Abstract
Noninvasive mucosal vaccines are attractive alternatives to parenteral vaccines. Although the conjugation of vaccine antigens with the B subunit of cholera toxin (CTB) is one of the most promising strategies for vaccine delivery to mucosal immune systems, the molecule cannot tolerate large-protein fusion, as it severely impairs pentamerization and loses affinity for GM1-ganglioside. Here we report a new strategy, in which steric hindrance between CTB-antigen fusion subunits is significantly reduced through the integration of unfused CTB “molecular buffers” into the pentamer unit, making them more efficiently self-assemble into biologically active pentamers. In addition, the chimeric protein took a compact configuration, becoming small enough to be secreted, and one-step affinity-purified proteins, when administered through a mucosal route, induced specific immune responses in mice. Since our results are not dependent on the use of a particular expression system or vaccine antigen, this strategy could be broadly applicable to bacterial enterotoxin-based vaccine design.
Mucosal immunizations through the oral or nasal route have recently attracted much attention because of their ease of administration and the ability to induce protective immunity, particularly against mucosal pathogens (17, 20, 33, 38). However, it has been reported by many investigators that intranasal or oral delivery of recombinant vaccines without the use of a delivery vehicle or mucosal adjuvant like cholera toxin (CT), heat-labile enterotoxin (LT) of Escherichia coli, or pertussis toxin often resulted in extremely weak or no immune response, emphasizing the necessity of mucosal adjuvants or delivery vehicles for efficient induction of an immune response. On the contrary, coadministration of recombinant vaccine antigens with such powerful mucosal adjuvants significantly augmented the immune response, often at comparable or even higher levels in comparison with parenteral vaccines. However, their enterotoxicity or potential hazardous effects on olfactory nerves have raised safety concerns for clinical use of these adjuvants (13).
One approach to circumvent the toxicity problem associated with the holotoxin is to use the completely nontoxic B subunit of these toxins: its safety has been evaluated in clinical trials (24). The B subunit of CT (CTB) and LT (LTB) are highly efficient carrier molecules for chemically or genetically conjugated antigens for eliciting mucosal and systemic antibody responses (3), as well as mucosal tolerance for prophylactic vaccines against autoimmune diseases (1, 29, 39). Conjugation greatly reduces the dose required for T-cell activation by more than 10,000-fold, compared with free antigen (12). In addition, the efficient antigen carrier effect of CTB for enhancement of mucosal and systemic antibody responses was not limited to in vivo vaccination regimens; CTB's dendritic cell (DC)-activating capacity mediated by upregulation of the major histocompatibility complex and secondary costimulatory molecules such as CD80 and CD86 on DC surfaces, as well as proinflammatory cytokine secretion, may provide a new potential technology for ex vivo DC-mediated vaccines (11). The precise mechanism underlining the immunostimulatory effect of CTB on DCs was not fully elucidated, but it is mainly associated with its binding affinity for the GM1-ganglioside receptor present on most nucleated cells including DCs (22). Although there is a large body of evidence indicating the advantages of conjugating antigens with CTB, it is also true for CT that conjugation further enhances the immune responses compared with administration of CT and antigen as a mixture (10, 11).
Genetic conjugation of vaccine antigens with CTB (or LTB) seems to be more convenient than chemical conjugation, which requires purification of two independent antigens and a subsequent chemical reaction for the linkage, followed by purification of the conjugated peptides. Small epitopes can be easily fused to the N or C terminus of the CTB molecule as secreted proteins, depending on the expression systems (9, 23, 31, 39), or they can even be embedded in the molecule without affecting pentamerization (12). In addition, CTB could also serve as a protein tag, similar to other frequently used tags such as GST, to stably express small oligopeptides as fusion proteins and to increase their water solubility. However, from a recombinant vaccine designing point of view, it is often desirable to conjugate a large oligopeptide to provide as many still-uncharacterized epitopes potentially involved in protective immunity as possible or to provide conformational epitopes only retainable as intact full-length proteins. Unfortunately, large-protein fusion to the N terminus was unsuccessful (29, 30), and large-polypeptide fusion with a molecular weight larger than CTB itself has been difficult even at its C terminus. Interference of pentamer assembly due to bulky antigen fusion seemed to be the major obstacle for genetic conjugation, because pentamerization is required to gain binding affinity for GM1-ganglioside, an essential property for CTB to serve as an effective antigen delivery vehicle. To overcome this hurdle, several investigators made a successful genetic conjugation of vaccine antigens with the A2 subunit of cholera toxin to construct holotoxin-like antigen-A2/CTB chimeric proteins, in which the antigen-A2 fusion protein binds noncovalently via the A2 alpha-helix structure to the central hole of the CTB pentamer (14). Although this type of chimera was reported to be effective for eliciting mucosal as well as systemic immunity when administered through mucosal routes (15, 26, 32, 33), low assembly efficiency between the A2 fusion and the CTB pentamer was often unable to produce intact holotoxin-like proteins in both in vivo and in vitro assembly systems (18, 37).
Here we demonstrated a potential new strategy, in which steric hindrance between each subunit of the CTB-antigen fusion molecule is significantly reduced, making the fusion proteins more efficiently self-assemble into biologically active pentamers in vivo. This enhanced pentamer assembly was achieved through the introduction of unfused free CTB “molecular buffers” into the pentamer unit, allowing the fusion molecules to assemble into pentamers within transformed cells and to assume a more compact configuration, becoming small enough to be secreted into culture medium from the host cells. The secretory nature of the heteropentameric CTB chimeric fusion construct made the antigen purification step much easier than steps required for intracellularly expressed antigens and allowed the circumvention of a tedious stepwise denaturation-renaturation process for assembly and of several subsequent purification steps usually required for intracellularly expressed antigens. Only a one-step affinity tag-based purification step under mild conditions can produce recombinant proteins that retain configurations as closely conserved as their native forms.
In this study we demonstrated the feasibility of the heteropentameric CTB chimeric fusion strategy by using a yeast Pichia pastoris gene expression system as a model system. P. pastoris cotransformed with two gene expression cassettes: one for CTB conjugated with a model vaccine antigen, the domain III of the Japanese encephalitis (JE) virus E glycoprotein, and another for the unfused CTB. Recombinant P. pastoris produced a heteropentameric CTB chimeric fusion protein as a secretory molecule, and the purified protein, when administered through the mucosal or parenteral route, induced JE virus-neutralizing serum antibodies. Since our results are not likely to be dependent on the use of a particular expression system or recombinant vaccine antigen, we expect that this strategy would broaden the applicability of bacterial enterotoxin subunit-based vaccines against infectious diseases.
MATERIALS AND METHODS
Construction of recombinant P. pastoris plasmid expression vectors for CTB and its fusion genes.
To construct P. pastoris expression vectors, CTB or CTB-antigen fusion genes were inserted downstream of the methanol-inducible AOX1 promoter of pAO815 (Invitrogen). A full-length CTB gene with a 375-bp open reading frame was PCR amplified from plasmid pM4 containing the CTA and CTB genes (a kind gift from Hiroshi Kiyono at the University of Tokyo) with primer pairs containing MunI restriction enzyme recognition sequences to generate cohesive ends compatible with an EcoRI recognition sequence. To enhance gene expression efficiency in eukaryotic cells, nucleotide sequences flanking the initiation codon were altered to the Kozac sequence (ACCATGG), except for the G immediately following the initiation codon (underlined); this residue was kept as A to have the original isoleucine instead of valine in the second amino acid of the full-length native CTB protein. The amplified fragment was inserted into the unique EcoRI site of the plasmid pAO815 to construct plasmid pB. The predicted amino acid sequence of the cloned CTB gene was identical to the B subunit of cholera toxin derived from Vibrio cholerae classical biotype 569B (GenBank accession no. U25679).
To construct CTB-antigen fusion gene expression vectors, the CTB gene was PCR amplified with the same 5′ primer used to construct the plasmid pB and a 3′ primer containing the hinge-encoding sequence (Gly-Pro-Gly-Pro) and MunI recognition site. The 3′ primer also contained an EcoRI recognition sequence between the hinge sequence and MunI recognition sequence. Insertion of the PCR-amplified fragment digested with MunI into the unique EcoRI site of plasmid pAO815 generated plasmid pBh, containing the full-length CTB gene fused in frame with the hinge-encoding sequence, the unique EcoRI site, and the stop codon. The C-terminal one-third of the E glycoprotein domain III reported to induce JE virus neutralization antibodies (6, 25, 35, 36) was amplified by reverse transcription-PCR (RT-PCR) from the JE virus RNA genome and inserted into the unique EcoRI site immediately downstream of the hinge-encoding sequence of plasmid pBh to construct plasmid pB:E, which encodes the CTB-JE virus E glycoprotein fusion with a predicted molecular mass of 33 kDa.
