Abstract
Bacillary angiomatosis (BA), one of the many clinical manifestations resulting from infection with the facultative intracellular bacterium Bartonella henselae, is characterized by angiogenic lesions. Macrophages have been identified as important effector cells contributing to the angiogenic process during B. henselae infection by infiltrating BA lesions and secreting vascular endothelial growth factor. Monocyte-macrophage chemoattractant protein 1 (MCP-1) recruits macrophages to sites of inflammation. In this study, we investigated the ability of B. henselae to upregulate MCP-1 gene expression and protein production in the human microvascular endothelial cell line HMEC-1. MCP-1 mRNA was induced at 6 and 24 h after treatment with bacteria, whereas protein production was elevated at 6, 24, and 48 h. This induction was not dependent on the presence of bacterial lipopolysaccharide or endothelial cell toll-like receptor 4. However, MCP-1 production was dependent on NF-κB activity. Outer membrane proteins of low molecular weight were able to upregulate MCP-1 production. Furthermore, supernatants from B. henselae-infected HMEC-1 were able to induce chemotaxis of THP-1 monocytes. These data suggest a mechanism by which the macrophage effector cell is recruited to the endothelium during B. henselae infection and then contributes to bacterial-induced angiogenesis.
Bartonella henselae is a fastidious gram-negative bacterial human pathogen of which the domestic cat is the natural reservoir. B. henselae can cause cat scratch disease, bacillary angiomatosis (BA), bacillary peliosis, bacteremia, and endocarditis (1, 3, 39, 48-50). BA is an opportunistic infection characterized by fever and angioproliferative lesions that may resemble Kaposi's sarcoma. Histological examination of BA lesions has revealed infiltration of polymorphonuclear leukocytes and macrophages (26, 27, 35). The exact mechanism of angiogenesis in BA is unknown; however, it has been shown that B. henselae-infected macrophages produce the pro-angiogenic mediator vascular endothelial growth factor (VEGF) (41). This VEGF production may be a crucial feature in the endothelial cell proliferation that is a hallmark of BA lesions. A paracrine loop has been proposed (41) in which the infected macrophage effector cell secretes VEGF and interleukin-8 (IL-8), which act as endothelial cell mitogens. In addition, the infected endothelial cell may upregulate expression and production of pro-angiogenic proteins such as chemokines, adhesion molecules (16), and cytokines (21, 41), while downregulating inducers of apoptosis such as caspases (22).
Pathogenic (tumor or inflammatory) angiogenesis provides a survival mechanism for the tissues of tumors and tumor-like lesions. The process of angiogenesis requires cooperation between cells, cytokines, growth factors, and matrix components. A sensitive balance between angiostatic and angiogenic factors must exist in order to control angiogenic activity; in tumors and tumor-like lesions, however, this tightly regulated system is upset (19). Angiogenesis is associated with conditions that involve inflammatory cell infiltrate (18), such as cancer, papopavirus infection, and herpesvirus infection (5, 7, 15, 29, 33). Angiogenesis and inflammation coordinate through common stimuli for endothelial cells and leukocytes; these stimuli include chemokines.
Angiogenic chemokines exert a direct effect on the endothelium and an indirect effect on angiogenic factor-expressing leukocytes (37, 52). Chemokines are induced by tumor necrosis factor (TNF) or IL-1 or by interaction with bacterial pathogens. Chemokines recruit leukocytes to sites of inflammation. Specifically, monocyte-macrophage chemoattractant protein 1 (MCP-1) stimulates chemotaxis of monocytes and macrophages to sites of inflammation (28). MCP-1 can also directly promote angiogenesis (37, 52). Thus, MCP-1 could play dual roles in the potential paracrine loop model of B. henselae-induced angiogenesis by acting in an autocrine-like manner on endothelial cells to promote angiogenesis, while recruiting macrophages, the effector cell in the model, to the site of infection.
Endothelial cells are critical for inflammation. They control vascular permeability, regulate coagulation and thrombosis, direct passage of leukocytes into areas of inflammation, and produce pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and adhesion molecules. Vascular endothelial cells line the luminal side of blood vessels and mediate interactions between blood and tissue (10, 23). Although they are not professional phagocytes, endothelial cells express toll-like receptors (tlr) which can be activated in response to pattern recognition molecules and therefore play a role in innate immunity. Human microvascular endothelial cells (HMEC-1) express tlr4, which recognizes lipopolysaccharide (LPS), endogenous HSP60, chlamydial HSP60, and other lipoproteins (17, 25, 38, 44, 51). Toll-like receptor signaling results in activation of NF-κB and transcription of genes such as chemokines and inflammatory cytokines. Since chemokines are important for angiogenesis and macrophage recruitment, toll-like receptor signaling in endothelial cells could contribute to B. henselae-induced angiogenesis.
In order to better understand the response of endothelial cells to the pathogen B. henselae, we examined the effect of B. henselae infection on expression and protein levels of the chemokine MCP-1 in HMEC-1. In addition, we examined the ability of conditioned media from B. henselae-infected HMEC-1 to cause chemotaxis of THP-1 monocytes. The data support a role for MCP-1 during B. henselae infection whereby MCP-1 mediates macrophage infiltration, leading to an angiogenic state.
(This work was presented in part at the 105th General Meeting of the American Society of Microbiology, Atlanta, Ga., 5 to 9 June 2005.)
MATERIALS AND METHODS
Bacterial strains.
Bartonella henselae Houston-1 (ATCC 49882) (40) strain was grown on chocolate agar prepared with heart infusion agar base (Difco, Detroit, MI) supplemented with 1% bovine hemoglobin (Becton Dickinson, Cockeysville, MD). Bacterial cultures were maintained at 37°C and 5% CO2 and humidity to saturation. For certain experiments, bacteria were heat killed at 100°C for 30 min as described previously (41). Escherichia coli strain JM109 was grown in Luria-Bertani broth or agar (Difco).
Cell lines.
The immortalized human microvascular cell line HMEC-1 (2) was cultured in MCDB131 cell culture media (Gibco BRL, Grand Island, NY) supplemented with 10% fetal calf serum (HyClone Laboratories, Logan, UT), 10 ng/ml epidermal growth factor, 1.461 g/liter l-glutamine, 1 μg/ml hydrocortisone, 50 μg/ml penicillin-streptomycin, 2.5 μg/ml amphotericin B (Sigma-Aldrich, St. Louis, MO), 2 mg/ml sodium bicarbonate, and 10 mM HEPES (Mediatech, Herndon, VA). Human THP-1 monocytes (54) were cultured in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% fetal calf serum, 5 μM 2-mercaptoethanol (Sigma-Aldrich), 10 μg/ml vancomycin (Sigma-Aldrich), and 1 μg/ml amphotericin B. Cells were subcultured every 2 days and were used in experiments at passages from 6 to 10. Cell cultures were maintained at 37°C and 5% CO2 and humidity to saturation.
