 scw) was equilibrated with SB, and the cleared supernatant was loaded onto the column. The column was washed with 10 column volumes (CV) of SB containing 20 mM imidazole (pH 7.4), and protein was eluted with 5 CV of SB containing 500 mM imidazole (pH 7.4). The Ni-NTA agarose column-eluted protein was refolded by a rapid 20-fold dilution in refolding buffer (20 mM sodium phosphate, 1 mM reduced glutathione [GSH], 0.05 mM oxidized glutathione [GSSG]; pH 8.0). After refolding (16 h, 22°C) in sealed bottles, the protein was bound to a Q-Sepharose resin column (0.6 ml gm−1 scw) pre-equilibrated with 20 mM sodium phosphate (pH 8.0). The Q-Sepharose resin was washed with 10 CV of 20 mM phosphate buffer (pH 8.0), followed by 5 CV of the same buffer containing 50 mM NaCl (pH 8.0). The PvMSP-142 or PcMSP-142 protein was eluted from the column in 20 mM sodium phosphate and 200 mM NaCl (pH 8.0). The fractions containing the protein were pooled and dialyzed against PBS. The endotoxin content of the final product was estimated by a chromogenic Limulus amebocyte lysate (LAL) endpoint assay (Associates of Cape Cod, Falmouth, MA) following manufacturer's instructions.
Formulation, lyophilization, and storage.
Purified proteins were quantified by optical density at 280 nm measurement, and the concentration was adjusted to a final 333 μg ml−1 in 3.15% sucrose, 5 mM sodium phosphate, 150 mM NaCl, pH 7.0. The protein was dispensed at 1.65 ml per vial, frozen, and lyophilized for 24 h (Flex-Dry MP; FTS System, Stone Ridge, NY). The lyophilized material was stored at −30°C except during transport from WRAIR to Lucknow, India, when they were stored at RT. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of lyophilized preparations of all four MSP-142 proteins indicated that they were stable if stored at −30°C for at least 2 years or at RT for more than 6 months (data not shown).
SDS-PAGE and Western blotting.
Purity of the final products was judged by SDS-PAGE using 4 to 12% Bis-Tris NuPage gels (Invitrogen, Carlsbad, CA) run under reducing and nonreducing conditions. For Western blotting, proteins were electrophoretically transferred to a nitrocellulose membrane followed by blocking with Casein blocker (Pierce, Rockford, IL). The blots were incubated in appropriate dilutions of primary and secondary antibodies for 2 h and then washed with PBST (PBS with 0.05% Tween-20) before development using BM-POD substrate (Roche, Indianapolis, IN). Monoclonal antibodies (MAb) 5.14 and 9.14 and the polyclonal rabbit serum against a PvMSP-119 protein produced in the baculovirus infected insect cell system (30) were used to confirm the correct folding of the products.
Animals.
Rhesus monkeys (Macaca mulatta) were housed in the primate care facility of the Central Drug Research Institute (CDRI), Lucknow, India, and immunization and challenge protocols were approved by the Institutional Animal Ethics Committee of CDRI and the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India. Monkeys were quarantined for 45 days and tested for the absence of common diseases, such as tuberculosis (by skin test and X-ray examination), and blood-borne parasites (by slide exam). Monkeys of either sex were distributed in groups of similar sex ratio and average weight (4.5 to 6.3 kg). Animals in each of the five groups were to receive one of the following preparations: soluble PvMSP-142 protein (PvS), refolded PvMSP-142 (PvR), soluble PcMSP-142 (PcS), refolded PcMSP-142 protein (PcR), or an adjuvant-only control (C).
Immunization schedule and bleeding.
Lyophilized protein in each vial was completely resuspended and dissolved in 1.65 ml of sterile distilled water followed by the addition of 3.85 ml of Montanide ISA720 adjuvant. Vials were vortexed for about 2 min to form thick emulsion, and 1.0 ml of the emulsion containing 100 μg of protein was vaccinated per dose. Control monkeys received 1.0 ml of PBS:Montanide ISA720 (30:70, vol/vol). The skin on the back of the monkeys was shaved, and the vaccine was administered subcutaneously at multiple sites. Three doses of the vaccine were administered at 4-week intervals. Animals were prebled 1 week prior to the first day of immunization and then bled every 2 weeks until the date of challenge. Immunization sites were monitored daily for adverse local reaction. The weights of the animals were recorded at 4-week intervals during the study.
Enzyme-linked immunosorbent assay (ELISA).
ELISA was performed in 96-well plates essentially as described before (41, 21). Briefly, the wells of the microtiter plates were coated with recombinant PvMSP-142 or PcMSP-142 (40 ng/100 μl/well) diluted in PBS by incubating the plates at 37°C for 1 h and then overnight at 4°C. The plates were blocked by incubation with 300 μl of 3% (wt/vol) nonfat dry milk powder (prepared in PBS) at 37°C for 2 h. After three washes with PBST, the plates were incubated with 100 μl of appropriately diluted antibodies (diluted in 1% milk-PBS) for 2 h at 37°C. The plates were washed thrice with PBST followed by 1.5 h of incubation with peroxidase-conjugated anti-monkey immunoglobulin G (1:2,000 diluted in 1% milk-PBS; Sigma). After washing the plates with PBST, the color was developed by adding the substrate solution (1 mg/ml O-phenylenediamine in citrate-phosphate buffer, pH 5.0, containing 1 μl/ml H2O2). The reaction was stopped after 10 min by adding 5 N H2SO4, and the absorbance was read at 490 nm using a Molecular Devices Spectromax Plus UVmax microplate ELISA reader.
Challenge and parasitemia records.
The monkeys in all five groups were challenged with 1 × 104 P. cynomolgi blood stage parasites 4 weeks after the last injection. The Giemsa-stained thick and thin blood smears prepared from individual monkeys were observed under a microscope using a 100× objective. The number of parasites was counted initially from thick smears and subsequently from thin smears and expressed in terms of parasites per cubed millimeter. The blood smears were prepared once daily from day 4 onwards until day 45. After 45 days, the monkeys were cured with chloroquine (34).
