Abstract
Understanding HLA-restricted adaptive host immunity to defined epitopes of malarial antigens may be required for the development of successful malaria vaccines. Fourteen epitopes of preerythrocytic malarial antigens known to mediate cytotoxic T-lymphocyte responses against target cells expressing HLA-A2-restricted epitopes were synthesized and pooled based on antigen: thrombospondin-related anonymous protein (TRAP), circumsporozoite protein (CSP), and export protein 1 (Exp-1) peptides. HLA-A2 supertype (*0201, *0202, *0205, *6802) peripheral blood mononuclear cells collected from 774 Malian children, aged 3 months to 14 years, with severe Plasmodium falciparum malaria matched to uncomplicated malaria or healthy controls were stimulated with the HLA-A2-restricted peptide pools. Significant gamma interferon production, determined by enzyme-linked immunospot assay to at least one of the three peptide pools, was observed in 24/58 (41%) of the severe malaria cases, 24/57 (42%) of the uncomplicated malaria cases, and 34/51 (67%) of the healthy controls. Significant lymphoproliferation to these peptides was observed in 12/44 (27%) of the severe malaria cases, 13/55 (24%) of the uncomplicated malaria cases, and 18/50 (36%) of the healthy controls. Responses to individual peptide pools were limited. These studies confirm the presence of adaptive cell-mediated immunity to preerythrocytic malaria antigens in volunteers from Mali and demonstrate that suballeles of the HLA-A2 supertype can effectively present antigenic epitopes. However, whether these immune responses to TRAP, CSP, and Exp-1 malarial proteins play a substantial role in protection remains a matter of controversy.
Despite technological advances, malaria remains a major threat to worldwide health, and the mechanisms underlying protective immunity to the causative parasite remain largely undefined. Naturally acquired sterile malaria immunity appears to be uncommon although sterile immunity to Plasmodium spp. has been achieved in mice, monkeys, and humans after irradiated-sporozoite injection (8, 21, 26). Irradiated-sporozoite development is arrested at the level of the hepatocyte, abrogating processing to the erythrocytic stage of parasite development. Evidence points to major histocompatibility complex-restricted CD8+ T-cell-mediated responses as being crucial in mediating immunity to malaria, though CD4+ T cells, cytokines, and antibodies may act in concert to prevent disease (18, 36).
Due to the impracticality of immunizing humans with irradiated sporozoites and the difficulties of including whole-length malaria proteins in current vaccines, the use of epitope-based, preerythrocytic-stage malaria vaccines is being evaluated. HLA polymorphisms complicate this approach. The presence of heterozygous class I alleles, variation in HLA binding affinities, and parasite diversity to escape recognition greatly increase the complexity of developing a successful vaccine. Nevertheless, the importance of HLA haplotypes in protection from natural infection is indirectly demonstrated by the association of HLA class I B53 and class II DRB1*1302-DQB1*0501 with protection against severe malaria in west Africa (17). The fact that HLA-B53 comprises ∼40% of West African haplotypic alleles while Caucasian and Asian populations have <1% penetration and the demonstration of LSA-1 and -3 antigen-specific cytotoxic T lymphocytes (CTL) restricted by this haplotype (2, 17) suggest that evolutionary pressure influences major histocompatibility complex polymorphism.
Many CTL epitopes are capable of binding several HLA class I alleles, and this overlap in peptide-binding specificity has led to the concept of HLA class I superfamilies. An A2-binding supertype comprising HLA-A*0201, -*0202, -*0203, -*0205, -*0206, -*6802, and -*6901 has been proposed based on common peptide-binding motifs (10). The phenotypic frequencies of HLA*0201, -*0202, -*0205, and -*6802 are greater than 5% in the black population, with negligible quantities of other HLA-A2 supertype alleles (7, 31). Immunological studies to evaluate the ability of A2 supertype alleles to present A2-restricted peptides to CD8+ T cells in malaria have not been reported.
Haplotypes of the HLA-A2 supertype range between 36 and 63% in African populations (6). Evidence of immunity to preerythrocytic-stage antigens restricted by HLA-A*0201 (1-3, 11, 12, 15, 19, 20, 24, 30, 35, 37), as well as other members of HLA-A2 superfamily, has been reported (3, 12). Natural infections display multiple CTL effector populations reactive to a variety of epitopes, including thrombospondin-related anonymous protein (TRAP), LSA-1 and -3, circumsporozoite protein (CSP), and export protein 1 (Exp-1) (1, 3, 12), suggesting that many immunodominant epitopes might be responsible for protective immunity. This raises the possibility that a subunit vaccine composed of multiple epitopes present in preerythrocytic antigens restricted by multiple HLA alleles could offer comprehensive coverage with sustained immunity. Individual CTL to an epitope may be weak, but cumulative CTL responses to multiple preerythrocytic epitopes may be protective. We sought, for the first time, to demonstrate cell-mediated immunity elicited in individuals with HLA-A2 supertype alleles upon stimulation with pools of malaria preerythrocytic epitopes. Identification of critical epitopes within these pools might provide further insights for the development of an epitope-based, preerythrocytic-stage malaria vaccine effective in individuals of diverse HLA haplotypes.
MATERIALS AND METHODS
Study site and enrollment.
Venous blood was obtained from Malian children (aged 3 months to 14 years) on enrollment into a study evaluating risk and protective factors for severe malaria. The study site of Bandiagara (population, 13,600) is located in Mali, West Africa, and has intense seasonal transmission (July to December) of Plasmodium falciparum malaria. Children (aged 3 months to 20 years) residing in Bandiagara have been determined to have between 0 and 4 symptomatic malaria infections per transmission season (mean, 1.54 episodes) (9). The dominant self-reported ethnic group is Dogon (81%) with Peuhl, Bambara, and other ethnic groups present. From October 1999 to January 2003, 253 index cases of severe malaria from Bandiagara and surrounding areas were admitted to the Bandiagara Malaria Clinic. Study personnel were available at the clinic 7 days a week and 24 hours a day. Cases were classified as severe malaria based on the following World Health Organization criteria (38) (definition includes baseline parasitemia and one or more of the listed features): coma (Blantyre coma scale [BCS] ≤ 2), seizure (one or more witnessed by the investigators; criteria were modified to allow for enrollment for children with one witnessed seizure rather than two to err on the side of safety in this rural setting), obtundation (depressed consciousness with BCS > 2), parasitemia of ≥500,000/mm3, lethargy or prostration (clinical judgment or child ≥7 months of age unable to sit unassisted), severe anemia (hemoglobin ≤ 5 g/dl), respiratory distress (intercostal muscle retraction, deep breathing, grunting), hypoglycemia (glucose ≤ 40 mg/dl), jaundice, renal insufficiency as indicated by lack of urination for >1 day, gross hematuria, state of shock (systolic blood pressure ≤ 50 mm Hg, rapid pulse, cold extremities), inability to eat or drink, and repeated vomiting (for the last two, the definition was included to preclude missing cases of impending severe illness although these criteria were not used as sole enrollment criteria). Each index case was age and residence matched to a case of uncomplicated malaria and a healthy control within 5 days of enrollment.
Age categories were defined as 3 to 5 months, 6 to 11 months, 1 year, 2 years, 3 to 4 years, 5 to 6 years, 7 to 8 years, 9 to 10 years, 11 to 12 years, and 13 to 14 years. Residence was defined as one of eight distinct sectors of Bandiagara or a specific outer village. Uncomplicated malaria was defined as P. falciparum parasitemia and axillary temperature ≥37.5°C detected by active surveillance, or parasitemia and symptoms leading to treatment seeking in the absence of other causes of fever on passive surveillance.
