Summary
Pulmonary microvascular dysfunction is central to the pathophysiology of pulmonary hypertension (PH), yet remains challenging to evaluate in clinical practice. This review outlines current and emerging methodologies for assessing the pulmonary microcirculation, including advanced imaging, computational modelling, and invasive haemodynamic techniques. Exercise right heart catheterisation, waveform analysis, and vasoreactivity testing provide indirect insights into microvascular health, while novel invasive approaches, such as pulmonary flow reserve and pulmonary microvascular resistance indices, offer the potential for more precise functional characterisation. Computational models incorporating CT-derived anatomical data and patient-specific haemodynamics may enhance early detection and phenotyping of PH. Although many of these tools remain in the research domain, their refinement and integration into clinical workflows could enable earlier diagnosis, personalised risk stratification, and monitoring of therapeutic response. Ultimately, translating these innovations into practice may allow for targeted interventions that address microvascular dysfunction at an earlier stage of disease progression.
Keywords: Pulmonary microcirculation, Magnetic resonance imaging, Computed tomography, Mathematical modelling, Invasive haemodynamics
Introduction
Right heart catheterisation (RHC) remains a critical tool for the diagnosis and monitoring of pulmonary hypertension (PH). The technique was first established in 1929 when Dr Forssmann famously self-catheterised his antecubital vein using a urinary catheter, and was later broadly introduced into clinical practice in the 1940s when Cournand and Richards established its clinical utility.1 By this method, PH is defined as a mean pulmonary artery pressure (mPAP) > 20 mmHg, with further division into pre- or post-capillary based on the pulmonary capillary wedge pressure (PCWP) cut-point of 15 mmHg and pulmonary vascular resistance (PVR) of 2 Wood units.2 While this definition is broadly accepted and specified in the guidelines, it fails to capture the early adverse functional and structural microvascular changes observed in PH.
In all forms of PH, adverse changes in the pulmonary microvasculature occur well before detectable macrovascular changes.3 This is intuitive, given that the microvasculature accounts for the large majority of the total pulmonary circulation, comprising almost 80%. Despite this, assessment of the structure and function of the pulmonary microcirculation in PH remains difficult and largely reserved to the research setting. Further research and development of these methodologies is critical for the early diagnosis of PH, which has important implications for optimising monitoring and treatment for these patients.
This review outlines current insights into the pulmonary microcirculation in PH, emphasising emerging imaging and haemodynamic technologies for early detection of adverse pulmonary remodelling. It focuses on group 1 pulmonary arterial hypertension, group 2 PH due to left heart disease and group 4 PH secondary to chronic thromboembolic PH (CTEPH), in which early diagnosis may improve outcomes.
Search strategy and selection criteria
A search of PubMed was performed on the 29th September 2024 using the search term (pulmonary microcirculat∗ OR lung microcirculat∗ OR pulmonary microvasculat∗ OR lung microvasculat∗) AND (pulmonary hypertension OR pulmonary arterial hypertension) identified 639 articles for review. After review of titles and abstracts, 167 full text articles remained. Following screening of full texts, the most common reasons for exclusion were: related to non-pulmonary microvascular beds, related to group 3 PH, or related to omics, which is outside of the scope of this review. Further full texts were identified through review of relevant reference lists. Only articles published in English were included.
Microscopic perspectives on the pulmonary microcirculation in PH
Interest in the pulmonary microcirculation spans decades, with early insights primarily derived from animal models. Electron microscopy studies in healthy humans have since established that as pulmonary vessels become more distal they lose their muscularity; smooth muscle cells (SMC) transition from a circumferential layer in the media to a partial helical layer, and vessels below 100 μm become largely non-muscular.4
Histological features of the pulmonary microcirculation share broad similarities across PH subtypes, but with important distinctions. Notably, in congenital heart disease (CHD) and idiopathic PH microvascular changes predominately effect arterioles, whereas in group 2 and group 4 PH, venulae involvement is also significant.3,5, 6, 7 Clinically, the implications of predominant arteriole versus venulae remodelling remain unclear, although these distinctions may contribute to the differential therapeutic responses observed.
While idiopathic PH and group 2 PH typically exhibit concentric laminar intimal fibrosis, CTEPH is characterised by eccentric and patchy fibrosis.8, 9, 10 Further, plexiform lesions and dilatations found in the microcirculation of idiopathic PH are absent in group 2 or 4 PH.8, 9, 10 Table 1 summarises these important features and distinctions.
Table 1.
Histological changes in the human pulmonary microcirculation in pulmonary hypertension identified via analysis of autopsy and biopsy specimens.
| Arteriole | Capillary | Venulae | |
|---|---|---|---|
| Group 1 PH3,9,11, 12, 13, 14 | |||
| IPAH | External + internal elastic lamina hyperplasia. Mild adventitial hypertrophy. Moderate-severe medial hypertrophy. Neomuscularisation. Severe concentric, laminar, intimal fibrosis. Fibrinoid vascular necrosis/rarefaction. Plexiform lesions. Dilatation lesions. |
Moderate to severe medial hyperplasia and areas of medial necrosis. Neomuscularisation. Luminal narrowing/obliteration. Fibroelastic septa. Rarefaction. |
No significant changes. |
| CHD | External + internal elastic lamina hyperplasia. Mild adventitial hypertrophy. Moderate medial hypertrophy. Neomuscularisation. Moderate-severe concentric intimal fibrosis. Luminal narrowing/obliteration. No plexiform or dilatation lesions. |
Medial hypertrophy/hyperplasia. Neomuscularisation. Intimal fibrosis. Luminal narrowing/obliteration. Fibroelastic septa. Recanalisation. Rarefaction in some cases. |
No significant changes. |
| CTD | External + internal elastic lamina hyperplasia. Intimal thickening Moderate medial hypertrophy. Neomuscularisation. Concentric, laminar or non-laminar, intimal fibrosis. Adventitial thickening less prominent. Plexiform lesions are observed in SLE-PAH and rarely in other forms of CTD-PH |
Medial hypertrophy/hyperplasia. Neomuscularisation. Intimal fibrosis. Luminal narrowing/obliteration. |
No significant changes |
| PVOD | Mild to moderate medial hypertrophy. Neomuscularisation. Moderate concentric intimal fibrosis. Fibrous webs and recanalisation. No plexiform or dilatation lesions. |
Moderate to severe concentric intimal fibrosis. Luminal obliteration. Rarefaction. Fibrous webs and recanalisation. Trivial medial hypertrophy. |
Venulae alterations predominate. Severe concentric intimal fibrosis—mostly dense fibrosis. Luminal narrowing/obliteration Fibrous webs and recanalisation. “Arterialisation” Trivial medial hypertrophy. |
| Group 2 PH6,10,15, 16, 17 | |||
| LHDa | Moderate medial hypertrophy. Neomuscularisation. Mild-moderate concentric intimal fibrosis. Luminal narrowing/obliteration. No plexiform or dilatation lesion. No webs or recanalisation. |
Concentric intimal fibrosis (significantly higher in HFpEF compared to HFrEF). Trivial medial hypertrophy. No webs or recanalisation |
Moderate to severe concentric intimal fibrosis—mostly loose fibrosis/hyalinosis. “Arterialisation” Trivial medial hypertrophy. No webs or recanalisation |
| Group 4 PH8,9,14,18 | |||
| CTEPH | Moderate to severe eccentric and patchy intimal fibrosis. Luminal narrowing/obliteration. Prominent fibrous septa and recanalisation. Trivial medial hypertrophy. Trivial neomuscularisation. No plexiform or dilatation lesions. |
Moderate-severe eccentric intimal fibrosis. Haemangiomatous-like foci Fibroelastic septa. |
Mild-moderate eccentric intimal fibrosis. Luminal narrowing/obliteration. Fibrous septa/webs and recanalisation to a lesser extent than the arteriole. |
Left heart disease, including heart failure with reduced and preserved ejection fraction, mitral stenosis and hypertrophic cardiomyopathy.