Coculture of endothelial cells and bacteria.
Prior to infection, the culture medium containing antibiotics was removed from cell cultures and replaced with MCDB131 without antibiotics or growth factors. Cells were permitted to adapt overnight. B. henselae Houston-1 cells were harvested from chocolate agar and suspended in cell culture media, and then the concentration of bacteria was determined spectrophotometrically. We have previously determined by plate counts that at an optical density of 600 nm, a reading of 0.5 is estimated to represent 109 bacteria. B. henselae bacteria were added to cells at a multiplicity of infection of 500 unless otherwise indicated. Cells were cocultured with Houston-1 cells at various time points.
For E. coli cocultures, JM109 bacteria were harvested into cell culture medium and added to cells at a density of 100 E. coli cells per HMEC-1 cell, also determined spectrophotometrically (43). During the experiments with longer time courses and during inhibition assays, E. coli LPS (Sigma-Aldrich) was used as a positive control for MCP-1 production in order to keep HMEC-1 cells alive, as E. coli JM109 infection was found to be cytotoxic for HMEC-1 cells at later time points.
Inhibition assays.
Polymyxin B sulfate (Sigma-Aldrich) was used to neutralize the bacterial LPS in some experiments. Houston-1 or E. coli JM109 cells or purified E. coli LPS was incubated with 30 μg/ml polymyxin B sulfate for 1 h at 37°C and 5% CO2 before being added to HMEC-1. In some experiments, HMEC-1 cells were preincubated with a mouse monoclonal antibody (HTA 125) specific for human toll-like receptor-4 (tlr-4) (e-Bioscience, San Diego, CA) in order to determine whether tlr-4 signaling was required for MCP-1 production. HMEC-1 cells were preincubated with 20 μg/ml anti-tlr4 for 1 h at 37°C with gentle shaking, and then cocultures proceeded as usual and supernatants were collected 8 h after addition of bacteria or LPS. Live E. coli JM109 cells were cultured with HMEC-1 during some experiments in order to ensure that the tlr4 monoclonal antibody would block activity from the LPS of live E. coli as well as from purified LPS. An isotype control (mouse immunoglobulin G2a, κ [IgG2a, κ]) (e-Bioscience) was used as a control antibody at 20 μg/ml. During NF-κB inhibition assays, HMEC-1 cells were incubated with 50 μM pyrrolidinedithiocarbamate (PDTC) (Sigma) or 100 μM N-tosyl-l-phenylalanine (TPCK) (Sigma) for 1 h at 37°C with 5% CO2 before infections. Inhibitors were maintained throughout the course of the assays.
Semiquantitative RT-PCR.
Reverse transcription-PCR (RT-PCR) was performed on HMEC-1 cocultured with B. henselae or E. coli at the indicated times after infection. Total RNA was extracted with TRIzol reagent (Sigma-Aldrich) according to the manufacturer's protocol. Total RNA was treated with RNase-free DNase (Ambion, Inc., Austin, TX) according to the manufacturer's instructions. The concentration of RNA was determined spectrophotometrically using a GeneQuant II system (Pharmacia Biotech, Cambridge, England). cDNA preparation and subsequent PCR amplification were carried out with a One-Step RT-PCR kit (QIAGEN, Inc., Valencia, CA) in the presence of gene-specific primers and 2 μg total RNA. The PCR conditions were 1 min at 95°C, 1 min at 58°C, and 1 min at 72°C for 35 cycles. Primer sequences for RT-PCR were as follows: for β-actin forward, 5′-AGAAAATCTGGCACCACACC-3′; for β-actin reverse, 5′-CCATCTCTTGCTCGAAGTCC-3′; for MCP-1 forward, 5′-TTCTCAAACTGAAGCTCGCACTCTCGCC-3′; and for MCP-1 reverse, 5′-TGTGGAGTGAGTGTTCAAGTCTTGGGAGTT-3′. PCR products were analyzed by electrophoresis through 2% agarose gels and were visualized by ethidium bromide staining. Amplicon sizes were 434 bp and 348 bp for β-actin and MCP-1 primers, respectively. RT-PCR data were analyzed by scanning densitometry of gel bands with Kodak 1D Image Analysis software and normalizing to β-actin signals obtained from the same time points. RT-PCRs included a no-template control and a no-reverse-transcriptase control to exclude DNA or RNA contamination.
MCP-1 ELISA.
MCP-1 levels in HMEC-1 supernatants were assayed with an MCP-1 DuoSet enzyme-linked immunosorbent assay (ELISA) development system (R & D Systems, Minneapolis, MN) according to the manufacturer's protocol. A 3,3′,5,5′-tetramethylbenzidine liquid substrate system (Sigma-Aldrich) was added, and the color was allowed to develop for 20 min. The reaction was stopped with 2 N sulfuric acid. ELISA plates were analyzed using a μQuant plate reader (Bio-Tek, Winooski, VT) at 450 nm.
Chemotaxis of THP-1 monocytes.
Chemotaxis of THP-1 monocytes was examined with modified Boyden chambers (Neuroprobe, Cabin John, MD) according to the manufacturer's instructions. Briefly, the lower well of the chamber was filled with 1.2 ml supernatants from untreated, B. henselae-stimulated, or E. coli-stimulated HMEC-1. A total 510 μl of THP-1 cell suspension (5 × 105 cells) was added to the upper well. A 5-μm-pore-size polyvinylpyrrolidone-free polycarbonate membrane (Neuroprobe) separated the two wells. Migration occurred while incubating the chambers at 37°C and 5% CO2 with humidity for 4 h. After 4 h, the upper side of the membrane was scraped three times with a sterile swab soaked in phosphate-buffered saline to remove nonmigrated cells, and the lower side of the membrane was fixed and stained with Hema-3 Stat Pack (Biochemical Sciences, Inc., Swedesbord, NJ) according to the manufacturer's instructions. Cells were counted in five high-powered fields per membrane, and these numbers were averaged. Cell counts ranged from 6 to 10 cells per high-powered field, with the exception of cells that migrated in response to untreated HMEC-1 supernatants, in which case zero to two cells were counted per microscope field as a result of reduced migration of THP-1 cells. Results are expressed as a chemotactic index, in which the average number of cells that migrated in response to untreated HMEC-1 supernatants was set at 1. A graph of the means of chemotactic indices for three experiments is shown.
Preparation of crude B. henselae LPS.
