Abstract
LcrV of Yersinia pestis is a major protective antigen proposed for inclusion in subunit plague vaccines. One way that anti-LcrV antibody is thought to protect is by inhibiting the delivery of toxins called Yops to host cells. The present study characterizes the relation between this inhibition and the phagocytosis of the bacteria. J774A.1 cells were infected with Y. pestis KIM5 in the presence of a protective polyclonal anti-LcrV antibody or a nonprotective polyclonal anti-YopM antibody, and delivery of YopH and YopE into the cytoplasm was assayed by immunoblotting. The ability to inhibit the delivery of these Yops depended upon having antibody bound to the cell surface; blocking conditions that prevented the binding of antibody to Fc receptors prevented the inhibition of Yop delivery. Anti-LcrV antibody also promoted phagocytosis of the yersiniae, whereas F(ab′)2 fragments did not. Further, anti-LcrV antibody could not inhibit the delivery of Yops into cells that were unable to phagocytose due to the presence of cytochalasin D. However, Yops were produced only by extracellular yersiniae. We hypothesize that anti-LcrV antibody does not directly inhibit Yop delivery but instead causes phagocytosis, with consequent inhibition of Yop protein production in the intracellular yersiniae. The prophagocytic effect of anti-LcrV antibody extended to mouse polymorphonuclear neutrophils (PMNs) in vitro, and PMNs were shown to be critical for protection: when PMNs in mice were ablated, the mice lost all ability to be protected by anti-LcrV antibody.
V antigen, or LcrV, of Yersinia pestis is a multifunctional virulence protein that is planned for inclusion in the generation of plague vaccines currently under development (26, 27). Within the bacterium, LcrV participates in controlling the activation of the Ysc type III secretion system when the bacterium contacts a host cell or is artificially activated by the absence of calcium in the medium (1, 14, 18). It is itself secreted by Ysc and is detectable on the surfaces of yersiniae incubated at 37oC to induce the expression of Ysc (7, 15). It is necessary for formation of the pore in the host cell membrane, through which six protein toxins called effector Yops are injected by the Ysc needle structure (7, 9, 11, 13). The effector Yops derange cellular signaling from bacterial binding, inactivate Rho GTPases and mitogen-activated protein kinases, and prevent the activation of NF-κB (3). Tissue culture cells intoxicated by Yops are unable to mobilize their actin cytoskeletons to engulf the yersiniae due to the synergistic effects of four of the Yops (YopH, -E, and -T and YpkA) (3, 8). This is thought to be a major antiphagocytic mechanism that the yersiniae use to prevent killing by polymorphonuclear neutrophils (PMNs) and macrophages. In contrast to the effector Yops, LcrV is released into the medium in significant amounts in tissue culture infection experiments; evidently, this release also happens during experimental plague in guinea pigs (23). Free LcrV can cause the release of the immunosuppressive cytokine interleukin-10 (IL-10) in mice (2, 12). In tissue culture, LcrV can elicit IL-10 production from monocytes/macrophages in a Toll-like receptor 2 (TLR2) and CD14-dependent manner, and TLR2−/− mice have increased resistance to an O:8 strain of Yersinia enterocolitica, which possesses LcrV, Yop, and Ysc proteins that are highly similar to those of Y. pestis (21, 22). LcrV also has been demonstrated to inhibit the chemotaxis of PMNs into sponges, both in vitro and in vivo (30).
LcrV is a potent protective antigen by both active and passive immunization and protects against both bubonic and pneumonic forms of plague (26, 27). However, it is not yet known how the protection is mediated. Given the multiple activities of LcrV, several mechanisms could be envisaged. Antibody against LcrV could opsonize the bacteria for phagocytosis; it could block delivery of Yops, thereby negating a major antiphagocytic effect and indirectly promoting phagocytosis; it could neutralize LcrV's ability to elicit IL-10 production; and it could neutralize the antichemotactic effect of LcrV. Previous studies showed that anti-LcrV antibody can promote phagocytosis by macrophage-like J774 cells and prevent downstream effects of Yop-deranged signaling (29). Protective anti-LcrV antibodies also were shown to decrease Yop-dependent cellular rounding due to the loss of actin microfilament function in infected HeLa cells (15). Our lab recently demonstrated that one mechanism whereby anti-LcrV antibody protects mice against systemic plague is independent of IL-10 (16). We hypothesized that antibody acted to inhibit the delivery of Yops. Consistent with this idea, anti-LcrV antibody was not able to enhance the clearance of a Y. pestis multiple-Yop mutant that is able to assemble a functional Ysc system and express and secrete LcrV but lacks the genes for the six effector Yops. However, previously we were unable to demonstrate an inhibitory effect of our protective anti-LcrV antibody on the delivery of Yops to HeLa cells (7), although we have verified that our anti-LcrV antibody can inhibit the delivery of Yops to J774A.1 cells (16).
In this study, we examined the relationship between phagocytosis and the inhibition of Yop delivery, and our experiments led to the explanation for why we had not been able to demonstrate an effect of our antibody on Yop delivery to HeLa cells. The data support the surprising conclusion that anti-LcrV antibody promotes phagocytosis with consequent inhibition of Yop production inside cells, rather than by directly blocking the delivery of Yops. Finally, we demonstrated that PMNs are the predominant mediator of protection by anti-LcrV antibody against plague in mice.
MATERIALS AND METHODS
Bacteria and their cultivation.
Y. pestis KIM5 (obtained from R. R. Brubaker, Michigan State University; KIM10 in his nomenclature) is virulent from an intravenous route of infection but not from peripheral routes, due to its chromosomal Δpgm mutation (28). Some experiments measuring the invasion of mammalian cells used Y. pestis KIM5 or KIM5-MYM expressing green fluorescent protein (GFP) from a plasmid (4). The latter strain lacks expression of all effector Yops due to in-frame deletions of their genes but otherwise has a normal type III secretion system and expresses and secretes the Yop delivery components, such as LcrV and YopD (16). In Y. pestis KIM5-MYM, the pGFP plasmid, obtained from Brendan Cormack, expressed GFPmut3 (2a). Y. pestis KIM5 expressed GFPmut3 weakly from a pTrc99A-based construct originally designed to fuse GFP to the C terminus of LcrV (pVGFP). However, Y. pestis KIM5 pVGFP did not express detectable LcrV-GFP fusion protein. Its expression and secretion of LcrV and Yops and its growth properties in vitro and in vivo in mice were identical to those of Y. pestis KIM5 (data not shown). Its weak fluorescence was sufficient for the purpose of our study. Yersiniae were grown for at least six generations at 26°C in exponential phase in heart infusion broth (Difco Laboratories, Detroit, MI) before being used to infect mammalian cells. They were washed once with phosphate-buffered saline (PBS; 135 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4; pH 7.4) by centrifugation and diluted into warm RPMI 1640 containing HEPES and l-glutamine (RPMI 1640; Life Technologies, Grand Island, NY) at the final desired bacterial concentration. Then 1-ml amounts were centrifuged for 5 min at 200 × g in wells of a six-well cluster dish, and the plates were incubated at 37°C in 5% CO2 for 2 h. Anti-LcrV or anti-YopM antibody was added to the wells at a final concentration of 5 or 40 μg/ml during the last 30 min of this incubation. In control tests, an anti-Yersinia antibody was used at 80 or 100 μg/ml. At infection time, the yersiniae were recovered with vigorous pipetting to remove any bacteria adherent to the dishes. Yersiniae given this incubation for 2 h at 37°C in RPMI 1640 following pregrowth at 26°C (26/37°C pregrowth) have previously been shown to have LcrV on their surfaces (7). Viable bacterial numbers recovered from infected tissue culture cells were determined as CFU counts after serially diluting the suspensions in PBS, plating them on tryptose blood agar plates (Difco Laboratories, Detroit, MI), and incubating the plates for 2 days at 30°C.
Preparation and infection of mammalian cells.
