Abstract
Silk fibroin from the silkworm, Bombyx mori, is a unique biomaterial that has been extensively studied for a variety of applications that utilize robust mechanical properties, biological compatibility, and controlled self-assembly properties. This study tested carbon–halogen (C–X) bond halogenation to alter the chemical composition of silk fibroin with the intention to generate novel functional materials. In brief, silk fibroin side-chain modification used halogen salts (NaX, X = Cl, Br, and I), hydrogen peroxide (H2O2), and the vanadium-dependent haloperoxidase from Curvularia inaequalis to produce primarily halogenated tyrosine residues along the amorphous regions of the silk fibroin protein. Halogenation was confirmed with various methods, including 1D 1H NMR, X-ray photoelectron spectroscopy, and analysis of chymotrypsin peptide digests by Q-TOF Liquid Chromatography–Mass Spectrometry. Secondary structure analysis by FTIR-ATR, circular dichroism, and Raman spectroscopy revealed increase in helical conformation of solubilized halogenated silk fibroin, while dried film functionality demonstrated higher abundance of β-sheet structures by maintenance of random coil content. Evaluation by contact angle measurement demonstrated increased hydrophilicity on silk fibroin films following addition of halogens by supporting the formation of water insoluble hydrogels after treatment with various organic and salt solvents. This study is the first to characterize the effects of enzymatic halogenations on the properties of silk fibroin, and this post-translational modification will be useful for the addition of non-natural small molecules or ligands, introducing new material types afforded by the silk fibroin structure.

Introduction
Silk fibroin fibers, fromBombyx mori silkworm cocoons, have been used for centuries to create strong and versatile textiles. Due to its special composition, silk fibroin has additional uses as a robust biopolymer fibrous material with a myriad of biomedical, electrochemical, and optical material applications. − Silk fibroin contains repetitive sequences of glycine, alanine, and serine that form antiparallel β-sheet structures that contribute to the strength, biocompatibility, and reinforced mechanical properties of fibroin-containing biomaterials. Since these amino acids make up >85% of the protein, the low reactivity of these residues makes chemical-based structure alteration difficult. However, several methods have been developed that modify amino acid side chains that modulate the chemical properties and functionality of the fibroin polymer. − Tyrosine is an attractive amino acid for modification due its reactivity and distribution along the protein, making up ∼5% of the silk fibroin sequence, with most of the tyrosine residues being in the amorphous regions of silk fibroin. The function of tyrosine has been studied and shown to support the formation of β-sheet structures through enhancement of intra- or intermolecular hydrogen bonding, as well as π-stacking of the aromatic rings, which promotes molecular stabilization, and β-crystallite formation. With this knowledge, modifications of this residue have been used to change the properties of silk fibroin materials through induced β-sheet formation, increased or decreased hydrophilicity, and the creation of an interchain network to form hydrogels. ,,
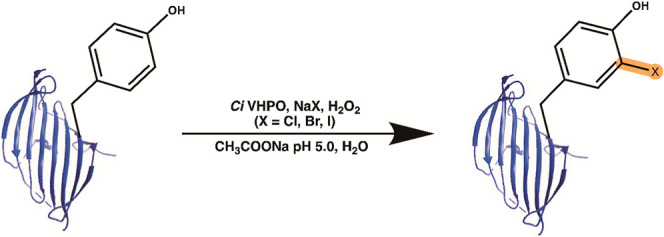
With the discovery and application of halogenating enzymes, enzymatic halogenation of compounds is beginning to rival synthetic approaches due to nature’s ability to yield useful products in aqueous environments, at high quantities, and at a moderate pH. Vanadium-dependent haloperoxidases (VHPOs) are a particular class of halogenases that employ an orthovanadate cofactor (VO4 3–), hydrogen peroxide (H2O2), and a halogen salt (NaX, X = Cl, Br, I) for halogenation. VHPOs generate a hypohalous acid (HOX) that nonspecifically reacts with electron-rich substrates to add a halogen atom (Cl, Br, I) through electrophilic substitution to vinyl- or aromatic group-containing compounds. VHPOs are found in both marine and terrestrial environments, and the vanadium-dependent haloperoxidase (VHPO) used in this study is derived from Curvularia inaequalis, a terrestrial fungus. Traditionally, VHPOs are used by organisms for either microbial defense or stress signaling; however, C. inaequalis is a parasite that uses halogenation to attack plant roots by breaking open host cell walls. , The biological and chemical practicality of this VHPO’s halogenating function has been advantageous for the production of commercially relevant chemicals in the biotechnological, pharmaceutical, and chemical industries. , This study investigates the modification of silk fibroin using a halogenating enzyme to understand how halogens change protein self-assembly, and material properties.
