Abstract
Malaria infection is initiated when the insect vector injects Plasmodium sporozoites into a susceptible vertebrate host. Sporozoites rapidly leave the circulatory system to invade hepatocytes, where further development generates the parasite form that invades and multiplies within erythrocytes. Previous experiments have shown that the thrombospondin-related adhesive protein (TRAP) plays an important role in sporozoite infectivity for hepatocytes. TRAP, a typical type-1 transmembrane protein, has a long extracellular region, which contains two adhesive domains, an A-domain and a thrombospondin repeat. We have generated recombinant proteins of the TRAP adhesive domains. These TRAP fragments show direct interaction with hepatocytes and inhibit sporozoite invasion in vitro. When the recombinant TRAP A-domain was used for immunoprecipitation against hepatocyte membrane fractions, it bound to α2-Heremans-Schmid glycoprotein/fetuin-A, a hepatocyte-specific protein associated with the extracellular matrix. When the soluble sporozoite protein fraction was immunoprecipitated on a fetuin-A-adsorbed protein A column, TRAP bound this ligand. Importantly, anti-fetuin-A antibodies inhibited invasion of hepatocytes by sporozoites. Further, onset of malaria infection was delayed in fetuin-A-deficient mice compared to that in wild-type C57BL/6 mice when they were challenged with Plasmodium berghei sporozoites. These data demonstrate that the extracellular region of TRAP interacts with fetuin-A on hepatocyte membranes and that this interaction enhances the parasite's ability to invade hepatocytes.
Malaria is a deadly parasitic disease which affects nearly 40% of the world's population. In any given year 300 to 500 million clinical cases occur, with more than a million deaths. Malaria is caused by species of the protozoan parasite Plasmodium. The infection is initiated when a female Anopheles mosquito injects Plasmodium sporozoites into a susceptible vertebrate host. The sporozoites migrate to the liver, where they undergo many rounds of schizogony in the hepatocytes. The resulting merozoites then invade and multiply within erythrocytes, causing the clinical symptoms of malaria.
Understanding the pathogenesis of malaria requires a keen understanding of the molecular mechanisms used by Plasmodium to recognize and invade host cells. Invasion is mediated by interactions between specific parasite molecules and complementary ligands on the host cells. Plasmodium has three invasive stages: (i) sporozoites (sporogonic cycle), which invade the salivary glands of the mosquito; (ii) salivary gland sporozoites (exoerythrocytic cycle), which invade hepatocytes in the vertebrate; and (iii) merozoites (erythrocytic cycle), which invade erythrocytes. The invasion of hepatocytes by sporozoites has been known to require two parasite proteins: circumsporozoite protein (CS) and thrombospondin-related adhesive protein (TRAP) (6, 28, 30, 33, 34, 44). These proteins are conserved in all Plasmodium species examined to date (26, 40) and are present on the parasite surface and in the micronemes (11, 29, 34). CS uniformly covers the external surfaces of sporozoites and represents up to 15% of the total protein synthesized by sporozoites obtained from salivary glands of mosquitoes (43). It contains a highly conserved 18-residue motif homologous to a portion of the type 1 repeat of thrombospondin. This motif, named region II plus, binds to heparin sulfate proteoglycans associated with hepatocyte membranes (7, 12, 31, 37). TRAP is expressed on the surfaces of salivary gland sporozoites (9, 34), where it displays a patchy distribution. TRAP contains a region II plus-like motif that binds to sulfated glycoconjugates (28, 32) and a region of ∼200 residues that displays homology to the A domain of von Willebrand factor (14). The A-domains are present in many proteins involved in cell-cell, cell-matrix, and matrix-matrix interactions via a divalent metal-binding domain, known as the metal ion-dependent adhesion site (MIDAS) motif, found in a variety of integrins, numerous collagen types, and matrilins (8).
Gene disruption experiments with Plasmodium berghei have demonstrated that TRAP is necessary for motility and invasion of sporozoites into both salivary glands in the mosquito and liver hepatocytes in the mouse (38). Specific point mutations in conserved residues of the A-domain and thrombospondin repeat (TSR) domains of TRAP have identified motifs necessary for mediating entry into both vertebrate and insect cells (25, 41). In Toxoplasma spp., MIC2, the TRAP family member, is expressed in all invasive stages, and indirect evidence suggests it is essential for invasion (19). It has been shown that A-domains in TRAP and MIC2 bind to heparin-like molecules, and interactions with these glycosaminoglycans are likely important for parasite recognition of host cells (16, 27). In mammalian systems, A-domains mediate binding to a variety of matrix and cell surface proteins, which raises the possibility that similar interactions may be important for parasite A-domain-containing proteins.
The main goal of the present study was to identify the receptor molecule on host hepatocytes that interacts with TRAP to mediate invasion. Using a recombinant TRAP A-domain protein, we show that it specifically binds to the α2-Heremans-Schmid (HS) glycoprotein/fetuin-A protein on hepatocyte membranes. This association promotes TRAP interaction with additional unidentified molecules on the hepatocyte membrane, eventually leading to the invasion of the host cell.
MATERIALS AND METHODS
Cell lines and cell culture.
HepG2 human hepatoma cells (American Type Culture Collection, Manassas, VA) were maintained in Dulbecco's modified Eagle medium (Gibco/BRL, Gaithersburg, MD), 10% fetal calf serum (HyClone Laboratories, Logan, UT), and penicillin-streptomycin-glutamine (Gibco/BRL, Gaithersburg, MD) in a humidified atmosphere with 5% CO2 at 37°C. P. berghei strain NK65 was used in this study.
Generation and expression of the GST-TRAP A-domain construct.
A DNA fragment encoding the P. berghei TRAP A-domain (residues 84 to 725), was generated by PCR from NK65 genomic DNA by using primers 5′-CGCGGATCCGGTCAGGAAATTCTTGACGAA-3′ and 5′-CCGGAATTCAAGAGCAACTTTTTCTACTTC-3′; for the TRAP repeat region (residues 854 to 1634), primers were 5′-CGCGGATCCCCACCAAAACCGGTAGCTCCTCCT-3′ and CCGGAATTCGTTATTAGATTTAGACTGCTTTT. The A-domain mutant PAdoT contains a mutation that changes the conserved Thr126 residue to alanine (25). The cloned PCR product for PAdoT was generated using TRAP A-domain primers. The PCR fragments for the A-domain, repeat region, and PAdoT were cloned into the pGEX-2T expression vector, creating an in-frame fusion of glutathione S-transferase (GST) with the TRAP A-domain, TRAP repeat region, and PAdoT. These clones were purified according to the standard procedure of the manufacturer (Amersham Biosciences, Piscataway, NJ). Briefly, Escherichia coli strain BL21(DE3) was transformed with pGEX-2T-A-domain, pGEX-2T-repeat region, or pGEX-2T-PAdoT, and cells were grown to mid-log phase at 37°C before induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 to 4 h. The cell pellet from 1 liter of culture was lysed using 40 to 60 ml of B-Per bacterial protein extraction reagent (Pierce, Rockford, IL), and the GST fusion proteins were purified using 500 to 600 μl glutathione Sepharose. The GST fusion protein was eluted from the glutathione Sepharose beads using 500 μl solution containing 10 mM reduced glutathione (Sigma, St. Louis, MO) in 50 mM Tris-Cl, pH 8.8. The purified fusion proteins were cleaved from the GST tag using a biotinylated thrombin kit according to the manufacturer's instructions (Novagen Inc., Stamford, CT).