Statistical analysis.
Group averages were compared by Fischer's least significant difference method (38). Pearson's coefficient of correlation (R2) was calculated by linear regression.
Nucleotide sequence accession number. The sequence determined in the course of this work has been deposited in GenBank under accession no. AY869723.
RESULTS
Sequence analysis of the PcMSP-142 gene.
The 380-amino-acid-long P. cynomolgi subsp. bastianelli MSP-142 protein had 17 residue differences from the 379-amino-acid-long P. cynomolgi subsp. ceylonensis MSP-142 protein (95% similarity) (31). All of the 17 differences occurred in the MSP-133 portion of the gene, the MSP-119 portion being fully conserved. The P. cynomolgi subsp. bastianelli MSP-142 sequence was the same size (380 residues) as the P. vivax SalI MSP-142 sequence, and the two proteins shared 77% sequence similarity (18). Only 11 of the 86 differences occurred in the C-terminal MSP-119 portion. The 11 cysteine residues of PcMSP-142 were positionally conserved in PvMSP-142.
Expression and purification of recombinant proteins.
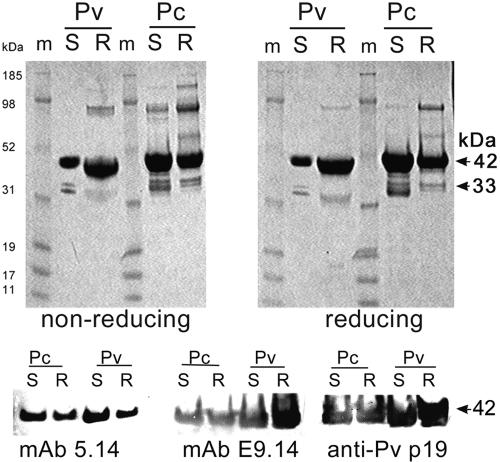
In the E. coli host strain Origami(DE3), both PvMSP-142 and PcMSP-142 proteins were produced as soluble molecules, and both antigens were purified to homogeneity using an identical two-step purification process. In the E. coli BL21(DE3) host, both PvMSP-142 and PcMSP-142 proteins were localized in the inclusion body fraction. The insoluble proteins were obtained by isolation of the inclusion bodies, resuspension in urea, partial purification on an Ni-NTA column, and refolding using a GSH-GSSH redox couple solution to gain optimal reactivity to a panel of conformation-specific monoclonal antibodies. The ratio of concentration of GSH to GSSG used during the in vitro refolding step was optimized for PvMSP-142 refolding using a checker board of concentration ratios and the reactivity to conformational monoclonal antibodies as an indicator of correct folding (data not shown). The refolded PvMSP-142 and PcMSP-142 proteins were further purified by ion exchange on a Q-Sepharose resin. Figure 1 (top panel) shows the SDS-PAGE analysis of all four MSP-142 products. A major band ∼45 to 50 kDa represented the MSP-142 monomer. A ∼33-kDa doublet represented a C-terminal degradation product of MSP-142 as confirmed by N-terminal sequencing and by Western blot analysis using antibodies against the N-terminal 6× His tag. A minor band at ∼90 kDa on the gels corresponded to the dimer of MSP-142. The combined purity of MSP-1-specific bands in all four products exceeded >99% by laser densitometry. The major band in each of the four lanes migrated as a discreet band under reducing and nonreducing conditions, indicating conformational homogeneity of the disulfide-bonded products. The residual endotoxin content of all four protein preparations was <50 EU/100-μg dose. The soluble and refolded PvMSP-1 and PcMSP-1 proteins reacted on a Western blot with PvMSP-119-specific conformational MAbs 5.14 and E9.14 and with a rabbit polyclonal serum against PvMSP-119 (Fig. 1, bottom panel).
FIG. 1.
Top panel, Coomassie blue-stained SDS-PAGE gel showing ∼5 μg of the soluble (S) and refolded (R) forms of the P. vivax (Pv) and P. cynomolgi (Pc) MSP-142 products under nonreducing and reducing conditions. The MSP-1-specific bands at 42 and 33 kDa are indicated by arrows. Bottom panel, Western blot reactivity of the four proteins with conformation dependent PvMSP-1-specific mouse MAbs 5.14 and E9.14 and with a rabbit polyclonal serum against PvMSP-119. m, molecular mass marker lanes.
Immunogenicity of PvMSP-142 and PcMSP-142 proteins.
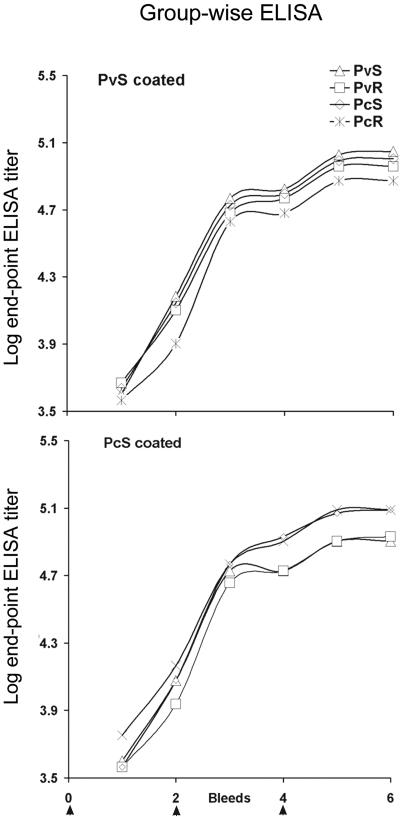
The E. coli-produced MSP-142 proteins emulsified in Montanide ISA720 showed no significant local adverse reaction at the site of vaccination. The weight of the animals was similar to others housed in the same animal care facility during the period of the trial. One of the animals (no. 9539) receiving PvS protein died after the last dose of vaccine but before challenge. After physical and necropsy examination it was determined that the cause of death was due to a respiratory infection not associated with the vaccination. For all animals the antibody end-point titers in the six bleeds collected at 2-week intervals are shown in Fig. 2. Table 1 shows the endpoint titer (×1,000) at the time of challenge. The adjuvant control monkeys had no detectable antibodies after three immunizations (not plotted). All animals immunized with MSP-142 seroconverted following the first vaccination, and the antibody titers were boosted following the second and third immunizations (indicated by arrows in Fig. 2). The PcMSP-142- and PvMSP-142-specific antibodies cross-reacted on ELISA with the heterologous MSP-142 protein. There was no statistically significant difference in the mean end-point titers among all four groups tested with either soluble PcMSP-142 or PvMSP-142 coated on plates. No significant difference was found between the mean titer of PcS or PvS groups when compared to PcR or the PvR groups, respectively, or when the PvS group was compared to PcS group and PvR group compared to PcR group.