B. henselae LPS was isolated as described previously with modifications (56). Briefly, 3-day-old bacteria were harvested from chocolate agar and suspended in phosphate-buffered saline. The bacterial pellet was washed three times with sterile water. Bacteria were lysed with lysis buffer (6% sodium dodecyl sulfate [SDS], 60 mM Tris, 6% β-mercaptoethanol, 10 mM dithiothreitol) at 100°C. Proteins were digested by proteinase K treatment. Crude LPS was dialyzed against sterile endotoxin-free water for 4 days. These crude extracts were concentrated with Centricon YM-3 (Millipore Corp., Bedford, MA). Crude B. henselae LPS was separated by Tricine-SDS-polyacrylamide gel electrophoresis and visualized by silver staining as described previously (56, 58). No proteins were visualized upon Coomassie blue staining. The concentration of B. henselae LPS was determined by separating both B. henselae and E. coli LPS (1 mg/ml) by SDS-polyacrylamide gel electrophoresis and silver staining and then scanning the gel and determining the concentration by densitometry.
Enrichment of outer membrane proteins from B. henselae and fractionation by molecular weight.
B. henselae outer membrane proteins (OMPs) were enriched as previously described (6, 14). The sarkosyl-insoluble pellet was resuspended in 10 mM HEPES (pH 7.4). Protein concentrations were determined using the Lowry protein assay (30). Protein (600 μg) in 400 μl Laemmli sample buffer with 142 mM 2-mercaptoethanol was heated at 95°C for 5 min and separated in a single large well of a two-dimensional 4 to 12% Bis-Tris NuPAGE gel (Invitrogen, Carlsbad, CA). Four sections of the gel were excised corresponding to 3 to 33 kDa (OMP-1), 34 to 52 kDa (OMP-2), 53 to 97 kDa (OMP-3), and 98+ kDa (OMP-4). Each section was minced, and proteins were eluted using a Model 422 Electro-Eluter (Bio-Rad, Hercules, CA) running at 30 milliamps for 3 h in elution buffer consisting of 25 mM Tris base, 192 mM glycine, and 0.1% SDS. Buffer was then exchanged using Microcon-YM-3 filters (Millipore Corp). Protein concentrations were determined using the Lowry protein assay (30). During some experiments, the lower-molecular-weight fraction of OMPs (OMP-1) was treated with polymyxin B sulfate (30 μg/ml) or proteinase K. OMP-1 was added to proteinase K (10 μg/ml) and incubated at 55°C for 3 h. Proteinase K was inactivated at 100°C for 15 min.
Statistics.
Significance was determined by a Student's t test with two-tailed distribution. P values less than 0.05 were considered significant. All experiments were performed in triplicate. Data are presented as the mean ± 1 standard deviation.
RESULTS
Induction of MCP-1 gene expression and protein production.
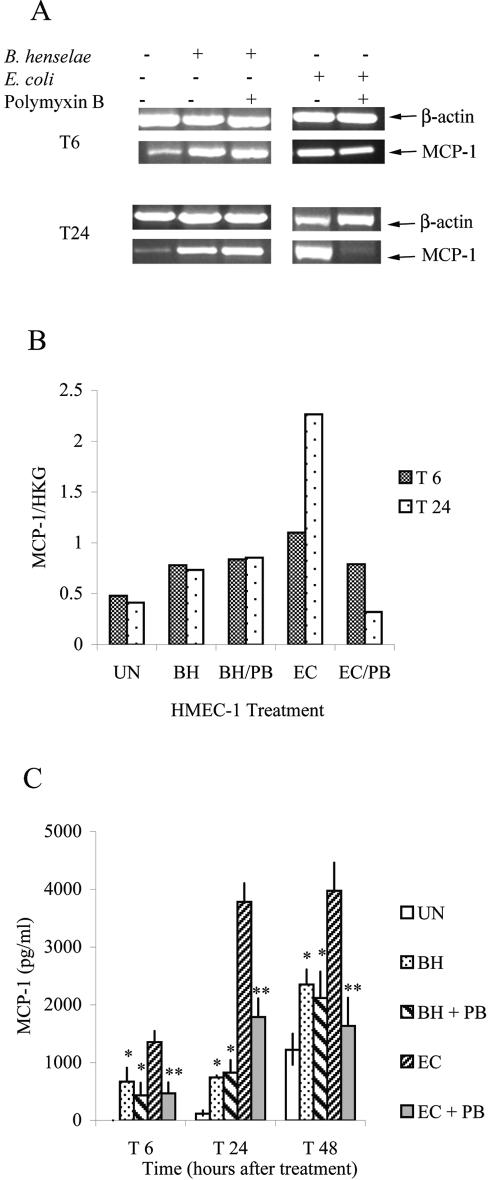
We investigated the effect of B. henselae coculture on MCP-1 expression and production in HMEC-1. MCP-1 transcript levels were assayed by semiquantitative RT-PCR at 6 and 24 h after infection (Fig. 1A). HMEC-1 cultured in the presence of B. henselae had 1.6 times higher levels of MCP-1 transcript than untreated controls at 6 h after treatment (T6) and 1.8 times higher levels of MCP-1 transcript 24 h after treatment (T24) (Fig. 1A and B). By 48 h after treatment, the MCP-1 message levels for uninfected HMEC-1 began to approach the MCP-1 message levels of B. henselae-infected HMEC-1 (data not shown). This was most likely due to the absence of growth factors in culture media; after 48 h of culture with no growth factors, cells lose viability and MCP-1 levels increase.
FIG. 1.
B. henselae induces MCP-1 in HMEC-1. (A) Cells were stimulated with B. henselae or E. coli. Some bacteria were preincubated with polymyxin B sulfate. The levels of MCP-1 mRNA in HMEC-1 were assayed by RT-PCR at 6 and 24 h after treatment (T6, T24). (B) RNA levels of MCP-1 in cells from experiments whose results are depicted in panel A were determined by scanning densitometry and normalized by comparison to the “housekeeping gene” (HKG) β-actin RNA levels. (C) MCP-1 protein production was determined by ELISA at 6, 24, and 48 h after treatment. (UN = untreated cells; BH = B.henselae-treated cells; PB = polymyxin B sulfate; EC = E. coli-stimulated cells; * = P < 0.007 [B. henselae treatment compared to untreated cells]; ** = P < 0.007 [untreated E. coli compared to E. coli treated with polymyxin B sulfate]). Results shown are those of one experiment representative of three similar experiments.
