Abstract
We previously described that DNA vaccination with the gene encoding amastigote surface protein 2 (ASP-2) protects approximately 65% of highly susceptible A/Sn mice against the lethal Trypanosoma cruzi infection. Here, we explored the possibility that bacterial recombinant proteins of ASP-2 could be used to improve the efficacy of vaccinations. Initially, we compared the protective efficacy of vaccination regimens using either a plasmid DNA, a recombinant protein, or both sequentially (DNA priming and protein boosting). Survival after the challenge was not statistically different among the three mouse groups and ranged from 53.5 to 75%. The fact that immunization with a recombinant protein alone induced protective immunity revealed the possibility that this strategy could be pursued for vaccination. We investigated this possibility by using six different recombinant proteins representing distinct portions of ASP-2. The vaccination of mice with glutathione S-transferase fusion proteins representing amino acids 261 to 500 or 261 to 380 of ASP-2 in the presence of the adjuvants alum and CpG oligodeoxynucleotide 1826 provided remarkable immunity, consistently protecting 100% of the A/Sn mice. Immunity was completely reversed by the in vivo depletion of CD8+ T cells, but not CD4+ T cells, and was associated with the presence of CD8+ T cells specific for an epitope located between amino acids 320 and 327 of ASP-2. We concluded that a relatively simple formulation consisting of a recombinant protein with a selected portion of ASP-2, alum, and CpG oligodeoxynucleotide 1826 might be used to cross-prime strong CD8+-T-cell-dependent protective immunity against T. cruzi infection.
In spite of the significant reduction in transmission over the past 20 years in countries such as Argentina, Brazil, Chile, and Uruguay, Chagas' disease (American trypanosomiasis) is still a major problem for many Latin American countries. The disease afflicts more than 15 million individuals, with an annual death toll of approximately 45,000 (reviewed in reference 18). The efficacy of conventional chemotherapy with nifurtimox or benznidazole is low and varies widely according to the infection status (acute or chronic) and the region of endemicity. Because of the low efficacy of treatment, the chronic phase of this infection usually persists for life. Approximately one-third of infected persons progress slowly to the symptomatic forms of the disease, characterized by cardiomyopathy and/or megagastrointestinal syndromes. Asymptomatic, chronically infected persons transmit the disease through blood transfusions and tissue transplantation. The lack of efficient drug treatment increases the importance of the development of new strategies to prevent or reduce the disease.
A number of studies have been published over the past 10 years demonstrating that recombinant proteins or plasmid DNA can elicit protective immunity against experimental Trypanosoma cruzi infection (2, 3, 4, 5, 7, 8, 12, 16, 20, 21, 23, 24, 28, 31, 32, 33, 34; reviewed in references 17 and 22). Vaccinated mice develop a strong cellular immune response mediated by CD4+ Th1 and CD8+ Tc1, thus surviving otherwise lethal acute infection. In addition to surviving acute infection, chronic phase pathologies of vaccinated mice were significantly reduced (4, 8, 28). Although in most studies, prophylactic vaccinations were performed, a recent report showed that therapeutic vaccination can also be effective in mice chronically infected with certain T. cruzi strains (4). We consider studies on vaccination against experimental Chagas' disease important, as an efficacious vaccine may complement other strategies such as vector control and chemotherapy for prevention and treatment of the disease.
Most experiments related to vaccination against T. cruzi infection have used BALB/c or C57BL/6 mice. Although these mice die when challenged with the infective trypomastigotes of certain parasite strains, they are not as susceptible to infection with T. cruzi as A/Sn mice. In order to study the mechanisms of protective immunity required for vaccination, we have been using A/Sn mice. This mouse strain is highly susceptible to infection with different strains of T. cruzi, and 100% of the animals die after a challenge with relatively small doses of the parasite. Due to its high susceptibility, we believe that this mouse strain provides an interesting model to study the immunological mechanisms required to induce a high degree of protective immunity against infection.
Using highly susceptible A/Sn mice, we have recently described that vaccination with a plasmid expressing amastigote surface protein 2 (ASP-2) generates specific CD4+ Th1 and CD8+ Tc1 immune responses. Most importantly, immunization with the asp-2 gene alone promotes the survival of approximately 65% of the A/Sn mice against a lethal Trypanosoma cruzi infection (28). Protective immunity could be improved if the animals were vaccinated simultaneously with the genes of ASP-2 and trans-sialidase (28).
Based on the optimistic results obtained with the asp-2 gene, we attempted to further improve protective immunity in this mouse model of infection. To this end, the initial goal of the present study was to compare the protective efficacy of vaccination regimens using either plasmid DNA, recombinant protein, or both sequentially (DNA priming and protein boosting). Our initial assumption was based on a number of reports showing that a DNA priming/protein boosting strategy could improve the protective efficacy in different vaccination models (14, 19, 26, 27).
During the course of these experiments, we observed that protective immunity was similar following immunization with either a plasmid DNA or a recombinant protein adsorbed to alum or when both types were combined (DNA priming/protein boosting). The fact that immunization with a recombinant protein alone was partially protective was unexpected; however, it revealed the possibility that this strategy could be pursued for experimental vaccination. Toward that goal, we used several recombinant proteins representing distinct portions of ASP-2. These proteins confirmed the protective potential of recombinant antigens when used in a relatively simple adjuvant formulation. Also, they identified a region containing highly protective epitopes capable of providing protective immunity to 100% of the vaccinated A/Sn mice.
MATERIALS AND METHODS
Mice and parasites.
Female 5- to 8-week-old A/Sn mice were purchased from the University of São Paulo. Parasites of the Y strain of T. cruzi were used in this study (25). Bloodstream trypomastigotes were obtained from mice infected 7 days earlier. The concentration of parasites was estimated and adjusted to 1,250 trypomastigotes per ml. Each mouse was inoculated intraperitoneally (i.p.) with 0.2 ml (250 trypomastigotes). Parasite development was monitored by counting the number of bloodstream trypomastigotes in 5 μl of fresh blood collected from the tail vein (13).
Plasmid and recombinant proteins based on the sequence of the asp-2 gene.
The plasmid encoding the asp-2 gene, ASP-2 DNA, was created and purified as previously described by Boscardin et al. (2). This plasmid contains the nucleotides encoding the mouse immunoglobulin κ chain signal peptide in frame with the nucleotide sequence encoding amino acids (aa) 67 to 694 of ASP-2.