Studies in high flow states (E.g.: CHD) confirm that microvascular remodelling occurs before resting pulmonary artery pressures rise.11,19 Irrespective of PH aetiology, adverse microvascular remodelling leads to significant functional impairment, characterised by endothelial dysfunction and impaired vasodilator mediator release.5,20
Functional perspectives on the pulmonary microcirculation
Historically, the pulmonary microcirculation was viewed as a passive conduit for blood flow. However, animal models have shown it plays an active regulatory role. In the late 1980s, it was demonstrated that the arterial microcirculation is the major source of recruitable vascular surface area under metabolic demand.7,21 Early experiments in lamb and sheep found that increased pulmonary flow with elevated left atrial pressure raised lymphatic flow without altering PVR or protein concentration, suggesting active microvascular recruitment.22,23
Exercise studies, using continuous monitoring of distal wedge, left atrial, and pulmonary pressures showed a reduction in PVR despite rising cardiac output, thus implying microvascular dilatation and recruitment, though these were indirect observations.24 Direct evidence came in 1994, when Nagasaka et al. used the servo-null micropuncture method in a cat model to show that increased pulmonary flow reduced total PVR via microvascular recruitment, without changes in arterial or venous resistance.25 This finding demonstrates that the pulmonary microcirculation is the main determinant of resistance under different conditions of flow.
Canine models further confirmed that fibrosis-induced microvascular obliteration led to higher PVR, steeper pressure-flow relationships, and markedly reduced pulmonary compliance.26 Additionally, large animal studies comparing proximal main pulmonary artery constriction with distal microvascular injury found that microvascular injury, but not proximal constriction, elevated diastolic pulmonary artery pressure due to increased wave-reflections,27 underscoring the microcirculation's role in resistance and compliance.
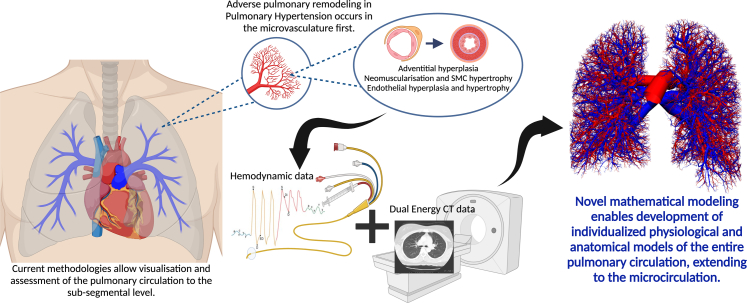
Combined CT-derived anatomical data with invasive haemodynamics and mathematical modelling (Fig. 1) further supports these findings, showing that small emboli <170 μm disproportionately increase PVR compared to larger emboli of similar volume.28 In human CTEPH, it is presumed that the degree of pulmonary microvascular dysfunction is the major determinant of persistent PH, and thus mortality, post pulmonary endarterectomy.29
Fig. 1.
Representation of the future direction of pulmonary microvascular functional and structural imaging enabling in-depth patient specific models of the pulmonary circulation to the level of the microcirculation, where adverse remodelling primarily occurs in pulmonary hypertension. This novel methodology involves combination of invasive haemodynamic, computed tomography derived anatomical data and complex mathematical modelling. Created in BioRender. Dagan, M. (2025) https://BioRender.com/u54k818. CT pulmonary circulation image used with permission from Burrowes et al., Journal of Applied Physiology 2005 Vol. 99 Issue 2 Pages 731–738, https://doi.org/10.1152/japplphysiol.01033.2004.
Together, experimental, clinical and emerging mathematical modelling technologies highlight the important functional role of the pulmonary microcirculation in determining resistance and compliance. However, direct assessment of pulmonary microvascular structure and function in vivo remains challenging. This underscores the need for novel and emerging imaging and haemodynamic techniques capable of detecting early microvascular remodelling.
Current and future approaches to pulmonary microcirculation imaging
Synchrotron-based vascular imaging
Advanced technologies such as synchrotron microangioradiography using K-edge subtraction and iodine based contrast offer high-resolution imaging of the pulmonary microcirculation, visualising vessels as small as 80 μm with low radiation exposure.30,31 This technology allows dynamic assessment of vessel calibre, blood flow velocity and volume over time, offering a unique insight into microvascular function.31,32 While preclinical models using this methodology have successfully demonstrated structural and functional changes in PH, such as reduced branching of small vessels and exaggerated vasoconstriction, clinical translation remains unfeasible at this stage. This is primarily due to the need for large beamlines (>140 m for a human heart) or continuous subject motion, making it impractical in the human setting.32
Nailfold videocapillaroscopy
While nailfold vidoecapillaroscopy (NVC) assesses peripheral capillary networks, changes in these small vessels have been associated with distal organ involvement, including the pulmonary microvasculature.33 Therefore, this non-invasive methodology has emerged as a promising tool for the assessment of PH. Beyond evaluating capillary density, NVC assessments reveal key morphological abnormalities in patients with PH compared to controls, such as irregularly enlarged loops and microhaemorrhages in group 1 PH, and capillary thrombi in group 4 PH.34 In systemic sclerosis, abnormal NVC patterns have been associated with the development of PH, and progression from early to late NVC changes correlates with disease severity, with worse NVC scores linked to elevated invasively derived mPAP.20,33 While NVC shows promise as a screening tool, in particular for systemic sclerosis related PH, it remains limited by subjective interpretation, small study populations, and lack of validated associations with clinical outcomes.
Pulmonary gas exchange
Carbon monoxide diffusing capacity (DLCO) is a readily available clinical investigation that estimates gas-exchange efficiency across the alveolar-capillary membrane and indirectly reflects pulmonary microvascular function. First introduced in 1915 and adopted clinically in the 1950s, DLCO is now a routine part of pulmonary function testing.35 Its utility stems from carbon monoxide's high affinity for haemoglobin, enabling calculation alveolar-capillary diffusion.
Reduced DLCO has been associated with mortality in various forms of PH. However, in group 1 and group 2 PH this appears to be driven by comorbidities such as increasing age, coronary artery disease and smoking exposure, thus limiting its specificity for pulmonary microvascular pathology.36,37 In contrast, DLCO may play a more important role in group 4 PH. In patients undergoing pulmonary endarterectomy (PEA) or balloon pulmonary angioplasty, a low pre-procedure DLCO predicts poorer haemodynamic improvement and higher mortality.38,39 This highlights DLCO's potential role in pre-procedural risk stratification by identifying patients with more advanced pulmonary microvascular remodelling.
