Abstract
Cholera toxin (CT), besides causing intestinal hypersecretion after intragastric administration or during cholera infection, affects a multitude of regulatory mechanisms within the gut mucosal network, including T cells. By use of microarray screening, real-time PCR, and immunohistochemistry, we demonstrate here a rapid depletion of jejunal CD8+ intraepithelial lymphocytes (IEL) in rats after intragastric CT challenge. This depletion may depend on CT-induced migration of IEL, since it was associated with a progressive decrease of CD8+ cells in the epithelium and a contemporary transient increase of such cells, preferentially at the base of the villi, in the lamina propria. A significant decrease in the total number of villous CD8+ cells at 6 and 18 h after CT challenge was detected; this possibly reflects an efflux from the jejunal mucosa. The kinetics of the CD8+ IEL demonstrate the return to normal intraepithelial position at original numbers already 72 h after the single CT dose. The induced migration seems to be dependent on the enzymatic A-subunit of CT, since challenge with neither sorbitol nor CT B-subunit did mimic the effects of CT on CD8+ IEL. Furthermore, a decrease in the level of both RANTES transcript and protein was detected, most likely as a consequence of the CT-induced migration of CD8+ IEL. These results point to a complex interaction between CT, epithelial cells, and IEL, resulting in a disturbance of the gut homeostasis, which might have relevance for the strong immunomodulatory effects of intragastrically administered CT.
Cholera toxin (CT) is an enterotoxin, produced by the bacterium Vibrio cholerae and is composed of an A-subunit (CTA) with ADP-ribosyl transferase activity and five B-subunits (CTBs) that bind to GM1-ganglioside receptors (14). CT affects the mammalian intestine by inducing salt and water secretion, resulting in severe diarrhea. Further, CT is known to possess strong immunomodulatory properties that can be taken advantage of by using CT as a mucosal adjuvant (16, 23, 34). The ability to break oral tolerance further proves that CT is capable of affecting the regulatory mechanisms in the intestinal mucosa (7, 37).
T cells are believed to be the main regulators of mucosal immune responses. The T cells in closest contact with the lumen, and thus with orally administered antigens, are the intraepithelial lymphocytes (IEL). Because of their strategic positioning, making up the first line of defense together with the epithelial cells, they are proposed to play an important role in mucosal immunity in relation to both immune defense and intestinal homeostasis (13, 44). One problem in studying IEL is the lack of a specific cell marker, although it is known that the cell surface protein RT6, restricted to mature T lymphocytes and a subpopulation of natural killer cells, is expressed at a remarkably high density on IEL (9). In addition, integrin αE seems to be a promising marker for IEL, although expression has also been detected on other T-cell subsets (2, 6). Still, it has been reported from several groups that the major subpopulation of IEL in the rat small intestine is T-cell receptor αβ+ (TCRαβ+) CD8+ (12, 18, 24, 39, 40). The majority of these cells are CD4− and express CD8 as an αβ heterodimer (12, 18, 24).
In spite of its strong stimulatory effects in vivo, it has been shown that CT is a potent inhibitor of both mitogen- and antigen-induced T-cell activation in vitro (8, 15, 41). In addition, it is known from in vitro experiments that the closely related heat-labile toxin (LT), produced by the bacterium Escherichia coli, can induce caspase-3-mediated apoptosis of mature CD8+ T lymphocytes (11, 27). Elson et al. have suggested that CT may exert such apoptotic effect on mouse IEL in vivo (8). In that study, a depletion of IEL, observed in horizontal sections of intestinal villi, was explained by CT-induced apoptosis.
In the present study we used a microarray technique combined with immunohistochemistry in order to screen for early cellular changes in the intestinal mucosa of the rat in response to intragastric CT challenge. The results show that CT has the ability to rapidly affect CD8+ IEL within the intestinal mucosa, leading to a transient depletion, with the evidence indicating that it is due to cell migration rather than cell death, followed by a repopulation of the epithelium within 72 h. Such specific and pronounced effects might be of great importance for the immunomodulatory properties of mucosally administered CT.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley strain rats (ALAB, Sollentuna, Sweden) that were 8 to 12 weeks old were used in all experiments. The animals were housed in an environmental room at 22°C and 58 to 65% relative humidity, with a controlled 12-h light-dark cycle and with free access to a standard pellet diet and tap water. For at least 7 days before the experiments, the rats were kept in cages containing up to five animals. All experiments were performed with approval from the local research ethical committee (122-2002/Stefan Lange).
Intragastric challenge.
Rats were given 100 μg of CT (List Biological Laboratories, Inc., Campbell, Calif.), 67 μg of recombinant CTB (molar equality to the CT dose) (22) or 4% sorbitol intragastrically by use of a baby-feeding catheter (optical density at 600 nm = 1.7 mm) under Isofluorhane anesthesia. CT and CTB were dissolved in 5 ml of phosphate-buffered saline (PBS) containing 6% NaHCO3 and 0.5% albumin, whereas sorbitol was dissolved in 5 ml of PBS only. Controls were given vehicle alone intragastrically. This dose of CT was chosen since in previous work it has been shown to induce, without any noticeable adverse effects, a transient small-intestinal hypersecretion and a strong intestinal mucosal immune response (21).
RNA isolation.
Rats were sacrificed 2, 6, 18, or 72 h after intragastric administration, and the small intestine was excised. The jejunum was opened longitudinally, and the proximal part was scraped with a knife with the start point some 40 cm from the pylorus to obtain the upper two-thirds of the villi. Microscopic examination revealed that only villi were scraped off; the base of the villi and the crypt region remained intact (Fig. 1). These tissue samples, which contained the upper mucosal layers, including lamina propria lymphocytes, were immediately homogenized in lysis buffer from the GenElute Mammalian Total RNA Kit (Sigma, St. Louis, Mo.), and total RNA was isolated according to the manufacturer's instructions. The RNA concentration was measured spectrophotometrically, and the quality was checked with an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Palo Alto, Calif.). Total RNA was used for real-time PCR (RT-PCR) analysis and in some cases for microarray analysis.