Matched uncomplicated-malaria controls were enrolled from the population of children presenting to the clinic daily. Healthy controls were enrolled after traveling to the residence of the severe malaria case and following a standardized protocol until an eligible control was identified. These children generally resided in close proximity to the index severe malaria case and were judged to have nearly identical living conditions and exposure to malaria. Responses to a series of background questions assessing modes of protection from malaria acquisition (i.e., nets, coils, insecticide) were also obtained, and no significant difference between study groups was noted. Children were enrolled as healthy controls if they were asymptomatic for acute illness, had no evidence or history of chronic illness, and were found to be aparasitemic.
Study protocols were reviewed and approved by the Malian Institutional Review Board (IRB) as well as the University of Maryland IRB. Village assent was obtained from village chiefs, government officials, and traditional healers prior to study initiation. Individual informed consent was obtained from the legal guardian of each child prior to enrollment though care for malaria was offered regardless of study participation.
PBMC collection.
Patient whole blood was collected at the study clinic into sterile EDTA tubes on admission, prior to institution of antimalarial therapy, and immediately refrigerated. Blood was processed by density centrifugation, within 2 hours of acquisition, utilizing lymphocyte separation medium (ICN Biomedical Inc., Aurora, OH) following standard techniques (23). Peripheral blood mononuclear cells (PBMC) were resuspended in media and linear rate frozen using isopropyl alcohol containers (Nalgene) to −70°C at the field site before transfer to liquid nitrogen storage containers for transportation to the University of Maryland at Baltimore. Follow-up PBMC were collected at a 6-month postenrollment appointment during the dry season.
HLA determination.
Class I HLA haplotypes were determined by PCR analysis and sequence-specific oligonucleotide probe hybridization (PCR-SSOP) on PBMC as previously described (5). For typing alleles of the HLA-A, -B, and -C loci, extracted DNA was amplified using intronic-locus-specific primers that span exons 1, 2, and 3. PCR products were immobilized onto positively charged nylon membranes and hybridized with probes matching polymorphic sequences of HLA-A, -B, and -C alleles. The hybridization signal was detected with chemiluminescent substrate on radiography film. Alleles were assigned based on the hybridization patterns with sets of known oligonucleotide probes as well as unpublished probe sequences (K. Cao, unpublished) to identify all genotypes of HLA class I alleles unambiguously. All genotype ambiguities were resolved using group-specific amplification and SSOP hybridization methods with the exception of alleles presenting differences outside exons 2 and 3. All groups of alleles differing only by synonymous nucleotide substitutions were collapsed into a four-digit assignment.
Epitope synthesis.
Fourteen 9- to 10-mer epitopes of preerythrocytic malarial antigens reported to mediate HLA-A2 superfamily-restricted CTL responses against target cells were synthesized at the University of Maryland Biopolymer Laboratory (Table 1). Aliquots of peptides suspended in dimethyl sulfoxide (DMSO) or sterile water (20 mg/ml) were stored at −70°C until use. For use as antigenic stimulation, peptides were combined into pools. Pool 1 consisted of five TRAP peptides, pool 2 consisted of four CSP peptides (one protein with both COOH and CONH2 terminal ends), and pool 3 consisted of four Exp-1 peptides combined at 10 to 20 μg/ml/peptide. Two HLA-A*03-restricted peptides (LSA-1 aa94-102 [QTNFKSLL] and aa59-68 [HVLSHNSYEK]) (12) were used as controls in HLA-A2, non-HLA-A*03 volunteers. Reconstituted lyophilized malaria peptides were tested for the presence of endotoxin (Biowhittaker Kinetic QCL chromogenic LAL). All samples contained less than 0.005 EU/ml of endotoxin, i.e., they were below the lowest standard and detectable limits of the endotoxin assay.
TABLE 1.
Malaria antigen peptide pools of TRAP, CSP, and Exp-1 and amino acid sequences of synthesized peptides
| Pool | Malaria antigen | Peptide (aa) | HLA restriction | Sequence | Terminal | Dilution | Reference(s) |
|---|---|---|---|---|---|---|---|
| 1 | TRAP | 50-59 | A2 | YLLMDCSGSI | COOH | DMSO | 37 |
| 1 | TRAP | 132-140 | A2 | NLTDALLQV | COOH | DMSO | 37 |
| 1 | TRAP | 136-144 | A2 | ALLQVRKHL | COOH | H2Ob | 37 |
| 1 | TRAP | 3-11 [tr26] | A2.1 | HLGNVKYLV | COOH | DMSO | 1 |
| 1 | TRAP | 500-508 [tr39] | A2.1 | GIAGGLALL | COOH | DMSO | 1 |
| 2 | CSP | A2.386-394 | A2 supertype | GLIMVLSFL | CONH2 | DMSO | 12, 35 |
| 2 | CSPa | A2.319-327 or 334-342 | A2.1 | YLNKIQNSL | COOH | DMSO | 3, 35 |
| 2 | CSPa | A2.319-327 or 334-342 | A2.1 | YLNKIQNSL | CONH2 | H2O | 3, 35 |
| 2 | CSP | A2.1-10 | A2 supertype | MMRKLAILSV | COOH | DMSO | 3, 35 |
| 2 | CSP | A2.7-16 | A2.1 | ILSVSSFLFV | COOH | H2O | 12 |
| 3 | Exp-1 | 2-10 | A2 supertype | KILSVFFLA | COOH | DMSO | 12 |
| 3 | Exp-1 | 80-88 | A2 supertype | VLAGLLGNV | COOH | DMSO | 12 |
| 3 | Exp-1 | 83-91 | A2 supertype | GLLGNVSTV | COOH | H2O | 12 |
| 3 | Exp-1 | 91-100 | A2 supertype | VLLGGVGLVL | COOH | DMSO | 12 |
Denotes peptide sequences with terminal chain differences (COOH or CONH2).
H2O, sterile water.
CMI assays. (i) IFN-γ ELISPOT.
Gamma interferon (IFN-γ)-secreting cells were quantified using a modified IFN-γ enzyme-linked immunospot (ELISPOT) assay (29). Fifty thousand and 100,000 cells/well were plated in duplicate on 96-well plates (Millipore MAHA, Bedford, MA) coated with 5 μg/ml human anti-IFN-γ (clone 2G1; Endogen, Rockford, IL) and incubated overnight with malaria peptide pools or control peptides (10 μg/ml). Anti-CD28/CD49d monoclonal antibodies (mAbs; clones 28.2 and 9F10, respectively; Pharmingen, San Diego, CA) at 1 μg/ml were added as costimulants (34). Aim-V media (catalog no. 12055-091; Gibco Invitrogen Corp.), and HLA-A*3-restricted peptides (in non-HLA-A*3 volunteers) were used as negative controls. Anti-CD3/anti-CD28 beads at 3 μl/ml were used as positive controls. Bound IFN-γ was detected using biotinylated anti-IFN-γ mAb (2 μg/ml; clone B133.5; Endogen) followed by a 1-hour incubation with avidin peroxidase (1:400). A 50:1 dilution of True Blue substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was added for 15 min at 50 μl per well, followed by a distilled H2O wash. Two observers read plates with stringent criteria applied to identify responders: (i) stimulant wells had to have >8 spots per well, (ii) the number of spots in stimulant wells had to be more than twice media controls, and (iii) the mean number of spots in stimulant wells had to differ significantly from media controls by paired t test (one-tailed, P < 0.05). Responders were defined as subjects with detectable response to pooled epitopes at either the 100,000-cell/plate concentration or at both the 50,000- and 100,000-cell/plate concentrations. Results were expressed as spot-forming cells (SFC)/106 PBMC.
(ii) Lymphoproliferation.
