Abstract
This study used a major histocompatibility complex class I tetramer reagent to track antigen-specific CD8 T cells in the lungs of mice immunized with the tuberculosis vaccine candidate Mtb72F. The results show that CD8 T cells recognizing an immunodominant Mtb32-specific epitope could be detected in significant numbers over the course of infection in mice exposed to low-dose aerosol challenge with Mycobacterium tuberculosis and that prior vaccination substantially increased the numbers of these cells early in the lungs. The effector phenotype of the cells was shown by the demonstration that many secreted gamma interferon, but very few contained granzyme B. As the course of the infection progressed, many activated CD8 T cells down-regulated expression of CD45RB and upregulated expression of the interleukin-7 receptor alpha chain, indicating a transition of these cells to a state of memory. These data support the hypothesis that M. tuberculosis-specific CD8 T cells can be targeted by vaccination with the Mtb72F polyprotein.
The facultative intracellular pathogen Mycobacterium tuberculosis currently infects one-third of the world's population, and the 2 to 3 million deaths per year make it the leading cause of death by a single bacterial agent (11, 42). Bacillus Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is currently the only available prophylactic vaccine (6, 8). Although vaccination with BCG is widespread throughout the world, it has been shown in clinical studies to possess variable protective efficacy that can also decline with age (8, 13, 14, 17). While BCG has been shown to protect children from disseminated tuberculosis (TB), it often fails to prevent adult pulmonary TB (3, 39). Due to the global prevalence of tuberculosis and the limitations of the current vaccine, a major research effort is currently under way to identify new vaccine candidates (1, 28, 31, 32).
One new candidate that has undergone substantial evaluation is the fusion or polyprotein designated Mtb72F, derived from the M. tuberculosis proteins Mtb32 and Mtb39 (5, 37). Previous studies have shown that mice immunized with Mtb72F, either as a protein and adjuvant formulation or in the form of a DNA vaccine, were protected against aerosol challenge with M. tuberculosis H37Rv at a level comparable to that of BCG (37). Furthermore, when used as a boost in guinea pigs primed with BCG, survival of these animals to aerosol challenge was doubled from 1 to 2 years compared to animals given BCG only (5). These animals had substantial evidence of lesion resolution and very low bacterial numbers in the lungs.
Although the nature of protective immunity to TB is not completely understood (4, 25, 30), CD4 T cells have been shown to be essential mediators of protection (7, 33, 35). Production of gamma interferon (IFN-γ) is essential, as shown by increased susceptibility in IFN-γ or IFN-γ receptor gene-disrupted animal models, as well as in humans possessing a rare mutation in the IFN-γ receptor (9, 15, 20). In addition to this, however, there has been a growing realization over the past decade or more that CD8 T cells also contribute significantly to protective immunity. Adoptive transfer of immune CD8 T cells can confer protection in mice (27, 29), and such cells accumulate in the lungs of aerosol-infected mice, where they secrete IFN-γ (12, 36). Antibody depletion of CD8 T cells during infection, as well as the use of mouse models possessing gene disruptions that result in significantly reduced numbers of CD8 T cells, also supports a role for CD8 T cells in protective immunity (2, 16, 19, 26, 38, 41).
The vaccine candidate Mtb72F contains an Mtb32-derived major histocompatibility complex (MHC) class I epitope, GAPINSATAM, which is strongly recognized by cytolytic and IFN-γ-secreting CD8 T cells (37). Since the precise role that CD8 T cells play during infection with M. tuberculosis has not been completely defined, we decided to examine the Mtb32 antigen-specific CD8-T-cell response to this peptide epitope in the lungs of mice. Using an MHC class I tetramer reagent containing the GAPINSATAM peptide, we were able to track CD8 T cells recognizing this epitope in the lungs of mice vaccinated with a DNA vaccine encoding Mtb72F and/or challenged by aerosol exposure to M. tuberculosis. The results of this study show that significant numbers of tetramer-positive CD8 T cells accumulate in the lungs of vaccinated mice and expand further in response to challenge infection. These cells expressed markers of cellular activation, and a subpopulation up-regulated expression of a recently identified early memory cell marker (22), the interleukin-7 receptor alpha chain (IL-7Rα). Approximately one-third of the CD8 T cells in the lungs expressed IL-7Rα when Mtb72F protein was delivered in a novel liposomal formulation aimed at inducing CD8-T-cell responses. These data show that antigen-specific CD8 T cells traffic to the lungs after vaccination and respond to subsequent infection with M. tuberculosis.
MATERIALS AND METHODS
Mice.