Similarly, in patients with systemic sclerosis, a DLCO <60% is associated with progression to group 1 CTD-PH and may serve as a non-invasive early screening tool.40,41 However, reduced DLCO in this population may also reflect evolving interstitial lung disease, limiting its specificity.
Overall, while DLCO provides an accessible, indirect measure of pulmonary microvascular integrity, it is influenced by several factors affecting gas exchange, including alveoli damage, capillary blood volume and flow, haemoglobin levels and ventilation-perfusion matching. As such, DLCO lacks specificity to the pulmonary microvasculature.
Stress testing
In many cases, haemodynamic perturbations become apparent only during exercise, suggesting that such abnormalities may reflect early pulmonary microvascular dysfunction and loss of dynamic pulmonary vasodilatory capacity. In this context, there has been interest in cardiopulmonary exercise testing (CPET) and stress echocardiography as non-invasive screening tests that may identify these early abnormalities.42
Impaired ventilatory efficiency, reflected by an elevated VE/VCO2 slope and reduced peak VO2 on CPET, have been associated with greater pulmonary vasoconstriction, elevated pulmonary pressures (mPAP and PVR), and more severe right ventricular dysfunction in systemic sclerosis–associated PH, idiopathic Group 1 PH, Group 4 PH and heart failure.42, 43, 44 Unfortunately, access to CPET clinically is largely limited to quaternary transplant or research centres.
Similarly, stress echocardiography for the identification of an abnormal pulmonary vasodilatory capacity with exercise has been explored, in particular in systemic sclerosis and HFpEF.2 Stress echocardiography has been proposed as a non-invasive test to clarify diagnosis of group 1 versus group 2 PH, however, there is a lack of validated criteria and only modest correlation with invasive measures.2,45
Both CPET and stress echocardiography are indirect measures of pulmonary functional capacity, and the degree to which abnormalities in these tests reflect microvascular versus macrovascular abnormalities is not known. Furthermore, the limited sensitivity and specificity of these investigations restrict their utility to screening, serving primarily to identify candidates for further invasive testing.
Nuclear imaging
Nuclear imaging has traditionally been used to evaluate perfusion defects in suspected pulmonary embolism, with technetium-99m-labelled macroaggregated albumin (Tc-99m MAA) remaining a clinically relevant tracer that localises vessels down to 20–100 μm.46 More recent efforts have explored tracers that assess pulmonary microvascular function more specifically. Iodine-123-iodoamphetamine (123I-IMP)46 and iodine-123-metaiodobenxylguanidine (123I-MIBG)47,48 are actively taken up by pulmonary endothelial cells and offer insight into pulmonary microvascular integrity and function.
123I-IMP has demonstrated superior sensitivity to Tc-99m MAA in detecting smaller perfusion defects.46 In a cohort of patients with CTEPH undergoing PEA, 123I-IMP revealed improved post-procedure microvascular uptake that was not detected with Tc-99m MAA.49 However, it's clinical interpretation is limited by its lipophilic nature, which reflects lipid solubility in addition to binding site availability.50
123I-MIBG, a noradrenaline analogue taken up via energy-dependent transport, has shown promise as a more specific marker of pulmonary microvascular endothelial function.50 Studies comparing 123I-MIBG uptake between patients with group 1 PH, group 2 PH, and CTEPH relative to healthy controls found reduced uptake in group 1 PH despite similar invasive haemodyanmics.48,51 This finding suggests there is early pulmonary microvascular dysfunction that is not captured by standard RHC metrics. A single case report also found that reduced pulmonary uptake of 123I-MIBG may precede clinical PH in patients with scleroderma, further emphasising the capability of this technique to capture early pulmonary microvascular dysfunction.47 However, these were small studies (combined n = 43) and further research is needed to determine clinical significance and its role in risk stratification.
Another investigational tracer targets adrenomedullin receptors, which are highly expressed on pulmonary microvascular endothelium, and may provide a means to assess microvascular endothelial dysfunction. Using Single Photon Emission Computed Tomography (SPECT) imaging with a Tc-99m labelled adrenomedullin ligand, studies have demonstrated heterogenous tracer uptake in group 1 PH and segmental perfusion defects in group 4 PH.52,53 While promising, this technology lacks the temporal and spatial resolution of MRI and CT, limiting current clinical utility.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) has emerged as a promising modality for evaluating pulmonary microvascular function due to its non-invasive nature and absence of ionising radiation. Dynamic contrast-enhanced (DCE) MRI using gadopentetate allows quantification of regional perfusion through measurement of mean transit times, pulmonary blood flow and blood volume.54 Early studies found that reduced pulmonary perfusion in patients with group 1 PH correlated inversely with PVR.55 However, these earlier methods are limited by manual processing and simplified mathematical modelling assumptions.
Recent advances have introduced automated lung segmentation and sophisticated reconstruction techniques, including compressed sensing and parallel imaging, enhancing efficiency and resolution.54,56 Despite these improvements, DCE-MRI still assesses the microcirculation indirectly via extrapolation from segmental artery-level data.
Hyperpolarised xenon-129 (HP 129Xe) combined with one point Dixon MRI represents a significant innovation in directly probing pulmonary microvascular function. This technique measures fluctuations in the xenon signal within red blood cells caused by cardiac contractions, allowing regional assessment of changes in pulmonary microvascular blood volume throughout the cardiac cycle and providing insight into microvascular compliance.57,58 Fig. 2 depicts the novel outputs from this technique. Compared to healthy controls, patients with group 1 PH and group 4 PH demonstrated skewed oscillation patterns toward low frequencies, reflecting impaired pulmonary microvascular compliance.57 In contrast, patients with idiopathic pulmonary fibrosis demonstrate high-frequency oscillations, representing preserved cardiac output into a reduced microvascular bed.58
Fig. 2.
Quantitative mapping of cardiopulmonary oscillations using hyperpolarized 129Xe gas exchange MRI. A) and B) demonstrate 129Xe MRI RBC transfer and oscillation imaging for a CTEPH patient with cpcPH, A) at baseline and B) post pulmonary thromboendarterectomy surgery. RBC oscillation maps indicate regions of both decreased and increased oscillation (arrows) (D-defect; L-low; H-high). C) and D) demonstrate comparison of metrics obtained from RBC oscillation maps in the healthy cohort versus patients with CTEPH, while E) and F) demonstrate these parameters pre and post pulmonary thromboendarterectomy in the same patient. Image used with permission from Lu et al., Magnetic Resonance in Med, Volume: 91, Issue: 4, Pages: 1541–1555, 2024, https://doi.org/10.1002/mrm.29965.