Generation of anti-A-domain antibodies.
The recombinant A-domain protein was used to immunize two rabbits to generate polyclonal antisera. The rabbits were immunized subcutaneously three times, either with 100 μg in complete Freund's adjuvant (first immunization) or with 50 μg of protein in incomplete Freund's adjuvant. Sera were collected 14 days after the last injection. Preimmune sera were obtained before immunization. The antibodies against the A-domain and preimmune sera were purified by incubation with glutathione Sepharose 4B for 2 h at 4°C. After centrifugation at 12,000 rpm for 2 min at 4°C, the supernatant was collected and the pellet discarded.
ELISA.
Binding of HepG2 cells to the recombinant TRAPs was carried out as described by Sinnis (36) with modifications. Briefly, 1 × 106 HepG2 cells were plated in 96-well plates and allowed to grow for 24 h. The cells were then fixed with 4% paraformaldehyde, washed with phosphate-buffered saline (PBS), and blocked for 1 h with 1% bovine serum albumin (BSA) in 0.1% PBS-Tween 20. The cells were incubated for 2 h with the appropriate concentration of recombinant protein. After a wash, cells were incubated with antibodies directed against TRAP (1:1,000), followed by an anti-rabbit immunoglobulin G secondary antibody conjugated to horseradish peroxidase (HRP) for an enzyme-linked immunosorbent assay (ELISA) (1:5,000; Sigma, St. Louis, MO). Bound enzyme for the ELISA was revealed by the addition of the substrate 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate) (ABTS), according to the manufacturer's instructions (Sigma, St. Louis, MO). After 1 h, absorbance was read at 405 nm in an ELISA plate reader. The binding of HepG2 cells to the recombinant TRAP A-domain was also checked in the presence of the divalent cations Mg2+ and Mn2+ at concentrations between 0 and 10 mM with or without 10 mM EDTA or EGTA. The experiment was performed as described above.
Assays for inhibition of sporozoite invasion (ISI).
The recombinant proteins were also tested for their abilities to inhibit hepatocyte infectivity by P. berghei sporozoites in vitro. The methodology for assaying P. berghei sporozoite invasion and development in cultured hepatocytes was carried out as previously described (18), with modifications. Briefly, HepG2 cells (4 × 105/well) were plated in each well of an eight-chamber slide (catalog no. 4808; Lab-Tek, Naperville, IL) and allowed to grow for 24 to 48 h. P. berghei sporozoites were dissected from the mosquito salivary glands, collected, and resuspended in Dulbecco's modified Eagle medium supplemented with fetal calf serum. The medium was removed from the cells, and 5,000 to 40,000 P. berghei sporozoites were added to each well. The recombinant TRAP A-domain protein was added at concentrations of 250 to 1,000 ng per well. The sporozoites were incubated for 2 h at 37°C under 5% CO2. The unattached sporozoites and medium were aspirated from the wells. The cells were then fixed with cold methanol, blocked with 1% BSA in Tris-buffered saline, and incubated with a monoclonal antibody against the CS protein (10). Wells were washed with Tris-buffered saline and incubated with an anti-mouse immunoglobulin conjugated to fluorescein isothiocyanate (Roche, Branchburg, NJ). The slides were washed, mounted in mounting medium (Roche, Branchburg, NJ), and counted microscopically. The percentage of inhibition was calculated using control cultures that had received a dilution of the native GST. The ISI50, defined as the concentration of the recombinant protein that reduces invasion by 50%, was determined as described by Hollingdale et al. (18). Each reaction was performed in triplicate.
Purification of plasma membrane from HepG2 cells.
We used the human hepatoma cell line HepG2 to extract hepatocyte membranes. Membrane extracts were prepared from these cells according to the method described by Hoeg et al. (17) with modifications. Briefly, HepG2 cells (5 × 108 to 109 cells/ml) were washed in ice-cold PBS containing 200 mM of the mild, nondenaturing detergent n-octyl glucoside, 0.25 M sucrose, and a protease inhibitor cocktail tablet (Bio-Rad Laboratories, Hercules, CA). After a wash, cells were homogenized and centrifuged at 1,000 × g, followed by ultracentrifugation at 100,000 × g for 1 h. The pellet from this ultracentrifugation was resuspended in a buffer containing 150 mM NaCl-10 mM Tris (pH 8.0) and flushed through a 22-gauge needle several times. This solution was recentrifuged at 100,000 × g. The purity of these membrane fractions was assessed using standard membrane markers such as the Na+/K ATPase and Mg2+/ATPase assay (5).
Immunoprecipitation assays.
Immunoprecipitation was performed using the Seize protein A immunoprecipitation kit (Pierce, Rockford, IL). Briefly, 100 μg of the recombinant A-domain of TRAP with 50 μg anti-A-domain antibodies was attached to a protein A column. One milligram of solubilized HepG2 membrane proteins was incubated on the column overnight at 4°C. The bound membrane protein was eluted using the elution buffer from the kit. The eluted fraction was run on a 4 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and stained with silver stain as described previously (23).
Characterization of the eluted proteins.
Immunoprecipitated eluted protein was detected using in situ tryptic digestion of proteins after the elution from the gel. This was performed at the Harvard University Microchemistry Laboratory under the direction of William Lane using microcapillary reverse-phase high-performance liquid chromatography nanoelectrospray tandem mass spectrometry (μLC/MS/MS) on a Finnigan LCQ DECA quadrupole ion trap mass spectrometer. The sequences of the proteins identified were used to search the databases from Sequest for homologous sequences.
Overlay binding assay.