Studies were performed using 8- to 10-week-old specific-pathogen-free female C56BL/6 mice purchased from Charles River Laboratories (Wilmington, MA). All mice were maintained within a biosafety level III facility at Colorado State University for the duration of the experiments and had free access to sterile water and standard mouse chow. The specific-pathogen-free nature of the mouse colonies was demonstrated by testing sentinel animals, which were shown to be negative for 13 known mouse pathogens. All experimental procedures were approved by the Colorado State University Animal Care and Use Committee.
Bacterial strains.
M. tuberculosis strain H37Rv, originally obtained from the Trudeau Mycobacteria Collection (TMCC 102), was grown from low-passage seed lots in Proskauer-Beck liquid medium containing 0.05% Tween 80 to mid-log phase, and 1.5-ml aliquots were frozen at −70°C until use.
Immunizations and aerosol infection.
Mice were immunized three times at 3-week intervals with either 100 μg of Mtb72F DNA or phosphate-buffered saline (PBS) via the intramuscular (tibialis) route. For each immunization, mice were injected with a total volume of 100 μl/mouse using 50 μl/leg. Three weeks following the last immunization, mice were infected via the aerosol route using a Glas-Col aerosol generator (Glas-Col, Terre Haute, IN), such that ∼100 viable bacteria were deposited in the lungs of each animal. The number of viable bacteria in the lungs was determined at specific time points by plating 10-fold serial dilutions of individual partial organ homogenates on nutrient Middlebrook 7H11 agar and counting bacterial colonies after 21 days of incubation at 37°C.
Preparation of cationic liposome-DNA complexes.
Liposomes were prepared by dissolving the cationic lipid DOTIM [octadecenolyoxy(ethyl-2-heptadecenyl-3 hydroxyethyl) imidazolinium chloride; Sigma-Aldrich, St. Louis, MO] and cholesterol (Avanti Polar Lipids, Alabaster, AL) in chloroform and adding equimolar concentrations to round-bottom 15-ml glass tubes. The solution was then dried overnight in a vacuum desiccator to a thin film. The lipids were rehydrated in 5% dextrose in water to form liposomes at a final concentration of 20 mM. Liposomes were incubated at 50°C for 50 min, followed by incubation for 2 h at room temperature. The liposomes were then extruded through a series of 1-μm, 0.45-μm, and 0.20-μm filters to form the final liposomes, as described previously (40). Noncoding plasmid DNA was prepared under very-low-endotoxin conditions (<0.25 endotoxin units/mg; Althea Technologies). Complexes of liposomes and noncoding plasmid DNA were prepared by first dissolving liposomes in 5% dextrose in water at a concentration of 100 μl liposomes per 1 ml dextrose solution. Next, plasmid DNA was added to the liposome solution at a final concentration of 100 μg DNA per ml liposome solution and complexes were formed spontaneously by gentle pipetting. To prepare vaccines, recombinant Mtb72F protein (kindly provided by the Corixa Corporation and GlaxoSmithKline) was added to preformed liposome-DNA complexes and mixed by gentle pipetting. After preparation, vaccines were kept at room temperature and administered within 30 min. For immunizations using cationic liposome-DNA complexes, mice were immunized two times, 14 days apart, via the intraperitoneal route using 10 μg/mouse of either Mtb72F protein or ova albumin (Sigma-Aldrich) in a total volume of 200 μl of liposome formulation.
Single-cell suspension preparation.
Animals were sacrificed by carbon dioxide asphyxiation and were perfused through the heart with 10 ml cold PBS containing 3 U/ml heparin (Sigma-Aldrich). The lungs were removed from the pulmonary cavity and placed in cold Dulbecco's minimal essential medium (DMEM; Cellgro, Herndon, VA). The lung tissue was disrupted using sterile razor blades and incubated for 30 min at 37°C in a final volume of 2 ml DMEM containing 0.7 mg/ml collagenase D (Roche Applied Science, Indianapolis, IN) and 30 μg/ml DNase (Sigma-Aldrich). Following incubation, the digested lung tissue was gently passed through a sterile 70-μm nylon screen; rinsed using 8 ml cold DMEM supplemented with 10% heat-inactivated endotoxin low fetal bovine serum (Atlas Biologicals, Fort Collins, CO), 10 mM HEPES buffer (Sigma-Aldrich), 2 mM l-glutamine (Sigma-Aldrich), 2% MEM-nonessential amino acids (100×; Sigma-Aldrich), and 50 μM 2-mercaptoethanol (Sigma-Aldrich); and centrifuged at 200 × g. Red blood cells were lysed using Gey's solution (0.15 M NH4Cl, 10 mM KHCO3), and the samples were centrifuged and resuspended in DMEM plus supplements. Cells were counted using a hemacytometer, and aliquots were taken for flow cytometric analysis.