FIG. 2.
Group wise mean of the log ELISA titers (y axis) at each of the six bleeds (x axis). The immunization events are denoted by arrows at the bottom. Bleeds were collected at 2-week intervals, and immunizations were administered at 4-week intervals (arrows).
TABLE 1.
Endpoint titers at the time of challenge
| Group | Animal no. | Wt change (kg) | ELISA titer (103)
|
No. of parasites/mm3
|
Day of onset of parasitemia | No. of days of parasitemia of >10.000/mm3 | |||
|---|---|---|---|---|---|---|---|---|---|
| PvS | PcS | Peak | Secondary | Avg | |||||
| Control | 9540 | −0.2 | 0 | 0 | 138,700 | 118,125 | 31,476 | 7 | 15 |
| 9550 | 0 | 0 | 0 | 156,400 | 101,270 | 30,261 | 6 | 16 | |
| 9551 | −0.4 | 0 | 0 | 153,750 | 94,710 | 18,765 | 7 | 9 | |
| 9557 | 0.7 | 0 | 0 | 88,472 | 45,450 | 14,366 | 6 | 11 | |
| 9562 | 0 | 0 | 0 | 86,050 | 46,840 | 14,198 | 6 | 12 | |
| 9563 | 0.5 | 0 | 0 | 97,376 | 42,284 | 14,296 | 7 | 13 | |
| PcS | 9537 | 0.2 | 128 | 256 | 65,410 | 43,065 | 12,719 | 6 | 11 |
| 9541 | −0.1 | 64 | 128 | 62,090 | 25,344 | 11,680 | 6 | 11 | |
| 9546 | 0.1 | 64 | 128 | 49,032 | 22,275 | 8,589 | 8 | 10 | |
| 9554 | 0.1 | 128 | 64 | 20,025 | 12,727 | 4,632 | 7 | 9 | |
| 9559 | 1.3 | 96 | 128 | 56,730 | 50,799 | 11,097 | 7 | 10 | |
| 9565 | 0.2 | 64 | 256 | 50,556 | 34,692 | 11,691 | 7 | 12 | |
| PcR | 9548 | 0 | 128 | 128 | 28,275 | 24,576 | 6,489 | 7 | 9 |
| 9549 | 0 | 64 | 128 | 52,011 | 41,185 | 11,400 | 6 | 11 | |
| 9556 | 0.5 | 64 | 64 | 51,954 | 35,084 | 9,970 | 6 | 10 | |
| 9558 | 0 | 64 | 128 | 55,472 | 29,900 | 10,771 | 7 | 11 | |
| 9560 | −0.1 | 128 | 128 | 19,669 | 12,994 | 3,686 | 7 | 7 | |
| 9564 | 0.1 | 64 | 128 | 15,288 | 3,913 | 3,640 | 7 | 6 | |
| PvS | 9535 | −0.3 | 128 | 64 | 12,702 | 12,502 | 2,437 | 7 | 3 |
| 9538 | −0.5 | 128 | 128 | 20,114 | 8,544 | 3,776 | 7 | 4 | |
| 9543 | 0.3 | 128 | 128 | 56,100 | 18,810 | 8,284 | 6 | 9 | |
| 9555 | −0.2 | 64 | 64 | 53,748 | 34,532 | 9,366 | 6 | 10 | |
| 9561 | −0.1 | 128 | 128 | 13,884 | 5,785 | 2,381 | 7 | 2 | |
| 9539a | 0.1 | 96 | 96 | ||||||
| PvR | 9536 | 0.1 | 64 | 64 | 51,707 | 30,422 | 10,821 | 6 | 13 |
| 9544 | 0.2 | 128 | 128 | 40,050 | 28,925 | 8,330 | 6 | 11 | |
| 9545 | −0.4 | 128 | 128 | 58,560 | 15,132 | 9,329 | 7 | 7 | |
| 9547 | −0.7 | 128 | 128 | 51,185 | 31,684 | 9,281 | 7 | 8 | |
| 9552 | 0.5 | 128 | 64 | 61,040 | 49,824 | 12,952 | 7 | 11 | |
| 9553 | 0.3 | 128 | 128 | 31,239 | 21,850 | 6,792 | 7 | 8 | |
Animal no. 9539 died prior to challenge.
Efficacy of PcMSP-142 and PvMSP-142 vaccine.
