Abstract
The ability of the intracellular bacterium Legionella pneumophila to cause disease is totally dependent on its ability to modulate the biogenesis of its phagosome and to replicate within alveolar cells. Upon invasion, L. pneumophila activates caspase-3 in macrophages, monocytes, and alveolar epithelial cells in a Dot/Icm-dependent manner that is independent of the extrinsic or intrinsic pathway of apoptosis, suggesting a novel mechanism of caspase-3 activation by this intracellular pathogen. We have shown that the inhibition of caspase-3 prior to infection results in altered biogenesis of the L. pneumophila-containing phagosome and in an inhibition of intracellular replication. In this report, we show that the preactivation of caspase-3 prior to infection does not rescue the intracellular replication of L. pneumophila icmS, icmR, and icmQ mutant strains. Interestingly, preactivation of caspase-3 through the intrinsic and extrinsic pathways of apoptosis in both human and mouse macrophages inhibits intracellular replication of the parental stain of L. pneumophila. Using single-cell analysis, we show that intracellular L. pneumophila induces a robust activation of caspase-3 during exponential replication. Surprisingly, despite this robust activation of caspase-3 in the infected cell, the host cell does not undergo apoptosis until late stages of infection. In sharp contrast, the activation of caspase-3 by apoptosis-inducing agents occurs concomitantly with the apoptotic death of all cells that exhibit caspase-3 activation. It is only at a later stage of infection, and concomitant with the termination of intracellular replication, that the L. pneumophila-infected cells undergo apoptotic death. We conclude that although a robust activation of caspase-3 is exhibited throughout the exponential intracellular replication of L. pneumophila, apoptotic cell death is not executed until late stages of the infection, concomitant with the termination of intracellular replication.
Legionella pneumophila is the causative agent of Legionnaires' disease, a potentially life-threatening bacterial pneumonia that is associated with high morbidity and mortality (1). Following invasion of the host cell, L. pneumophila diverts its phagosome from the “default” endosomal-lysosomal pathway to form a rough endoplasmic reticulum (ER)-derived replicative niche (28, 29, 46, 56, 60). The L. pneumophila Dot/Icm type IV secretion system plays essential roles in both diverting the L. pneumophila-containing phagosome (LCP) from the endosomal-lysosomal pathway and the biogenesis of the rough ER-derived LCP (46, 50, 51, 56, 57). Biogenesis of the LCP involves the interception of ER early secretory vesicles (31, 47) and recruitment of the small GTP-binding protein Rab1 and the v-SNARE Sec22b (16, 32), which are involved in vesicular trafficking between the ER and the Golgi apparatus (7). However, within gamma interferon-activated macrophages, the LCP fuses to lysosomes (48). During late stages of intracellular replication, the LCP membrane becomes disrupted, followed by bacterial escape into the cytoplasm, where mitochondria and host cell vesicles are dispersed among the bacteria that continue to replicate in the cytoplasm (38). This transient replication in the cytoplasm is followed by lysis of the plasma membrane and bacterial egress from the host cell (36-38).
Evasion of the endosomal-lysosomal pathway and biogenesis of the LCP are mediated by bacterial effectors that are exported into the host cell through the Dot/Icm secretion system (12, 35, 41). The L. pneumophila Dot/Icm secretion system is encoded by 25 dot/icm genes that are located at two chromosomal loci (51, 58). Most of these genes encode membrane proteins that are believed to be structural components of the secretion apparatus (6). The icmS, -R, -Q, and -W genes encode cytoplasmic proteins with chaperone-like properties and are thought to be required for the export of effectors through the Dot/Icm secretion system (11, 17). Many Dot/Icm effectors, such as RalF, LidA, SidC, LepA, and LepB, have been identified to be exported into the host cell (9, 12, 35, 41). Surprisingly, these effectors play a minor role, if any, in the intracellular replication of L. pneumophila.
Several bacterial pathogens induce apoptosis in mammalian cells by activating specific components of the apoptotic pathways (23, 42). Caspases play essential roles in apoptotic cell death (8, 15, 59). Two major apoptotic pathways, designated the extrinsic and intrinsic pathways, mediate the activation of apoptotic caspases in response to various extra- and intracellular apoptotic stimuli (10, 52, 53, 55). The two main apoptotic pathways converge on caspase-3 activation (45). Caspase-3 plays a major role in apoptosis through the cleavage of various structural and nonstructural proteins to dismantle the cell (19, 45).
Apoptotic caspases have also been shown to be involved in nonapoptotic cellular activities such as cell cycle regulation, proliferation, differentiation, motility, and receptor internalization (2, 49). For example, caspase-3 activation has been shown to be required for platelet production by megakaryoctes and for skeletal muscle differentiation (14, 20). During the process of platelet production, a limited activation of caspase-3 has been shown to be associated with the cleavage of the actin regulator gelsolin in the absence of DNA fragmentation (14). The activation of caspase-3 during skeletal muscle differentiation has been shown to result in the cleavage and activation of a key kinase required for this process, without DNA fragmentation. Inhibition of caspase-3 has been shown to interfere with both processes (14, 20). Such nonapoptotic functions of caspase-3 may involve both limited and compartmentalized activation of the caspase that results in a selective cleavage of specific target proteins without dismantling the cell (14, 49). The mechanism(s) that keeps the activity of caspase-3 from cleaving certain substrates to commit the cell to apoptosis is still unclear (49).
L. pneumophila activates caspase-3 in various cell types during early stages of infection (21, 39). The activation of caspase-3 by L. pneumophila is completely dependent on the functional integrity of the Dot/Icm secretion system (39, 62), since replication-deficient dot/icm mutants of L. pneumophila do not activate caspase-3 (39, 62). Inhibition of caspase-3 activity during early stages of infection by L. pneumophila blocks the ability of the LCP to evade the endosomal-lysosomal pathway (39). Early activation of caspase-3 by the Dot/Icm secretion system of L. pneumophila is associated with the cleavage of rabaptin-5 (39), which is a major effector of Rab-5 that controls the fusion of early endosomes (54, 61).
Since the initiator caspases 8 and 9 are not activated during early stages of infection, the mechanism by which L. pneumophila activates caspase-3 does not involve the intrinsic or the extrinsic pathway of apoptosis and may instead be novel (39). In this study, we show that the preactivation of caspase-3 and early induction of apoptosis do not rescue the intracellular replication of L. pneumophila icmS, -R, and -Q mutant strains of L. pneumophila. Pharmacological activation of caspase-3 at any stage of the infection results in apoptosis and a cessation of intracellular replication of the parental strain of L. pneumophila. Interestingly, a single-cell analysis of infected macrophages reveals that L. pneumophila induces a robust activation of caspase-3 throughout the intracellular replication period without apparent apoptotic cell death. Instead, apoptosis is triggered at late stages of infection, concomitant with the termination of intracellular replication.
MATERIALS AND METHODS
Bacterial strains and eukaryotic cell lines.
The parental L. pneumophila strain AA100 and its isogenic dotA, icmS, icmR, and icmQ mutant strains have been described previously (37, 62). All L. pneumophila strains were grown for 3 days at 37°C on buffered charcoal-yeast extract-agar plates. The plates used for the cultivation of dotA, icmS, icmR, and icmQ mutant strains were supplemented with kanamycin at a concentration of 50 μg/ml.