Recombinant TRAP proteins (the A-domain, the repeat region, GST, and the A-domain mutant PAdoT) were dotted onto 6-mm sections of nitrocellulose membranes. The membranes were saturated with 3% BSA and 1% Tween 20 in PBS at room temperature for 1 h. Fetuin-A was biotinylated using the reagent EZ-Link sulfo-(NHS-LC-biotin) succinimidyl-6-biotinamide hexanoate according to the manufacturer's instructions (Pierce, Rockford, IL). The biotinylated fetuin was overlaid on the blotted nitrocellulose membrane to a final concentration of 0.1 μg/ml and incubated for 30 min. After a wash with PBS, the membranes were incubated with 0.1 μg/ml of streptavidin-HRP in 1% BSA-0.05% Tween 20 in PBS and washed, and ligand binding was detected by ECL autoradiography (Amersham Biosciences, Piscataway, NJ).
Sporozoites and infected-hepatocyte preparations.
Plasmodium berghei (strain NK65) sporozoites were obtained from dissection of the salivary glands of Anopheles stephensi mosquitoes 18 to 20 days after they had taken an infective blood meal from mice. Prior to injection, sporozoites were centrifuged at 1,000 × g for 3 min at 4°C and resuspended in PBS with 1% BSA. A total of 10,000 to 15,000 sporozoites were injected intravenously into C57BL/6 (control) and fetuin-A knockout (20, 39) mice. Mice were examined daily for the presence of malaria parasites in their peripheral blood by thin blood smears stained with Giemsa stain.
RT-PCR of P. berghei liver parasites.
Wild-type (n = 8) and fetuin-A knockout (n = 8) mice (20, 39) were injected intravenously in the tail vein with 10,000 to 15,000 P. berghei sporozoites. Mice were followed by daily examination of blood smears to detect blood-stage parasitemia. The eight fetuin-A knockout mice and eight C57BL/6 control mice were used to determine Plasmodium liver stage load. At 40 h after injection, livers were removed and processed for RNA extraction to perform reverse transcription-PCR (RT-PCR) (4). Briefly, 40 h after sporozoite injection, the mice were scarified, and their livers were harvested and frozen in liquid nitrogen. The livers were homogenized in PowerGen with 8 to 10 ml of TRI-reagent (Sigma, St. Louis, MO) and vortexed. To 1 ml of homogenate, 1 ml of buffer-saturated phenol and 250 μl of chloroform-isoamyl alcohol were added, and the mixture was incubated on ice for 5 min and centrifuged at 14,000 × g or 12,000 rpm for 15 min at 4°C. The aqueous phase was collected and precipitated in 1 volume of isopropanol for 30 min at −20°C. The RNA was spun for 20 min at 12,000 rpm and 4°C, and the supernatant was carefully drained. A 500-μl volume of cold 75% ethanol was then added, and the pellet was vortexed for 5 seconds. The RNA was spun for 5 min at 12,000 rpm at 4°C. The supernatant was carefully drained, and the pellet was air dried. The pellet was dissolved in Ultraspec water and rocked overnight at 4°C. RT-PCR was performed using a SuperScript One-Step RT-PCR kit with Platinum Taq (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA was quantified by absorbance at 260 nm, and the RT reaction was performed with 1 μg of total RNA and random hexamers supplied by the manufacturer. PCR of the cDNA was performed using parasite rRNA primers that recognize Plasmodium berghei-specific sequences within the 18S rRNA as described previously by Briones et al. (4). The forward primer used was 5′-AGGATGTATTCGCTTTATTTAATGC-′3, and the reverse primer was 5′-TCTTGTCCAAACAATTCATCATATC-′3. As a positive control for the integrity of RNA samples, we detected mouse β-actin mRNA using the specific forward primer 5′-ACAGTGTGGGTGACCCCGTC-′3 and reverse primer 5′-CCCTGTGCTGCTCACCGA-′3. One-fifth of the amplification product was analyzed by horizontal gel electrophoresis on 2% agarose in 0.04 M Tris-acetate-0.001 M EDTA, stained with 0.5 μg/ml ethidium bromide, and photographed under long-wavelength UV light.
Statistical analysis.
A skewness/kurtosis normality test was used to determine if the data sets were parametric or nonparametric. A one-tailed Student t test and a Kruskal-Wallis test were performed on the parametric and nonparametric data sets, respectively, to determine the statistical significance of the variation in the prepatent period and in mean parasitemia between the wild-type controls and fetuin-A knockout mice on various days of the challenge. The P values indicate that the data points we are measuring have a high probability of falling into the range described in the 95% confidence interval (P = 0.0048). STATA software was used to perform all of the statistical analyses.
RESULTS
Generation and expression of TRAP recombinant proteins.
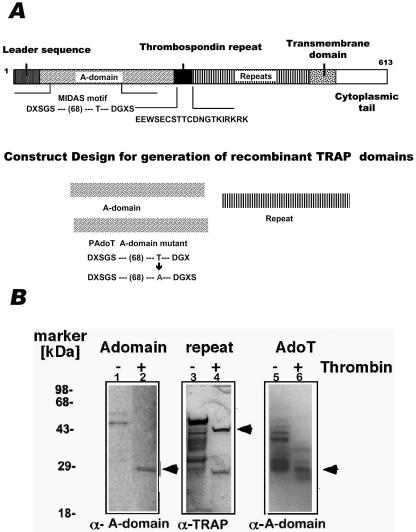
Plasmodium sporozoites enter hepatocytes of the vertebrate host shortly after being inoculated into the blood circulation by an infected mosquito. Genetic evidence has shown that the type I transmembrane protein TRAP, which bears two adhesive domains, is required both for motility and for cell invasion by Plasmodium sporozoites (38). Toward defining hepatocyte molecules that interact with the adhesive domain of TRAP, we generated P. berghei TRAP recombinant proteins. We produced fragments that contained either the A-domain, the repeat region of TRAP, or the A-domain mutant PAdoT (Fig. 1A). All constructs were expressed as GST fusion proteins. The GST tags were eventually cleaved at the thrombin cleavage site from the purified fusion proteins.
FIG. 1.
Generation and expression of TRAP recombinant proteins. (A) Schematic drawing of P. berghei TRAP indicating the various domains and the design of the construct for the generation of recombinant TRAP proteins. (B) Western blot showing TRAP recombinant proteins probed using an anti-A-domain antibody (lanes 1, 2, 5, and 6) or anti-TRAP (lanes 3 and 4). Lane 1, 5 to 10 μg of GST-TRAP A-domain fusion protein; lane 2, 1 to 2 μg of TRAP A-domain after thrombin cleavage; lane 3, 5 to 10 μg of GST-TRAP repeat fusion protein; lane 4, 1 to 2 μg of TRAP repeat after thrombin cleavage; lane 5, 5 to 10 μg of GST-TRAP/PAdoT (mutant of TRAP A-domain) fusion protein; lane 6, 1 to 2 μg of TRAP/PAdoT protein after thrombin cleavage.