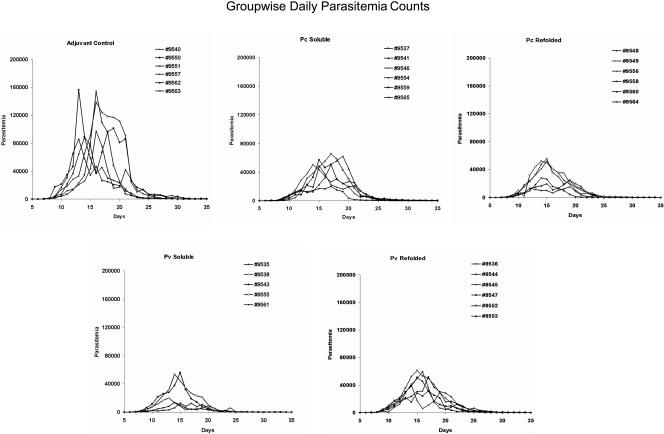
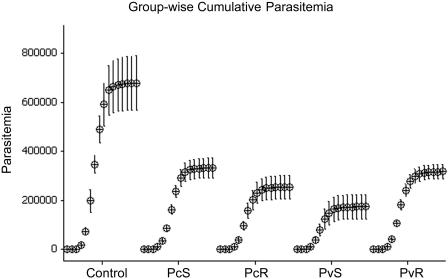
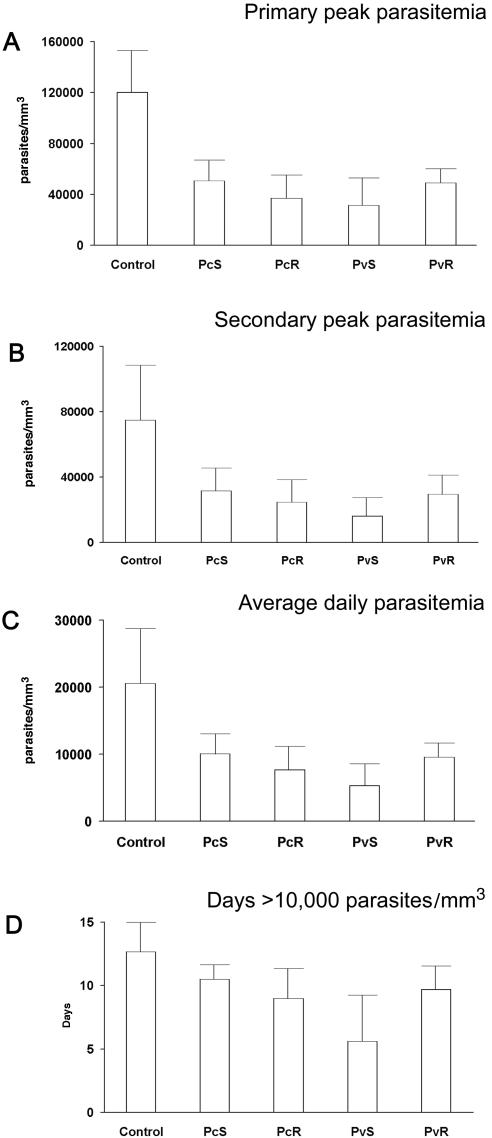
All the animals were challenged with blood stages of P. cynomolgi subsp. bastianelli 4 weeks following the last immunization. The MSP-142-Montanide ISA720 combination failed to induce a sterile protective response, and likewise no delay in patency was observed in any of the vaccinated groups (Fig. 3, Table 1). However, the magnitude and duration of the parasitemia profile of the individual monkeys was significantly affected by the vaccines (Fig. 3). Animals in the control and test groups showed a similar initial rise in parasitemia between days 8 to 10; however, after day 10, all four vaccinated groups were able to control parasite development as demonstrated by the lower rate of increase in parasitemia (Fig. 3). The mean group-wise cumulative parasitemia on day 35 in all MSP-142-vaccinated groups was lower than the adjuvant control group (P < 0.05) (Fig. 4). The group of monkeys receiving soluble PvMSP-142 protein (PvS group) demonstrated the strongest control over parasite growth (P = 0.009) when compared to the control group. The coefficients of correlation (R2) between mean cumulative parasitemia on day 35 and the anti-PvS and anti-PcS ELISA titers were 0.514 and 0.229, respectively. Using a typical bimodal parasitemia curve of P. cynomolgi subsp. bastianelli in the rhesus monkey model (e.g., monkey no. 9550 in Fig. 3), we were able to establish four parameters to represent the parasite burden in each group: (A) primary peak parasitemia, (B) secondary peak parasitemia, (C) average daily parasitemia, and (D) number of days with parasitemia of >10,000 parasites mm−3.
FIG. 3.
Daily parasitemia records of individual monkeys (parasites mm−3, y axis) plotted against days following the day of challenge (x axis). Groups shown from left to right in the top row are adjuvant control, P. cynomolgi MSP-142 soluble, and P. cynomolgi MSP-142 refolded; the bottom row shows P. vivax MSP-142 soluble and P. vivax MSP-142 refolded.
FIG. 4.
Group-wise average cumulative parasitemia (parasites mm−3) of the 35-day follow-up was plotted for all immunized groups. Only odd-day parasitemias were plotted, and the bars represent the standard errors calculated from the means.
Primary peak parasitemia.
All four MSP-142-vaccinated groups had significantly lower primary peak parasitemia compared to the adjuvant control group (analysis done on log-transformed data, P < 0.05) (Fig. 5A). The peak parasitemia in the PvS group was lower than the PvR and PcS groups (P < 0.05); however, it was notable that the PvS group had five instead of six animals. The coefficient of correlation (R2) of the primary peak parasitemia with a PvS-specific ELISA titer was 0.599, and a PcS-specific ELISA was 0.320. The secondary peak parasitemia is the second highest peak in the parasitemia profile (excluding the parasitemia on the day and day + 1 on which primary peak parasitemia was observed). All four MSP-142-vaccinated groups had significantly lower secondary peak parasitemia compared to the control group (P < 0.05). The PvS group had significantly lower secondary peak parasitemia compared to the PcS group (P < 0.05), the other MSP-142 vaccinated groups being similar (Fig. 5B). The R2 with PvS- and PcS-specific ELISAs were 0.454 and 0.260, respectively. All four MSP-142 immunized groups had significantly lower average daily parasitemias compared to the control group (P < 0.05). As in case of the primary peak parasitemia, the average daily parasitemia was also lower in the PvS group compared to PvR and PcS groups (P < 0.05) (Fig. 5C). The R2 with PvS- and PcS-specific ELISAs were 0.514 and 0.229, respectively. The number of days when parasitemia above 10,000 mm−3 was recorded was lower in the PvS, PvR, and PcR groups compared to the controls (P < 0.05). The PvS group had the smallest number of days with this cutoff parasitemia compared to other MSP-142-vaccinated groups (Fig. 5D). The R2 with PvS- and PcS-specific ELISA were 0.395 and 0.080, respectively. Based on the above analysis, all four MSP-142 vaccines appeared to significantly reduce the parasite burden in this challenge model, with the PvS group showing the lowest parasite burden.