Both the human U937 and mouse J774A.1 macrophage cell lines were maintained in RPMI-1640 tissue culture medium (Gibco BRL) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL) and were grown at 37°C in the presence of 5% CO2. U937 cells were differentiated for 48 h using phorbol 12-myristate 13-acetate (Sigma, St. Louis, MO) as described previously (62), and J774A.1 macrophages were allowed to adhere for at least 12 h before infection. For bacterial intracellular growth kinetic experiments, U937 and J774A.1 cells were seeded into 96-well plates (Becton Dickinson, NJ) at a concentration of 1 × 105 cells per well. For caspase-3 activity assays, the macrophages were seeded into opaque 96-well plates (Corning Inc., NY).
Kinetics of staurosporin-TNF-α-induced caspase-3 activity assays.
Differentiated U937 cells and J774A.1 cells were treated with staurosporin (Sigma) at a concentration of either 0.5 or 1.0 μM for U937 cells and 10 μM for J774A.1 cells. The treatment was carried out for 1, 3, 5, 7, 10, and 16 h for U937 cells and 1, 3, 8, 12, and 16 h for J774A.1 cells. At each of the above-mentioned time points, the caspase-3 activities of treated and untreated cells were monitored with a fluorometric caspase-3 assay kit (BioVision Inc., Mountain View, CA) as described previously (39). The level of caspase-3 enzymatic activity was measured in arbitrary fluorescent units (AFU) by using a Perkin-Elmer fluorescence plate reader with excitation at 400 nm and emission at 505 nm. For tumor necrosis factor alpha (TNF-α)-induced caspase-3 activity, differentiated U937 cells were treated with either human recombinant TNF-α (10 ng/ml) (BD Pharmingen, San Jose, CA) alone or TNF-α with 1.0, 0.1, or 0.01 μg/ml cycloheximide (Sigma, MO). The caspase-3 activity was determined after 5 h of treatment. The experiments were carried out in triplicate for each of the treatment time points; the level of caspase-3 activity in treated cells was compared to that in untreated cells, as described above.
Growth kinetics of L. pneumophila and caspase-3 activation in U937 and J774A.1 macrophages.
Differentiated U937 cells and J774A.1 macrophages were treated with staurosporin at a concentration of either 0.5 or 1.0 μM for U937 cells and 10 μM for J774A.1. After 1 h of treatment of U937 cells and 3 h of treatment of J774A.1 cells, both the treated (in the presence of staurosporin) and untreated monolayers were infected with L. pneumophila strains at a multiplicity of infection (MOI) of 10. The infection was carried out for 1 h, followed by three washes to remove extracellular bacteria. After the washes, staurosporin-treated cell culture medium was restored to the monolayers that were treated with staurosporin prior to infection. Differentiated U937-cell monolayers were treated with TNF-α (10 ng/ml) in the presence of 0.05 μg/ml cycloheximide. After 5 h of treatment, both treated and untreated monolayers were infected with L. pneumophila AA100 as mentioned above. After washing of the cells, TNF-α-cycloheximide-treated cell culture medium was added to the monolayers that were treated with TNF-α-cycloheximide prior to infection. The numbers of CFU in the treated and untreated monolayers were enumerated at 2, 24, 48, and 72 h postinfection for U937 macrophages and at 2, 24, and 48 h postinfection for J774A.1 macrophages, as described previously (24). To study the effect of preinduction of caspase-3 activity on intracellular replication, the growth kinetics of intracellular replication were compared between both the treated and untreated monolayers.
For a study of the effect of staurosporin- or TNF-α-mediated apoptosis induction at late stages of L. pneumophila intracellular replication, differentiated U937-cell monolayers were infected with L. pneumophila AA100 at an MOI of 10. Infection was carried out for 1 h, followed by a 1-h gentamicin treatment (50 μg/ml) to kill extracellular bacteria. At 8 h postinfection, some of the infected monolayers were treated with either 0.5 μM staurosporin or TNF-α (10 ng/ml) in the presence of 0.05 μg/ml cycloheximide. The numbers of CFU in the treated and untreated monolayers were enumerated at 2, 24, 48, and 72 h postinfection, as described previously (24).
Detection of caspase-3 activation and apoptosis in L. pneumophila-infected U937 cells.
Differentiated U937 cells seeded onto glass coverslips in 12-well plates were infected with the parental strain AA100 at an MOI of 5 for 1 h, followed by three washes to remove extracellular bacteria and further incubation for various time periods. At the end of each incubation, the cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.2) for 30 min at room temperature, followed by three washes with PBS. The fixed cells were then permeabilized on ice with ice-chilled 0.1% Triton X-100 and 0.01% sodium citrate in PBS for 15 min, followed by three washes with ice-chilled PBS. Fluorescence labeling of apoptotic nuclei was carried out using fluorescein isothiocyanate-conjugated terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) using an in situ cell death detection kit as recommended by the manufacturer (Roche, Indianapolis, IN) (11, 39). The fixation of cells and labeling for laser scanning confocal microscopy were done as described previously (11, 39). Immunofluorescence labeling of active caspase-3 and intracellular bacteria was carried out as follows. The TUNEL-labeled cells were blocked with 3% bovine serum albumin in RPMI with 10% fetal bovine serum for 1 h at 37°C. Immunofluorescence labeling was carried out in blocking buffer for 1 h at 37°C. Rabbit polyclonal anti-active caspase-3 antibodies (BD Pharmingen, San Jose, CA), used at a 1:1,000 dilution, and the mouse monoclonal anti-L. pneumophila antibody MAb 3/1 (26), used at a 1:2,000 dilution, were used as the primary antibodies, followed by three washes with PBS (11, 39). After 1 h of blocking at 37°C, the cells were treated for 1 h with a 1:4,000 dilution of the secondary antibodies, Alexa fluor 647-conjugated donkey anti-rabbit immunoglobulin G (IgG) and Alexa fluor 555-conjugated donkey anti-mouse IgG (Molecular Probes Inc., Eugene, OR). Uninfected macrophages and macrophages treated with 1 μM staurosporin for 4 h were labeled for intracellular bacteria, active caspase-3, and apoptotic nuclei as mentioned above. An analysis of macrophages for the presence of intracellular bacteria, active caspase-3, and apoptotic nuclei (TUNEL positive) was carried out using Zeiss Axiovert 100 M laser scanning confocal microscopy, as described previously (11, 39). On average, 8 to 15 0.2-μm serial sections of each image were captured and stored for further analyses, using Adobe Photoshop 6.0 (Adobe Photoshop, Inc.). The level of caspase-3 activation was quantified by measuring the fluorescence intensity of active caspase-3 in uninfected cells, staurosporin-treated cells, and Legionella-infected cells using LSM 510 software. For the Legionella-infected cells, cells were analyzed at 2, 4, 8, 12, and 18 h postinfection. The kinetics of caspase-3 activation in cells was presented in a histogram by plotting the values of the arbitrary fluorescent units of active caspase-3 throughout the cell. The average and standard deviation of the arbitrary fluorescence intensities of the cells were then calculated.
RESULTS
Preactivation of caspase-3 does not rescue intracellular replication of L. pneumophila icmS, -R, and -Q mutants.