One hundred thousand to 150,000 cells/well were plated in triplicate and incubated with media, control peptide, or malaria peptide pools at 10 μg/ml for 5 days, and [3H]thymidine incorporation was determined as described previously (32). Stimulation indices (SI) were calculated by dividing the mean counts per minute of triplicate wells poststimulation by the mean cpm observed in media controls. Values that fell outside the 95% confidence interval (CI) of replicate wells were excluded from analysis. Positive samples were defined as those with (i) mean of wells with malarial pools differing significantly from media controls by paired t test (one-tailed, P < 0.05), (ii) SI of >2, and (iii) mean cpm that exceeded the media control by >1,000.
CMI results were excluded if anti-CD3/CD28-stimulated cells failed to exhibit strong (>800 SFC/106 PBMC) responses. PBMC from four HLA-A*0201-positive U.S. citizens without previous exposure to malaria or history of travel to malaria-endemic areas, as well as eight HLA-A2-negative Malian children from the study (with the following HLA-A alleles: *2301, *2601, *3001, *3002, and *3303) were used as negative controls.
Statistical analysis.
Analyses of differences in IFN-γ production by ELISPOT or proliferation of cells between clinical groups were performed using X2 analysis (EpiInfo; Centers for Disease Control and Prevention; 2000). A two-sided Student t test was used for analysis of continuous variables with equal variance and χ2 test for categorical variables using SPSS 10.0 (2000; SPSS Corp., Chicago, IL). Spearman rank order correlation (SigmaStat 3.0) was used to analyze the correlation of nonnormally distributed CMI responses obtained in the transmission season versus the dry season. The level for statistically significant differences was set at a P of <0.05.
RESULTS
Patients and HLA determinations.
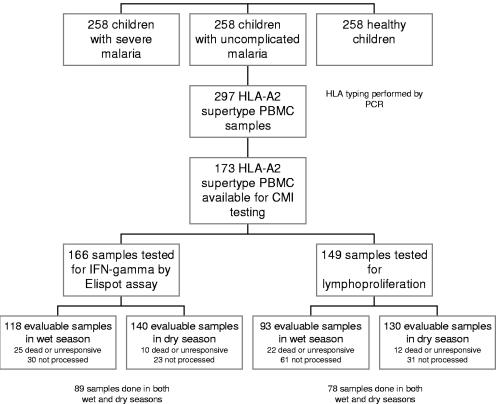
PBMC were available from 726 of 759 enrolled study children (242 severe malaria cases, 239 uncomplicated malaria cases, and 245 healthy children). Severe-malaria criteria included hyperparasitemia (38%), cerebral malaria (41%), severe anemia (21%), and other (2.0%) (more than one diagnosis possible). Case fatality was 19/242 (8%). HLA haplotypes were determined in 698 of 726 submitted samples. Twenty-eight samples could not be typed utilizing published and unpublished oligonucleotide probes. The A*0201, A*0202, A*0205, and A*6802 haplotypes comprised at least one allele in 105 (15.4%), 107 (15.7%), 27 (4%), and 58 (8.5%) samples, respectively. Two hundred ninety-seven (43.4%) samples demonstrated at least one allele corresponding to the HLA-A2 superfamily. Cell-mediated immunity testing was performed in 173 individuals (Table 2). All individuals with HLA-A*0201 were processed, as well as a proportion of those with subtype alleles. Reasons for not performing immunologic assays included cell loss during transit, collection of insufficient cell numbers, utilization of cells in alternate experiments, death of study participants, and inability to recruit study participants in the dry season (Fig. 1)
TABLE 2.
Patient characteristics of PBMC samples with HLA-A2 supertype in which CMI was tested (n = 173)d
| Characteristic | Value for:
|
||
|---|---|---|---|
| Severe malaria case | Uncomplicated malaria case | Healthy control | |
| No. | 64 | 58 | 51 |
| No. female (%) | 25 (39) | 28 (48) | 22 (43) |
| Age (mo) (range) | 41.4 (3.8-135.5) | 40.8 (9.6-126.3) | 39.3 (4.5-118.7) |
| Hemoglobin (g/dl) (range) | 8.69 (4.0-12.9)a | 9.2 (5.3-13.7)a | 10.7 (7.9-14.5) |
| Parasite densityc | 190,934b | 9,190a | 0 |
Paired t-test significance (P < 0.05) was determined between children with severe or uncomplicated malaria compared to healthy controls. All analysis was performed with paired Student's t test.
Paired t-test significance (P < 0.05) was determined between children with severe malaria and uncomplicated malaria controls.
Geometric mean parasite density per mm3.
As part of a larger study, children with severe malaria (n = 258) were matched by age and residence to an uncomplicated malaria control and a healthy control at enrollment (total n = 759).
FIG. 1.
Trial profile demonstrates enrollment numbers, HLA-A2 supertype determination, and quantity of specimens available for immunologic assays. A portion of sample PBMC did not survive the freeze process and were noted to be dead upon thaw. Unresponsive cells appeared intact but did not release IFN-γ in response to positive stimulant (anti-CD3/anti-CD28 beads). Reasons for not performing an immunologic assay included utilization of cells in alternate experiments, cell loss during transit, collection of insufficient cell numbers, and death or loss of follow-up of study participants.
CMI measurements. (i) IFN-γ ELISPOT.
ELISPOT assays were performed on PBMC from 166 of the 173 unique HLA-A2 study participants (58 severe malaria cases, 57 uncomplicated malaria cases, and 51 healthy participants) including 119 unique samples obtained at enrollment (malaria transmission [wet] season) and 140 obtained during the dry season. Eighty-nine individuals had ELISPOT assays performed at both time points. Assays could not be performed in 88 volunteers (wet season, n = 55; dry season, n = 33). ELISPOT assay results were analyzed independently in the wet and dry seasons and also in a combined format indicating a positive result in at least one season. Results were stratified by HLA subtype (Table 3). No differences were observed between the numbers of positive assays in the wet versus the dry season. Positive assays were noted in all HLA subtypes, to all pooled epitopes, and in all enrollment groups. Statistical significance was achieved only when the positive responses to CSP (any allele) in uncomplicated malaria were compared to those of healthy controls (wet season: odds ratio [OR] = 0.26 [95% CI, 0.07 to 0.93], P = 0.02; dry season: OR = 0.47 [95% CI, 0.15 to 1.45], P = 0.14; combined: OR = 0.34 [95% CI, 0.13 to 0.88], P = 0.01). After stratification by HLA-A2 allele, significant immunoreactivity (of combined results) was noted to CSP by the healthy group compared to the uncomplicated-malaria group in HLA-A*0202 (OR = 0.17 [95% CI, 0.03 to 0.94], P = 0.016) and to Exp-1 between the healthy and severe malaria groups in HLA-A*0201 (OR = 0.09 [95% CI, 0.0 to 0.96], P = 0.016). The overall number of positive assays in the wet season was similar to that in the dry season although individual responses did not necessarily correlate between seasons (Spearman correlations: healthy, 0.14, P = 0.18; uncomplicated malaria, −0.176, P = 0.068; severe malaria, −0.0952, P = 0.44). No difference in reactivity was noted among individuals with HLA-A2 homozygosity (n = 16, data not shown).
TABLE 3.