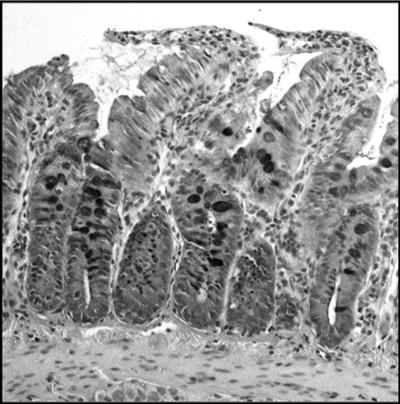
FIG. 1.
Histology after intestinal scrapings. A PAS-stained jejunal section, counterstained with hematoxylin, is shown. The remaining jejunal tissue after scraping consists of tissues comprising the intestinal wall, excluding, approximately, the upper two-thirds of the villi, which constitute the samples for gene expression analyses. Magnification, ×10.
Microarray experiments.
The microarray experiments were performed with groups of three rats. RNA from the following three groups was examined: control, 6-h CT challenge, and 18-h CT challenge. RGU34A GeneChips (Affymetrix, Inc., Santa Clara, Calif.) were used in all experiments. The RNA was converted into labeled target cRNA and hybridized to the GeneChip, followed by washing, staining, and scanning according to the GeneChip expression analysis manual (Affymetrix, Inc.). The resulting images were analyzed with Affymetrix Microarray Suite 5.0 software. All probe sets were used for scaling and normalization. Default values were used during the analysis. All CT-challenged samples were compared to all control samples run on the same occasion, which generated a total of nine comparisons for each group. A mean value was calculated from the signal log2 ratios for each gene and group.
Relative quantification by RT-PCR.
A total of 5 μg of total RNA for each sample was converted into cDNA. The cDNA synthesis and the analyses with the LightCycler (Roche Diagnostics, Basel, Switzerland) were performed as described earlier (10). Oligonucleotide primers purchased from Scandinavian Gene Synthesis (Köping, Sweden) were used for the relative quantification by RT-PCR (Table 1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene in all experiments. Due to interexperimental variation, only samples processed on the same day were compared to each other. The relative levels of transcripts as ratios between control and challenged rats were calculated for each target gene by the formula described by Pfaffl (30). The ratios were converted into log2 values.
TABLE 1.
Primers and MgCl2 concentrations used for RT-PCR
| Gene | Primer sequence
|
MgCl2 concn (nM) | |
|---|---|---|---|
| Forward | Reverse | ||
| CD8α | 5′-GGCTCAGTGGAGGGAATGGGATTG-3′ | 5′-GAGTGATGACCAGGGACAGCAGAAGAAC-3′ | 4 |
| CD8β | 5′-GCCCACGACACTGACAGCCTGCTAC-3′ | 5′-CTCCAGGTAACGTGCCTGACCATTCAC-3′ | 4 |
| CD4 | 5′-TGGTTTTCACGGGGCTCTGCATC-3′ | 5′-GGAGCACTGGCAAGTCTTCTTCTCACTG-3′ | 4 |
| RT6 | 5′-AATTTTCCTGGACTCCCCGAAGAGGAAG-3′ | 5′-GCTGGACCAGAAGACTGGTGAGAACCAC-3′ | 5 |
| Integrin αE | 5′-GAGTTCCAGACATCCGAGGAAAGGAGG-3′ | 5′-TGGATCTCAGCGATGCAGAACACTTTG-3′ | 5 |
| GAPDH | 5′-GAGAAGGCTGGGGCTCACCTGAAG-3′ | 5′-GCATTGCTGACAATCTTGAGGGAGTTG-3′ | 5 |
Immunohistochemistry.
Rats were sacrificed 2, 6, 18, and 72 h after CT challenge. Groups of four to nine animals were used. Samples from the jejunum were fixed in 4% buffered formaldehyde, embedded in paraffin, and sectioned at 4 μm. Antigen retrieval was performed by heating the sections in a microwave oven in 1 mmol of EDTA buffer (pH 9.0). The sections were incubated with a monoclonal antibody against rat CD8 (MRC OX-8; Serotec, Ltd., Oxford, United Kingdom). The PAP procedure (Dako, Glostrup, Denmark) was used as secondary reagents, and the sections were developed with diaminobenzidine as substrate. The sections were lightly counterstained with hematoxylin, dehydrated, and mounted. The total number of CD8+ cells per villi and the percentage of the CD8+ cells that were localized to an intraepithelial position were calculated.
Sections from control animals and animals exposed to CT for 2 or 6 h were incubated with a polyclonal antibody against active caspase-3 (R&D Systems Europe, Ltd,. Abingdon, United Kingdom). The immunoreaction was visualized by an alkaline phosphatase-conjugated secondary antibody (Chemicon International, Harrow, United Kingdom) and a substrate solution containing nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate disodium salt (Roche Diagnostics, Mannheim, Germany). To verify that this antibody would detect caspase-3 in apoptotic rat CD8+ lymphocytes, we isolated the latter type of cells from the mesenteric lymph nodes, induced apoptosis by incubating the cells with 1 mM H2O2 (Sigma, St. Louis, Mo.), and then after treatment with Triton X-100 (Sigma) to permeabilize the cells stained the cells as described above. The results showed that at least 80% of the cells were strongly stained.
ELISA.
Small-intestinal mucosa samples were excised as described above 18 h after CT challenge and weighed before storage at −70°C in a PBS buffer containing one Complete Protease inhibitor cocktail tablet (Roche Diagnostics, Basel, Switzerland) for each 10 ml. The samples were thawed and permeabilized with saponin (Sigma, St. Louis, Mo.) at a final concentration of 2% (wt/vol) in PBS at 4°C overnight. The samples were then centrifuged at 16,000 × g for 5 min, and the supernatants were analyzed for RANTES content with a Duoset cytokine enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems, Inc. (Minneapolis, Minn.), according to the manufacturer's recommendations. Recombinant Rat RANTES (Peprotech, Ltd., London, United Kingdom) was used as a standard.