Cultures of HeLa epithelioid cells or J774A.1 macrophage-like cells (ATCC, Manassas, VA) were initiated at 1 × 105 cells per well in six-well cluster dishes containing 2 ml RPMI 1640 plus 10% fetal bovine serum (FBS) per well. Duplicate wells were allowed for each treatment/infection. When microscopic analysis was to be performed, the wells contained glass coverslips that could be removed individually, and two coverslips were allowed per treatment. The dishes were incubated at 37°C with 5% CO2 for 2 to 3 days to achieve near confluence (5 × 105 to 9 × 105 cells per well) on the day of the experiment. Thirty minutes before infection, the medium was removed, and the cells were washed twice with warm PBS. In some experiments designed to test the effect of antibody on the delivery of Yops, the mammalian cells were pretreated with an Fc receptor-blocking agent to saturate Fc receptors. Three blocking protocols were used. In one, called HgG, 500 μg/ml human gamma globulin (HgG; Sigma-Aldrich Chemical Co., St. Louis, MO) in RPMI 1640 without FBS was added to the cells 30 min prior to infection. Just before infection, the bacterial suspension containing anti-LcrV or anti-YopM antibodies was supplemented with HgG at 50 μg/ml. Then the RPMI 1640-HgG on the mammalian cells was removed, and 2 ml of bacterial suspension was added per well. In the second blocking treatment, called FcBM, cells were pretreated for 30 min to as long as 1.25 h (with longer times giving more-reliable results) prior to infection with FcBlock (rat monoclonal anti-mouse CD16 and CD32 [FcγIII and FcγII receptors] antibody 2.4G2; BD Biosciences-Pharmingen, San Diego, CA) at 10 μg/ml plus 1% (wt/vol) bovine serum albumin (BSA) in RPMI 1640 (in a total volume of 400 to 500 μl). Thirty minutes before infection, anti-LcrV or anti-YopM antibodies were added to the bacteria in RPMI 1640, along with mouse serum (MS; Sigma-Aldrich) at a final concentration of 1% (vol/vol). The MS was later found to be optional. At infection time, the RPMI 1640-FcBlock-BSA was removed from the mammalian cells, and the bacteria in RPMI 1640-MS-antibody were added. The third blocking protocol, called FMM, differed from FcBM only in that the mammalian cells were pretreated for 30 min to 1.25 h prior to infection with RPMI 1640 containing 10% (vol/vol) heat-inactivated FBS and 1% (vol/vol) MS. Unless otherwise indicated, the multiplicity of infection (MOI) was 10. Infection was initiated by centrifugation for 5 min at 200 × g at room temperature, and then the plates were incubated 4 h for measurement of Yop delivery by immunoblotting. For assays of bacterial adherence or invasion of HeLa and J774A.1 cells, the cells were cultured and infected as described above, but no HgG was added.
Elicited PMNs were obtained by peritoneal lavage of 5- to 8-week-old female C57BL/6 mice with RPMI 1640 5 h after intraperitoneal injection of 5 ml of 1% glycogen. The lavage fluid (5 ml of RPMI 1640) was centrifuged at 4°C, and erythrocytes were lysed with 150 mM NH4Cl (pH 7.0) or a 15-s shock in water, followed by the addition of 10× Hanks balanced salt solution. The cells were pelleted, resuspended in cold Hanks balanced salt solution, and fractionated by Percoll gradient centrifugation (Amersham Biosciences Corp., Piscataway, NJ). Microscopic evaluation of stained smears of the PMN fraction indicated undetectable (less than 1%) contamination by mononuclear cells. PMNs were used immediately in phagocytosis assays.
Decrease in phagocytosis by J774A.1 cells after blocking of macrophages FcγRIII and FcγRII.
To test the effectiveness of FcBlock in inhibiting phagocytosis through CD32 and CD16, we measured the phagocytosis of opsonized fluorescent microspheres after treatment of J774A.1 cells with FcBlock. Briefly, biotinylated fluorescent 1-μm microspheres (Molecular Probes Inc., Eugene, OR) were treated with antibiotin monoclonal antibody 2F5 (Molecular Probes) and incubated for 1 h at 37°C. The J774A.1 cells in RPMI 1640 were treated with FcBlock (made in PBS) at 2 mg per 106 cells or with the same volume of PBS and incubated 1 h at 37°C with 5% CO2. The cells were then washed with PBS, opsonized fluorescent microspheres in RPMI 1640 were added, and the cultures were incubated 1 h at 37°C with 5% CO2. Then the cells were washed five times with cold PBS. The amount of phagocytosis was measured by counting the cell-associated microspheres in 20 cells per field of view in 20 fields of view.
Antibodies and recombinant LcrV.
The protective polyclonal rabbit anti-hexahistidine-tagged LcrV immunoglobulin G (IgG) (anti-LcrV antibody) and nonprotective polyclonal rabbit anti-YopM IgG were described previously and were purified by using protein A-conjugated beads as previously described (6, 13). The concentration of antibody was determined by bicinchoninic acid protein assay (Pierce Chemical Co., Rockford, IL), and dilutions were made in PBS. Forty micrograms per milliliter was the concentration estimated to be present in mice receiving a protective 100-μg dose of anti-LcrV antibody and was used in most of the experiments in this study.
Anti-Yersinia antibody had been raised in New Zealand White rabbits against whole cells of Y. pestis KIM6 (Y. pestis KIM5 lacking the LcrV-encoding virulence plasmid pCD1) grown at 26°C in heart infusion broth plus 0.2% xylose plus 1 mM MgCl2. The yersiniae were washed with PBS, and formalin was added to give a concentration of 0.1% (vol/vol). Sera that reacted with the immunogen in a precipitin test were pooled and concentrated by ammonium sulfate precipitation. Before use, the antibody was further purified on protein A-conjugated beads. During the study, it was discovered that this antibody preparation apparently had been contaminated with antibodies able to detect LcrV in an immunoblot assay. This reactivity was removed by passing the antibody over a column containing hexahistidine-tagged LcrV bound to Ni- nitrilotriacetic acid resin. The adsorption with LcrV did not detectably alter the effects of this antibody on the phagocytosis of Y. pestis KIM5 by J774A.1 cells or the delivery of Yops by Y. pestis KIM5 to J774A.1 cells.
F(ab′)2 fragments of anti-LcrV antibody were prepared by digestion with immobilized pepsin (Pierce Chemical Co., Rockford, IL). Briefly, anti-LcrV antibody was dialyzed at 4°C for 3 h in 20 mM sodium acetate buffer (pH 4.5), the pH was confirmed, and immobilized pepsin was added and incubated for 6 h at 37°C with shaking at 350 rpm. The pepsin beads were removed, and the pH was adjusted with binding buffer for protein A purification (0.1% disodium EDTA, pH 8.0). The F(ab′)2 fragments were purified from undigested IgG and also from Fc fragments on a protein A column (Pierce). The F(ab′)2 fragment was dialyzed against PBS overnight at 4°C. To concentrate the preparation further and achieve more-complete elimination of Fc fragments, the F(ab′)2 fragments were concentrated with a Centricon YM-30 (Millipore Corp., Billerica, MA). The purity of the final preparation of F(ab′)2 fragments was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, staining with Coomassie brilliant blue, and immunoblotting with detection with horseradish peroxidase-conjugated goat anti-rabbit IgG-Fc (Bethyl, Inc., Montgomery, TX) followed by enhanced chemiluminescence (SuperSignal West Pico chemiluminescence substrate from Pierce). Their functionality was demonstrated in an immunoblot in which hexahistidine-tagged LcrV on the blot was probed with F(ab′)2 fragments or full-length anti-LcrV antibody, detected with alkaline-phosphatase-conjugated goat anti-rabbit IgG heavy plus light chains (Bethyl), and developed with Nitro Blue Tetrazolium - BCIP (5-bromo-4-chloro-3-indolylphosphate) (Sigma-Aldrich).
Assays for Yop delivery.