MCP-1 levels in supernatants of HMEC-1 were analyzed at 6, 24, and 48 h after treatment by ELISA. There was a significant increase in MCP-1 levels when HMEC-1 cells were cultured with B. henselae for 6, 24, or 48 h (P < 0.007) (Fig. 1C). In order to determine whether the bacterial stimulating molecule was heat stable, bacteria were heat killed by boiling at 100°C for 15 min before addition to HMEC-1. When bacteria were heat killed, MCP-1 levels were not significantly lowered (data not shown). These data indicate that a heat-stable molecule is responsible for MCP-1 production.
Induction of MCP-1 gene expression and protein production is independent of B. henselae LPS and endothelial cell tlr4.
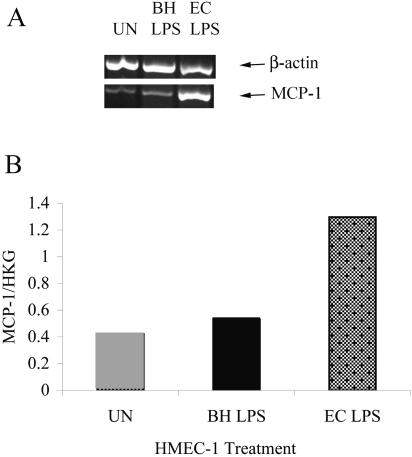
We investigated the role of B. henselae LPS in MCP-1 production from HMEC-1. Incubation of HMEC-1 with B. henselae LPS did not efficiently induce MCP-1 expression in HMEC-1 (Fig. 2A and B). We also investigated the effect of polymyxin B sulfate, an antibiotic known to neutralize LPS of many bacteria, including Bartonella spp. (34). Bacteria (B. henselae or E. coli) were treated with polymyxin B sulfate before addition to HMEC-1. Although incubation of E. coli with polymyxin B sulfate significantly lowered MCP-1 gene expression and protein production from HMEC-1 at T6, T24, and T48 (P ≤ 0.006), polymyxin B had little effect on B. henselae-induced MCP-1 expression and production (Fig. 1A, B, and C).
FIG. 2.
B. henselae LPS does not efficiently upregulate mcp-1 expression. (A) Cells were stimulated with 1 μg/ml B. henselae LPS or E. coli LPS. After 8 h, the levels of MCP-1 mRNA were assayed by RT-PCR. (B) RNA levels of MCP-1 in cells from experiments whose results are depicted in panel A were determined by scanning densitometry and normalized by comparison to housekeeping gene (HKG) β-actin levels. UN = untreated cells; BH = B. henselae-treated cells; EC = E. coli-stimulated cells.
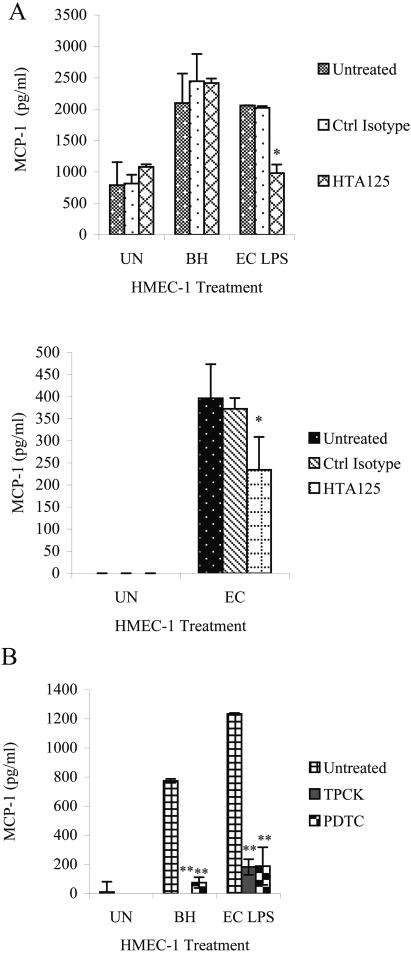
MCP-1 production can be mediated by tlr4 or tlr2 activation (53). Since HMEC-1 express tlr2 poorly (13), we investigated whether B. henselae-induced MCP-1 production is mediated through tlr4 on HMEC-1. We preincubated HMEC-1 with a tlr4 mouse monoclonal antibody (HTA125) or an isotype control (mouse IgG2a, κ) and then treated the cells with live B. henselae or E. coli LPS (1 μg/ml). Supernatants were collected 8 h after infection and assayed for MCP-1 by ELISA. While HTA125 reduced MCP-1 production caused by E. coli LPS (P < 0.002), B. henselae-induced MCP-1 production remained unchanged by the blocking antibody (P > 0.900) (Fig. 3A). E. coli LPS was used as a positive control for the activity of the blocking antibody; however, the induction of MCP-1 by live E. coli JM109 was also lowered in the presence of the blocking antibody (Fig. 3B; P < 0.01).
FIG. 3.
MCP-1 production during inhibition assays. Supernatants were collected 8 h after infection and analyzed by ELISA for MCP-1 levels. (A) Cells were incubated with anti-tlr4 (HTA125) or a control isotype (mouse IgG2a, κ) for 1 h before coculture with B. henselae or addition of E. coli LPS (1 μg/ml) (upper panel). Cells were incubated with HTA125 or a control isotype for 1 h before addition of live E. coli JM109 (lower panel). (B) HMEC-1 cells were cultured in the presence of the NF-κB inhibitor PDTC or TPCK before infection with B. henselae or addition of E. coli LPS (1 μg/ml). (UN = untreated HMEC-1; BH = HMEC-1 cocultured with B. henselae; EC LPS = HMEC-1 stimulated with E. coli LPS; EC = HMEC-1 stimulated with live E. coli; * = P < 0.05; ** = P < 2.00 × 10−4). A representative of three experiments is shown.
MCP-1 production requires NF-κB activation.
B. henselae outer membrane proteins have been shown to induce NF-κB in HUVEC. Recently, the virB (which encodes a putative type IV secretion pathway in B. henselae) has also been implicated as a primary factor in NF-κB activation by B. henselae in HUVEC (46). In order to determine whether the transcription factor NF-κB was responsible at least partly for MCP-1 production in HMEC-1 cultured with B. henselae, NF-κB inhibitors PDTC and TPCK were added to cells before coculture. MCP-1 production in response to both B. henselae and E. coli LPS (1 μg/ml) decreased significantly in the presence of either inhibitor (P < 0.0002) (Fig. 3C).
Low-molecular-weight outer membrane proteins (OMP-1) from B. henselae induce MCP-1 production in HMEC-1.