The nucleotide sequences corresponding to different portions of ASP-2 (clone 9) were obtained by PCR amplification using Platinum Taq High Fidelity DNA polymerase (Invitrogen). Oligonucleotides containing the EcoRI and BamHI restriction sites, purchased from Invitrogen, were used for amplification. The amplified fragments were treated with the restriction enzymes EcoRI and BamHI and ligated into the pGEX-3X vector (Amershan) treated with these same enzymes. The recombinant plasmids were transformed into Escherichia coli DH5α. The recombinant proteins were expressed and purified as described earlier (2). The purity of recombinant protein was determined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). SDS molecular weight markers (Sigma) were used as protein standards.
Immunization.
A/Sn mice were immunized according to protocols described earlier (3, 29). Each mouse received 3 doses of 100 μg of plasmid DNA injected intramuscularly at 0, 3, and 5 weeks. Immunization with recombinant His-65kDa consisted of 3 i.p. doses of 25 μg of protein adsorbed to alum (Alhydrogel 85 Superfos; Biosector, Vedbaek, Denmark) given at 0, 3, and 5 weeks. Efficient coupling of recombinant His-65kDa to alum was obtained by mixing 2.5 μl 2% (wt/vol) Al2O3 per μg of recombinant protein for 2 h. Our DNA priming/protein boosting regimen consisted of two doses of 100 μg of plasmid DNA injected at 0 and 3 weeks. A third dose was provided 2 weeks later and consisted of an i.p. injection of 25 μg of recombinant His-65kDa adsorbed to alum.
Immunization with the recombinant proteins His-65kDa, His-25kDa, glutathione S-transferase (GST)-P1-P3, GST-P4-P7, GST-P4-P5, and GST-P6-P7 was performed as described in the legend to Fig. 2. CpG oligodeoxynucleotide (ODN) 1826 (TCCATGACGTTCCTGACGTT) was synthesized with a nuclease-resistant phosphorothioate backbone (Coley Pharmaceutics, Wellesley, MA).
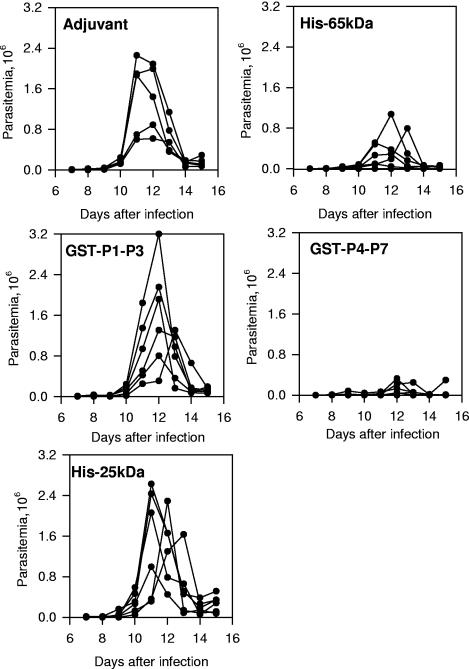
FIG. 2.
Trypomastigote-induced parasitemia in A/Sn mice immunized with recombinant protein His-65kDa, GST-P1-P3, GST-P4-P7, or His-25kDa. Groups of six mice were immunized at 0, 2, and 4 weeks with His-65kDa, GST-P1-P3, GST-P4-P7, His-25kDa, or adjuvant alone. Each mouse received i.p. 25 μg of recombinant protein adsorbed to alum in the presence of 10 μg of CpG ODN 1826. Two weeks after the last dose was administered, mice were challenged i.p. with 250 bloodstream trypomastigotes. The parasitemia for each individual mouse is represented. The peak parasitemias of mice immunized with His-65kDa or GST-P4-P7 were lower than the parasitemias of animals immunized with adjuvant only, GST-P1-P3, or His-25kDa (P < 0.01 in all cases by Tukey's HSD test). No significant difference was observed when we compared the peak parasitemias of mice immunized with His-65kDa or GST-P4-P7 (P > 0.05). Also, no difference was found when we compared the peak parasitemias of mice immunized with GST-P1-P3 or His-25kDa or adjuvant alone (P > 0.05).
One week after the last immunizing dose with plasmids and/or recombinant proteins, the blood was collected from the tail and the sera were analyzed for the presence of antibodies to His-65kDa. One week later, mice were challenged with bloodstream trypomastigotes. Results are representative of two or more independent experiments.
Immunological assays.
Anti-His-65kDa antibodies were detected by enzyme-linked immunosorbent assay as described earlier (2). Titers are reported as the reciprocals of the highest serum dilution producing an average optical density at 492 nm greater than 0.1. The enzyme-linked immunospot assay (ELISPOT assay) was performed essentially as described earlier (2).
Hemoculture and histopathological analysis.
Aliquots of 0.1 ml of blood were collected and cultured at 28°C for 1 month in 5 ml of axenic liver infusion tryptose medium. Parasite growth was monitored microscopically every week.
Tissue specimens were collected from vaccinated and infected mice at the indicated day after challenge and fixed in paraformaldehyde for further processing. Paraffin-embedded tissue sections were stained with hematoxylin and eosin stain and analyzed by optical microscopy. Slides from the heart and the quadriceps muscle of each mouse were analyzed in a blind fashion by one of us (C.R.F.M.). Areas of inflammatory infiltrates in the myocardium, pericardium, and endocardium or in the striated muscle were analyzed and semiquantified.
In vivo depletion of CD4+ and CD8+ T cells.
Ascites fluids containing GK1.5 and 53.6.7 monoclonal antibodies were produced in BALB/c nude mice, and the concentration of rat immunoglobulin G (IgG) was estimated as described previously (15). At days 3 and 4 before challenge with trypomastigotes, mice immunized with GST-P4-P7 were treated i.p. with a dose of 1 mg per day of rat IgG, anti-CD4, or anti-CD8. Seven days after challenge, each mouse received one more dose of 1 mg of rat IgG, anti-CD4, or anti-CD8. The efficacy of depletion of CD4+ or CD8+ spleen cells before challenge was commonly higher than 98 or 96%, respectively.
Peptide synthesis.