Materials and Methods
Expression of VClPO from Curvularia inaequalis
Vanadium-dependent chloroperoxidase (VClPO) synthetic gene was bought from Integrated DNA Technologies (Coralville, IA) and cloned into a pET15b vector using the Gibson Assembly protocol provided by NEB Laboratories. First, PCR was performed with Phusion High-Fidelity DNA Polymerase (New England BioLabs, Ipswich, MA) to add the 5′ and 3′ overhang sequences to the VClPO gene for vector assembly. Table S1 provides the forward and reverse primers used for PCR. Following the double digest protocol (NEB Laboratories) with restriction enzymes NdeI and XhoI (New England BioLabs, Ipswich, MA), the gene was inserted into the digestion site to create an N-terminal HisTag for enzyme purification. The resulting pET15b-VClPO construct was transformed into BL21 DE3 E. coli cells and grown at 220 rpm in 1 L LB broth solution with ampicillin (0.1 mg/mL, Gold Biotechnology Inc., St. Louis, MO) until 0.6 OD600 in a 37 °C shaker. At that point, IPTG (0.5 mM) (Gold Biotechnology Inc., St. Louis, MO) was added to induce protein expression, and the temperature was reduced to 18 °C under shaking for 16 h. Cultures were centrifuged at 8,000g for 10 min, 4 °C and lysed by sonication in lysis buffer (50 mM HEPES, 250 mM NaCl, 10% glycerol pH = 7.4). Cell debris was removed by centrifugation at 25,000g, 4 °C for 30 min. FPLC (Bio-Rad NGC Quest 10 Chromatography, Hercules, CA) fitted with an IMAC HisTag column (HisTrap FF Crude, 5 mL; Cytiva Life Sciences, Malborough, MA) was used for enzyme purification. The column was equilibrated with 0.22 μm filtered 18.2 MΩ water for 10 column volumes followed by sample application and washing with wash buffer (50 mM HEPES, 250 mM NaCl 30 mM imidazole, 10% glycerol pH = 7.4) for 5 column volumes. Protein was eluted in 5 mL fractions with wash buffer plus 500 mM imidazole for 10 column volumes followed by concentration with Amicon Ultra-15 Centrifugal Filter Units (10,000 NMWL) (EMD Millipore, Burlington, MA) spun at 5700 rpm for 10 min. The resulting concentration was ∼8 mg/mL. Protein was flash-frozen in liquid nitrogen and stored at −80 °C until used. Purified fractions were confirmed using SDS-PAGE gel electrophoresis (12% Tris-glycine) to contain VClPO from C. inaequalis (Figure S1). Phenol red activity assays were performed as published. Briefly, 50 μM phenol red was added to a solution of 10 mM NaBr, 0.1 mM sodium vanadate, 10 mM H2O2, 100 mM sodium acetate pH = 5.0, and 20 μL of purified enzyme. Assays were allowed to go to presumed completion when the assay solution turned from yellow to blue signifying all phenol red was brominated. Results are shown in Figure S2.
Preparation of Aqueous Silk Fibroin
Aqueous solutions of silk fibroin have been established in other publications. B. mori cocoons were processed and weighed out to be 7.5 g and boiled for 30 min in 3 L of 0.02 M sodium carbonate solution. The degummed silk fibers were then rinsed in 18.2 MΩ water three times and dried overnight. Silk fibers were dissolved in 9.3 M LiBr in a 20% w/v solution and heated to 60 °C for 2 h. The solution was dialyzed (Spectrum Repligen Spectra/Por Dialysis Membrane Tubing, 12–14 kDa MWCO, New Brunswick, NJ) against 2 L of 18.2 MΩ water at 4 °C for 2 days with six water changes. Silk solutions were then transferred into a 50 mL conical tube and centrifuged twice at 10,000g for 20 min to remove insoluble impurities. Concentrations of silk solutions were determined by comparing the aqueous silk mass to the dried silk mass. The resulting concentration was 6.5% w/v silk.