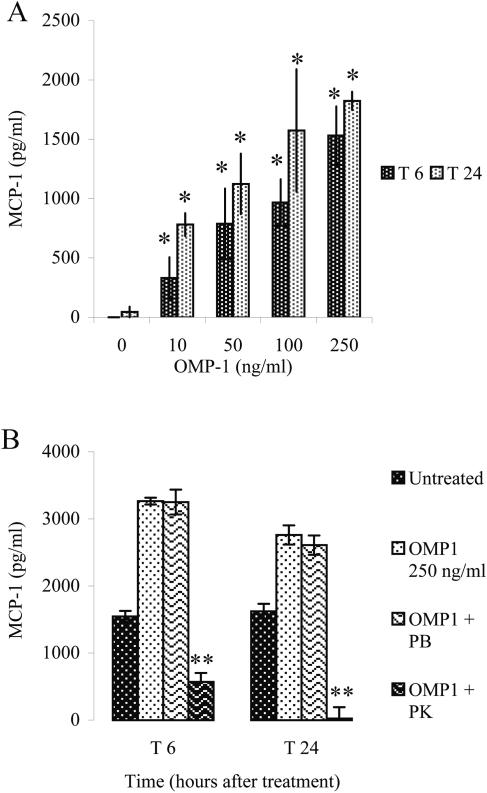
Since MCP-1 production in response to B. henselae appears to occur in an LPS-independent manner, we investigated whether OMPs of the bacteria stimulate MCP-1 production in HMEC-1. It has been shown that OMPs induce NF-κB activation in human umbilical cord vein endothelial cells (HUVEC) (16). Since NF-κB is one of the transcription factors that induce MCP-1 gene expression (42, 55), we investigated whether OMPs could induce production of MCP-1 from HMEC-1. Outer membrane proteins were enriched from B. henselae as previously described (6). The OMPs were separated into four molecular weight fractions (see Materials and Methods), and OMPs were added to HMEC-1 culture at 250 ng/ml. Out of the four fractions, only the lowest-molecular-weight fraction, fraction 1 (OMP-1), which corresponds to the OMPs of molecular mass between 3 and 33 kDa, significantly increased MCP-1 production in HMEC-1 at 6 and 24 h after addition of OMPs (P < 0.002) (Fig. 4A). OMP-1 also caused MCP-1 production from HMEC-1 in a dose-dependent manner (Fig. 4A). Preincubation of OMP-1 with polymyxin B sulfate did not abrogate its effect (P > 0.700) (Fig. 4B), suggesting that endotoxin contamination in the OMP-1 preparation is not responsible for MCP-1 induction. However, proteinase K treatment significantly lowered the effect of OMP-1 (P < 0.0002), revealing that the factor in OMP-1 which upregulates MCP-1 production is probably a protein or proteins.
FIG. 4.
MCP-1 production in response to OMP fraction 1. HMEC-1 cells were stimulated with OMP-1 with various dosages and under various conditions. Supernatants were collected at 6 and 24 (T6, T24) h after addition of OMP-1. MCP-1 levels were determined by ELISA. (A) Dose-dependent response of HMEC-1 to various OMP-1 concentrations. (B) Effects of polymyxin B sulfate (30 μg/ml) (PB) and proteinase K (10 μg/ml) (PK) on OMP-1-induced MCP-1 production in HMEC-1 cells. (* = P < 0.002; ** = P < 2.00 × 10−4). A representative of three experiments is shown.
Supernatants from B. henselae-treated HMEC-1 induce chemotaxis of THP-1 monocytes.
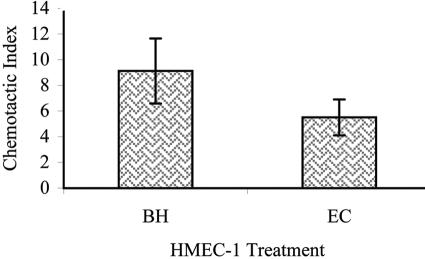
Since MCP-1 levels are elevated in response to B. henselae, we investigated whether supernatants from B. henselae-treated HMEC-1 could cause chemotactic migration of THP-1 monocytes. A modified Boyden chamber assay was used to assess the chemotactic response of THP-1 monocytes to chemoattractants present in the supernatant from B. henselae-treated HMEC-1. Migrated cells were fixed, stained, and counted in five high-powered fields. Supernatants from untreated HMEC-1 were used as negative controls, and supernatants from E. coli-treated HMEC-1 were used as positive controls. Supernatants from B. henselae-infected HMEC-1 induced THP-1 chemotaxis at a level at least 6 times that of supernatants from uninfected HMEC-1 (Fig. 5) and at levels similar to those of supernatants from E. coli-infected HMEC-1 (4 times that of uninfected supernatants).
FIG. 5.
THP-1 monocyte chemotaxis in response to supernatants from HMEC-1. Chemotaxis of THP-1 monocytes in response to supernatants from untreated HMEC-1, supernatants from HMEC-1 cultured with B. henselae (BH), and supernatants from HMEC-1 treated with live E. coli (EC). Results are expressed as a chemotactic index. The number of migrated THP-1 in five microscopic fields was averaged for supernatants from untreated, B. henselae-treated, and E. coli-treated HMEC-1. The chemotactic index for cells which migrated in response to uninfected supernatants was set at 1, and other samples were normalized accordingly. Results shown are the means of three separate experiments.
DISCUSSION
B. henselae causes disease characterized by vascular proliferative lesions (3) into which macrophages infiltrate during infection (26, 27, 35). In the paracrine loop model of angiogenesis, macrophages are implicated as effector cells; upon stimulation by B. henselae, they secrete VEGF and other endothelial cell mitogens (41). Concurrently, endothelial cells upregulate pro-angiogenic factors such as chemokines, inhibit apoptosis through inhibition of caspases (22), and upregulate adhesion molecules (16) which may promote proliferation. In this study we investigated the expression and production of the chemokine MCP-1 from B. henselae-stimulated HMEC-1. MCP-1 is a member of the C-C chemokine family and is produced and secreted by monocytes, fibroblasts, and vascular endothelial cells. MCP-1 then interacts with its CCR2B receptor on monocytes and macrophages to cause chemotaxis (8). MCP-1 can also directly promote angiogenesis. When tumor cells are transfected with the mcp-1 gene and injected into a murine model, angiogenesis is stimulated (37). In addition, MCP-1 implants induce angiogenesis in a rabbit cornea (52). During B. henselae infection, MCP-1 released from endothelial cells can cause chemotaxis of monocytes and macrophages to the site of infection, thereby promoting an angiogenic state by recruiting the effector cell.