Statistical analyses.
Statistical analysis of the data expressed as log2 ratios was performed by using the Wilcoxon signed-rank test with the hypothetical value set to zero. For CD8+ cell counts and ELISA analyses, the Mann-Whitney test was used.
RESULTS
Microarray experiments.
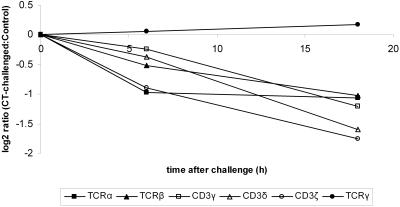
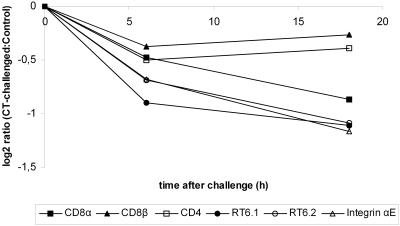
Microarray experiments were performed on samples collected from rats that had been challenged with CT for 6 and 18 h and from control rats given vehicle only. The samples comprised approximately the upper two-thirds of the jejunal villi (Fig. 1). No detected expression of the crypt markers Cdx1 (36) and Hes1 (17) further supports that the samples were not contaminated with crypt cells. The most striking finding of the differently expressed genes was that all transcripts represented on the chip associated with the TCRαβ-CD3 complex were downregulated 6 and 18 h after CT challenge in a similar pattern. The level of TCRγ transcripts was, however, unchanged between the groups (Fig. 2). Because the experiments were done with tissue samples containing a heterogeneous cell population, this opened two possibilities. The first was that the decrease in the level of TCRαβ-CD3 transcripts in the CT-exposed animals was due to an intracellular signaling pathway that prompts a coordinated downregulation of the transcription. The second possibility was that the altered expression levels were caused by changes in the T-cell population. Therefore, a number of well-known T-cell markers were checked on the array to see whether any of these proved to have the same expression pattern as the TCRαβ-CD3 complex. The array experiments showed decreased levels of CD8α, RT6.1, RT6.2, and integrin αE after both 6- and 18-h CT challenges in similarity to the TCRαβ-CD3 transcripts, suggesting an effect on CD8+ IEL. The levels of CD4 and CD8β transcripts on the other hand were unaffected (Fig. 3).
FIG. 2.
Levels of TCR-CD3 complex-associated transcripts after intragastric challenge with CT. Samples were prepared from the upper parts of the villi. Transcript abundance ratios (challenged to control) plotted over time were measured by the microarray technique. Each value represents the mean of a group of three animals. The different transcripts correspond to the following probe sets on the RGU34A array: M18853_g_at (TCRα), M18854_at (TCRβ), S79711_at (CD3γ), X53430_at (CD3δ), D13555_at (CD3ζ), and S75435_i_at (TCRγ).
FIG. 3.
Levels of T-cell marker transcripts after intragastric challenge with CT. Samples were prepared from the upper parts of the villi. Transcript abundance ratios (challenged to control) plotted over time were measured by the microarray technique. Each value represents the mean of a group of three animals. The different transcripts correspond to the following probe sets on the RGU34A array: X03015_at (CD8α), X04310_at (CD8β), M15768_at (CD4), X52082cds_s_at (RT6.1), M85193_at (RT6.2), and AF020046_s_at (integrin αE).
Microarray data were analyzed for changes in transcription level of cytokines. Most of the cytokines were expressed at a level that is too low to be examined by the microarray technique. Still, RANTES transcripts reached above this threshold level and showed the same expression pattern as the TCRαβ-CD3 complex (data not shown).
It is also noteworthy that there was no detectable increase in the very low basal level of Fas ligand transcripts at any time after CT treatment (data not shown).
RT-PCR.
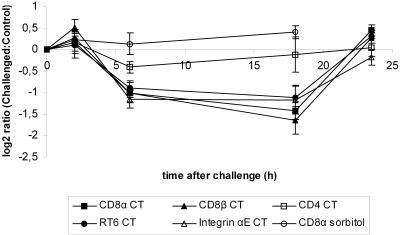
In order to validate the expression pattern of the T-cell markers, we used RT-PCR. Since RT6.1 and RT6.2 showed very similar expression patterns in the microarray analysis, we used a primer pair for RT6 that was not serotype specific. The PCR confirmed the findings for CD8α, RT6, integrin αE, and CD4. However, the PCR data showed, in contradiction to the microarray results, that CD8β also had an expression pattern similar to the TCRαβ-CD3 complex (Fig. 4). Thus, the levels of CD8α, CD8β, RT6, and integrin αE transcripts were decreased at both 6 h (P < 0.05) and 18 h (P < 0.05) in the CT-treated rats, whereas the level of CD4 transcripts was just modestly decreased at 6 h and unchanged at 18 h, indicating that CD8+ but not CD4+ T cells were affected.
FIG. 4.
Levels of T-cell marker transcripts after intragastric challenge with either CT or 4% sorbitol. Samples were prepared from the upper parts of the villi. Transcript abundance ratios (challenged to control) plotted over time were measured by RT-PCR. Each value represents the mean ± the standard error of the mean (SEM) of a group of four to six animals.
Two extra time points, at 2 and 72 h, were added for the PCR analysis. This was done to study the kinetics in more detail. At neither of these time points were significant differences between the control and the CT-challenged groups in the levels of CD8α, CD8β, RT6, integrin αE, and CD4 transcripts detected (Fig. 4).