For the attempt to produce heteropentameric CTB chimeric fusion proteins, a P. pastoris multigene expression plasmid was constructed for coexpression of the CTB-E glycoprotein fusion gene and unfused CTB gene. The entire CTB gene expression cassette, obtained by double digestion of the plasmid pB with BglII and BamHI, was inserted into the unique BamHI site of plasmid pB:E to construct plasmid pB:E/B. The orientation of the two expression cassettes within plasmid pB:E/B was determined, and the plasmid having the two cassettes in the same orientation was used for yeast transformation and subsequent protein expression experiments.
To fuse six-His tags with the heteropentameric constructs for purification of the chimera from the culture supernatant, additional 5′ and 3′ PCR primers containing the six-His tag-encoding sequence (CATCATCATCATCATCAT) and the sequence specific for the domain III-encoding regions were synthesized. PCR with the 5′ primer with the His-tag-encoding sequence and the 3′ primer without the tag sequence previously used for the domain III amplification generated a fragment of a six-His domain III fusion gene. The E glycoprotein domain III-encoding sequence was removed from plasmid pB:E/B by digesting the plasmid with EcoRI, and it was replaced with the six-His domain III fusion fragment to generate plasmid pB:E-His/B, which includes an internal His tag immediately downstream of the hinge (GPGP). Similarly, a combination of the 5′ and the 3′ primers both containing His-tag-encoding sequences generated plasmid pB:E-His/B, containing both the internal and the C-terminal His tags. His tags were added only to the CTB-E glycoprotein fusion gene, but not to the unfused CTB gene, for purification of only the CTB pentamer proteins carrying E glycoprotein domain III, thus minimizing contamination with CTB pentamers without the fused proteins.
For construction of a CTB gene fused with an oligonucleotide encoding a T-cell epitope of human insulin B chain (B:9-23), two single-stranded oligonucleotides were synthesized and annealed to produce double-stranded oligonucleotides for insertion into the unique EcoRI site of the plasmid pBh to construct plasmid pB:(B:9-23) with one epitope and plasmid pB:(B:9-23)2 with two epitopes connected in tandem.
DNA sequences of the CTB and its fusion genes were confirmed by a DNA sequencer (ABI Genetic Analyzer 3100), and each construct was introduced into P. pastoris GS115 by electroporation (1.5 kV, 200 Ω, 25 μF) (Gene Pulser; Bio-Rad) after the plasmid vectors were linearized by SalI digestion to enhance integration of the expression cassettes into the yeast chromosome by homologous recombination, according to the manufacturer's instructions (Invitrogen). Stable transgene integration was confirmed by PCR with a gene-specific primer set by using transformed yeast genomic DNA preparations as templates. Transcription of the integrated genes was also confirmed by RT-PCR with the same primer set by using total RNA preparations as templates.
Expression analysis of heteropentameric chimeric fusion proteins.
Integrated genes were expressed by methanol induction from transformed P. pastoris GS115 according to the manufacturer's instructions (Invitrogen). For analysis of intracellularly accumulated recombinant proteins, yeast cell numbers were estimated (1 optical density at 600 nm [OD600] unit is ∼5 × 107 cells/ml), and cells were lysed by incubating the cell pellet with a yeast protein extraction reagent (Pierce) supplemented with dithiothreitol (final concentration, 0.1 M) and phenylmethylsulfonyl fluoride (final concentration, 1 mM) at room temperature for 30 min with occasional vortexing, according to the manufacturer's instructions. Samples were centrifuged at 18,000 × g for 10 min to remove insoluble debris, and the supernatant was used as a recombinant protein source for analysis. To determine whether expressed gene products were secreted into culture medium, culture supernatant was directly subjected to 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For analysis of monomeric CTB fusion proteins, the samples were boiled for 3 min prior to SDS-PAGE analysis. The CTB moieties of blotted protein bands were recognized by using a 1:1,000 dilution of rabbit anti-cholera toxin antiserum (Sigma) and a 1:1,000 dilution of alkaline phosphatase-conjugated anti-rabbit immunoglobulin (Sigma), followed by colorimetric detection with alkaline phosphatase substrate (Sigma). The E glycoprotein moieties of the chimeric proteins were similarly recognized by anti-JE virus rabbit immune serum and the anti-rabbit antibody. The anti-JE immune serum was prepared by immunizing a rabbit intramuscularly twice with the formalin-inactivated JE vaccine with a 1-month interval, and 1 week after the second immunization, the animal was sacrificed for preparation of the immune serum.
A GM1-enzyme-linked immunosorbent assay (GM1-ELISA) was conducted to determine the expression levels and specific binding affinity of CTB and its fusion proteins for GM1-ganglioside, a natural receptor of cholera toxin. Briefly, a 96-well microtiter plate (Sumitomo Bakelite Co., Ltd.) was coated with 5 μg/ml of monosialoganglioside GM1 (Sigma) diluted in bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3; 50 μl/well), pH 9.6, and the plates were incubated at 4°C overnight. After wells were washed with PBST (phosphate-buffered saline with 0.05% Tween 20) three times, wells were blocked with PBS containing 1% bovine serum albumin (BSA). Crude yeast cell extract or culture supernatant was applied to wells and washed with PBST, and the same primary and secondary antibody sets used for detection of CTB or E glycoprotein by Western blotting were used. Plates were incubated at 37°C for 20 min after the addition of alkaline phosphatase substrate (Sigma) to wells, and the OD415 was measured by microplate reader (Bio-Rad Model 680). CTB pentamer protein expression levels were estimated based on the standard curves generated by serial dilutions of known amounts of CTB protein (Sigma). Statistical significance of the differences in protein expression levels was determined by a Mann-Whitney U test.
For obtaining immunization materials, heteropentameric CTB chimeric fusion proteins fused with six-histidine tags were purified from the culture supernatant by using a Ni2+ column, according to the manufacturer's instructions (Amersham).
Animals, immunization, and sample collection.
Groups of five to seven female BALB/c mice at the age of 6 weeks (SEAC, Inc., Japan) were immunized with the heteropentameric CTB chimeric fusion proteins purified by the Ni2+ column either through the intranasal, subcutaneous, or intraperitoneal route, with or without the use of incomplete Freund's adjuvant (IFA) or CT (Sigma). One microgram and 0.05 μg of CT were used for intranasal and subcutaneous immunization, respectively. For intranasal immunization, mice were immunized without anesthesia in both external nares with a standard volume of 20 μl of the recombinant proteins calculated to contain approximately 15 μg of the E glycoprotein as priming immunization. Mice were given booster immunizations at weekly intervals a total of four times, with each booster containing approximately 30 μg of the E glycoprotein. Mice were similarly immunized with the recombinant proteins through the intraperitoneal or subcutaneous route. As positive controls, mice were immunized intranasally with approximately 2 × 106 PFU of a formalin-inactivated Beijing-1 strain of JE vaccine (Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan), for a total of three times at weeks 0, 3, and 5, and the immune sera were collected 1 week after the last immunization. Mice were also immunized intraperitoneally or subcutaneously with approximately 1 × 107 PFU of the JE vaccine at weeks 0 and 3, and the immune sera were collected 1 week after the second immunization.
For analysis of nasal secretory antibodies, nasal cavities of sacrificed mice were washed several times with 200 μl of PBS, and after centrifugation the supernatant was collected for detection of specific antibodies. Intestinal secretions were also collected for antibody analysis. Briefly, the ileal region of the small intestine was excised (approximately 3 cm long), and the intestinal tubes were opened by cutting the tubes perpendicularly with scissors. The excised tubes were immersed in 500 μl of PBS containing a protease inhibitor cocktail (Sigma) and vigorously vortexed. The samples were centrifuged to recover the supernatant for antibody analysis.
Serum and mucosal antibody analysis.
An indirect ELISA was performed for antibody analysis. Briefly, flat-bottom 96-well microtiter plates (Sumitomo Bakelite Co., Ltd.) were coated with the formalin-inactivated JE vaccine (∼5 × 106 PFU/well) diluted in bicarbonate buffer (50 μl/well), pH 9.6, by incubating at 4°C overnight, and wells were blocked with PBS containing 1% BSA. Mouse immune serum serially diluted with PBS containing 0.5% BSA was applied to antigen-coated wells in duplicate and incubated for 2 h at 37°C. Anti-mouse immunoglobulin G (IgG) conjugated with alkaline phosphatase, followed by its substrate addition, resulted in color development that was measured by a microplate reader (Bio-Rad) at OD415. Endpoint titers of specific antibodies against the virus or CTB protein were determined based on the highest serum dilution giving an OD value equivalent to that for preimmune serum. Mucosal antibodies of the IgA isotype were analyzed in nasal and intestinal secretions, and results were expressed as the mean OD415 obtained from individual mice. Statistical significance of differences in endpoint titers or OD415 was determined by a Mann-Whitney U test.
DTH response analysis.
For a measure of cellular immunity, the delayed-type hypersensitivity (DTH) response against the formalin-inactivated JE vaccine was analyzed in mice immunized intranasally or intraperitoneally with the purified heteropentameric proteins or the formalin-inactivated JE vaccine as a positive control. Approximately 5 × 106 PFU of the JE vaccine in a volume of 50 μl was injected into the footpad of a hind leg 1 week after the last immunization, and the thickening of the footpad was measured after 24 h. The DTH responses were expressed as differences in footpad thicknesses between the right and the left footpad injected with the JE vaccine and PBS, respectively. Statistical significance of differences in DTH responses was determined by a Mann-Whitney U test.
JE virus and neutralization assay.
A virus neutralization test was performed using an indirect immunostaining method as described previously, with slight modifications (27). JE virus (Beijing-1 strain) was propagated in an Aedes albopictus cell clone C6/36 (21) and cultured at 28°C in growth medium supplemented with 0.2 mM nonessential amino acids; prepared virus solution was stored at −70°C until use. Fifty microliters of infectious JE virus (50 PFU) was mixed with an equal volume of mouse immune serum diluted 10-fold with 2% Dulbecco's modified Eagle's medium. Immune sera were heated at 56°C for 30 min prior to mixing with the virus for complement inactivation. The mixture was incubated at 28°C for 2 h and applied to six-well plates (50 μl/well) in which BHK-21 cells had grown to confluency in Eagle's minimal essential medium with 10% fetal calf serum. Plates were incubated at 37°C for 2 h in a CO2 incubator (5% CO2) to allow virus to attach cell surfaces. A total of 100 μl of 0.04% DEAE dextrall and 0.8% methyl cellulose was added to each well and incubated at 37°C for 24 h at 5% CO2. After a 24-h incubation period, cells were fixed with methanol, and infected cells were visualized by the addition of hyperimmune rabbit anti-JE antiserum, followed by anti-rabbit IgG conjugated with horseradish peroxidase and its substrate. The number of infected cells was counted for each immune serum sample under a light microscope, and virus neutralization levels were determined based on the percent reduction of infected cells compared with nonimmune control serum. Statistical significance of differences in neutralization levels was determined by a Mann-Whitney U test. Differences were considered significant at a P value of <0.05.
RESULTS
Construction of CTB expression vectors and their gene product analysis.
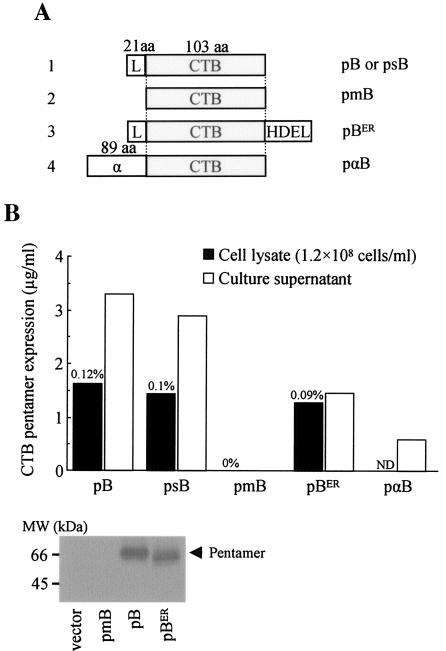
Prior to construction of CTB-antigen fusion genes and their expression analysis, we evaluated whether P. pastoris was a suitable model organism for expression of CTB genes. For this purpose we constructed several CTB expression plasmid vectors (Fig. 1a) and evaluated their gene products by GM1-ELISA and Western blotting (Fig. 1b). We found that the full-length CTB gene with its 21-amino-acid original leader sequence (pB) was expressed as secreted proteins that retained specific binding affinity for GM1-ganglioside (Fig. 1b). We also found pentamer proteins in cell lysate. CTB pentamers detected in the cell extract were probably the ones involved in a process of intracellular trafficking for secretion, because the attempt to accumulate pentamers in the cytoplasmic compartment by deleting the leader sequence (pmB) was unsuccessful. The complete absence of pentamer formation both in intra- and extracellular compartments of yeast transformed with the plasmid pmB was most likely the result of a posttranscriptional mechanism (e.g., absence of translation or rapid degradation of proteins in the cytoplasm), because comparable levels of CTB gene transcripts from pB- and pmB-transformed cells were detected (data not shown). This observation led us to conclude that efficient pentamer formation could not take place within the yeast cytoplasm and suggested that translocation of proteins into the lumen of the endoplasmic reticulum (ER) was a process required for pentamer assembly. Then, we hypothesized that translocation into the ER compartment and subsequent intracellular trafficking to cell surface, but not secretion per se, are important for subunit assembly and the subsequent stability of the protein complex. To test this hypothesis, we constructed an ER-retention form of the CTB protein by fusing a tetrapeptide yeast microsomal retention signal (HDEL) at the C terminus of CTB (pBER) (8). The addition of the retention signal significantly suppressed pentamer secretion without affecting intracellular pentamer accumulation levels compared with the parental construct (pB) (Fig. 1b), indicating that protein passage through the lumen of the ER and/or the Golgi apparatus during the secretion process is important for pentamer assembly. This could be due to the increase of the monomer subunit concentration within the lumen of intracellular organelles, which makes them self-assemble more efficiently into pentamers, a mechanism closely resembling the holotoxin assembly process taking place within the periplasmic space of gram-negative bacteria like V. cholera and E. coli (19).
FIG. 1.
Construction and expression analysis of CTB genes in P. parstoris. (A) A schematic representation of five CTB gene constructs: pB, a full-length CTB gene (375-bp open reading frame) containing a 21-amino-acid original leader sequence; psB, P. pastoris codon-optimized full-length synthetic CTB gene; pmB, matured form of CTB gene devoid of the leader sequence but provided with the initiation codon immediately in front of the first amino acid residue of the matured CTB protein; pBER, the full-length CTB gene containing a tetrapeptide yeast microsomal retention signal (HDEL) at the C terminus; pαB, matured form of CTB gene fused in frame with the S. cerevisiae alpha-factor secretion signal at the N terminus. (B) Intra- and extracellular CTB pentamer expressions were analyzed by GM1-ELISA from cellular extract and culture supernatant, respectively. For cellular extract, 1 ml of sample was prepared from approximately 1.2 × 108 cells, determined by the OD600. Estimated percent levels of intracellular CTB pentamer proteins in yeast total soluble protein are provided. Data are representative of a clone with the highest expression level screened from up to 50 independent transformants. The lower panel shows secreted expression of CTB pentamer proteins detected by the Western blotting of crude culture supernatant. aa, amino acids; ND, not determined.
To evaluate whether a yeast secretion signal functions better than CTB's leader peptide, we replaced the original 21-amino-acid CTB leader with an 89-amino-acid alpha-factor secretion signal of Saccharomyces cerevisiae (pαB) (5). In contrast to our expectation, it decreased the secreted expression levels, probably because the bulky N-terminal alpha-factor polypeptide fusion, even until its cleavage, may have interfered with the assembly process or because the cellular endopeptidase was insufficient to cleave off the factor for the large amount of fusion proteins being made, consequently reducing pentamer levels (Fig. 1b). Finally, to evaluate whether codon optimization would contribute to increased CTB expression, we constructed a P. pastoris codon-optimized synthetic CTB gene (psB) based on a codon usage database provided by Kazusa DNA Research Institute (Chiba, Japan) and tested in parallel the expression levels with the native CTB gene (pB). However, we did not find significant differences in the expression levels between the two constructs (Fig. 1b).
Immunoblot analysis confirmed secreted expression of CTB pentamers from pB- and pBER- but not from pmB-transformed recombinant yeast (Fig. 1b, lower panel). The apparent molecular mass of the CTB pentamer (approximately 65 kDa) was larger than that of the bacterially expressed protein (45 kDa), probably due to uncleaved signal peptide remaining at the N terminus and/or the glycosylation of the CTB protein. Although bacterial and yeast CTB pentamers were different in molecular weight, we did not find substantial differences in the ganglioside binding patterns of several CTB pentamer proteins expressed in P. pastoris compared with bacterially produced proteins (data not shown). Taken together, our preliminary experiments indicated that the original CTB gene was the most suited form as starting material for expression of CTB chimeric fusion genes in P. pastoris.
Construction of CTB fusion gene expression vectors and their gene product analysis.
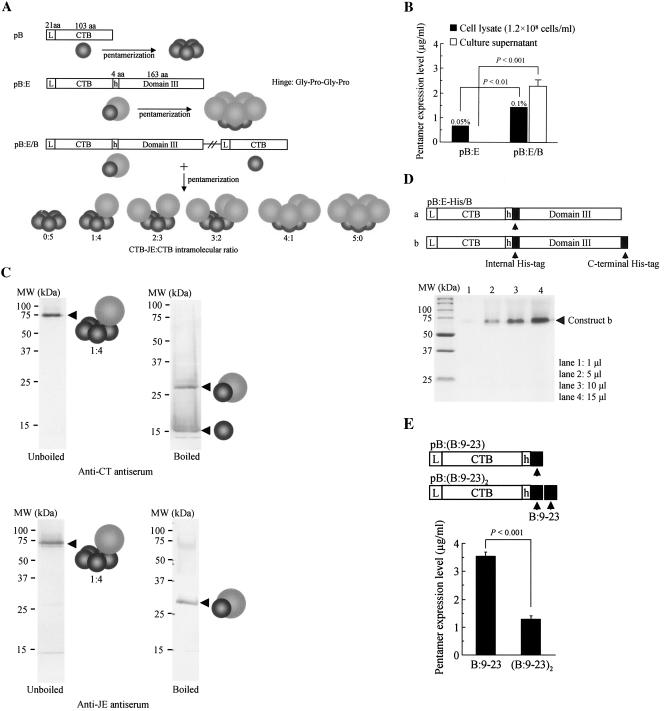
Two separate CTB-JE virus E glycoprotein fusion gene expression plasmid vectors were engineered based on the plasmids pBh and pB: (i) plasmid pB:E encoding the CTB fusion protein and (ii) plasmid pB:E/B encoding both the fused and unfused CTB protein (Fig. 2a). In theory, transformation of yeast with plasmid pB:E produces a homopentameric CTB chimeric fusion protein, and cointegration of the two expression cassettes by plasmid pB:E/B into the yeast chromosome produces a series of heteropentameric CTB chimeric fusion proteins, as shown in Fig. 2a. For these two constructs, transgene expressions were analyzed by GM1-ELISA, and their expression levels were determined (Fig. 2b; for method, see the legend of Fig. 1b). We first confirmed that hyperimmune rabbit anti-JE virus antiserum was specific and not reactive with CTB proteins, yeast cellular extract, or culture supernatant. Transformation with plasmid pB:E in an attempt to produce a homopentameric CTB fusion protein resulted in significantly reduced intracellular pentamers and, more importantly, the secretory property of the CTB pentamer completely vanished (compare with the construct pB shown in Fig. 1b). Replacement of the GPGP hinge with a longer sequence with 15 amino acids [(G4S)3] did not improve the result (data not shown). Surprisingly, however, transformation with plasmid pB:E/B significantly increased the levels of intracellular pentamers reactive with anti-JE virus antiserum, and more importantly, the secretory property was almost completely restored to a level close to that of the pB construct (Fig. 2b).
FIG. 2.
Construction and expression analysis of heteropentameric CTB chimeric fusion genes in P. pastoris. (A) A schematic representation of three CTB-related gene constructs and their expected gene products. Two separate CTB fusion expression plasmid vectors were constructed based on the plasmid pBh and pB: pB:E, CTB gene fused in frame with a sequence encoding one-third of the C-terminal region of the JE virus E glycoprotein (domain III; 163 amino acids); pB:E/B, plasmid containing two expression cassettes for CTB fusion and unfused proteins derived from pB:E and pB, respectively. Expected single subunit gene products and their pentamer molecules were schematically drawn for each construct. In theory, coexpression of the fused and the unfused CTB subunits by the plasmid pB:E/B within a single cellular compartment results in the production of six types of pentameric molecular species (i.e., 0:5 to 5:0). (B) Intra- and extracellular CTB chimeric fusion proteins were analyzed by GM1-ELISA as described in the legend of Fig. 1b. Estimated percent levels of intracellularly expressed pentamer proteins in yeast total soluble protein are provided. Data are expressed as the average concentration of pentamers ± standard errors obtained from several clones, with the highest expression levels screened from up to 50 independent transformants. (C) Western blot analysis of the culture supernatant of recombinant yeast transformed with the plasmid pB:E/B. Both boiled (two right panels) and unboiled (two left panels) samples were reacted with anti-CT (two upper panels) or anti-JE antiserum (two lower panels). Schematic drawings for protein bands indicated by arrowheads are the predicted molecular species based on their molecular size and specific reactivity of immune sera. (D) Based on the observation that the heteropentameric construct, unlike homopentamers, was secreted into culture medium, two additional plasmids for expression of heteropentamers with six-His tags were engineered (pB:E-His/B), and secreted proteins purified with the N2+ column were analyzed by SDS-PAGE, followed by Coomassie brilliant blue staining. For conciseness, only the CTB fusion genes of the heteropentameric constructs were drawn. The gel picture shows the purified protein bands of the construct (b) containing two histidine tags (both internal and the C-terminal), with increasing volumes of eluted samples from the N2+ column. (E) Effects on the secretory property of homopentamers by fusion of a short oligopeptide to the CTB molecule were evaluated. A 15-amino-acid T-cell epitope of human insulin B chain (B:9-23) was selected as a model oligopeptide to be fused at the C terminus of the CTB molecule. Two separate expression plasmid vectors were engineered: the CTB gene fused with an oligonucleotide encoding the B:9-23 epitope [pB:(B:9-23)] and the CTB gene fused with two tandemly linked epitope genes [pB:(B:9-23)2]. Secreted expressions were analyzed as described in the legends of Fig. 1b and 2b. Data are expressed as the average concentrations of secreted pentamer proteins ± standard errors from 10 clones, with the highest expression levels screened by GM1-ELISA from up to 50 independent transformants. Statistical significance of the differences in pentamer expression levels was determined by a Mann-Whitney U test. aa, amino acids.
For the purpose of analyzing the secreted pentamer proteins from yeast transformed with plasmid pB:E/B, heat-treated or untreated culture supernatant was subjected to 12.5% SDS-PAGE and Western blot analysis by using anti-CT or anti-JE antiserum (Fig. 2c). For unboiled samples, both antisera recognized the same major protein band with an apparent molecular mass of approximately 75 kDa, a smaller value than the calculated molecular mass of the heteropentameric CTB chimeric fusion molecule consisting of one fusion molecule and four unfused CTB molecules (“1:4-type” heteropentamer; calculated molecular mass, 87.2 kDa). However, the smaller apparent molecular mass of the heteropentameric construct was consistent with our observation that the CTB pentamer exhibited higher gel mobility than its actual molecular mass, probably because its compact configuration was retainable even in the presence of SDS (Fig. 1b). Boiling the samples for 3 min resulted in almost the complete dissociation of pentamers into the fused CTB monomers (calculated molecular mass, 31.6 kDa) reactive with both anti-CT and anti-JE antisera and the unfused CTB monomers (calculated molecular mass, 13.9 kDa) recognizable only by anti-CT antiserum (Fig. 2c).
Based on the observation that the heteropentameric CTB chimeric construct, unlike homopentamers, was efficiently secreted into the culture medium as intact pentameric molecules retaining the original binding affinity for GM1-ganglioside and native antigenicity of both CTB and the E glycoprotein, we inserted a six-His tag-encoding sequence just behind the hinge region (internal His tag), or both at the internal and the C-terminal (pB:E-His/B) His tags for convenient purification of the chimeric proteins by using an N2+ affinity column (Fig. 2d, upper panel). The histidine tags were inserted only into the fusion gene and not into the “buffer CTB” gene to purify only pentameric proteins carrying the vaccine protein, excluding even a minute amount of the 0:5 type of unfused CTB pentamer secreted into culture medium. One-step affinity purification from yeast culture medium by the N2+ column resulted in a fairly pure 1:4 type of heteropentameric protein with a molecular mass close to 75 kDa (Fig. 2d, lower panel), a value consistent with the data obtained from the Western blot analysis of the crude culture supernatant (Fig. 2c). We found that purification efficiency was not significantly different between the two types of histidine-tagged constructs (data not shown).