Bacterial pathogens such as E. coli, Orientia tsutsugamushi, and Porphyromonas gingivalis increase the production and secretion of chemokines such as IL-8 and MCP-1 (9, 24, 59). MCP-1 is induced in HMEC-1 in response to B. henselae. Both mRNA and protein levels are upregulated; mRNA levels are higher than those of untreated controls in B. henselae-treated HMEC-1 at 6 and 24 h posttreatment, while protein levels in treated cells are higher at 6, 24, and 48 h posttreatment. Furthermore, supernatants from B. henselae-stimulated HMEC-1 caused chemotaxis of THP-1 monocytes. Thus, the levels of MCP-1 produced by HMEC-1 in response to B. henselae in vitro are sufficient to function as a chemoattractant for monocytes. Results also reveal that the bacterial factor which causes MCP-1 production is probably a heat-stable molecule.
The lipopolysaccharide of B. henselae has recently been characterized as containing a lipid A possessing features known to reduce endotoxicity, including a pentaacyl lipid A and a long-chain fatty acid (58). B. henselae LPS induces tlr4 levels 1,000-fold lower than Salmonella enterica sv. Friedenau LPS (58). In addition, LPS from B. quintana, which is likely quite similar to B. henselae LPS, induces IL-8 (GRO-CINC-1 in rats) but not TNF in rats or human whole blood (34). B. henselae LPS also does not induce TNF in cats (34). In this study, the addition of polymyxin B sulfate to B. henselae before addition to HMEC-1 did not reduce MCP-1 production; however, polymyxin B sulfate had a significant lowering effect on E. coli-induced MCP-1 production. Moreover, the LPS from B. henselae does not induce upregulation of MCP-1 expression in HMEC-1. These data corroborate with the low endotoxicity of LPS from Bartonella spp. to imply a limited or nonexistent role for LPS in B. henselae-induced MCP-1 production.
Toll-like receptors activated by various microbial products can cause expression and production of chemokines (11, 12, 31), including MCP-1 (45). LPS, a tlr4 agonist, causes MCP-1 production in a tlr4-dependent manner (53). Most studies confirm that MCP-1 production is tlr4 mediated and usually caused by LPS in a bacterial infection. However, recently it was discovered that tlr4-deficient and tlr4-competent mice have the same MCP-1 response to infection by Leishmania major (4), which is known to cause chemokine production early in infection (20). Our findings indicate that MCP-1 production in response to B. henselae is not tlr4 dependent. In contrast, E. coli LPS-induced MCP-1 production was lowered in the presence of a tlr4 monoclonal antibody. These data suggest the possibility of an alternate pathway to tlr4 activation for the MCP-1 production from B. henselae-treated HMEC-1. Furthermore, these results again exclude B. henselae LPS from a role in MCP-1 production. HMEC-1 express tlr1, tlr3, tlr4, and tlr5 but express tlr2 very weakly, which is why they are unresponsive to tlr2 ligands (13). Thus, the MCP-1 production investigated in this study is probably not tlr4 or tlr2 mediated. Other tlr or similar receptor pathways must be investigated to pinpoint the exact mechanism of MCP-1 induction in HMEC-1 in response to B. henselae.
The mcp-1 gene contains binding sites for both NF-κB and AP-1 (42, 55), and both transcription factors have been implicated in mcp-1 expression (9, 57). It has been established that B. henselae induces NF-κB-dependent upregulation of adhesion molecules in HUVEC independent of LPS (16). In this study, we used two NF-κB inhibitors to determine whether MCP-1 protein production requires NF-κB activation. Diverse NF-κB inhibitors have been used with HMEC-1 previously in similar experiments (9). PDTC is an antioxidant that inhibits the phosphorylation of IκB (36, 47), and TPCK inhibits proteosome-dependent degradation of inhibitory peptides (32). Consequently, through the use of these inhibitors, we demonstrated that MCP-1 production caused by B. henselae in HMEC-1 is NF-κB dependent.
OMPs of B. henselae are important for pathogenesis (6, 16). OMPs have been implicated in the NF-κB-dependent upregulation of adhesion molecules in HUVEC (16). However, it has also been determined that NF-κB-dependent proinflammatory response to B. henselae in HUVEC is mediated primarily by virB (46), the operon which encodes a putative type IV secretion system in B. henselae, indicating a role for secreted and transported proteins in NF-κB activation. However, data presented here reveal the ability of B. henselae Houston-1 OMPs, specifically OMPs of molecular mass between 3 and 33 kDa, to enhance production of the C-C chemokine MCP-1 from HMEC-1. We have also shown that the MCP-1 induction seen here is NF-κB dependent through the use of two NF-κB inhibitors. Thus, bacterial-induced NF-κB upregulation may occur from a combination of mechanisms. Incubation of OMP-1 with polymyxin B sulfate before addition to HMEC-1 reveals that this MCP-1 induction is LPS independent. These data point to a heat-stable low-molecular-weight OMP of B. henselae Houston-1 that contributes at least in part to B. henselae-induced MCP-1 production from endothelial cells. Further studies are needed in order to specify the putative OMP that causes MCP-1 upregulation in endothelial cells.
We have described upregulation of gene expression and protein production of the chemokine MCP-1 in response to B. henselae. This stimulation of HMEC-1 is independent of B. henselae LPS and toll-like receptor 4 but dependent on NF-κB activity. MCP-1 produced by infected HMEC-1 most likely contributes to the ability of conditioned media from these cells to induce monocyte chemotaxis. The recruitment of macrophages by MCP-1 produced from infected endothelial cells could have broad implications on mechanisms of angiogenesis during this infection. Specifically, the macrophage effector cell which secretes VEGF and other angiogenic factors is brought to the site of infection. Pathogenic angiogenesis provides actively growing target cells for B. henselae in an enriched vascularized microenvironment, and while the specific role of MCP-1 induction in this phenomenon is not completely understood, we suggest that recruitment of the monocyte/macrophage effector cell is an important component of the pathway.
Acknowledgments
This research was supported by Public Health Service Grant R01-AI38178 from the National Institutes of Health.
We thank Cathy Newton and the laboratory of Thomas Klein for supplying E. coli LPS. We also thank Kurt Musselman and John Hassell's lab at the Shriners Hospital for Children for their kind donation of four Boyden chemotaxis chambers. We thank Thomas Lawley of Emory University and The Biological Products Branch, Centers for Disease Control and Prevention, for providing the HMEC-1 cell line used in these studies.