Silk Fibroin Halogenation Assays
Halogenation assays, as shown in Figure A, were developed from published protocols. 40 nM of VClPO was added to a 2% w/v silk solution with either 2 mM of NaCl, NaBr, or NaI, 0.1 mM sodium vanadate, 213 mM H2O2, and 25 mM sodium acetate at pH = 5.0. Blank assays included all materials without the enzyme, and assays were left overnight. The next day, assay solutions were added to a Slide-A-Lyzer MINI Dialysis Device, 20 K MWCO, (Thermo Scientific) and dialyzed against 18.2 MΩ water for 1 day with two water changes. Dialyzed solutions were then removed from devices and added to 1.5 mL Eppendorf tubes. Samples were then stored at 4 °C until used.
1.

(A) Proposed halogenation pathway for functionalization of ortho-positioned H on tyrosine rings. 1H NMR spectra of unmodified (black) and halogenated samples (blue = chlorinated, red = brominated, and purple = iodinated) of silk fibroin. (B) LC-QTOF FASTA Sequence Alignment with silk fibroin light chain. Sequences of silk fibroin light chain given in one-letter amino acid code. Halogenated amino acids are printed in bold. The detected halogenation of Cl, DiCl, Br, DiBr, I, and DiI is marked above the identified halogenated amino acids. Numbers on side mark amino acid positions.
1H NMR
Silk solutions were subjected to 1H NMR to investigate the aromatic signals from tyrosine that appear at 6.75 ppm (br s, 2H, Tyrφ) and 7.03 ppm (br s, 2H Tyrφ) in unmodified samples. Samples were prepared by adding 500 μL of the protein sample to coaxial 5 mm tubes (Wilmad-LabGlass) with the inset filled with D2O for frequency stabilization. The data were acquired using the WATERGATE pulse sequence for solvent suppression.
Protein Digest with Chymotrypsin and LC–MS
After the corresponding halogenation, samples were digested for 24 h at 37 °C with α-chymotrypsin (Sigma-Aldrich) in 0.1 M ammonium bicarbonate solution at a ratio of 1:100 enzyme-to-silk fibroin. Samples were dried using a stream of nitrogen gas, then redissolved in 100 μL of LC–MS grade water and 0.1% formic acid. Two sets of each Cl, Br, and I samples were tested in triplicate runs. Samples were loaded at 0.1 μg/μL with a 10 μL total load onto an Agilent 1290 Infinity II HPLC. A Poroshell Stable bond 300 SB-C8, 2.1 mm × 75 mm, 5 μm, 300 Å, 400 bar column (cat. No. 660750-906, Agilent, CA) was used for separation with a flow rate of 0.4 mL/min and a temperature of 60 °C. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The column flow rate was 0.4 mL/min, and the following gradient conditions were used: 0.5 min 5% B ramped up to 40% B at 15 min and to 90% B at 18 min which was kept at this concentration for 2 min postrun. Following HPLC separation, samples were analyzed in an Agilent 6546 Q-TOF under a positive-ion mode using the auto ms/ms settings at the m/z acquisition range of 100–1700 m/z and a scan rate 3.0 spectra/second. Instrument parameters: gas temp 290 °C, gas flow 13 l/min, nebulizer 35 psig, sheath gas temp 275 °C, sheath gas flow 12 l/min, and source parameters: VCap 4000, Nozzle voltage 2000, Fragmentor 175, skimmer 65, and Octopole RFPeak 750. Samples were analyzed using Agilent Bioconfirm 10.0 under the nonreducing protein digest settings by choosing the enzyme chymotrypsin, and the corresponding special modifications by halogenation, e.g., Cl, DiCl, Br, DiBr, I, and DiI. The identified compounds were aligned with the following B. mori silk fibroin FASTA files: sp|P05790|FIBH_BOMMO Fibroin heavy chain OS = B. mori OX = 7091 GN = FIBH PE = 1 SV = 4, and tr|Q7JYG3|Q7JYG3_BOMMO Fibroin light chain OS = B. mori OX = 7091 PE = 2 SV = 1. The FASTA sequence alignment reports of all observed peptide compounds with halogen modifications are listed in the Supporting Information file Seq Alignment Supporting Information
X-ray Photoelectron Spectroscopy of Silk Films
Films were prepared on silicon wafers (University Wafer, Mechanical grade, South Boston, MA) and subjected to UV/ozone treatment (Novascan UV Ozone Cleaner, Novascan Technologies, Boone, IA) for 10 min. Silk solutions were drop cast and allowed to dry overnight with no further treatment. Survey scans and high-resolution XPS spectra were collected on an M-Probe Surface Science XPS spectrometer. Spectra were collected using a spot size of 800 mm, 0.01 eV step, and averaged over 75 scans. Atomic % was determined by integration of individual peaks in survey scans using analysis software provided by M-probe Surface Science.
