Abstract
Persistence of borreliae within the vertebrate host depends on the fate of interactions between the spirochetes and target cells. The present work demonstrates the direct binding of the Borrelia burgdorferi outer surface proteins OspA and OspB to CR3 and that this binding is independent of iC3b.
Borrelia burgdorferi is the etiological agent of Lyme disease. One of the factors contributing to Borrelia dissemination and therefore to persistent infection is the capacity of this microorganism to interact with several cellular targets. The process of microorganism attachment to cells and subsequent phagocytosis is thought to be rate limiting and is therefore considered to be a major factor in determining the outcome of cell infections regarding their clearance or the development of pathogenic responses. Once internalized by phagocytes, these spirochetes can be killed by agents released by the host cell (10, 19). Infection-triggered host cell responses, among them antigen processing, eventually result in immune protection and/or in inflammatory phenomena or the development of autoimmune mechanisms (11).
In previous studies, we have shown that, in the absence of specific antibodies, B. burgdorferi binds to different domains of the complement receptor 3 (CR3) of polymorphonuclear leukocytes (PMNs) (3). OspA and OspB are two major lipoprotein components of the outer membranes (OM) of B. burgdorferi within unfed ticks. Soon after B. burgdorferi leaves a vertebrate host and enters a tick vector, OspA and OspB start to be expressed. OspA mediates the attachment and colonization of the vector midgut (25). When infected nymphs feed on vertebrates, continuing the natural transmission cycle, OspA and OspB on the surface of B. burgdorferi are down-regulated, and OspC starts to be expressed. A recent publication demonstrates that OspA/B function is not required for B. burgdorferi infection and dissemination in mice (37), but the results do not exclude a role for OspA in the pathogenesis of late-stage Lyme disease. During persistent infection of humans, OspA and OspB seem to be expressed to some degree, since OspA and OspB antibodies are detectable in some patients early after disease onset and also later during arthritic illness (2, 15) or during antibiotic-resistant Lyme arthritis (2, 18). A European study reports as much as 70 to 80% recognition of OspA and/or OspB antigens by sera from acrodermatitis chronica atrophicans and arthritis patients (1). This suggests that OspA can be present on borreliae, albeit at lower levels, during infection of the human host (7). In a model of experimental murine borreliosis, antibodies to OspA were weakly present during the early immune response and, most importantly, spirochetes expressing OspA could be detected within the first 2 weeks of infection (23). Cell-mediated immunity involving OspA has also been reported during early Lyme borreliosis (17).
Additionally, OspA shows a proinflammatory effect by inducing priming and activation pathways in human PMNs (24), B-cell mitogenesis, and cytokine production (20). OspA and OspB are also involved in B. burgdorferi adherence to endothelial cells (6, 33). A Borrelia mutant with reduced expression of a truncated form of OspB has been shown to be less capable of penetrating human endothelial cells in vitro and of infecting murine organs (30). In keeping with these observations, Sellati et al. (32) have found that neutrophils can migrate better through endothelial cell monolayers that had been previously coincubated with B. burgdorferi.
On the basis of the information available to date, a distinction needs to be drawn between the factors leading to infection by B. burgdorferi and those leading to the development of immune responses. Even though OspA/B may not have a role for B. burgdorferi infectivity of mammals, these surface proteins may be involved in immune mechanisms during late disease events, through interaction with antigen-presenting phagocytes.
CR3 (also known as Mac-1, CD11b/CD18, αMβ2 integrin, and gp165,95) belongs to the integrin family of transmembrane proteins. These proteins consist of a unique α subunit associated to a common β chain. They play a fundamental role in cell-to-cell and cell-to-extracellular-matrix adhesion and can also function as receptors for pathogenic agents (12). One of the main CR3 ligands in neutrophils is the iC3b fragment of the complement component C3 (35). The borrelia surface proteins OspA and OspC have been found to up-regulate CR3 in PMNs (4, 36), an observation related to the activation of neutrophil transendothelial migration mentioned above (32).