Editor: J. T. Barbieri
REFERENCES
- 1.Adal, K. A., C. J. Cockerell, and W. A. Petri, Jr. 1994. Cat scratch disease, bacillary angiomatosis, and other infections due to Rochalimaea. N. Engl. J. Med. 330:1509-1515. [DOI] [PubMed] [Google Scholar]
- 2.Ades, E. W., F. J. Candal, R. A. Swerlick, V. G. George, S. Summers, D. C. Bosse, and T. J. Lawley. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 99:683-690. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, B. E., and M. A. Neuman. 1997. Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 10:203-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniazi, S., H. P. Price, P. Kropf, M. A. Freudenberg, C. Galanos, D. F. Smith, and I. Muller. 2004. Chemokine gene expression in toll-like receptor-competent and -deficient mice infected with Leishmania major. Infect. Immun. 72:5168-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbeit, J. M., D. C. Olson, and D. Hanahan. 1996. Upregulation of fibroblast growth factors and their receptors during multi-stage epidermal carcinogenesis in K14-HPV16 transgenic mice. Oncogene 13:1847-1857. [PubMed] [Google Scholar]
- 6.Burgess, A. W., and B. E. Anderson. 1998. Outer membrane proteins of Bartonella henselae and their interaction with human endothelial cells. Microb. Pathog. 25:157-164. [DOI] [PubMed] [Google Scholar]
- 7.Carbone, M., H. I. Pass, P. Rizzo, M. Marinetti, M. Di Muzio, D. J. Mew, A. S. Levine, and A. Procopio. 1994. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene 9:1781-1790. [PubMed] [Google Scholar]
- 8.Charo, I. F., S. J. Myers, A. Herman, C. Franci, A. J. Connolly, and S. R. Coughlin. 1994. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc. Natl. Acad. Sci. USA 91:2752-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho, N. H., S. Y. Seong, M. S. Choi, and I. S. Kim. 2001. Expression of chemokine genes in human dermal microvascular endothelial cell lines infected with Orientia tsutsugamushi. Infect. Immun. 69:1265-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cines, D. B., E. S. Pollak, C. A. Buck, J. Loscalzo, G. A. Zimmerman, R. P. McEver, J. S. Pober, T. M. Wick, B. A. Konkle, B. S. Schwartz, E. S. Barnathan, K. R. McCrae, B. A. Hug, A. M. Schmidt, and D. M. Stern. 1998. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91:3527-3561. [PubMed] [Google Scholar]
- 11.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17:251-263. [DOI] [PubMed] [Google Scholar]
- 12.Fan, J., and A. B. Malik. 2003. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat. Med. 9:315-321. [DOI] [PubMed] [Google Scholar]
- 13.Faure, E., O. Equils, P. A. Sieling, L. Thomas, F. X. Zhang, C. J. Kirschning, N. Polentarutti, M. Muzio, and M. Arditi. 2000. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 275:11058-11063. [DOI] [PubMed] [Google Scholar]
- 14.Filip, C., G. Fletcher, J. L. Wulff, and C. F. Earhart. 1973. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J. Bacteriol. 115:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394:588-592. [DOI] [PubMed] [Google Scholar]
- 16.Fuhrmann, O., M. Arvand, A. Göhler, M. Schmid, M. Krüll, S. Hippenstiel, J. Seybold, C. Dehio, and N. Suttorp. 2001. Bartonella henselae induces NF-κB-dependent upregulation of adhesion molecules in cultured human endothelial cells: possible role of outer membrane proteins as pathogenic factors. Infect. Immun. 69:5088-5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golenbock, D. T., R. Y. Hampton, N. Qureshi, K. Takayama, and C. R. Raetz. 1991. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J. Biol. Chem. 266:19490-19498. [PubMed] [Google Scholar]
- 18.Griffioen, A. W., and G. Molema. 2000. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol. Rev. 52:237-268. [PubMed] [Google Scholar]
- 19.Hanahan, D., and J. Folkman. 1996. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86:353-364. [DOI] [PubMed] [Google Scholar]
- 20.Ji, J., J. Sun, and L. Soong. 2003. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 71:4278-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempf, V. A. J., B. Volkmann, M. Schaller, C. A. Sander, K. Alitalo, T. Reiss, and I. B. Autenrieth. 2001. Evidence of a leading role for VEGF in Bartonella henselae-induced endothelial cell proliferations. Cell. Microbiol. 3:623-632. [DOI] [PubMed] [Google Scholar]
- 22.Kirby, J. E., and D. M. Nekorchuk. 2002. Bartonella-associated endothelial proliferation depends on inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 99:4656-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkpatrick, C. J., F. Bittinger, C. L. Klein, S. Hauptmann, and B. Klosterhalfen. 1996. The role of the microcirculation in multiple organ dysfunction syndrome (MODS): a review and perspective. Virchows Arch. 427:461-476. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi-Sakamoto, M., E. Isogai, and K. Hirose. 2003. Porphyromonas gingivalis modulates the production of interleukin 8 and monocyte chemotactic protein 1 in human vascular endothelial cells. Curr. Microbiol. 46:109-114. [DOI] [PubMed] [Google Scholar]
- 25.Kol, A., A. H. Lichtman, R. W. Finberg, P. Libby, and E. A. Kurt-Jones. 2000. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 164:13-17. [DOI] [PubMed] [Google Scholar]
- 26.LeBoit, P. E., T. G. Berger, B. M. Egbert, J. H. Beckstead, T. S. Yen, and M. H. Stoler. 1989. Bacillary angiomatosis. The histopathology and differential diagnosis of a pseudoneoplastic infection in patients with human immunodeficiency virus disease. Am. J. Surg. Pathol. 13:909-920. [PubMed] [Google Scholar]
- 27.LeBoit, P. E., T. G. Berger, B. M. Egbert, T. S. Yen, M. H. Stoler, T. A. Bonfiglio, J. A. Strauchen, C. K. English, and D. J. Wear. 1988. Epithelioid haemangioma-like vascular proliferation in AIDS: manifestation of cat scratch disease bacillus infection? Lancet i:960-963. [DOI] [PubMed]
- 28.Leonard, E. J., and T. Yoshimura. 1990. Human monocyte chemoattractant protein-1 (MCP-1). Immunol. Today 11:97-101. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Ocejo, O., A. Viloria-Petit, M. Bequet-Romero, D. Mukhopadhyay, J. Rak, and R. S. Kerbel. 2000. Oncogenes and tumor angiogenesis: the HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene 19:4611-4620. [DOI] [PubMed] [Google Scholar]
- 30.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 31.Luster, A. D. 2002. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 14:129-135. [DOI] [PubMed] [Google Scholar]