Flow cytometry.
Single-cell suspensions from individual mice were resuspended in PBS containing 0.1% sodium azide (Fisher Chemicals, Fairlawn, NJ). Cells were incubated in the dark for 20 min at 4°C with specific antibody (directly conjugated to fluorescein isothiocyanate [FITC], phycoerythrin [PE], peridinin chlorophyll a protein [PerCP], or allophycocyanin [APC] at 3 μg/ml), followed by 2 washes using PBS with sodium azide. Cells were analyzed immediately using a FACSCalibur dual-laser flow cytometer (BD Biosciences, Mountain View, CA) and the data were analyzed using CellQwest software (BD Biosciences, San Jose, CA) and Summit software (DakoCytomation, Fort Collins, CO). Viable lymphocytes were gated based upon their forward- and side-scatter characteristics, and cell populations were analyzed by expression of fluorochrome-labeled markers. All analyses were performed with a minimum acquisition of 200,000 events.
Cell surface markers were analyzed with the following specific antibodies: FITC anti-CD44 (clone IM7), anti-CD3ɛ (clone 145-2C11), anti-CD62L (clone MEL-14), anti-CD45RB (clone 16A), PerCP anti-CD8α (clone 53-6.7), APC anti-CD3ɛ (clone 145-2C11), and anti-CD127 (clone A7R34; eBiosciences, San Diego, CA). Cell suspensions were also stained with a PE-Mtb32 H-2Db MHC class I tetramer (Beckman Coulter Immunomics, San Diego, CA) containing the peptide GAPINSATAM.
For intracellular antibody staining, single-cell suspensions were resuspended in DMEM with supplements and stimulated with 0.1 μg/ml anti-CD3ɛ (clone 145-2C11) and 1 μg/ml anti-CD28 (clone 37.51), in the presence of 3 μM monensin for 4 h at 37°C at 5% CO2. Cells were stained with antibodies for cell surface molecules as described above, before permeabilization with BD Cytoperm/Cytofix (BD PharMingen, San Diego, CA) according to the manufacturer's instructions. Cells were incubated with FITC-conjugated anti-IFN-γ (clone XMG1.2) or APC-conjugated anti-granzyme B (clone GB12; Caltag Laboratories, Burlingame, CA) for 20 min at 4°C, washed twice, and resuspended in PBS with sodium azide before analysis. Appropriate isotype controls were prepared simultaneously and were included in each analysis. Unless otherwise noted, all antibodies were purchased from BD PharMingen.
RESULTS
Kinetics of accumulation of tetramer-specific CD8 cells in the lungs.
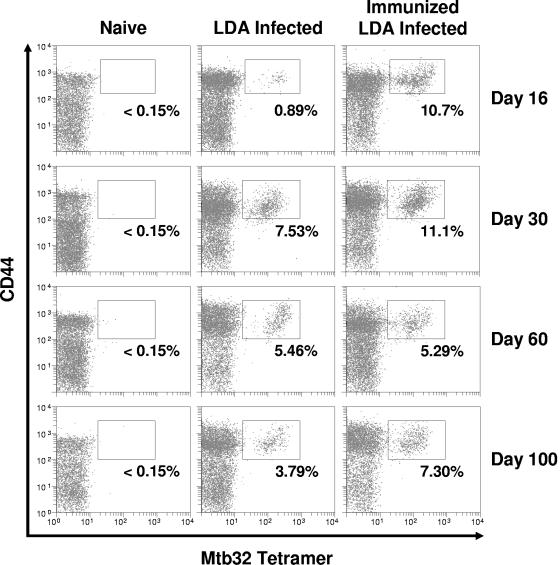
To determine if antigen-specific CD8 T cells were generated either by vaccination or challenge infection resulting in their accumulation in the lungs, lung cells were analyzed at various times by flow cytometry. Cells were gated on the CD3/CD8-positive population and further analyzed for CD44 expression and tetramer staining. As shown in Fig. 1, at all time points background tetramer staining in naïve mice was <0.15%. In infected mice, a small but detectable number of antigen-specific CD8 T cells could be found by day 16. These cells increased by day 30 and then slowly declined. In contrast, in mice vaccinated with Mtb72F prior to challenge, by day 16 of infection ∼11% of CD8 T cells in the lungs recognized the Mtb72F epitope GAPINSATAM. The number of these cells also slowly declined over time.