In an attempt to investigate whether the effects on CD8+-cell-associated transcripts were a result of the physiological conditions or a more CT-specific response, we did control experiments where rats were challenged with sorbitol instead of CT. Sorbitol, like CT, gives rise to a very fast and dramatic hypersecretory response, which makes it a good control. The RT-PCR analysis showed that the CT-induced effects could not be mimicked by sorbitol (Fig. 4).
Rats were also given CTB to see whether this was enough to elicit the same effects observed with CT holotoxin. This was investigated 18 h after challenge, the time point at which the most pronounced effects were seen after CT treatment. No difference in the level of CD8α transcripts was observed between the untreated control and the CTB-challenged group (data not shown).
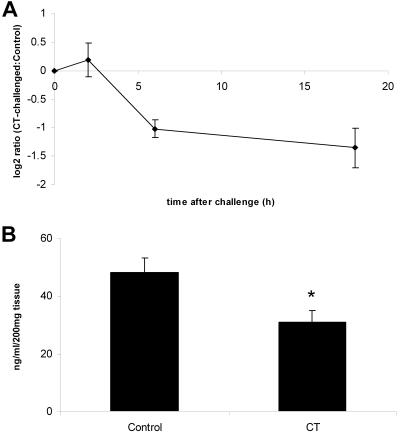
Also, the microarray results for RANTES were confirmed by RT-PCR. Decreased levels of RANTES transcripts were detected at 6 h (P < 0.05) and 18 h (P < 0.05) in the CT-treated rats (Fig. 5A). In accordance with this, a decrease in the level of RANTES protein, measured by sandwich ELISA, was seen 18 h after CT challenge (P < 0.05) (Fig. 5B).
FIG. 5.
Levels of RANTES transcripts (A) and protein (B) after intragastric challenge with 100 μg of CT. Samples were prepared from the upper parts of the villi. Each value represents the mean of a group of four to six animals; error bars correspond to the SEM. Transcript abundance ratios, CT-challenged:control, plotted over time were measured by RT-PCR. Protein levels were measured by sandwich ELISA 18 h after CT challenge. A significant decrease (P < 0.05) compared to the control is denoted by an asterisk.
Immunohistochemistry.
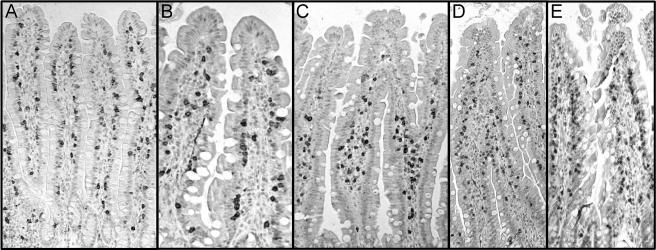
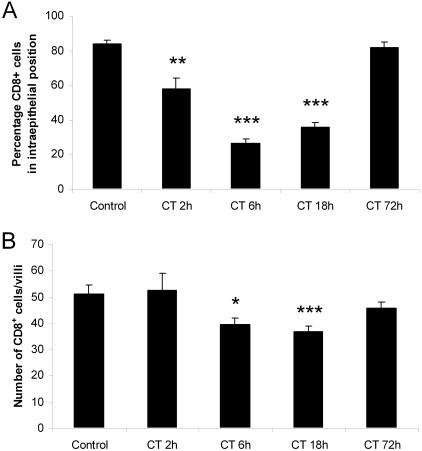
In order to study the CD8+ cells in the jejunal mucosa, we performed immunohistochemistry analyses. The monoclonal antibody MRC OX-8 (anti-CD8) was applied to stain for CD8+ cells at 2, 6, 18, and 72 h after CT challenge. For comparison, we also stained for CD8+ cells 18 h after challenge with CTB. In the control samples, taken from rats given vehicle only, cells within the epithelium, representing IEL, and a few cells in the lamina propria were stained (Fig. 6A). A difference could be seen already 2 h after CT challenge in the tip of the villi, with the percentage of CD8+ cells that were localized to intraepithelial positions decreasing from 84 to 58% (P < 0.01) (Fig. 6B and 7A). After 6 h (Fig. 6C) and 18 h (not shown) dramatic changes were detected. The CD8+ cells in intraepithelial positions were much fewer, and a considerable proportion of the stained cells were seen in subepithelial positions in the lamina propria, especially in the lower parts of the villi. At these time points the percentages of stained cells in intraepithelial positions had dropped to 26 and 36%, respectively (P < 0.001) (Fig. 7A). In contrast, 18 h after CTB treatment no difference was seen compared to the control (Fig. 6E). When we looked at stained CD8+ cells 72 h after CT challenge, such cells were back into normal intraepithelial positions (Fig. 6D and 7A). The redistribution of the CD8+ cells was accompanied by a significant decrease in total villous CD8+ cells. The total number of CD8+ cells per villi was reduced with 20% at 6 h (P < 0.05) and with 25% at 18 h (P < 0.001) in the CT-treated rats. At 2 and 72 h no significant differences compared to the control were detected (Fig. 7B). Altogether, this pattern correlates with the gene expression analysis showing a marked but transient decrease in CD8+ IEL-associated transcripts in samples comprising the upper parts of the villi. The results suggest that CD8+ IEL migrate into the lamina propria and away from the villi after CT challenge, followed by a rapid rerecruitment of CD8+ cells back into the epithelium.
FIG. 6.
Immunolocalization of CD8+ cells in the jejunum. All panels are light photomicrographs of sections immunostained with a monoclonal CD8 antibody. The tissues were taken from rats given vehicle only (A) and from rats challenged with either CT for 2 h (B), 6 h (C), or 72 h (D) or CTB for 18 h (E). Magnification: ×10 (A, C, D and E); ×20 (B).
FIG. 7.
Percentage of CD8+ cells that were localized in an intraepithelial position (A) and total number of CD8+ cells per villi (B), counted in immunostained sections of whole villi, at different time points after intragastric challenge with CT. Each value represents the mean of a group of four to nine animals; error bars correspond to the SEM. A significant decrease compared to the control is denoted as follows: ✽, P < 0.05; ✽✽, P < 0.01; or ✽✽✽, P < 0.001.