The purified TRAP A-domain fragment was used to immunize two rabbits to generate polyclonal antisera. These antisera showed strong reactivity against total protein from P. berghei sporozoites, the purified wild-type A-domain, and the A-domain mutant pAdoT (Fig. 1B). Anti-TRAP antisera used in our previous TRAP knockout study (38) reacted well with the recombinant TRAP repeat fragment (Fig. 1B).
Ability of TRAP recombinant proteins to bind HepG2 hepatocytes.
Once we established the expression, solubility, and purification of the recombinant A-domain, the repeat region, and the PAdoT mutant protein of the TRAP, we developed an in vitro binding assay to assess the binding of these recombinant proteins to cultured hepatocytes. The recombinant A-domain fragment was examined for binding to hepatocytes over a range of concentrations. Our results in Fig. 2A show a dose-response relationship in the binding between the recombinant TRAP A-domain and hepatocytes. This response was saturable at concentrations of 8 to 10 μg of protein. In comparison, GST protein showed negligible levels of adhesion to hepatocytes at all concentrations (Fig. 2A).
FIG. 2.
Binding of purified recombinant TRAP constructs with HepG2 cells. All the recombinant proteins were cleaved from GST using the thrombin cleavage site. (A) Binding of various concentrations of the purified recombinant TRAP A-domain and GST (as a control) to 1 × 106 HepG2 cells. (B) Binding of 1 μg recombinant TRAP A-domain in the presence of various concentrations of MgCl2, MgCl2 plus 10 mM EDTA, or MgCl2 plus 10 mM EGTA to 1 × 106 HepG2 cells. (C) Binding of 1 μg of the purified recombinant TRAP A-domain to HepG2 cells in the presence of MnCl2, MnCl2 plus 10 mM EDTA, or MnCl2 plus 10 mM EGTA to 1 × 106 HepG2 cells. (D) Binding of various concentrations of the recombinant TRAP repeat protein to 1 × 106 HepG2 cells. Data are averages ± standard deviations from three experiments.
Specific binding of A-domains is dependent on the presence of divalent cations that are coordinated in MIDAS. To test the binding specificity of the recombinant A-domain to HepG2 cells, 1 μg of soluble recombinant A-domain protein was allowed to bind HepG2 cells in the presence of the divalent cation Mn2+ or Mg2+ ranging in concentration from 0 to 10 mM. Our results showed enhanced binding in the presence of divalent cations, which was also saturated at concentrations of 8 to 10 mM (Fig. 2B and C). The divalent cation Mn2+ showed enhanced binding in comparison to Mg2+. We also showed (Fig. 2B and C) that in the presence of 10 mM EDTA/EGTA with Mn2+ or Mg2+ ions, the binding of the TRAP A-domain to HepG2 cells was substantially aborted. In comparison, GST protein showed negligible levels of adhesion to hepatocytes in the presence of a divalent cation (data not shown). This suggests that the binding of the recombinant TRAP A-domain is highly specific to HepG2 cells in the presence of divalent cations. Therefore, the TRAP A-domain binding was specific to hepatocytes. The binding of the recombinant TRAP repeat protein with hepatocytes was similar to TRAP A-domain binding (Fig. 2D). However, the binding did not improve compared to TRAP A-domain binding in the presence of divalent cations (data not shown). This further proves the effect of the MIDAS motif on the TRAP A-domain.
A functional TRAP A-domain inhibits Plasmodium sporozoite invasion.
Having demonstrated the specific binding of the TRAP A-domain to hepatocytes, we tested the recombinant protein for its ability to inhibit P. berghei sporozoite invasion of hepatocytes in vitro.
It has been well established previously and also shown by our experiments that invasive and exoerythrocyte forms (EEFs) of P. berghei sporozoites invade HepG2 cells (37). We examined the ISI and the formation of EEFs of sporozoites in HepG2 cells by using various concentrations of the recombinant TRAP A-domain. In the presence of 1 μg of recombinant protein, 90% of sporozoites were unable to invade or form EEFs in HepG2 cells in comparison with the control (Fig. 3A and B). These results suggest that specific receptors for sporozoite TRAP binding exist on hepatocytes that can be saturated with active recombinant TRAP A-domain fragments.
FIG. 3.
The recombinant TRAP A-domain inhibits in vitro invasion by sporozoites and the development of EEFs in HepG2 cells. (A) A sporozoite invasion inhibition assay was performed using 0, 250, 500, and 1,000 ng per 100 μl of recombinant TRAP A-domain protein on 1 × 105 HepG2 cells. (B) Inhibition of EEF development was assayed by counting the number of EEFs in the presence of 0, 250, 500, and 1,000 ng per 100 μl of the recombinant TRAP A-domain protein on 1 × 105 HepG2 cells. (C) A sporozoite invasion inhibition assay was performed using 0, 100, 5,000, and 1,000 ng per 100 μl of recombinant TRAP PAdoT protein on 1 × 105 HepG2 cells. (D) Inhibition of EEF development was assayed using 0, 100, 500, and 1,000 ng per 100 μl of recombinant TRAP PAdoT protein on 1 × 105 HepG2 cells. The experiment was performed with the purified recombinant TRAP A-domain or TRAP PAdoT protein cleaved from GST using the thrombin cleavage site. The number of invading sporozoites and the number of EEFs formed were counted in 20 fields, and the data are averages ± standard deviations from three experiments.
To assess the specificity of TRAP A-domain binding, we tested the PAdoT mutant, which contains a mutated MIDAS motif, for its ability to inhibit sporozoite invasion and development of EEFs in HepG2 cells. Our results showed negligible inhibition of sporozoite invasion and EEF formation by using concentrations of 100, 500, and 1,000 ng of purified recombinant PAdoT per 100 μl of reaction mixture in comparison to the active TRAP A-domain (Fig. 3C and D).
Identification of the TRAP receptor on hepatocytes by immunoprecipitation.
The immunoprecipitation technology using the Seize protein A immunoprecipitation kit (Pierce, Rockford, IL) was established for the purification of specific HepG2 membrane proteins that interacted with the purified recombinant TRAP A-domain. The experiment was performed as described in Materials and Methods. Briefly, the soluble hepatocyte membrane protein fraction was loaded onto a protein A column coupled with the purified recombinant TRAP A-domain with anti-TRAP A-domain antibodies. The eluted fraction was run on a 4 to 20% SDS-PAGE gel. The results showed a 66-kDa protein eluted out by immunoprecipitation (Fig. 4A). This protein was cleaved from the gel and subjected to μLC/MS/MS analysis as described in Materials and Methods, and Sequest databases were searched for protein sequences homologous to the sequences obtained. The proteins identified from the database was α2-HS glycoprotein/fetuin-A, with three peptide sequences having high scores. Fetuin-A is a hepatic protein that belongs to the cystatin superfamily (2). We found by an immunofluorescence assay that fetuin-A was present in the cytoplasm and in the extracellular vicinity of HepG2 cells (data not shown).