Although CR3 clearly seems to be among the adhesion molecules involved in the phagocyte-borrelia interaction, evidence is indirect since it is based on the blocking effect of antibodies or on the mere upregulation of CR3 on cell surfaces. B. burgdorferi could actually bind to receptors other than CR3, and either lateral interaction of these receptors with CR3 or secondary effects exerted through the mobilization of CR3-containing intracellular compartments could then result in CR3-mediated phenomena not having necessarily involved the direct binding of the microorganisms to CR3. The present study was therefore designed to identify biochemically, in a direct manner, the B. burgdorferi surface molecules able to bind to the integrin CR3 and whether the binding is dependent on iC3b. The strategy followed was as follows: (i) to purify CR3 from mammalian cells expressing it and link it covalently to Sepharose (affinity column); (ii) to prepare outer membranes from a strain of Borrelia garinii; and (iii) to isolate and identify what proteins among those in solubilized outer membranes bind to the CR3 affinity column, in the absence or presence of iC3b.
Human leukocyte CR3 was purified according to methods previously described (8, 13) but starting from THP-1 macrophage-like cells. Monocytic THP-1 cells were first differentiated to macrophages by treatment with 20 nM phorbol-myristate-acetate for 3 days. Cell lysates (100 mM Tris-HCl [pH 7.8], 150 mM NaCl, 2 mM MgCl2, 1% Triton X-100, 0.025% NaN3, 5 mM iodoacetamide, and proteinase inhibitors [Complete; Roche]) were cleared by successive centrifugations at 10,000 × g and 100,000 × g, preadsorbed with human immunoglobulin G coupled to Sepharose CL-4B and then purified using an M1/70 (rat anti-mouse CD11b) immunoaffinity column (13). The anti-CD11b monoclonal antibody (MAb) had been purified and concentrated from hybridoma cell supernatants by use of HiTrap Protein G HP columns (Amersham Biosciences). Fractions eluted from the CD11b immunoaffinity column (8) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The fractions containing CR3 (94% purity, based on gel imaging in a Bio-Rad VersaDoc system and quantitation by means of the Quantity One program) were combined and dialyzed using Vivaspin tubes (Sartorius) (5,000, molecular weight cutoff) to eliminate salts and replace the existing buffer with 20 mM potassium phosphate (pH 7.0). The resulting material was employed to prepare two affinity columns (containing 230 μg covalently bound CR3 each) by reacting CR3 with CNBr-activated Sepharose 4FF (Amersham Biosciences) according to the manufacturer's instructions.
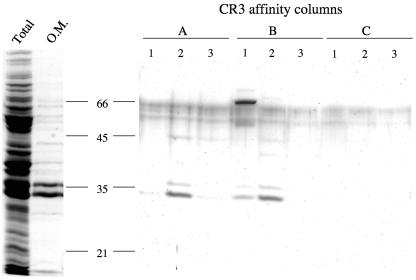
OM from the BITS strain of B. garinii were prepared from 1011 cells grown in BSK medium as described previously (3). The procedure involved the centrifugation of a microorganism suspension in OM buffer (10 mM HEPES, 150 mM NaCl, 1 mM MgCl2, 20% sucrose) containing Complete proteinase inhibitors (Roche) on a 20 to 60% ([wt/wt]) (in OM buffer) sucrose continuous gradient, according to Radolf et al. (29). Outer membrane (upper band) and total Borrelia (lower band) fractions were characterized by reducing SDS-PAGE (Fig. 1, left panel) and by immunoblotting using monoclonal antibodies directed against the major outer membrane components OspA, OspB, and OspC, kindly provided by A. G. Barbour (Univ. of California Irvine). This demonstrated a protein pattern characteristic of borrelia outer surface proteins (29) containing OspA, -B, and -C. Purified outer membranes were solubilized in 20 mM Tris-HCl (pH 7.5)-200 mM NaCl-1 mM EDTA-0.5% Triton X-100 and subjected to CR3 affinity separations. Before outer membrane extracts were used for cell binding experiments, Triton X-100 was eliminated by passage through Extracti-Gel D columns (Pierce).
FIG. 1.
B. burgdorferi outer membrane proteins that bind to CR3 affinity columns. Images of silver-stained gels (12% SDS-PAGE) are shown. Left-hand side: whole borrelia and borrelia outer membranes (O.M.). Right-hand side: fractions 1, 2, and 3 (elution with 1 M KCl) from each of the affinity columns A (CR3-Sepharose), B (CR3-Sepharose with bound iC3b), and C (Sepharose without CR3). The background staining observed in the area from 55 to 65 kDa across the whole gel is nonspecific and unrelated to the proteins contained in the samples loaded.