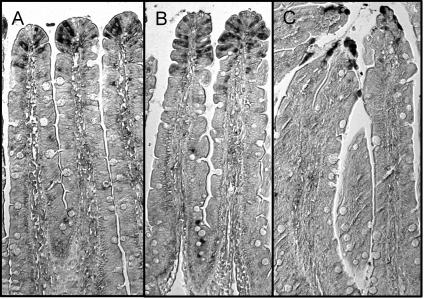
To examine whether the detected effect was due to an apoptotic event, we stained for cells positive for active caspase-3 in sections taken from control rats and from rats challenged with CT for 2 or 6 h with an anti-caspase-3 antibody, which was checked to efficiently stain apoptotic CD8+ rat lymphocytes. As expected, epithelial cells in the outermost tip of the villi were stained in all of the samples, thus working as an internal positive control in these experiments; however, only very few lymphocytes were stained, and their number did not differ between the CT-treated and the control rats (Fig. 8).
FIG. 8.
Immunodetection of active caspase-3 in the jejunum. All panels are light photomicrographs of sections immunostained with a polyclonal antibody against active caspase-3. The tissues were taken from rats given vehicle only (A) and from rats challenged with CT for 2 h (B) or 6 h (C). Magnification, ×10.
DISCUSSION
Our study shows that intragastric challenge with CT induces a rapid and marked depletion of CD8+ IEL in the jejunal villi. These cells are, however, back in their intraepithelial positions at original numbers within 72 h. The specific experiments identifying these events were undertaken based on the results from initial studies of the effects of CT treatment on intestinal mucosa using an oligonucleotide microarray technique. Our results show that the microarray technique not only is useful for examining expression pattern and transcriptional regulations but can also be used to screen for changes in different cell populations in a single experiment. It is obvious that when applying microarray techniques for detecting changes over time in a complete organ as the gut in vivo, any change in transcript level, specific for a certain cell type, could reflect either or both of a changed number or changed transcriptional activity of the cell type in question. This makes it necessary to combine the microarray experiments, as we have done here, with morphological techniques.
The CT-induced depletion of IEL was detected in samples prepared from the upper parts of the jejunal villi, as supported by both histological and biochemical evaluations. A marked decrease in the level of transcripts associated with the TCRαβ-CD3 complex was seen 6 and 18 h after CT challenge. No effect was seen on the level of TCRγ transcript, which indicates that the effect is restricted to TCRαβ+ cells with the TCRγδ+ cells being unaffected, even though there was no probe for TCRδ transcripts on the RGU34A GeneChip. Five different T-cell markers were examined by RT-PCR to further specify the affected cells. The analysis revealed that integrin αE, RT6, CD8α, and CD8β but not CD4 had expression patterns similar to that of the TCRαβ-CD3 complex after CT challenge. These results, except for the decrease in CD8β transcript level, were in accordance with the microarray data. The reason no decrease in the level of CD8β transcripts was detected by the microarray technique is most likely because CD8β is expressed at relatively low levels and was very close to the detection limit. At these ranges, the RT-PCR technique is much more accurate and is more likely to pick up differences in transcript levels. The decrease in RT6 and integrin αE transcripts gives a good indication that IEL are affected, since it has been shown that IEL express RT6 and integrin αE to a much greater extent than lamina propria lymphocytes (6, 9, 40). The results may also allow a tentative identification of the type of IEL affected. Since both CD8α and CD8β, but not CD4, were downregulated in the CT-treated rats it seems the effect is restricted to CD8+ cells. Whether only the cells that express CD8 as an αβ heterodimer or whether both CD8αα+ and CD8αβ+ cells are affected cannot be concluded. However, it is evident that the major subpopulation of rat IEL expresses CD8 as an αβ heterodimer (12, 18, 24).
Immunohistochemical analyses with an anti-CD8 monoclonal antibody confirmed that CD8+ IEL are affected after CT challenge. The analyses show that CD8+ cells in intraepithelial positions were fewer in the CT-treated animals at 2, 6, and 18 h and that the depletion effect was most apparent in the tip of the villi. In contrast, the number of stained cells in the lamina propria increased at the same time with the highest number in the lower parts of the villi. Together with the finding that the total number of villous CD8+ cells were decreased 6 and 18 h after CT challenge, it suggests that the CD8+ IEL migrates into the lamina propria and away from the villi.
For immunohistochemistry and PCR analyses, extra time points, compared to the microarray experiments, were added in order to see whether the CT-induced effects were lasting over a longer period of time. Elson et al. found that CD8+ IEL in the jejunum of mouse were depleted 72 h after intragastric CT challenge (8). In addition, Penney et al. observed a decrease in the number of both TCRαβ+ and TCRγδ+ IEL in the mouse duodenum 48 h after challenge with 100 μg of CT, although no decrease in IEL was detected when a lower dose (10 μg) was used (28). The single toxin dose used in the present study, 100 μg, would rather correspond to the lower dose used in mice by Penney et al., which also was the dose used by Elson et al., with regard to its ability to induce intestinal hypersecretion and an intestinal mucosal immune response (20, 21). Our results indicate that there is a rapid rerecruitment of CD8+ IEL back into the jejunal epithelium. The immunohistochemical analyses show, in accordance with the PCR results, that the CD8+ cells are back in their intraepithelial positions at the original numbers within 72 h after CT challenge. It should be noted that 72 h is the turnover time for intestinal epithelial cells, and CT-induced effects mediated via these cells should be diminished at this time point. Whether the recruited cells display the same cell characteristics as the originals or are unrelated CD8+ cells remains to be examined. However, it has been shown that the 50% population renewal times for TCRαβ+ IEL in the mouse duodenum and ileum are 12 to 36 days and 9 to 11 days, respectively (29). If the regeneration time is similar in the rat jejunum, it is tempting to speculate that cells related to the original IEL are responsible for the rapid reoccupation of the epithelium.