Yop delivery during infection of cells in culture was measured by detecting the Yops directly in the mammalian cell cytosol by immunoblotting or indirectly through cytotoxicity (retracting of cellular processes and rounding up due to the combined effects of YopH, YopE, and YopT and YpkA). For immunoblot assays, one of two replicate wells received 1 ml of 300-μg/ml trypsin (giving a 100-μg/ml final concentration), and then both wells received a protease inhibitor, such as Pefabloc (Boehringer Mannheim, Indianapolis, IN) at 900 μg/ml, or a mixture of 30 μg/ml each (final concentration) of Pefabloc, aprotinin, and leupeptin (Boehringer Mannheim). Then, as previously described (6), medium and soluble cytoplasmic and debris fractions were obtained in the presence of protease inhibitors. The debris containing yersiniae, membranes, and large organelles was discarded. Other fractions were trichloroacetic acid precipitated, and equivalent amounts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 12% (wt/vol) acrylamide, transferred to Immobilon P (Millipore Inc., Billerica, MA), probed with a mouse anti-YopH antibody and a rabbit antibody to YopE, and detected with alkaline-phosphatase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG as previously described (6). In a modification of this assay with J774 cells only, the cells were infected and washed as described above, but after 30 min, the medium was replaced with one of the following: RPMI 1640 containing gentamicin (Gm) at 7.5 μg/ml to inhibit Yop synthesis in extracellular yersiniae; RPMI 1640 containing a mixture of Gm (500 μg/ml), streptomycin (500 μg/ml), and ofloxacin (200 μg/ml) to kill all yersiniae; or RPMI 1640 with no additions. After 1 h, the cells were washed, overlaid with fresh RPMI 1640 containing antibodies as described previously, and incubated an additional 2.5 h (for a total of 4 h) before being lysed and analyzed as described earlier. Previous tests had shown that treatment with the antibiotic mixture in the identical cell infection configuration reduces viable numbers of Y. pestis organisms by more than 5 orders of magnitude. In a second protocol, the J774 cells were pretreated for 30 min with cytochalasin D in amounts ranging from 0.5 μM to 2.5 μM (Sigma-Aldrich), and the drug was present throughout the subsequent 3-h infection. Cytochalasin D (2.5 μM) was shown to inhibit phagocytosis of opsonized fluorescein isothiocyanate-labeled zymosan particles (Molecular Probes) by 100% (data not shown).
Cytotoxicity was assessed by cellular morphology in phase-contrast micrographic images. Images were obtained with a Nikon Eclipse E800 microscope fitted with a Plan Fluor Phase 100× objective (Nikon USA via Fryer Co., Inc., Cincinnati, OH) and a Photometrics CoolSNAP cf charge-coupled device camera (Image Processing, Inc., North Reading, MA). Images were acquired and processed by using MetaMorph software (Universal Imaging Corp., Downingtown, PA).
Adherence and phagocytosis/invasion assays.
Adherence was determined as previously described (4) at 15 min after infection of triplicate wells per antibody treatment (anti-LcrV or anti-YopM). Briefly, after the 15-min incubation in the CO2 incubator, the infected cells were washed three times with PBS and then subjected to water lysis. The recovered yersiniae were enumerated as CFU. Percent adherence was calculated as the number of cell-associated CFU divided by the number of input CFU times 100%.
Two methods were used to measure invasion or phagocytosis. For Gm protection assays, two sets of triplicate wells per antibody treatment were infected at an MOI of 3. After 15 min at 37°C with 5% CO2, the cells were washed three times with PBS, covered with fresh RPMI 1640 plus anti-LcrV or anti-YopM antibody, and returned to the CO2 incubator. After 1 h, the RPMI 1640 plus antibody for one set was replaced with RPMI 1640 plus 7.5 μg/ml Gm, and the incubation was continued for 1 h. Then both sets of wells were washed three times with PBS and subjected to water lysis and enumeration of CFU. Percent invasion was calculated as the number of cell-associated CFU in the presence of Gm divided by the number of CFU in the absence of Gm times 100%.
To measure invasion by microscopy, Y. pestis KIM5 pVGFP was used for infection as described above, where the mammalian cells were seeded in six-well cluster dishes containing coverslips. After 1 h of incubation at 37°C in a CO2 incubator, the coverslips were removed and the cells were fixed for 30 min at room temperature with 2% paraformaldehyde (pH 7.4) in PBS. An alternate protocol employed J774A.1 cells grown in suspension in six-well dishes coated with 1% agarose and assayed in microcentrifuge tubes. Solution changes were made by centrifugation. Freshly isolated PMNs also were tested for phagocytosis in microcentrifuge tubes. In both protocols, the fixed but nonpermeabilized cells were treated with an anti-Yersinia antibody and then a Texas Red-labeled goat anti-rabbit IgG (Molecular Probes) to specifically label surface-bound yersiniae. The cells from assays done in suspension were deposited onto slides in a cytocentrifuge (Shandon Cytospin 3; Thermo Electric Corp.). The coverslips or cytospin slides were mounted in Vectashield (Vector Laboratories, Inc., Burlingame, CA) and evaluated in a Zeiss Axiophot microscope (Carl Zeiss, Inc., Batavia, IL) for intracellular (green due to GFP alone) or extracellular (red due to Texas Red at 630 nm) yersiniae. Images were captured using a Spot digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI) and managed using KS 400 Imaging System release 3.0 software.
Indirect immunofluorescence of J774 cells treated with anti-LcrV and anti-YopM antibody.
J774A.1 cells were seeded in six-well cluster dishes as described above. They were washed twice with PBS and then overlaid with ice-cold RPMI 1640 on ice for 5 min. They were then treated with the FcBM or FMM blocking solution for 30 min on ice. After being blocked, the cells were washed once with ice-cold PBS. The cells were then covered with anti-LcrV or anti-YopM antibody at 40 μg/ml in ice-cold PBS containing 1% mouse serum for 30 min on ice. They were washed twice with ice-cold PBS and then treated for 30 min on ice with Oregon Green 488-conjugated goat anti-rabbit heavy-plus-light-chain IgG (Molecular Probes) in PBS. They were then fixed with 2% paraformaldehyde at room temperature for 30 min, washed with PBS, and examined microscopically as described above for invasion. Fluorescence was analyzed at 515 to 555 nm (fluorescein isothiocyanate filter).
Ablation of PMNs and infection of mice.
The anti-mouse monoclonal 1A8 antibody selectively binds to the Ly-6G protein, expressed on the surfaces of circulating neutrophils (PMNs), but not monocytes, lymphoids, or erythroid cells. For ablation of PMNs, we treated mice with 250 μg of rat anti-mouse Ly-6G monoclonal antibody (BD Biosciences) 24 h before infection. Rat IgG (Sigma-Aldrich) was injected at the same time and in the same amount into control mice. Six hours later, the mice received anti-LcrV or anti-YopM antibody. At 24 h after the ablation of PMNs, we infected mice with Y. pestis KIM5. Viable bacterial numbers in liver and spleen were measured after 48 and 72 h. To confirm the PMN ablation condition during the experiment, we collected blood and bone marrow from mice subjected and not subjected to ablation and detected the levels of PMNs (Ly-6G-positive cells) and monocytes and macrophages (detected with allophycocyanin-conjugated anti-mouse F4/80 from eBioscience, San Diego, CA) after 24 h and 48 h by fluorescence-activated cell sorting.
Statistics.
All experiments were done at least twice. Significance of differences was evaluated by an unpaired two-tailed Student t test.
RESULTS
Anti-LcrV antibody partially inhibits Yop delivery to the cytosol of J774A.1 cells but does not inhibit the delivery of Yops to HeLa cells.
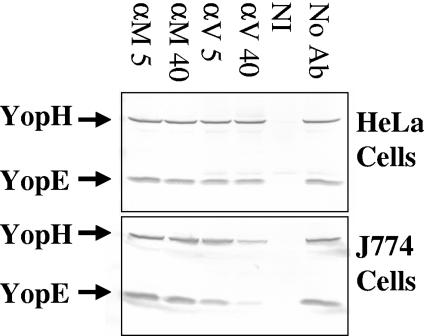
The present study was initiated to resolve discrepancies in the literature concerning the ability of protective anti-LcrV antibodies to inhibit the delivery of antiphagocytic Yops. Pettersson et al. found that anti-LcrV antibody could partially inhibit Yop-dependent rounding up of HeLa cells (15), while Weeks et al. had found that anti-LcrV antibody would inhibit YopJ-mediated apoptosis of J774A.1 cells after infection with Y. pestis CO92 (29), and we recently corroborated their findings with Y. pestis KIM5 (16). However, we had previously been unable to demonstrate an inhibitory effect of our protective anti-LcrV antibody on the delivery of Yops to HeLa cells (7). We revisited the question of whether anti-LcrV antibody could inhibit the delivery of Yops to HeLa cells. Our protective anti-LcrV antibody failed to inhibit Yop delivery to HeLa cells, but in tests run in parallel with J774A.1 cells, some inhibition was clearly seen when anti-LcrV antibody was present, compared to results from cultures containing the nonprotective anti-YopM antibody (Fig. 1). There also was little if any inhibition of cytotoxicity in HeLa cells by anti-LcrV (data not shown; see below), even though LcrV is necessary for delivery of Yops to HeLa cells by Y. pestis (14). These data showed that the same experiment performed with the two cell types gave different findings.
FIG. 1.