FIG. 4.
Immunoprecipitation of hepatocyte membrane proteins (1 mg) using 100 μg of the purified recombinant TRAP A-domain and GST protein (control) coupled to a protein A column with 50 μg of anti-A-domain and anti-GST antibodies. (A) Silver-stained 4 to 20% gradient gel showing protein eluted from the purified TRAP A-domain recombinant protein column coupled to protein A with anti-A-domain antibodies (lane 1) and no protein eluted from a GST protein column coupled to protein A with anti-GST antibodies (lane 2). (B) Immunoprecipitation of 500 μg of sporozoite soluble protein bound to 100 μg fetuin-A coupled to a protein A column and 50 μg of anti-fetuin A antibodies. Western blotting was performed using anti-TRAP antibodies at a 1:1,000 dilution. TRAP elution from the soluble sporozoite fraction is shown. Lane 1, sporozoite soluble fraction; lane 2, elute 1 after immunoprecipitation; lane 3, elute 2 after immunoprecipitation; lane 4, CS in the soluble fraction of sporozoites before immunoprecipitation; lane 5, no CS detection after immunoprecipitation. For lanes 4 and 5, Western blotting was performed using a 1:1,000 dilution of 3D11 (anti-CS antibodies).
Identification of sporozoite proteins that bind to fetuin-A by immunoprecipitation.
To determine a possible interaction between fetuin-A and sporozoite proteins, we used commercially available fetuin-A protein (Calbiochem, La Jolla, CA) and goat anti-human fetuin-A antibodies (DiaSorin, Stillwater, MN) for immunoprecipitation. The assay was performed by immunoprecipitating the soluble sporozoite proteins on a protein-A column coupled with fetuin-A protein with anti-fetuin-A antibodies. The eluted fraction was run on a 4 to 20% SDS-PAGE gel, and Western blotting was subsequently performed using anti-TRAP A-domain and anti-CS (3D11) antibodies.
The TRAP A-domain antibodies recognized a 66-kDa (the correct size for full-length TRAP) protein, demonstrating that fetuin-A pulled out soluble TRAP from the sporozoite soluble protein fraction (Fig. 4B, lanes 1 to 3). On the other hand, anti-CS antibodies could not detect anything on the Western blot, suggesting that fetuin-A binding was specific for the native TRAP of P. berghei sporozoites (Fig. 4B, lanes 4 and 5).
Binding of fetuin-A to TRAP.
The commercially available anti-fetuin-A antibodies (DiaSorin, Stillwater, MN) were examined for their ability to inhibit sporozoite invasion and development of EEFs in HepG2 cells. Our results indicated that as little as 250 ng/100 μl of anti-fetuin-A with 1 × 105 HepG2 cells was sufficient to inhibit P. berghei sporozoite invasion by 50%, whereas 1 μg/100 μl of anti-fetuin-A showed 90% inhibition in comparison to the controls (Fig. 5A). We also observed formation of proportionately fewer EEFs inside the hepatocytes (Fig. 5B). To further confirm the binding of fetuin-A to the recombinant TRAP A-domain, we performed an overlay binding assay as described in Materials and Methods. Our results showed that fetuin-A specifically binds to the TRAP A-domain in comparison with the recombinant TRAP repeat region, GST, or PAdoT (A-domain mutant) protein (Fig. 5C).
FIG. 5.
Fetuin-A antibodies inhibit sporozoite invasion of hepatocytes and development of EEFs. (A) ISI assay using 0, 250, 500, and 1,000 ng of anti-fetuin-A antibodies per 100 μl on 1 × 105 HepG2 cells. (B) Inhibition of EEF development using 0, 250, 500, and 1,000 ng of anti-fetuin-A antibodies per 100 μl on 1 × 105 HepG2 cells. Data are averages ± standard deviations from three experiments. (C) Overlay binding assay showing the interaction of purified recombinant TRAP constructs with fetuin-A. One milligram of each recombinant protein was dotted onto the nitrocellulose paper and overlaid with 1 mg/ml biotin-labeled fetuin-A. The blot was detected using 0.1 μg/ml of streptavidin-HRP. Recombinant protein was cleaved from GST using the thrombin cleavage site. Lane 1, recombinant TRAP A-domain; lane 2, recombinant TRAP repeat region; lane 3, recombinant TRAP PAdoT; lane 4, GST.
Infectivity of Plasmodium berghei sporozoites in fetuin-A-deficient mice.
Our results described above indicate that fetuin-A binds to TRAP of P. berghei and also blocks sporozoite invasion and formation of EEFs in HepG2 cells. Therefore, we were interested in testing whether this molecule was necessary for entry of sporozoites or for their further development in the hepatocyte in vivo. To examine this, we first determined the infectivity of P. berghei sporozoites in fetuin-A knockout mice (20, 39). Our results indicated that fetuin-A-deficient mice showed a 2-day increase in the prepatent period of malaria infection compared to wild-type control C57BL/6 mice (P = 0.0048) (Table 1). The data show that P. berghei infectivity was significantly lower for fetuin-A-deficient mice than for wild-type mice. Differences were most pronounced at days 5 and 6 postinfection, with P values from 0.0173 to 0.0154, respectively. The mean parasitemia on day 8 was 0.65% for fetuin-A-deficient mice compared to 1.76% for wild-type controls; the difference did not reach statistical significance. This finding indicates a permissive role for fetuin-A during blood passage and initial hepatocyte attachment/entry of sporozoites but not at later stages of parasite development. Thus, at days 7 and 8 postinfection, infectivities for wild-type C57BL/6 and fetuin A knockout mice were not statistically different (P = 0.5375 and 0.7128, respectively).
TABLE 1.
Infectivity of Plasmodium berghei sporozoites for fetuin-A-deficient micea
| Mice | No. infected/no. inoculated | Prepatent period (mean days ± SD) | 95% Confidence interval |
|---|---|---|---|
| Wild type | 8/8 | 3.6 ± 0.92 | 2.86-4.39 |
| Fetuin-A knockout | 8/8 | 5.2 ± 1.49 | 4.01-6.49 |
Fetuin-A knockout mice show less susceptibility to infection by P. berghei sporozoites. Wild-type C57BL/6 and fetuin-A knockout mice were injected with 10,000 to 15,000 sporozoites intravenously in the tail vein. Mice were examined daily for the presence of malaria parasites in their peripheral blood. The prepatent period corresponds to the delay between inoculation and the appearance of blood-stage parasites in blood smears. Parasitemia was defined as the percentage of infected erythrocytes.