Two CR3 affinity columns (vol = 1 ml) were run to isolate CR3-binding proteins from borrelia outer membrane extracts, namely, without (Fig. 1, column A) or with (column B) iC3b previously bound to CR3. Binding of iC3b (Calbiochem) to column B was performed by passage of 230 μg of iC3b (three successive cycles) followed by washing with 6 column volumes of CR3 column buffer (20 mM Tris-HCl [pH 7.5], 66 mM NaCl, 1 mM EDTA, and 0.5% Triton X-100). Additionally, a third column (column C; control, no bound CR3) was prepared by processing CNBr-activated Sepharose 4FF exactly as described above for the covalent binding of CR3 but omitting CR3. The control column was used to detect the nonspecific binding of Borrelia proteins to Sepharose. Each of the three columns was loaded with equal amounts of solubilized borrelia outer membranes whose ionic composition had been adjusted to achieve that of CR3 column buffer. The columns were washed with 5 volumes of the CR3 column buffer, and bound proteins were detached with a high-salt solution (20 mM Tris-HCl [pH 7.5], 1 M KCl, 1 mM EDTA, 0.5% Triton X-100). The electrophoretic analysis of the starting material and eluted fractions (Fig. 1) demonstrated the following: (i) the CR3 affinity columns had bound proteins selectively, since only a weak 45-kDa protein band was observed in the control column C, and (ii) proteins bound to CR3 or CR3+iC3b (columns A and B) were eluted in the first three fractions, in correspondence with the KCl wave. A comparison between the proteins eluted from columns A (CR3) and B (CR3+iC3b) showed the following. (i) The presence of iC3b (68 kDa) (27) and of another protein of about 50 kDa in fraction 1 (but not 2) of column B was revealed. This indicates that both iC3b and the 50-kDa protein had been eluted by a lower KCl concentration than that of the proteins found in fractions 2 and 3. In fact, the 50-kDa protein might be bound to iC3b. The separation between proteins in fraction 1 and fraction 2 or 3 is not likely to be a gel filtration effect, since the Sepharose 4 FF matrix (exclusion limit [Mr] > 2 × 107) is not able to discriminate between molecules of 68 and 35 kDa (technical specifications are available from Amersham Biosciences). (ii) The presence of two protein bands positioned above and below the 35-kDa molecular mass marker in fractions 1, 2, and 3, with a clear maximum in fraction 2, from both columns A and B (Fig. 1) was also revealed. The mobility of these two proteins coincides with those of two major components of borrelia outer membranes, OspA and OspB (29). Their binding to CR3 requires a higher KCl concentration to be disrupted than that of iC3b, which is eluted earlier (see column B). The identity of the 36- and 34-kDa proteins as OspB and OspA, respectively, was assessed by immunoblotting (Fig. 2). No reaction was obtained when Western blots were probed with anti-OspC (not shown).
FIG. 2.