IFN-γ-secreting cells determined by ELISPOT in PBMC from HLA-A2 (*0201, *0202, *0205, *6802)-positive children with severe malaria and age- and residence-matched children with uncomplicated malaria and healthy controls stimulated with HLA-A2-restricted malaria peptide poolse
| HLA | Group | Pool | Resulta,d (%)
|
||
|---|---|---|---|---|---|
| Wet season | Dry season | Combinedb | |||
| *6802 | Healthy | 1 | 3/7 (43) | 0/5 (0) | 3/7 (43) |
| 2 | 2/7 (29) | 1/5 (20) | 3/7 (43) | ||
| 3 | 2/7 (29) | 0/5 (0) | 2/7 (29) | ||
| Uncomplicated | 1 | 0/6 (0) | 2/8 (25) | 2/9 (22) | |
| 2 | 0/6 (0) | 1/8 (13) | 1/9 (11) | ||
| 3 | 0/6 (0) | 1/8 (13) | 1/9 (11) | ||
| Severe | 1 | 2/8 (25) | 3/11 (27) | 5/13 (38) | |
| 2 | 0/7 (0) | 3/11 (27) | 5/13 (38) | ||
| 3 | 1/8 (13) | 2/11 (18) | 3/13 (23) | ||
| *0201 | Healthy | 1 | 4/10 (40) | 4/18 (22) | 8/20 (40) |
| 2 | 2/10 (20) | 4/18 (22) | 6/20 (30) | ||
| 3 | 0/10 (0) | 1/18 (6) | 1/20 (5) | ||
| Uncomplicated | 1 | 4/20 (20) | 3/18 (17) | 7/23 (30) | |
| 2 | 3/20 (15) | 4/18 (22) | 2/23 (9) | ||
| 3 | 2/20 (10) | 2/18 (11) | 3/23 (13) | ||
| Severe | 1 | 2/12 (17) | 1/19 (5) | 3/21 (14) | |
| 2 | 3/12 (25) | 2/19 (11) | 5/21 (24) | ||
| 3 | 1/12 (8) | 2/19 (11) | 3/21 (14) | ||
| *0202 | Healthy | 1 | 4/14 (29) | 7/18 (39) | 8/19 (42) |
| 2 | 5/14 (36) | 7/18 (39) | 10/19 (53) | ||
| 3 | 5/14 (36) | 1/18 (6) | 6/19 (32) | ||
| Uncomplicated | 1 | 4/18 (22) | 2/15 (13) | 6/19 (32) | |
| 2 | 2/18 (11) | 2/15 (13) | 3/19 (16) | ||
| 3 | 5/18 (28) | 0/15 (0) | 5/19 (26) | ||
| Severe | 1 | 1/10 (10) | 4/18 (22) | 5/21 (24) | |
| 2 | 1/11 (9) | 4/18 (22) | 4/21 (19) | ||
| 3 | 1/11 (9) | 4/18 (22) | 5/21 (24) | ||
| *0205 | Healthy | 1 | 1/5 (20) | 1/5 (20) | 2/5 (40) |
| 2 | 2/5 (40) | 1/5 (20) | 2/5 (40) | ||
| 3 | 2/5 (40) | 3/5 (60) | 3/5 (60) | ||
| Uncomplicated | 1 | 2/4 (50) | 0/4 (0) | 2/6 (33) | |
| 2 | 0/4 (0) | 0/4 (0) | 0/6 (0) | ||
| 3 | 0/4 (0) | 1/4 (25) | 1/6 (17) | ||
| Severe | 1 | 1/2 (50) | 0/1 (0) | 1/3 (33) | |
| 2 | 1/2 (50) | 0/1 (0) | 1/3 (33) | ||
| 3 | 1/2 (50) | 1/1 (100) | 2/3 (67) | ||
| Allc | Healthy | 1 | 12/36 (33) | 12/46 (26) | 21/51 (41) |
| 2 | 11/36 (31) | 13/46 (28) | 21/51 (41) | ||
| 3 | 7/35 (20) | 5/46 (11) | 12/51 (24) | ||
| Uncomplicated | 1 | 10/49 (20) | 7/45 (16) | 17/57 (30) | |
| 2 | 5/49 (10) | 7/45 (16) | 11/57 (19) | ||
| 3 | 7/49 (14) | 4/45 (9) | 11/57 (19) | ||
| Severe | 1 | 6/33 (18) | 8/49 (16) | 14/58 (24) | |
| 2 | 5/33 (15) | 9/49 (18) | 13/58 (22) | ||
| 3 | 4/33 (12) | 9/49 (18) | 13/58 (22) | ||
Results are depicted as number of positive assays/total number of assays performed.
Combined results represent a positive assay for either the wet-season PBMC or the dry-season PBMC.
All, summary results of all HLA-A2 subtypes combined.
Boldface indicates significantly reduced immune response between the severe or uncomplicated malaria groups compared to healthy controls in response to the peptide stimulant denoted. The level of significance was set at a P of <0.05.
Pool 1, TRAP; pool 2, CSP; pool 3, Exp-1.
The number of individuals demonstrating significant IFN-γ release to any of the peptide pools was calculated (Table 4). Combining wet and dry season results, a reduced number of individuals reacting to any of the stimulant pools were observed in the severe malaria group (24/58, 41%) and uncomplicated malaria group (24/57, 42%) compared to healthy controls (34/51, 67%) (severe malaria versus healthy: OR = 0.35 [95% CI, 0.15 to 0.83], P = 0.008; uncomplicated malaria versus healthy: OR = 0.36 [95% CI, 0.15 to 0.86], P = 0.01). Similarly, reduced responses were noted in severe (7/33, 21%) and uncomplicated malaria (16/49, 33%) cases during the wet season compared to healthy controls (21/36, 58%) (severe malaria versus healthy: OR = 0.19 [95% CI, 0.06 to 0.63], P = 0.002; uncomplicated malaria versus healthy: OR = 0.35 [95% CI, 0.13 to 0.92], P = 0.02). Reduced responses were noted between malaria cases with HLA-A*0202 and -A*6802 alleles compared to healthy controls, but these results were not significant.
TABLE 4.
Summary of cell-mediated immune responses by HLA-A2 (*0201, *0202, *0205, *6802)-positive children with severe malaria and age- and residence-matched children with uncomplicated malaria and healthy controls stimulated with HLA-A2-restricted malaria peptide poolsc
| HLA | Group | ELISPOT resultb(%)
|
Proliferation resultb(%)
|
||||
|---|---|---|---|---|---|---|---|
| Wet | Dry | Combined | Wet | Dry | Combined | ||
| *6802 | Healthy | 4/7 (57) | 1/5 (20) | 5/7 (71) | 3/7 (43) | 0/5 (0) | 3/7 (43) |
| Severe | 2/8 (25) | 6/11 (55) | 8/13 (62) | 0/2 (0) | 3/7 (43) | 3/8 (38) | |
| Uncomplicated | 0/6 (0) | 2/8 (25) | 2/9 (22) | 2/7 (29) | 0/8 (0) | 2/8 (25) | |
| *0201 | Healthy | 5/10 (50) | 6/18 (33) | 11/20 (55) | 2/14 (14) | 7/15 (47) | 7/19 (37) |
| Severe | 3/12 (25) | 3/19 (16) | 6/21 (29) | 2/5 (40) | 4/16 (25) | 5/18 (28) | |
| Uncomplicated | 7/20 (35) | 5/18 (28) | 11/23 (48) | 2/14 (14) | 5/21 (24) | 6/24 (25) | |
| *0202 | Healthy | 9/14 (64) | 11/18 (61) | 14/19 (74) | 2/14 (14) | 6/18 (33) | 8/19 (42) |
| Severe | 1/11 (9) | 7/18 (39) | 8/21 (38) | 1/5 (20) | 3/15 (20) | 4/15 (27) | |
| Uncomplicated | 7/18 (39) | 2/15 (13) | 8/19 (42) | 2/16 (13) | 2/15 (13) | 4/18 (22) | |
| *0205 | Healthy | 3/5 (60) | 3/5 (60) | 4/5 (80) | 0/4 (0) | 0/5 (0) | 0/5 (0) |
| Severe | 1/2 (50) | 1/1 (100) | 2/3 (67) | 0/2 (0) | 0/1 (0) | 0/3 (0) | |
| Uncomplicated | 2/4 (50) | 1/4 (25) | 3/6 (50) | 1/4 (25) | 0/4 (0) | 1/5 (20) | |
| Alla | Healthy | 21/36 (58) | 21/46 (46) | 34/51 (67) | 7/39 (18) | 13/43 (30) | 18/50 (36) |
| Severe | 7/33 (21) | 17/49 (35) | 24/58 (41) | 3/14 (21) | 10/39 (26) | 12/44 (27) | |
| Uncomplicated | 16/49 (33) | 10/45 (22) | 24/57 (42) | 7/40 (18) | 7/48 (15) | 13/55 (24) | |
All, summary of the results of all HLA-A2 subtypes combined.