FIG. 5.
Parameters chosen to represent parasite burden (open bars). Group-wise mean of primary peak parasitemia (A); secondary peak parasitemia (B); average daily parasitemia (C); number of days when >10,000 parasites mm−3 were recorded (D). Standard deviation within group is plotted as a line above each bar.
DISCUSSION
Correct folding, purity, and yield of the final product are important criteria for the process development of a vaccine antigen. The soluble and refolded forms of PvMSP-142 possess important elements of correct structure as evidenced by the presence of disulfide bond-stabilized conformational epitopes. Both products also had comparable final yields (8 to 10 mg/liter) and purity (>99%). The process for the production of soluble PvMSP-142 would be easier to scale up because it does not require a refolding step. On the other hand, we have obtained highly purified preparations of the refolded PvMSP-142 product by directly refolding washed inclusion bodies, bypassing the Ni-NTA chromatography; this would make the refolded PvMSP-142 more cost-effective to manufacture compared to the soluble PvMSP-142. In the present study we took advantage of the close phylogenic relationship between P. cynomolgi and P. vivax and used the P. cynomolgi-rhesus model to compare the immunogenicity and efficacy of the two P. vivax MSP-142 products. It was encouraging to determine that the soluble and the refolded PvMSP-142 products showed similar immunogenicity and efficacy in this model. Immunization of monkeys in both the homologous and heterologous challenge groups showed similar reduction in parasite burden compared to the adjuvant control groups. Between the two P. cynomolgi MSP-142-immunized groups the refolded protein group appeared to be marginally better at lowering the parasitemia, while between the P. vivax antigens the soluble product appeared to induce a slightly better immune and protective response. Overall, the P. vivax soluble protein appeared to be the best antigen in this trial, although a statistical bias resulting from five instead of six animals in the group cannot be ruled out.
A similar level of protection induced by both homologous and heterologous MSP-142 antigens was not surprising given their immunological and structural relatedness. Monoclonal and polyclonal antibodies against several antigens of P. cynomolgi and P. vivax have been shown to be cross-reactive (20, 46), and infection induced by P. cynomolgi sporozoite injection protects humans against a P. vivax challenge (39). Mouse monoclonal and naturally acquired human antibodies against PvMSP-1 also cross-react with a baculovirus recombinant PcMSP-1 protein; in that study deglycosylation of the MSP-1 product improved the cross-reactivity of antibodies (19). Since E. coli-produced PvMSP-142 was naturally nonglycosylated, it was an attractive alternative to employ the P. cynomolgi-rhesus model for the purpose of evaluating the protective efficacy of P. vivax MSP-142 products. The E. coli-produced PvMSP-142- and PcMSP-142-induced cross-reacting antibodies and vaccination with PvMSP-142 induced a similar level of partial protection against P. cynomolgi parasites, as seen in the homologous vaccination groups. While there are few reports of cross-species protection in malaria, the results of this trial suggest the importance of this model for evaluating immune responses to other conserved vaccine candidates of P. vivax.
The protective response to MSP-1 has been shown to be antibody mediated using in vitro invasion inhibition and passive transfer experiments (44). Within the 42-kDa MSP-1, protective epitopes have been mapped to the two epidermal growth factor (EGF)-like domains in P. falciparum MSP-119 (1). Data presented here confirmed a positive correlation between PvMSP-142- and PcMSP-142-specific antibody titers and parameters chosen to indicate parasite burden. Analysis of the amino acid differences between PcMSP-142 and PvMSP-142 might reveal some clues toward the cross-species protection and indicate the importance of equivalent amino acid positions for the binding of invasion inhibitory antibodies. Out of the 86 amino acid differences between PvMSP-142 and PcMSP-142, 11 differences occur in the EGF domains containing the MSP-119 region. Previous studies have used binding of inhibitory and noninhibitory P. falciparum and P. yoelii MSP-119-specific MAbs to PfMSP-1 or PyMSP-119 protein constructs to identify amino acid positions on MSP-119 that are critical for the binding of invasion inhibitory antibodies. In an attempt to understand the cross-species protection observed in this trial, we analyzed each of the 11 amino acid differences between PvMSP-119 and PcMSP-119 in light of the results from previous studies with P. falciparum and P. yoelii MSP-1. The following amino acid differences were seen between PcMSP-119 and PvMSP-119: R6-T, E14-D, Y33-T, D37-E, A38-G, P45-S, M47-V, K52-N, N65-T, K67-S, and E69-K, respectively (amino acids were numbered as reported with the crystal structure of P. cynomolgi MSP-119 [8]). The differences at amino acid positions 14, 37, 38, and 47 are side chain conservative changes. A nonconservative change in P. falciparum MSP-1 at the position corresponding to R6 has been shown to have no effect on the binding of P. falciparum MSP-1-specific inhibitory MAbs 12.8, 12.10, and 5B1 (40). The E14 corresponding position in PyMSP-119 has been shown to be critical for the binding of inhibitory PyMSP-119 MAbs F5 and B6 (5); this position also represents one of the four amino acid polymorphic sites in MSP-119 of P. falciparum. Since the challenge with the P. falciparum FVO allele results in higher protection in the FVO-vaccinated Aotus monkeys compared to the 3D7 MSP-119-vaccinated group (24), we hypothesize that a conserved difference at the E14 position between PcMSP-119 and PvMSP-119 was critical for allowing cross-species protection seen in this study. The A38 corresponding residue in PyMSP-119 is G; an inhibitory MAb, G3, failed to bind PyMSP-119 from isolates that had a nonconservative change, N, at this position (5). We speculate that a conserved amino acid difference, A38-G, at this position may also be critical for allowing cross-protection in this study. The radical charge difference K67-S between PcMSP-119 and PvMSP-119 was similar to the R-G difference seen between the 3D7 and FVO alleles of P. falciparum. The lack of species specificity observed in this trial, despite the presence of a nonconserved change at this position, suggests that allele-specific protective response between the 3D7 and FVO strains of PfMSP-1 (24) may not be a result of a radical substitution at this position. The difference E69-K between PcMSP-119 and PvMSP-119 is also the only known polymorphism reported in the field isolates of PvMSP-119 (35); based on the observations presented here, we suggest that this allelic polymorphism in the field isolates of P. vivax may be immunologically silent. Residues corresponding to all other observed differences between PvMSP-119 and PcMSP-119 were not reported to be targets of inhibitory PfMSP-1 or PyMSP-1 MAbs in the published literature. The three-dimensional crystal structure of PcMSP19 shows that all but one amino acid difference between PcMSP119 and PvMSP119 are concentrated at one end within the EGF domain, and, interestingly, the residues shown to be targets of PfMSP119 inhibitory MAbs 12.8 and 12.10 are not located at the same end of the molecule (8, 40). While the above analysis is an indirect approach towards understanding the lack of species specificity observed in this trial, direct experiments confirming these inferences are required to tie this data to the existing views on the structure-function of MSP-119.