The Dot/Icm system is essential for the activation of caspase-3, and the inhibition of caspase-3 blocks the intracellular replication of L. pneumophila (39). The induction of caspase-3 activity prior to infection by the dotA mutant does not rescue the defect in intracellular replication (39). However, DotA is a structural component of the Dot/Icm secretion system, and therefore its defect is expected to result in a defect in the export of all Dot/Icm effectors. The L. pneumophila icmS, -R, and -Q genes encode cytoplasmic proteins with chaperone-like properties that are thought to be involved in exporting specific Dot/Icm effectors into the host cell (11, 17). Mutagenesis of icmR or -Q abrogates the intracellular replication of L. pneumophila and its ability to activate caspase-3 (11, 39, 50). However, a defect in icmS results in a partial defect in both caspase-3 activation and intracellular replication (11, 39). Unlike the dotA mutant that is expected to be defective in all export through the Dot/Icm system, the icmS, -R, and -Q mutants are expected to be defective in the export of specific Dot/Icm effectors (11, 39, 50). Therefore, we hypothesized that the induction of caspase-3 activity prior to infection may rescue the intracellular replication of these mutants, since they are defective in the export of specific effectors and also in the activation of caspase-3. To test this hypothesis, U937 macrophages were pretreated with either 0.5 or 1.0 μM of the protein kinase inhibitor staurosporin to induce caspase-3 activity through the intrinsic pathway of apoptosis (5, 30, 43). After 1 h of staurosporin treatment, both the treated and untreated U937 cells were infected with the icmS, -R, or -Q mutant strain. In addition, untreated U937 cells were infected with the parental strain AA100 and the dotA mutant as positive and negative controls for the intracellular replication of L. pneumophila, respectively. Growth kinetics data showed that the defect in intracellular replication of both the icmR and icmQ mutants was not rescued by preactivation of caspase-3 by either 0.5 or 1.0 μM staurosporin (Fig. 1A and B). Moreover, the data revealed that by 72 h postinfection, the icmS mutant showed an about 10-fold increase in CFU in untreated monolayers. However, intracellular replication of the icmS mutant was completely abrogated in the U937 macrophages pretreated with either 0.5 or 1.0 μM staurosporin (Fig. 1C). The parental strain AA100 showed an about 3-log increase in CFU by 72 h postinfection, and the dotA mutant strain was completely defective in intracellular replication, as shown previously (Fig. 1D) (4).
FIG. 1.
Effect of preinduction of caspase-3 activity on intracellular replication of icmS, icmR, and icmQ mutants in U937 macrophages. The graphs show the growth kinetics of icmR (A), icmQ (B), icmS (C), A100 (D), and dotA strains in untreated U937 macrophages or macrophages treated with 0.5 or 1.0 μM staurosporin (St.) for 1 h prior to infection. Infections were performed for 1 h using an MOI of 10 (see Materials and Methods). The cells were lysed at different time intervals, and the numbers of bacteria in the monolayers were enumerated after growth on agar plates. The results are representative of three independent experiments. The experiments were done in triplicate, and error bars represent standard deviations (some of the error bars are too small to be displayed).
Intrinsic pathway-mediated preactivation of caspase-3 blocks intracellular replication of L. pneumophila.
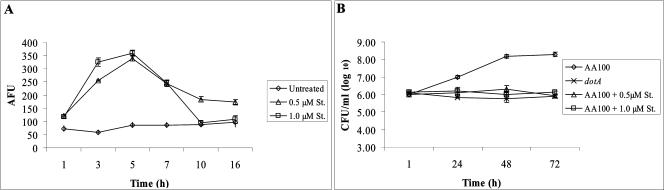
Based on the growth kinetics results for the icmS mutant, we hypothesized that the preactivation of caspase-3, and thus the early induction of apoptosis by agents other than L. pneumophila, could be detrimental to the intracellular replication of L. pneumophila. Accordingly, we examined the effect of preactivation of caspase-3 on the intracellular replication of the parental strain AA100. Initially, we examined the kinetics of caspase-3 activation in U937 macrophages pretreated with either 0.5 or 1.0 μM staurosporin. The data showed that by 1 h of staurosporin treatment, there was an approximately threefold increase in caspase-3 activity compared to that in untreated cells (Fig. 2A). By 5 h of staurosporin treatment, caspase-3 activity peaked, with an ∼5.5-fold increase compared to that in untreated cells. However, beyond the 5-h time point, the caspase-3 activity in staurosporin-treated cells showed a gradual decline.
FIG. 2.
Kinetics of staurosporin-induced caspase-3 activity and growth kinetics of AA100 in staurosporin-treated U937 macrophages. (A) Kinetics of caspase-3 activity in U937 cells treated with 0.5 and 1.0 μM staurosporin (St.) for different time periods. Caspase-3 activity was determined using a fluorogenic substrate specific for caspase-3 and is expressed in AFUs. (B) Growth kinetics of AA100 in U937 macrophages treated with either 0.5 or 1.0 μM staurosporin for 1 h prior to infection. Infections were performed for 1 h using an MOI of 10 (see Materials and Methods). The cells were lysed at different time intervals, and the numbers of bacteria in the monolayers were enumerated after growth on agar plates. The results are representative of three independent experiments. The experiments were done in triplicate, and error bars represent standard deviations.
Based on the above results, untreated U937 macrophages or macrophages pretreated for 1 h with either 0.5 or 1.0 μM staurosporin were infected with the parental strain AA100. The intracellular replication of AA100 in both treated and untreated monolayers was examined by enumerating the number of CFU at 1, 24, 48, and 72 h postinfection. The growth kinetics results revealed that by 48 h postinfection, the number of CFU of AA100 in untreated U937 macrophages showed an ∼100-fold increase (Fig. 2B). However, the wild-type strain AA100 did not replicate in U937 macrophages pretreated with either 0.5 or 1.0 μM staurosporin at 24, 48, or 72 h postinfection. Thus, the staurosporin-mediated early activation of caspase-3, and thus the early induction of apoptosis, correlated with an inhibition of intracellular replication of L. pneumophila.
Extrinsic pathway-mediated preactivation of caspase-3 activation blocks intracellular replication of L. pneumophila.
In order to test the hypothesis that the inability of staurosporin-treated host cells to support L. pneumophila replication was because of an early induction of apoptosis and not because of the inhibition of protein kinases by staurosporin (5, 30), we decided to test the effect of TNF-α-induced caspase-3 activation on the intracellular replication of L. pneumophila. The binding of TNF-α to TNF-α receptor 1 initiates two signaling pathways, one of which promotes cell survival through the activation of the transcription factors NF-κB and AP-1 and the other of which promotes cell death, in the absence of de novo RNA or protein synthesis, through the activation of caspase-8 (3).
Initially, in order to optimize the concentration of the protein synthesis inhibitor needed to enhance TNF-α-induced caspase-3 activation, U937 macrophages were treated with either TNF-α (10 ng/ml) alone or in addition to 1.0, 0.1, or 0.01 μg/ml cycloheximide. After 5 h of treatment, the caspase-3 activity in treated U937 macrophages was determined and compared to that in untreated cells (Fig. 3A). Treatment with TNF-α alone showed only a onefold increase in caspase-3 activity compared to that in untreated cells. The caspase-3 activity level in U937 macrophages treated with cycloheximide alone was found to be approximately equal to that in the untreated cells (0.01 μg/ml of cycloheximide) or onefold (0.1 μg/ml of cycloheximide) or twofold (1.0 μg/ml of cycloheximide) higher than that in untreated cells (Fig. 3A). The level of caspase-3 activity in U937 cells treated with TNF-α (10 ng/ml) in the presence of either 0.01, 0.1, or 1.0 μg/ml cycloheximide was approximately two-, three-, or fivefold higher than that in untreated cells, respectively (Fig. 3A). Accordingly, we used the same concentration of TNF-α (10 ng/ml) in the presence of 0.05 μg/ml cycloheximide. The rationale behind the selection of this concentration of cycloheximide was to obtain the optimal maximum effect of cycloheximide on TNF-α-induced apoptosis.