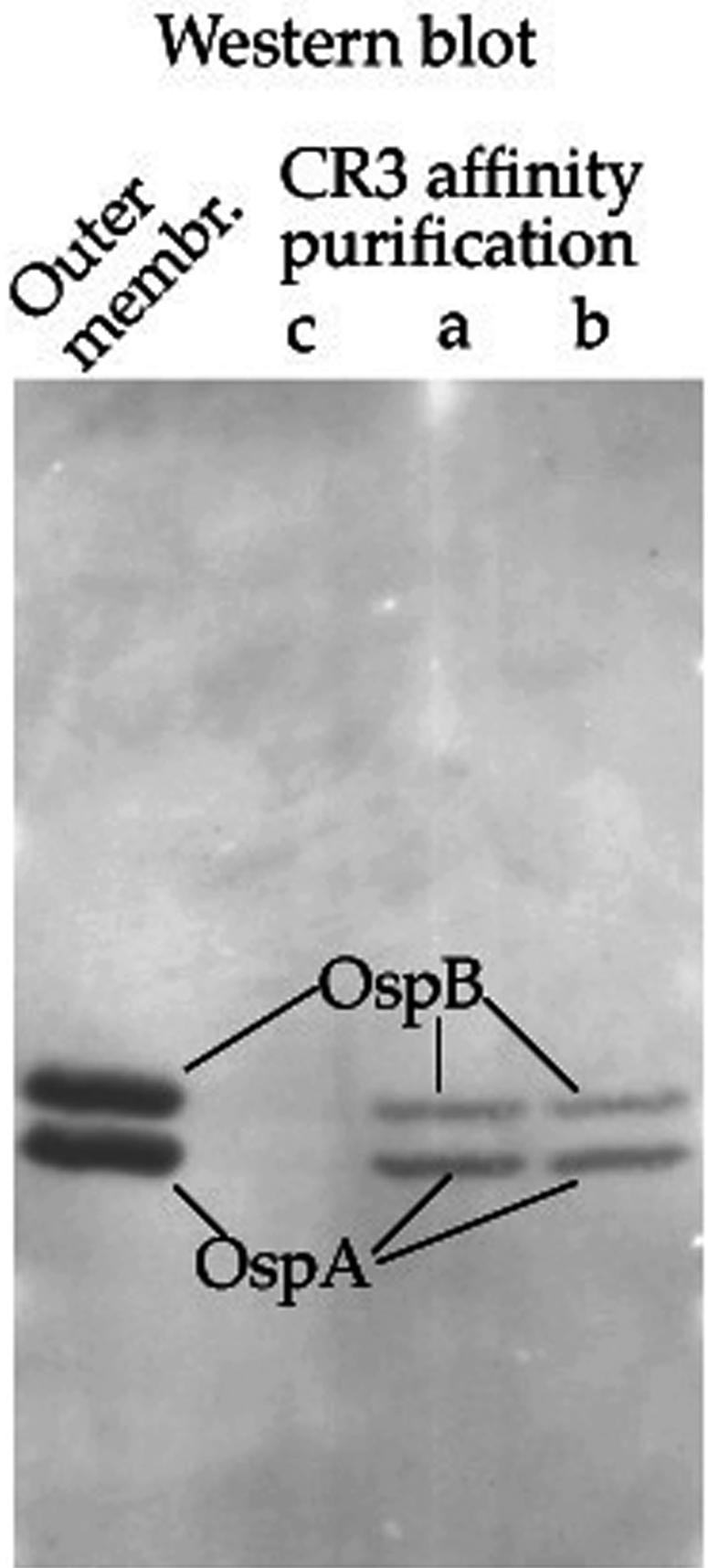
OspA and OspB are CR3-binding proteins. Borrelia outer membranes (starting material) and fraction 2 from each of the affinity columns shown in Fig. 1 were run on 12% SDS-PAGE gels. Lanes: a, CR3; b, CR3+iC3b; c, no CR3. A Western blot from the gel was probed sequentially with anti-OspA (MAb H5332) and anti-OspB (MAb H6831), detecting each time the reactive bands by means of enhanced chemiluminescence reactions. These anti-Osp antibodies had been previously tested individually and shown to be specific for OspA and OspB.
The fact that OspA and OspB bind in the same amounts to the CR3 affinity columns independently of the presence of iC3b (Fig. 1, columns A and B) indicates that (i) iC3b is not necessary as a mediator for the association of OspA/B to CR3, a conclusion strengthened by the fact that iC3b and OspA/B do not elute simultaneously from column B, and (ii) the binding of OspA/B to CR3 does not seem to involve the I domain of CR3. If that had been the case, the presence of iC3b bound to the I domain of CR3 (Fig. 1, column B) would have had the effect of decreasing the amount of OspA and OspB bound to CR3, since they would have been competing for the same site. In fact, since OspA and OspB are glycosylated (31), it is likely that they are binding to the lectin-like domain of CR3 (34).
The finding that the interaction between OspA/B and CR3 is direct and not iC3b mediated indicates that the eventual generation of iC3b as a result of the action of factor H bound to borreliae (16) would not influence the interaction between the microorganisms and phagocyte membrane CR3. Therefore, on the one hand, factor H protects against complement lysis of the spirochetes; on the other, the microorganisms can still be taken up and/or eventually killed and/or processed by host phagocytes.