It was clearly demonstrated that heteropentamers had distinct advantages over homopentamers in terms of the pentamerization efficiency and the ease of purification (Fig. 2b). Then, we decided to determine how large-antigen fusion could be tolerated by the CTB molecule to retain the homopentameric structure without affecting the secretory nature of the complex. For this purpose a 15-amino-acid T-cell epitope of human insulin B chain (B:9-23) was chosen as a model oligopeptide to be fused at the C terminus of the CTB. Two types of CTB fusion genes were engineered based on plasmid pBh: (i) the CTB gene fused with a synthetic oligonucleotide encoding the B:9-23 epitope [pB:(B:9-23)] and (ii) the CTB gene fused with two tandemly connected epitope genes [pB:(B:9-23)2] (Fig. 2e). The fusion of one epitope did not negatively affect secreted expression levels compared with the unfused CTB pentamer (compare Fig. 1b and 2e); however, a two-epitope fusion resulted in a potent negative effect, indicating that the secretory property of the homopentamer was severely impaired even with a slight elongation of the fused oligopeptides, which is not likely to occur for heteropentameric constructs.
Immunogenicity analysis of the heteropentameric CTB chimeric fusion proteins.
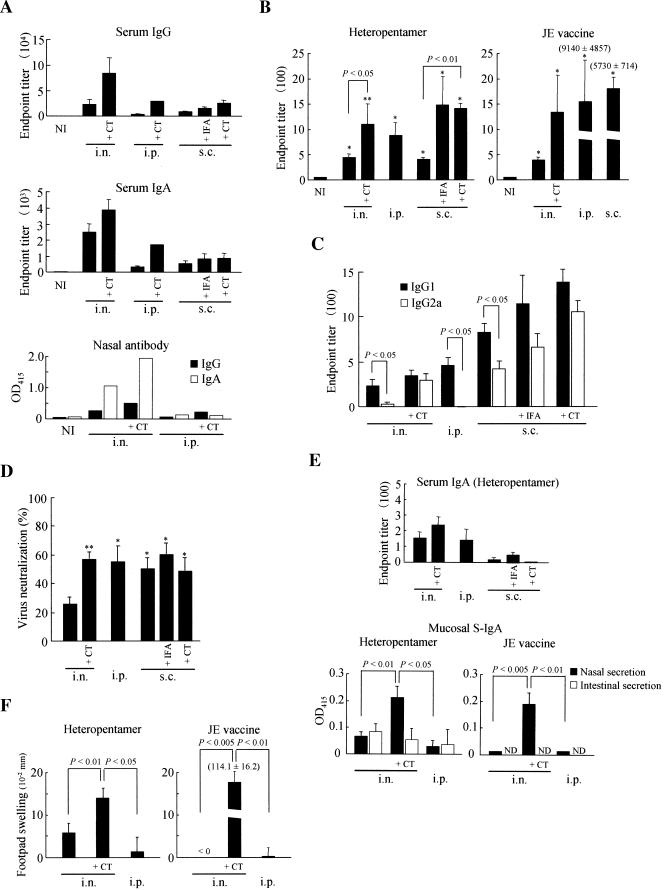
Prior to immunogenicity analysis of the purified heteropentameric CTB chimeric fusion proteins, we confirmed the potential mucosal immunogenicity of a P. pastoris-produced CTB pentamer (the pB construct) by orally immunizing mice with crude or partially purified proteins, and we found that the immunization induced systemic as well as local humoral immune responses against CTB (unpublished data). To evaluate the immunogenicity of the purified heteropentameric chimeric proteins (Fig. 2d, construct b), BALB/c mice were immunized through the intranasal, subcutaneous, or intraperitoneal route with or without the use of IFA or CT as adjuvant. One microgram and 0.05 μg of CT were used for intranasal and subcutaneous immunization, respectively. We first confirmed that immunization with the heteropentameric construct induced specific serum as well as mucosal antibody responses against CTB (Fig. 3a). Intranasal immunization induced the highest CTB-specific responses of both IgG and IgA isotypes in serum and nasal secretions, and CT coadministered as adjuvant exhibited the most profound immunogenicity when given through the intranasal route in comparison with the other two parenteral routes of administration examined, which showed similar levels of response against CTB. Consistent with this observation, the parenteral route was a less efficacious inductive site than the intranasal route for CTB-specific antibody responses in nasal secretions. As we expected, serum had a higher specific IgG than IgA, but the nasal secretions showed the opposite tendency, with higher IgA than IgG levels.
FIG. 3.
Immunogenicity analysis of purified heteropentameric CTB chimeric fusion proteins. (A) Serum and mucosal antibody responses against CTB were examined in BALB/c mice immunized with the heteropentameric protein through the intranasal, intraperitoneal, or subcutaneous route in the presence or absence of indicated adjuvant. Both IgG and IgA isotypes of immunoglobulins were examined from serum and nasal secretions. The data are expressed as endpoint titers ± standard errors for serum antibodies and as the average OD415 for secretory antibodies in the pooled nasal washings. NI, nonimmune samples obtained from unimmunized mice. (B) JE virus-specific serum IgG responses were determined for mice immunized with the heteropentameric protein or the formalin-inactivated JE vaccine. The data are expressed as endpoint titers ± standard errors. NI, nonimmune samples obtained from unimmunized mice. *, P < 0.01 versus NI. **, P < 0.001 versus NI. (C) Virus-specific serum IgG1 and IgG2a subclasses were determined for immune sera from mice immunized with the heteropentameric protein. The data are expressed as endpoint titers ± standard errors as described for panel b. (D) JE virus neutralization antibodies were analyzed in mice immunized with the heteropentameric protein with or without the use of adjuvant as indicated. The data are expressed as percent reductions in PFU ± standard errors for each immune serum diluted 10-fold with PBS. *, P < 0.05; **, P < 0.01 versus intranasal immunization with the heteropentameric protein alone. (E) Virus-specific serum IgA responses were determined for mice immunized with the heteropentameric protein (upper panel). The data are expressed as endpoint titers ± standard errors. Nasal and intestinal washings were analyzed for the presence of virus-specific serum IgA (S-IgA), and the data are expressed as the average OD415 after subtraction of the background levels of samples obtained from naïve mice. Undiluted and fourfold-diluted samples were used in assays for nasal washings of mice immunized with the heteropentameric protein and the JE vaccine, respectively. ND, not determined. (F) DTH responses induced against the formalin-inactivated JE vaccine were determined. The data are expressed as the differences in footpad thickness ± standard errors between the right and the left footpads injected with the JE vaccine and PBS, respectively. Statistical significance of differences was determined by a Mann-Whitney U test. i.n., intranasal; i.p., intraperitoneal; s.c., subcutaneous.
Next, we determined whether the heteropentameric construct could induce a specific serum IgG response against JE virus (Fig. 3b). For all immunization routes tested, the chimeric proteins induced specific serum IgG against JE virus, and the use of IFA or CT adjuvant had obvious effects on the response. Intranasal administration of the chimeric proteins induced comparable levels of serum IgG compared with the formalin-inactivated JE vaccine; however, the vaccine exhibited much stronger immunogenicity when administered through the intraperitoneal or subcutaneous route than the chimeric proteins given through the same routes. Serum IgG subclass analysis indicated that the response was somewhat skewed toward IgG1 production for all immunization routes examined, which is indicative of a Th2-type response (Fig. 3c). However, intranasal coadministration of CT augmented the IgG2a response to a level comparable to that of IgG1. We also determined whether the induced serum antibodies had a JE virus neutralization capacity (Fig. 3d). Intranasal administration of the chimeric constructs resulted in weak but significant levels of virus neutralization, and the efficacy was enhanced by the addition of CT, reaching the levels attained by intraperitoneal or subcutaneous immunization. In contrast, immunization with the formalin-inactivated JE vaccine through the intranasal or intraperitoneal route induced significantly higher levels of virus neutralization, typically reaching more than 90%, by using the same assay method as described in the legend of Fig. 3d (data not shown), indicating the superior protective efficacy of the currently available inactivated JE vaccine compared to the recombinant E glycoprotein domain III fused with the CTB pentamer.