The reported effects after CT challenge are probably not a result caused by the CT-induced intestinal hypersecretion as such, seeing that the strong hypersecretory response stimulated by administration of 4% sorbitol did not give rise to any change in the level of CD8α transcripts. Neither is it explained by the mere binding to GM1 receptors, since the binding CTB protein did not mimic the action of CT holotoxin. The fact that we did not observe any effect on the CD8+ IEL after CTB challenge contradicts results in mice reported by Elson et al., demonstrating a decrease in the level of CD8+ IEL after oral administration of 100 μg of CTB (8). A possible explanation for the divergent results may be the source of CTB. We used recombinant CTB, which is totally free from CTA, whereas commercially available preparations of CTB often contain small amounts of holotoxin. The origin of the CTB used by Elson et al. could not be established; however, the CTB dose was 10-fold higher than the CT dose used in these experiments. We used molar equivalent doses of CT and CTB. Thus, the most likely explanation is that the effects we observed on CD8+ IEL are dependent on the enzymatic activity of CTA and mediated by cyclic AMP released by CT in the epithelial cells. How this signal is transferred to IEL is an open question. However, CT has been shown to enhance the production of interleukin-1 (IL-1), IL-6, IL-8, and IL-10 by intestinal epithelial cells (3, 25, 35). Still, it should be pointed out that these effects, apart from the rapid IL-6 secretion by epithelial cells shown by McGee et al. (25), have been detected after 24 h of CT treatment, whereas we see effects on IEL already after a 6-h treatment period. However, in a mouse ileal loop, induction of IL-6 and IL-10 transcription and secretion of IL-6 have been reported within 6 h after CT challenge (19). Another possibility is that IEL are affected by a CT-induced release of 5-hydroxytryptamine from enterochromaffin cells in the epithelium, either by a direct action or via the enteric nervous system or by prostaglandin E2, known to be released in the small intestine of rat upon CT challenge (1, 5).
In earlier studies, both Elson et al. and Penney et al. suggested that a decrease in the number of IEL by CT treatment is caused by CT-induced apoptosis (8, 28), a view supported by in vitro experiments showing that the closely related LT enterotoxin induces apoptosis when tested on isolated CD8+ lymphocytes (27). However, the LT-mediated apoptosis in the latter experiment was first detected 12 h after direct addition of the B-subunit of the toxin onto the CD8+ lymphocytes. In our study we saw a migration of CD8+ IEL and a decrease in the total number of villous CD8+ cells within 6 h after CT challenge and smaller changes already at 2 h, whereas we saw no effect on CD8+ IEL after challenge with CTB alone. Moreover, we used longitudinal sections of villi to study IEL compared to the horizontal sections studied by Elson et al. This allowed us to map the stained cells along the longitudinal villus axis, and our findings suggest that the decrease in IEL after CT challenge may be explained by an induced migration of CD8+ cells from intraepithelial positions toward the villus core and possibly also out of the villus with the afferent lymph. Further, very few lymphocytes were stained for active caspase-3, an effector molecule known to be important in the apoptosis of T cells, including, as confirmed by us in the present study, CD8+ rat lymphocytes (11, 32, 42), at either of the time points examined, and no difference could be detected compared to the control. In addition, the microarray data did not show any increase in the level of Fas ligand, a gene that has been shown to become activated in many forms of T-cell apoptosis (31), after CT challenge.
Although difficult to study directly, the depletion of CD8+ IEL may be important for CT's adjuvanticity, since these cells seem to be important for the maintenance of intestinal homeostasis. It has been reported that IEL have an inhibitory action on lymphocyte proliferation, although the majority of this action seems to be restricted to the CD8αα+ subset (38). In addition, it was recently shown that primed CD8αβ+ TCRαβ+ IEL interact with lamina propria CD4+ T lymphocytes to downregulate their production of inflammatory cytokines and reduce their proliferative activity in a Toxoplasma gondii infection model (26). The same group reported earlier that primed IEL downregulate the production of inflammatory chemokines by infected enterocytes (4). This inhibitory action might be reversed by CT since the IEL are no longer in contact with their normal cellular milieu, including epithelial cells, various antigen-presenting cells, other lymphocytes, and local enteric neurons. Another possibility is that IEL get the opportunity to exert their inhibitory action on cells located more distantly from the epithelial layer.
A more obvious consequence of a depletion is that there will be changes in the cytokine environment. In this report we have shown a decrease in the level of the chemokine RANTES in the upper parts of the jejunal villi of CT-treated animals. This effect may be explained by the CT-induced depletion of IEL, since it has been reported that RANTES is highly expressed by intestinal IEL (33). Depletion of IEL is likely to cause other changes in the cytokine milieu, since rat intestinal IEL have been shown to produce a number of other cytokines including gamma interferon and transforming growth factor β (33, 43). However, because its sensitivity is too low, none of these prospective changes could be detected by the microarray technique.
In conclusion, we have been able to identify changes in a specific CD8+-cell population after intragastric CT challenge by using a combination of microarray and immunohistochemical techniques. The studied depletion of CD8+ IEL might be important for the strong mucosal adjuvanticity of orally administered CT. We suggest that the depletion, at least in part, is due to cell migration and conclude that CD8+ IEL constitute a very dynamic cell population. The rapid rerecruitment of similar cells after CT-induced depletion points out that CD8+ IEL play an important role, although not well understood, in the mucosal network.
Acknowledgments
The study was supported by grants from The Swedish Research Council (Medicine), project K2003-06 X3382, and from the Swedish state during the LUA agreement (I33913).