FIG. 3.
TNF-α-mediated preactivation of caspase-3 blocks intracellular replication of AA100 in U937 macrophages. (A) TNF-α-induced caspase-3 activity in U937 macrophages in the presence of different concentrations of cycloheximide (CHX). Caspase-3 activity was determined using a fluorogenic substrate specific for caspase-3 and is expressed in AFUs. (B) Growth kinetics of AA100 and the dotA mutant in untreated macrophages and of AA100 in macrophages treated with TNF-α-cycloheximide for 5 h prior to infection. Infections were performed for 1 h using an MOI of 10 (as described in Materials and Methods). The cells were lysed at different time intervals, and the numbers of bacteria in the monolayers were enumerated after growth on agar plates. The results are representative of three independent experiments. The experiments were done in triplicate, and error bars represent standard deviations. Some of the error bars are too small to be displayed.
To study the effect of TNF-α-mediated preactivation of caspase-3 on the intracellular replication of L. pneumophila, U937 macrophages were pretreated for 5 h with TNF-α (10 ng/ml) in the presence of 0.05 μg/ml cycloheximide (10, 52, 53, 55). The intracellular replication of AA100 in both treated and untreated cells was monitored by determining the numbers of CFU at 1, 24, 48, and 72 h postinfection. The growth kinetics data showed that while the intracellular replication of AA100 in untreated U937 macrophages showed an almost 3-log increase in CFU by the 48-h time point, AA100 failed to replicate in the TNF-α-cycloheximide-treated cells (Fig. 3B). TNF-α (10 ng/ml) alone or cycloheximide (0.05 μg/ml) alone had no significant effect on either the intracellular or in vitro replication of AA100 (data not shown). These data support our findings that the preactivation of caspase-3, and thus the early induction of apoptosis, correlates with an inhibition of intracellular replication of L. pneumophila.
Inhibition of L. pneumophila intracellular replication by preactivation of caspase-3 in mouse macrophages.
Previous work from our laboratory and others has shown that L. pneumophila activates caspase-3 in different types of human cells (22, 25, 40). However, it has never been tested whether L. pneumophila activates caspase-3 in mouse macrophages, which are widely used to study Legionella pathogenesis (22, 25, 40).
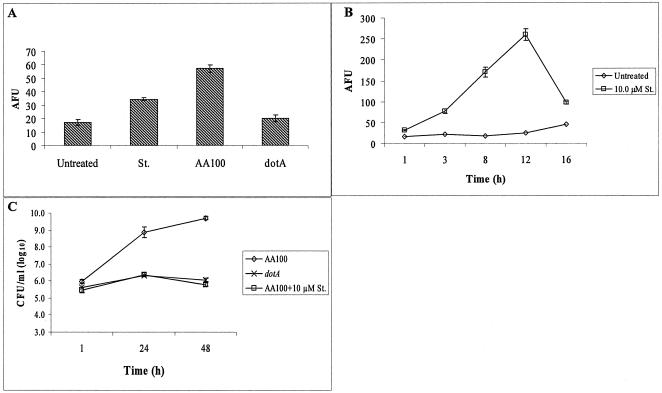
The BALB/c mouse-derived macrophage cell line J774A.1 supports the intracellular replication of L. pneumophila (27, 33). To test whether L. pneumophila activates caspase-3 in J774A.1 macrophages during the early stages of infection, monolayers of J774A.1 macrophages were infected with AA100 or the dotA mutant strain for 1 h, followed by further incubation for 5 h. A caspase-3 activity assay showed that while AA100-infected macrophages had an approximately threefold increase in caspase-3 (P < 0.01) activity compared to uninfected cells, the dotA mutant strain failed to activate caspase-3 (Fig. 4A). Thus, L. pneumophila activates caspase-3 in mouse macrophages in a Dot/Icm-dependent manner, similar to the case in human cells.
FIG. 4.
Effect of preactivation of caspase-3 on intracellular replication of AA100 in mouse macrophages. (A) Caspase-3 activity in J774A.1 macrophages at 6 h postinfection with AA100 or the dotA mutant using an MOI of 10 for 1 h compared to that after treatment with staurosporin (St.). (B) Kinetics of caspase-3 activity in J774A.1 macrophages at different time points after treatment with staurosporin. Caspase-3 activity was determined using a fluorogenic substrate specific for caspase-3 and is expressed in AFUs. (C) Growth kinetics of AA100 and the dotA mutant in untreated J774A.1 macrophages and of AA100 in J774A.1 macrophages treated with staurosporin for 2 h prior to infection at an MOI of 10 for 1 h. The cells were lysed at different time intervals, and the numbers of bacteria in the monolayers were enumerated after growth on agar plates. The results are representative of three independent experiments. The experiments were done in triplicate, and error bars represent standard deviations. Some of the error bars are too small to be displayed.
The effect of staurosporin-mediated preactivation of caspase-3 on the intracellular replication of L. pneumophila in J774A.1 macrophages was also examined. We initially examined the kinetics of staurosporin-induced caspase-3 activity in J774A.1 macrophages treated with 10 μM staurosporin. The kinetics of caspase-3 activity showed that by 3, 8, and 12 h post-staurosporin treatment, there were ∼3-, 7-, and 12-fold increases in caspase-3 activity, respectively, compared to that in untreated macrophages (Fig. 4B).
To study the effect of preactivation of caspase-3 on the intracellular replication of AA100 in J774A.1 macrophages, the monolayers were pretreated for 2 h with 10 μM staurosporin. The intracellular replication of AA100 was followed by determining the numbers of CFU at 1, 24, and 48 h postinfection. The data showed that while AA100 had an approximately 3-log increase in the number of CFU by 48 h in untreated J774A.1 macrophages, AA100 failed to replicate in staurosporin-treated macrophages (Fig. 4C). Taken together, these data allowed us to conclude that the preactivation of caspase-3 and early induction of apoptosis abrogate the intracellular replication of L. pneumophila in both human and mouse macrophages.
Induction of caspase-3 by TNF-α or staurosporin during intracellular exponential-phase replication block furthers proliferation.
It has been proposed that at early stages of infection, L. pneumophila induces a limited activation of caspase-3 which is essential for arresting trafficking of the LCP through the endosomal-lysosomal pathway (39). The data described above showed that the preactivation of caspase-3 through the intrinsic or the extrinsic pathway of apoptosis prior to infection, and thus the early induction of apoptosis, abrogated the intracellular replication of L. pneumophila in human and mouse macrophages. Therefore, we examined the effect of staurosporin and TNF-α-cycloheximide treatment on the intracellular replication of L. pneumophila at later stages of infection.
