Abstract
The resolution of primary and secondary chlamydial genital infection in immunoglobulin A (IgA)-deficient (IgA−/−) mice was not different from that in IgA+/+ mice. Furthermore, depletion of either CD4+ or CD8+ T cells prior to reinfection of IgA+/+ or −/− mice had limited impact on immunity to reinfection. Thus, although antibody contributes importantly to immunity to chlamydial genital tract reinfection, IgA antibodies are not an absolute requirement of that protective response.
Sexually transmitted infections due to Chlamydia trachomatis cause considerable morbidity and socioeconomic burden worldwide. Four million new cases of chlamydial sexually transmitted diseases are reported annually in the United States, and costs associated with the management of these infections exceed $2 billion (7). Vaginal inoculation of the female mouse with Chlamydia muridarum (C. trachomatis strain mouse pneumonitis) closely mimics acute genital infection of women and provides a reasonable model in which to study adaptive immunity (8). Female mice develop a self-limiting infection that originates in the lower genital tract, ascends to infect the urine horns and oviducts, and resolves in approximately 4 weeks (1, 9). Mice that resolve infection are markedly resistant to reinfection (9), and CD4+ Th1 T-cell responses are arguably the most vital elements of protective immunity (3, 4, 6, 10, 11, 15, 20). Recently, however, we have demonstrated that antibodies (B cells) play a key role in adaptive immunity to genital tract reinfection (10, 11).
Chlamydiae predominantly infect mucosal epithelial cells and cause disease at mucosal surfaces, and thus the mucosal immune response has long been predicted to be important in antichlamydial adaptive immunity. Antichlamydial immunoglobulin A (IgA) antibodies are found in both the serum and genital tract secretions following murine chlamydial genital infection (9), and antichlamydial IgA antibodies have been associated with resolution of infection in women (2). Our previous studies reveal an important role for antibody in adaptive immunity to chlamydial genital tract reinfection (10, 11). In those studies we demonstrate that mice deficient in both CD4+ T cells and antibody are unable to resolve secondary chlamydial infection, whereas mice deficient in only CD4+ T cells or B cells resolve chlamydial reinfection. Those results clearly define a previously unrecognized role for antibody in immunity to chlamydial genital tract reinfection. However, the results could not distinguish the relative contribution of IgA in immune protection because the antibody deficiency was panspecific (i.e., absence of all classes of immunoglobulins). Knowing whether the protective efficacy of the antichlamydial antibody response is solely dependent on IgA antibodies is of importance not only because chlamydia cause mucosal infection, but also because the composition of experimental chlamydial vaccines and vaccination protocols will be impacted by the need to elicit antichlamydia IgA responses. In the present study we evaluated the role of IgA antibodies in adaptive immunity to chlamydial reinfection using mice with a targeted disruption in the switch region and α-heavy chain locus (IgA−/−).
Breeding pairs of C57BL/6 × 129 IgA-deficient (IgA−/−) mice and C57BL/6 × 129 F2 (IgA+/+) mice (wild-type control) were generated as previously described (5) and provided as a kind gift by I. N. Mbawuike, Baylor College of Medicine, Houston, Tex. All animal procedures were in accordance with institutional policies for animal health and well-being and were approved by the institutional animal care and use committee. The targeted mutation was confirmed as described previously (22). Methodologies used for infection, enumeration of inclusion forming units (IFUs), T-cell subpopulation depletion, and antichlamydial antibody titration have be reported in detail previously (9, 11) and are only briefly described here. Eight- to 12-week-old female mice were treated with Depo-Provera 5 days prior to infection. Mice were inoculated vaginally with 100 50% infective doses of C. muridarum (5 × 104 IFUs), and infection was followed by enumeration of IFUs from vaginal-cervical swabs collected at various times throughout the course of infection. To assess the role of IgA in adaptive immunity to chlamydial reinfection, mice that had resolved primary infection were depleted of either CD4+ or CD8+ T cells prior to secondary infectious challenge. Groups of mice were injected with anti-CD4, anti-CD8, or phosphate-buffered saline (PBS) on days 56, 57, 58, 61, 64, 67, 70, 73, 76, 79, 82, 85, and 88 after primary infection. Depo-Provera-treated mice (day 57 after primary infection) were rechallenged (secondary infection) on day 62 after primary infection. The T-cell depletion scheme described above has been shown to effectively deplete CD4+ and CD8+ T-cell subpopulations prior to infectious challenge and throughout the course of the study period (9) and was confirmed for these studies (data not shown). Antichlamydial antibody titers were determined using a C. muridarum elementary body enzyme-linked immunosorbent assay and isotype-specific detection antibodies (9).