The adjuvant Montanide ISA720 has been used in several human vaccine trials (42, 27). PvMSP-142-Montanide ISA720 adjuvant combination has been shown to induce both B- and T-cell responses in mice (14, 36). We have also reported the induction of high-titer invasion inhibitory antibodies to Montanide ISA720 formulated Apical Membrane Antigen-1 of P. falciparum (15). Encouraged by these findings, we chose to proceed with Montanide ISA720 for the purpose of this trial. As expected, high levels of MSP-1-specific antibodies were induced in all the animals in each of the four MSP-142-vaccinated groups. This immune response, while not resulting in sterile protection, did significantly lower parasitemia in both the homologous and heterologous challenge groups compared to the adjuvant control group. Immunization with MSP-1 along with immunostimulators permissible for human use are known to induce a protective response in the rodent models of malaria (28). MSP-1-induced protection in the new world monkey model has, however, been shown to require the use of Freunds Complete Adjuvant (FCA) (24, 12, 37). Indeed, a high degree of sterile protection has been previously reported using baculovirus-produced P. cynomolgi MSP-142; however, that vaccine was also formulated with FCA (33). The results of this study clearly indicate the ability of MSP-142 with Montanide ISA720 to induce a partially protective immune response in primates using an adjuvant acceptable for human use instead of FCA. Similar partial protection has been reported in two other challenge trials using PvMSP-119 and a nonionic block copolymer in the splenetomized Saimiri monkey model against P. vivax challenge (45, 11).
In summary, we report that E. coli-derived soluble and refolded MSP-142 antigens of P. vivax and P. cynomolgi produced using scalable production processes had comparable immunogenicity in rhesus monkeys. We also show that both P. cynomolgi and P. vivax MSP-142 protein vaccination using Montanide ISA720 adjuvant can induce a partially protective response against a P. cynomolgi challenge. A vaccination induced reduction in parasite burden similar to that reported in this study may be sufficient in reducing mortality in the endemic areas while still not impeding the development of age-related natural immunity against malaria. An Origami strain of E. coli that is sensitive to kanamycin has recently become available. With the knowledge that the soluble and refolded products are immunologically equivalent, we are in the process of comparing the reproducibility of the production process, cost of manufacture, and quality of the final product as criteria to down-select and advance into GMP production of a PvMSP-142-based vaccine.
Acknowledgments
This study was funded by grants no. 990352 and no. 990339 from the WHO to D.C.K. and D.E.L.
We thank C. M. Gupta, Director, CDRI, for his support in this international collaborative effort. We thank Shirley Longacre, Pasteur Institute, France, for P. vivax-specific monoclonal and polyclonal antibodies, and Evelina Angov from WRAIR for the expression plasmid used in the study and Jeff Lyon and COL Chris Ockenhouse and for their critical suggestions. We thank Craig Morrissette, WRAIR, for statistical analysis.
The views expressed here are those of the authors and should not be construed to represent those of the U.S. Department of the Army or the U.S. Department of Defense.
Editor: W. A. Petri, Jr.
Footnotes
CDRI communication no. 6711.
REFERENCES
- 1.Ahlborg, N., I. T. Ling, W. Howard, A. A. Holder, and E. M. Riley. 2002. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infect. Immun. 70:820-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aikawa, M., L. H. Miller, and J. Rabbege. 1975. Caveola-vesicle complexes in the plasmalemma of erythrocytes infected by Plasmodium vivax and P. cynomolgi. Unique structures related to Schuffner's dots. Am. J. Pathol. 79:285-300. [PMC free article] [PubMed] [Google Scholar]
- 3.Angov, E., B. M. Aufiero, A. M. Turgeon, M. Van Handenhove, C. F. Ockenhouse, K. E. Kester, D. S. Walsh, J. S. McBride, M. C. Dubois, J. Cohen, J. D. Haynes, K. H. Eckels, D. G. Heppner, W. R. Ballou, C. L. Diggs, and J. A. Lyon. 2003. Development and pre-clinical analysis of a Plasmodium falciparum merozoite surface protein-1(42) malaria vaccine. Mol. Biochem. Parasitol. 128:195-204. [DOI] [PubMed] [Google Scholar]
- 4.Ballou, W. R., M. Arevalo-Herrera, D. Carucci, T. L. Richie, G. Corradin, C. L. Diggs, P. Druilhe, B. K. Giersing, A. Saul, D. G. Heppner, K. E. Kester, D. E. Lanar, J. Lyon, A. V. Hill, W. Pan, and J. D. Cohen. 2004. Update on the clinical development of candidate malaria vaccines. Am. J. Trop. Med. Hyg. 71(Suppl. 2):239-247. [PubMed] [Google Scholar]
- 5.Benjamin, P. A., I. T. Ling, G. Clottey, L. M. Valero, S. A. Ogun, S. L. Fleck, D. Walliker, W. D. Morgan, B. Birdsall, J. Feeney, and A. A. Holder. 1999. Antigenic and sequence diversity at the C-terminus of the merozoite surface protein-1 from rodent malaria isolates, and the binding of protective monoclonal antibodies. Mol. Biochem. Parasitol. 104:147-156. [DOI] [PubMed] [Google Scholar]