U937 macrophages infected with the parental strain AA100 were treated with staurosporin (0.5 μM) or TNF-α (10 ng/ml)-cycloheximide (0.05 μg/ml) at 8 h postinfection. The intracellular replication of AA100 in both treated and untreated cells was followed by enumerating the CFU at 1, 24, 48, and 72 h postinfection. The data showed that by 24 h postinfection, AA100 had an approximately 2-log increase in CFU in both treated and untreated macrophages (Fig. 5A and B). In the untreated macrophages, AA100 showed ∼3- and ∼5-log increases in CFU by 48 and 72 h postinfection, respectively (Fig. 5A and B). In contrast, in the treated macrophages, the number of CFU did not show any further increase at 48 or 72 h postinfection (Fig. 5A and B). These data suggested that although L. pneumophila requires caspase-3 activity to arrest trafficking of its phagosome through the endosomal-lysosomal pathway during the early stages of infection, the induction of apoptosis at any stage of intracellular replication of L. pneumophila coincides with a cessation of intracellular replication.
FIG. 5.
Effect of caspase-3 activation during early stages on exponential replication of L. pneumophila. The graphs show the amount of intracellular replication of AA100 in U937 macrophages that were either untreated or treated at 8 h postinfection with 0.5 μM staurosporin (St.) (A) or TNF-α-cycloheximide (CHX) (B). Infections were performed for 1 h using an MOI of 10 (see Materials and Methods). The cells were lysed at different time intervals, and the numbers of bacteria in the monolayers were enumerated after growth on agar plates. The results are representative of three independent experiments. The experiments were done in triplicate, and error bars represent standard deviations. Some of the error bars are too small to be displayed.
Robust activation of caspase-3 by L. pneumophila without apoptosis during exponential replication.
Our data described above showed that the preactivation of caspase-3 and the subsequent induction of apoptosis through the intrinsic or extrinsic pathway prior to infection coincide with a termination of intracellular bacterial replication. With few exceptions (49), the activation of caspase-3 in mammalian cells results in rapid apoptosis and dismantling of the cell. It is rather puzzling that although caspase-3 activity is essential for the intracellular replication of L. pneumophila during early stages of infection, the induction of apoptosis coincides with the termination of intracellular replication. Therefore, it was essential to perform a single-cell analysis to examine how the activation of caspase-3 in each infected cell is coordinated with the subsequent induction of apoptosis and termination of intracellular replication. We examined by in situ single-cell analysis the caspase-3 activity, cell apoptosis, and bacterial replication at the single-cell level. We used laser scanning confocal microscopy for a single-cell analysis of U937 macrophages infected with the parental strain A100 or treated with staurosporin. At different time points after infection, the infected U937 macrophages were fixed, permeabilized, and fluorescently labeled for apoptotic nuclei by TUNEL, for activated caspase-3, and for intracellular bacteria (see Materials and Methods). Any nuclei labeled by TUNEL were considered apoptotic, regardless of the intensity of staining, since any detectable level of DNA fragmentation is a clear sign of the demise of the cell. To establish a quantitative temporal relationship between the activation of caspase-3 and the appearance of TUNEL-positive nuclei, we quantitated the average AFUs of active caspase-3/cell. Uninfected cells had a background level of approximately 50 AFUs of active caspase-3/cell, and therefore we considered a minimum of 100 AFUs/cell as a positive result for active caspase-3.
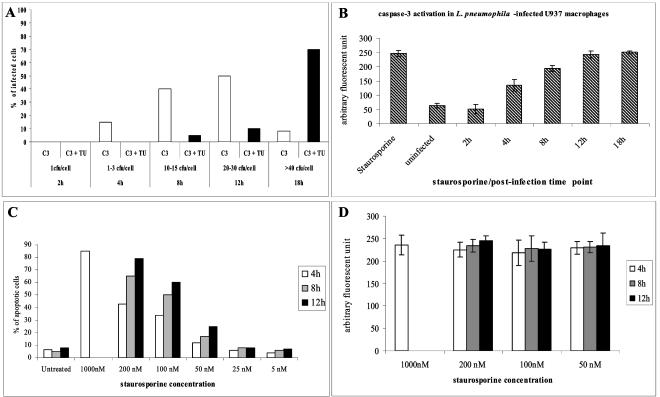
Two hours after infection, the majority (>90%) of the infected cells contained one bacterium (Fig. 6). None of the infected cells showed apoptotic nuclei, and the staining for activated caspase-3 in these cells was similar to the background level of staining in uninfected cells (Fig. 6 and 7). Four hours after infection, 87% of the infected cells contained one to three bacteria (Fig. 6). None of the infected cells showed apoptotic nuclei, but 15% of the infected cells contained active caspase-3. Quantitation of active caspase-3 at the single-cell level showed that the AFU value for the cells that contained active caspase-3 at 4 h was ∼150, compared to ∼50 AFUs at 2 h postinfection or in uninfected cells (Fig. 6). Eight hours after infection, 85% of the infected cells contained 10 to 15 bacteria, and 40% of these cells showed caspase-3 activity in the absence of apoptotic nuclei. The average AFU value for active caspase-3 in the TUNEL-negative cells that contained active caspase-3 was ∼200, compared to ∼50 AFUs in uninfected cells (Fig. 6). Only 5% of the infected cells showed activated caspase-3 in the presence of apoptotic nuclei, and the AFU value for these cells was ∼250, similar to that for cells treated with 1 μM staurosporin (Fig. 6 and 7). Twelve hours after infection, 80% of the infected cells contained 20 to 30 bacteria, among which 50% showed a robust caspase-3 activity level. The average AFU value for active caspase-3 in the TUNEL-negative cells that contained active caspase-3 at 12 h was ∼250, similar to that for staurosporin (1 μM)-treated cells (Fig. 6). Only 10% of infected cells showed activated caspase-3 in the presence of apoptotic nuclei, and the AFU value for active caspase-3 was also similar to that for staurosporin-treated cells (Fig. 6 and 7). Eighteen hours after infection, 85% of the infected cells contained >40 bacteria, among which 70% showed activated caspase-3 in the presence of apoptotic nuclei and 8% showed only active caspase-3 (Fig. 6 and 7). In both TUNEL-positive and TUNEL-negative cells at 18 h, the AFU value for active caspase-3 was ∼250, similar to that for staurosporin-treated cells (Fig. 6 and 7). Importantly, >90% of staurosporin-treated cells with activated caspase-3 had apoptotic nuclei at any time point tested after staurosporin treatment, and the average AFU value for active caspase-3 was ∼250 (Fig. 6).
FIG. 6.
Kinetics of caspase-3 activation and apoptosis induction in L. pneumophila-infected and staurosporin-treated U937 macrophages. L. pneumophila-infected cells (MOI of 5 for 1 h) were fixed and labeled 2 to 18 h after infection. Staurosporin-treated cells were fixed and labeled 4, 8, and 12 h after treatment. (A) For each time point, ∼100 macrophages were analyzed by laser scanning confocal microscopy for the number of intracellular bacteria, for active caspase-3 (C3), and for apoptotic nuclei detected by TUNEL (TU) assays. (B) AFUs of caspase-3 activity in nonapoptotic L. pneumophila-infected cells compared to those in cells treated with 1 μM staurosporin. (C) Percentages of apoptotic nuclei in cells treated with different concentrations of staurosporin. (D) Average AFUs/cell for active caspase-3. The experiments were done in triplicate, and the results are representative of three independent experiments.