Further quantification of P. berghei hepatic development in vivo was done after 40 hours of sporozoite injections into the animals by amplifying a 393-bp fragment of 18S rRNA from the livers of mice by using RT-PCR as described by Briones et al. (4). Our results confirmed that fewer P. berghei EEFs developed in fetuin-A knockout mice than in C57BL/6 mice (see Fig. S2 in the supplemental material). As a positive control for the integrity of RNA in all the animals, β-actin mRNA was measured by RT-PCR. Figure S2, lane 1, in the supplemental material shows the amplification of a 231-bp amplicon in a fetuin-A knockout mouse. Similar results were obtained for mRNA fractions of all eight fetuin-A knockout and all eight C57BL/6 mice, demonstrating that the differences measured by RT-PCR reflected mRNA amount and not mRNA quality (see Fig. S1 in the supplemental material).
DISCUSSION
Sporozoites of Plasmodium are transmitted by the mosquito vector to the vertebrate host and need to invade multiple cell types during their lives. They traverse the secretory cells in the mosquito salivary glands to reach the salivary duct, and once inoculated into the vertebrate host, they invade hepatocytes, where they differentiate into the next parasite stage. Studies with Plasmodium berghei, a species that infects rodents, have shown that the sporozoite-specific transmembrane protein TRAP is essential for sporozoite gliding, cell invasion, and in vivo infectivity (38). TRAP contains an A-domain in its extracellular portion which is known to be involved in cell-cell, cell-matrix, and matrix-matrix interactions. The A-domain consists of approximately 200 amino acids that include a conserved DXSXS motif involved in the coordination of divalent cation binding (25). We have expressed the recombinant TRAP A-domain with the aim of identifying its ligand on hepatocytes. Using a solid-phase assay, we showed that the recombinant A-domain binds to HepG2 cells in vitro. This binding was concentration dependent and saturable, consistent with a specific receptor-ligand interaction. In addition, we found that binding of the TRAP A-domain to HepG2 cells increased two- to threefold in the presence of Mg2+ and Mn2+, respectively, and was blocked by the addition of EDTA/EGTA (Fig. 2). The mutant protein PAdoT, which contains a mutated MIDAS motif, did not show strong binding compared to the control TRAP A-domain recombinant protein (data not shown). Our results also suggest that the MIDAS motif in the presence of divalent cations contributes strongly to binding of the A-domain to hepatocytes, confirming previous reports (8). The recombinant repeat protein of TRAP showed only a low level of binding with HepG2 cells, and this binding did not change with increasing concentrations of protein or in the presence of divalent cations. This binding behavior could be due to the acidic nature of the asparagine/proline-rich repeat region (40). In many cases, proline-rich regions are involved in intramolecular interactions rather than in receptor-ligand interactions (26, 22).
It has been shown previously that TRAP knockout sporozoites are less infective to hepatocytes than to wild-type sporozoites (38). To further confirm the role of TRAP in sporozoite invasion, the recombinant TRAP A-domain was tested for its ability to inhibit invasion of HepG2 cells by P. berghei sporozoites in vitro. The results presented here showed 90% inhibition of sporozoite invasion and formation of EEFs in HepG2 cells when we used 1 μg/100 μl of recombinant protein. These results prove that the recombinant TRAP A-domain was functional and capable of binding its receptor on the hepatocyte membrane, thus blocking further interactions between the parasite and the hepatocyte receptor in vitro.
We used the recombinant A-domain of TRAP and anti-TRAP A-domain antibodies for immunoprecipitation studies which resulted in the isolation of a 66-kDa protein from the soluble membrane fraction of HepG2 cells. This protein fraction was analyzed by μLC/MS/MS on a Finnigan LCQ DECA quadrupole ion trap mass spectrometer, and the MS/MS spectra were then searched in the databases from Sequest for homologous sequences. The peptide sequences were identified with α2-HS glycoprotein/fetuin-A, which is synthesized and secreted by the liver into the circulation. Fetuin-A is a major serum protein which can bind diverse ligands in vitro. Thus, fetuin-A has been demonstrated to interact with the insulin receptor, inhibiting insulin receptor autophosphorylation and tyrosine kinase activity (13, 15). Fetuin-A also mediates lipid transport and opsonization. Regarding its major physiological function, fetuin-A has, however, been unequivocally shown to inhibit pathological calcification at the systemic level (35) and to regulate bone mineralization (39). As previously reported, fetuin-A is the human homologue of bovine fetuin, with an approximate molecular weight of 60 kDa (1, 21, 24, 42). Human fetuin-A has a two-chain form whose N-terminal heavy chain is disulfide bonded to the C-terminal light chain. It is an acidic glycoprotein with three N-linked and three O-linked oligosaccharide chains, whose terminal sugar residues are rich in sialic acid (N-acetylneuraminic acid). Nearly 20% of the circulating fetuin-A pool is phosphorylated at serine-120 and serine-130 (15). Fetuin-A synthesis is down-regulated significantly during injury, infection, inflammation, and malignancy (21, 24). The promoter for fetuin-A has been characterized, and its down-regulation during the acute phase of infection has been attributed to transcriptional inactivation (3, 13).
Commercially available antibodies against fetuin-A also reacted with the eluted protein. In addition, the TRAP protein was pulled out when a soluble protein fraction of sporozoites was immunoprecipitated with fetuin-A and fetuin-A antibodies. Further, the anti-fetuin-A antibodies at a 1-μg/ml concentration inhibited invasion by sporozoites and formation of EEFs in HepG2 cells (Fig. 5A and B). These results further confirmed the interaction of TRAP with fetuin-A. How does fetuin-A interact with TRAP to mediate sporozoite invasion of hepatocytes? Our results suggest that the interaction between the TRAP A-domain and fetuin-A occurs in vitro and may have a functional role in invasion. To test if the TRAP A-domain-fetuin-A interaction is necessary for sporozoite attachment, invasion, or subsequent development in hepatocytes, we infected fetuin-A knockout mice with malaria parasites (20, 39). Fetuin-A-deficient mice showed delayed infectivity compared to C57BL/6 control mice, with significant statistical differences on days 5 and 6 (Table 1). These results indicate that fetuin-A is necessary for a robust infection, and its deletion provides partial resistance. Fetuin-A is, however, not the only molecule involved in sporozoite invasion. It is quite likely that fetuin-A is part of a scaffold that includes one or more cell surface molecules. Binding of TRAP to fetuin-A may bring TRAP into close proximity with a receptor that ultimately allows it to enter the hepatocyte. However, in the absence of fetuin-A, other molecules can take over the function of fetuin-A, enabling parasite entry, albeit less efficiently. Further experiments aimed at identifying molecules that interact with fetuin-A at the hepatocyte surface will shed light on the precise mechanism by which sporozoites gain entry into the liver cell.