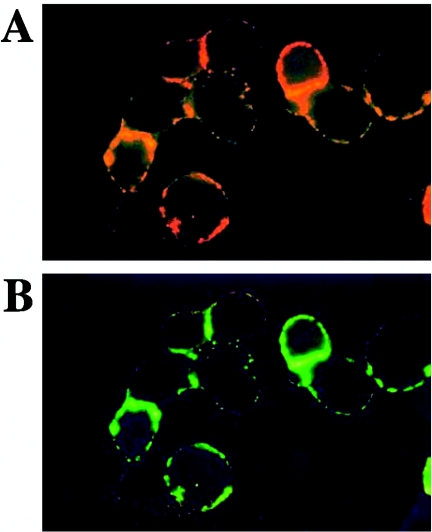
Lastly, mono- and double-immunofluorescence labeling (CR3 or CR3/OspB) was performed using CHO cells expressing Mac-1, obtained from D. M. Mosser (13). These cells were treated first with a detergent-free extract from borrelia outer membranes and then with either anti-CR3 alone or with anti-CR3 followed by anti-OspB. The results show the colocalization of these two molecules (Fig. 3), a result consistent with the CR3 binding column experiments. An entirely similar colocalization of CR3 and OspA was observed (not shown).
FIG. 3.
Colocalization of OspB with CR3 on cell surfaces. CHO cells stably transfected with human CR3 (clone CHO-Mac-1-1.35) (13) were cultured as previously described (3) and treated at 4°C with a Borrelia outer membrane extract (20 μg/ml) free of Triton X-100 by passage through an Extracti-Gel D column (Pierce). Cells were washed and incubated at 4°C first with anti-CD11b MAb M1/70 (1 μg/ml) and then with tetramethyl rhodamine isocyanate-labeled anti-rat antibody (Sigma) and were finally taken to 37°C for CR3 clustering. An aliquot of the CR3-tagged cells was left as was (A), and another was incubated with anti-OspB, at 4°C, and then with a fluorescein isothiocyanate-labeled anti-mouse antibody (Sigma) (B). Immunolabeled cells were washed, cytocentrifuged, and fixed with 2% paraformaldehyde in PBS. Fluorescence was observed by Zeiss 250-CF fluorescence microscopy (Jenalmar, Jena, Germany). (A) CR3 clustering (red); (B) OspB binding superimposed on CR3 (green).
The present results demonstrate in a direct way and for the first time the binding of the B. burgdorferi outer surface proteins OspA and OspB to CR3 and that this binding is independent of iC3b. They do not exclude, though, the possibility that borrelia molecules other than OspA/B may also be able to bind CR3. Other receptors, such as CD14, have been found to be involved in the process of attachment of borreliae to target cells and their successive engulfment (36). Nevertheless, it has been concluded that CD14 facilitates but is not the ligand-specific receptor responsible for the activation of neutrophils by Borrelia lipoproteins. These results are consistent with ours and point to the fact that even if OspA and OspB can bind directly to CR3, as demonstrated here, receptors other than CR3 might play regulatory functions regarding the binding capacity and/or affinity of target cells toward borreliae. Cross-regulations could occur, e.g., (i) through an increased mobilization toward the cell surface of an intracellular pool of vesicles containing CR3 (22), triggered by interaction between receptors other than CR3 and their ligands; or (ii) by CR3 clustering and activation as a result of surface stimulation of receptors such as FcγR (14). Outer envelope molecules other than OspA/B are also involved in the attachment of Borrelia to host cells, such as Bgp, Vsp, and decorin-binding proteins (DbpA/B), which interact with glycosaminoglycans (9, 21, 26), a fibronectin-binding protein (28), and an integrin ανb3-binding protein (5).
The overall balance of surface expression and activation of adhesion/binding molecules on both host cells and spirochetes would then determine the fate of the latter in late inflammatory processes, at the level of joints and the central nervous system. At this stage, OspA/B are expressed and act as antigens (1, 2, 15, 18). Therefore, OspA/B may not play a role in B. burgdorferi infectivity of mammals (37) but may be involved in late-disease immune mechanisms by virtue of their interaction with CR3 of phagocytes and/or antigen-presenting cells.
Additionally, on the basis of having established that OspA/B can interact directly with CR3, it can be speculated that the fact that borreliae down-regulate OspA/B when they enter the human host could have been the result of a selective pressure mechanism by which those bacteria able to avoid CR3-mediated interactions with killer cells were able to survive in higher proportions and therefore prevail through successive transmission cycles.