IgA isotype analysis indicated that weak but significant JE virus-specific responses were induced in both serum and mucosal secretions (Fig. 3e). However, in contrast to serum IgG, which was somewhat more efficiently induced by subcutaneous immunization (Fig. 3b, left panel), the serum IgA response was higher for intranasal immunization (Fig. 3e, upper panel). We also found that intranasal immunization with the chimeric construct or the JE vaccine induced mucosal IgA responses in nasal secretions, and the responses were significantly augmented by coadministration of CT (Fig. 3e, lower panels). Additionally, we found weak but detectable secretory IgA in intestinal secretions of mice immunized intranasally with the chimeric construct, but the adjuvant effect of CT was not as clearly observed as for nasal secretions (Fig. 3e, lower left panel). The JE vaccine showed patterns of secretory IgA responses similar to immunization with the chimeric construct, demonstrating that the adjuvant effect of CT was independent of coadministered heterologous antigens (Fig. 3e, lower right panel).
Finally, we analyzed the DTH response-inducing capacity of the heteropentameric construct (Fig. 3f). Intranasal immunization induced weak but detectable levels of a DTH response, which was significantly augmented by coadministration of CT (Fig. 3f, left panel). In contrast, intranasal immunization with the JE vaccine did not induce a DTH response in the absence of CT, but the response became extremely strong when CT was coadministered (Fig. 3f, right panel), demonstrating that nasal tissue was a highly efficient inductive site for the DTH response if a strong mucosal adjuvant like CT was used. Consistent with this observation, intraperitoneal immunization did not induce the response for the JE vaccine or the heteropentameric construct, and coadministration of CT or another potentially strong adjuvant like pertussis toxin could augment the response, but much less potently than seen for intranasal immunization (unpublished data).
DISCUSSION
The vast majority of pathogens initiate infection through mucosal portals of entry and replicate at or initiate their systemic spread from the mucosal surface of the gastrointestinal, respiratory, or urogenital tract. Nevertheless, most vaccines currently licensed for use in humans and animals are subcutaneously or intramuscularly injectable ones, with a few exceptions including the oral polio vaccine. However, it is often difficult with parenteral vaccines to provide local protective immunity mediated in part by secretory IgA in mucosal tissues. Thus, noninvasive mucosal prophylactic vaccines became attractive alternatives to conventional injection-based vaccines, particularly against mucosal pathogens. Mucosal vaccines, particularly ones that can be administered nasally, are often effective in priming a full range of local as well as systemic immune responses against protective antigenic epitopes (16, 17). In addition, the needle-free, noninvasive nature of this type of topically administrable vaccine may be safer than needle injection-type vaccines because it reduces the risk of infection from blood-borne pathogens and may also be cost-effective because administration does not require highly trained medical or veterinary personnel. In spite of the attractive features of mucosal vaccines, their targets have essentially been limited to mucosal infections, and their potential applicability to nonmucosal pathogens such as arthropod vector-borne viruses and parasites has been neglected until recently (34). However, our previous studies with malaria transmission-blocking vaccines (2) and the formalin-inactivated JE vaccine (unpublished data) justified the investigation of noninvasive mucosal vaccines against mucosa-unrelated pathogens. An equally important finding we made from these studies was that the efforts to develop potentially safer mucosal carriers and adjuvants are necessary for effective vaccine design.
It seems critically important for CT (or CTB) to cross the mucosal barrier via binding to the cell surface receptor GM1-ganglioside in order to be effective as a mucosal antigen and adjuvant (22). Our recent study showed that CT (or CTB) coadministered with GM1-ganglioside through the intranasal or parenteral route (i.e., subcutaneous or intramuscular) significantly reduced the systemic IgG response against itself (unpublished results). The inhibitory effect of GM1 was much more prominent with the intranasal than the parenteral route, completely preventing the response when given intranasally, while the blocking effect was not fully functional for parenteral immunization. This result clearly indicates that receptor-mediated mucosal uptake of CT (or CTB) is an essential step required for its immunogenicity and strongly suggests that the adjuvant and carrier functions of CT (or CTB) are critically controlled by its binding affinity for cell receptors. From this observation one can speculate that pentamerization, which in turn confers GM1 binding affinity, is the essential process that allows CTB to act as a carrier (for CTA in the case of CT or fused antigens) to cross the mucosal barrier. This unique characteristic of CTB or its related molecules including CTB and A2/CTB fusion chimeras may become less obvious for parenteral immunization regimens.
In this study we demonstrated a potential new strategy to genetically engineer CTB-based mucosal vaccines to enhance the immunogenicity of fused antigens. Homopentameric assembly between each fusion subunit was significantly affected by the size of the fused antigens, especially for the N terminus fusion to CTB. An alternative technique to overcome this problem is to take advantage of the molecular structure of CT (or LT) holotoxin, in which the bulky A1 subunit of approximately 22 kDa is located above the ring of the CTB pentamer unit by noncovalent interaction of the A2 alpha-helix with the central hole of the pentamer ring (40). In theory, replacement of the A1 subunit with any heterologous foreign antigen could assemble into holotoxin-like molecules, in which the antigen is located above the pentamer ring (14, 16, 18, 26, 32, 33), circumventing high steric hindrance between each pentamer subunit. However, the relatively low frequency of antigen-A2 fusion protein assembly with the pentamer unit seemed to be the problem (18). In addition, these holotoxin-like chimeras were intracellularly produced, requiring whole-cell lysis or periplasmic protein preparations, because the replacement of the intact A1 subunit with foreign antigens may impair the translocation process across the outer membrane of the bacterial host.
The heteropentameric fusion strategy described in this study may provide a solution to the problems associated with homopentamer and A2 fusion strategies. In addition, heteropentameric CTB chimeric fusion proteins may have the following features.
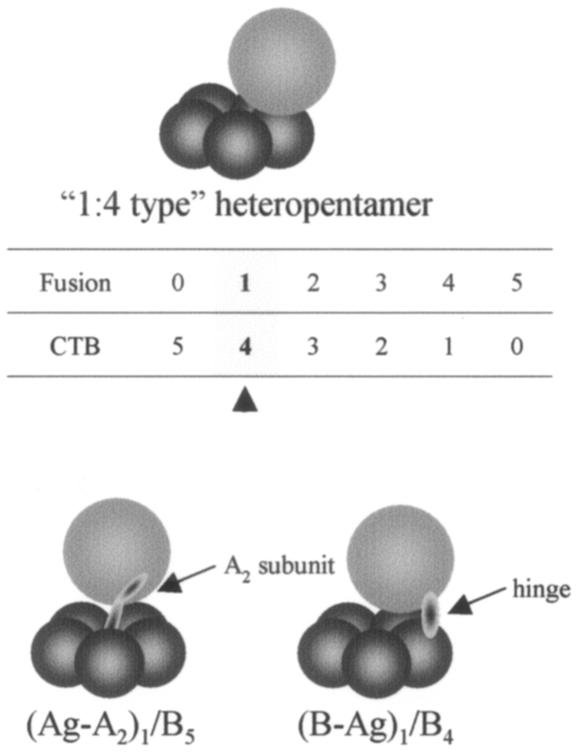
(i) The molecular size of a fused antigen may determine the molecular ratio between the fused and the unfused CTB subunit within a pentamer unit. The complex would automatically avoid configurations that cannot tolerate high steric hindrance; however, the molecular ratio between the fused antigen and the pentamer may become greater than 1 and may go up to 5 (i.e., 5:0-type homopentamer), depending on the size of the fused antigen. This would not be expected with a holotoxin-like chimera in which the ratio does not exceed 1 unless fused antigens are tandemly connected. However, it is speculated that the molecular structure of the secreted 1:4-type heteropentamer observed in this study (Fig. 2C) may resemble that of the holotoxin-like chimera, where the fused antigen is placed above the CTB pentamer ring. This speculation is based on the molecular structure of the CTB in which the C terminus is located close to the top of the ring structure (41) (Fig. 4). The role played by the A2 subunit that connects the antigen to the pentamer is replaced with the hinge placed between the antigen and the B subunit.
FIG. 4.
Schematic representation of a secreted molecular species of a heteropentameric CTB chimeric fusion protein from recombinant P. pastoris and its predicted structural resemblance to the Ag-A2/B holotoxin-like chimeric protein. The numeric table shows all six possible combinations of the fused and the unfused CTB molecules within a single pentameric structure. The arrowhead indicates the major molecular species of heteropentameric protein having an intramolecular subunit ratio of 1:4, which was efficiently secreted into the culture medium in our experimental model system. The role played by the A2 subunit connecting the antigen to the pentamer ring is replaced with the hinge in a 1:4-type heteropentamer molecule. For both types of chimeric constructs, the bulky fused antigens are located above the pentamer ring, possibly avoiding high steric hindrance between each B subunit to assemble into a stable pentamer structure.