Anti-LcrV antibody partially inhibits the delivery of YopH and YopE to J774A.1 cells but has no effect on the delivery of these Yops to HeLa cells. HeLa and J774A.1 cells were infected at an MOI of 10 with 26/37°C-grown Y. pestis KIM5 in the presence of anti-YopM (αM) or anti-LcrV (αV) antibody and the mild HgG blocking agent. After 4 h, delivery of YopH and YopE to the cytosol was measured by immunoblotting. Lanes: αM and αV 5, 5 μg/ml antibody; αM and αV 40, 40 μg/ml antibody; NI, noninfected; No Ab, cells were infected in the absence of antibody.
Blocking conditions determine whether anti-LcrV or anti-YopM antibody binds to J774A.1 cells.
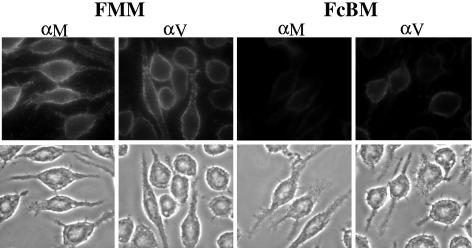
We wondered if the ability of anti-LcrV antibody to affect Yop delivery in J774A.1 cells may lie in the way that anti-LcrV, but not anti-YopM, antibody interacts with the surfaces of the cells. To test this idea, we treated J774A.1 cells with anti-LcrV or anti-YopM antibody and determined by indirect immunofluorescence whether there was specific labeling by anti-LcrV antibody. In Fig. 2, left panels show that both anti-LcrV and anti-YopM antibody strongly labeled the surfaces of the J774A.1 cells. However, to see this labeling, it mattered what blocking agent was used. For the left panels of Fig. 2, the cells were pretreated with a mixture of 10% heat-inactivated FBS and 1% MS to prevent nonspecific binding of antibody to the cells, and the antibodies were applied in the presence of 1% MS (protocol FMM; see Materials and Methods). If instead, the blocking agent was a mixture of 1% BSA and FcBlock, there was only weak staining of either cell type with either antibody in the presence of 1% MS (protocol FcBM; see Materials and Methods) (Fig. 2, right panels). FcBlock prevents non-antigen-specific binding to both the FcγII and FcγIII receptors on mouse cells (and was in fact raised against J774 cells). These findings indicate that the labeling of J774A.1 cells by anti-LcrV (and anti-YopM) antibody required binding by the Fc portion of the antibody to the Fc receptors on the cells and that anti-LcrV did not recognize a component on J774A.1 cells in an antigen-specific manner (unless this was one of the Fc receptors themselves).
FIG. 2.
Effects of two blocking treatments on the binding of anti-LcrV and anti-YopM antibody to J774A.1 cells. Binding of anti-YopM (αM) or anti-LcrV (αV) antibody to J774A.1 cells was determined for two blocking regimens. Then the cells were treated with Oregon Green 488-conjugated secondary antibody in PBS and visualized by fluorescence (top panels) or phase-contrast (bottom panels) microscopy. FMM, 10% FBS with 1% MS pretreatment for 30 min, and then 40 μg/ml αM or αV in the presence of 1% MS; FcBM, FcBlock (rat monoclonal antibody against a common epitope in the extracellular domains of FcγRII and FcγRIII) with 1% BSA pretreatment for 30 min, and then 40 μg/ml αM or αV in the presence of 1% MS.
FcBlock abrogates the ability of anti-LcrV antibody to inhibit the delivery of Yops to J774 cells.
The finding that the antibodies could bind to J774A.1 cells in the presence of a mild blocking agent (as in protocol FMM) suggested the possibility that the surface localization of anti-LcrV on J774A.1 cells as well as to LcrV on the bacterial surface might be what permitted anti-LcrV antibody to partially block the delivery of Yops to these cells. If so, then the FcBM protocol should eliminate the effect of anti-LcrV antibody on Yop delivery, whereas the FMM protocol should not. FcBlock treatment does effectively block FcγRII and FcγRIII, because numbers of cell-associated opsonized fluorescent beads in J774A.1 cells treated with FcBlock were much lower than those for nontreated control cells: 3 (±2.6) versus 23 (±5.0), respectively (Fig. 3A). To test our hypothesis, we treated J774A.1 cells with the FcBM or the FMM blocking protocol, infected the cells with Y. pestis KIM5, and assayed the delivery of Yops by immunoblotting (Fig. 3B). As predicted, there was little to no decrease in the amount of YopH delivered to the J774A.1 cell cytosol in the presence of anti-LcrV, compared to anti-YopM, antibody when the cells were blocked per the FcBM protocol. In contrast, YopH delivery to J774A.1 cells blocked with the FMM protocol was similar to that without the use of any blocking regimen, and anti-LcrV antibody caused less YopH to be delivered to the J774A.1 cytosol than when anti-YopM antibody was present.
FIG. 3.
Specific blocking of Fc receptors on J774A.1 cells abrogates the inhibition of YopH delivery by anti-LcrV antibody. Panel A: control test for efficacy of FcBlock in inhibiting phagocytosis mediated by IgG1. J774A.1 cells were treated with Fc Block or PBS for 1 h and then allowed to engulf opsonized fluorescent beads for 1 h. The image shows an overlay of the fluorescent image of the beads onto the phase-contrast image to reveal the cellular outlines. Panel B: J774A.1 cells were given the indicated blocking regimens and infected with Y. pestis KIM5 for 4 h at 37°C in the presence of 40 μg/ml anti-YopM (αM) or anti-LcrV (αV) antibody. Delivery of YopH into the J774A.1 cells was determined by immunoblotting of samples of the soluble cytoplasmic fraction. Blocking treatments: FcBM, RPMI 1640 plus FcBlock plus 1% BSA pretreatment for 1.25 h at 37°C and then infection with yersiniae suspended in RPMI 1640 containing αM or αV and 1% MS; FMM, RPMI 1640 plus 10% FBS plus 1% MS pretreatment for 1.25 h at 37°C and then infection with yersiniae suspended in RPMI 1640 containing αM or αV and 1% MS; None, pretreatment with RPMI 1640 at 37°C for 1.25 h followed by infection with yersiniae suspended in RPMI 1640 containing αM or αV.
Anti-LcrV antibody, but not anti-YopM antibody, can delay cytotoxicity in HeLa cells if no blocking agent is present.
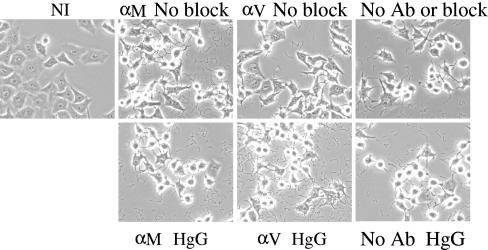
The finding that the effectiveness of blocking Fc receptors affects whether anti-LcrV antibody inhibits Yop delivery prompted us to reevaluate the protocol we used routinely in studies of Yop delivery to HeLa cells in the presence of anti-LcrV antibody (7). In those experiments, we used HgG (see Materials and Methods) to prevent nonspecific binding of antibody to FcγIII receptors present on HeLa cells (25). Like FMM, the HgG blocking protocol has no effect on the ability of anti-LcrV antibody to inhibit Yop delivery to J774A.1 cells (Fig. 1). However, perhaps HeLa cells have a lower density of Fc receptors than do J774A.1 cells, and milder blocking procedures might be effective for HeLa cells. To test this idea, we infected HeLa cells treated with no blocker or subjected to the HgG protocol and examined them microscopically for retracting and rounding up as evidence of “cytotoxicity” due to the delivery of Yops. This is a highly sensitive assay, and we used it to allow direct comparison with the study results of Pettersson et al., where anti-LcrV antibody was found to partially block cytotoxicity in HeLa cells infected by Y. pseudotuberculosis (15). As in that study, we used an MOI of 2 and monitored cytotoxicity at hourly intervals. At 2 to 3 h after infection, we saw a picture similar to Fig. 1 of Pettersson et al. (15), where HeLa cells not given any blocking treatment, but treated with anti-LcrV antibody, showed less cytotoxicity than ones treated with HgG (Fig. 4). Cells treated with anti-YopM antibody were equally cytotoxic, whether or not HgG was present, and resembled cells treated with HgG and anti-LcrV antibody. After 4 h of infection, all cells were cytotoxic. This experiment resolved the conflict in the literature by showing that if no Fc-blocking treatment is given, anti-LcrV antibody can delay the onset of Yop-mediated cytotoxicity in HeLa cells infected with Y. pestis KIM5. However, there remained the intriguing finding that the ability of anti-LcrV antibody to inhibit Yop delivery and cytotoxicity appeared to depend on the ability of the antibody to bind to the mammalian Fc receptor.