The course of primary chlamydial genital tract infection of IgA−/− mice was indistinguishable from that of IgA+/+ mice (Fig. 1). Neither the shedding of infectious chlamydiae nor the duration of infection was different between the strains at any of the time points analyzed. The finding that IgA−/− mice resolved primary infection comparably to IgA+/+ mice was not unexpected. We had previously shown the resolution of primary infection in antibody-deficient mice was nearly identical to that in antibody-positive wild-type mice (21) and thus anticipated that animals deficient in only IgA would respond similarly.
FIG. 1.
Primary C. muridarum genital tract infection of female IgA+/+ and IgA−/− mice. Depo-Provera-treated mice were infected intravaginally with approximately 50,000 IFUs of C. muridarum elementary bodies. Infection was monitored by swabbing the vaginal vault and enumerating IFUs on HeLa cell monolayers (9). Data are presented as log10 IFUs and represent the means ± standard errors of the means of triplicate determinations of 18 mice per group. IFUs recovered from IgA+/+ and IgA−/− mice were not statistically different at any time point (Student's t test).
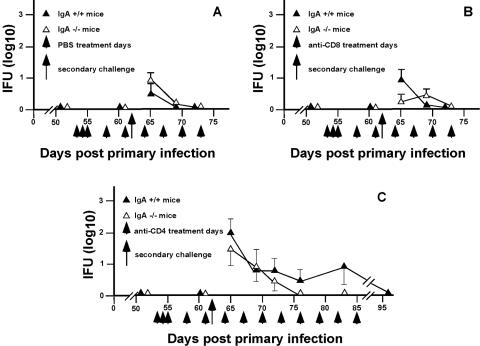
The protective role of antibody in murine chlamydial genital infection has only been clearly established in immunity to reinfection (10, 11). The experimental approach used previously for demonstrating the role of antibody in immunity to reinfection was used in the present study to determine if the protective effect of antibody was limited to IgA or if other immunoglobulin classes contributed to the protective response. The ability of IgA+/+ and IgA−/− mice to resolve a secondary infectious challenge was evaluated in nondepleted (PBS-treated), CD4+ T-cell-depleted (anti-CD4-treated), or CD8+ T-cell-depleted (anti-CD8-treated) mice that had resolved primary infection. Both IgA+/+ and IgA−/− mice were markedly immune to reinfection (PBS treated; Fig. 2A). Similarly, depletion of CD8+ T cells had no effect on the course of secondary infection (Fig. 2B). Although depletion of CD4+ T cells extended the duration of secondary infection (Fig. 2C) (compared to nondepleted and CD8-depleted mice), in both IgA+/+ and IgA−/− mice, CD4-depleted mice shed far fewer infectious bacteria than during primary infection (4 to 5 log10 lower IFU counts) and resolved secondary infection in the absence of CD4+ T cells. A greater number of CD4-depleted mice remained culture positive for a longer duration than CD8-depleted or nondepleted mice (Table 1), but the magnitude of the chlamydial shedding was far less than that observed following primary infection.
FIG. 2.