(ii) Whether the heteropentameric proteins are secreted and (if secreted) which molecular species are selected to be secreted from the host cells may be influenced by the size of the fused antigens as well as the nature of the expression systems. In the present study we found that the majority of secreted chimeric proteins carrying domain III of the JE virus E glycoprotein were the 1:4-type complex (Fig. 2), suggesting that secreted expression of pentamers carrying two or more antigens with the size of domain III would be difficult to predict in the model expression system we used in this study. The question of why the majority of secreted heteropentameric chimeras were in 1:4- but not in 0:5-type configurations (i.e., unfused CTB pentamer) may simply be explained by a factor of chance associated with the frequency of encounter between the molecules. If it is assumed that both the fused and the unfused CTB molecules exist at equal molar concentrations with equal encountering frequency between any subunits, a chance that all five subunits consisted of the unfused CTB is 1/25 (∼3%), but a chance that a molecule has a 1:4 configuration is 1/25 × 5 (∼16%). However, in reality, steric hindrance between the fused molecules may interfere with the assembly process, resulting in a larger population of molecular species with a lower ratio of fused to unfused molecules. For example, complexes are more likely to have 1:4 rather than 4:1 configuration, even though the two species have mathematically equal chances of being made. Additionally, this also raises the possibility of intentionally unbalancing the molar concentrations of fused and unfused CTB molecules to optimize production of the desired species of heteropentamers.
(iii) It has been difficult with a conventional CTB fusion method to fuse antigens at the C terminus to maintain the molecular configuration needed for effective vaccines. However, it may become feasible with the heteropentameric fusion strategy, because the complex may be able to maintain a proper pentamer configuration even in the presence of one or a few N-terminally fused CTB subunits without significantly affecting the binding affinity for GM1-ganglioside.
(iv) The heteropentameric CTB fusion strategy could also be applied to other gene expression systems including DNA vaccines (4). Having a similar mechanism of protein secretion for yeast and mammalian cells, heteropentameric constructs may have advantages over homopentamers or holotoxin-like molecules; their secretory nature may increase cellular uptake of the fusion chimera by adjacent cells or by the professional antigen-presenting cells for increased major histocompatibility complex class I and/or class II presentation to elicit enhanced humoral as well as cellular immune responses (possibly including mucosal responses) (7, 28).
(v) This strategy could be applied to a multivalent vaccine design, in which different protective antigens are integrated into a single complex molecule.
Our present study demonstrated that the heteropentameric CTB chimeric molecules fused with the domain III of the JE virus E glycoprotein were secreted from yeast P. pastoris and, after affinity tag-based purification and subsequent immunization with the purified protein, induced systemic as well as mucosal immune responses with virus neutralizing capacity. However, the fact that the use of CT significantly augmented the mucosal and systemic immune responses (Fig. 3) indicated the weak immunogenicity of the heteropentameric construct. One possible reason for the lower immunogenicity of the chimeric construct than the formalin-inactivated JE vaccine is that domain III represents only one-third of the entire E glycoprotein and also possibly that the same JE vaccine material was used for immunogenicity assays, i.e., ELISA (Fig. 3b, c, and e), virus neutralization (Fig. 3d), and the DTH response (Fig. 3f); thus, better responses were expected for the inactivated JE vaccine. We also observed that anti-JE mucosal responses were relatively low following intranasal immunization with either heteropentamers or inactivated JE vaccine and that the response levels were not significantly higher than seen in mice immunized by the systemic route (Fig. 3e). However, the addition of CT significantly augmented the response especially in nasal secretions. This seems to be a unique characteristic of antigens with weak mucosal immunogenicity and is consistent with the observation that the E glycoprotein domain III was a relatively weak mucosal immunogen even if it was conjugated with CTB, requiring the help of CT (or other potent mucosal adjuvant) to become a strong mucosal immunogen. We also observed low mucosal responses in the intestinal secretions of mice immunized by the intranasal route with the construct, even in the presence of CT (Fig. 3e, lower-left panel). Intestinal mucosa seemed to be less effective as an inductive and/or effector site than nasal mucosa. Consistent with this observation are our recent unpublished results indicating that administration of much higher amounts of the JE vaccine than used in this study or administration of the heteropentameric construct directly into the intestinal ileal region induced systemic as well as local humoral responses but to a lesser extent compared with intranasal immunization. In addition, the same antigens administered intragastrically were even less efficacious than intraileal administration, indicating that there are obvious hierarchies in the levels of immune responses attainable, depending on the site of mucosal administration with nonreplicating antigens.
Although at present there seems to be much room to improve the mucosal immunogenicity of heteropentameric constructs, we expect our findings will provide a contribution to the field of mucosal vaccines. Further studies are in progress to evaluate this strategy using various recombinant antigens and testing whether the incorporation of antigens into the constructs increases their mucosal immunogenicity. A heteropentameric fusion strategy, which would not depend on a particular vaccine antigen or expression system as indicated in our preliminary studies with heterologous antigens produced as secreted proteins (unpublished data), may provide an advancement in antigen delivery systems or adjuvants based on bacterial toxin-derived molecules including LT and other molecules with potential carrier functions. These vaccines may become effective against important diseases of humans and animals including mucosa-unrelated infections, as well as for therapeutic tumor vaccines (10, 11) and prophylactic and even therapeutic vaccines against autoimmune diseases (1, 29, 39).
Acknowledgments
This work was supported by basic science research grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and the Bio-Oriented Technology Research Advancement Institution (BRAIN).
We thank Masaru Nagamine at the Center of Molecular Biosciences, University of the Ryukyus, for his useful comments on this study.
Editor: J. D. Clements
REFERENCES
- 1.Arakawa, T., J. Yu, D. K. Chong, J. Hough, P. C. Engen, and W. H. Langridge. 1998. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat. Biotechnol. 16:934-938. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa, T., T. Tsuboi, A. Kishimoto, J. Sattabongkot, N. Suwanabun, T. Rungruang, Y. Matsumoto, N. Tsuji, H. Hisaeda, A. Stowers, I. Shimabukuro, Y. Sato, and M. Torii. 2003. Serum antibodies induced by intranasal immunization of mice with Plasmodium vivax Pvs25 co-administered with cholera toxin completely block parasite transmission to mosquitoes. Vaccine 21:3143-3148. [DOI] [PubMed] [Google Scholar]
- 3.Areas, A. P., M. L. Oliveira, E. N. Miyaji, L. C. Leite, K. A. Aires, W. O. Dias, and P. L. Ho. 2004. Expression and characterization of cholera toxin B-pneumococcal surface adhesin A fusion protein in Escherichia coli: ability of CTB-PsaA to induce humoral immune response in mice. Biochem. Biophys. Res. Commun. 321:192-196. [DOI] [PubMed] [Google Scholar]
- 4.Arrington, J., R. P. Braun, L. Dong, D. H. Fuller, M. D. Macklin, S. W. Umlauf, S. J. Wagner, M. S. Wu, L. G. Payne, and J. R. Haynes. 2002. Plasmid vectors encoding cholera toxin or the heat-labile enterotoxin from Escherichia coli are strong adjuvants for DNA vaccines. J. Virol. 76:4536-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brake, A. J., J. P. Merryweather, D. G. Coit, U. A. Heberlein, F. R. Masiarz, G. T. Mullenbach, M. S. Urdea, P. Valenzuela, and P. J. Barr. 1984. Alpha-factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 81:4642-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chia, S. C., P. S. Leung, C. P. Liao, J. H. Huang, and S. T. Lee. 2001. Fragment of Japanese encephalitis virus envelope protein produced in Escherichia coli protects mice from virus challenge. Microb. Pathog. 31:9-19. [DOI] [PubMed] [Google Scholar]