In healthy individuals, xenon oscillation maps show a clear anterior-to-posterior signal intensity gradient, reflecting normal gravitational variation in pulmonary microvascular perfusion. This gradient is lost in individuals with group 1 PH and idiopathic pulmonary fibrosis, suggesting early disruption in regional pulmonary microvascular function.58
Interestingly, mean red blood cell oscillation was strongly negatively correlated with DLCO (r = −0·64, p ≤ 0.001), and changes in oscillation amplitude preceded the reduction in DLCO, supporting its potential as an early marker of pulmonary microvascular dysfunction.58 Further, in follow up imaging of patients with group 4 PH after PEA, the proportion of low oscillation regions decreased significantly (from 37% to 23%), while high oscillation regions remained unchanged, and mean oscillation amplitude overall did not significantly differ.57 These findings suggest that while PEA effectively reduces proximal obstruction, persistent distal microvascular abnormalities may contribute to ongoing haemodynamic impairment and incomplete clinical recovery. This highlights the potential role of HP 129Xe MRI in patient selection for PEA.
While promising for early detection of pulmonary microvascular dysfunction and guiding treatment decisions, clinical adoption of HP 129Xe MRI remains constrained by technical and logistical challenges. These include the need for prolonged breath-holding, which is often not feasible for patients with PH, and the limited global supply plus high cost of HP 129Xe gas.
Another rapidly advancing MRI technique is 4D flow imaging, an evolving technology that enables visualisation of pulmonary artery velocity and flow rate, with the use of deep learning models to optimise outputs.59 It has demonstrated the potential to differentiate between isolated post-capillary PH and combined pre- and post-capillary PH in individuals with group 2 PH, though with modest specificity (∼55%).60 Limitations include lower spatial and temporal resolution compared to CT, longer acquisition times, and dependency on radiographer expertise. Nonetheless, with rapid advancements in artificial intelligence-driven reconstruction, 4D flow MRI may play a role in detailed haemodynamic phenotyping in PH in the near future.
CT-based computational modelling
Computational models that incorporate both anatomical imaging and physiological data have been developed to better understand heterogeneity in pulmonary microvascular perfusion across health and various forms of PH. One of the earliest modelling approaches applied the Windkessel model in individuals with group 4 PH as a proof of concept.61 Despite its simplification, with exclusion of pulsatile flow, wave reflections and regional variation, it demonstrated that changes in post-stenotic pressure could help estimate the contribution of microvascular dysfunction to overall haemodynamics.61 Although limited in scope, these early models laid the groundwork for more anatomically and physiologically realistic simulations of microvascular dynamics.
To improve on this, anatomically detailed models were developed by Burrowes et al. incorporating the complex branching structure of the pulmonary microcirculation.62 Unlike previous symmetrical models, this approach accounted for the asymmetrical geometry and the contribution of supernumerary vessels, which are thought to represent the recruitable portion of the pulmonary microcirculation. Using contrast enhanced multidetector row computed tomography (MDCT) with spatial resolution of 0.68 mm, Burrowes et al. mapped large vessels and used a volume filling branching (VFB) algorithm to computationally generate arterial and venous tree down to 5–10 μm.62 This resulted in high-fidelity patient-specific models comprising ∼375,000 arterial segments and ∼497,000 venous segments, all connected by symmetric capillary “sheets”.62,63 These models enable patient-specific simulation of perfusion across the entire pulmonary circulation, including microvascular domains, while preserving anatomical realism.
Expanding on this foundation, Clark and Tawhai integrated wave transmission theory with a sheet flow impedance model to simulate temporal flow dynamics and regional variation in the pulmonary microcirculation.64 Their model used a typical pulmonary artery pressure waveform at a heart rate of 75 beats per minute and computed impedance and wave reflections across the entire vascular tree, including the microcirculation (represented in Fig. 1). When comparing a symmetric vascular model to one incorporating asymmetric branching and gravitational influences, wave reflections were observed exclusively in the asymmetric model, highlighting the importance of anatomical realism in computational modelling. Flow heterogeneity was demonstrated across both the cardiac cycle and the gravitational axis. Interestingly, clusters of microvascular units with flow patterns independent of gravitational gradients emerged, primarily driven by differences in path lengths between the right and left heart.64,65 When the model was adjusted to mimic PH by uniformly reducing vascular compliance, it produced non-uniform redistribution of blood flow and greater regional perfusion heterogeneity, underscoring the role of pulmonary microvascular stiffness in driving localised flow abnormalities.64
This same model has been adapted to reflect structural changes in group 1 PH and CTEPH using histologically-informed perturbations.66,67 Simulated impedance spectra in the main pulmonary artery showed distinct signatures for each disease. For example, group 1 PH exhibited a right-shifted high frequency impedance, while CTEPH revealed proximal wave reflections that dampen signal transmission.67 Wave intensity metrics, such as wave speed, forward compression wave intensity, and backward wave intensity, showed promise in distinguishing between group 1 PH and CTEPH, although non-linear relationships may complicate clinical application. Notably, pressure criteria for PH (mPAP >20 mmHg) were only reached at advanced remodelling stages, aligning with the concept that early microvascular remodelling precedes detectible elevation in mPAP.65,66
Alternative modelling strategies have also been used to simulate group 2 PH by increasing left atrial pressure and reducing cardiac output.68,69 These models, based on asymmetrical vascular trees and 1D fluid dynamics, showed reductions in wall shear stress and increased cyclic stretch within the pulmonary microcirculation—factors implicated in vascular remodelling.69 Quantification of uncertainty and sensitivity revealed that pulmonary microcirculation density was a key determinant of model output.70 However, unlike the Clark and Tawhai model, these simulations lack invasive pressure waveforms, limiting their ability to reflect patient-specific haemodynamics.64
Overall, CT-based computational models offer a powerful platform to simulate pulmonary microvascular anatomy and physiology across PH phenotypes. By integrating patient-specific anatomical and haemodynamic data, these models have the potential to support early diagnosis, stratify disease severity, monitor disease progression and evaluate response to therapy, thereby bridging the gap between physiological insight and clinical decision making.
Invasive approaches to the evaluation of pulmonary microcirculation
Exercise right heart catheterisation
The 2022 re-introduction of exercise PH into clinical guidelines has renewed interest in exercise RHC as a diagnostic tool. This technique can unmask early pulmonary vascular disease and assist in differentiating Group 1 PH from HFpEF or dynamic mitral regurgitation. Although direct correlations with histology are lacking, a steep rise in mPAP relative to cardiac output during exercise is thought to reflect early pulmonary microvascular remodelling and a loss of vascular recruitability.71 This interpretation is supported by data in individuals with systemic sclerosis, in whom exercise PH predicts future development of resting PH and is associated with worse outcomes.71,72 Elevated mPAP/CO slope in individuals with chronic thromboembolic disease may also identify those who could benefit from pulmonary endarterectomy.73 However, in such cases, these haemodynamic changes likely reflect macrovascular obstruction rather than true microvascular pathology, underscoring the limitations of exercise RHC in isolating microcirculatory abnormalities. Additionally, data regarding the prognostic relevance of exercise PH in Group 2 PH is lacking.