Effect of anti-CD4 or anti-CD8 T-cell subpopulation depletion on the resolution of a secondary C. muridarum genital tract infection of IgA+/+ and IgA−/− mice. Following the resolution of primary infection, groups of mice were treated with PBS (nondepleted group) (A), anti-CD8 (B), or anti-CD4 (C) and rechallenged (day 62 after primary infection) with infectious chlamydia (5 × 104 IFUs). Infection was monitored as described in the Fig. 1 legend (9). Data are presented as log10 IFU and represent the means plus standard errors of the means of triplicate determination of five to seven mice per group. No significant differences between IgA+/+ and IgA−/− mice were found (Students t test).
TABLE 1.
Genital tract reinfection of wild-type (IgA+/+) and IgA-deficient (IgA−/−) mice
| Treatmenta | No. of culture-positive mice/total no. of micec on indicated day postchallengeb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgA+/+
|
IgA−/−
|
|||||||||||
| 3 | 7 | 10 | 14 | 21 | 42 | 3 | 7 | 10 | 14 | 21 | 42 | |
| PBS | 3/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 5/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Anti-CD8 | 4/6 | 1/6 | 0/6 | 0/6 | 0/6 | 0/6 | 1/5 | 3/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Anti-CD4 | 5/6 | 5/6 | 4/6 | 4/6 | 2/6 | 0/6 | 4/7 | 3/7 | 2/7 | 0/7 | 0/7 | 0/7 |
Mice were treated with either PBS, anti-CD8, or anti-CD4 as described in the Fig. 1 legend.
Days following secondary infectious challenge.
Number of culture-positive mice/total number of mice rechallenged with infectious chlamydiae.
Antichlamydial antibody responses were evaluated in the groups of IgA+/+ and IgA−/− mice following chlamydial infection (data not shown). Briefly, all mice developed high titers of antichlamydial antibody. IgA −/− mice had somewhat higher total antichlamydial antibody titers than IgA+/+ mice, 65,536 versus 16,384, respectively. Analysis of the immunoglobulin isotype specificity of the antichlamydial responses confirmed the IgA deficiency in IgA−/− mice and indicated heightened antichlamydial IgG2a and IgG2b responses in those mice. Heightened IgG responses have been reported previously for IgA−/− mice (5, 12). IgG1 responses were less than a titer of 16 in both strains of mice, which is typical for murine chlamydial genital tract infection (9).
Previous studies have convincingly shown that B cells play an important role in immunity to chlamydial genital tract reinfection in the mouse (10, 11), and a protective role for antibody has been demonstrated in the guinea pig model of chlamydial genital tract infection (17-19). The purpose of the present study was to specifically address the relative contribution of IgA in this protective response by using IgA-deficient mice and the experimental design used previously to demonstrate the role of antibody in immunity to chlamydial reinfection (11). We reasoned that, because chlamydia are mucosal pathogens and antichlamydial IgA has been associated with lower infectious burdens in women (2), IgA may play a dominant role in protective immunity to reinfection. Our results demonstrate that antichlamydial IgA is not a required component of adaptive antibody-mediated immunity to reinfection. However, the possibility that antichlamydial IgA antibodies function in immunity in the immunocompetent host cannot be ruled out by the results of this study. Thus, the data should not be interpreted to imply that IgA antibodies are not involved in immunity to reinfection in the immunocompetent host. Rather IgA may function in immunity to reinfection, but, because other antibody subclasses also confer protective immunity to reinfection, the elimination of a single antibody class (e.g., IgA) minimally impacts the course of secondary infection. Similarly, if the principal function of IgA is to prevent the establishment of intracellular infection by blocking the attachment of chlamydiae to host cells, we cannot completely rule out the possibility that the challenge dose used for reinfection overwhelmed the neutralizing function of IgA. The contribution of both antichlamydial IgG and IgA to protective immunity is supported by numerous in vitro studies showing the neutralizing capabilities of those antibodies (13, 14, 16, 23, 24) and in vivo data showing antichlamydial IgA as well as IgG in genital tract secretions (2, 9). In summary, the protective efficacy of the antichlamydial antibody response to murine chlamydial genital tract reinfection is not solely dependent on IgA and other classes of antibodies effectively protect against reinfection.