FIG. 4.
Anti-LcrV antibody can delay cytotoxicity in infected HeLa cells if no blocking agent is present. HeLa cells given the indicated blocking regimens were infected with Y. pestis KIM5 at an MOI of 2 in RPMI 1640 containing 40 μg/ml anti-YopM antibody (αM), 40 μg/ml anti-LcrV antibody (αV), or no antibody (No Ab). At various times after infection, cytotoxicity due to delivery of Yops was assessed by phase-contrast microscopy. The images shown were taken at 3 h postinfection. For blocking with HgG, the HeLa cells were pretreated for 30 min at 37°C with RPMI 1640 containing 500 μg/ml HgG and then were infected with yersiniae in RPMI 1640 containing αM or αV and 50 μg/ml HgG; for experiments with no blocking agent, the cells were pretreated in RPMI 1640 and infected with yersiniae in RPMI 1640 containing anti-YopM or anti-LcrV antibody. Ab, antibody; NI, not infected.
Anti-LcrV antibody does not alter the amount of adherence of Y. pestis KIM5 to HeLa and J774A.1 cells.
Intimate contact is necessary for delivery of proteins through type III secretion mechanisms. One possible way that antibody against LcrV could inhibit Yop delivery to J774A.1 cells would be by decreasing the number of bacteria that stably adhered to the cells. We tested whether 40 μg/ml anti-LcrV affected the cell association of Y. pestis KIM5 with J774A.1 cells more than did anti-YopM, which does not bind to the bacterial surface (data not shown). The combined results of 11 experiments on different days were that 33% (±3%) and 31% (±11%) of the input bacteria became cell associated for bacteria pretreated with anti-LcrV and anti-YopM antibody, respectively. Accordingly, anti-LcrV did not significantly alter the adherence of the bacteria, consistent with the findings of others for their anti-LcrV antibodies and strains of Y. pestis (29) and Y. enterocolitica (19). One similar experiment with HeLa cells also showed no difference in bacterial adherence in the presence of anti-LcrV from that with anti-YopM antibody. Accordingly, although these experiments did not rule on whether the adherence varied in efficacy for the delivery of Yops, they did show that bacterial adherence per se was not the mechanism through which anti-LcrV antibody inhibited Yop delivery when Fc receptors were not blocked.
Anti-LcrV antibody promotes the phagocytosis of Y. pestis KIM5.
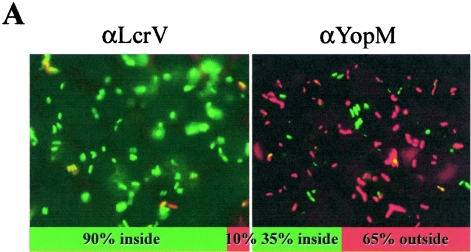
Rosqvist et al. had shown that Y. pestis EV76, made to efficiently enter HeLa cells, failed to produce Yops (20). Accordingly, another way that anti-LcrV antibody might affect Yop delivery to J774A.1 cells is through affecting uptake of the bacteria by the cells. We tested this idea by Gm protection assay in J774A.1 cells but found no effect of anti-LcrV antibody: in five experiments on different days, 157% (±44%) (anti-LcrV present) and 140% (±78%) (anti-YopM present) of the yersiniae survived the treatment with Gm. Taken at face value, the data indicated that all of the yersiniae were intracellular, regardless of which antibody was present. This result was unexpected and prompted us to seek confirmation by a different assay for invasion, a double-fluorescent-labeling technique analogous to the one used by Weeks et al. (29) but taking advantage of the fluorescence from GFP in Y. pestis KIM5 pVGFP. This assay gave a different picture: consistent with the findings of Weeks et al. with Y. pestis CO92, we found that for infected J774A.1 cells, 30 to 35% of yersiniae were intracellular in the presence of the nonprotective anti-YopM antibody (or no antibody) and 65 to 70% were extracellular (Fig. 5). When anti-LcrV antibody was present, 85 to 95% of the bacteria were intracellular. In contrast, Gm protection assays performed in parallel showed no killing of extracellular bacteria (e.g., 116% or 114% of cell-associated yersiniae were “protected” from Gm when anti-LcrV or anti-YopM, respectively, was present in one such experiment), even though the concentration of Gm used had been demonstrated to reduce viable numbers by more than 3 orders of magnitude under the same conditions but lacking J774A.1 cells. The hypothesis that Yops were responsible for inhibiting the phagocytosis of Y. pestis KIM5 by J774A.1 cells was supported by the finding that a multiple-Yop mutant of Y. pestis KIM5 lacking the genes for all six effector Yops and expressing GFP was almost completely intracellularly located in J774A.1 cells, even in the presence of anti-YopM antibody (data not shown). These findings indicated that the partial inhibition by anti-LcrV antibody of Yop delivery to J774 cells correlated with abrogation of an antiphagocytic effect. They also revealed that Gm protection does not always give reliable results. The disparity between the two assays for phagocytosis appeared to be much smaller for HeLa cells (data not shown), indicating that the Gm protection assay varied in efficacy for Y. pestis interacting with different mammalian cell types.
FIG. 5.
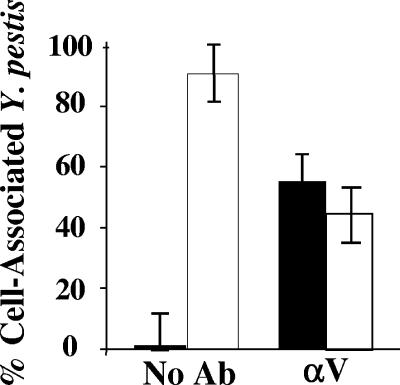
Full-length anti-LcrV antibody, but not F(ab′)2 fragments, promotes the phagocytosis of Y. pestis KIM5 pVGFP in J774A.1 cells. J774A.1 cells were infected at an MOI of 3 with Y. pestis KIM5 pVGFP in the presence of 40 μg/ml anti-LcrV, 100 μg/ml anti-LcrV F(ab′)2, or no antibody. After 1 h, the cells were fixed, and extracellular yersiniae were stained with anti-Yersinia primary antibodies and Texas Red-conjugated secondary antibody. Intracellular bacteria would not be stained and were green due to the expression of GFP. Numbers of extracellular and intracellular yersiniae were counted in 20 cells in 20 fields. Panel A shows fluorescence micrographs from such assays to illustrate the discrimination of intracellular and extracellular yersiniae. αLcrV, anti-LcrV; αYopM, anti-YopM. Panel B shows the data obtained for F(ab′)2 fragments of anti-LcrV antibody [αV F(ab′)2], PBS (No Ab), and anti-LcrV antibody (αV). Filled bars: intracellular yersiniae; open bars: extracellular yersiniae. Error bars show ±1 standard deviation (SD) from the mean numbers per J774A.1 cell. The results from the treatment with F(ab′)2 fragments of anti-LcrV antibody differed significantly from those from the treatment with full-length anti-LcrV at a P value of 0.05 (*).
Yops are delivered by extracellular yersiniae.