Waveform analysis
Waveform analysis using a dual-tipped pressure and Doppler velocity sensor wire allows simultaneous acquisition of pulmonary artery pressure and flow velocity in the segmental pulmonary artery.74 This approach can provide detailed insight into pulmonary microvascular dynamics and is particularly valuable for assessing compliance and wave reflection patterns. Parameters derived include wave speed, forward compression wave, forward decompression wave, backward wave and reservoir-excess pressure. Reservoir-excess pressure is especially promising as it can be calculated from pressure tracings alone, eliminating the need for Doppler flow measurements that are rarely measured in clinical practice.74 In healthy controls, reservoir-excess pressure and velocity waveforms closely align. In contrast, individuals with group 1 PH demonstrate a significantly higher reservoir-excess pressure and a rapid mid-systolic fall in velocity consistent with prominent backwards wave reflections.74 This pattern represents the reduction in pulmonary microvascular compliance observed in PH.
Similarly, analysis of pressure decay curves during RHC is another invasive haemodynamic measure that has been investigated for its utility in identifying microvascular dysfunction in patients with CTEPH.18 By isolating the pressure decay between the pulmonary artery and wedge pressure, both upstream (microvasculature) and downstream (macrovascular) resistance components can be calculated.18 In individuals with CTEPH, upstream (microvascular) resistance independently predicted poor outcomes post PEA.18
These waveform based metrics offer clinically accessible ways to assess pulmonary microvasculature involvement and compliance abnormalities in PH. However, broader application will require standardised reference values and validation in larger cohorts.
Vasoreactivity testing
Acute pulmonary vasoreactivity testing is guideline-recommended in individuals with group 1 PH to identify vaso-responders who may benefit from long-term calcium channel blocker therapy.2 The response to inhaled vasodilators (most commonly nitric oxide or iloprost) primarily reflects pulmonary microvasculature reactivity. While such testing is not recommended in other PH groups, and may be harmful in group 2 PH, its role is being explored in group 4 PH, where persistent microvascular dysfunction contributes to residual elevation in PVR and increased mortality post PEA.18,75 In patients with CTEPH undergoing PEA, a >10% reduction in mPAP during nitric oxide challenge was associated with improved long term outcomes.75 Though these findings are preliminary, vasoreactivity could potentially aid in risk stratification and assessment of fixed pulmonary microvascular damage beyond group 1 PH.
Indices of pulmonary flow reserve and microvascular resistance
Advances in coronary microvascular diagnostics may be translatable to the pulmonary circulation. In the coronary system, assessments of microvascular function using Doppler flow velocity or bolus thermodilution have become guideline-recommended for angina with non-obstructive coronary arteries.76 Continuous thermodilution offers direct, reproducible quantification of absolute coronary flow and microvascular resistance, with advantages over bolus thermodilution methods.77
The pulmonary circulation shares many anatomical and physiological features with the coronary circulation,7 and animal models have successfully adapted these coronary techniques to the pulmonary microcirculation.78,79 In primate models, both Doppler and thermodilution methods demonstrated feasibility. Pulmonary artery diameter and flow velocity remained stable during saline infusion at 10–15 ml/min, allowing baseline flow measurement without hyperaemia.77,79 Additionally, vasodilators such as adenosine and acetylcholine produced dose dependent increase in pulmonary Doppler flow velocity, with no accompanying changes in vessel calibre or systemic haemodynamics, consistent with a distal endothelial-dependent microvascular action.79 Intrapulmonary nitroprusside on the other hand, increased both Doppler pulmonary flow velocity and pulmonary artery diameter, suggesting mixed endothelial-dependent and independent vasodilatory mechanism.80 Importantly, thermal stability along the vessel course confirmed that thermodilution remains a valid surrogate of flow in the pulmonary circulation.78
These methods enable calculation of pulmonary flow reserve and pulmonary index of microvascular resistance or microvascular resistance reserve, depending on bolus or continuous thermodilution method. With induction of microvascular obstruction in primate models, a significant reduction in pulmonary flow reserve and increase in microvascular resistance was observed before overt haemodynamic changes.78 In children with group 1 PH, PFR measurements using acetylcholine showed that non-responders to vasodilation had significantly lower PFR than responders.81 This demonstrates that invasive indices of pulmonary microvascular function may aid in phenotyping and early detection.
While promising, these invasive techniques have not yet been translated to clinical pulmonary practice. Notably, the continuous thermodilution method with its more accurate and direct quantification of coronary flow and microvascular resistance, has yet to be evaluated in the pulmonary microcirculation.
Conclusion
The pulmonary microcirculation is a critical determinant of pathophysiological changes observed in pulmonary hypertension, regardless of aetiology. Although our understanding of pulmonary microvascular structure and function has advanced substantially, most clinical assessment tools remain confined to the research setting. Current approaches are often indirect or are limited by technical, logistical and financial barriers.
Recent innovations show promise. CT-based computational modelling offers the ability to construct patient-specific anatomical and physiological models, while invasive techniques such as pulmonary flow reserve and microvascular resistance indices could enable detailed phenotyping in PH. These emerging methodologies may allow for earlier diagnosis, identification of patient-specific microvascular changes and improved monitoring of disease progression and treatment response. As these tools are refined and validated, they may pave the way for targeted interventions and personalised approaches to pulmonary vascular disease.
Outstanding questions
The pulmonary microcirculation has long been recognised as a central determinant of pathophysiological changes across all forms of PH. However, clinical assessment of pulmonary microvascular structure and function remains limited by current diagnostic modalities. Whether advanced CT-based computational modelling can be reliably applied to generate patient-specific assessments of pulmonary microvascular perfusion, resistance, and compliance remains to be determined. Similarly, the potential of invasive techniques such as pulmonary flow reserve and pulmonary microvascular resistance to be validated and standardised for routine clinical use across PH subtypes is yet to be realised. Another key consideration is whether early identification of pulmonary microvascular dysfunction will enable timely therapeutic intervention, improve symptom management, or slow disease progression. It is also critical to understand the risks posed by premature adoption of unvalidated technologies, particularly in vulnerable PH populations. Answering these questions is essential for transitioning novel diagnostic strategies from the research setting into tools that benefit individuals living with or at risk of pulmonary hypertension.
Contributors
All authors made a substantial contribution to the conceptualisation of this review. MD conducted the literature search and draughted the manuscript and figures. SN, WC, DCM, and DMK critically reviewed the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Declaration of interests
David M Kaye has received funding from Pfizer, Novartis, Abbott, Novo Nordisk and Corvia Medical for educational events and meeting support and is on the Advisory Board for Novo Nordisk and the Heart Foundation of Australia. No other authors have declarations. No funders had any role in paper design, data collection, interpretation, or writing of this review.
Acknowledgements
David M Kaye is supported by a National Health and Medical Research Council of Australia Grant and a Medical Research Future Fund of Australia Grant. Misha Dagan is supported by an National Health and Medical Research Council of Australia postgraduate scholarship.