- 7.Decroix, N., H. Hocini, C. P. Quan, B. Bellon, M. D. Kazatchkine, and J. P. Bouvet. 2001. Induction in mucosa of IgG and IgA antibodies against parenterally administered soluble immunogens. Scand. J. Immunol. 53:401-409. [DOI] [PubMed] [Google Scholar]
- 8.Denecke, J., R. De Rycke, and J. Botterman. 1992. Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 11:2345-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dertzbaugh, M. T., D. L. Peterson, and F. L. Macrina. 1990. Cholera toxin B-subunit gene fusion: structural and functional analysis of the chimeric protein. Infect. Immun. 58:70-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson, K., J. B. Sun, I. Nordstrom, M. Fredriksson, M. Lindblad, B. L. Li, and J. Holmgren. 2004. Coupling of antigen to cholera toxin for dendritic cell vaccination promotes the induction of MHC class I-restricted cytotoxic T cells and the rejection of a cognate antigen-expressing model tumor. Eur. J. Immunol. 34:1272-1281. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson, K., M. Fredriksson, I. Nordstrom, and J. Holmgren. 2003. Cholera toxin and its B subunit promote dendritic cell vaccination with different influences on Th1 and Th2 development. Infect. Immun. 71:1740-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George-Chandy, A., K. Eriksson, M. Lebens, I. Nordstrom, E. Schon, and J. Holmgren. 2001. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen-presenting cells. Infect. Immun. 69:5716-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagiwara, Y., T. Iwasaki, H. Asanuma, Y. Sato, T. Sata, C. Aizawa, T. Kurata, and S. Tamura. 2001. Effects of intranasal administration of cholera toxin (or Escherichia coli heat-labile enterotoxin) B subunits supplemented with a trace amount of the holotoxin on the brain. Vaccine 19:1652-1660. [DOI] [PubMed] [Google Scholar]
- 14.Hajishengallis, G., S. K. Hollingshead, T. Koga, and M. W. Russell. 1995. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol. 154:4322-4332. [PubMed] [Google Scholar]
- 15.Hajishengallis, G., S. M. Michalek, and M. W. Russell. 1996. Persistence of serum and salivary antibody responses after oral immunization with a bacterial protein antigen genetically linked to the A2/B subunits of cholera toxin. Infect. Immun. 64:665-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haneberg, B., and J. Holst. 2002. Can nonliving nasal vaccines be made to work? Expert Rev. Vaccines 1:227-232. [DOI] [PubMed] [Google Scholar]
- 17.Harandi, A. M., J. Sanchez, K. Eriksson, and J. Holmgren. 2003. Recent developments in mucosal immunomodulatory adjuvants. Curr. Opin. Investig. Drugs 4:156-161. [PubMed] [Google Scholar]
- 18.Hatic, S. O., II, J. A. McCann, and W. D. Picking. 2001. In vitro assembly of novel cholera toxin-like complexes. Anal. Biochem. 292:171-177. [DOI] [PubMed] [Google Scholar]
- 19.Hirst, T. R., and J. Holmgren. 1987. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc. Natl. Acad. Sci. USA 84:7418-7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmgren, J., C. Czerkinsky, K. Eriksson, and A. Mharandi. 2003. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine 21(Suppl. 2):S89-S95. [DOI] [PubMed] [Google Scholar]
- 21.Igarashi, A. 1978. Isolation of a Singh's Aedes albopictus cell clone sensitive to dengue and chikungunya virus. J. Gen. Virol. 40:531-544. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura, Y. I., R. Kawashima, Y. Shirai, R. Kato, T. Hamabata, M. Yamamoto, K. Furukawa, K. Fujihashi, J. R. McGhee, H. Hayashi, and T. Dohi. 2003. Cholera toxin activates dendritic cells through dependence on GM1-ganglioside which is mediated by NF-κB translocation. Eur. J. Immunol. 33:3205-3212. [DOI] [PubMed] [Google Scholar]
- 23.Lebens, M., J. B. Sun, H. Sadeghi, M. Backstrom, I. Olsson, N. Mielcarek, B. L. Li, A. Capron, C. Czerkinsky, and J. Holmgren. 2003. A mucosally administered recombinant fusion protein vaccine against schistosomiasis protecting against immunopathology and infection. Vaccine 21:514-520. [DOI] [PubMed] [Google Scholar]
- 24.Levine, M. M., J. B. Kaper, D. Herrington, J. Ketley, G. Losonsky, C. O. Tacket, B. Tall, and S. Cryz. 1988. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet 2:467-470. [DOI] [PubMed] [Google Scholar]
- 25.Lin, C. W., and S. C. Wu. 2003. A functional epitope determinant on domain III of the Japanese encephalitis virus envelope protein interacted with neutralizing-antibody combining sites. J. Virol. 77:2600-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin, M., G. Hajishengallis, D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2001. Recombinant antigen-enterotoxin A2/B chimeric mucosal immunogens differentially enhance antibody responses and B7-dependent costimulation of CD4+ T cells. Infect. Immun. 69:252-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuno, Y., T. Fukunaga, M. Tadano, Y. Okamoto, T. Ohnishi, and M. Takagi. 1985. Rapid focus reduction neutralization test of Japanese encephalitis virus in microtiter system. Arch. Virol. 86:129-135. [DOI] [PubMed] [Google Scholar]
- 28.Pamonsinlapatham, P., N. Decroix, L. Mihaila-Amrouche, A. Bouvet, and J. P. Bouvet. 2004. Induction of a mucosal immune response to the streptococcal M protein by intramuscular administration of a PADRE-ASREAK peptide. Scand. J. Immunol. 59:504-510. [DOI] [PubMed] [Google Scholar]
- 29.Sadeghi, H., S. Bregenholt, D. Wegmann, J. S. Petersen, J. Holmgren, and M. Lebens. 2002. Genetic fusion of human insulin B-chain to the B-subunit of cholera toxin enhances in vitro antigen presentation and induction of bystander suppression in vivo. Immunology 106:237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez, J., A. M. Svennerholm, and J. Holmgren. 1988. Genetic fusion of a non-toxic heat-stable enterotoxin-related decapeptide antigen to cholera toxin B-subunit. FEBS Lett. 241:110-114. [DOI] [PubMed] [Google Scholar]
- 31.Song, H., L. Zhou, W. Fang, Y. Li, X. Wang, H. Fang, X. Li, M. Wu, and B. Qiu. 2004. High-level expression of codon optimized foot-and-mouth disease virus complex epitopes and cholera toxin B subunit chimera in Hansenula polymorpha. Biochem. Biophys. Res. Commun. 315:235-239. [DOI] [PubMed] [Google Scholar]
- 32.Sultan, F., L. L. Jin, M. G. Jobling, R. K. Holmes, and S. L. Jr. Stanley. 1998. Mucosal immunogenicity of a holotoxin-like molecule containing the serine-rich Entamoeba histolytica protein (SREHP) fused to the A2 domain of cholera toxin. Infect. Immun. 66:462-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toida, N., G. Hajishengallis, H. Y. Wu, and M. W. Russell. 1997. Oral immunization with the saliva-binding region of Streptococcus mutans AgI/II genetically coupled to the cholera toxin B subunit elicits T-helper-cell responses in gut-associated lymphoid tissues. Infect. Immun. 65:909-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, L., L. Kedzierski, S. L. Wesselingh, and R. L. Coppel. 2003. Oral immunization with a recombinant malaria protein induces conformational antibodies and protects mice against lethal malaria. Infect. Immun. 71:2356-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu, K. P., C. W. Wu, Y. P. Tsao, T. W. Kuo, Y. C. Lou, C. W. Lin, S. C. Wu, and J. W. Cheng. 2003. Structural basis of a flavivirus recognized by its neutralizing antibody: solution structure of the domain III of the Japanese encephalitis virus envelope protein. J. Biol. Chem. 278:46007-46013. [DOI] [PubMed] [Google Scholar]
- 36.Wu, S. C., C. H. Yu, C. W. Lin, and I. M. Chu. 2003. The domain III fragment of Japanese encephalitis virus envelope protein: mouse immunogenicity and liposome adjuvanticity. Vaccine 21:2516-2522. [DOI] [PubMed] [Google Scholar]
- 37.Yu, J., and W. H. Langridge. 2001. A plant-based multicomponent vaccine protects mice from enteric diseases. Nat. Biotechnol. 19:548-552. [DOI] [PubMed] [Google Scholar]
- 38.Yuki, Y., and H. Kiyono. 2003. New generation of mucosal adjuvants for the induction of protective immunity. Rev. Med. Virol. 13:293-310. [DOI] [PubMed] [Google Scholar]
- 39.Yuki, Y., Y. Byun, M. Fujita, W. Izutani, T. Suzuki, S. Udaka, K. Fujihashi, J. R. McGhee, and H. Kiyono. 2001. Production of a recombinant hybrid molecule of cholera toxin-B-subunit and proteolipid-protein-peptide for the treatment of experimental encephalomyelitis. Biotechnol. Bioeng. 74:62-69. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, R. G., D. L. Scott, M. L. Westbrook, S. Nance, B. D. Spangler, G. G. Shipley, and E. M. Westbrook. 1995. The three-dimensional crystal structure of cholera toxin. J. Mol. Biol. 251:563-573. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, R. G., M. L. Westbrook, E. M. Westbrook, D. L. Scott, Z. Otwinowski, P. R. Maulik, R. A. Reed, and G. G. Shipley. 1995. The 2.4 A crystal structure of cholera toxin B subunit pentamer: choleragenoid. J. Mol. Biol. 251:550-562. [DOI] [PubMed] [Google Scholar]