Supplementary Material
Acknowledgments
We thank Kai Matuschewski, Kevin Millitello, Manoj Duraisingh, Shankar Chinta, and Suman Dhar for critical reading of the manuscript. We thank the Victor Nussenzweig lab at NYU and the Marcelo Jacobs-Lorena lab at Johns Hopkins for provided us with Anopheles stephensi mosquito eggs. We also thank Photini Sinnis, Kai Matuschewski, and Ute Frevert for providing us with antibodies, reagents, and protocols. We also thank Karine Kaiser of NYU for helping D.J. with dissection of mosquito salivary glands and Johanna Patricia Daily of HSPH for helping with statistical analysis.
This study was supported by NIH grant R01-AI 050689-02 (to A.A.S.) and by the Deutsche Forschungsgemeinschaft (to W.J.-D.).
Editor: J. F. Urban, Jr.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Akhoundi, C., N. Rochet, B. Ferrua, and B. Rossi. 1994. Production and characterization of monoclonal and polyclonal antibodies to human α2-HS: development of a two-site ELISA test. J. Immunol. Methods 172:189-196. [DOI] [PubMed] [Google Scholar]
- 2.Arnaud, P., and L. Kalabay. 2002. α2-HS glycoprotein: a protein in search of a function. Diabetes Metab. Res. Rev. 18:311-314. [DOI] [PubMed] [Google Scholar]
- 3.Banine, F., C. Gangneux, L. Mercier, A. Le Cam, and J. P. Salier. 2000. Positive and negative elements modulate the promoter of the human liver-specific α2-HS-glycoprotein gene. Eur. J. Biochem. 267:1214-1222. [DOI] [PubMed] [Google Scholar]
- 4.Briones, M. R., M. Tsuji, and V. Nussenzweig. 1996. The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes. Mol. Biochem. Parasitol. 77:7-17. [DOI] [PubMed] [Google Scholar]
- 5.Cefaratti, C., A. Romani, and A. Scarpa. 2002. Differential localization and operation of distinct Mg2+ transporters in apical and basolateral sides of rat liver plasma membrane. J. Biol. Chem. 275:3772-3780. [DOI] [PubMed] [Google Scholar]
- 6.Cerami, C., U. Frevert, P. Sinnis, B. Takacs, P. Clavijo, M. J. Santo, and V. Nussenzweig. 1992. The basolateral domain of hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell 70:1021-1033. [DOI] [PubMed] [Google Scholar]
- 7.Cerami, C., F. Kwakye-Berko, and V. Nussenzweig. 1992. Binding of malarial CS protein to sulfate and cholesterol sulfate: dependency on disulfide bond formation between cysteines in region II. Mol. Biochem. Parasitol. 54:1-12. [DOI] [PubMed] [Google Scholar]
- 8.Colombatti, A., P. Bonaldo, and R. Doliana. 1993. Type A modules: interacting domains found in several nonfibrillar collagens and in other extracellular matrix proteins. Matrix 13:297-306. [DOI] [PubMed] [Google Scholar]
- 9.Cowan, G., S. Krishna, A. Crisanti, and K. Robson. 1992. Expression of thrombospondin-related anonymous protein in Plasmodium falciparum sporozoites. Lancet 339:1412-1423. [DOI] [PubMed] [Google Scholar]
- 10.Eichinger, D. J., D. E. Arnot, J. P. Tam, V. Nussenzweig, and V. Enea. 1986. Circumsporozoite protein of Plasmodium berghei: gene cloning and identification of the immunodominant epitopes. Mol. Cell. Biol. 6:3965-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine, E., M. Aikawa, A. H. Cochrane, and R. S. Nussenzweig. 1984. Immuno-electron microscopic observations on Plasmodium knowlesi sporozoites: localization of protective antigen and its precursors. Am. J. Trop. Med. Hyg. 33:220-226. [DOI] [PubMed] [Google Scholar]
- 12.Frevet, U., P. Sinnis, C. Cerami, W. Shreffler, B. Takacs, and V. Nussenzweig. 1993. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J. Exp. Med. 177:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangneux, C., M. Daveau, M. Hiron, C. Derambure, J. Papaconstantinou, and J. P. Salier. 2003. The inflammation-induced down-regulation of plasma fetuin-A (α2-HS-glycoprotein) in liver results from the loss of interaction between long C/EBP isoforms at two neighboring binding sites. Nucleic Acids Res. 31:5957-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girma, J. P., D. Meyer, C. L. Verweij, H. Pannekoek, and J. J. Sixma. 1987. Structure-function relationship of human von Willebrand factor. Blood 70:605-611. [PubMed] [Google Scholar]
- 15.Håglund, A. C., B. Ek, and, P. Ek. 2001. Phosphorylation of human plasma α2-Heremans-Schmid glycoprotein (human fetuin) in vivo. Biochem. J. 357:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper, J. M., E. F. Hoff, and V. B. Carruthers. 2004. Multimerization of the Toxoplasma gondii MIC2 integrin-like A-domain is required for binding to heparin and human cells. Mol. Biochem. Parasitol. 4:201-212. [DOI] [PubMed] [Google Scholar]
- 17.Hoeg, J. M., S. J. Demosky, E. J. Schaefer, T. E. Starzl, and H. B. Brewer. 1984. Characterization of hepatic low-density lipoprotein binding protein and cholesterol metabolism in normal and homozygous familial hypercholesterolmic subjects. J. Clin. Investig. 73:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollingdale, M. R., E. H. Nardin, S. Tharavanij, A. L. Schwartz, and R. S. Nussenzweig. 1984. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J. Immunol. 132:909-913. [PubMed] [Google Scholar]
- 19.Huynh, M. H., K. E. Rabenau, J. M. Harper, W. L. Beatty, L. D. Sibley, and V. B. Carruthers. 2003. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 22:2082-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahnen-Dechent, W., T. Schinke, A. Trindl, W. Muller-Esterl, F. Sablitzky, S. Kaiser, and M. Blessing. 1997. Cloning and targeted deletion of the mouse fetuin gene. J. Biol. Chem. 272:31496-31503. [DOI] [PubMed] [Google Scholar]
- 21.Kalabay, L., L. Jakab, Z. Prohaszka, G. Fust, Z. Benko, L. Telegdy, Z. Lorincz, P. Zavodszky, P. Arnaud, and B. Fekete. 2002. Human fetuin/α2HS-glycoprotein level as a novel indicator of liver cell function and short-term mortality in patients with liver cirrhosis and liver cancer. Eur. J. Gastroenterol. Hepatol. 14:389-394. [DOI] [PubMed] [Google Scholar]