Acknowledgments
We acknowledge Alan G. Barbour of the Departments of Microbiology and Molecular Genetics and Medicine, University of California Irvine, for kindly providing the monoclonal antibodies H5332 (anti-OspA), H6831 (anti-OspB), and L221F8 (anti-OspC) and David Mosser for the CHO-Mac-1-1.35 cell clone.
Editor: J. T. Barbieri
REFERENCES
- 1.Batsford, S., C. Rust, and U. Neubert. 1998. Analysis of antibody response to the outer surface protein family in Lyme borreliosis patients. J. Infect. Dis. 178:1676-1683. [DOI] [PubMed] [Google Scholar]
- 2.Chen, J., J. A. Field, L. Glickstein, P. J. Molloy, B. T. Huber, and A. C. Steere. 1999. Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer surface protein A of Borrelia burgdorferi. Arthritis Rheum. 42:1809-1812. [DOI] [PubMed] [Google Scholar]
- 3.Cinco, M., R. Murgia, G. Presani, and S. Perticarari. 1997. Integrin CR3 mediates the binding of non-specifically opsonized Borrelia burgdorferi to human phagocytes and mammalian cells. Infect. Immun. 65:4784-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinco, M., E. Panfili, G. Presani, and S. Perticarari. 2000. Interaction with Borrelia burgdorferi causes increased expression of the CR3 integrin and increased binding affinity to fibronectin via CR3. J. Mol. Microbiol. Biotechnol. 2:575-579. [PubMed] [Google Scholar]
- 5.Coburn, J., and C. Cugini. 2003. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin ανβ3. Proc. Natl. Acad. Sci. USA 100:7301-7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comstock, L. E., E. Fikrig, R. J. Schoberg, R. A. Flavell, and D. D. Thomas. 1993. A monoclonal antibody to OspA inhibits association of Borrelia burgdorferi with human endothelial cells. Infect. Immun. 61:423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Silva, A. M., and E. Fikrig. 1997. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J. Clin. Investig. 99:377-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond, M. S., D. E. Staunton, A. R. de Fougerolles, S. A. Stacker, J. Garcia-Aguilar, M. L. Hibbs, and T. A. Springer. 1990. ICAM-1 (CD54): a counter receptor for Mac-1 (CD11b/CD18). J. Cell Biol. 111:3129-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer, J. R., N. Parveen, L. Magoun, and J. M. Leong. 2003. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 100:7307-7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia, R., L. Gusmani, R. Murgia, C. Guarnaccia, M. Cinco, and G. Rottini. 1998. Elastase is the only human neutrophil granule protein that alone is responsible for in vitro killing of Borrelia burgdorferi. Infect. Immun. 66:1408-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross, D. M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z. A. Nagy, J. A. Field, A. C. Steere, and B. T. Huber. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281:703-706. [DOI] [PubMed] [Google Scholar]
- 12.Hauck, C. R. 2002. Cell adhesion receptors—signaling capacity and exploitation by bacterial pathogens. Med. Microbiol. Immunol. (Berlin) 191:55-62. [DOI] [PubMed] [Google Scholar]
- 13.Hondalus, M. K., M. S. Diamond, L. A. Rosenthal, T. A. Springer, and D. M. Mosser. 1993. The intracellular bacterium Rhodococcus equi requires Mac-1 to bind to mammalian cells. Infect. Immun. 61:2919-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jongstra-Bilen, J., R. Harrison, and S. Grinstein. 2003. Fcγ-receptors induce Mac-1 (CD11b/CD18) mobilization and accumulation in the phagocytic cup for optimal phagocytosis. J. Biol. Chem. 278:45720-45729. [DOI] [PubMed] [Google Scholar]
- 15.Kalish, R. A., J. M. Leong, and A. C. Steere. 1995. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect. Immun. 63:2228-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2002. Immune evasion of Borrelia burgdorferi: insufficient killing of the pathogens by complement and antibody. Int. J. Med. Microbiol. 291(Suppl. 33):141-146. [DOI] [PubMed] [Google Scholar]