To assess how the location of the bacteria related to the delivery of Yops, we tested whether Yops are delivered only at the cell surface when Y. pestis binds by its natural ligands to J774A.1 cells. We infected replicate cultures of J774A.1 cells with Y. pestis KIM5 in the presence of 40 μg/ml anti-LcrV or anti-YopM antibody. After 30 min to allow internalization of yersiniae, one culture was treated with Gm, one was given a mixture of Gm, streptomycin, and ofloxacin at high concentrations, and one did not receive any antibiotics. After 1 h, the antibiotics were replaced by fresh medium with antibody, and the incubation was continued for 2.5 h, for a total of 4 h (commonly used in assays for the delivery of Yops). The antibiotic mixture was designed to kill all yersiniae in the culture and has been shown to kill essentially 100% of yersiniae in infection assays within 15 min (data not shown). The only Yops that would be delivered to J774A.1 cells in this culture would be those delivered within the first 30 to 45 min. The Gm treatment was provided to inhibit Yop synthesis by extracellular, but not intracellular, yersiniae. In this case, we were not depending on the ability of Gm to kill the yersiniae, and a control test showed that Gm added immediately after the addition of bacteria to J774A.1 cells would abolish essentially all YopH delivery to J774A.1 cells (data not shown). Prior to drug treatment, the yersiniae in the culture with Gm would deliver the same amount of Yops as those in the culture with the bactericidal antibiotic mixture; there would then be 3.5 h of incubation in which any live intracellular yersiniae could deliver Yops. The culture without antibiotics provided a reference for the amount of Yops that would be delivered by non-antibiotic-treated yersiniae, both extracellular and intracellular, in the entire 4 h. Figure 6 shows that the same amount of YopH was delivered in the culture that received Gm as in the counterpart that received the bactericidal mixture for all three antibody treatment groups, and this was significantly less than that in J774A.1 cells infected in the absence of antibiotics. This finding indicated that no detectable delivery of Yops occurred after the first 30 to 45 min of infection, even in cultures where at least 30% of the yersiniae were intracellular (Fig. 5). Significantly, there was much less YopH delivered in the cultures treated with antibiotics when anti-LcrV was present than when anti-YopM or no antibody was present, and there was no difference between the two antibiotic treatments. Accordingly, even though anti-LcrV causes most of the yersiniae to become intracellular, where they should be protected from Gm inhibition of protein synthesis, these yersiniae did not deliver more Yops than did yersiniae that were killed after 30 min. These results argue that Y. pestis KIM5 does not deliver Yops from within J774A.1 cells, in full agreement with findings for Y. pestis EV76 within HeLa cells (see reference [20] and Discussion).
FIG. 6.
YopH is not delivered into J774A.1 cells by intracellular Y. pestis. Three sets of three J774A.1 cultures were infected with Y. pestis KIM5 in the presence of 40 μg/ml anti-LcrV antibody (αV), anti-YopM antibody (αM), or no antibody (No Ab). After 30 min, one culture of each group received Gm, one received a mixture of antibiotics at high concentration (Mix), and one was not treated with any antibiotics (NT). The molecular weight (mw) of the prestained marker shown was 47,500; an extract from a noninfected J774A.1 culture (NI) also was included. After 1 h, the cells were washed and given fresh medium lacking any antibiotics but containing antibodies as described above, and incubation was continued for an additional 2.5 h. The cytoplasmic fraction of the J774A.1 cells was recovered and analyzed for YopH by immunoblotting.
Anti-LcrV antibody does not inhibit the delivery of Yops by extracellular yersiniae in the presence of cytochalasin D.
We next asked whether the decreased delivery of Yops to J774A.1 cells was direct or indirect; i.e., does anti-LcrV antibody prevent Yop delivery and then the yersiniae are vulnerable to phagocytosis, or does anti-LcrV antibody promote phagocytosis, and the resulting intracellular yersiniae then fail to produce Yops? To test whether anti-LcrV antibody can directly block Yop delivery, we determined whether anti-LcrV antibody would inhibit Yop delivery by J774A.1 cells that were unable to phagocytose due to disruption of their actin microfilaments with cytochalasin D. Figure 7 shows that the inhibitory effect of anti-LcrV antibody on the delivery of YopH and YopE to the J774A.1 soluble cellular fraction was progressively abolished as the concentration of cytochalasin D increased. This showed that functional actin is necessary for anti-LcrV to block the delivery of Yops and suggests that anti-LcrV antibody acts by promoting phagocytosis, with inhibition of Yop delivery occurring secondarily.
FIG. 7.
Functional actin is required for anti-LcrV antibody to inhibit the delivery of YopH and YopE into J774A.1 cells. J774A.1 cells pretreated with different concentrations of cytochalasin D (Cyto D) were infected with Y. pestis KIM5 for 4 h in the presence of 40 μg/ml anti-LcrV antibody (αV), anti-YopM antibody (αM), or no antibody (No Ab), and delivery of YopH and YopE into the soluble cytoplasmic fraction was assayed by immunoblotting.
F(ab′)2 fragments of anti-LcrV antibody do not promote the phagocytosis of Y. pestis KIM5.
If anti-LcrV antibody directly promoted phagocytosis, one way this could occur would be that antibody clustered on LcrV at the bacterial surface engages receptors by its Fc domain and triggers bacterial uptake. Accordingly, we tested whether F(ab′)2 fragments of anti-LcrV antibody would promote the phagocytosis of Y. pestis. These were generated by cleavage of anti-LcrV antibody by pepsin, which results in dimeric antigen-binding sites lacking the Fc domain. If antibody worked by directly blocking LcrV's function in Yop delivery, F(ab′)2 fragments would still be expected to be effective. Figure 5B shows that whereas the full-length anti-LcrV antibody promoted phagocytosis, the F(ab′)2 anti-LcrV fragment had little effect. We know that the F(ab′)2 fragments were functional, because they were as effective as full-length anti-LcrV antibody in binding hexahistidine-tagged LcrV as demonstrated in immunoblots and in neutralizing the IL-10-eliciting effect of LcrV (data not shown and A. V. Philipovskiy and S. C. Straley, unpublished data). These data showed that the Fc region of anti-LcrV antibody was crucial for promoting phagocytosis, in agreement with findings by Weeks et al. (29).
Based on these findings, we hypothesized that any antibody that could promote phagocytosis would cause decreased delivery of Yops. We tested this idea by carrying out the phagocytosis and Yop delivery assays in the presence of 80 or 100 μg/ml anti-Yersinia antibody, which we previously showed binds evenly over the surface of 26/37°C-grown Y. pestis KIM5 (7). Anti-Yersinia antibody did reduce the delivery of YopE and YopH (Fig. 8) as well as delay cellular rounding (data not shown), though never as effectively as did anti-LcrV antibody. Correspondingly, anti-Yersinia antibody weakly promoted phagocytosis (64% ± 12% of yersiniae were intracellular, based on three experiments). These results show that reduction of Yop delivery can occur as a consequence of opsonization by an antibody other than anti-LcrV.
FIG. 8.
Anti-Yersinia antibody also reduces the delivery of YopH and YopE. J774A.1 cells in six-well cluster dishes were infected at an MOI of 10 with Y. pestis KIM5(pGFP) in the presence of 40 μg/ml anti-LcrV or anti-Yersinia antibody or of PBS. Delivery of YopH and YopE to the soluble J774A.1 cellular fraction was assayed by immunoblotting. Lanes: PBS, no antibody; αYersinia, anti-Yersinia antibody; α-V, anti-LcrV antibody.
PMNs are major mediators of protection by anti-LcrV antibody.
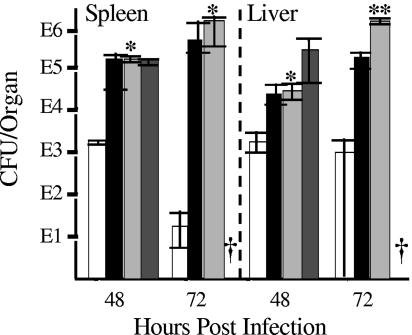
The findings in this study indicated that promotion of phagocytosis may be an important way that anti-LcrV antibody protects against plague and point to a major role of phagocytic cells as mediators of protection. In a previous study, we found that macrophages contribute to the control of bacterial numbers in the liver but are dispensable or redundant in the spleen (16). We proposed that another cell type is the major mediator of protection and that a likely candidate is the PMN. To test this idea, we evaluated protection in mice depleted of PMNs by treatment with anti-Gr-1 (Ly-6G) antibody (Fig. 9). These mice were not significantly depleted for macrophages or other leukocytes (data not shown). Mice depleted of PMNs were unable to control Y. pestis numbers in either the liver or spleen, despite the presence of anti-LcrV antibody. This finding showed that PMNs are critical for protection against plague by anti-LcrV antibody.
FIG. 9.
PMNs are essential for early protection by anti-LcrV antibody. C57BL/6 mice were given the anti-Ly-6G monoclonal antibody 1A8 at −18 h and on days 1 and 2 postinfection to ablate PMNs. Groups of these mice and of control mice whose PMNs were not ablated were also treated with a protective 100-μg dose of anti-LcrV antibody at −18 h. Additional control C57BL/6 mice were given a mock treatment with PBS at this time. All mice were infected retroorbitally (intravenously) on day 0 with 3 × 104 Y. pestis KIM5 organisms. Groups of three mice per treatment were analyzed for CFU counts in the liver and spleen on days 2 and 3 postinfection. Open bars, control mice given anti-LcrV antibody; filled bars, control mice given PBS; light-grey bars, PMN-depleted mice given anti-LcrV antibody; dark-grey bars, PMN-depleted mice given PBS. Error bars indicate ±1 SD from the mean. PMN-depleted mice given PBS died before the 72-h point (†). Viable numbers in PMN-depleted mice given anti-LcrV antibody differed significantly from those in control mice given anti-LcrV antibody at P values of <0.05 (*) and <0.01 (**).