Circular Dichroism of Modified Silk Fibroin
CD spectra were collected on a Jasco J-815 CD spectrometer (Jasco, Easton, MD) using a quartz cuvette with a 1 cm path length from 300 to 190 nm at a scan rate of 20 nm/min and averaged over three repetitive scans. Unmodified and halogenated samples were diluted to 0.5 mg/mL with 18.2 MΩ water. Spectra were plotted with OriginLab Pro 2020 software. Compositions of spectra were deconvoluted using BESTSEL software.
Fourier Transform Infrared Analysis of Silk Films
Silk films were prepared on silicon wafers that were treated with UV/Ozone for 10 min. Next, silk solutions were drop cast onto silicon wafers and allowed to dry overnight. Two films were made per sample with one film being treated with methanol for 10 min to induce crystallization. Fourier transform infrared spectroscopy (FTIR) analysis was performed on a Bruker Lumos spectrometer by using the ATR attachment. Liquid nitrogen was added to the system to provide nitrogen gas for reduction of the water vapor contribution from the atmosphere. Each measurement had 64 scans coadded with 4 cm–1 resolution and the wavenumber ranged from 600 cm–1 to 4000 cm–1. The sample’s spectra were plotted in OriginLab Pro 2020 software and were normalized. Peak deconvolution of the Amide I region (1600 cm–1–1700 cm–1) was also performed on OriginLab Pro 2020 software using Fourier Self-Deconvolution (FSD) v1.00 and Peak Deconvolution v1.50 applications available from the app center. FSD was done by plotting the Amide I region individually from the whole spectrum and applying the application with a smoothing factor of 0.15. A Lorentzian function was applied to each curve to distinguish the contribution of each element (β-sheets, β-turns, side chains) to the Amide I peak. Each spectrum had 7 peaks individually picked based on FSD and were plotted underneath the deconvoluted spectrum. Peak assignments were given based on previous data for silk fibroin. ,
Raman Spectroscopy of Silk Films
Raman spectra were obtained on a Renishaw inVia Raman Microscope (Renishaw, Gloucestershire, United Kingdom) with a 785 nm laser and a 100× objective lens. Laser power was set to 10% with 6 reads per sample. Each sample (unmodified, chlorinated, brominated, and iodinated) was drop cast on gold microscope slides (Gold Seal Microscope Slides, Clay Adams) because silicon wafers had interference in the Raman spectra and were tested in three different areas on the slide. The wavenumber range was set to 800 cm–1–1800 cm–1 to collect all necessary peaks related to proteins.
Contact Angle Measurements of Silk Fibroin Films
Silk samples were spin-coated onto silicon wafers that were treated as described previously. The spin coater system used was a Spin-coater Model P6700 (Specialty Coating Systems, Indianapolis, IN) and done by the following procedure: a silicon wafer was placed on spinning mechanism and had 60 μL of silk solution added to the middle of the wafer. Films were spun by initially increasing the spin rate to 1000 rpm for 30 s and a 30 s hold at 1000 rpm. Films were allowed to dry for 1 h, followed by treatment with methanol for 1 h, and drying overnight. Next, silk films were analyzed on a Biolin Scientific Attension Theta (Biolin Scientific, Gothenburg, Sweden) with oneAttension software using the sessile drop technique with a 2 μL drop size of distilled water. Two films from each sample were made and analyzed. Each film had three measurements performed, and the data points were averaged together for a final measurement.
Silk Fibroin Film Fabrication
Silk fibroin aqueous samples were cast into 5 mm diameter polypropylene molds and allowed to air-dry overnight. Dry films were removed from the molds and dissolved in 200 μL of either ddH2O (control), 30% ammonium sulfate, acetone, or methanol for 20 min to induce crystallite formation. Films were washed twice in ddH2O for 5 min and ddH2O was removed, and the films were allowed to air-dry overnight. Pictures of films were taken to compare the treatments of different solvents to unmodified and halogenated samples.