- 6.Bessette, P. H., F. Aslund, J. Beckwith, and G. Georgiou. 1999. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc. Natl. Acad. Sci. USA 96:13703-13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackman, M. J., I. T. Ling, S. C. Nicholls, and A. A. Holder. 1991. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 49:29-33. [DOI] [PubMed] [Google Scholar]
- 8.Chitarra, V., I. Holm, G. A. Bentley, S. Petres, and S. Longacre. 1999. The crystal structure of C-terminal merozoite surface protein 1 at 1.8 A resolution, a highly protective malaria vaccine candidate. Mol. Cell 3:457-464. [DOI] [PubMed] [Google Scholar]
- 9.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, P. J. Barr, B. T. Yokota, and G. S. Hut. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitnis, C. E., and M. J. Blackman. 2000. Host cell invasion by malaria parasites. Parasitol. Today 16:411-415. [DOI] [PubMed] [Google Scholar]
- 11.Collins, W. E., D. C. Kaslow, J. S. Sullivan, C. L. Morris, G. G. Galland, C. Yang, A. M. Saekhou, L. Xiao, and A. A. Lal. 1999. Testing the efficacy of a recombinant merozoite surface protein MSP-1(19) of Plasmodium vivax in Saimiri boliviensis monkeys. Am. J. Trop. Med. Hyg. 60:350-356. [DOI] [PubMed] [Google Scholar]
- 12.Darko, C. A., E. Angov, W. E. Collins, E. S. Bergmann-Leitner, A. S. Girouard, S. L. Hitt, J. S. McBride, C. L. Diggs, A. A. Holder, C. A. Long, J. W. Barnwell, and J. A. Lyon. 2005. The clinical-grade 42-kilodalton fragment of merozoite surface protein 1 of Plasmodium falciparum strain FVO expressed in Escherichia coli protects Aotus nancymai against challenge with homologous erythrocytic-stage parasites. Infect. Immun. 73:287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta, S., P. Malhotra, and V. S. Chauhan. 1995. Sequence analysis of apical membrane antigen 1 (AMA-1) of Plasmodium cynomolgi bastianelli. Mol. Biochem. Parasitol. 73:267-270. [DOI] [PubMed] [Google Scholar]
- 14.Dutta, S., L. W. Ware, A. Barbosa, C. F. Ockenhouse, and D. E. Lanar. 2001. Purification, characterization, and immunogenicity of a disulfide cross-linked Plasmodium vivax vaccine candidate antigen, merozoite surface protein 1, expressed in Escherichia coli. Infect. Immun. 69:5464-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta, S., P. V. Lalitha, L. A. Ware, A. Barbosa, J. K. Moch, M. A. Vassell, B. B. Fileta, S. Kitov, N. Kolodny, D. G. Heppner, J. D. Haynes, and D. E. Lanar. 2002. Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli. Infect. Immun. 70:3101-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galinski, M. R., D. E. Arnot, A. H. Cochrane, J. W. Barnwell, R. S. Nussenzweig, and V. Enea. 1987. The circumsporozoite gene of the Plasmodium cynomolgi complex. Cell 48:311-319. [DOI] [PubMed] [Google Scholar]
- 17.Galinski, M. R., C. C. Medina, P. Ingravallo, and J. W. Barnwell. 1992. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell 69:1213-1226. [DOI] [PubMed] [Google Scholar]
- 18.Gibson, H. L., J. E. Tucker, D. C. Kaslow, A. U. Krettli, W. E. Collins, M. C. Kiefer, I. C. Bathurst, and P. J. Barr. 1992. Structure and expression of the gene for Pv200, a major blood-stage surface antigen of Plasmodium vivax. Mol. Biochem. Parasitol. 50:325-333. [DOI] [PubMed] [Google Scholar]
- 19.Holm, I., F. Nato, K. N. Mendis, S. Longacre. 1997. Characterization of C-terminal merozoite surface protein-1 baculovirus recombinant proteins from Plasmodium vivax and Plasmodium cynomolgi as recognized by the natural anti-parasite immune response. Mol. Biochem. Parasitol. 89:313-319. [DOI] [PubMed] [Google Scholar]
- 20.Kamboj, K. K., J. W. Barnwell, R. S. Nussenzweig, and A. H. Cochrane. 1988. Characterization of cross-reactive blood-stage antigens of the Plasmodium cynomolgi complex using anti-Plasmodium vivax monoclonal antibodies. J. Parasitol. 74:403-408. [PubMed] [Google Scholar]
- 21.Kaushal, D. C., and N. A. Kaushal. 2002. Diagnosis of malaria by detection of plasmodial lactate dehydrogenase with an immunodot enzyme assay. Immunol. Investig. 31:93-106. [DOI] [PubMed] [Google Scholar]
- 22.Kocken, C. H., M. A. Dubbeld, A. Van Der Wel, J. T. Pronk, A. P. Waters, J. A. Langermans, and A. W. Thomas. 1999. High-level expression of Plasmodium vivax apical membrane antigen 1 (AMA-1) in Pichia pastoris: strong immunogenicity in Macaca mulatta immunized with P. vivax AMA-1 and adjuvant SBAS2. Infect. Immun. 67:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krotoski, W. A., W. E. Collins, R. S. Bray, P. C. Garnham, F. B. Cogswell, R. W. Gwadz, R. Killick-Kendrick, R. Wolf, R. Sinden, L. C. Koontz, and P. S. Stanfill. 1982. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am. J. Trop. Med. Hyg. 31:1291-1293. [DOI] [PubMed] [Google Scholar]
- 24.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Guevara Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuvin, S. F., H. K. Beye, F. Stohlman, Jr., P. G. Contacos, and G. R. Coatney. 1963. Malaria in man. Infection by Plasmodium vivax and the B strain of Plasmodium cynomolgi. JAMA 184:1018-1020. [DOI] [PubMed] [Google Scholar]