Accordingly, it was important to know whether anti-LcrV antibody promotes phagocytosis in PMNs. Figure 10 shows that it did. Interestingly, in the absence of antibody, the yersiniae were almost all (90 to 95%) extracellular, indicating that the antiphagocytic properties of 26/37°C-grown Y. pestis are highly effective against mouse PMNs. Anti-LcrV antibody caused 55% of the yersiniae to be phagocytosed.
FIG. 10.
Anti-LcrV antibody promotes phagocytosis by PMNs. Glycogen-elicited PMNs from C57BL/6 mice were infected in suspension at an MOI of 3 with Y. pestis KIM5 pVGFP in the presence of 40 μg/ml anti-LcrV antibody (αV) or PBS (No Ab). Phagocytosis was assayed after 1 h as described in the legend to Fig. 5. Numbers of extracellular and intracellular yersiniae were counted in 20 cells in 20 fields. Filled bars, intracellular yersiniae; open bars, extracellular yersiniae. Error bars show ±1 SD from the mean numbers per PMN. The data for αV treatment differed significantly from those for PBS at a P value of <0.0001.
DISCUSSION
This work was part of an ongoing effort in our laboratory to improve our understanding of LcrV activities in the pathogenesis of Y. pestis and clarify how antibody against it protects against plague. This predominantly in vitro study was initiated to resolve conflicts in the literature concerning anti-LcrV antibody's ability to inhibit the delivery of Yops to HeLa cells. Our experiments with J774A.1 cells provided a critical insight that led us to the resolution. We confirmed findings by others (15, 29) that anti-LcrV antibody at least partially inhibits the delivery of Yops to J774A.1 cells and promotes the phagocytosis of Y. pestis KIM5 (Fig. 1 and 5). A critical finding was that the cells had to be able to phagocytose for anti-LcrV antibody to inhibit Yop delivery (Fig. 7). Moreover, intracellular Y. pestis KIM5 apparently does not produce Yops (Fig. 6), in agreement with the results of the experiment by Rosqvist et al. (20), in which Y. pestis EV76 was made to be mostly localized within HeLa cells, due to expression of invasin, or at the surfaces, due to expression of YadA. It was important for us to make a test with J774A.1 cells and Y. pestis KIM5, because Y. pestis KIM5, associated with human macrophages, was previously shown to exhibit strong yop gene expression (17), in seeming conflict with later findings for EV76 and HeLa cells. That earlier study was made before contact activation of type III secretion had been discovered and before it was appreciated that Yops were delivered into host cells, with resulting inhibition of phagocytosis. The yersiniae in that study had been grown at 37°C in the presence of calcium, and those that bound to the macrophages would have been primed for rapid Yop delivery and subsequent induction of yop gene expression and very likely were mostly extracellularly located. We do not yet know what causes the shutdown of Yop production upon phagocytosis. Some of the bacteria may be killed, especially by PMNs; however, in J774A.1 cells, all of the bacteria remain viable throughout the assay, even when they are mostly intracellular (as found in the Gm protection tests). This is an important question for future study, because it has implications for the regulation of Ysc and for the cell biology of any intracellular phase of Y. pestis infection. However, for the purpose of this study, this finding coupled with the requirement of actin function in the host cell for inhibition of Yop delivery led to the conclusion that anti-LcrV antibody likely directly causes phagocytosis, which then inhibits Yop production and delivery, instead of the other way around.
Meanwhile, we had found that blocking conditions affected whether anti-LcrV antibody could inhibit Yop delivery to J774 cells. We had been using mild blocking agents such as 1% MS or 50 μg/ml HgG to prevent non-LcrV interactions of anti-LcrV antibody with the J774A.1 cells, and these had little effect on Yop delivery or the ability of anti-LcrV antibody to inhibit Yop delivery to these cells. However, stronger blocking conditions typically used for immunofluorescence did interfere with the effect of anti-LcrV if they were effective in preventing binding of the antibody to the cells (Fig. 2 and 3). Coupled with the indication that anti-LcrV antibody may directly promote phagocytosis, this finding prompted the test of whether the Fc portion of the antibody was essential for anti-LcrV antibody to promote phagocytosis. F(ab′)2 fragments of anti-LcrV antibody were unable to promote phagocytosis of the yersiniae, implicating a role for the Fc portion of the antibody in the mechanism whereby anti-LcrV antibody inhibits Yop delivery. This finding is in agreement with one by Weeks et al. (29), namely, that F(ab′)2 fragments of their rabbit polyclonal anti-LcrV antibody were ineffective at promoting the phagocytosis of Y. pestis CO92 by J774A.1 cells.
These experiments led to clarification of why we had previously been unable to demonstrate an effect of anti-LcrV antibody on Yop delivery to HeLa cells (see reference 7 and Fig. 1), in contrast to the findings by Pettersson et al. (15). We had been using HgG during our infection experiments, whereas Pettersson et al. had not used a blocking agent. When we omitted the HgG, we were able to replicate their experiment. Our interpretation of this finding is that the HgG had been sufficient to block the Fc receptors on HeLa cells, thereby preventing anti-LcrV antibody from promoting phagocytosis. It should be noted that the inhibitory effect of anti-LcrV antibody on Yop effects in HeLa cells is subtle, and we had to use a very low MOI to see a partial effect (Fig. 4), as had Pettersson et al. This likely reflects the relatively weak phagocytosis of yersiniae by HeLa cells compared to that by J774A.1 cells. In HeLa cells, a small fraction of the yersiniae would be engulfed, resulting in a small diminution of Yop delivery, detectable most easily by a sensitive assay such as one detecting a delay in the development of cytotoxicity (Fig. 4). An implication of these results is that anti-LcrV antibody may not block the delivery of Yops and hence the effects of Yops in all cells that may come in contact with Y. pestis in vivo.
The requirement that the Fc portion of anti-LcrV antibody be present for phagocytosis to be promoted implicates the Fc receptor of phagocytes in the inhibition of Yop delivery, as noted by Weeks et al. (29). However, our adherence studies, consistent with the findings of other groups (19, 29), failed to indicate a role of anti-LcrV antibody in mediating the adherence of the bacteria, despite the fact that the yersiniae had been grown so as to express LcrV on their surfaces (7). Y. pestis is able to adhere to many cell types and likely has multiple adhesins that dominate small effects of opsonization by anti-LcrV antibody through the small amount of surface LcrV. We hypothesize that the Fc receptors play a signaling role that coordinates with signaling due to clustering of receptors by Y. pestis adhesins to overcome the local effects of small amounts of Yops that are delivered instantly upon bacterial contact. Such an effect would require antibody bound to the bacterial surface, and indeed, anti-YopM antibody, which does not bind to nonpermeabilized Y. pestis, did not promote phagocytosis. However, anti-Yersinia antibody, which does bind to the Y. pestis surface, only weakly promoted phagocytosis and the consequent reduction of Yop delivery to J774A.1 cells. In the study by Weeks et al. (29), a similar polyclonal anti-Yersinia antibody preparation also gave only a partial enhancement of the phagocytosis of Y. pestis CO92(P) by J774A.1 cells, but the effect of this antibody on the delivery of Yops was not reported. It is likely that the extent to which an antibody against a Yersinia surface component promotes phagocytosis depends on the balance of multiple factors, as analyzed for invasin (10). For example, the anti-LcrV and anti-Yersinia antibodies in our study are distributed differently on the bacterial surface, with anti-LcrV being punctate and anti-Yersinia being diffusely distributed; perhaps this affects the strength of prophagocytic signaling. In a previous study, opsonization of Y. pseudotuberculosis with an anti-Yersinia antibody did not promote phagocytosis by J774A.1 cells (5). However, the antibody did promote the phagocytosis of a strain lacking both invasin and the virulence plasmid that encodes the Yops and type III secretion system, and it caused decreased phagocytosis of an invasin mutant that contained the virulence plasmid. We speculate that the anti-Yersinia antibody in that study was able to promote both adherence and phagocytosis when Yops were absent but did not signal strongly enough to overcome effects of Yops, and indeed, increased adherence could have caused greater delivery of Yops (and consequently stronger antiphagocytosis) by the plasmid-containing strain. Analogously, YadA expressed by Y. pestis EV76 promoted surface localization of the bacteria on HeLa cells and strong expression of Yops, whereas uptake of Y. pestis mediated by invasin resulted in weak Yop expression (20). However, further study is needed before concluding that phagocytosis by any means results in the inhibition of Yop expression and delivery.