- 17.Krause, A., G. R. Burmester, A. Rensing, C. Schoerner, U. E. Schaible, M. M. Simon, P. Herzer, M. D. Kramer, and R. Wallich. 1992. Cellular immune reactivity to recombinant OspA and flagellin from Borrelia burgdorferi in patients with Lyme borreliosis. J. Clin. Investig. 90:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lengl-Janssen, B., A. F. Strauss, A. C. Steere, and T. Kamradt. 1994. The T helper cell response in Lyme arthritis: differential recognition of Borrelia burgdorferi outer surface protein A (OspA) in patients with treatment-resistant or treatment-responsive Lyme arthritis. J. Exp. Med. 180:2069-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lusitani, D., S. E. Malawista, and R. R. Montgomery. 2002. Borrelia burgdorferi are susceptible to killing by a variety of human polymorphonuclear leukocyte components. J. Infect. Dis. 185:797-804. [DOI] [PubMed] [Google Scholar]
- 20.Ma, Y., and J. J. Weis. 1993. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-mitogenic and cytokine-stimulatory properties. Infect. Immun. 61:3843-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magoun, L., W. R. Zuckert, D. Robbins, N. Parveen, K. R. Alugupalli, T. G. Schwan, A. G. Barbour, and J. M. Leong. 2000. Variable small protein (Vsp)-dependent and Vsp-independent pathways for glycosaminoglycan recognition by relapsing fever spirochaetes. Mol. Microbiol. 36:886-897. [DOI] [PubMed] [Google Scholar]
- 22.Miller, L. J., D. F. Bainton, N. Borregaard, and T. A. Springer. 1987. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J. Clin. Investig. 80:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery, R. R., S. E. Malawista, K. J. Feen, and L. K. Bockenstedt. 1996. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J. Exp. Med. 183:261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison, T. B., J. H. Weis, and J. J. Weis. 1997. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J. Immunol. 158:4838-4845. [PubMed] [Google Scholar]
- 25.Pal, U., A. M. de Silva, R. R. Montgomery, D. Fish, J. Anguita, J. F. Anderson, Y. Lobet, and E. Fikrig. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parveen, N., Caimano, M., Radolf, J. D., and J. M. Leong. 2003. Adaptation of the Lyme disease spirochaete to the mammalian host environment results in enhanced glycosaminoglycan and host cell binding. Mol. Microbiol. 47:1433-1444. [DOI] [PubMed] [Google Scholar]
- 27.Pfrommer, G. S., S. M. Dickens, M. A. Wilson, B. J. Young, and T. R. Kozel. 1993. Accelerated decay of C3b to iC3b when C3b is bound to the Cryptococcus neoformans capsule. Infect. Immun. 61:4360-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Probert, W. S., and B. J. Johnson. 1998. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 30:1003-1015. [DOI] [PubMed] [Google Scholar]
- 29.Radolf, J. D., M. S. Goldberg, K. Bourell, S. I. Baker, J. D. Jones, and M. V. Norgard. 1995. Characterization of outer membranes isolated from Borrelia burgdorferi, the Lyme disease spirochete. Infect. Immun. 63:2154-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadziene, A., A. Barbour, P. A. Rosa, and D. D. Thomas. 1993. An OspB mutant of Borrelia burgdorferi has been found to show a reduced invasiveness in vitro and reduced infectivity in vivo. Infect. Immun. 61:3590-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambri, V., C. Stefanelli, and R. Cevenini. 1992. Detection of glycoproteins in Borrelia burgdorferi. Arch. Microbiol. 157:205-208. [DOI] [PubMed] [Google Scholar]
- 32.Sellati, T. J., M. J. Burns, M. A. Ficazzola, and M. B. Furie. 1995. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect. Immun. 63:4439-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, D. D., and L. E. Comstock. 1989. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect. Immun. 57:1324-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thornton, B. P., V. Vetvicka, M. Pitman, R. C. Goldman, and G. D. Ross. 1996. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 156:1235-1246. [PubMed] [Google Scholar]
- 35.Todd, R. F., III. 1996. The continuing saga of complement receptor type 3 (CR3). J. Clin. Investig. 98:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wooten, R. M., T. B. Morrison, J. H. Weis, S. D. Wright, R. Thieringer, and J. J. Weis. 1998. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 160:5485-5492. [PubMed] [Google Scholar]
- 37.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]