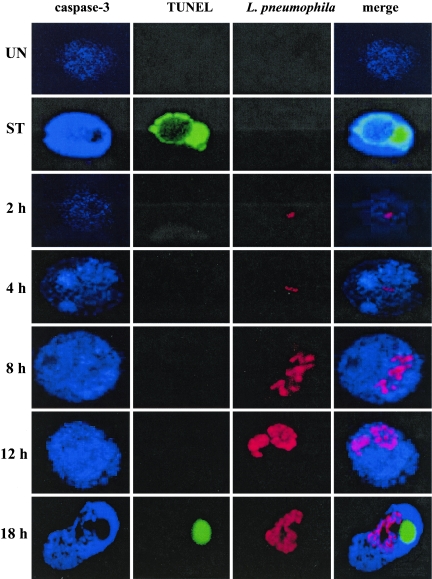
FIG. 7.
Representative laser scanning confocal microscopy images of L. pneumophila-infected macrophages. U937 macrophages were infected with the parental strain AA100 at an MOI of 5. Infected macrophages were fixed and permeabilized 2, 4, 8, 12, and 18 h after infection. Apoptotic nuclei were labeled using TUNEL (green), intracellular bacteria were labeled using mouse monoclonal anti-L. pneumophila antibodies followed by Alexa fluor 555-conjugated secondary antibodies (red), and active caspase-3 was labeled using rabbit polyclonal anti-active caspase-3 antibodies followed by Alexa fluor 647-conjugated secondary antibodies (blue). The experiments were done in triplicate, and the results are representative of three independent experiments.
To examine whether the temporal and spatial activation of caspase-3 and the delayed appearance of apoptosis in L. pneumophila-infected cells were distinct from other apoptotic stimuli, U937 macrophages were treated with different concentrations of staurosporin for 4 h, 8 h, or 12 h and examined for apoptotic nuclei by TUNEL and for activated caspase-3 (see Materials and Methods). Single-cell analysis by laser scanning confocal microscopy showed that there was a dose- and time-dependent induction of apoptosis (Fig. 6C). At all time points, <10% of the cells treated with 50 to 1,000 nM staurosporin showed activated caspase-3 without the presence of apoptotic nuclei. Analyses of cells with apoptotic nuclei showed averages of 220 to 250 AFUs of activated caspase-3 (Fig. 6D). The ∼10% of the cells with activated caspase-3 but without apoptotic nuclei showed averages of 210 to 240 AFUs (data not shown). The data showed that the gradual increase in the concentration of staurosporin as well as the gradual increase in the treatment time resulted in an increase in the percentage of apoptotic cells but not the level of caspase-3 activity (Fig. 6C). Unlike L. pneumophila-infected cells, which showed a gradual increase in caspase-3 activation throughout the course of intracellular replication, we were unable to detect a gradual increase in AFUs of activated caspase-3 in staurosporin-treated cells (Fig. 6D). In contrast, by 12 h after infection, >50% of L. pneumophila-infected cells showed activated caspase-3, with an AFU value of about 250 (Fig. 6B). However, most of the infected cells did not reveal the presence of apoptotic nuclei until about 18 h after infection (Fig. 6A). These data showed that although L. pneumophila-infected cells had a complete activation of caspase-3 (∼250 AFUs) by 12 h after infection (Fig. 6A and B), the appearance of apoptotic nuclei was not exhibited until 18 h postinfection (Fig. 6A). We concluded that there is a delay in the appearance of apoptotic nuclei in L. pneumophila-infected macrophages in the presence of a robust activation of caspase-3, indicating that during exponential-phase bacterial replication, the organism interferes with late stages of the execution of apoptosis.
Interestingly, during the early stages of infection (4 to 12 h), infected cells with activated caspase-3 in the absence of apoptotic nuclei exhibited a punctate pattern of activated caspase-3 throughout the cytosol (Fig. 7). Moreover, this punctate pattern of activated caspase-3 showed a gradual increase in its intensity and distribution throughout the cytosol that correlated with the increase in the number of intracellular bacteria (Fig. 6 and 7). However, at late stages of infection (18 h), infected cells exhibited a diffuse pattern of activated caspase-3 throughout the cytosol, together with the presence of apoptotic nuclei, similar to the case for staurosporin-treated cells (Fig. 6). This diffuse pattern of activated caspase-3 in the presence of apoptotic nuclei was observed in 90% of U937 macrophages treated with staurosporin (Fig. 6). Punctate patterns of activated caspase-3 have been suggested to represent a “limited” or “compartmentalized” activation of caspase-3 (14).
DISCUSSION
Recent studies have revealed that apoptotic caspases are involved in several nonapoptotic cellular processes in which limited and compartmentalized activation of caspases may result in the selective cleavage of specific target proteins without driving the cell into apoptosis (2, 49). Caspase-3 activation by L. pneumophila during early stages of infection has been shown to play a key role in arresting the trafficking of the LCP through the endosomal-lysosomal pathway (39). L. pneumophila activates caspase-3 during early stages of infection in a Dot/Icm-dependent manner that is independent of the extrinsic and intrinsic pathways of apoptosis (21, 39). This suggests that the activation of caspase-3 by L. pneumophila may be directly mediated by a Dot/Icm effector(s) (39, 62).
L. pneumophila icmS and -R encode cytoplasmic proteins with chaperone-like properties that are believed to be involved in the export of the Dot/Icm effector(s) into the host cell (11, 17). The IcmQ protein has been shown to possess a pore-forming activity and is thought to form the channel in the host cell membranes through which Dot/Icm effectors are exported (18). The level of the defect in intracellular replication of L. pneumophila icmS, -R, and -Q mutants correlates with the ability of these mutants to activate caspase-3 (39). The icmR and -Q mutations result in severe attenuation in intracellular replication, while the icmS mutation results in a partial defect in intracellular replication (11). Since caspase-3 activity is essential for phagosome biogenesis, we have examined whether the activation of caspase-3 prior to infection by the icmS, -R, and -Q mutants will rescue their defect in intracellular replication. Our data show that the activation of caspase-3 prior to infection through the intrinsic or extrinsic pathway of apoptosis (3, 13) does not rescue the intracellular replication of L. pneumophila icmR, icmS, and icmQ mutants. Several Dot/Icm effectors have been shown to be exported into the host cell (9, 12, 35, 41). Thus, a lack of IcmS, IcmR, and IcmQ may interfere with exporting the Dot/Icm effectors necessary for caspase-3 activation as well as other events required for biogenesis of the L. pneumophila replicative niche. Importantly, the activation of caspase-3 prior to infection through the intrinsic or extrinsic pathway of apoptosis abrogates intracellular replication of the parental strain of L. pneumophila. In addition, the activation of caspase-3 by pharmacological agents at any stage of infection results in apoptosis and the abrogation of intracellular replication. Thus, the data show that the effects of caspase-3 activation by L. pneumophila and by apoptosis-inducing agents on intracellular replication are distinct. These studies suggest a unique pattern of caspase-3 activation by L. pneumophila.
The activation of caspase-3 through the intrinsic or extrinsic pathway of apoptosis shows that the majority of treated U937 macrophages become apoptotic within 5 h of treatment. Moreover, the majority of the macrophages have apoptotic nuclei in addition to a high level of active caspase-3 that appears diffusely distributed throughout the cytosol. During early stages of infection (4 h), L. pneumophila-infected cells show a low level of active caspase-3 that exhibits a punctate distribution throughout the cytosol in the absence of apoptotic nuclei. The punctate distribution of active caspase-3 has been previously described to indicate a compartmentalized pattern of capsase-3 activation (14). Importantly, the presence of apoptotic nuclei is not detectable until late stages of infection (12 to 18 h), despite a robust activation of caspase-3. To establish a temporal relationship between the activation of caspase-3 and the appearance of TUNEL-positive nuclei, we quantitated the average AFUs of active caspase-3. Our data clearly show the appearance of active caspase-3, but with a delay in the appearance of TUNEL-positive nuclei. In contrast, in staurosporin-treated cells, the activation of caspase-3 is always associated with TUNEL-positive nuclei. Accordingly, the induction of apoptosis is delayed after the activation of caspase-3 in L. pneumophila-infected cells. Thus, the induction of apoptosis in L. pneumophila-infected cells coincides with the termination of intracellular replication. This prediction is supported by (i) the cessation of intracellular replication following induction of apoptosis prior to infection or during exponential intracellular replication and (ii) our finding that treatment of the L. pneumophila-infected cells with staurosporin or TNF-α during exponential intracellular replication is concomitant with a cessation of intracellular replication.
Recently, it has been shown that the induction of apoptosis in macrophages restricts the intracellular replication of Mycobacterium avium (44). It has been suggested that this restriction of intracellular replication occurs as a consequence of an interruption in intracellular vesicular trafficking (44). Biogenesis of the LCP involves recruitment of the ER's early secretory vesicles (31, 57). Apoptosis has been shown to inhibit the trafficking of secretory vesicles (34). Based on this, it is possible that an early induction of apoptosis interferes with the biogenesis of the LCP.
It has been suggested that during cellular processes that require nonapoptotic limited activities of caspases, antiapoptotic factors are thought to keep the level of the enzymatic activity of activated caspases in check and thus protect the cell from going into apoptosis (49). During early stages of infection, L. pneumophila induces a limited or compartmentalized activation of caspase-3 to arrest trafficking of the LCP through the endosomal-lysosomal pathway without driving the host cell into apoptosis. We speculate that there is an induction of antiapoptotic processes in L. pneumophila-infected cells that limit the activity of caspase-3 from dismantling the infected cell. However, during late stages of infection, apoptosis commences as a result of either further activation of caspase-3 or weakening of the antiapoptotic cellular mechanisms.
In summary, we have shown that the activation of caspase-3 prior to or during the early stages of infection is correlated with an inhibition of intracellular replication of L. pneumophila. During the early stages of infection and exponential replication, L. pneumophila induces the activation of caspase-3 by a Dot/Icm-dependent process without driving the infected cell into apoptotic death. At any stage of infection, when apoptosis is triggered in the host cell, either by L. pneumophila or upon caspase-3 activation by pharmacological agents, intracellular replication is ceased. Our data show an intricate and delicate balance mediated by the Dot/Icm system to induce a limited activation of caspase-3 but not apoptotic death of the host cell until late stages of intracellular replication, when the bacteria are ready to escape the spent host cell.
Acknowledgments
Y.A.K. is supported by Public Health Service awards RO1AI43965 and R21AI038410-06A1. This work was supported by the Commonwealth of Kentucky Research Challenge Trust Fund (R.D.S. and J.S.).
Editor: J. T. Barbieri
REFERENCES
- 1.Abu Kwaik, Y. 1998. Fatal attraction of mammalian cells to Legionella pneumophila. Mol. Microbiol. 30:689-696. [DOI] [PubMed] [Google Scholar]
- 2.Algeciras-Schimnich, A., B. C. Barnhart, and M. E. Peter. 2002. Apoptosis-independent functions of killer caspases. Curr. Opin. Cell Biol. 14:721-726. [DOI] [PubMed] [Google Scholar]
- 3.Baud, V., and M. Karin. 2001. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11:372-377. [DOI] [PubMed] [Google Scholar]
- 4.Berger, K. H., J. Merriam, and R. R. Isberg. 1994. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol. Microbiol. 14:809-822. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand, R., E. Solary, P. O'Connor, K. W. Kohn, and Y. Pommier. 1994. Induction of a common pathway of apoptosis by staurosporine. Exp. Cell Res. 211:314-321. [DOI] [PubMed] [Google Scholar]
- 6.Bitar, D. M., M. Molmeret, and Y. Abu Kwaik. 2004. Molecular and cell biology of Legionella pneumophila. Int. J. Med. Microbiol. 293:519-527. [DOI] [PubMed] [Google Scholar]
- 7.Brittle, E. E., and M. G. Water. 2000. Cell biology. ER-to-Golgi traffic—this bud's for you. Science 289:403-404. [DOI] [PubMed] [Google Scholar]
- 8.Chang, H. Y., and X. Yang. 2000. Proteases for cell suicide: functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 64:821-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, J., K. S. de Felipe, M. Clarke, H. Lu, O. R. Anderson, G. Segal, and H. A. Shuman. 2004. Legionella effectors that promote nonlytic release from protozoa. Science 303:1358-1361. [DOI] [PubMed] [Google Scholar]
- 10.Chen, M., and J. Wang. 2002. Initiator caspases in apoptosis signaling pathways. Apoptosis 7:313-319. [DOI] [PubMed] [Google Scholar]
- 11.Coers, J., J. C. Kagan, M. Matthews, H. Nagai, D. M. Zuckman, and C. R. Roy. 2000. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol. Microbiol. 38:719-736. [DOI] [PubMed] [Google Scholar]
- 12.Conover, G. M., I. Derre, J. P. Vogel, and R. R. Isberg. 2003. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol. Microbiol. 48:305-321. [DOI] [PubMed] [Google Scholar]
- 13.Cross, T. G., D. Scheel-Toellner, N. V. Henriquez, E. Deacon, M. Salmon, and J. M. Lord. 2000. Serine/threonine protein kinases and apoptosis. Exp. Cell Res. 256:34-41. [DOI] [PubMed] [Google Scholar]
- 14.De Botton, S., S. Sabri, E. Daugas, Y. Zermati, J. E. Guidotti, O. Hermine, G. Kroemer, W. Vainchenker, and N. Debili. 2002. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood 100:1310-1317. [DOI] [PubMed] [Google Scholar]
- 15.Degterev, A., M. Boyce, and J. Yuan. 2003. A decade of caspases. Oncogene 22:8543-8567. [DOI] [PubMed] [Google Scholar]
- 16.Derre, I., and R. R. Isberg. 2004. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect. Immun. 72:3048-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumenil, G., and R. R. Isberg. 2001. The Legionella pneumophila IcmR protein exhibits chaperone activity for IcmQ by preventing its participation in high-molecular-weight complexes. Mol. Microbiol. 40:1113-1127. [DOI] [PubMed] [Google Scholar]
- 18.Dumenil, G., T. P. Montminy, M. Tang, and R. R. Isberg. 2004. IcmR-regulated membrane insertion and efflux by the Legionella pneumophila IcmQ protein. J. Biol. Chem. 279:4686-4695. [DOI] [PubMed] [Google Scholar]
- 19.Enari, M., H. Sakahira, H. Yokoyama, K. Okawa, A. Iwamatsu, and S. Nagata. 1998. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43-50. [DOI] [PubMed] [Google Scholar]
- 20.Fernando, P., J. F. Kelly, K. Balazsi, R. S. Slack, and L. A. Megeney. 2002. Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl. Acad. Sci. USA 99:11025-11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, L.-Y., and Y. Abu Kwaik. 1999. Activation of caspase-3 in Legionella pneumophila-induced apoptosis in macrophages. Infect. Immun. 67:4886-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao, L.-Y., and Y. Abu Kwaik. 1999. Apoptosis in macrophages and alveolar epithelial cells during early stages of infection by Legionella pneumophila and its role in cytopathogenicity. Infect. Immun. 67:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao, L.-Y., and Y. Abu Kwaik. 2000. Hijacking the apoptotic pathways of the host cell by bacterial pathogens. Microb. Infect. 2:1705-1719. [DOI] [PubMed] [Google Scholar]
- 24.Gao, L.-Y., O. S. Harb, and Y. Abu Kwaik. 1998. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect. Immun. 66:883-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hägele, S., J. Hacker, and B. C. Brand. 1998. Legionella pneumophila kills human phagocytes but not protozoan host cells by inducing apoptotic cell death. FEMS Microbiol. Lett. 169:51-58. [DOI] [PubMed] [Google Scholar]
- 26.Helbig, J. H., P. C. Luck, Y. A. Knirel, W. Witzleb, and U. Zahringer. 1995. Molecular characterization of a virulence-associated epitope on the lipopolysaccharide of Legionella pneumophila serogroup 1. Epidemiol. Infect. 115:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higa, F., N. Kusano, M. Tateyama, T. Shinzato, N. Arakaki, K. Kawakami, and A. Saito. 1998. Simplified quantitative assay system for measuring activities of drugs against intracellular Legionella pneumophila. J. Clin. Microbiol. 36:1392-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobsen, M. D., M. Weil, and M. C. Raff. 1996. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J. Cell Biol. 133:1041-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagan, J. C., and C. R. Roy. 2002. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4:945-954. [DOI] [PubMed] [Google Scholar]
- 32.Kagan, J. C., M. P. Stein, M. Pypaert, and C. R. Roy. 2004. Legionella subvert the functions of rab1 and sec22b to create a replicative organelle. J. Exp. Med. 199:1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kura, F., K. Suzuki, H. Watanabe, Y. Akamatsu, and F. Amano. 1994. Difference in Legionella pneumophila growth permissiveness between J774.1 murine macrophage-like JA-4 cells and lipopolysaccharide (LPS)-resistant mutant cells, LPS1916, after stimulation with LPS. Infect. Immun. 62:5419-5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe, M., J. D. Lane, P. G. Woodman, and V. J. Allan. 2004. Caspase-mediated cleavage of syntaxin 5 and giantin accompanies inhibition of secretory traffic during apoptosis. J. Cell Sci. 117:1139-1150. [DOI] [PubMed] [Google Scholar]
- 35.Luo, Z. Q., and R. R. Isberg. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 101:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molmeret, M., and Y. Abu Kwaik. 2002. How does Legionella pneumophila exit the host cell? Trends Microbiol. 10:258-260. [DOI] [PubMed] [Google Scholar]
- 37.Molmeret, M., O. A. T. Alli, S. Zink, A. Flieger, N. P. Cianciotto, and Y. Abu Kwaik. 2002. icmT is essential for pore formation-mediated egress of Legionella pneumophila from mammalian and protozoan cells. Infect. Immun. 70:69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molmeret, M., D. M. Bitar, L. Han, and Y. A. Kwaik. 2004. Disruption of the phagosomal membrane and egress of Legionella pneumophila into the cytoplasm during the last stages of intracellular infection of macrophages and Acanthamoeba polyphaga. Infect. Immun. 72:4040-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molmeret, M., S. D. Zink, L. Han, A. Abu-Zant, R. Asari, D. M. Bitar, and Y. Abu Kwaik. 2004. Activation of caspase-3 by the Dot/Icm virulence system is essential for arrested biogenesis of the Legionella-containing phagosome. Cell Microbiol. 6:33-48. [DOI] [PubMed] [Google Scholar]
- 40.Muller, A., J. Hacker, and B. Brand. 1996. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect. Immun. 64:4900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679-682. [DOI] [PubMed] [Google Scholar]
- 42.Navarre, W. W., and A. Zychlinsky. 2000. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol. 2:265-273. [DOI] [PubMed] [Google Scholar]
- 43.Omura, S., Y. Iwai, A. Hirano, A. Nakagawa, J. Awaya, H. Tsuchya, Y. Takahashi, and R. Masuma. 1977. A new alkaloid AM-2282 of Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. (Tokyo) 30:275-282. [DOI] [PubMed] [Google Scholar]
- 44.Pais, T. F., and R. Appelberg. 2004. Induction of Mycobacterium avium growth restriction and inhibition of phagosome-endosome interactions during macrophage activation and apoptosis induction by picolinic acid plus IFNgamma. Microbiology 150:1507-1518. [DOI] [PubMed] [Google Scholar]
- 45.Porter, A. G., and R. U. Janicke. 1999. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6:99-104. [DOI] [PubMed] [Google Scholar]
- 46.Roy, C. R., K. H. Berger, and R. R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663-674. [DOI] [PubMed] [Google Scholar]
- 47.Roy, C. R., and L. G. Tilney. 2002. The road less traveled: transport of Legionella to the endoplasmic reticulum. J. Cell Biol. 158:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santic, M., M. Molmeret, and Y. Abu Kwaik. 2005. Maturation of the Legionella pneumophila-containing phagosome into a phagolysosome within gamma interferon-activated macrophages. Infect. Immun. 73:3166-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwerk, C., and K. Schulze-Osthoff. 2003. Non-apoptotic functions of caspases in cellular proliferation and differentiation. Biochem. Pharmacol. 66:1453-1458. [DOI] [PubMed] [Google Scholar]
- 50.Segal, G., and H. A. Shuman. 1997. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect. Immun. 65:5057-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segal, G., and H. A. Shuman. 1998. How is the intracellular fate of the Legionella pneumophila phagosome determined. Trends Microbiol. 6:253-255. [DOI] [PubMed] [Google Scholar]
- 52.Shi, Y. 2002. Apoptosome: the cellular engine for the activation of caspase-9. Structure (Cambridge) 10:285-288. [DOI] [PubMed] [Google Scholar]
- 53.Shi, Y. 2002. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9:459-470. [DOI] [PubMed] [Google Scholar]
- 54.Stenmark, H., G. Vitale, O. Ullrich, and M. Zerial. 1995. Rabaptin-5 is a direct effector of the small GTPase rab5 in endocytic membrane fusion. Cell 83:423-432. [DOI] [PubMed] [Google Scholar]
- 55.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 56.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tilney, L. G., O. S. Harb, P. S. Connelly, C. G. Robinson, and C. R. Roy. 2001. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell Sci. 114:4637-4650. [DOI] [PubMed] [Google Scholar]
- 58.Vogel, J. P., H. L. Andrews, S. K. Wong, and R. R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873-876. [DOI] [PubMed] [Google Scholar]
- 59.Wang, J., and M. J. Lenardo. 2000. Roles of caspases in apoptosis, development, and cytokine maturation revealed by homozygous gene deficiencies. J. Cell Sci. 113:753-757. [DOI] [PubMed] [Google Scholar]
- 60.Wiater, L. A., K. Dunn, F. R. Maxfield, and H. A. Shuman. 1998. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect. Immun. 66:4450-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107-117. [DOI] [PubMed] [Google Scholar]
- 62.Zink, S. D., L. Pedersen, N. P. Cianciotto, and Y. Abu Kwaik. 2002. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect. Immun. 70:1657-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]