Recently we reported the unexpected finding that, in studies of systemic plague, anti-LcrV antibody has little if any effect on viable yersinia numbers in organs during the first 6 h of infection (16). In that study, Y. pestis KIM5 had been grown as in the present study, so as to have LcrV but no F1 capsule on the surface. The yersiniae, injected intravenously, mainly seeded the liver and spleen. Once there, Yops were crucial for bacterial growth, and anti-LcrV antibody acted to prevent an increase in viable yersinia numbers and to promote clearance. Macrophages and dendritic cells are cells that are likely to encounter the yersiniae when they first arrive in liver and spleen, but these cells were not major mediators of protection by anti-LcrV antibody (16). They may be responsible for some bacterial killing, notably in the liver, but they also could be niches for the protected intracellular growth of the yersiniae. The present study demonstrated that PMNs are major mediators of protection by anti-LcrV antibody. These cells are expected to migrate into infected organs within hours of seeding by the yersiniae and have been shown to accumulate in foci of acute inflammation, at least by 12 h after infection of mice (e.g., reference 24). We hypothesize that PMNs stimulated by the inflammatory milieu are able to phagocytose and kill both free yersiniae and yersiniae as they are released from any intracellular niche and that anti-LcrV antibody contributes to bacterial clearance by promoting phagocytosis. This keeps bacterial numbers low and may prevent the accumulation of secreted LcrV. Antibody may also neutralize the immunosuppressive effects of the free LcrV, but this effect appears not to be the dominant protective mechanism of anti-LcrV antibody, if direct control over bacterial numbers also indirectly limits the amount of LcrV released (16). It remains to be determined if neutralization of LcrV's immunosuppressive effect is important in a situation where bacterial numbers are higher, such as in postexposure prophylaxis.
In summary, our findings support the new hypothesis that anti-LcrV antibody inhibits the delivery of Yops to mammalian cells by directly promoting phagocytosis, with a consequent inhibition of Yop production. The data implicate the Fc portion of anti-LcrV antibody as a direct participant in this process and hence as essential for protection. This study also demonstrated that PMNs are major mediators of protection by anti-LcrV antibody. Coupled with previous data demonstrating the importance of the inhibition of Yop effects in vivo (16), the findings in this study support the hypothesis that a major protective mechanism of anti-LcrV antibody is to inhibit Yop production by promoting phagocytosis by PMNs.
Acknowledgments
We thank Donald A. Cohen (University of Kentucky) for many helpful discussions.
This study was supported by PHS (NIAID) grant AI21017.
Editor: D. L. Burns
REFERENCES
- 1.Bergman, T., S. Håkansson, Å. Forsberg, L. Norlander, A. Macellaro, A. Bäckman, I. Bölin, and H. Wolf-Watz. 1991. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J. Bacteriol. 173:1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brubaker, R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 3.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan, C., H. A. Jones, Y. H. Kaya, R. D. Perry, and S. C. Straley. 2000. Invasion of epithelial cells by Yersinia pestis: evidence for a Y. pestis-specific invasin. Infect. Immun. 68:4523-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fällman, M., K. Andersson, S. Håkansson, K.-E. Magnusson, O. Stendahl, and H. Wolf-Watz. 1995. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 63:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fields, K. A., and S. C. Straley. 1999. LcrV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism. Infect. Immun. 67:4801-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields, K. A., M. L. Nilles, C. Cowan, and S. C. Straley. 1999. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect. Immun. 67:5395-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosdent, N., I. Maridonneau-Parini, M.-P. Sory, and G. R. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70:4165-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmström, A., J. Olsson, P. Cherepanov, E. Maier, R. Nordfelth, J. Pettersson, R. Benz, H. Wolf-Watz, and Å. Forsberg. 2001. LcrV is a channel size-determining component of the Yop translocon of Yersinia. Mol. Microbiol. 39:620-632. [DOI] [PubMed] [Google Scholar]
- 10.Isberg, R. R., and P. Barnes. 2001. Subversion of integrins by enteropathogenic Yersinia. J. Cell Sci. 114:21-28. [DOI] [PubMed] [Google Scholar]
- 11.Marenne, M.-N., L. Journet, L. J. Mota, and G. R. Cornelis. 2003. Genetic analysis of the formation of the Ysc-Yop translocation pore in macrophages by Yersinia enterocolitica: role of LcrV, YscF, and YopN. Microb. Pathog. 35:243-258. [DOI] [PubMed] [Google Scholar]
- 12.Nedialkov, Y. A., V. L. Motin, and R. R. Brubaker. 1997. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect. Immun. 65:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemeth, J., and S. C. Straley. 1997. Effect of Yersinia pestis YopM on experimental plague. Infect. Immun. 65:924-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilles, M. L., K. A. Fields, and S. C. Straley. 1998. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J. Bacteriol. 180:3410-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettersson, J., A. Holmström, J. Hill, S. Leary, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, Å. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface-exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961-976. [DOI] [PubMed] [Google Scholar]
- 16.Philipovskiy, A. V., C. Cowan, C. R. Wulff-Strobel, S. H. Burnett, E. J. Kerschen, D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2005. Antibody against V antigen prevents Yop-dependent growth of Yersinia pestis. Infect. Immun. 73:1532-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollack, C., S. C. Straley, and M. S. Klempner. 1986. Probing the phagolysosomal environment of human macrophages with a Ca2+-responsive operon fusion in Yersinia pestis. Nature 322:834-836. [DOI] [PubMed] [Google Scholar]
- 18.Price, S. B., C. Cowan, R. D. Perry, and S. C. Straley. 1991. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J. Bacteriol. 173:2649-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roggenkamp, A., L. Leitritz, A. Sing, V. A. J. Kempf, K. Baus, and J. Heesemann. 1999. Anti-recombinant V antigen serum promotes uptake of Yersinia enterocolitica serotype 08 by macrophages. Med. Microbiol. Immunol. 188:151-159. [DOI] [PubMed] [Google Scholar]
- 20.Rosqvist, R., Å. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol. 4:657-667. [DOI] [PubMed] [Google Scholar]
- 21.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 168:1315-1321. [DOI] [PubMed] [Google Scholar]
- 22.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, H., J. Keppie, E. C. Cocking, and K. Witt. 1960. The chemical basis of the virulence of Pasteurella pestis. 1. The isolation and aggressive properties of Past. pestis and its products from infected guinea pigs. Br. J. Exp. Pathol. 41:452-459. [PMC free article] [PubMed] [Google Scholar]
- 24.Straley, S. C., and M. L. Cibull. 1989. Differential clearance and host-pathogen interactions of YopE− and YopK− YopL− Yersinia pestis in BALB/c mice. Infect. Immun. 57:1200-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su, H., G. J. Spangrude, and H. D. Caldwell. 1991. Expression of FcγRIII on HeLa 229 cells: possible effect on in vitro neutralization of Chlamydia trachomatis. Infect. Immun. 59:3811-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titball, R. W., and E. D. Williamson. 2001. Vaccination against bubonic and pneumonic plague. Vaccine 19:4175-4184. [DOI] [PubMed] [Google Scholar]
- 27.Titball, R. W., and E. D. Williamson. 2004. Yersinia pestis (plague) vaccines. Expert Opin. Biol. Ther. 4:965-973. [DOI] [PubMed] [Google Scholar]
- 28.Une, T., and R. R. Brubaker. 1984. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect. Immun. 43:895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weeks, S., J. Hill, A. Friedlander, and S. Welkos. 2002. Anti-V antigen antibody protects macrophages from Yersinia pestis-induced cell death and promotes phagocytosis. Microb. Pathog. 32:227-237. [DOI] [PubMed] [Google Scholar]
- 30.Welkos, S., A. Friedlander, D. McDowell, J. Weeks, and S. Tobery. 1998. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb. Pathog. 24:185-196. [DOI] [PubMed] [Google Scholar]