Results and Discussion
Characterization of Halogenation on Tyrosine Residues
Reconstituted silk fibroin was exposed to VClPO as described above and analyzed by 1H NMR to understand how the proton environment on the aromatic rings changed during halogenation. During these assays, there was also a notable color change from a clear aqueous solution to a yellow and slightly opaque color. The 1H NMR spectrum in Figure B shows unmodified silk fibroin aromatic peaks at 6.75 and 7.03 ppm corresponding to the tyrosine protons at positions 3, 5 and 2, 6, respectively. The spectra also show the signals from slowly exchanging amide protons in the region between 7.8 to 8.8 ppm. Silk fibroin that has been exposed to VClPO showed a complete loss in tyrosine aromatic signals and a change in line shape and intensity in the amide region. We cannot eliminate the possibility that the signals from monohalogenated tyrosines overlap with amide peaks. The liquid chromatography–mass spectrometry (LC–MS) conditions were optimized with the corresponding mass-specific standards for l-tyrosine, 3-chloro-l-tyrosine, 3,5-dichloro-l-tyrosine, 3-bromot-l-tyrosine, 3,5-dibromo-l-tyrosine, 3-monoiodo-l-tyrosine, and 3,5-diiodo-l-tyrosine (Figure S3). All standards demonstrated good ionization under the set conditions. Regarding detection of halogenation, standards were ambiguous: all contained nonhalogenated tyrosine together with halogenated compounds including mono- and dihalogenated tyrosine forms. The presence of VClPO-dependent halogenation was confirmed in hydrochloric acid proteolyzed silk fibroin samples (Figure S4). While the nonhalogenated silk fibroin sample clearly showed no signs of any halogenation, all other halogenated samples had both mono- and dihalogenated forms. The detection abundance of the halogenated tyrosine compounds was not as high as the abundance of the standards, which might be due to impurities and lack of complete proteolysis from cleanup of the analyzed samples. In a different approach, to determine if the halogenation was equally distributed throughout both the light and heavy chain of the silk fibroin molecule, samples were treated with chymotrypsin, and the proteolytic digests were analyzed by LC–MS/MS using a 6546 LC-Q-TOF MS instrument (Agilent, CA). Proteomics analysis was done using Agilent Bioconfirm 10.0 assisted FASTA sequence alignment with the B. mori silk fibroin FASTA files (Fibroin Heavy Chain-sp|P05790|FIBH_BOMMO, and Fibroin Light Chain-tr|Q7JYG3|Q7JYG3_BOMMO). The fibroin light chain was analyzed by searching for halogen modifications, including Cl, DiCl, Br, DiBr, I, and DiI. Analysis of the nonpolymeric fibroin light chain demonstrated existence of Cl, Br, and I halogenation at tyrosine residues throughout the whole sequence (Figure B). In contrast, only specific tyrosine residues including tyrosine 110, 167, and 196 demonstrated either single or dual halogenation. There was also evidence of brominated tryptophan at residue 47, and chlorination or bromination at phenylalanine 130. Halogenation of the polymeric silk fibroin heavy chain seemed to be limited to the initial region of the protein between amino acid residues 1 and 25 and the last region of the protein between amino acid residues 5236 and 5263 in all samples, including the Cl sample, Br sample, and I sample (Table S2).
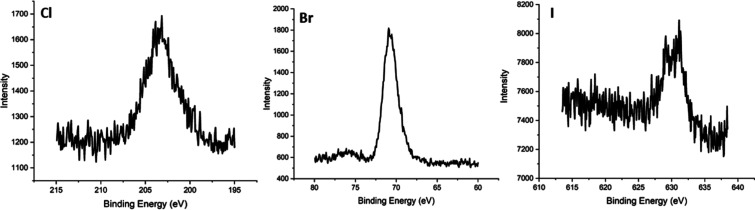
The LC–MS analysis provided coverage for the complete heavy chain silk molecule (Seq Alignment Supplemental Data). The enhanced detection of halogenation at the C- and N-terminals of the protein might be due to existing intramolecular disulfide bonds between cysteines 10, 11, 5244, 5260, and 5263 that promote peptide fragment stability during MS/MS fragmentation analysis. In contrast to the Br and I samples, the Cl sample showed peptide fragment halogenation throughout the middle of the molecule, even without the presence of disulfide bonds. Lastly, XPS was employed to determine the elemental composition of silk fibroin films before and after enzyme-mediated halogenation. Figure shows survey scans of orbital energies of unmodified and halogenated silk fibroin samples after halogenation. Enzyme-treated silk fibroin films showed the presence of 2.7% Br, 0.8% Cl, and 0.25% I (Table S3). High-resolution XPS of Br 3d5/2, I 3d5/2, and Cl 2p peaks confirms the halogenation of silk fibroin films, Figure . