Abstract
Johne's disease in ruminants results in chronic enteritis caused by the pathogenic bacterium Mycobacterium avium subsp. paratuberculosis. This study examined two M. avium subsp. paratuberculosis strains (JD3 and W), using different doses and routes of infection, to establish the optimal time postchallenge when predictable levels of infection, gut lesions, and clinical disease occur in a large proportion of sheep. While a small proportion (25%) of sheep challenged with a low-passage-number laboratory culture of M. avium subsp. paratuberculosis (strain W) became infected, no infection was found in animals exposed to a high-passage-number culture isolate of strain W. In contrast, a primary tissue homogenate of M. avium subsp. paratuberculosis (JD3) resulted in high (90%) infection rates and gut histopathology following oral or intratonsillar challenge. The optimal conditions necessary to produce Johne's disease involve oral inoculation of 3-month-old lambs with four doses of 5 × 108 CFU of M. avium subsp. paratuberculosis isolated directly from the gut lymphatic tissues of clinically affected sheep. This resulted in consistent gut histopathology at 9 months and the onset of clinical disease by 11 months postchallenge.
Johne's disease is a widespread and economically important chronic bacterial infection found in domesticated and wild ruminants. The disease is caused by infection with Mycobacterium avium subsp. paratuberculosis, and it can take years before clinical signs are evident. The disease is manifested as a chronic enteritis leading to reduced production, weight loss, and eventually death (23). Chronic enteritis causes malabsorption of protein and essential nutrients across the gut and in the later stages results in net protein loss (13). Johne's disease has been observed in sheep, cattle, and more recently, farmed deer (2, 17). In New Zealand, the first reported case of Johne's disease in sheep was in 1952 (1), and since that time it has become recognized as one of the most important production-limiting infections of ruminants. Currently available diagnostic tests and vaccines are less than optimal (23).
Few studies have used an experimental model of Johne's disease in sheep using the “ovine” strain of M. avium subsp. paratuberculosis to establish infection and disease. There is a need to develop an infection model that reliably produces clinical disease so that improved immunodiagnostics and vaccine efficacy studies for the prevention of infection, pathology, and clinical disease can be better understood. Most sheep models use oral inoculation as the preferred route of challenge, as this closely mimics naturally occurring infection or disease (7, 26, 33, 40, 41). The dose of the inoculum and the number of doses used have varied markedly between the different infection models used to date, with doses from 103 CFU to 1010 CFU reported to cause infection (7, 35). While lower doses can establish infection in 50% of challenged animals, they do not appear to produce clinical disease. Sheep challenged with M. avium subsp. paratuberculosis isolated from cattle, deer, and sheep become infected or develop clinical disease (3, 4, 7, 16, 24, 40). The infecting bacteria can be either a direct tissue isolate (22, 24, 44) or passaged by serial laboratory culture (42, 44) in vitro. While “bovine” strains of M. avium subsp. paratuberculosis are known to infect many species, including sheep (2, 41), naturally occurring Johne's disease in New Zealand and Australian sheep flocks appears to be predominantly due to the ovine strain, which seems to be more host species specific (31). Reddacliff and Whittington (40) have used an IS1311-typed ovine strain of M. avium subsp. paratuberculosis and demonstrated high infection rates (100%) in merino lambs given 107 CFU and necropsied within 14 weeks of challenge. Stewart et al. (41) found that five sheep given 1010 CFU of a direct tissue isolate of ovine M. avium subsp. paratuberculosis became fecal culture positive and that one developed clinical disease. In contrast, none of five animals were fecal culture positive or developed clinical disease following exposure to an equivalent dose of laboratory-cultured M. avium subsp. paratuberculosis (41).
Routes of infection have included oral challenge directly or via a stomach tube, inoculation into the crypt of the tonsil, and intravenous injection (15, 24, 27, 34, 39). These approaches have shown various results, with dissemination of M. avium subsp. paratuberculosis throughout the body, including the gut, and in some studies clinical disease was observed (24, 27, 34, 39). Variations in the findings from different studies may have been influenced by both the species and breed of animal used and also by variations in the strain and dose of M. avium subsp. paratuberculosis used to establish experimental infection.
The main objective of this study was to establish an optimal challenge protocol for ovine M. avium subsp. paratuberculosis that would result in predictable levels of infection and disease at defined time points postchallenge. A secondary objective was to examine the immune profiles of animals from infection to the onset of clinical disease.
MATERIALS AND METHODS
Ethical approvals.
The animal experiments carried out for this study were approved by the Invermay AgResearch Animal Ethics Committee (approval numbers P453, P499, P518, and P594).
Experimental animals.
The experimental sheep comprised 152 merino lambs, all of which were castrated males. The lambs were selected from flocks in which no Johne's disease had been previously observed and were held with ewes until weaning at 3 months of age. The absence of M. avium subsp. paratuberculosis infection in the foundation flocks was confirmed by fecal culture. After weaning, lambs were randomly allocated to experimental treatment groups as required. The lambs were managed under conventional New Zealand sheep farming conditions in open paddocks.
Challenge strains of M. avium subsp. paratuberculosis.
Two different strains of M. avium subsp. paratuberculosis (JD3 and W) were used to challenge sheep in this study. Both strains were obtained from clinically diseased sheep after necropsy, determined to be IS900 positive by PCR, and typed as ovine strains by IS1311 PCR-restriction enzyme analysis (31). The JD3 strain was isolated from the jejunal and ileocecal lymph nodes of a clinically diseased sheep from Central Otago, New Zealand. This strain was given to the challenged sheep as a direct tissue homogenate. The W strain was isolated from a diseased sheep in Southland, New Zealand, and used as a laboratory culture. A low-passage-number (3 passages) culture of strain W was compared with a continuously passaged (>10 passages) culture to monitor experimental infection. The numbers of organisms present in tissue homogenates or cultures were estimated by microscopic counting with phase contrast, and the numbers of CFU of bacteria were confirmed retrospectively by plate culture. There were consistently low levels of bacterial contamination when M. avium subsp. paratuberculosis was obtained directly from lymphatic tissues recovered aseptically from animals at necropsy.
Establishment of pathology in sheep exposed to high-dose challenge with M. avium subsp. paratuberculosis.
An experiment was designed to establish the minimum time necessary between infectious challenge and the development of histopathological changes within the intestines of artificially challenged lambs. A high-dose challenge was chosen to ensure that predictable disease outcomes would be found. A group of 30 12-week-old lambs were challenged orally with four doses, each containing 1 × 109 CFU, of M. avium subsp. paratuberculosis (JD3) obtained from tissue homogenates from the gut lymphatics of a clinically affected sheep. Doses were delivered to the lambs at 3-day intervals over a 2-week period. While the experimental protocol premised the use of inoculating doses of 5 × 108 CFU, retrospective plate counts confirmed that the inoculum contained 1 × 109 CFU. A group of 10 unchallenged control lambs were kept separate from the challenged sheep. The lambs were bled prior to infection and at regular intervals thereafter. Animals were necropsied electively at 6, 9, and 10 months postinfection, at which time histopathological examination and culture for M. avium subsp. paratuberculosis were performed.
Chronological development of clinical disease.
In an attempt to establish clinical disease in lambs following the experimental challenge, a group of 30 lambs were infected and their immune responses monitored until disease became evident through significant weight loss and a failure to thrive. Each lamb received an oral dose of 5 × 108 CFU of the direct tissue homogenate of M. avium subsp. paratuberculosis (JD3) as a 1-ml inoculum suspended in pasteurized homogenized milk. Further challenges were given 2 and 3 weeks after the initial infectious dose. Ten unchallenged control animals were used as “sentinels” and managed in direct contact with the group of challenged lambs. All animals were weighed and serial blood samples were taken at challenge and at monthly intervals until slaughter. A group of six randomly selected animals were culled at 13 months postchallenge to screen for subclinical pathology. An elective slaughter protocol was used to target all clinically affected animals. Any animals that lost 10% of their maximum weight while still growing were culled. Animals losing 15% of their weight when fully grown were culled, except during winter, when the limit was raised to 18%. A further group of 30 lambs were challenged orally with a high dose (1 × 109 CFU) of a continuously passaged (>10 passages) laboratory culture of M. avium subsp. paratuberculosis (W).
Route and strain of challenge.
We explored whether the oral route of challenge was mandatory or if an oropharyngeal challenge via the tonsil could produce a representative infection and disease in the infection model. We also explored what impact different strains of M. avium subsp. paratuberculosis might have on disease outcomes. Forty-two merino lambs were randomly assigned to four groups: three groups of 12 were challenged with M. avium subsp. paratuberculosis, and a group of six unchallenged animals were used as sentinels. The first group of 12 animals were given a 1-ml oral dose of 5 × 107 CFU/ml of M. avium subsp. paratuberculosis isolated directly from gut tissue homogenates from an infected merino sheep (JD3). The second group was challenged by injecting a 200-μl dose (2.5 × 108 CFU/ml) of the direct tissue homogenate of M. avium subsp. paratuberculosis (JD3) into the tonsillar crypt using a blunt 18-gauge needle. The third group of 12 animals were given a 1-ml oral dose of 1 × 109 CFU/ml of a low-passage-number (three passages) laboratory culture of M. avium subsp. paratuberculosis strain W. It was hypothesized that laboratory cultures of strain W could have reduced virulence, as an earlier study showed that a challenge with continuously passaged (>10 passages) M. avium subsp. paratuberculosis strain W (1 × 1010 CFU) failed to establish infection or disease. As a result of this, a high dose (1 × 109 CFU) of low-passage-number strain W was used and compared with a lower dose (5 × 107 CFU) of the direct tissue homogenate (JD3). One month later, all three groups were given a second challenge. The animals were electively culled at 7, 12, 14, and 16 months postchallenge to determine when representative pathology first became evident.
Necropsy.
All animals were euthanized humanely using either a captive bolt gun or injection with barbiturate into the jugular vein. Samples taken included several sections of the jejunal lymph nodes (anterior, middle, and posterior JJLN), the ileocecal lymph node (ICLN), three sections of jejunum (anterior, middle, and posterior JJ), the terminal ileum, the ileocecal valve, the spleen, and the prescapular lymph node.
Gross visible lesions.
Gross macroscopic pathological changes were classified into one of the following three categories. Minor lesions are defined as a small number of palpable granules (<10) in the jejunal lymphatic (lacteal) ducts, with slight thickening of the jejunum or enlargement of the JJLN. Moderate lesions are defined as many (>10) granules (usually) present in the jejunal lymphatic ducts, with enlarged JJLN, ICLN, and/or a thickened ileum or jejunum. The mesentery may also be thickened with some cording apparent. Severe lesions are granulomatous lesions in the lymphatic ducts or gut lymph nodes, enlarged JJLN and ICLN, and/or thickening of the jejunum and ileum, with cording of the mesentery and numerous lymphatic granules.
Histology.
Once samples were removed from the animal, they were placed in 10% buffered formalin. Sections were cut to 4 to 5 μm using a rotary microtome. Duplicate slides were stained using an automatic stainer (Shandon Linisatin GLX) with hematoxylin and eosin and Ziehl-Neelsen stain. Histological lesions were graded on a numerical scale from 0 to 3, using the criteria outlined by Perez et al. (37), as follows: grade 1, focal granulomata within the Peyer's patch lymphoid tissue or the lamina propria only; grade 2, focal granulomata within the Peyer's patch lymphoid tissue, with extension into the surrounding lamina propria; and grade 3, gross lesions evident upon macroscopic examination, with widespread inflammatory infiltrates throughout the lamina propria and submucosae.
Lymphocyte transformation (LT) assay using peripheral blood leukocytes.
A 10-ml heparinized Vacutainer of blood was centrifuged at 1,260 × g for 15 min. The buffy coat was removed and washed by centrifugation in 45 ml of phosphate-buffered saline (PBS). The cells were resuspended in 5 ml of 10% sheep serum (Gibco BRL, Grand Island, NY) made up in RPMI 1640 (Gibco BRL) with l-glutamine and gentamicin (Gibco BRL). One hundred microliters of cells were plated in a U-bottomed 96-well plate (Nunc Denmark), and 50 μl of antigen or mitogen was added to replicate wells. The concentrations of mitogen and antigen used for the assay were as follows: concanavalin A (Sigma), 50 μg/ml; and purified protein derivative from M. avium subsp. paratuberculosis (PPDj; CSL), 50 μg/ml. The plates were then incubated under humidified conditions for 3 days at 37°C in a 5% CO2 atmosphere. Fifty microliters of [H3]thymidine (Amersham Pharmacia, Piscataway, NJ) with a specific activity of 10 μCi/ml was added to each well, after which the cells were incubated for a further 18 h and harvested (Cambridge Harvester, Watertown, MA) and the radioactivity counted in a Wallac 1205 Betaplate counter (Turku Finland). Results were expressed in counts per minute.
Isolation of mononuclear leukocytes from lymph nodes for lymphocyte transformation assays.
Lymph nodes were removed aseptically from the animal immediately following slaughter, placed into a petri dish with 30 ml of RPMI containing 2% fetal calf serum (FCS; Gibco BRL), and macerated using a scalpel and forceps. The medium containing the cells was poured through a 70-μm cell strainer (Becton Dickinson) into a 50-ml conical tube and held on ice during transportation back to the laboratory. The cells from the lymph nodes were layered onto 7.5 ml of Histopaque (Sigma) (δ 1.083) and centrifuged at 450 × g for 60 min. The cells at the interface were removed, washed in 35 ml of PBS, and centrifuged at 340 × g for 15 min. The live mononuclear cells from all tissues were counted and adjusted to 2.5 × 106 cells/ml in RPMI supplemented with 10% FCS. Mitogens and antigens were used at the following concentrations: concanavalin A, 25 μg/ml; pokeweed mitogen (Sigma), 6.25 μg/ml; and PPDj, 25 μg/ml. The plates were incubated for 4 days at 37°C in 5% CO2 before the addition of [H3]thymidine (10 μCi/ml).
ELISA.