The peptides KEGSKSGV (aa 94 through 101), AESLFVYI (aa 121 through 128), HEHNLFGI (aa 130 through 137), YEIVAGYI (aa 140 through 147), AESWPSIV (aa 150 through 157), TEWETGQI (aa 320 through 327), and PETLGHEI (aa 650 through 658) were prepared with standard Nα[9-fluorenylmethyloxycarbonyl] on a PSSM8 multispecific peptide synthesizer (Shimadzu, Kyoto, Japan) by solid-phase synthesis with a scale of 30 μmol. Peptides were purified by high-pressure liquid chromatography in a Shimadzu system. Peptides were analyzed in a C18 Vydac column (10 by 250 mm, 5-μm particle diameter). The peptides were obtained in a range of 80 to 90% purity. Their identities were confirmed by using the Micro quantitative time of flight mass spectrometer equipped with an electrospray ionization source (Micromass, United Kingdom).
Statistical analysis.
The values of peak parasitemia of each individual mouse were log transformed before being compared by one-way analysis of variance followed by Tukey's honestly significant difference (HSD) tests available at the site http://faculty.vassar.edu/lowry/VassarStats.html. The log rank test was used to compare the mouse survival rate after challenge with T. cruzi. Fisher's exact test was used to compare the different proportions of mice that survived infection. The differences were considered significant when the P value was <0.05.
RESULTS
Initially, we compared the protective immune responses of A/Sn mice immunized according to the group protocols pcDNA3 (I), alum (II), ASP-2 DNA (III), Alum/His-65kDa (IV), or ASP-2 DNA (V), followed by Alum/His-65kDa (DNA priming/protein boosting). After a challenge with bloodstream trypomastigotes of T. cruzi, we observed a significant reduction in the peak parasitemia of animals from mouse groups III, IV, and V compared to the control mice of groups I and II (P < 0.05 in all cases) (Table 1). The peak parasitemias of animals from mouse groups III, IV, and V were not statistically different (P > 0.05).
TABLE 1.
Trypomastigote-induced parasitemia and mortality in mice immunized with either ASP-2 DNA or recombinant protein administered individually or sequentially (DNA priming/protein boosting)
| Mouse group no. | Immunogena | Peak parasitemia (log ± SD)b | No. of mice alive/total no. challengedc | % Protection |
|---|---|---|---|---|
| I | pcDNA3 | 6.35 ± 0.08 | 0/16 | 0 |
| II | Alum | 6.53 ± 0.05 | 0/8 | 0 |
| III | ASP-2 DNA | 5.42 ± 0.09 | 12/16 | 75.00 |
| IV | Alum/His-65kDa | 5.62 ± 0.32 | 8/15 | 53.31 |
| V | ASP-2 DNA plus alum/His-65kDa | 5.55 ± 0.23 | 11/15 | 73.74 |
Groups of seven or eight mice were immunized at 0, 3, and 5 weeks with pcDNA3, ASP-2 DNA, alum, or the formulation alum/His-65kDa. A fifth group of mice was injected with two doses of ASP-2 DNA at 0 and 3 weeks. A third immunizing dose was administered after 5 weeks and consisted of the formulation alum/His-65kDa.
Two weeks after the last dose, mice were challenged i.p. with 250 bloodstream trypomastigotes. The values of peak parasitemia of mouse groups III, IV, and V were lower than those for mouse groups I and II (P < 0.05 in all cases). The peak parasitemias of groups III, IV, and V were not statistically different (P > 0.05).
The number of mice alive was recorded 60 days after challenge. Animals from groups III, IV, and V survived longer than mice from groups I and II (P < 0.0001 in all cases by the log rank test). There was no significant statistical difference among groups III, IV, and V (P > 0.05).
We also recorded the mouse mortality among the different mouse groups after the challenge with T. cruzi. Survival at day 60 was 75, 53.31, or 73.74% in mouse groups III, IV, or V, respectively. Statistical comparisons were performed by the log rank, chi-square, or Fisher exact probability tests. In all cases, we failed to detect a significant difference among these three mouse groups.
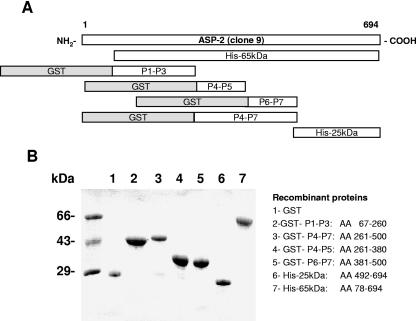
Based on the results that immunization with a recombinant protein adsorbed to alum provided protective immunity, we used six different recombinant proteins representing different portions of ASP-2 for subsequent studies of vaccination. Figure 1A and 1B displays a schematic representation and the SDS-PAGE analysis of the different recombinant proteins, respectively.
FIG. 1.
Schematic representation and SDS-PAGE analysis of the recombinant proteins based on the ASP-2 antigen. (A) Schematic representation of the recombinant proteins. (B) SDS-PAGE of recombinant proteins stained with Coomassie blue. The amount of protein loaded was approximately 1 μg per lane.
Initially, A/Sn mice were immunized with the recombinant proteins His-65kDa, GST-P1-P3, GST-P4-P7, and His-25kDa. These recombinant proteins were adsorbed to alum and injected i.p. in the presence of CpG ODN 1826. Control mice were immunized with the adjuvants only (alum and CpG ODN 1826). To determine whether the immunizations were performed properly, after the third immunizing dose, the presence of specific antibodies was determined by enzyme-linked immunosorbent assay using the recombinant protein His-65kDa as an antigen. The stronger antibody responses were detected in animals immunized with recombinant protein His-65kDa or His-25kDa. Antibody titers of mice immunized with GST-P4-P7 were higher than the titers of mice immunized with GST-P1-P3. Sera from control mice injected with adjuvant only had no detectable specific antibodies to His-65kDa (data not shown).
After challenge with bloodstream trypomastigotes in mice immunized with His-65kDa or GST-P4-P7, we observed a significant reduction in the peak parasitemia compared to animals immunized with adjuvant only, GST-P1-P3, or His-25kDa (Fig. 2) (P < 0.01 in all cases). No significant difference in statistics was observed when we compared the peak parasitemia of mice immunized with His-65kDa or GST-P4-P7 (Fig. 2) (P > 0.05). Also, no difference was found when we compared the peak parasitemias of mice immunized with GST-P1-P3 or His-25kDa or adjuvant alone (Fig. 2) (P > 0.05).
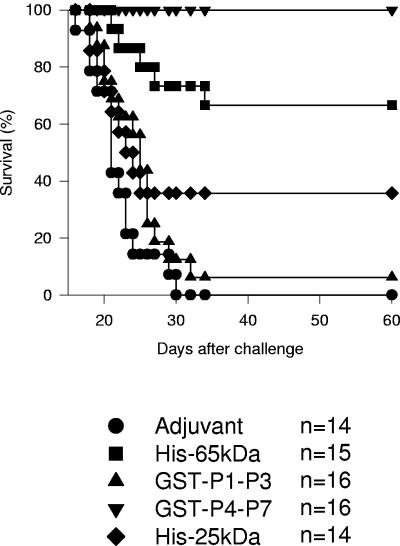
Also important was the fact that immunization with His-65kDa or GST-P4-P7 dramatically reduced mouse mortality after a challenge. While 100% of the control mice injected with adjuvant died before the 30th day after the challenge, 66.66% or 100% of the mice immunized with His-65kDa or GST-P4-P7, respectively, survived the challenge (Fig. 3). In contrast, survival was limited in mice immunized with recombinant proteins GST-P1-P3 or His-25kDa. In these cases, mouse survival was 6.25% or 35.74%, respectively (Fig. 3).
FIG. 3.
Trypomastigote-induced mortality in A/Sn mice immunized with recombinant proteins His-65kDa, GST-P1-P3, GST-P4-P7, or His-25kDa. The graph shows the Kaplan-Meier curves for the survival of 14 to 16 mice per group. Statistical analysis revealed significant differences in the survival of mice immunized with GST-P4-P7 compared to that of mice immunized with other recombinant proteins or adjuvant only (P < 0.05 in all cases by the log rank test). Mice immunized with His-65kDa displayed a survival rate significantly higher than that for other mouse groups with the exception of the animals that received GST-P4-P7 (P < 0.05 in all cases). Mice immunized with His-25kDa had a survival rate higher than that for the control animals that received adjuvant only (P < 0.05). In contrast, the survival of mice injected with GST-P1-P3 or adjuvant only was not statistically different (P > 0.05).
Statistical analysis revealed significant differences in the survival of mice immunized with GST-P4-P7 compared to mice immunized with other recombinant proteins or adjuvant only (P < 0.05 in all cases). Mice immunized with His-65kDa displayed a survival rate significantly higher than that for other mouse groups, with the exception of the animals that received GST-P4-P7 (P < 0.05 in all cases). Mice immunized with His-25kDa had a survival rate higher than that for the control animals that received adjuvant only (P < 0.05). In contrast, the survival of mice injected with GST-P1-P3 or adjuvant only was not statistically different (P > 0.05).
The mice vaccinated with GST-P4-P7 that survived the challenge were rechallenged 56 days later. Seven of eight mice survived this second challenge, while all negative control mice immunized with adjuvant alone died (n = 6). Parasitological studies were performed on the surviving mice 77 days after the second challenge. We found that 57.1% of the mice (four of seven) harbored living parasites that could be isolated from their blood. These results indicated that the majority of the vaccinated mice were not able to completely eliminate the parasites.
We also evaluated the presence of parasite nests and the level of inflammatory response in their heart and striated muscle. Histological analyses failed to detect the presence of parasite nests in the heart or striated muscle of mice vaccinated with GST-P4-P7. No inflammatory lesions were found in the striated muscle of vaccinated mice. In their heart tissue, either no lesions or minimal lesions were found. We concluded that most vaccinated A/Sn mice not only survived two challenges with T. cruzi but also controlled the inflammatory reaction in the skeletal and heart muscles (data not shown).
The recombinant protein GST-P4-P7 expresses aa 261 to 500 of ASP-2. In an attempt to further narrow the protective epitopes present in this recombinant protein, we immunized mice with two other proteins representing aa 261 to 380 (GST-P4-P5) or aa 381 to 500 (GST-P6-P7) of ASP-2. After challenged with trypomastigotes, mice vaccinated with GST-P4-P7 or GST-P4-P5 displayed a peak parasitemia significantly lower than that for animals injected with GST-P6-P7 or adjuvant alone (Table 2) (P < 0.01). No statistically significant difference was observed when we compared the peak parasitemia of mice immunized with GST-P4-P7 or GST-P4-P5 (Table 2) (P > 0.05). Also, no difference was found when we compared the peak parasitemia of mice immunized with GST-P6-P7 or adjuvant alone (Table 2) (P > 0.05).
TABLE 2.
Trypomastigote-induced parasitemia and mortality in A/Sn mice immunized with recombinant protein GST-P4-P7, GST-P4-P5, or GST-P6-P7
| Immunogena | Peak parasitemia (log ± SD)b | No. of mice alive/total no. challengedc | % Protection |
|---|---|---|---|
| Adjuvant | 6.06 ± 0.16 | 0/6 | 0 |
| GST-P4-P7 | 4.67 ± 0.61 | 5/5 | 100 |
| GST-P4-P5 | 4.96 ± 0.34 | 6/6 | 100 |
| GST-P6-P7 | 6.13 ± 0.21 | 0/6 | 0 |
Groups of five or six mice were immunized at 0, 2, and 4 weeks with GST-P4-P7, GST-P4-P5, GST-P6-P7, or adjuvant alone as described in the legend to Fig. 2. Two weeks after the last dose was given, mice were challenged i.p. with 250 bloodstream trypomastigotes.
The peak parasitemias in mice immunized with either GST-P4-P7 or GST-P4-P5 were lower than those for animals immunized with GST-P6-P7 or adjuvant alone (P < 0.01 in all cases by Tukey's HSD test). The peak parasitemias of mice immunized with either GST-P4-P7 or GST-P4-P5 were not statistically different (P > 0.05).
Mouse survival was recorded at day 60 after infection. Animals immunized with GST-P4-P7 or GST-P4-P5 survived longer than mice injected with GST-P6-P7 or adjuvant alone (P < 0.0001 by the log rank test). There was no significant statistical difference between the survival curves of mice vaccinated with GST-P4-P7 or GST-P4-P5.
Statistical analysis revealed a significant difference in the survival of mice immunized with GST-P4-P7 or GST-P4-P5 when compared to mice immunized with GST-P6-P7 or adjuvant only (P < 0.0001 in all cases). The survival of mice injected with GST-P6-P7 or adjuvant only was not statistically different (P > 0.05) (Table 2). These results clearly indicated that the protective epitopes present in GST-P4-P7 were located at the aa 261 to 380 present in the recombinant protein GST-P4-P5.
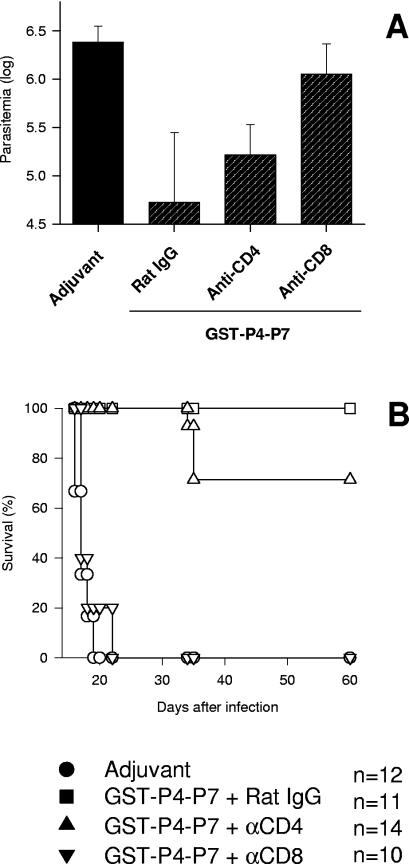
To determine the participation of either CD4+, CD8+, or both types of T cells in the protective immunity observed after immunization with the recombinant protein GST-P4-P7, we treated vaccinated mice with anti-CD4 or anti-CD8 antibodies prior to the challenge with T. cruzi. Treatment with monoclonal antibody anti-CD8 dramatically increased mouse susceptibility to infection compared to animals that received only rat IgG. The peak parasitemia of mice treated with anti-CD8 was significantly higher than that for vaccinated mice that received rat IgG or anti-CD4 (P < 0.05 in both cases) (Fig. 4A).
FIG. 4.
Trypomastigote-induced parasitemia and mortality in mice immunized with GST-P4-P7 and treated with anti-CD4 or anti-CD8 antibodies. Groups of five or six mice were immunized as described in the legend to Fig. 2 with the recombinant protein GST-P4-P7 or adjuvant alone. Mice immunized with the recombinant protein were treated with rat IgG, anti-CD4, or anti-CD8 as described in Materials and Methods. (A) The peak parasitemias in animals immunized with GST-P4-P7 and treated with rat IgG or anti-CD4 were significantly lower than the parasitemias of immunized animals treated with anti-CD8 (P < 0.01 in all cases by Tukey's HSD test). The values of parasitemia of mice immunized with GST-P4-P7 and treated with anti-CD8 were not different from those of control mice injected with adjuvant only (P > 0.05). (B) The graph shows Kaplan-Meier curves for the survival of 10 to 14 mice per group. Animals immunized with GST-P4-P7 and treated with rat IgG or anti-CD4 survived longer than mice treated with anti-CD8 (P < 0.0001 by the log rank test). There was no significant statistical difference between the survival curves of mice vaccinated with the recombinant protein GST-P4-P7 and treated with anti-CD8 or control animals injected with adjuvant alone (P > 0.05).
While 100% of the mice vaccinated with GST-P4-P7 and treated with rat IgG survived the infection, 100% (10 of 10) of anti-CD8-treated mice died (P < 0.0001). In contrast, only 4 of the 14 mice vaccinated with GST-P4-P7 and treated with anti-CD4 died after the challenge (P > 0.05) (Fig. 4B). We concluded from these experiments that, in vivo, CD8+ T cells were critical to the efficient reduction of peak parasitemia and to mouse survival after vaccination with the recombinant protein GST-P4-P7.
Our results established that CD8+ T cells provide the protective immunity observed after immunization with GST-P4-P7. In order to determine whether we could detect specific CD8+ T cells in the spleens of mice immunized with the different recombinant proteins, we synthesized seven peptides based on the predicted amino acid sequence of the asp-2 gene. Peptides were selected by the presence of putative sites for binding to A/Sn mouse major histocompatibility complex class I gene product H-2Kk. Peptides with putative sites for binding to H-2Dd were not present in the predicted amino acid sequence of the asp-2 gene (2).
The spleen cells of immunized mice were tested in the ELISPOT assay for their ability to secrete gamma interferon (IFN-γ) upon in vitro stimulation in the presence of the different peptides. During repeated experiments, we detected the presence of peptide-specific IFN-γ-secreting cells in the spleens of mice immunized with His-65kDa or GST-P4-P7 specific for the epitope represented by the synthetic peptide TEWETGQI (Table 3). In contrast, in mice immunized with GST-P1-P3 or His-25kDa, we were unable to detect the presence of specific IFN-γ-secreting cells to the peptides tested (Table 3). The epitope TEWETGQI is located between aa 320 and 327 of ASP-2. These amino acids are represented between GST-P4-P7 and GST-P4-P5.
TABLE 3.
ELISPOT assay for the detection of peptide-specific IFN-γ-secreting cells
| Peptideb in culture | No. of IFN-γ-secreting cells/106 spleen cells of mice immunized with recombinant proteinsa:
|
||||
|---|---|---|---|---|---|
| Adjuvantc | His-65kDa | GST-P1-P3 | GST-P4-P7 | His-25kDa | |
| No peptide | 3.0 ± 4.2 | 6.0 ± 0.0 | 5.0 ± 4.2 | 5.0 ± 1.4 | 5.0 ± 4.2 |
| KEGSKSGV | 2.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 5.0 ± 4.2 | 4.0 ± 2.8 |
| AESLFVYI | 1.0 ± 1.4 | 1.0 ± 1.4 | 0.0 ± 0.0 | 3.0 ± 1.4 | 5.0 ± 1.4 |
| HEHNLFGI | 5.0 ± 1.4 | 3.0 ± 1.4 | 1.0 ± 1.4 | 8.0 ± 2.8 | 2.0 ± 0.0 |
| YEIVAGYI | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.0 ± 0.0 | 8.0 ± 2.8 | 0.0 ± 0.0 |
| AESWPSIV | 0.0 ± 0.0 | 1.0 ± 1.4 | 0.0 ± 0.0 | 5.0 ± 1.4 | 1.0 ± 1.4 |
| TEWETGQI | 1.0 ± 1.4 | 29.0 ± 1.4d | 1.0 ± 1.4 | 113.0 ± 9.8d | 0.0 ± 0.0 |
| PETLGHEI | 3.0 ± 1.4 | 2.0 ± 2.8 | 0.0 ± 0.0 | 4.0 ± 2.8 | 4.0 ± 0.0 |
The number of IFN-γ-secreting cells was determined by the ELISPOT assay.
The final concentration of each peptide in culture was 10 μg/ml.
A/Sn mice were immunized with the indicated recombinant protein as described in the legend to Fig. 2. Two weeks after the last immunization, pooled spleen cells were collected from three mice.
The number of IFN-γ-secreting cells in the cultures containing the peptide TEWETGQI were higher than the number in cultures containing no peptide or other peptides (P < 0.01 by Tukey's HSD test).
The phenotype of IFN-γ-producing cells specific for the peptide TEWETGQI was established using a selective in vivo depletion approach. Mice immunized with GST-P4-P7 were treated with either anti-CD4 or anti-CD8 or rat IgG. The number of peptide-specific IFN-γ-producing cells per 106 spleen cells of mice treated with rat IgG, anti-CD4, or anti-CD8 was 170.0 ± 16.9, 174.6 ± 6.0, or 36.0 ± 8.7, respectively. Therefore, we concluded that the in vivo depletion of CD8+ cells reduced the number of peptide-specific IFN-γ-producing cells by 79%. In contrast, depletion of CD4 cells had no impact on the number of cells specific to the epitope TEWETGQI.
To determine whether there was a correlation between the protective immunity observed after immunization with the recombinant proteins His-65kDa, GST-P4-P7, GST-P1-P3, or His-25kDa and the immune response to the peptide TEWETGQI, we evaluated the number of IFN-γ-secreting cells in the spleens of individual mice immunized with each recombinant protein. The number of IFN-γ-secreting cells specific to the peptide TEWETGQI per 106 spleen cells in mice immunized with GST-P4-P7 or His-65kDa were 147.11 ± 28.87 and 41.66 ± 11.86, respectively (n = 3, P < 0.01, by Tukey's HSD test). In mice immunized with GST-P1-P3, His-25kDa, or adjuvant only, we could not detect IFN-γ-secreting cells specific for the peptide TEWETGQI.
DISCUSSION
The aim of our study was to explore the possibility of using bacterial recombinant proteins based on the ASP-2 antigen for vaccination against experimental T. cruzi infection. Our initial hypothesis was that a recombinant protein based on the ASP-2 antigen could be useful as a booster injection in mice previously immunized with DNA. We reasoned that this regimen could enhance the protective immune response observed with DNA vaccination alone. Contrary to this hypothesis, we found that the survival of mice immunized with DNA alone or with a DNA priming/protein boosting regimen was nearly identical (75 or 73.74%, respectively).
Based on the partial protection that we observed, we decided to pursue the question of whether recombinant proteins of ASP-2 could be used to elicit protective immunity against a T. cruzi infection. In the subsequent experiments, we added a second adjuvant, CpG ODN 1826, to alum. We selected this adjuvant based on previous studies showing that CpG ODN 1826 could improve Th1 immune response and protective immunity to a T. cruzi infection induced by a purified native protein (6). At this point, the precise relevance of CpG ODN 1826 in our system is not clear. It is certainly not critical because we can see significant protection in the absence of CpG ODN 1826 after vaccination with His-65kDa or GST-P4-P7 (Table 1 and data not shown, respectively). At the same time, the presence of CpG ODN 1826 did help protective efficacy. It is possible that the presence of CpG ODN 1826 increases the activation of specific CD8+ T cells. This hypothesis remains to be tested.
To map the protective determinants present within His-65kDa, we used three proteins containing different domains of ASP-2. Immunization with recombinant proteins GST-P1-P3 or His-25kDa representing the N- or the C-terminal domains of ASP-2, respectively, provided limited protective immunity compared to His-65kDa. In contrast, when we used a recombinant protein expressing aa 261 to 500 of ASP-2 (GST-P4-P7) for vaccination, we obtained 100% protection against a lethal challenge with T. cruzi trypomastigotes. Statistical analysis revealed that the protective efficacy of the recombinant protein GST-P4-P7 was higher than that of His-65kDa. The results obtained with GST-P4-P7 not only confirmed the protective potential of recombinant proteins based on the ASP-2 of T. cruzi but also mapped highly protective epitope(s) to the central domain of this molecule. Using a shorter recombinant protein (GST-P4-P5), we were able to narrow this highly protective epitope(s) to the region between aa 261 and 380. Whether smaller recombinant proteins, or even synthetic peptides, can be used to vaccinate A/Sn mice remains to be evaluated.
Protective immunity obtained after immunization with GST-P4-P7, alum, and CpG ODN 1826 was remarkable compared to other protocols of immunization. This formulation provided a high degree of protective immunity compared to ASP-2 DNA, His-65 kDa, or both in the same protocol of vaccination. Until now, more than 50 mice immunized with this formulation have been challenged. To date, all of these immunized mice have survived T. cruzi challenge. Detailed studies on the longevity of protective immune responses induced with GST-P4-P7 vaccination have yet to be performed. Nevertheless, vaccinated A/Sn mice that survived the first challenge were rechallenged 56 days later. Seven of eight mice survived infection, proving that this protective immunity is highly efficient.
Although protective immunity elicited by immunization with recombinant protein GST-P4-P7 drastically reduced the peak parasitemia and promoted mouse survival, we did not obtain sterile immunity in most animals. In spite of the fact that sterile immunity was not achieved, we found little or no inflammation in the heart or striated muscle of mice challenged twice in the course of 133 days. Our observations suggest that vaccination controls acute-phase parasitemia, survival, and the development of inflammatory responses in the heart and striated muscle. Our results reinforce previously described observations that immunity induced by T. cruzi ASP-2 encoding the DNA vaccine gene drastically reduce the inflammatory responses in the heart and skeletal muscle of A/Sn mice (28).
While vaccination with GST-P4-P7 protected 100% of the mice against infection, immunization with His-65kDa or ASP-2 DNA never achieved this level of protective immunity. The fact that immunization with GST-P4-P7 was more efficient than the vaccination protocol using a larger recombinant protein correlated with the number of IFN-γ-producing cells specific to the peptide TEWETGQI (Table 3). Therefore, it is plausible to propose that the shorter GST-P4-P7 recombinant protein can induce greater numbers of protective CD8+ T cells specific for the peptide TEWETGQI. The specific reason for these results is currently unknown. It is possible that the protective epitope TEWETGQI may be better processed by antigen-presenting cells after immunization with GST-P4-P7. Also, the presence of GST may provide greater T-cell help to the epitope TEWETGQI. Alternatively, we should evaluate whether His-65kDa contains an epitope(s) that activates regulatory T cells which can inhibit the CD8+-T-cell immune response to TEWETGQI.
Through depletion studies, we found that treatment with anti-CD8, but not with anti-CD4 antibodies, completely reversed the protective immunity elicited by immunization with GST-P4-P7. The fact that depletion of CD4+ T cells had a limited impact on protective immunity was not fully expected. Our previous study has shown that when treated with anti-CD4 antibodies, mice vaccinated simultaneously with asp-2 and ts genes were as susceptible to infection as anti-CD8-treated mice (28). The results described here cannot be attributed only to an inefficient elimination of CD4+ T cells because immunized mice were treated with the same antibodies and schedule used in the previous report (28). Also, the extension of depletion was monitored by fluorescence-activated cell sorter analysis before and after challenge. However, the most important observation was the fact that mice treated with anti-CD8 were as susceptible to infection as control mice injected with adjuvant only. Therefore, it seems that in the absence CD8+ T cells, CD4+ T cells were not able to provide a significant degree of protective immunity. These results suggest a different balance in these two T-cell populations after immunization with DNA or the recombinant protein GST-P4-P7.
Previous studies have shown that formulations containing distinct recombinant proteins could elicit protective immunity in BALB/c or C57BL/6 mice against a lethal challenge with T. cruzi (16, 20, 23, 32, 34). Depletions of CD4+ or CD8+ T cells before challenge were not performed in animals vaccinated with cruzipain or TC52 (20, 23). In the case of vaccination with recombinant paraflagellar rod proteins, mice genetically deficient in CD8 expression failed to develop protective immunity against T. cruzi infection (34). Our results concur with these latter results, and both studies confirm that CD8+ T cells play a critical role in protective immunity elicited by recombinant proteins, as well as DNA vaccines (12, 28; reviewed in references 17 and 22).
How are these protective CD8+ T cells primed by the formulation containing recombinant protein, alum, and CpG ODN 1826? It is plausible to speculate that this formulation uses the cross-priming route of antigen presentation (reviewed in references 1 and 9). In fact, formulations containing CpG ODN 1826 are described as capable of inducing specific CD8+ T cells by the cross-priming route (10, 11; reviewed in reference 30). Whether this route of antigen presentation is operating in our case should be tested; this may provide an interesting model to study the development of an anti-T. cruzi vaccine.
In summary, our data on the protective immune response elicited by recombinant proteins of the ASP-2 antigen created a new model to study how recombinant proteins can be used to elicit CD8+-T-cell-mediated protective immune responses to this intracellular pathogen. Also, we have confirmed and extended the results of several other studies that have described the protective potential of this antigen (2, 5, 8, 28). Together, they emphasize the importance of the immune response to ASP-2 for the development of immunity to T. cruzi infection. Studies on the immune response to ASP-2 may aid our understanding of the immunopathological mechanisms leading to Chagas' disease and the development of new strategies for the prevention or treatment of the disease.
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo, CNPq, FAPEMIG, Brazil (EDT 24.000), and The National Institutes of Health, Bethesda, Md. (RO1 AI040196).
We thank Fanny Tzelepis for helping with the ELISPOT assay and Gisela Sasso for the histology. A.F.S.A., M.I.H., R.T.G., and M.M.R. are recipients of fellowships from CNPq. J.R.C.V., S.B.B., and B.C.G.D.A. were recipients of fellowships from FAPESP.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Ackerman, A. L., and P. Cresswell. 2004. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 5:678-684. [DOI] [PubMed] [Google Scholar]
- 2.Boscardin, S. B., S. S. Kinoshita, A. E. Fujimura, and M. M. Rodrigues. 2003. Immunization with cDNA expressed by amastigotes of Trypanosoma cruzi elicits protective immune response against experimental infection. Infect. Immun. 71:2744-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa, F., G. Franchin, V. L. Pereira-Chioccola, M. Ribeirão, S. Schenkman, and M. M. Rodrigues. 1998. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine 16:768-774. [DOI] [PubMed] [Google Scholar]
- 4.Dumonteil, E., J. Escobedo-Ortegon, N. Reyes-Rodriguez, A. Arjona-Torres, and M. J. Ramirez-Sierra. 2004. Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice. Infect. Immun. 72:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fralish, B. H., and R. L. Tarleton. 2003. Genetic immunization with LYT1 or a pool of trans-sialidase genes protects mice from lethal Trypanosoma cruzi infection. Vaccine 21:3070-3080. [DOI] [PubMed] [Google Scholar]
- 6.Frank, F. M., P. B. Petray, S. I. Cazorla, M. C. Munoz, R. S. Corral, and E. L. Malchiodi. 2003. Use of a purified Trypanosoma cruzi antigen and CpG oligodeoxynucleotides for immunoprotection against a lethal challenge with trypomastigotes. Vaccine 22:77-86. [DOI] [PubMed] [Google Scholar]
- 7.Fujimura, A. E., S. S. Kinoshita, V. L. Pereira-Chioccola, and M. M. Rodrigues. 2001. DNA sequences encoding CD4+ and CD8+ T-cell epitopes are important for efficient protective immunity induced by DNA vaccination with a Trypanosoma cruzi gene. Infect. Immun. 69:5477-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg, N., and R. Tarleton. 2002. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect. Immun. 70:5547-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath, W. R., G. T. Belz, G. M. Behrens, C. M. Smith, S. P. Forehan, I. A. Parish, G. M. Davey, N. S. Wilson, F. R. Carbone, and J. A. Villadangos. 2004. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199:9-26. [DOI] [PubMed] [Google Scholar]
- 10.Heit, A., K. M. Huster, F. Schmitz, M. Schiemann, D. H. Busch, and H. Wagner. 2004. CpG-DNA aided cross-priming by cross-presenting B cells. J. Immunol. 172:1501-1507. [DOI] [PubMed] [Google Scholar]
- 11.Heit, A., T. Maurer, H. Hochrein, S. Bauer, K. M. Huster, D. H. Busch, and H. Wagner. 2003. Toll-like receptor 9 expression is not required for CpG DNA-aided cross-presentation of DNA-conjugated antigens but essential for cross-priming of CD8 T cells. J. Immunol. 170:2802-2805. [DOI] [PubMed] [Google Scholar]
- 12.Katae, M., Y. Miyahira, K. Takeda, H. Matsuda, H. Yagita, K. Okumura, T. Takeuchi, T. Kamiyama, A. Ohwada, Y. Fukuchi, and T. Aoki. 2002. Coadministration of an interleukin-12 gene and a Trypanosoma cruzi gene improves vaccine efficacy. Infect. Immun. 70:4833-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krettli, A. U., and Z. Brener. 1976. Protective effects of specific antibodies in Trypanosoma cruzi infections. J. Immunol. 116:755-760. [PubMed] [Google Scholar]
- 14.Letvin, N. L., D. C. Montefiori, Y. Yasutomi, H. C. Perry, M. E. Davies, C. Lekutis, M. Alroy, D. C. Freed, C. I. Lord, L. K. Handt, M. A. Liu, and J. W. Shiver. 1997. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc. Natl. Acad. Sci. USA 94:9378-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, S., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, M. Esteban, P. Palese, R. S. Nussenzweig, and F. Zavala. 1993. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. USA 90:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luhrs, K. A., D. L. Fouts, and J. E. Manning. 2003. Immunization with recombinant paraflagellar rod protein induces protective immunity against Trypanosoma cruzi infection. Vaccine 21:3058-3069. [DOI] [PubMed] [Google Scholar]
- 17.Martin, D., and R. Tarleton. 2004. Generation, specificity, and function of CD8+ T cells in Trypanosoma cruzi infection. Immunol. Rev. 201:304-317. [DOI] [PubMed] [Google Scholar]
- 18.Morel, C. M., and J. Lazdins. 2003. Chagas disease. Nat. Rev. Microbiol. 1:14-15. [DOI] [PubMed] [Google Scholar]
- 19.Nyika, A., A. F. Barbet, M. J. Burridge, and S. M. Mahan. 2002. DNA vaccination with map1 gene followed by protein boost augments protection against challenge with Cowdria ruminantium, the agent of heartwater. Vaccine 20:1215-1225. [DOI] [PubMed] [Google Scholar]
- 20.Ouaissi, A., E. Guilvard, Y. Delneste, G. Caron, G. Magistrelli, N. Herbault, N. Thieblemont, and P. Jeannin. 2002. The Trypanosoma cruzi Tc52-released protein induces human dendritic cell maturation, signals via Toll-like receptor 2, and confers protection against lethal infection. J. Immunol. 168:6366-6374. [DOI] [PubMed] [Google Scholar]
- 21.Planelles, L., M. C. Thomas, C. Alonso, and M. C. Lopez. 2001. DNA immunization with Trypanosoma cruzi HSP70 fused to the KMP11 protein elicits a cytotoxic and humoral immune response against the antigen and leads to protection. Infect. Immun. 69:6558-6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues, M. M., S. B. Boscardin, J. R. Vasconcelos, M. I. Hiyane, G. Salay, and I. S. Soares. 2003. Importance of CD8 T cell-mediated immune response during intracellular parasitic infections and its implications for the development of effective vaccines. An. Acad. Bras. Cienc. 75:443-468. [DOI] [PubMed] [Google Scholar]
- 23.Schnapp, A. R., C. S. Eickhoff, D. Sizemore, R. Curtiss III, and D. F. Hoft. 2002. Cruzipain induces both mucosal and systemic protection against Trypanosoma cruzi in mice. Infect. Immun. 70:5065-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sepulveda, P., M. Hontebeyrie, P. Liegeard, A. Mascilli, and K. A. Norris. 2000. DNA-Based immunization with Trypanosoma cruzi complement regulatory protein elicits complement lytic antibodies and confers protection against Trypanosoma cruzi infection. Infect. Immun. 68:4986-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva, L. H. P., and V. Nussenzweig. 1953. Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Folia Clin. Biol. 20:191-203. [Google Scholar]
- 26.Song, M. K., S. W. Lee, Y. S. Suh, K. J. Lee, and Y. C. Sung. 2000. Enhancement of immunoglobulin G2a and cytotoxic T-lymphocyte responses by a booster immunization with recombinant hepatitis C virus E2 protein in E2 DNA-primed mice. J. Virol. 74:2920-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanghe, A., S. D'Souza, V. Rosseels, O. Denis, T. H. Ottenhoff, W. Dalemans, C. Wheeler, and K. Huygen. 2001. Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect. Immun. 69:3041-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasconcelos, J. R., M. I. Hiyane, C. R. F. Marinho, C. Claser, A. M. Vieira-Machado, R. T. Gazinelli, O. Bruña-Romero, J. M. Alvarez, S. B. Boscardin, and M. M. Rodrigues. 2004. Protective immunity against Trypanosoma cruzi infection in a highly susceptible mouse strain following vaccination with genes encoding the amastigote surface protein-2 and trans-sialidase. Hum. Gene Ther. 15:878-886. [DOI] [PubMed] [Google Scholar]
- 29.Vasconcelos, J. R., S. B. Boscardin, M. I. Hiyane, S. S. Kinoshita, A. E. Fujimura, and M. M. Rodrigues. 2003. A DNA-priming protein-boosting regimen significantly improves type 1 immune response but not protective immunity to Trypanosoma cruzi infection in a highly susceptible mouse strain. Immunol. Cell Biol. 81:121-129. [DOI] [PubMed] [Google Scholar]
- 30.Wagner, H., A. Heit, F. Schmitz, and S. Bauer. 2004. Targeting split vaccines to the endosome improves vaccination. Curr. Opin. Biotechnol. 15:538-542. [DOI] [PubMed] [Google Scholar]
- 31.Wizel, B., N. Garg, and R. Tarleton. 1998. Vaccination with trypomastigotes surface antigen1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect. Immun. 66:5073-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrightsman, R. A., B. D. Dawson, D. L. Fouts, and J. E. Manning. 1994. Identification of immunodominant epitopes in Trypanosoma cruzi trypomastigote surface antigen-1 protein that mask protective epitopes. J. Immunol. 153:3148-3154. [PubMed] [Google Scholar]
- 33.Wrightsman, R. A., and J. E. Manning. 2000. Paraflagellar rod proteins administered with alum and IL-12 or recombinant adenovirus expressing IL-12 generates antigen-specific responses and protective immunity in mice against Trypanosoma cruzi. Vaccine 18:1419-1427. [DOI] [PubMed] [Google Scholar]
- 34.Wrightsman, R. A., K. A. Luhrs, D. Fouts, and J. E. Manning. 2002. Paraflagellar rod protein-specific CD8+ cytotoxic T lymphocytes target Trypanosoma cruzi-infected host cells. Parasite Immunol. 24:401-412. [DOI] [PubMed] [Google Scholar]