- 32.Mackman, N. 1994. Protease inhibitors block lipopolysaccharide induction of tissue factor gene expression in human monocytic cells by preventing activation of c-Rel/p65 heterodimers. J. Biol. Chem. 269:26363-26367. [PubMed] [Google Scholar]
- 33.Majewski, S., A. Szmurlo, M. Marczak, S. Jablonska, and W. Bollag. 1994. Synergistic effect of retinoids and interferon alpha on tumor-induced angiogenesis: anti-angiogenic effect on HPV-harboring tumor-cell lines. Int. J. Cancer 57:81-85. [DOI] [PubMed] [Google Scholar]
- 34.Matera, G., M. C. Liberto, A. Quirino, G. S. Barreca, A. G. Lamberti, M. Iannone, E. Mancuso, E. Palma, F. A. Cufari, D. Rotiroti, and A. Foca. 2003. Bartonella quintana lipopolysaccharide effects on leukocytes, CXC chemokines and apoptosis: a study on the human whole blood and a rat model. Int. Immunopharmacol. 3:853-864. [DOI] [PubMed] [Google Scholar]
- 35.Monteil, R. A., J. F. Michiels, P. Hofman, M. C. Saint-Paul, C. Hitzig, C. Perrin, and J. Santini. 1994. Histological and ultrastructural study of one case of oral bacillary angiomatosis in HIV disease and review of the literature. Eur. J. Cancer 30:65-71. [DOI] [PubMed] [Google Scholar]
- 36.Munoz, C., D. Pascual-Salcedo, M. C. Castellanos, A. Alfranca, J. Aragones, A. Vara, M. J. Redondo, and M. O. de Landazuri. 1996. Pyrrolidine dithiocarbamate inhibits the production of interleukin-6, interleukin-8, and granulocyte-macrophage colony-stimulating factor by human endothelial cells in response to inflammatory mediators: modulation of NF-kappa B and AP-1 transcription factors activity. Blood 88:3482-3490. [PubMed] [Google Scholar]
- 37.Nakashima, E., N. Mukaida, Y. Kubota, K. Kuno, K. Yasumoto, F. Ichimura, I. Nakanishi, M. Miyasaka, and K. Matsushima. 1995. Human MCAF gene transfer enhances the metastatic capacity of a mouse cachectic adenocarcinoma cell line in vivo. Pharm. Res. 12:1598-1604. [DOI] [PubMed] [Google Scholar]
- 38.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164:558-561. [DOI] [PubMed] [Google Scholar]
- 39.Raoult, D., P. E. Fournier, M. Drancourt, T. J. Marrie, J. Etienne, J. Cosserat, P. Cacoub, Y. Poinsignon, P. Leclercq, and A. M. Sefton. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann. Intern. Med. 125:646-652. [DOI] [PubMed] [Google Scholar]
- 40.Regnery, R. L., B. E. Anderson, J. E. Clarridge III, M. C. Rodriguez-Barradas, D. C. Jones, and J. H. Carr. 1992. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J. Clin. Microbiol. 30:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resto-Ruiz, S. I., M. Schmiederer, D. Sweger, C. Newton, T. W. Klein, H. Friedman, and B. E. Anderson. 2002. Induction of a potential paracrine angiogenic loop between human THP-1 macrophages and human microvascular endothelial cells during Bartonella henselae infection. Infect. Immun. 70:4564-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roebuck, K. A., L. R. Carpenter, V. Lakshminarayanan, S. M. Page, J. N. Moy, and L. L. Thomas. 1999. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-kappaB. J. Leukoc. Biol. 65:291-298. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual,2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Sasu, S., D. LaVerda, N. Qureshi, D. T. Golenbock, and D. Beasley. 2001. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ. Res. 89:244-250. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer, T. M., K. Desouza, J. V. Fahey, K. W. Beagley, and C. R. Wira. 2004. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology 112:428-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmid, M. C., R. Schulein, M. Dehio, G. Denecker, I. Carena, and C. Dehio. 2004. The VirB type IV secretion system of Bartonella henselae mediates invasion, proinflammatory activation and antiapoptotic protection of endothelial cells. Mol. Microbiol. 52:81-92. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt, K. N., E. B. Traenckner, B. Meier, and P. A. Baeuerle. 1995. Induction of oxidative stress by okadaic acid is required for activation of transcription factor NF-kappa B. J. Biol. Chem. 270:27136-27142. [DOI] [PubMed] [Google Scholar]
- 48.Schwartzman, W. A. 1992. Infections due to Rochalimaea: the expanding clinical spectrum. Clin. Infect. Dis. 15:893-900. [DOI] [PubMed] [Google Scholar]
- 49.Slater, L. N., D. F. Welch, D. Hensel, and D. W. Coody. 1990. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N. Engl. J. Med. 323:1587-1593. [DOI] [PubMed] [Google Scholar]
- 50.Slater, L. N., D. F. Welch, and K. W. Min. 1992. Rochalimaea henselae causes bacillary angiomatosis and peliosis hepatis. Arch. Intern. Med. 152:602-606. [PubMed] [Google Scholar]
- 51.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 52.Toi, M., A. L. Harris, and R. Bicknell. 1991. Interleukin-4 is a potent mitogen for capillary endothelium. Biochem. Biophys. Res. Commun. 174:1287-1293. [DOI] [PubMed] [Google Scholar]
- 53.Tsuboi, N., Y. Yoshikai, S. Matsuo, T. Kikuchi, K. Iwami, Y. Nagai, O. Takeuchi, S. Akira, and T. Matsuguchi. 2002. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J. Immunol. 169:2026-2033. [DOI] [PubMed] [Google Scholar]
- 54.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 55.Ueda, A., Y. Ishigatsubo, T. Okubo, and T. Yoshimura. 1997. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J. Biol. Chem. 272:31092-31099. [DOI] [PubMed] [Google Scholar]
- 56.van den Akker, W. M. 1998. Lipopolysaccharide expression within the genus Bordetella: influence of temperature and phase variation. Microbiology 144(Pt. 6):1527-1535. [DOI] [PubMed] [Google Scholar]
- 57.Wang, Y., G. K. Rangan, B. Goodwin, Y. C. Tay, and D. C. Harris. 2000. Lipopolysaccharide-induced MCP-1 gene expression in rat tubular epithelial cells is nuclear factor-kappaB dependent. Kidney Int. 57:2011-2022. [DOI] [PubMed] [Google Scholar]
- 58.Zahringer, U., B. Lindner, Y. A. Knirel, W. M. van den Akker, R. Hiestand, H. Heine, and C. Dehio. 2004. Structure and biological activity of the short-chain lipopolysaccharide from Bartonella henselae ATCC 49882T. J. Biol. Chem. 279:21046-21054. [DOI] [PubMed] [Google Scholar]
- 59.Zhao, B., R. A. Bowden, S. A. Stavchansky, and P. D. Bowman. 2001. Human endothelial cell response to gram-negative lipopolysaccharide assessed with cDNA microarrays. Am. J. Physiol. Cell Physiol. 281:C1587-C1595. [DOI] [PubMed] [Google Scholar]