- 22.Kay, B. K., M. P. Williamson, and M. Sudol. 2002. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 14:231-241. [PubMed] [Google Scholar]
- 23.Krishnan, V., T. Narasimhan, and S. Safe. 1992. Development of gel staining techniques for detecting the secretion of procathepsin D (52-kDa protein) in MCF-7 human breast cancer cells. Anal. Biochem. 204:137-142. [DOI] [PubMed] [Google Scholar]
- 24.Mathews, S. T., D. D. Deutsch, G. Iyer, N. Hora, B. Pati, J. Marsh, and G. Grunberger. 2002. Plasma α2-HS glycoprotein concentrations in patients with acute myocardial infarction quantified by a modified ELISA. Clin. Chim. Acta 319:27-34. [DOI] [PubMed] [Google Scholar]
- 25.Matuschewski, K. A. C. Nunes, V. Nussenzweig, and R. Menard. 2002. Plasmodium sporozoite invasion into insect and mammalian cells is directed by the same dual binding system. EMBO. J. 21:1597-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick, C. J., D. S. Tuckwell, A. Crisanti, M. J. Humphries, and M. R. Hollingdale. 1999. Identification of heparin as a ligand for the A-domain of Plasmodium falciparum thrombospondin-related adhesion protein. Mol. Biochem. Parasitol. 100:111-124. [DOI] [PubMed] [Google Scholar]
- 27.Menard, R. 2001. Gliding motility and cell invasion by Apicomplexa: insights from the Plasmodium sporozoite. Cell Microbiol. 2:63-73. [DOI] [PubMed] [Google Scholar]
- 28.Müller, H. M., I. Reckmann, M. R. Hollingdale, H. Bujard, K. J. Robson, and A. Crisanti. 1993. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO J. 28:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagasawa, H., M. Aikawa, P. M. Procell, G. H. Campbell, W. E. Collins, and C. C. Campbell. 1988. Plasmodium malariae: distribution of circumsporozoite protein in midgut oocysts and salivary gland sporozoites. Exp. Parasitol. 66:27-34. [DOI] [PubMed] [Google Scholar]
- 30.Nussenzweig, V., and R. S. Nussenzweig. 1985. Circumsporozoite proteins of malaria parasites. Cell 42:401-403. [DOI] [PubMed] [Google Scholar]
- 31.Pancake, S. J., G. D. Holt, S. Mellouk, and S. L. Hoffman,. 1992. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J. Cell Biol. 117:1351-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robson, K. J., U. Frevert, I. Reckmann, G. Cowan, J. Beier, I. G. Scragg, K. Takehara, D. H. Bishop, G. Pradel, R. Sinden, S. Saccheo, H. M. Müller, and A. Crisanti. 1995. Thrombospondin-related adhesive protein (TRAP) of Plasmodium falciparum: expression during sporozoite ontogeny and binding to human hepatocytes. EMBO J. 14:3883-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robson, K. J. H., J. R. S. Hall, M. W. Jennings, T. J. R. Harris, K. Marsh, C. I. Newbold, V. E. Tate, and D. J. Weatherall. 1988. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature 335:79-82. [DOI] [PubMed] [Google Scholar]
- 34.Rogers, W. O., A. Malik, S. Mellouk, K. Nakamura, M. D. Roberts, A. Szarfman, D. M. Gordon, A. Nussler, M. Aikawa, and S. L. Hoffman. 1992. Characterization of Plasmodium falciparum sporozoite surface protein 2. Proc. Natl. Acad. Sci. USA 89:9176-9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schäfer, C., A. Heiss, A. Schwarz, R. Westenfeld, M. Ketteler, M. J. Floege, W. Müller-Esterl, T. Schinke, and W. Jahnen-Dechent. 2003. The serum protein α2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J. Clin. Investig. 112:357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinnis, P. 1998. An immunoradiometric assay for the quantification of Plasmodium sporozoite invasion of HepG2 cells. J. Immunol. Methods 221:17-23. [DOI] [PubMed] [Google Scholar]
- 37.Sinnis, P., P. Clavijo, D. Fenyo, B. T. Chait, C. Cerami, and V. Nussenzweig. 1994. Structural and functional properties of region II-plus of the malaria circumsporozoite protein. J. Exp. Med. 180:297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sultan, A. A., V. Thathy, U. Frevert, K. J. Robson, A. Crisanti, V. Nussenzweig, R. S. Nussenzweig, and R. Ménard. 1997. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell 90:511-522. [DOI] [PubMed] [Google Scholar]
- 39.Szweras, M., D. Liu, E. A. Partridge, J. Pawling, B. Sukhu, C. Clokie, W. Jahnen-Dechent, H. C. Tenenbaum, C. J. Swallow, M. D. Grynpas, and J. W. Dennis. 2002. α2-HS glycoprotein/fetuin, a transforming growth factor-β/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J. Biol. Chem. 277:19991-19997. [DOI] [PubMed] [Google Scholar]
- 40.Templeton, T. J., and D. C. Kaslow. 1997. Cloning and cross-species comparison of the thrombospondin-related anonymous protein (TRAP) gene from Plasmodium knowlesi, Plasmodium vivax, and Plasmodium gallinaceum. Mol. Biochem. Parasitol. 84:13-24. [DOI] [PubMed] [Google Scholar]
- 41.Wengelnik, K., R. Spaccapelo, S. Naitza, K. J. Robson, C. J. Janse, F. Bistoni, A. P. Waters, and A. Crisanti. 1999. The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J. 18:5195-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeh, T. C., W. Li, G. A. Keller, and R. A. Roth. 1998. Disruption of a putative SH3 domain and the proline-rich motifs in the 53-kDa substrate of the insulin receptor kinase does not alter its subcellular localization or ability to serve as a substrate. J. Cell. Biochem. 68:139-150. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida, N., P. Potocnjak, V. Nussenzweig, and R. S. Nussenzweig. 1981. Biosynthesis of Pb44, the protective antigen of sporozoites of Plasmodium berghei. J. Exp. Med. 154:1225-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida, N., R. S. Nussenzweig, P. Potocnjak, M. Aikawa, and V. Nussenzweig. 1980. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 207:71-75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.