References
- 1.Callan P., Clark A.L. Right heart catheterisation: indications and interpretation. Heart. 2016;102(2):147–157. doi: 10.1136/heartjnl-2015-307786. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2023;44(15):1312. doi: 10.1093/eurheartj/ehad018. [DOI] [PubMed] [Google Scholar]
- 3.Heath D., Whitaker W. Hypertensive pulmonary vascular disease. Circulation. 1956;14(3):323–343. doi: 10.1161/01.cir.14.3.323. [DOI] [PubMed] [Google Scholar]
- 4.Reid L., Meyrick B. Hypoxia and pulmonary vascular endothelium. Ciba Found Symp. 1980;78:37–60. doi: 10.1002/9780470720615.ch3. [DOI] [PubMed] [Google Scholar]
- 5.Madonna R., Biondi F., Ghelardoni S., D'Alleva A., Quarta S., Massaro M. Pulmonary hypertension associated to left heart disease: phenotypes and treatment. Eur J Intern Med. 2024;129:1–15. doi: 10.1016/j.ejim.2024.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Hunt J.M., Bethea B., Liu X., et al. Pulmonary veins in the normal lung and pulmonary hypertension due to left heart disease. Am J Physiol Lung Cell Mol Physiol. 2013;305(10):L725–L736. doi: 10.1152/ajplung.00186.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guntheroth W.G., Luchtel D.L., Kawabori I. Functional implications of the pulmonary microcirculation. An update. Chest. 1992;101(4):1131–1134. doi: 10.1378/chest.101.4.1131. [DOI] [PubMed] [Google Scholar]
- 8.Dorfmüller P., Günther S., Ghigna M.R., et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J. 2014;44(5):1275–1288. doi: 10.1183/09031936.00169113. [DOI] [PubMed] [Google Scholar]
- 9.Wagenvoort C.A., Wagenvoort N. Primary pulmonary hypertension. Circulation. 1970;42(6):1163–1184. [Google Scholar]
- 10.Delgado J.F., Conde E., Sánchez V., et al. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail. 2005;7(6):1011–1016. doi: 10.1016/j.ejheart.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Meyrick B., Reid L. Ultrastructural findings in lung biopsy material from children with congenital heart defects. Am J Pathol. 1980;101(3):527–542. [PMC free article] [PubMed] [Google Scholar]
- 12.Favoino E., Prete M., Liakouli V., et al. Idiopathic and connective tissue disease-associated pulmonary arterial hypertension (PAH): similarities, differences and the role of autoimmunity. Autoimmun Rev. 2024;23(4) doi: 10.1016/j.autrev.2024.103514. [DOI] [PubMed] [Google Scholar]
- 13.Galambos C., Sims-Lucas S., Abman S.H., Cool C.D. Intrapulmonary bronchopulmonary anastomoses and plexiform lesions in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;193(5):574–576. doi: 10.1164/rccm.201507-1508LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjornsson J., Edwards W.D. Primary pulmonary hypertension: a histopathologic study of 80 cases. Mayo Clin Proc. 1985;60(1):16–25. doi: 10.1016/s0025-6196(12)65277-x. [DOI] [PubMed] [Google Scholar]
- 15.Fayyaz A.U., Edwards W.D., Maleszewski J.J., et al. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation. 2018;137(17):1796–1810. doi: 10.1161/CIRCULATIONAHA.117.031608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maron B.A., Kleiner D.E., Arons E., et al. Evidence of advanced pulmonary vascular remodeling in obstructive hypertrophic cardiomyopathy with pulmonary hypertension. Chest. 2023;163(3):678–686. doi: 10.1016/j.chest.2022.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodale F., Sanchez G., Friedlich A.L., Scannell J.G., Myers G.S. Correlation of pulmonary arteriolar resistance with pulmonary vascular changes in patients with mitral stenosis before and after valvulotomy. N Engl J Med. 1955;252(23):979–983. doi: 10.1056/NEJM195506092522303. [DOI] [PubMed] [Google Scholar]
- 18.Gerges C., Gerges M., Friewald R., et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: hemodynamic phenotyping and histomorphometric assessment. Circulation. 2020;141(5):376–386. doi: 10.1161/CIRCULATIONAHA.119.041515. [DOI] [PubMed] [Google Scholar]
- 19.Rabinovitch M., Haworth S.G., Vance Z., et al. Early pulmonary vascular changes in congenital heart disease studied in biopsy tissue. Hum Pathol. 1980;11(5 Suppl):499–509. [PubMed] [Google Scholar]
- 20.Dara A., Arvanitaki A., Theodorakopoulou M., Athanasiou C., Pagkopoulou E., Boutou A. Non-invasive assessment of endothelial dysfunction in pulmonary arterial hypertension. Mediterr J Rheumatol. 2021;32(1):6–14. doi: 10.31138/mjr.32.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson W.L., Emhardt J.D., Bartek J.P., et al. Site of recruitment in the pulmonary microcirculation. J Appl Physiol. 1989;66(5):2079–2083. doi: 10.1152/jappl.1989.66.5.2079. [DOI] [PubMed] [Google Scholar]
- 22.Teague W.G., Berner M.E., Bland R.D. Effect of pulmonary perfusion on lung fluid filtration in young lambs. Am J Physiol. 1988;255(6 Pt 2):H1336–H1341. doi: 10.1152/ajpheart.1988.255.6.H1336. [DOI] [PubMed] [Google Scholar]
- 23.Coates G., O'Brodovich H., Jefferies A.L., Gray G.W. Effects of exercise on lung lymph flow in sheep and goats during normoxia and hypoxia. J Clin Invest. 1984;74(1):133–141. doi: 10.1172/JCI111393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman J.H., Cochran C.P., Roselli R.J., Parker R.E., King L.S. Pressure and flow changes in the pulmonary circulation in exercising sheep: evidence for elevated microvascular pressure. Am Rev Respir Dis. 1993;147(4):921–926. doi: 10.1164/ajrccm/147.4.921. [DOI] [PubMed] [Google Scholar]
- 25.Nagasaka Y., Ishigaki M., Okazaki H., et al. Effect of pulmonary blood flow on microvascular pressure profile determined by micropuncture in perfused cat lungs. J Appl Physiol. 1994;77(4):1834–1839. doi: 10.1152/jappl.1994.77.4.1834. [DOI] [PubMed] [Google Scholar]
- 26.Michel R.P., Hakim T.S., Freeman C.R. Distribution of pulmonary vascular resistance in experimental fibrosis. J Appl Physiol. 1988;65(3):1180–1190. doi: 10.1152/jappl.1988.65.3.1180. [DOI] [PubMed] [Google Scholar]
- 27.Calvin J.E., Jr., Baer R.W., Glantz S.A. Pulmonary artery constriction produces a greater right ventricular dynamic afterload than lung microvascular injury in the open chest dog. Circ Res. 1985;56(1):40–56. doi: 10.1161/01.res.56.1.40. [DOI] [PubMed] [Google Scholar]
- 28.Clark A.R., Burrowes K.S., Tawhai M.H. The impact of micro-embolism size on haemodynamic changes in the pulmonary micro-circulation. Respir Physiol Neurobiol. 2011;175(3):365–374. doi: 10.1016/j.resp.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Jamieson S.W., Kapelanski D.P., Sakakibara N., et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76(5):1457–1462. doi: 10.1016/s0003-4975(03)00828-2. [DOI] [PubMed] [Google Scholar]