IFN-γ-secreting cells were determined by ELISPOT, and lymphoproliferation was measured by [H3]thymidine incorporation. Results are reported as positive responses to any of the three malaria peptide pools/total number of assays performed. Combined results represent a positive response in either the transmission (wet) or dry season. Boldface indicates significantly reduced immune response noted between severe or uncomplicated groups compared to healthy controls within haplotype with level of significance set at P of <0.05.
Pool 1, TRAP; pool 2, CSP; pool 3, Exp-1.
Eighty-nine individuals had assays performed in both the wet and dry seasons. Responses to each of the three peptide pools were evaluated, with each individual having three evaluable results. A total of 51 positive responses were noted at study enrollment (wet season). Forty-four responses reverted to negative by the time of the dry-season analysis, while 7 remained positive (3 to TRAP, 2 to CSP, and 2 to Exp-1). All seven responses that remained positive were from the healthy control group. There were 32 newly positive reactions detected in the dry season that were not detectable at the time of study enrollment (results not shown).
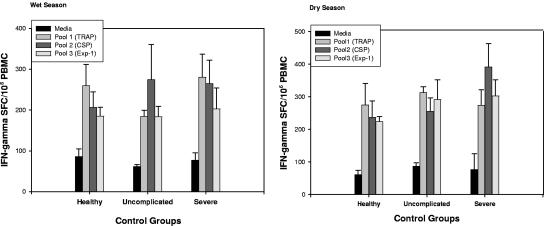
The number of IFN-γ SFC/106 PBMC in response to stimulants was stratified by enrollment group and season collected (Fig. 2). No differences were noted in the magnitude of the responses between seasons. Likewise, no differences were observed in the magnitude of the responses between malaria groups and healthy controls or in the number of SFC noted after stimulation with positive control anti-CD3/anti-CD28 beads (data not shown).
FIG. 2.
Bar graph depiction of IFN-γ-secreting cells determined by ELISPOT as SFC per 106 PBMC in HLA-A2 (*0201, *0202, *0205, *6802)-positive children with severe malaria and age- and residence-matched uncomplicated-malaria cases or healthy controls. Production of IFN-γ in response to pooled HLA-A2-restricted malaria peptide pools or media was measured (pool 1, thrombospondin-related anonymous protein; pool 2, circumsporozoite protein; pool 3, export protein 1) in both the wet and dry seasons. Standard errors are depicted.
Severe cases were stratified by predominant disease presentation (hyperparasitemia, cerebral manifestations, severe anemia, or other), by gender, and by age. The mean age of children with positive responses to at least one stimulant was 40.6 months. No significant differences in assay reactivity were noted after disease stratification, or after stratification by age or sex (data not shown). No responses to any of the peptide pools were observed using PBMC from four malaria-naive HLA-A*0201 U.S. citizens or from the eight HLA-A2-negative study participants.
(ii) Lymphoproliferation.
Proliferation assays were performed with PBMC from 149 HLA-A2 study participants (44 severe malaria cases, 55 uncomplicated malaria cases, and 50 healthy participants) including 93 assays performed on wet-season samples and 130 on dry-season samples. Seventy-eight individuals had proliferation assays performed in both seasons. Assay results were assessed by season or in a combined format indicating a positive result in at least one season. Results were stratified by HLA-A2 subtype (Tables 4 and 5). Limited responses were noted in both the wet- and dry-season samples (0 to 40%), with no difference observed between the numbers of responders between the wet and dry seasons. Stratification of results by HLA-A2 subtype and by stimulant pools revealed that CSP elicited the fewest proliferative responses in any enrollment group in either season (combined results, 4/149), while Exp-1 elicited the most (combined results, 30/149), at both time points and in all enrollment groups. Combined wet- and dry-season results revealed that PBMC from 12/44 (27%) of the severe malaria cases, 13/55 (24%) of the uncomplicated malaria cases, and 18/50 (36%) of the healthy group proliferated significantly to at least one of the three peptide pools. No correlations between positive results were noted between enrollment groups or between seasons. Stratification of severe cases by enrollment criteria revealed no association between proliferative responses and level of disease severity (data not shown). No proliferation to any of the peptide pools were observed using PBMC from four malaria-naive HLA-A*0201 U.S. citizens or from the eight HLA-A2-negative study participants.
TABLE 5.
Lymphoproliferation as measured by [H3]thymidine incorporation in PBMC from HLA-A2 (*0201, *0202, *0205, *6802)-positive children with severe malaria and age- and residence-matched children with uncomplicated malaria and healthy controls stimulated with HLA-A2-restricted malaria peptide poolsd
| HLA | Group | Pool | Resulta(%)
|
||
|---|---|---|---|---|---|
| Wet season | Dry season | Combinedb | |||
| *6802 | Healthy | 1 | 1/7 (14) | 0/5 (0) | 1/7 (14) |
| 2 | 0/7 (0) | 0/5 (0) | 0/7 (0) | ||
| 3 | 2/7 (28) | 0/5 (0) | 2/7 (28) | ||
| Uncomplicated | 1 | 1/7 (14) | 0/8 (0) | 1/8 (13) | |
| 2 | 0/7 (0) | 0/8 (0) | 0/8 (0) | ||
| 3 | 1/7 (14) | 1/8 (13) | 1/8 (13) | ||
| Severe | 1 | 0/2 (0) | 0/7 (0) | 0/8 (0) | |
| 2 | 0/2 (0) | 1/6 (17) | 1/7 (14) | ||
| 3 | 0/2 (0) | 1/6 (17) | 1/7 (14) | ||
| *0201 | Healthy | 1 | 1/14 (7) | 1/15 (7) | 3/19 (16) |
| 2 | 0/14 (0) | 1/15 (7) | 1/19 (5) | ||
| 3 | 1/14 (7) | 5/15 (33) | 5/19 (26) | ||
| Uncomplicated | 1 | 0/14 (0) | 0/21 (0) | 0/24 (0) | |
| 2 | 0/14 (0) | 0/21 (0) | 0/24 (0) | ||
| 3 | 1/14 (7) | 5/21 (24) | 5/24 (21) | ||
| Severe | 1 | 2/5 (40) | 2/16 (13) | 4/18 (22) | |
| 2 | 0/5 (0) | 0/16 (0) | 0/18 (0) | ||
| 3 | 1/5 (20) | 3/16 (19) | 3/18 (17) | ||
| *0202 | Healthy | 1 | 0/14 (0) | 0/18 (0) | 1/19 (5) |
| 2 | 2/14 (14) | 0/18 (0) | 2/19 (11) | ||
| 3 | 1/14 (7) | 5/18 (28) | 5/19 (26) | ||
| Uncomplicated | 1 | 1/16 (6) | 0/15 (0) | 1/18 (6) | |
| 2 | 0/16 (0) | 0/15 (0) | 0/18 (0) | ||
| 3 | 2/16 (13) | 3/15 (20) | 5/18 (28) | ||
| Severe | 1 | 0/5 (0) | 0/15 (0) | 0/15 (0) | |
| 2 | 0/5 (0) | 0/15 (0) | 0/15 (0) | ||
| 3 | 1/5 (20) | 2/15 (13) | 3/15 (20) | ||
| *0205 | Healthy | 1 | 0/4 (0) | 0/5 (0) | 0/5 (0) |
| 2 | 0/4 (0) | 0/5 (0) | 0/5 (0) | ||
| 3 | 0/4 (0) | 0/5 (0) | 0/5 (0) | ||
| Uncomplicated | 1 | 1/4 (25) | 0/4 (0) | 1/5 (20) | |
| 2 | 0/4 (0) | 0/4 (0) | 0/5 (0) | ||
| 3 | 0/4 (0) | 0/4 (0) | 0/5 (0) | ||
| Severe | 1 | 0/2 (0) | 0/1 (0) | 0/3 (0) | |
| 2 | 0/2 (0) | 0/1 (0) | 0/3 (0) | ||
| 3 | 0/2 (0) | 0/1 (0) | 0/3 (0) | ||
| Allc | Healthy | 1 | 2/39 (5) | 1/43 (2) | 5/50 (10) |
| 2 | 2/39 (5) | 1/43 (2) | 3/50 (6) | ||
| 3 | 4/39 (10) | 10/43 (23) | 12/50 (20) | ||
| Uncomplicated | 1 | 3/40 (8) | 0/48 (0) | 3/55 (5) | |
| 2 | 0/40 (0) | 0/48 (0) | 0/55 (0) | ||
| 3 | 4/40 (10) | 9/48 (19) | 11/55 (20) | ||
| Severe | 1 | 2/14 (14) | 2/39 (5) | 4/44 (9) | |
| 2 | 0/14 (0) | 1/38 (3) | 1/43 (2) | ||
| 3 | 2/14 (14) | 6/38 (16) | 7/43 (16) | ||
Results are depicted as number of positive assays/total number of assays performed.
Combined results represent a positive assay in PBMC collected during either the wet or dry season.
All, summary of the results of all HLA-A2 subtypes combined.
Pool 1, TRAP; pool 2, CSP; pool 3, Exp-1.
Of the 78 individuals with assays performed in both the wet and dry seasons, results were analyzed for each of the three malaria peptide pools. There were 20 positive results in the wet season. Of these, 16 reverted to negative by the time of the dry-season analysis while four remained positive (1 to TRAP and 3 to Exp-1). There were 15 newly positive reactions that were detected in the dry season that were not detectable at the time of study enrollment (results not shown).
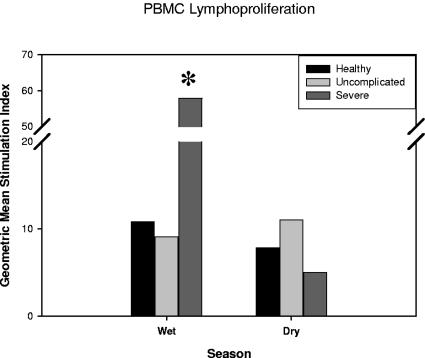
The intensity of proliferative responses in positive samples was also examined. Results were stratified by wet and dry season among enrollment groups and compared to media control (Fig. 3). Significant increases in SI were noted in severe malaria cases at the time of illness compared to the dry season (geometric mean SI, 57.9 versus 4.9; P = 0.04) although the number of samples proliferating to stimuli was low (wet season, n = 4; dry season, n = 7). No other differences in SI intensity were observed between the enrollment groups or between the wet and dry seasons.
FIG. 3.
Geometric mean stimulation indices of proliferating PBMC derived from children with severe or uncomplicated malaria and healthy controls matched by age and residence. Lymphoproliferation as determined by [H3]thymidine incorporation after stimulation with HLA-A2-restricted malaria peptide pools or media was measured (pool 1, thrombospondin-related anonymous protein; pool 2, circumsporozoite protein; pool 3, export protein 1) in both the wet (transmission) and dry seasons. Results are depicted as a combination of the responses to all three pools in each enrollment group. The asterisk depicts significant difference between wet and dry seasons (P < 0.05).
DISCUSSION
The goals of this study were to investigate whether HLA-A2 allelic subtypes might effectively present malaria peptides eliciting a T-cell-mediated immune response and to evaluate whether there are differences in these responses in matched volunteers with severe or uncomplicated malaria and healthy controls. After determining HLA haplotypes by sensitive PCR techniques, we demonstrated for the first time that alleles of HLA-A2-restricted PBMC are capable of recognizing HLA-A2 preerythrocytic malaria antigenic epitopes. We found that 43% of Malian individuals have an HLA-A2 supertype phenotype (A*0201, A*0202, A*0205, or A*6802 allele), similar to estimated frequencies of 36 to 63% in other African ethnicities (6, 7). We observed IFN-γ responses to at least one peptide pool in 42 to 67% of all volunteers tested (depending upon disease state) after stimulating PBMC from HLA-A2-positive children with HLA-A2-restricted epitope pools of CSP, TRAP, and Exp-1 malaria proteins. Although proliferative responses were observed to all peptide pools, these responses were limited. These results might have important implications in that they demonstrate that a larger segment of the population than previously thought could be responsive to malaria subunit vaccines.
After stratifying participants according to the four HLA-A2 subtype alleles and analyzing CMI responses, no measurable differences were noted between children with different subtypes. Due to the limited number of subjects with A*0205 and A*6802 alleles, the effect of structural allelic differences may not be clearly illustrated. The specificity of the responses to malaria peptide pools was confirmed by the absence of CMI to malaria epitopes when PBMC from four malaria-naive HLA-A*0201 controls and eight HLA-A2-negative study participants were tested (data not shown).
We were encouraged to observe that subtype alleles could effectively present HLA-A2-restricted peptides and anticipated that a high proportion of individuals would have demonstrable CMI responses to malaria antigens. However, despite stimulating known HLA-A2 PBMC with HLA-A2-restricted malaria peptides, the majority of children did not mount a detectable immune response either during the acute illness (wet season) or after disease recovery (dry season). Among the enrollment groups, IFN-γ production as determined by ELISPOT was highest in healthy volunteers. When allelic subtypes were combined, healthy volunteers demonstrated 33% reactivity to TRAP (versus 41% in Kenyan studies [14]), 31% to CSP, and 20% to Exp-1. In contrast, significantly lower reactivity rates were observed in children with uncomplicated or severe malaria. Similarly, we found that PBMC from healthy controls had increased proliferative responses upon malaria peptide stimulation compared to matched cases of severe and uncomplicated malaria in combined wet- and dry-season analysis. We observed limited overall proliferative responses (0 to 23%) when allelic subtypes were combined into one group and analyzed as a whole, complicating conclusions made regarding differences among individual allelic subtypes. The few severe cases that did proliferate (to any peptide pool) had a vigorous response. The finding of increased immunoreactivity among healthy volunteers may reflect blunted immune responses in patients with malaria, as has been reported in cases of severe disease (4) and/or indirect evidence for stronger host responsiveness in healthy controls endowing them with the ability to mount these, as well as yet-undefined, immune responses. No changes were observed in the magnitude of responses to control stimulant (anti-CD3/CD28 beads) among enrollment groups to suggest immunologic blunting. Of interest, all of the ELISPOT responses that remained positive from the wet to the dry season were documented in healthy volunteers (n = 7).
Of the peptide pools tested, TRAP consistently elicited the greatest IFN-γ responses in all study groups while Exp-1 elicited the least. These findings were nearly identical during the dry season follow-up. This contrasts with proliferation results where Exp-1 elicited the most activity in all enrollment groups in both seasons with almost no detectable proliferation to CSP, suggesting that different effector immune responses are elicited by different malarial proteins. Studies have demonstrated little correlation between cellular IFN-γ secretion and lymphoproliferation in response to malaria peptides (13, 27, 28). This is likely due to the fact that these assays measure different CMI responses, i.e., effector T-cell function for ex vivo IFN-γ production and largely memory T cells for lymphoproliferative responses.