We gratefully acknowledge help with the microarray analyses from the SweGene microarray unit at Lund University, supported by the Knut and Alice Wallenberg Foundation, which also provided financial core support to GUVAX. We also thank Marianne Lindblad for providing recombinant CTB.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Beubler, E., G. Kollar, A. Saria, K. Bukhave, and J. Rask-Madsen. 1989. Involvement of 5-hydroxytryptamine, prostaglandin E2, and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology 96:368-376. [DOI] [PubMed] [Google Scholar]
- 2.Brenan, M., and D. J. Rees. 2000. Sequence analysis or rat integrin alphaE1 and alphaE2 subunits: tissue expression reveals phenotypic similarities between intraepithelial lymphocytes and dendritic cells in lymph. Eur. J. Immunol. 30:1527-1537. [DOI] [PubMed] [Google Scholar]
- 3.Bromander, A. K., M. Kjerrulf, J. Holmgren, and N. Lycke. 1993. Cholera toxin enhances alloantigen presentation by cultured intestinal epithelial cells. Scand. J. Immunol. 37:452-458. [DOI] [PubMed] [Google Scholar]
- 4.Buzoni-Gatel, D., H. Debbabi, F. J. Mennechet, V. Martin, A. C. Lepage, J. D. Schwartzman, and L. H. Kasper. 2001. Murine ileitis after intracellular parasite infection is controlled by TGF-β-producing intraepithelial lymphocytes. Gastroenterology 120:914-924. [DOI] [PubMed] [Google Scholar]
- 5.Cassuto, J., M. Jodal, R. Tuttle, and O. Lundgren. 1982. 5-Hydroxytryptamine and cholera secretion: physiological and pharmacological studies in cats and rats. Scand. J. Gastroenterol. 17:695-703. [DOI] [PubMed] [Google Scholar]
- 6.Cerf-Bensussan, N., A. Jarry, N. Brousse, B. Lisowska-Grospierre, D. Guy-Grand, and C. Griscelli. 1987. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur. J. Immunol. 17:1279-1285. [DOI] [PubMed] [Google Scholar]
- 7.Elson, C. O., and W. Ealding. 1984. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J. Immunol. 133:2892-2897. [PubMed] [Google Scholar]
- 8.Elson, C. O., S. P. Holland, M. T. Dertzbaugh, C. F. Cuff, and A. O. Anderson. 1995. Morphologic and functional alterations of mucosal T cells by cholera toxin and its B subunit. J. Immunol. 154:1032-1040. [PubMed] [Google Scholar]
- 9.Fangmann, J., R. Schwinzer, and K. Wonigeit. 1991. Unusual phenotype of intestinal intraepithelial lymphocytes in the rat: predominance of T-cell receptor α/β+/CD2− cells and high expression of the RT6 alloantigen. Eur. J. Immunol. 21:753-760. [DOI] [PubMed] [Google Scholar]
- 10.Flach, C. F., S. Lange, E. Jennische, and I. Lonnroth. 2004. Cholera toxin induces expression of ion channels and carriers in rat small intestinal mucosa. FEBS Lett. 561:122-126. [DOI] [PubMed] [Google Scholar]
- 11.Fraser, S. A., L. de Haan, A. R. Hearn, H. K. Bone, R. J. Salmond, A. J. Rivett, N. A. Williams, and T. R. Hirst. 2003. Mutant Escherichia coli heat-labile toxin B subunit that separates toxoid-mediated signaling and immunomodulatory action from trafficking and delivery functions. Infect. Immun. 71:1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorczynski, R. M., Z. Chen, Z. Cohen, H. Plapler, and D. Wojcik. 1996. Phenotypic and functional assessment of intraepithelial lymphocytes (IEL) isolated from rat colon and small bowel. Immunol. Lett. 50:131-137. [DOI] [PubMed] [Google Scholar]
- 13.Hayday, A., E. Theodoridis, E. Ramsburg, and J. Shires. 2001. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat. Immunol. 2:997-1003. [DOI] [PubMed] [Google Scholar]
- 14.Holmgren, J. 1981. Actions of cholera toxin and the prevention and treatment of cholera. Nature 292:413-417. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren, J., L. Lindholm, and I. Lonnroth. 1974. Interaction of cholera toxin and toxin derivatives with lymphocytes. I. Binding properties and interference with lectin-induced cellular stimulation. J. Exp. Med. 139:801-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmgren, J., N. Lycke, and C. Czerkinsky. 1993. Cholera toxin and cholera B subunit as oral-mucosal adjuvant and antigen vector systems. Vaccine 11:1179-1184. [DOI] [PubMed] [Google Scholar]
- 17.Kayahara, T., M. Sawada, S. Takaishi, H. Fukui, H. Seno, H. Fukuzawa, K. Suzuki, H. Hiai, R. Kageyama, H. Okano, and T. Chiba. 2003. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 535:131-135. [DOI] [PubMed] [Google Scholar]
- 18.Kearsey, J. A., and A. W. Stadnyk. 1996. Isolation and characterization of highly purified rat intestinal intraepithelial lymphocytes. J. Immunol. Methods 194:35-48. [DOI] [PubMed] [Google Scholar]
- 19.Klimpel, G. R., M. Asuncion, J. Haithcoat, and D. W. Niesel. 1995. Cholera toxin and Salmonella typhimurium induce different cytokine profiles in the gastrointestinal tract. Infect. Immun. 63:1134-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange, S., and J. Holmgren. 1978. Protective antitoxic cholera immunity in mice: influence of route and number of immunizations and mode of action of protective antibodies. Acta Pathol. Microbiol. Scand. C 86C:145-152. [DOI] [PubMed] [Google Scholar]
- 21.Lange, S., I. Lonnroth, and H. Nygren. 1984. Protection against experimental cholera in the rat. A study on the formation of antibodies against cholera toxin and desensitization of adenylate cyclase after immunization with cholera toxin. Int. Arch. Allergy Appl. Immunol. 75:143-148. [PubMed] [Google Scholar]