FIG. 1.
Kinetic analysis of the Mtb32 antigen-specific CD8-T-cell response in nonimmunized mice and in mice immunized with Mtb72F followed by low-dose aerosol (LDA) infection with M. tuberculosis. C57BL/6 mice were immunized three times, at 3-week intervals, via the intramuscular route. Three weeks following the last immunization, mice were challenged. Time points represent the number of days after infection. Lung cells were stained with the Mtb32 H-2Db tetramer and antibodies specific for CD3, CD8, and CD44. Lymphocytes were gated based upon characteristic size and granularity and further gated based upon concurrent CD3 and CD8 expression. The data are from one mouse (representative of n = 4 mice). Percentages represent the number of CD44hi tetramer-positive cells as compared to the total number of CD3/CD8-positive cells. In all cases, background tetramer staining in naïve mice was <0.15%.
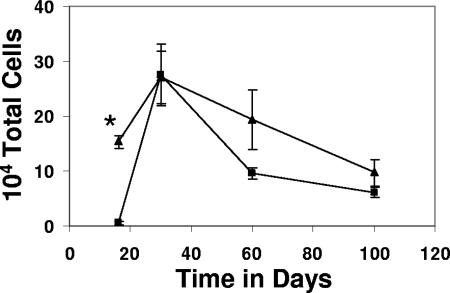
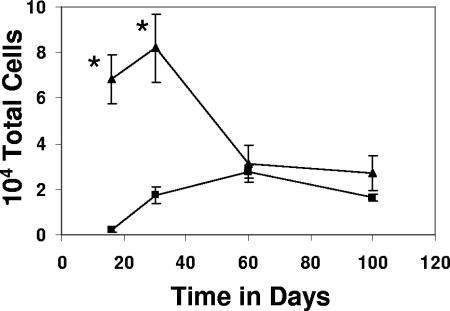
Relative cell numbers are shown in Fig. 2. These data indicated that vaccinated mice generated much higher numbers of antigen-specific CD8 T cells in the lungs earlier during infection, but that by day 30 the numbers of such cells are much the same. This coincides with immunological control of M. tuberculosis replication in the lungs of mice and occurs when we consistently observe a 1-log10 reduction in bacterial numbers between vaccinated and nonvaccinated animals (37).
FIG. 2.
Immunization with Mtb72F resulted in greater numbers of antigen-specific CD8 T cells in the lungs of mice following infection. Data are expressed as the means ± the standard errors of the means and are from four mice per group per time point. Triangles, Mtb72F-immunized mice; squares, nonimmunized mice. Statistical significance based upon a comparison of Mtb72F-immunized and nonimmunized groups was determined using Student's t test and is indicated by an asterisk (P < 0.005).
Functional characteristics of antigen-specific CD8 T cells.
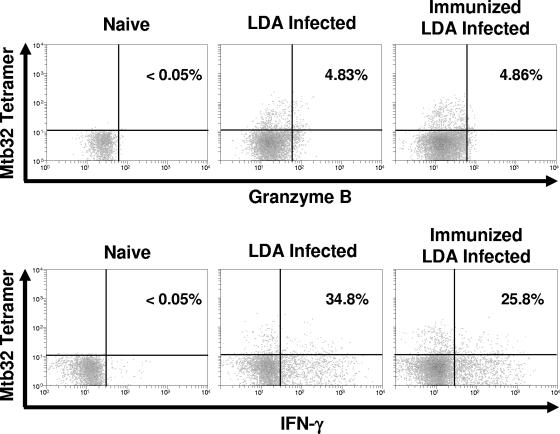
To further determine the functional nature of the lung CD8 T cells, lung cells were stained using the tetramer and also by intracellular staining with antibodies for IFN-γ or granzyme B. As shown in Fig. 3, a large percentage of tetramer-positive cells were also positive for IFN-γ, whereas only a very small percentage were positive for the cytocidal molecule granzyme B.
FIG. 3.
Intracellular cytokine staining of lung-derived cells for granzyme B and IFN-γ at 30 days following aerogenic infection suggests that the tetramer-positive cells are primarily IFN-γ producing rather than cytolytic. The data are from one mouse (representative of n = 4 mice). Percentages represent the number of antigen-specific IFN-γ-positive or granzyme B-positive cells as compared to the total number of tetramer-positive cells.
Kinetics of emergence of CD8 T cells expressing the IL-7 receptor.