An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies was adapted from a method previously described by Griffin et al. (18). Flat-bottomed microtiter plates (Nunc Maxisorp immunoplate) were coated with 50 μl of the antigen PPDj (12.5 μg/ml). Following washing (six times) of the plates, 50 μl of plasma, diluted 1:100 with wash buffer, was added to duplicate wells. Plates were incubated at 37°C for 1 hour and then washed six times in wash buffer. A horseradish peroxide-labeled rabbit anti-sheep antibody (Dako), diluted 1:2,000 in wash buffer, was used to detect the primary antibody. After incubation at 37°C for 30 min, the plates were washed again. One hundred microliters of substrate solution (citric acid plus NaHPO4 plus o-phenylenediamine dihydrochloride [Sigma] plus H2O2) was added to each well. The plates were left in the dark for 20 min at room temperature, and then 50 μl of 4 M H2SO4 was added to each well to stop the reaction. The plates were read in an ELISA reader (Bio-Rad model 3550 microplate reader) at 490 nm, and the results were expressed in ELISA units (EU) as follows: EU = (sample optical density − negative control optical density) × 100.
IFN-γ assay.
The gamma interferon (IFN-γ) assay used a proprietary Bovigam kit (CSL) to detect IFN-γ. Briefly, 1.5 ml of blood or cells (isolated from lymph nodes at 2.5 × 106 cells/ml) was placed into each of four wells of a 24-macrowell plate (BD Falcon). Each well was stimulated with 100 μl of one of the following antigens or mitogen: saline control, PPDa (300 μg/ml), PPDj (300 μg/ml), and pokeweed mitogen (100 μg/ml). After 24 h of whole-blood culture at 37°C, the plasma supernatant was removed and frozen at −20°C until required. An analysis of IFN-γ was performed using the standard protocol outlined for the Bovigam ELISA kit.
Flow cytometric analysis of isolated cells.
Isolated leukocytes were resuspended to a concentration of 1 × 106 cells/ml in PBS containing 2% FCS. The cells were centrifuged at 340 × g for 7 min, after which the medium was removed and antibodies to the following cell surface markers were added: CD4 (44.97), CD8 (38.65), γδ T-cell receptor (86D), CD25 (9.14) (all supplied from the Centre for Animal Biotechnology, University of Melbourne), and a B-cell marker (BAQ155A; supplied by Veterinary Medical R&D, Pullman, Washington). The cells and antibodies were mixed well and placed on ice to incubate for 30 min. Unbound antibodies were removed by washing with 2% FCS in PBS. A fluorescein isothiocyanate-labeled secondary antibody (donkey anti-mouse immunoglobulin G; Jackson Immunochemicals) was diluted 1/40 with 2% FCS in PBS. Ten microliters of the diluted secondary antibody was added to the cells. The cells and antibody were mixed and incubated on ice in the dark for 30 min. The cells were then washed once with 2% FCS in PBS and again with fluorescence-activated cell sorting (FACS) buffer (5% FCS and 1% sodium azide in PBS). After the final wash, the cells were resuspended in 500 μl of FACS buffer and 500 μl of 1% paraformaldehyde in PBS. The stained cells were then analyzed in a FACScalibur instrument (Becton Dickinson).
Microbial culture of M. avium subsp. paratuberculosis.
Once removed from the animal, samples for culture were kept frozen at −20°C until required. Using aseptic technique, roughly 2 g of gut tissue (ileocecal valve) was homogenized in 1 to 2 ml of water using a mortar and pestle. The homogenized tissue was placed in a 15-ml conical tube (BD Falcon) with 10 ml of 0.75% hexadecylpyridinium chloride and left to stand at room temperature for 48 h. Samples from lymph nodes (posterior JJLN) were homogenized using 1 ml of water. The sediment was collected, and 50 μl was inoculated onto two Middlebrook 7H11 agar slopes containing 20% egg yolk and mycobactin J (0.5-mg/ml ferric mycobactin J; Allied Monitor). The following antibiotics were added to one 7H11 agar slope: amphotericin B (25 μg/ml; Sigma), naladixic acid (50 μg/ml; Sigma), and vancomycin (50 μg/ml; Sigma). The second agar slope remained antibiotic-free. Cultures were then incubated at 37°C in 5% CO2 for up to 20 weeks. This culture method was used for the first two experiments, while the automated Bactec culture system (Becton Dickinson) (14, 45) was used for the final experiment, where the samples were incubated for a maximum of 60 days.
Statistical analysis.
Statistical differences between the groups were analyzed using GenStat for Windows, release 6.1 2002, to take into account genetic heterogeneity and treatment differences. Data were modeled using residual maximum likelihood (36). The error for repeated measurements on the same animal was modeled by a first-order autoregressive process with heterogeneity of variance at the time points. Tests of significance were performed using the Wald statistic (43).
RESULTS
Establishment of gut pathology in sheep exposed to high-dose challenge.
The time required for the development of Johne's disease pathology in experimentally infected sheep is shown in Table 1. Pathological changes were assessed by the development of gross visible lesions of gut tissues at necropsy and by histopathological examination. At 6 months postchallenge, none of 10 electively necropsied sheep showed typical gut histopathology, although 2 were positive by culture. All 10 animals in the group necropsied at 9 months were culture positive, 20% had gross visible lesions, and 90% had minor histological lesions. The necropsies carried out at 10 months postchallenge showed that 60% of the animals had gross visible lesions with more severe histopathology than was evident at 9 months postchallenge. At no time throughout this study did the animals show any weight loss consistent with clinical Johne's disease.
TABLE 1.
Incidence of infection and disease in sheep at different time intervals following oral infectious challenge with M. avium subsp. paratuberculosis
| Time (mos) from challenge to necropsya | No. of animals with gross visible lesions at necropsy/total no. of animals | No. of animals with positive histology/total | Mean histology grade | No. of animals with positive culture/total |
|---|---|---|---|---|
| 6 | 0/10 | 0/10 | 0.0 | 2/10 |
| 9 | 2/10 | 9/10 | 1.3 | 10/10 |
| 10 | 6/10 | 8/10 | 2.1 | 9/10 |
The challenge comprised four doses of 1 × 109 CFU of M. avium subsp. paratuberculosis (JD3) administered orally over 2 weeks. No weight loss was evident in any of the animals.
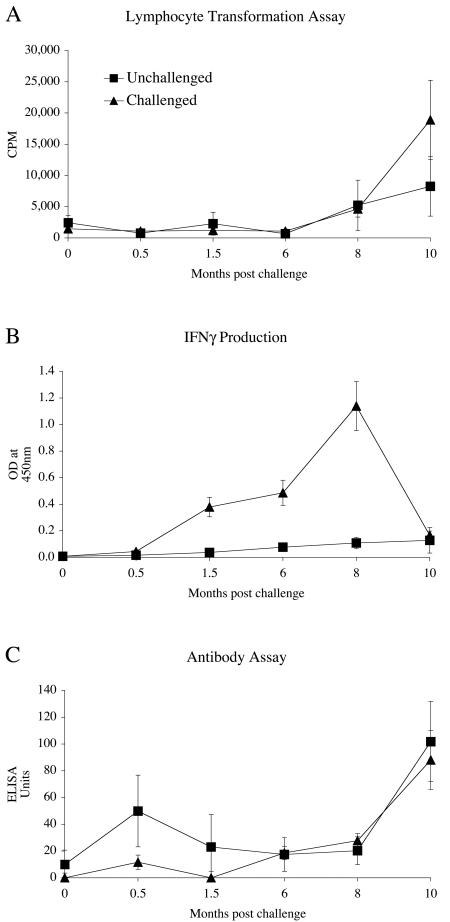
The immunological profiles seen for the experimentally infected sheep are shown in Fig. 1. There was no evidence of PPDj-specific LT responses (Fig. 1A) in M. avium subsp. paratuberculosis-challenged animals or unchallenged control sheep for the first 6 months postinfection. After 8 months, the group mean responses for challenged animals increased markedly. The low level of lymphoproliferative reactivity seen in the orally challenged animals for the first 6 months parallels the low levels of infection seen at this time (Table 1).
FIG. 1.
Immune response profiles of unchallenged controls and sheep challenged orally with a tissue homogenate of M. avium subsp. paratuberculosis (JD3). (A) Mean lymphocyte transformation response following PPDj stimulation of peripheral blood mononuclear cells (PBMC). (B) IFN-γ production in PPDj-stimulated blood. (C) PPDj-specific antibody levels, as measured by ELISA. Mean data ± standard errors of the means (SEM) are shown (n = 30 challenged and 10 unchallenged animals).
The IFN-γ assay was the only test to detect early M. avium subsp. paratuberculosis-specific reactivity which had positive results 6 to 7 weeks after the experimental challenge (Fig. 1B). The amount of specific IFN-γ, first detectable at 1.5 months postchallenge, increased rapidly between 6 and 8 months with a large drop at 10 months, while that of the unchallenged animals remained at background levels. There was little difference in antibody production between the M. avium subsp. paratuberculosis-challenged animals and the unchallenged controls (Fig. 1C). At 10 months postchallenge, both the unchallenged and challenged animals had increases in PPDj-specific antibodies.
Chronological development of clinical disease.
A study was designed to establish the temporal patterns of clinical disease for two groups of 30 lambs challenged orally with a moderately high dose (5 × 108) of either a tissue homogenate of M. avium subsp. paratuberculosis (JD3) or a continuously passaged microbial culture of M. avium subsp. paratuberculosis (W). None of the animals challenged with the continuously passaged culture of M. avium subsp. paratuberculosis were found to be infected or had any gut histopathology. A subgroup of the JD3-challenged animals (n = 8) were necropsied (Table 2) between 11 and 12 months because of a failure to thrive. Groups of six animals were selected at random for necropsy at 13 months to establish the spectrum of pathology. The 10 clinically affected JD3-challenged animals necropsied during the 14- to 21-month period included several animals that had severe histological lesions that were culture negative. At the completion of the study (22 months), six sheep remained clinically unaffected and continued to thrive. At necropsy, three had minor histology lesions (average histology score of 1), but none were culture positive.
TABLE 2.
Histology and culture results for animals challenged orally with M. avium subsp. paratuberculosis (JD3)
| Parameter | No. of positive animals/total no. of animals
|
|||
|---|---|---|---|---|
| Necropsied at 11 to 12 mos | Necropsied at 13 mosb | Necropsied at 14 to 21 mos | Necropsied at 22 mos | |
| Histology | 8/8* | 5/6 | 6/10* | 3/6 |
| Culture | 7/8 | 6/6 | 3/10 | 0/6 |
Challenge with JD3-HD-O (direct tissue homogenate, high dose, administered orally) consisted of 5 × 108 CFU administered as three doses at weekly intervals. *, Animals were necropsied due to weight loss associated with clinical disease.
Animals culled at 13 months were chosen randomly.
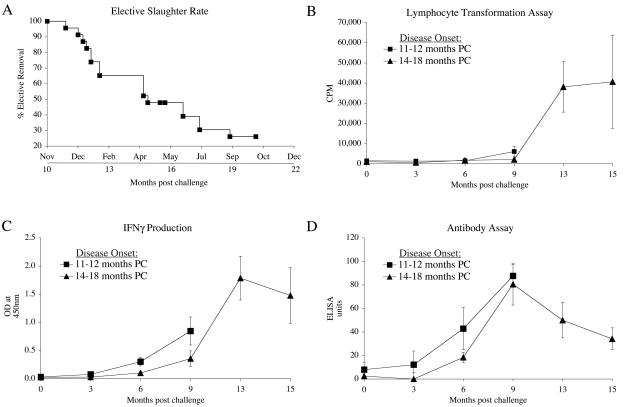
Figure 2A shows the rate at which animals exposed to the challenge were selectively removed from 11 months to 21 months postchallenge. It was not until 11 months postchallenge that the first animal was necropsied due to a loss of weight. Immunological data from cohorts of animals (6) that went on to develop clinical disease at different times were grouped and examined retrospectively after necropsy to identify if the immune profiles of the animals were altered as a precursor to disease development (Fig. 2). The LT responses of animals selectively removed due to clinical disease at 14 to 18 months postchallenge first showed reactivity at 13 months postchallenge. Little reactivity was seen in animals slaughtered at 11 to 12 months postchallenge. Animals that had an earlier onset of disease initially showed a trend towards higher IFN-γ responses than those animals that developed clinical disease at 14 to 18 months postchallenge. Another trend observed was a higher group mean antibody level at 6 months postchallenge for animals culled selectively due to the onset of clinical disease at 11 to 12 months postchallenge.
FIG. 2.
Cull rates and immune profiles of sheep challenged orally with M. avium subsp. paratuberculosis (JD3). (A) Elective slaughter rates of challenged sheep due to weight loss starting at 10 months postchallenge. (B) PBMC lymphocyte transformation levels after PPDj stimulation in cells from animals electively slaughtered due to clinical disease at different times after challenge (PC). (C) IFN-γ levels in blood after stimulation with PPDj for animals electively slaughtered due to clinical disease at different times postchallenge (PC). (D) Specific antibody levels to PPDj in electively slaughtered animals. Squares represent animals that developed disease early (at 11 to 12 months postchallenge [PC]). Triangles represent animals that developed disease later (at 14 to 18 months postchallenge [PC]). Mean responses ± SEM are shown for each group (n = 6).
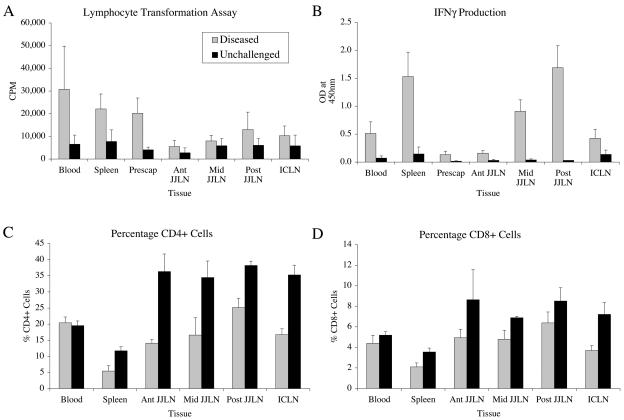
The blood, spleen, and prescapular lymph node mononuclear cells obtained at necropsy from nine animals that developed clinical disease gave strong LT responses that were greater than that found in the gut lymph nodes (Fig. 3A). When the PPDj-specific immune responses of different lymphoid tissue samples from the diseased animals were assessed, the levels seen in the blood, spleen, and prescapular (peripheral) lymph nodes were three to six times greater than those in unchallenged controls. The level of reactivity seen in the posterior jejunal lymph node cells was two times greater than that in unchallenged controls. The reactivities in the other gut lymphatic tissue cells were only marginally greater than the values from unchallenged animals.
FIG. 3.
Immune profiles for different tissues from clinically diseased or unchallenged animals following necropsy. (A) Lymphocyte proliferation following PPDj stimulation of isolated cells. (B) IFN-γ production following PPDj stimulation of isolated cells. (C) Proportions of CD4+ cells from different tissues, as detected by FACS analysis. (D) Proportions of CD8+ cells. Measurements are expressed as mean counts or percentages (n = 9 diseased and 3 unchallenged animals) plus the SEM.
The unchallenged group produced significantly lower levels of IFN-γ in vitro (P < 0.001) than those in tissues from diseased animals (Fig. 3B). Strikingly, high levels were seen in the spleen compared to the blood, but low levels were seen in the peripheral lymphatics (prescapular lymph nodes) of diseased animals. Higher levels of reactivity (IFN-γ) could be seen in the posterior jejunal lymph nodes of diseased animals, which gradually decreased in samples taken closer to the proximal end of the jejunal lymphatic chain.
There was a significantly lower (P < 0.001) percentage of CD4+ T cells (Fig. 3C) in lymphatic tissues of diseased sheep than in tissues from unchallenged controls. There was also a significantly lower percentage of CD4+ T cells in the spleen than in the other tissues examined. The gut lymph nodes from the clinically diseased animals had a lower proportion of CD4+ T cells than those from unchallenged controls. The posterior JJLN had a consistently higher percentage of CD4+ T cells than the other gut-associated lymphoid tissues from diseased animals. The percentage of CD8+ T cells was significantly lower (P < 0.001) in the lymphoid tissues of diseased sheep than in those from unchallenged controls (data not shown). The proportion of CD8+ T cells did not differ significantly between the various lymphatic tissues examined.
There were no significant differences in the percentages of B cells or γδ T cells found in tissues obtained from diseased animals compared with those from unchallenged animals (data not shown). The spleens from diseased animals tended to have the highest percentages of B cells, and the blood had the lowest percentages of B cells. The percentage of γδ T cells found in the blood was significantly different from that seen in the lymphatic tissues (P < 0.001), and the proportion found in the blood of unchallenged animals was higher (P < 0.001) than that seen in diseased animals.
Route and strain of challenge.
The lambs challenged with the primary tissue homogenate (JD3) of M. avium subsp. paratuberculosis via either the tonsillar or oral route showed a trend towards higher levels of infection and disease than those challenged orally with the laboratory cultured strain W. The high prevalence of histopathology seen early (7 months) in the animals infected by the intratonsil route was a particular feature of this experiment (Table 3). None of the animals in this study challenged with JD3 either orally or via the intratonsillar route developed clinical disease, as measured by weight loss, within the 16 months of the study. The results given in Table 3 show that primary tissue homogenates of M. avium subsp. paratuberculosis produced more predictable levels of infection and disease than did the significantly higher (20×) dose of the low-passage-number laboratory culture of M. avium subsp. paratuberculosis strain W.
TABLE 3.
Histolopathological and microbiological status of animals challenged by different infection protocolsa
| Route of infection | Strain of organism | Treatment designation | Dose (CFU) | No. of positive animals/total no. of animals (histopathology result; culture result)
|
|||
|---|---|---|---|---|---|---|---|
| Necropsied at 7 mos | Necropsied at 12 mos | Necropsied at 14 to 16 mos | Total | ||||
| Intra tonsill | JD3 (direct tissue homogenate) | JD3-IT | 5 × 107 | 5/7, 7/7 | 1/2, 0/2 | 1/3, 1/3 | 7/12, 8/12 |
| Oral | JD3 (direct tissue homogenate) | JD3-O | 5 × 107 | 4/6, 6/6 | 1/2, 0/2 | 4/4, 2/4 | 9/12, 8/12 |
| Oral | W strain (laboratory cultured) | W-O | 1 × 109 | 1/6, 3/6 | 0/2, 0/2 | 0/4, 0/4 | 1/12, 3/12 |
Each group received two doses at 1 month apart. No animals had clinical disease, as measured by weight loss.
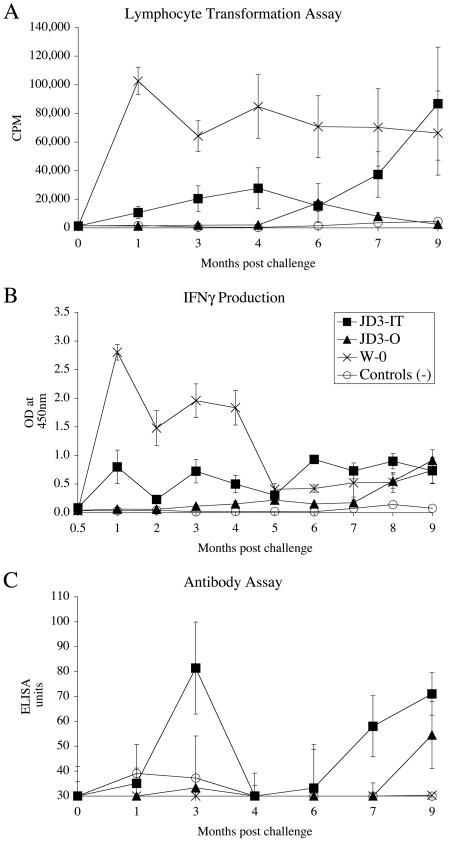
The immunological profiles (Fig. 4A) of animals exposed to different infection protocols showed distinct patterns. The animals challenged orally with the tissue homogenate of JD3 (JD3-O group) did not elicit LT responses much greater than those seen in the unchallenged control animals. The animals exposed to tissue homogenates of bacteria via the intratonsillar route (JD3-IT group) gave a stronger response than the animals challenged with a similar dose of bacteria by the oral route, especially in the first 4 months postchallenge. The animals challenged orally with the laboratory cultured isolate W (W-O group) gave consistently high LT reactivities from 1 month postchallenge and throughout the duration of the experiment.
FIG. 4.
Immune profiles of animals after experimental challenge via the tonsil with the JD3 tissue homogenate (JD3-IT), represented by closed squares. Animals challenged orally with the tissue homogenate JD3 (JD3-O) are represented by closed triangles. Animals challenged orally with the W strain (W-O) are represented by crosses, and unchallenged controls are represented by open circles. (A) Mean lymphoproliferative responses of PBMC to PPDj. (B) IFN-γ responses in whole blood after stimulation with PPDj. (C) Plasma antibody levels specific to PPDj, as measured by ELISA. Mean responses ± SEM are shown (n = 12).
The IFN-γ response of the W-O group was higher than that of any of the other treatment groups (Fig. 4B). The animals in this group showed a strong response within 1 month postchallenge, which began to decrease after 5 months to the levels seen in the other challenged groups. This pattern mirrored the LT responses shown in Fig. 4A, except that the IFN-γ levels dropped to low levels after 5 months. Animals exposed via the tonsillar route (JD3-IT group) produced a stronger response than JD3-O group animals and the unchallenged control animals.
The specific antibody profiles (Fig. 4C) show that the JD3-IT group produced more antibody than the other treatment groups, with a peak at 3 months and again at 7 months onwards. Animals in the JD3-O group showed increasing levels of antibody at 9 months postchallenge. The unchallenged controls or animals in the W-O group produced minimal amounts of antibody throughout the experiment.
DISCUSSION
The main objective of this study was to develop a reliable, standardized M. avium subsp. paratuberculosis infection model in sheep that would predictably produce clinical Johne's disease and which could be used to evaluate new vaccination protocols and diagnostic assays. Published data using sheep as a model have used strains of M. avium subsp. paratuberculosis sourced from cattle (24), deer (3, 4), and sheep (20, 40). This may explain in part the differing outcomes, as bovine and ovine strains of M. avium subsp. paratuberculosis are known to be phenotypically different in vivo (31, 41) and to express different properties when grown in vitro (45). A feature of culturing the laboratory strain (W) was that its doubling time decreased dramatically with continuous passaging. To date, very few experimental infection studies with sheep have used typed ovine strains of M. avium subsp. paratuberculosis. Reddacliff and Whittington (40) recently used a typed ovine strain of M. avium subsp. paratuberculosis to infect merino lambs. Whereas they established infection consistently, the animals were slaughtered at 14 weeks postchallenge, prior to the development of gross or microscopic lesions.
The model used here resulted in infection in a high proportion of experimentally challenged animals, with typical histopathology and clinical disease. The initial experiment was designed to study the chronological development of infection, histopathological lesions, and clinical disease after challenge (at 6, 9, and 10 months). The findings from this study are compatible with the results obtained by Reddacliff and Whittington (40), who used merino lambs experimentally challenged with 7 × 107 CFU of an ovine strain of M. avium subsp. paratuberculosis, from which they isolated M. avium subsp. paratuberculosis from a range of tissues at 7 to 14 weeks postchallenge. Other experimental challenge studies reported that histological lesions may be found in the first few months following challenge (24). This was not seen in the present study or that published by Reddacliff and Whittington (40). The development of lesions within months of experimental infection may require higher doses to infect the sheep or the use of the bovine strain of M. avium subsp. paratuberculosis for experimental challenge. Although the doses used in the present experiments were higher than those used by Reddacliff and Whittington (40), there was no histopathology or clinical Johne's disease seen 6 months following challenge. The high infection rates seen in the gut tissues of animals at 9 and 10 months postchallenge would imply that animals necropsied at 6 months postchallenge were likely to have been actively infected.
A second study used moderate doses of M. avium subsp. paratuberculosis (>108 CFU) to establish the time course for the onset of clinical disease. While disease was evident from 11 months onwards, a small proportion (20%) of animals showed no signs of clinical disease at 22 months postchallenge. These may represent a subgroup of innately resistant animals within the challenged group. No infection or disease was seen using a high-dose challenge (1010 CFU) with a continuously passaged laboratory culture of M. avium subsp. paratuberculosis (W).
Challenges using different routes of inoculation resulted in infection and disease similar to those reported previously (24). Oral inoculations using primary tissue homogenates of M. avium subsp. paratuberculosis (JD3) produced results similar to those seen with other experimental infection models (20-22). The intratonsillar route of infection produced a pathology typical of Johne's disease, as seen by histological analysis of the intestine and associated lymph nodes. This suggested that while lymphatic spread had occurred, there was a propensity for M. avium subsp. paratuberculosis to traffic to the intestines. There is evidence from deer naturally infected with M. avium subsp. paratuberculosis that the retropharyngeal lymph nodes may become infected with M. avium subsp. paratuberculosis (14). Earlier studies using an experimental Mycobacterium bovis infection of deer (19) have established that the draining retropharyngeal lymph nodes serve as a primary target of infection following installation of bacteria into the tonsillar crypt.
Lambs experimentally challenged orally with moderate doses of the tissue homogenate (JD3) of M. avium subsp. paratuberculosis showed no LT reactivity until 6 months postchallenge. Experimental challenge studies with sheep (bighorn × mouflon hybrid or domestic sheep given 50 mg [wet weight] M. avium subsp. paratuberculosis) have shown increased LT reactivity 5 months after challenge (46). Antibody levels in animals exposed orally to medium or high doses of tissue homogenates of M. avium subsp. paratuberculosis remained at background levels until at least 6 to 9 months postchallenge. Peak levels of antibody were recorded at 9 months postinfection (Fig. 2D and 4C) for challenged sheep, just before clinical disease became evident. The elective removal of animals showing clinical signs (weight loss) may have eliminated animals producing large amounts of antibody associated with disease. Recent literature has shown that sheep experimentally challenged with ovine and bovine strains of M. avium subsp. paratuberculosis that develop clinical disease have low antibody levels (41). The IFN-γ test was the first assay to detect sensitization in sheep orally challenged with the primary tissue homogenate of M. avium subsp. paratuberculosis. The observation in this study of an early IFN-γ response followed later by antibody and LT responses is comparable to previously published data on the kinetics of immune reactivity to M. avium subsp. paratuberculosis infections in cattle and goats (5, 40, 42, 44). Unchallenged control animals showed increased immune reactivity between 8 and 15 months into the experiment. The most logical explanation was that the sheep may have been exposed to large numbers of environmental mycobacteria associated with seasonal changes and feeding rotations in the winter-spring period (September to October).
The infection studies which compared the challenge of sheep with M. avium subsp. paratuberculosis (JD3) isolated from tissue homogenates to that with the cultured strain (W) showed that high LT and IFN-γ responses occurred within 2 months postchallenge in the absence of antibody. The protective immune response to M. avium subsp. paratuberculosis is considered to require strong cell-mediated immunity (CMI) and low levels of antibody (10), which is similar to the response seen in sheep challenged with laboratory cultures of M. avium subsp. paratuberculosis (W). Although three of the animals challenged with a low-passage culture of M. avium subsp. paratuberculosis (W) were culture positive and one had histopathology, they had similar immune profiles to the remainder of the group that were challenged but uninfected. Stewart et al. (41) observed that sheep challenged with cultured ovine M. avium subsp. paratuberculosis did not become infected and had lower immune responses, as measured by IFN-γ and absorbed ELISA, than sheep challenged with tissue homogenates (44).
Published data show that different routes of experimental challenge result in different patterns of immune reactivity. Intravenously and intratracheally challenged animals produce increased serological titers compared to those in animals exposed orally (33). The immune responses seen for animals challenged by the intratonsillar route (JD3-IT group) were higher overall than those found for the orally challenged (JD3-O) group (Fig. 4). Eight of the nine sheep that developed clinical disease (Fig. 3) following challenge with tissue homogenates of M. avium subsp. paratuberculosis (JD3) had multibacillary lesions at necropsy. Previously published data suggest that sheep with multibacillary disease produce minimal CMI responses (8, 9, 12, 13, 38). These findings do not correlate with the results obtained in the present study. The mean peripheral blood LT response at necropsy from the experimentally challenged diseased sheep had a stimulation index of 18, in contrast with earlier findings (9), where naturally infected (multibacillary) animals had a stimulation index of 2.7. In the previous study, only one section of the mesenteric (jejunal) lymph node was examined, whereas the results from the present study show that different regions within the jejunal lymph node gave dramatically different immune readouts. This study corroborates independent findings that clinically diseased animals have lower numbers of CD4+ T cells and a reduction in the CMI response in gut lymphatics (11, 25). Diseased animals in the present study had higher LT and IFN-γ responses in the posterior JJLN than the other regions of the jejunal lymph nodes. This suggests that the specific immune response associated with Johne's disease is greatest at the site where the most severe lesions occur in the gut lymph nodes. The localized increase in Johne's disease-specific CMI responses can be correlated with the region with the largest number of CD4+ T cells. M. avium subsp. paratuberculosis-reactive CD4+ T cells from within the gut or peripheral lymphatics may migrate to the more severely affected sites (posterior JJLN) in an attempt to contain the infection (6, 28-30, 32). The increased levels of IFN-γ seen in gut lymph nodes could be explained by these tissues containing larger numbers of johnin-reactive CD4+ T cells. However the number of CD25+ T cells (activated T cells) present in these tissues did not increase, as would be expected if a large number of johnin-reactive CD4+ T cells had either proliferated in or trafficked into the lymph node with the most severe disease. Although CD25 expression is variable in chronic infections such as those with M. avium subsp. paratuberculosis (42), it should be expected that a small increase in this cell population would be seen with increased numbers of reactive CD4+ T cells in the diseased lymph node.
This study has resulted in the development of a robust experimental sheep model in which Johne's disease occurs in a large proportion of challenged animals. Critical time points for the establishment of infection or disease have been determined. This model can be applied in the future to evaluate the protective efficacy of vaccines or to more critically chart immunological profiles that are associated with infection, disease, or protective immunity. It may also be relevant to the development of improved diagnostic tests to identify patterns of immunity during the early stages of infection with M. avium subsp. paratuberculosis.
Acknowledgments
We thank the following individuals for their contributions to this work: Phil Farquhar (AgResearch Invermay) for animal husbandry, Gary Clark and Dylan Turner for histology, Pam Crosbie (University of Otago) and Gary Yates (AgResearch Wallaceville) for culture of M. avium subsp. paratuberculosis, and Peter Johnstone (AgResearch Invermay) for statistical analysis of the data.
This research was supported by grants from the Foundation of Research, Science & Technology (FoRST) and the University of Otago Research Committee.
Editor: J. L. Flynn
REFERENCES
- 1.Armstrong, M. C. 1956. Johne's disease of sheep in the South Island of New Zealand. N. Z. Vet. J. 4:56-59. [Google Scholar]
- 2.Bakker, D., P. T. Willemsen, and F. G. van Zijderveld. 2000. Paratuberculosis recognized as a problem at last: a review. Vet. Q. 22:200-204. [DOI] [PubMed] [Google Scholar]
- 3.Begara-McGorum, I., L. A. Wildblood, C. J. Clarke, K. M. Connor, K. Stevenson, C. J. McInnes, J. M. Sharp, and D. G. Jones. 1998. Early immunopathological events in experimental ovine paratuberculosis. Vet. Immunol. Immunopathol. 63:265-287. [DOI] [PubMed] [Google Scholar]
- 4.Begara-McGorum, I. M., L. A. Wildblood, and D. G. Jones. 1997. Early immune events following experimental infection of lambs with Mycobacterium avium subspecies paratuberculosis. Biochem. Soc. Trans. 25:279S. [DOI] [PubMed] [Google Scholar]
- 5.Billman-Jacobe, H., M. Carrigan, F. Cockram, L. A. Corner, I. J. Gill, J. F. Hill, T. Jessep, A. R. Milner, and P. R. Wood. 1992. A comparison of the interferon gamma assay with the absorbed ELISA for the diagnosis of Johne's disease in cattle. Aust. Vet. J. 69:25-28. [DOI] [PubMed] [Google Scholar]
- 6.Bradley, L. M., and S. R. Watson. 1996. Lymphocyte migration into tissue: the paradigm derived from CD4 subsets. Curr. Opin. Immunol. 8:312-320. [DOI] [PubMed] [Google Scholar]
- 7.Brotherston, J. G., N. J. Gilmour, and J. Samuel. 1961. Quantitative studies of Mycobacterium johnei in the tissues of sheep. J. Comp. Pathol. 71:286-299. [DOI] [PubMed] [Google Scholar]
- 8.Burrells, C., C. J. Clarke, A. Colston, J. M. Kay, J. Porter, D. Little, and J. M. Sharp. 1999. Interferon-gamma and interleukin-2 release by lymphocytes derived from the blood, mesenteric lymph nodes and intestines of normal sheep and those affected with paratuberculosis (Johne's disease). Vet. Immunol. Immunopathol. 68:139-148. [DOI] [PubMed] [Google Scholar]
- 9.Burrells, C., C. J. Clarke, A. Colston, J. M. Kay, J. Porter, D. Little, and J. M. Sharp. 1998. A study of immunological responses of sheep clinically-affected with paratuberculosis (Johne's disease): the relationship of blood, mesenteric lymph node and intestinal lymphocyte responses to gross and microscopic pathology. Vet. Immunol. Immunopathol. 66:3-4. [DOI] [PubMed] [Google Scholar]
- 10.Chiodini, R. J. 1996. Immunology: resistance to paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 12:313-343. [DOI] [PubMed] [Google Scholar]
- 11.Chiodini, R. J., and W. C. Davis. 1993. The cellular immunology of bovine paratuberculosis: immunity may be regulated by CD4 super(+) helper and CD8 super(+) immunoregulatory T lymphocytes which down-regulate gamma/delta super(+) T-cell cytotoxicity. Microb. Pathog. 14:355-367. [DOI] [PubMed] [Google Scholar]
- 12.Clarke, C. J., A. Colston, D. Little, J. Kay, H. M. Alzuherri, J. M. Sharp, and C. Burrells. 1996. The immune response in paratuberculosis infection of small ruminants. Vet. Immunol. Immunopathol. 54:1-4.9082648 [Google Scholar]
- 13.Clarke, C. J., and D. Little. 1996. The pathology of ovine paratuberculosis: gross and histological changes in the intestine and other tissues. J. Comp. Pathol. 114:419-437. [DOI] [PubMed] [Google Scholar]
- 14.de Lisle, G. W., G. F. Yates, and R. H. Montgomery. 2003. The emergence of Mycobacterium paratuberculosis in farmed deer in New Zealand—a review of 619 cases. N. Z. Vet. J. 51:58-62. [DOI] [PubMed] [Google Scholar]
- 15.Gilmour, N. J., D. I. Nisbet, and J. G. Brotherston. 1965. Experimental oral infection of calves with Mycobacterium johnei. J. Comp. Pathol. 75:281-286. [DOI] [PubMed] [Google Scholar]
- 16.Gilmour, N. J. L., K. W. Angus, and B. Mitchell. 1977. Intestinal infection and host response to oral administration of Mycobacterium johnei in sheep. Vet. Microbiol. 2:223-235. [Google Scholar]
- 17.Gilmour, N. J. L., and J. F. C. Nyange. 1989. Paratuberculosis (Johne's disease) in deer. Practice 11:193-196. [Google Scholar]
- 18.Griffin, J. F. T., J. P. Cross, D. N. Chinn, C. R. Rodgers, and G. S. Buchan. 1994. Diagnosis of tuberculosis due to Mycobacterium-bovis in New Zealand red deer (Cervus-elaphus) using a composite blood-test and antibody-assays. N. Z. Vet. J. 42:173-179. [DOI] [PubMed] [Google Scholar]
- 19.Griffin, J. F. T., C. G. Mackintosh, and G. S. Buchan. 1995. Animal-models of protective immunity in tuberculosis to evaluate candidate vaccines. Trends Microbiol. 3:418-424. [DOI] [PubMed] [Google Scholar]
- 20.Gwozdz, J. M., and K. G. Thompson. 2002. Antigen-induced production of interferon-gamma in samples of peripheral lymph nodes from sheep experimentally inoculated with Mycobacterium avium subsp. paratuberculosis. Vet. Microbiol. 84:243-252. [DOI] [PubMed] [Google Scholar]
- 21.Gwozdz, J. M., K. G. Thompson, and B. W. Manktelow. 2001. Lymphocytic neuritis of the ileum in sheep with naturally acquired and experimental paratuberculosis. J. Comp. Pathol. 124:317-320. [DOI] [PubMed] [Google Scholar]
- 22.Gwozdz, J. M., K. G. Thompson, B. W. Manktelow, A. Murray, and D. M. West. 2000. Vaccination against paratuberculosis of lambs already infected experimentally with Mycobacterium avium subspecies paratuberculosis. Aust. Vet. J. 78:560-566. [DOI] [PubMed] [Google Scholar]
- 23.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kluge, J. P., R. S. Merkal, W. S. Monlux, A. B. Larsen, K. E. Kopecky, F. K. Ramsey, and R. P. Lehmann. 1968. Experimental paratuberculosis in sheep after oral, intratracheal, or intravenous inoculation lesions and demonstration of etiologic agent. Am. J. Vet. Res. 29:953-962. [PubMed] [Google Scholar]
- 25.Koets, A., V. Rutten, A. Hoek, F. van Mil, K. Muller, D. Bakker, E. Gruys, and W. van Eden. 2002. Progressive bovine paratuberculosis is associated with local loss of CD4(+) T cells, increased frequency of gamma delta T cells, and related changes in T-cell function. Infect. Immun. 70:3856-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen, A. B., R. S. Merkal, and R. C. Cutlip. 1975. Age of cattle as related to resistance to infection with Mycobacterium paratuberculosis. Am. J. Vet. Res. 36:255-257. [PubMed] [Google Scholar]
- 27.Larsen, A. B., J. M. Miller, and R. S. Merkal. 1977. Subcutaneous exposure of calves to Mycobacterium paratuberculosis compared with intravenous and oral exposures. Am. J. Vet. Res. 38:1669-1671. [PubMed] [Google Scholar]
- 28.Mackay, C. R. 2000. Follicular homing T helper (Th) cells and the Th1/Th2 paradigm. J. Exp. Med. 192:F31-F34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackay, C. R. 1993. Homing of naive, memory and effector lymphocytes. Curr. Opin. Immunol. 5:423-427. [DOI] [PubMed] [Google Scholar]
- 30.Mackay, C. R. 1992. Migration pathways and immunologic memory among T lymphocytes. Semin. Immunol. 4:51-58. [PubMed] [Google Scholar]
- 31.Marsh, I., R. Whittington, and D. Cousins. 1999. PCR-restriction endonuclease analysis for identification and strain typing of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium based on polymorphisms in IS1311. Mol. Cell. Probes 13:115-126. [DOI] [PubMed] [Google Scholar]
- 32.Meeusen, E. N. 1998. Differential migration of Th1 and Th2 cells—implications for vaccine and infection studies. Vet. Immunol. Immunopathol. 63:157-166. [DOI] [PubMed] [Google Scholar]
- 33.Merkal, R. S., A. B. Larsen, K. E. Kopecky, J. P. Kluge, W. S. Monlux, R. P. Lehmann, and L. Y. Quinn. 1968. Experimental paratuberculosis in sheep after oral, intratracheal, or intravenous inoculation: serologic and intradermal tests. Am. J. Vet. Res. 29:963-969. [PubMed] [Google Scholar]
- 34.Merkal, R. S., W. S. Monlux, J. P. Kluge, A. B. Larsen, K. E. Kopecky, L. Y. Quinn, and R. P. Lehmann. 1968. Experimental paratuberculosis in sheep after oral, intratracheal, or intravenous inoculation: histochemical localization of dehydrogenase activities. Am. J. Vet. Res. 29:971-982. [PubMed] [Google Scholar]
- 35.Nisbet, D. I., N. J. Gilmour, and J. G. Brotherston. 1962. Quantitative studies of Mycobacterium johnei in tissues of sheep. III. Intestinal histopathology. J. Comp. Pathol. 72:80-91. [DOI] [PubMed] [Google Scholar]
- 36.Patterson, H. D., and R. Thompson. 1971. Recovery of inter-block information when block sizes are unequal. Biometrika 58:545-554. [Google Scholar]
- 37.Perez, V., J. F. Garcia Marin, and J. J. Badiola. 1996. Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. J. Comp. Pathol. 114:107-122. [DOI] [PubMed] [Google Scholar]
- 38.Perez, V., J. Tellechea, J. M. Corpa, M. Gutierrez, and J. F. Garcia Marin. 1999. Relation between pathologic findings and cellular immune responses in sheep with naturally acquired paratuberculosis. Am. J. Vet. Res. 60:123-127. [PubMed] [Google Scholar]
- 39.Rankin, J. D. 1959. The estimation of doses of Mycobacterium johnei suitable for the production of Johne's disease in cattle. J. Pathol. Bacteriol. 77:638-642. [DOI] [PubMed] [Google Scholar]
- 40.Reddacliff, L. A., and R. J. Whittington. 2003. Experimental infection of weaner sheep with S strain Mycobacterium avium subsp. paratuberculosis. Vet. Microbiol. 96:247-258. [DOI] [PubMed] [Google Scholar]
- 41.Stewart, D. J., J. A. Vaughan, P. L. Stiles, P. J. Noske, M. L. Tizard, S. J. Prowse, W. P. Michalski, K. L. Butler, and S. L. Jones. 2004. A long-term study in Merino sheep experimentally infected with Mycobacterium avium subsp. paratuberculosis: clinical disease, faecal culture and immunological studies. Vet. Microbiol. 104:165-178. [DOI] [PubMed] [Google Scholar]
- 42.Storset, A. K., H. J. Hasvold, M. Valheim, H. Brun-Hansen, G. Berntsen, S. K. Whist, B. Djonne, C. M. Press, G. Holstad, and H. J. Larsen. 2001. Subclinical paratuberculosis in goats following experimental infection. An immunological and microbiological study. Vet. Immunol. Immunopathol. 80:271-287. [DOI] [PubMed] [Google Scholar]
- 43.Wald, A. 1943. Tests of statistical hypotheses concerning several parameters when the number of observations is large. Trans. Am. Math. Soc. 54:426. [Google Scholar]
- 44.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whittington, R. J., I. Marsh, S. McAllister, M. J. Turner, D. J. Marshall, and C. A. Fraser. 1999. Evaluation of modified BACTEC 12B radiometric medium and solid media for culture of Mycobacterium avium subsp. paratuberculosis from sheep. J. Clin. Microbiol. 37:1077-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams, E. S., J. C. DeMartini, and S. P. Snyder. 1985. Lymphocyte blastogenesis, complement fixation, and fecal culture as diagnostic tests for paratuberculosis in North American wild ruminants and domestic sheep. Am. J. Vet. Res. 46:2317-2321. [PubMed] [Google Scholar]