- 26.Kuvin, S. F., H. K. Beye, F. Stohlman Jr., P. G. Contacos, and G. R. Coatney. 1962. Clinical and physiological responses in sporozoite-induced B strain Plasmodium cynomolgi and Plasmodium vivax infections in normal volunteers. Trans. R Soc. Trop. Med. Hyg. 56:371-378. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence, G., Q. Q. Cheng, C. Reed, D. Taylor, A. Stowers, N. Cloonan, C. Rzepczyk, A. Smillie, K. Anderson, D. Pombo, A. Allworth, D. Eisen, R. Anders, and A. Saul. 2000. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine 18:1925-1931. [DOI] [PubMed] [Google Scholar]
- 28.Ling, I. T., S. A. Ogun, P. Momin, R. L. Richards, N. Garcon, J. Cohen, W. R. Ballou, and A. A. Holder. 1997. Immunization against the murine malaria parasite Plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine 15:1562-1567. [DOI] [PubMed] [Google Scholar]
- 29.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasite Immunol 16:63-67. [DOI] [PubMed] [Google Scholar]
- 30.Longacre, S., K. N. Mendis, and P. H. David. 1994. Plasmodium vivax merozoite surface protein 1 C-terminal recombinant proteins in baculovirus. Mol. Biochem. Parasitol. 64:191-205. [DOI] [PubMed] [Google Scholar]
- 31.Longacre, S. 1995. The Plasmodium cynomolgi merozoite surface protein 1 C-terminal sequence and its homologies with other Plasmodium species. Mol. Biochem. Parasitol. 74:105-111. [DOI] [PubMed] [Google Scholar]
- 32.Okenu, D. M., P. Malhotra, P. V. Lalitha, C. E. Chitnis, and V. S. Chauhan. 1997. Cloning and sequence analysis of a gene encoding an erythrocyte binding protein from Plasmodium cynomolgi. Mol. Biochem. Parasitol. 89:301-306. [DOI] [PubMed] [Google Scholar]
- 33.Perera, K. L., S. M. Handunnetti, I. Holm, S. Longacre, and K. Mendis. 1998. Baculovirus merozoite surface protein 1 C-terminal recombinant antigens are highly protective in a natural primate model for human Plasmodium vivax malaria. Infect. Immun. 66:1500-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puri, S. K., K. K. Kamboj, and G. P. Dutta. 1989. Infectivity studies on Anopheles stephensi using P. cynomolgi B infection in rhesus monkeys. Trop. Med. Parasitol. 40:409-411. [PubMed] [Google Scholar]
- 35.Putaporntip, C., S. Jongwutiwes, N. Sakihama, M. U. Ferreira, W. G. Kho, A. Kaneko, H. Kanbara, T. Hattori, and K. Tanabe. 2002. Mosaic organization and heterogeneity in frequency of allelic recombination of the Plasmodium vivax merozoite surface protein-1 locus. Proc. Natl. Acad. Sci. USA 99:16348-16353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachdeva, S., G. Ahmad, P. Malhotra, P. Mukherjee, and V. S. Chauhan. 2004. Comparison of immunogenicities of recombinant Plasmodium vivax merozoite surface protein 1 19- and 42-kilodalton fragments expressed in Escherichia coli. Infect. Immun. 72:5775-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh, S., M. C. Kennedy, C. A. Long, A. J. Saul, L. H. Miller, and A. W. Stowers. 2003. Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1. Infect. Immun. 71:6766-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snedecor, G. W., and W. G. Cochran. 1980. Statistical methods, p. 234-245. Iowa State University Press, Ames, Iowa.
- 39.Tobie, J. E., S. M. Wolff, and G. M. Jeffery. 1966. Immune response of man to inoculation with Plasmodium cynomolgi and challenge with P. vivax. Lancet ii:300-302. [DOI] [PubMed] [Google Scholar]
- 40.Uthaipibull, C., B. Aufiero, S. E. Syed, B. Hansen, J. A. Guevara Patino, E. Angov, I. T. Ling, K. Fegeding, W. D. Morgan, C. Ockenhouse, B. Birdsall, J. Feeney, J. A. Lyon, and A. A. Holder. 2001. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307:1381-1394. [DOI] [PubMed] [Google Scholar]
- 41.Voller, A., D. E. Bidwell, and A. Bartlett. 1976. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull. W.H.O. 53:55-65. [PMC free article] [PubMed] [Google Scholar]
- 42.Walther, M., S. Dunachie, S. M. Keating, J. Vuola, T. Berthoud, A. Schmidt, C. Maier, L. F. Andrews, R. Andersen, S. Gilbert, I. Poulton, D. Webster, F. Dubovsky, E. Tierney, P. Sarpotdar, S. Correa, A. Huntcooke, G. Butcher, J. E. Williams, R. B. Sinden, G. Thornton, and A. V. S. Hill. 2005. A. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine 23:857-864. [DOI] [PubMed] [Google Scholar]
- 43.Waters, A. P., D. G. Higgins, and T. F. McCutchan. 1993. Evolutionary relatedness of some primate models of Plasmodium. Mol. Biol. Evol. 10:914-923. [DOI] [PubMed] [Google Scholar]
- 44.Wipasa, J., H. Xu, M. Makobongo, M. Gatton, A. Stowers, and M. F. Good. 2002. Nature and specificity of the required protective immune response that develops postchallenge in mice vaccinated with the 19-kilodalton fragment of Plasmodium yoelii merozoite surface protein 1. Infect. Immun. 70:6013-6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, C., W. E. Collins, J. S. Sullivan, D. C. Kaslow, L. Xiao, and A. A. Lal. 1999. Partial protection against Plasmodium vivax blood-stage infection in Saimiri monkeys by immunization with a recombinant C-terminal fragment of merozoite surface protein 1 in block copolymer adjuvant. Infect. Immun. 67:342-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang, C., W. E. Collins, and P. Millet. 1994. Immunologic characterization of Plasmodium vivax antigens using Plasmodium cynomolgi liver stage-primed immune sera. Am. J. Trop. Med. Hyg. 51:365-371. [DOI] [PubMed] [Google Scholar]