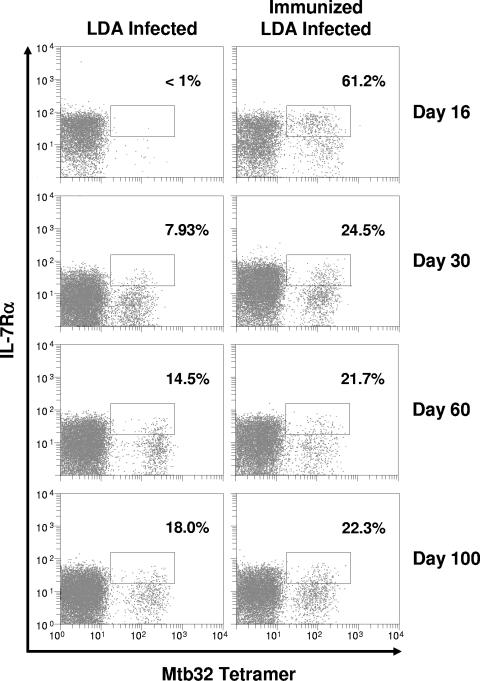
There is increasing evidence to show that CD8 T cells in a state of memory immunity express high levels of the IL-7Rα (22). To determine if expression of this receptor could be detected on CD8 T cells entering the lungs, cells were further analyzed for tetramer staining and staining for IL-7Rα. As shown in Fig. 4, cells expressing this memory marker could be detected in nonimmunized infected mice by day 30 and the numbers increased steadily through day 100. In mice vaccinated with Mtb72F prior to challenge, more than half of the antigen-specific CD8 T cells expressed IL-7Rα by day 16 and this declined slowly through day 100. Relative total cell numbers are shown in Fig. 5 and illustrate the large numbers of such cells present in the lungs over the initial stage of the infection where vaccinated mice showed approximately a 1-log10 reduction in the lung bacterial load (data not shown).
FIG. 4.
Mtb32 tetramer-positive CD8 T cells in the lung express high levels of IL-7Rα following immunization with Mtb72F and subsequent aerogenic challenge. At multiple time points, lung cells were stained with the H-2Db tetramer and antibodies specific for CD3, CD8, and IL-7Rα. Lymphocytes were gated based upon characteristic size and granularity and further gated based upon concurrent CD3 and CD8 expression. The data are from one mouse (representative of n = 4 mice). Percentages represent the number of IL-7Rαhi tetramer-positive cells as compared to the total number of tetramer-positive cells. In all cases, background tetramer staining in naïve mice was <0.15%.
FIG. 5.
Greater numbers of memory precursor cells were present in the lungs of Mtb72F-immunized mice prior to day 60 as compared to nonimmunized mice following M. tuberculosis infection. Data are expressed as the means ± the standard errors of the means (n = 4 mice/group/time point). Triangles, Mtb72F-immunized mice; squares, nonimmunized mice. Statistical significance based upon a comparison of Mtb72F-immunized and nonimmunized groups was determined using Student's t test and is indicated by asterisks (P < 0.05).
Cationic liposomal immunization induces antigen-specific CD8 T cells.
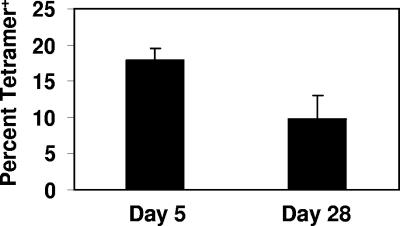
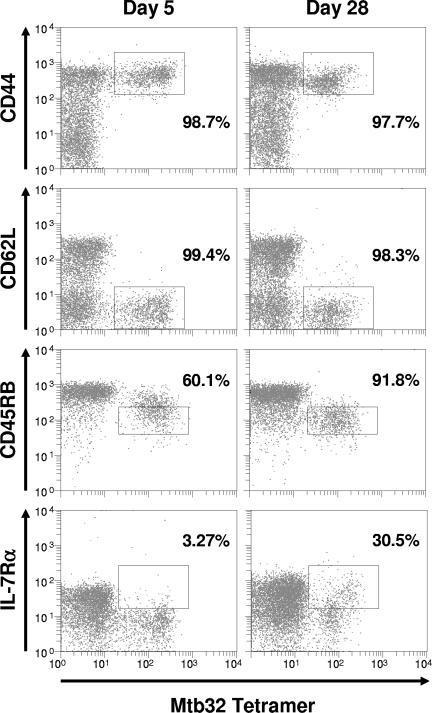
In a final study, we wished to determine if Mtb72F delivered as protein could generate a detectable antigen-specific CD8 response in the lung, even prior to infection. For these studies, we used a novel cationic liposome adjuvant formulated with noncoding plasmid DNA and Mtb72F protein. Figure 6 shows that 5 days after the second boosting vaccine 17% of all CD8 T cells in the lungs were tetramer positive, which decreased to 10% on day 28 postimmunization. As shown in Fig. 7, virtually all of these cells had an activated phenotype (CD44hi CD62Llo). By day 28, the majority had a CD45RBmid phenotype, indicating a transition from an activated to an early memory state. Very few tetramer-positive cells expressed the IL-7Rα initially, but by day 28 nearly a third possessed this phenotype.
FIG. 6.
Immunization with Mtb72F protein in conjunction with cationic liposome complexes and noncoding DNA results in a strong pulmonary antigen-specific CD8-T-cell response in the absence of infection. C57BL/6 mice (n = 4 mice/group/time point) were immunized two times, 14 days apart, via the intraperitoneal route. Five days and 28 days following the second immunization, lung cells were isolated and stained with the H-2Db tetramer and antibodies specific for CD3, CD8, and CD44. Lymphocytes were gated based upon characteristic size and granularity and further gated based upon concurrent CD3 and CD8 expression. Data are expressed as the means ± the standard errors of the means.
FIG. 7.
Mtb32-specific CD8 T cells isolated from lungs of mice following immunization possess an activated phenotype. Mice (n = 4 mice/group/time point) were immunized two times, 14 days apart via the intraperitoneal route with cationic liposomes plus Mtb72F protein. Five days and 28 days following the second immunization, lung cells were isolated and stained with the H-2Db tetramer and antibodies specific for CD3, CD8, and surface activation markers. Lymphocytes were gated based upon characteristic size and granularity and further gated based upon concurrent CD3 and CD8 expression. Representative dot plots are shown where the percentages represent the number of tetramer-positive cells falling within the indicated region as compared to the total number of tetramer-positive cells. In all cases, background tetramer staining in naïve mice was <0.15%.
DISCUSSION
The results of this study showed that infection of mice by aerosol exposure resulted in the accumulation in the lung tissues of large numbers of CD8 T cells that were specific for a 10-mer peptide of the M. tuberculosis protein Mtb32. This peptide, which is highly immunogenic for CD8 T cells capable of cytolysis and cytokine secretion in vitro, is a major component of the effective tuberculosis vaccine candidate Mtb72F (5, 37). In this regard, when mice were vaccinated with Mtb72F DNA prior to challenge, a much larger and more rapid accumulation of antigen-specific CD8 T cells was observed. While it is not known if the antigen-specific cells identified in this study actually contribute to protective immunity to the challenge infection, determination of that was not the purpose of the current study. The purpose instead was to determine if T cells recognizing a dominant MHC class I-restricted epitope could traffic to the lungs after intramuscular inoculation of the Mtb72F candidate tuberculosis vaccine, and this indeed proved to be the case. Studies are currently under way to see if cell sorted tetramer-positive CD8 T cells can adoptively protect recipient mice following aerosol infection.
The data further showed that the antigen-specific CD8 T cells in the lungs of infected mice were capable of producing IFN-γ, which is known to be essential for protection following aerosol challenge with M. tuberculosis. In contrast, the majority of these cells did not produce granzyme B early during infection, suggesting that CD8 T cells entering the sites of infection in the lungs at early time points are more likely to control M. tuberculosis infection indirectly through activation of local macrophages via IFN-γ secretion, rather than to directly lyse infected target cells by secretion of perforin and granzyme B. This is in keeping with the observation that perforin gene knockout mice are not less resistant to aerosol challenge (10, 24). These results, however, do not preclude cytolytic activity at time points later during the infectious process, as observed by others (23, 34). Further experiments utilizing an in vivo cytotoxic assay will delineate the lytic function of these antigen-specific CD8 T cells late in infection. That antigen-specific CD8 T cells capable of producing IFN-γ preferentially accumulate in the lungs of mice following aerosol infection confirms the findings of a recent paper by Kamath et al., which examined the response to two vaccine candidates, CFP10 and Mtb10.4 (23).
Using IL-7Rα as a marker, we found that a subset of M. tuberculosis-specific CD8 T cells that accumulated in the lungs of infected mice gradually assumed a memory precursor phenotype over the course of the infection. In contrast to the nonimmunized mice, if the mice were first vaccinated with Mtb72F, then memory precursor CD8 T cells comprised a major component of the early cellular response in the lungs after challenge. The actual numbers (and percentages) of these cells then declined for unknown reasons, but might indicate transition of these cells to an effector phenotype.
Whether the antigen-specific cells entered the lungs following infection or were already there prior to challenge was not addressed in the Mtb72F DNA vaccination experiments. However, pilot studies using a BCG infection model indicated a substantial establishment of activated protective T cells (A. Kipnis and I. Orme, unpublished data) similar to those demonstrated in chronic infection (21). Furthermore, recruitment of activated T cells to the lungs following immunization was demonstrated here by studies in which we examined the lungs of mice vaccinated with Mtb72F protein delivered in a CD8-directed liposomal formulation. This study showed that a strong antigen-specific CD8-T-cell response could be elicited in the lungs of mice in the absence of infection and that 1 month after two immunizations, about one-third of the antigen-specific CD8 T cells in the lungs expressed the IL-7Rα. Moreover, by this time most of these cells had begun to reduce the expression of CD45RB from high to moderate, which is a further suggestion of transition to a memory state (18).
The data presented here demonstrate preferential recruitment and accumulation of activated, antigen-specific CD8 T cells into the lungs of mice following aerosol challenge with M. tuberculosis. Furthermore, that these cells were capable of producing IFN-γ upon restimulation suggests that they could be actively participating in the control of bacterial replication. It was also shown that vaccination with the Mtb72F DNA vaccine significantly increased the number of these cells in the lungs following infection, which indicates that the CD8-T-cell component of adaptive immunity can be specifically targeted by vaccination. Further studies currently in progress will quantify the amount of protection conferred by this population of Mtb32-specific CD8 T cells, to further define the relevance of the CD8-T-cell response to M. tuberculosis infection. These experiments, as well as others in progress, will ultimately determine whether CD8-T-cell epitopes should be included in future M. tuberculosis candidate vaccines.
Acknowledgments
This work was supported by NIH grants AI-40488 and AI-45707.
We are very grateful to Yves Lobet (GlaxoSmithKline) for providing the Mtb72F recombinant protein.
Editor: J. D. Clements
REFERENCES
- 1.Andersen, P. 2001. TB vaccines: progress and problems. Trends Immunol. 22:160-168. [DOI] [PubMed] [Google Scholar]
- 2.Behar, S. M., C. C. Dascher, M. J. Grusby, C. R. Wang, and M. B. Brenner. 1999. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189:1973-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom, B. R., and C. J. Murray. 1992. Tuberculosis: commentary on a reemergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 4.Boom, W. H. 1996. The role of T-cell subsets in Mycobacterium tuberculosis infection. Infect. Agents Dis. 5:73-81. [PubMed] [Google Scholar]
- 5.Brandt, L., Y. A. Skeiky, M. R. Alderson, Y. Lobet, W. Dalemans, O. C. Turner, R. J. Basaraba, A. A. Izzo, T. M. Lasco, P. L. Chapman, S. G. Reed, and I. M. Orme. 2004. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect. Immun. 72:6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer, T. F., and G. A. Colditz. 1995. Relationship between bacille Calmette-Guerin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin. Infect. Dis. 20:126-135. [DOI] [PubMed] [Google Scholar]
- 7.Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 8.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 9.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, A. M., C. D'Souza, A. A. Frank, and I. M. Orme. 1997. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect. Immun. 65:1317-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. W.H.O. Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 12.Feng, C. G., A. G. D. Bean, H. Hooi, H. Briscoe, and W. J. Britton. 1999. Increase in gamma interferon-secreting CD8+, as well as CD4+, T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 67:3242-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine, P. E. 1989. The BCG story: lessons from the past and implications for the future. Rev. Infect. Dis. 11(Suppl. 2):S353-S359. [DOI] [PubMed] [Google Scholar]
- 14.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn, J. L., M. M. Goldstein, K. J. Triebold, B. Koller, and B. R. Bloom. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 89:12013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg, A. M. 1998. The tuberculosis epidemic. Scientific challenges and opportunities. Public Health Rep. 113:128-136. [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin, J. P., and I. M. Orme. 1994. Evolution of CD4 T-cell subsets following infection of naive and memory immune mice with Mycobacterium tuberculosis. Infect. Immun. 62:1683-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guleria, I., R. Mukherjee, and S. H. Kaufmann. 1993. In vivo depletion of CD4 and CD8 T lymphocytes impairs Mycobacterium w vaccine-induced protection against M. tuberculosis in mice. Med. Microbiol. Immunol. (Berlin) 182:129-135. [DOI] [PubMed] [Google Scholar]
- 20.Jouanguy, E., S. Lamhamedi-Cherradi, F. Altare, M. C. Fondaneche, D. Tuerlinckx, S. Blanche, J. F. Emile, J. L. Gaillard, R. Schreiber, M. Levin, A. Fischer, C. Hivroz, and J. L. Casanova. 1997. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J. Clin. Investig. 100:2658-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junqueira-Kipnis, A. P., J. Turner, M. Gonzalez-Juarrero, O. C. Turner, and I. M. Orme. 2004. Stable T-cell population expressing an effector cell surface phenotype in the lungs of mice chronically infected with Mycobacterium tuberculosis. Infect. Immun. 72:570-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 23.Kamath, A. B., J. Woodworth, X. Xiong, C. Taylor, Y. Weng, and S. M. Behar. 2004. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J. Exp. Med. 200:1479-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laochumroonvorapong, P., J. Wang, C.-C. Liu, W. Ye, A. L. Moreira, K. B. Elkon, V. H. Freedman, and G. Kaplan. 1997. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect. Immun. 65:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mogues, T., M. E. Goodrich, L. Ryan, R. LaCourse, and R. J. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller, I., S. P. Cobbold, H. Waldmann, and S. H. Kaufmann. 1987. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect. Immun. 55:2037-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orme, I. M. 1987. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J. Immunol. 138:293-298. [PubMed] [Google Scholar]
- 28.Orme, I. M. 2001. The search for new vaccines against tuberculosis. J. Leukoc. Biol. 70:1-10. [PubMed] [Google Scholar]
- 29.Orme, I. M., and F. M. Collins. 1984. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 84:113-120. [DOI] [PubMed] [Google Scholar]
- 30.Orme, I. M., and F. M. Collins. 1983. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J. Exp. Med. 158:74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orme, I. M., and A. A. Izzo. 2004. TB vaccine preclinical screening and development, p. 561-571. In W. R. Jacobs, Jr. (ed.), Tuberculosis and the tubercle bacillus. ASM Press, Washington, D.C.
- 32.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 9:115-118. [DOI] [PubMed] [Google Scholar]
- 33.Orme, I. M., A. D. Roberts, J. P. Griffin, and J. S. Abrams. 1993. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 151:518-525. [PubMed] [Google Scholar]
- 34.Romano, M., O. Denis, S. D'Souza, X. M. Wang, T. H. Ottenhoff, J. M. Brulet, and K. Huygen. 2004. Induction of in vivo functional Db-restricted cytolytic T cell activity against a putative phosphate transport receptor of Mycobacterium tuberculosis. J. Immunol. 172:6913-6921. [DOI] [PubMed] [Google Scholar]
- 35.Scanga, C. A., V. P. Mohan, K. Yu, H. Joseph, K. Tanaka, J. Chan, and J. L. Flynn. 2000. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serbina, N. V., and J. L. Flynn. 1999. Early emergence of CD8+ T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect. Immun. 67:3980-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skeiky, Y. A., M. R. Alderson, P. J. Ovendale, J. A. Guderian, L. Brandt, D. C. Dillon, A. Campos-Neto, Y. Lobet, W. Dalemans, I. M. Orme, and S. G. Reed. 2004. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 172:7618-7628. [DOI] [PubMed] [Google Scholar]
- 38.Sousa, A. O., R. J. Mazzaccaro, R. G. Russell, F. K. Lee, O. C. Turner, S. Hong, L. Van Kaer, and B. R. Bloom. 2000. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 97:4204-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sterne, J. A., L. C. Rodrigues, and I. N. Guedes. 1998. Does the efficacy of BCG decline with time since vaccination? Int. J. Tuberc. Lung Dis. 2:200-207. [PubMed] [Google Scholar]
- 40.Templeton, N. S., D. D. Lasic, P. M. Frederik, H. H. Strey, D. D. Roberts, and G. N. Pavlakis. 1997. Improved DNA:liposome complexes for increased systemic delivery and gene expression. Nat. Biotechnol. 15:647-652. [DOI] [PubMed] [Google Scholar]
- 41.Turner, J., C. D. D'Souza, J. E. Pearl, P. Marietta, M. Noel, A. A. Frank, R. Appelberg, I. M. Orme, and A. M. Cooper. 2001. CD8- and CD95/95L-dependent mechanisms of resistance in mice with chronic pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 24:203-209. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. 2004. The World Health Report 2004. World Health Organization, Geneva, Switzerland.