- 30.Tokunaga C., Matsushita S., Sakamoto H., et al. A new method for visualizing pulmonary artery microvasculature using synchrotron radiation pulmonary microangiography: the measurement of pulmonary arterial blood flow velocity in the high pulmonary blood flow rat model. Acta Radiol. 2018;59(12):1482–1486. doi: 10.1177/0284185118770892. [DOI] [PubMed] [Google Scholar]
- 31.Schwenke D.O., Pearson J.T., Umetani K., Kangawa K., Shirai M. Imaging of the pulmonary circulation in the closed-chest rat using synchrotron radiation microangiography. J Appl Physiol. 2007;102(2):787–793. doi: 10.1152/japplphysiol.00596.2006. [DOI] [PubMed] [Google Scholar]
- 32.Shirai M., Schwenke D.O., Eppel G.A., et al. Synchrotron-based angiography for investigation of the regulation of vasomotor function in the microcirculation in vivo. Clin Exp Pharmacol Physiol. 2009;36(1):107–116. doi: 10.1111/j.1440-1681.2008.05073.x. [DOI] [PubMed] [Google Scholar]
- 33.Xu C., Sellke F.W., Abid M.R. Assessments of microvascular function in organ systems. Am J Physiol Heart Circ Physiol. 2022;322(6):H891–H905. doi: 10.1152/ajpheart.00589.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arvanitaki A., Giannakoulas G., Triantafyllidou E., et al. Peripheral microangiopathy in precapillary pulmonary hypertension: a nailfold video capillaroscopy prospective study. Respir Res. 2021;22(1):27. doi: 10.1186/s12931-021-01622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes J.M.B., Bates D.V. Historical review: the carbon monoxide diffusing capacity (DlCO) and its membrane (Dm) and red cell (Θ·Vc) components. Respir Physiol Neurobiol. 2003;138(2):115–142. doi: 10.1016/j.resp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Trip P., Nossent E.J., de Man F.S., et al. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J. 2013;42(6):1575–1585. doi: 10.1183/09031936.00184412. [DOI] [PubMed] [Google Scholar]
- 37.Hoeper M.M., Meyer K., Rademacher J., Fuge J., Welte T., Olsson K.M. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4(6):441–449. doi: 10.1016/j.jchf.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Suda R., Tanabe N., Ishida K., et al. Prognostic and pathophysiological marker for patients with chronic thromboembolic pulmonary hypertension: usefulness of diffusing capacity for carbon monoxide at diagnosis. Respirology. 2017;22(1):179–186. doi: 10.1111/resp.12883. [DOI] [PubMed] [Google Scholar]
- 39.Minatsuki S., Hatano M., Hirose K., et al. Differential effects of balloon pulmonary angioplasty on chronic thromboembolic pulmonary disease. Heart. 2024;110(18):1133–1138. doi: 10.1136/heartjnl-2024-323883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allanore Y., Borderie D., Avouac J., et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum. 2008;58(1):284–291. doi: 10.1002/art.23187. [DOI] [PubMed] [Google Scholar]
- 41.Coghlan J.G., Denton C.P., Grünig E., et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73(7):1340. doi: 10.1136/annrheumdis-2013-203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovacs G., Bartolome S., Denton C.P., et al. Definition, classification and diagnosis of pulmonary hypertension. Eur Respir J. 2024;64(4) doi: 10.1183/13993003.01324-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guazzi M., Borlaug B.A. Pulmonary hypertension due to left heart disease. Circulation. 2012;126(8):975–990. doi: 10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 44.Dumitrescu D., Nagel C., Kovacs G., et al. Cardiopulmonary exercise testing for detecting pulmonary arterial hypertension in systemic sclerosis. Heart. 2017;103(10):774–782. doi: 10.1136/heartjnl-2016-309981. [DOI] [PubMed] [Google Scholar]
- 45.Škafar M., Ambrožič J., Toplišek J., Cvijić M. Role of exercise stress echocardiography in pulmonary hypertension. Life (Basel) 2023;13(6):1385. doi: 10.3390/life13061385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirose Y., Hayashida K., Ishida Y., Takamiya M., Nishimura T. I-123 iodoamphetamine lung scanning in patients with ventilation-perfusion mismatching. Clin Nucl Med. 1995;20(5):421–425. doi: 10.1097/00003072-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Higo K., Kubota K., Hiwatari S., et al. The potential for early diagnosis of pulmonary arterial hypertension using lung iodine-123-metaiodobenzylguanidine (123I-MIBG) uptake: a case report. Radiol Case Rep. 2020;15(8):1164–1167. doi: 10.1016/j.radcr.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higo K., Kubota K., Miyanaga S., et al. Impairment of iodine-123-metaiodobenzylguanidine ((123)I-MIBG) uptake in patients with pulmonary artery hypertension. Int Heart J. 2018;59(1):112–119. doi: 10.1536/ihj.16-629. [DOI] [PubMed] [Google Scholar]
- 49.Kume N., Hayashida K., Nakanishi N., Cho I., Suga K., Matsunaga N. Visualization of functional improvement by 123I-IMP lung SPET after thromboendarterectomy for chronic pulmonary embolism. Nucl Med Commun. 1999;20(3):247–253. doi: 10.1097/00006231-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Slosman D.O., Morel D.R., Alderson P.O. A new imaging approach to quantitative evaluation of pulmonary vascular endothelial metabolism. J Thorac Imaging. 1988;3(1):49–52. doi: 10.1097/00005382-198801000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Gimelli A., Pugliese N.R., Bertasi M., et al. Cardio-pulmonary involvement in pulmonary arterial hypertension: a perfusion and innervation scintigraphic evaluation. J Nucl Cardiol. 2021;28(2):546–556. doi: 10.1007/s12350-019-01689-w. [DOI] [PubMed] [Google Scholar]
- 52.Alonso Martinez L.M., Harel F., Létourneau M., et al. SPECT and PET imaging of adrenomedullin receptors: a promising strategy for studying pulmonary vascular diseases. Am J Nucl Med Mol Imaging. 2019;9(5):203–215. [PMC free article] [PubMed] [Google Scholar]
- 53.Harel F., Levac X., Nguyen Q.T., et al. Molecular imaging of the human pulmonary vascular endothelium using an adrenomedullin receptor ligand. Mol Imaging. 2015;14(5) doi: 10.2310/7290.2015.00003. [DOI] [PubMed] [Google Scholar]
- 54.Ley S., Ley-Zaporozhan J. Pulmonary perfusion imaging using MRI: clinical application. Insights Imaging. 2012;3(1):61–71. doi: 10.1007/s13244-011-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohno Y., Hatabu H., Murase K., et al. Primary pulmonary hypertension: 3D dynamic perfusion MRI for quantitative analysis of regional pulmonary perfusion. AJR Am J Roentgenol. 2007;188(1):48–56. doi: 10.2214/AJR.05.0135. [DOI] [PubMed] [Google Scholar]