Acknowledgments
This work was supported by U.S. Public Health Service grant AI38991 from the National Institutes of Health.
Editor: J. D. Clements
REFERENCES
- 1.Barron, A. L., H. J. White, R. G. Rank, B. L. Soloff, and E. B. Moses. 1981. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J. Infect. Dis. 143:63-66. [DOI] [PubMed] [Google Scholar]
- 2.Brunham, R. C., C.-C. Kuo, L. Cles, and K. K. Holmes. 1983. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect. Immun. 39:1491-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cain, T. K., and R. G. Rank. 1995. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 63:1784-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter, T. W., K. H. Ramsey, G. S. Miranpuri, C. E. Poulsen, and G. I. Byrne. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 65:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harriman, G. R., M. Bogue, P. Rogers, M. Finegold, S. Pacheco, A. Bradley, Y. Zhang, and I. N. Mbawuike. 1999. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J. Immunol. 162:2521-2529. [PubMed] [Google Scholar]
- 6.Igietseme, J. U., K. H. Ramsey, D. M. Magee, D. M. Williams, T. J. Kincy, and R. G. Rank. 1993. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg. Immunol. 5:317-324. [PubMed] [Google Scholar]
- 7.Institute of Medicine Committee on Prevention and Control of Sexually Transmitted Diseases. 1997. The hidden epidemic: confronting sexually transmitted diseases. National Academy Press, Washington, D.C.
- 8.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 70:2741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison, S. G., and R. P. Morrison. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect. Immun. 69:2643-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy, A. K., J. Sharma, J. J. Coalson, G. Zhong, and B. P. Arulanandam. 2004. Chlamydia trachomatis pulmonary infection induces greater inflammatory pathology in immunoglobulin A deficient mice. Cell. Immunol. 230:56-64. [DOI] [PubMed] [Google Scholar]
- 13.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 1997. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine 15:575-582. [DOI] [PubMed] [Google Scholar]
- 14.Peeling, R., I. W. Maclean, and R. C. Brunham. 1984. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect. Immun. 46:484-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry, L. L., K. Feilzer, and H. D. Caldwell. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J. Immunol. 158:3344-3352. [PubMed] [Google Scholar]
- 16.Peterson, E. M., X. Cheng, B. A. Markoff, T. J. Fielder, and L. M. de la Maza. 1991. Functional and structural mapping of Chlamydia trachomatis species-specific major outer membrane protein epitopes by use of neutralizing monoclonal antibodies. Infect. Immun. 59:4147-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rank, R. G., and A. L. Barron. 1983. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infect. Immun. 39:463-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rank, R. G., and B. E. Batteiger. 1989. Protective role of serum antibody in immunity to chlamydial genital infection. Infect. Immun. 57:299-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rank, R. G., H. J. White, and A. L. Barron. 1979. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect. Immun. 26:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su, H., and H. D. Caldwell. 1995. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 63:3302-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su, H., K. Feilzer, H. D. Caldwell, and R. P. Morrison. 1997. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect. Immun. 65:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, Y., S. Pacheco, C. L. Acuna, K. C. Switzer, Y. Wang, X. Gilmore, G. R. Harriman, and I. N. Mbawuike. 2002. Immunoglobulin A-deficient mice exhibit altered T helper 1-type immune responses but retain mucosal immunity to influenza virus. Immunology 105:286-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, Y.-X., S. Stewart, T. Joseph, H. R. Taylor, and H. D. Caldwell. 1987. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J. Immunol. 138:575-581. [PubMed] [Google Scholar]
- 24.Zhang, Y.-X., S. J. Stewart, and H. D. Caldwell. 1989. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect. Immun. 57:636-638. [DOI] [PMC free article] [PubMed] [Google Scholar]