A number of factors may have influenced the low percentage of immune responses detected in this study. The mechanism of preerythrocytic protective immunity in natural malaria remains unclear, hampered by the inability to examine liver CD8+ T cells directly. As a result, the immunologic assay(s) that best assesses host response to malaria infection and its relevance in measuring host protection from disease is uncertain. The low frequency of Plasmodium-specific CD8+ T cells, the relatively immature immune systems of these very young children (mean age 41 months), antigenic variation, and short-lived immunity may have affected our ability to detect malaria-induced immune responses. We found that the majority of positive CMI responses documented during active infection reverted to negative during a follow-up period with negligible transmission. This compares with Kenyan results in which short-lived IFN-γ immune responses to TRAP were documented when ELISPOT assays were performed at 1-year intervals (14). Moreover, peptides capable of inducing proliferative responses in one setting do not necessarily elicit similar responses in another (13, 16, 18, 33), nor does binding affinity necessarily correlate with IFN-γ responses (15). We did not perform longitudinal follow-up to quantify malaria exposure following enrollment, so it is unclear whether the immune responses present during the low-transmission season were due to long-lived immunity or to ongoing “low-level” antigenic exposure.
We wish to emphasize that, due to the small quantity of blood collected from severely ill children, immunologic studies could only be performed once with cryopreserved PBMC. This limited our analysis to the measurement of immune responses to pools of epitopes rather than individual epitopes. Variations in ELISPOT technique (ex vivo versus cultured PBMC) and the use of cryopreserved versus fresh cells could alter or limit the ability to detect low-level CD8+ T-cell secretion of IFN-γ (13, 25). To maximize the detection of ex vivo IFN-γ responses, we added anti-CD28 and anti-CD49d mAbs to the cultures. Anti-CD28 and -CD49d mAbs have been shown to increase the measured effector frequency of peripheral blood-derived CD4+ T cells (34). Anti-CD28 has recently been validated with peripheral CD8+ T cells (22). In extensive control experiments, the addition of these mAbs did not increase IFN-γ SFC in media control cultures from HLA-A2- and HLA-A3-positive children (results not shown). To our knowledge, this is the first time that an ex vivo IFN-γ ELISPOT assay optimized by the addition of costimulation has been utilized in PBMC from malaria-endemic areas.
In summary, we have demonstrated for the first time that HLA-A2 allelic subtypes can effectively present malaria peptides eliciting a T-cell-mediated immune response. Despite demonstrating allelic cross-reactivity, only a minority of children, regardless of disease state, exhibited detectable immune responses to malaria peptide pools. Additional research is needed to identify the individual epitopes responsible for eliciting detectable CMI responses and to determine whether these immune responses are critical in mediating protection from P. falciparum infection. These results suggest that immune responses to other epitopes in TRAP, CSP, Exp-1 or other uncharacterized proteins might be required to endow protection from infection and/or disease.
Acknowledgments
We acknowledge the dedication of the Bandiagara Malaria Project staff and the populace of Bandiagara, Mali, West Africa, who have been so supportive of research efforts and so in need of the benefits of malaria vaccines.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Aidoo, M., A. Lalvani, C. E. Allsopp, M. Plebanski, S. J. Meisner, P. Krausa, M. Browning, S. Morris-Jones, F. Gotch, and D. A. Fidock. 1995. Identification of conserved antigenic components for a cytotoxic T lymphocyte-inducing vaccine against malaria. Lancet 345:1003-1007. [DOI] [PubMed] [Google Scholar]
- 2.Aidoo, M., A. Lalvani, S. C. Gilbert, J. T. Hu, P. Daubersies, N. Hurt, H. C. Whittle, P. Druihle, and A. V. Hill. 2000. Cytotoxic T-lymphocyte epitopes for HLA-B53 and other HLA types in the malaria vaccine candidate liver-stage antigen 3. Infect. Immun. 68:227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum-Tirouvanziam, U., C. Servis, A. Habluetzel, D. Valmori, Y. Men, F. Esposito, L. Del Nero, N. Holmes, N. Fasel, and G. Corradin. 1995. Localization of HLA-A2.1-restricted T cell epitopes in the circumsporozoite protein of Plasmodium falciparum. J. Immunol. 154:3922-3931. [PubMed] [Google Scholar]
- 4.Brasseur, P., M. Agrapart, J. J. Ballet, P. Druilhe, M. J. Warrell, and S. Tharavanij. 1983. Impaired cell-mediated immunity in Plasmodium falciparum-infected patients with high-parasitemia and cerebral malaria. Clin. Immunol. Immunopathol. 27:38-50. [DOI] [PubMed] [Google Scholar]
- 5.Cao, K., M. Chopek, and M. A. Fernandez-Vina. 1999. High and intermediate resolution DNA typing systems for class I HLA-A, B, C genes by hybridization with sequence-specific oligonucleotide probes (SSOP). Rev. Immunogenet. 1:177-208. [PubMed] [Google Scholar]
- 6.Cao, K., J. Hollenbach, X. Shi, W. Shi, M. Chopek, and M. A. Fernandez-Vina. 2001. Analysis of the frequencies of HLA-A, B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Hum. Immunol. 62:1009-1030. [DOI] [PubMed] [Google Scholar]
- 7.Cao, K., A. M. Moormann, K. E. Lyke, C. Masaberg, O. P. Sumba, O. K. Doumbo, D. Koech, A. Lancaster, M. Nelson, D. Meyer, R. Single, R. J. Hartzman, C. V. Plowe, J. Kazura, D. L. Mann, M. B. Sztein, G. Thomson, and M. A. Fernandez-Vina. 2004. Differentiation between African populations is evidenced by the diversity of alleles and haplotypes of HLA class I loci. Tissue Antigens 63:293-325. [DOI] [PubMed] [Google Scholar]
- 8.Clyde, D. F., V. C. McCarthy, R. M. Miller, and R. B. Hornick. 1973. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am. J. Med. Sci. 266:398-403. [DOI] [PubMed] [Google Scholar]
- 9.Coulibaly, D., D. A. Diallo, M. A. Thera, A. Dicko, A. B. Guindo, A. K. Kone, Y. Cissoko, S. Coulibaly, A. Djimde, K. Lyke, O. K. Doumbo, and C. V. Plowe. 2002. Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali. Am. J. Trop. Med. Hyg. 67:604-610. [DOI] [PubMed] [Google Scholar]
- 10.del Guercio, M. F., J. Sidney, G. Hermanson, C. Perez, H. M. Grey, R. T. Kubo, and A. Sette. 1995. Binding of a peptide antigen to multiple HLA alleles allows definition of an A2-like supertype. J. Immunol. 154:685-693. [PubMed] [Google Scholar]
- 11.Doolan, D. L., and S. L. Hoffman. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165:1453-1462. [DOI] [PubMed] [Google Scholar]
- 12.Doolan, D. L., S. L. Hoffman, S. Southwood, P. A. Wentworth, J. Sidney, R. W. Chesnut, E. Keogh, E. Appella, T. B. Nutman, A. A. Lal, D. M. Gordon, A. Oloo, and A. Sette. 1997. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA-B supertype alleles. Immunity 7:97-112. [DOI] [PubMed] [Google Scholar]