- 56.Zöllner F.G., Daab M., Weidner M., et al. Semi-automatic lung segmentation of DCE-MRI data sets of 2-year old children after congenital diaphragmatic hernia repair: initial results. Magn Reson Imaging. 2015;33(10):1345–1349. doi: 10.1016/j.mri.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Lu J., Alenezi F., Bier E., et al. Optimized quantitative mapping of cardiopulmonary oscillations using hyperpolarized (129) Xe gas exchange MRI: digital phantoms and clinical evaluation in CTEPH. Magn Reson Med. 2024;91(4):1541–1555. doi: 10.1002/mrm.29965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niedbalski P.J., Bier E.A., Wang Z., Willmering M.M., Driehuys B., Cleveland Z.I. Mapping cardiopulmonary dynamics within the microvasculature of the lungs using dissolved (129)Xe MRI. J Appl Physiol. 2020;129(2):218–229. doi: 10.1152/japplphysiol.00186.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ponz I., Nuche J., Sanchez Sanchez V., et al. Non-invasive assessment of pulmonary vasculopathy. Heart. 2021;2(1):5–14. [Google Scholar]
- 60.Nuche J., Ponz I., Sánchez Sánchez V., et al. Four-dimensional magnetic resonance pulmonary flow imaging for assessing pulmonary vasculopathy in patients with postcapillary pulmonary hypertension. J Clin Med. 2025;14(3):929. doi: 10.3390/jcm14030929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spazzapan M., Sastry P., Dunning J., Nordsletten D., de Vecchi A. The use of biophysical flow models in the surgical management of patients affected by chronic thromboembolic pulmonary hypertension. Front Physiol. 2018;9:223. doi: 10.3389/fphys.2018.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burrowes K.S., Hunter P.J., Tawhai M.H. Anatomically based finite element models of the human pulmonary arterial and venous trees including supernumerary vessels. J Appl Physiol. 2005;99(2):731–738. doi: 10.1152/japplphysiol.01033.2004. [DOI] [PubMed] [Google Scholar]
- 63.Fung Y.C., Sobin S.S. Theory of sheet flow in lung alveoli. J Appl Physiol. 1969;26(4):472–488. doi: 10.1152/jappl.1969.26.4.472. [DOI] [PubMed] [Google Scholar]
- 64.Clark A.R., Tawhai M.H. Temporal and spatial heterogeneity in pulmonary perfusion: a mathematical model to predict interactions between macro- and micro-vessels in health and disease. ANZIAM J. 2018;59(4):562–580. [Google Scholar]
- 65.Burrowes K.S., Clark A.R., Tawhai M.H. Blood flow redistribution and ventilation-perfusion mismatch during embolic pulmonary arterial occlusion. Pulm Circ. 2011;1(3):365–376. doi: 10.4103/2045-8932.87302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ebrahimi B.S., Tawhai M.H., Kumar H., et al. A computational model of contributors to pulmonary hypertensive disease: impacts of whole lung and focal disease distributions. Pulm Circ. 2021;11(4) doi: 10.1177/20458940211056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebrahimi B.S., Tawhai M.H., Kumar H., Clark A.R. Wave reflection in an anatomical model of the pulmonary circulation in local and global hypertensive disease. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:4973–4976. doi: 10.1109/EMBC.2019.8857948. [DOI] [PubMed] [Google Scholar]
- 68.Qureshi M.U., Vaughan G.D., Sainsbury C., et al. Numerical simulation of blood flow and pressure drop in the pulmonary arterial and venous circulation. Biomech Model Mechanobiol. 2014;13(5):1137–1154. doi: 10.1007/s10237-014-0563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bartolo M.A., Qureshi M.U., Colebank M.J., Chesler N.C., Olufsen M.S. Numerical predictions of shear stress and cyclic stretch in pulmonary hypertension due to left heart failure. Biomech Model Mechanobiol. 2022;21(1):363–381. doi: 10.1007/s10237-021-01538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colebank M.J., Chesler N.C. Efficient uncertainty quantification in a spatially multiscale model of pulmonary arterial and venous hemodynamics. Biomech Model Mechanobiol. 2024;23(6):1909–1931. doi: 10.1007/s10237-024-01875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kovacs G., Herve P., Barbera J.A., et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50(5) doi: 10.1183/13993003.00578-2017. [DOI] [PubMed] [Google Scholar]
- 72.Zeder K., Avian A., Bachmaier G., et al. Exercise pulmonary resistances predict long-term survival in systemic sclerosis. Chest. 2021;159(2):781–790. doi: 10.1016/j.chest.2020.08.2110. [DOI] [PubMed] [Google Scholar]
- 73.Guth S., Wiedenroth C.B., Rieth A., et al. Exercise right heart catheterisation before and after pulmonary endarterectomy in patients with chronic thromboembolic disease. Eur Respir J. 2018;52(3) doi: 10.1183/13993003.00458-2018. [DOI] [PubMed] [Google Scholar]
- 74.Su J., Manisty C., Simonsen U., Howard L.S., Parker K.H., Hughes A.D. Pulmonary artery wave propagation and reservoir function in conscious man: impact of pulmonary vascular disease, respiration and dynamic stress tests. J Physiol. 2017;595(20):6463–6476. doi: 10.1113/JP274385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skoro-Sajer N., Hack N., Sadushi-Koliçi R., et al. Pulmonary vascular reactivity and prognosis in patients with chronic thromboembolic pulmonary hypertension: a pilot study. Circulation. 2009;119(2):298–305. doi: 10.1161/CIRCULATIONAHA.108.794610. [DOI] [PubMed] [Google Scholar]
- 76.Byrne R.A., Rossello X., Coughlan J.J., et al. 2023 ESC Guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC) Eur Heart J. 2023;44(38):3720–3826. doi: 10.1093/eurheartj/ehad191. [DOI] [PubMed] [Google Scholar]
- 77.Belmonte M., Gallinoro E., Pijls N.H.J., et al. Measuring absolute coronary flow and microvascular resistance by thermodilution. J Am Coll Cardiol. 2024;83(6):699–709. doi: 10.1016/j.jacc.2023.12.014. [DOI] [PubMed] [Google Scholar]
- 78.Ilsar R., Chawantanpipat C., Chan K.H., et al. Measurement of pulmonary flow reserve and pulmonary index of microcirculatory resistance for detection of pulmonary microvascular obstruction. PLoS One. 2010;5(3) doi: 10.1371/journal.pone.0009601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ilsar R., Chawantanpipat C., Chan K.H., et al. Measurement of pulmonary flow reserve in higher primates. Clin Exp Pharmacol Physiol. 2009;36(8):797–802. doi: 10.1111/j.1440-1681.2009.05160.x. [DOI] [PubMed] [Google Scholar]
- 80.Celermajer D.S., Cullen S., Deanfield J.E. In vivo detection of endothelium dependent and independent pulmonary artery relaxation in children. Br Heart J. 1993;69(4):298–302. doi: 10.1136/hrt.69.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimmermann R., Kreuder J., Michel-Behnke I., Voelkel N.F., Schranz D. Pulmonary flow reserve in children with idiopathic pulmonary arterial hypertension: implications for diagnosis and therapy. Eur J Med Res. 2006;11(5):208–213. [PubMed] [Google Scholar]