- 22.Lebens, M., S. Johansson, J. Osek, M. Lindblad, and J. Holmgren. 1993. Large-scale production of Vibrio cholerae toxin B subunit for use in oral vaccines. Biotechnology 11:1574-1578. [DOI] [PubMed] [Google Scholar]
- 23.Lycke, N. 1997. The mechanism of cholera toxin adjuvanticity. Res. Immunol. 148:504-520. [DOI] [PubMed] [Google Scholar]
- 24.Marquez, M. G., A. Galeano, S. Olmos, and M. E. Roux. 2000. Flow cytometric analysis of intestinal intraepithelial lymphocytes in a model of immunodeficiency in Wistar rats. Cytometry 41:115-122. [PubMed] [Google Scholar]
- 25.McGee, D. W., C. O. Elson, and J. R. McGhee. 1993. Enhancing effect of cholera toxin on interleukin-6 secretion by IEC-6 intestinal epithelial cells: mode of action and augmenting effect of inflammatory cytokines. Infect. Immun. 61:4637-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mennechet, F. J., L. H. Kasper, N. Rachinel, L. A. Minns, S. Luangsay, A. Vandewalle, and D. Buzoni-Gatel. 2004. Intestinal intraepithelial lymphocytes prevent pathogen-driven inflammation and regulate the Smad/T-bet pathway of lamina propria CD4+ T cells. Eur. J. Immunol. 34:1059-1067. [DOI] [PubMed] [Google Scholar]
- 27.Nashar, T. O., N. A. Williams, T. R. Hirst, and T. O. Nahar. 1996. Cross-linking of cell surface ganglioside GM1 induces the selective apoptosis of mature CD8+ T lymphocytes. Int. Immunol. 8:731-736. [DOI] [PubMed] [Google Scholar]
- 28.Penney, I., P. J. Kilshaw, and T. T. MacDonald. 1996. Increased division of alpha beta TCR+ and γδ TCR+ intestinal intraepithelial lymphocytes after oral administration of cholera toxin. Immunology 89:54-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penney, L., P. J. Kilshaw, and T. T. MacDonald. 1995. Regional variation in the proliferative rate and lifespan of αβ TCR+ and γδ TCR+ intraepithelial lymphocytes in the murine small intestine. Immunology 86:212-218. [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:E45-E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinkoski, M. J., and D. R. Green. 1999. Fas ligand, death gene. Cell Death Differ. 6:1174-1181. [DOI] [PubMed] [Google Scholar]
- 32.Sabbagh, L., S. M. Kaech, M. Bourbonniere, M. Woo, L. Y. Cohen, E. K. Haddad, N. Labrecque, R. Ahmed, and R. P. Sekaly. 2004. The selective increase in caspase-3 expression in effector but not memory T cells allows susceptibility to apoptosis. J. Immunol. 173:5425-5433. [DOI] [PubMed] [Google Scholar]
- 33.Shires, J., E. Theodoridis, and A. C. Hayday. 2001. Biological insights into TCRγδ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity 15:419-434. [DOI] [PubMed] [Google Scholar]
- 34.Simmons, C. P., M. Ghaem-Magami, L. Petrovska, L. Lopes, B. M. Chain, N. A. Williams, and G. Dougan. 2001. Immunomodulation using bacterial enterotoxins. Scand. J. Immunol. 53:218-226. [DOI] [PubMed] [Google Scholar]
- 35.Soriani, M., L. Bailey, and T. R. Hirst. 2002. Contribution of the ADP-ribosylating and receptor-binding properties of cholera-like enterotoxins in modulating cytokine secretion by human intestinal epithelial cells. Microbiology 148:667-676. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian, V., B. Meyer, and G. S. Evans. 1998. The murine Cdx1 gene product localizes to the proliferative compartment in the developing and regenerating intestinal epithelium. Differentiation 64:11-18. [DOI] [PubMed] [Google Scholar]
- 37.Sun, J. B., J. Holmgren, and C. Czerkinsky. 1994. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc. Natl. Acad. Sci. USA 91:10795-10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teitelbaum, D. H., B. C. Reyes, R. M. Merion, and L. Mosley. 1996. Intestinal intraepithelial lymphocytes: identification of an inhibitory subpopulation. J. Surg. Res. 63:123-127. [DOI] [PubMed] [Google Scholar]
- 39.Todd, D., A. J. Singh, D. L. Greiner, J. P. Mordes, A. A. Rossini, and R. Bortell. 1999. A new isolation method for rat intraepithelial lymphocytes. J. Immunol. Methods 224:111-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waite, D. J., M. C. Appel, E. S. Handler, J. P. Mordes, A. A. Rossini, and D. L. Greiner. 1996. Ontogeny and immunohistochemical localization of thymus-dependent and thymus-independent RT6+ cells in the rat. Am. J. Pathol. 148:2043-2056. [PMC free article] [PubMed] [Google Scholar]
- 41.Woogen, S. D., W. Ealding, and C. O. Elson. 1987. Inhibition of murine lymphocyte proliferation by the B subunit of cholera toxin. J. Immunol. 139:3764-3770. [PubMed] [Google Scholar]
- 42.Yada, S., H. Nukina, K. Kishihara, N. Takamura, H. Yoshida, K. Inagaki-Ohara, K. Nomoto, and T. Lin. 2001. IL-7 prevents both caspase-dependent and -independent pathways that lead to the spontaneous apoptosis of i-IEL. Cell. Immunol. 208:88-95. [DOI] [PubMed] [Google Scholar]
- 43.Yamada, K., Y. Kimura, H. Nishimura, Y. Namii, M. Murase, and Y. Yoshikai. 1999. Characterization of CD4+ CD8αα+ and CD4− CD8αα+ intestinal intraepithelial lymphocytes in rats. Int. Immunol. 11:21-28. [DOI] [PubMed] [Google Scholar]
- 44.Yoshikai, Y. 1999. The interaction of intestinal epithelial cells and intraepithelial lymphocytes in host defense. Immunol. Res. 20:219-235. [DOI] [PubMed] [Google Scholar]