- 13.Flanagan, K. L., E. A. Lee, M. B. Gravenor, W. H. Reece, B. C. Urban, T. Doherty, K. A. Bojang, M. Pinder, A. V. Hill, and M. Plebanski. 2001. Unique T cell effector functions elicited by Plasmodium falciparum epitopes in malaria-exposed Africans tested by three T cell assays. J. Immunol. 167:4729-4737. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan, K. L., T. Mwangi, M. Plebanski, K. Odhiambo, A. Ross, E. Sheu, M. Kortok, B. Lowe, K. Marsh, and A. V. Hill. 2003. Ex vivo interferon-gamma immune response to thrombospondin-related adhesive protein in coastal Kenyans: longevity and risk of Plasmodium falciparum infection. Am. J. Trop. Med. Hyg. 68:421-430. [PubMed] [Google Scholar]
- 15.Gonzalez, J. M., K. Peter, F. Esposito, I. Nebie, J. M. Tiercy, A. Bonelo, M. Arevalo-Herrera, D. Valmori, P. Romero, S. Herrera, G. Corradin, and J. A. Lopez. 2000. HLA-A*0201 restricted CD8+ T-lymphocyte responses to malaria: identification of new Plasmodium falciparum epitopes by IFN-γ ELISPOT. Parasite Immunol. 22:501-514. [DOI] [PubMed] [Google Scholar]
- 16.Good, M. F., D. Pombo, I. A. Quakyi, E. M. Riley, R. A. Houghten, A. Menon, D. W. Alling, J. A. Berzofsky, and L. H. Miller. 1988. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc. Natl. Acad. Sci. USA 85:1199-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill, A. V., J. Elvin, A. C. Willis, M. Aidoo, C. E. Allsopp, F. M. Gotch, X. M. Gao, M. Takiguchi, B. M. Greenwood, and A. R. Townsend. 1992. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature 360:434-439. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman, S. L., C. N. Oster, C. Mason, J. C. Beier, J. A. Sherwood, W. R. Ballou, M. Mugambi, and J. D. Chulay. 1989. Human lymphocyte proliferative response to a sporozoite T cell epitope correlates with resistance to falciparum malaria. J. Immunol. 142:1299-1303. [PubMed] [Google Scholar]
- 19.Lalvani, A., N. Hurt, M. Aidoo, P. Kibatala, M. Tanner, and A. V. Hill. 1996. Cytotoxic T lymphocytes to Plasmodium falciparum epitopes in an area of intense and perennial transmission in Tanzania. Eur. J. Immunol. 26:773-779. [DOI] [PubMed] [Google Scholar]
- 20.Malik, A., J. E. Egan, R. A. Houghten, J. C. Sadoff, and S. L. Hoffman. 1991. Human cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. Proc. Natl. Acad. Sci. USA 88:3300-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nussenzweig, R. S., J. Vanderberg, H. Most, and C. Orton. 1967. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature 216:160-162. [DOI] [PubMed] [Google Scholar]
- 22.Ott, P. A., B. R. Berner, B. A. Herzog, R. Guerkov, N. L. Yonkers, I. Durinovic-Bello, M. Tary-Lehmann, P. V. Lehmann, and D. D. Anthony. 2004. CD28 costimulation enhances the sensitivity of the ELISPOT assay for detection of antigen-specific memory effector CD4 and CD8 cell populations in human diseases. J. Immunol. Methods 285:223-235. [DOI] [PubMed] [Google Scholar]
- 23.Pasetti, M. F., R. J. Anderson, F. R. Noriega, M. M. Levine, and M. B. Sztein. 1999. Attenuated ΔguaBA Salmonella typhi vaccine strain CVD 915 as a live vector utilizing prokaryotic or eukaryotic expression systems to deliver foreign antigens and elicit immune responses. Clin. Immunol. 92:76-89. [DOI] [PubMed] [Google Scholar]
- 24.Plebanski, M., M. Aidoo, H. C. Whittle, and A. V. Hill. 1997. Precursor frequency analysis of cytotoxic T lymphocytes to pre-erythrocytic antigens of Plasmodium falciparum in west Africa. J. Immunol. 158:2849-2855. [PubMed] [Google Scholar]
- 25.Reece, W. H., M. Pinder, P. K. Gothard, P. Milligan, K. Bojang, T. Doherty, M. Plebanski, P. Akinwunmi, S. Everaere, K. R. Watkins, G. Voss, N. Tornieporth, A. Alloueche, B. M. Greenwood, K. E. Kester, K. P. McAdam, J. Cohen, and A. V. Hill. 2004. A CD4+ T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 10:406-410. [DOI] [PubMed] [Google Scholar]
- 26.Rieckmann, K. H., R. L. Beaudoin, J. S. Cassells, and K. W. Sell. 1979. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull. W. H. O. 57(Suppl. 1):261-265. [PMC free article] [PubMed] [Google Scholar]
- 27.Riley, E. M., S. J. Allen, S. Bennett, P. J. Thomas, A. O'Donnell, S. W. Lindsay, M. F. Good, and B. M. Greenwood. 1990. Recognition of dominant T cell-stimulating epitopes from the circumsporozoite protein of Plasmodium falciparum and relationship to malaria morbidity in Gambian children. Trans. R. Soc. Trop. Med. Hyg. 84:648-657. [DOI] [PubMed] [Google Scholar]
- 28.Riley, E. M., S. J. Allen, J. G. Wheeler, M. J. Blackman, S. Bennett, B. Takacs, H. J. Schonfeld, A. A. Holder, and B. M. Greenwood. 1992. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 14:321-337. [DOI] [PubMed] [Google Scholar]
- 29.Salerno-Goncalves, R., M. F. Pasetti, and M. B. Sztein. 2002. Characterization of CD8+ effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J. Immunol. 169:2196-2203. [DOI] [PubMed] [Google Scholar]
- 30.Sedegah, M., B. K. Sim, C. Mason, T. Nutman, A. Malik, C. Roberts, A. Johnson, J. Ochola, D. Koech, and B. Were. 1992. Naturally acquired CD8+ cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. J. Immunol. 149:966-971. [PubMed] [Google Scholar]
- 31.Sidney, J., S. Southwood, D. L. Mann, M. A. Fernandez-Vina, M. J. Newman, and A. Sette. 2001. Majority of peptides binding HLA-A*0201 with high affinity crossreact with other A2-supertype molecules. Hum. Immunol. 62:1200-1216. [DOI] [PubMed] [Google Scholar]
- 32.Tacket, C. O., M. B. Sztein, S. S. Wasserman, G. Losonsky, K. L. Kotloff, T. L. Wyant, J. P. Nataro, R. Edelman, J. Perry, P. Bedford, D. Brown, S. Chatfield, G. Dougan, and M. M. Levine. 2000. Phase 2 clinical trial of attenuated Salmonella enterica serovar Typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect. Immun. 68:1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troye-Blomberg, M., E. M. Riley, L. Kabilan, M. Holmberg, H. Perlmann, U. Andersson, C. H. Heusser, and P. Perlmann. 1990. Production by activated human T cells of interleukin 4 but not interferon-gamma is associated with elevated levels of serum antibodies to activating malaria antigens. Proc. Natl. Acad. Sci. USA 87:5484-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waldrop, S. L., K. A. Davis, V. C. Maino, and L. J. Picker. 1998. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J. Immunol. 161:5284-5295. [PubMed] [Google Scholar]
- 35.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 36.Weiss, W. R., S. Mellouk, R. A. Houghten, M. Sedegah, S. Kumar, M. F. Good, J. A. Berzofsky, L. H. Miller, and S. L. Hoffman. 1990. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. J. Exp. Med. 171:763-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wizel, B., R. Houghten, P. Church, J. A. Tine, D. E. Lanar, D. M. Gordon, W. R. Ballou, A. Sette, and S. L. Hoffman. 1995. HLA-A2-restricted cytotoxic T lymphocyte responses to multiple Plasmodium falciparum sporozoite surface protein 2 epitopes in sporozoite-immunized volunteers. J. Immunol. 155:766-775. [PubMed] [Google Scholar]
- 38.World Health Organization Communicable Diseases Cluster. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-90. [PubMed] [Google Scholar]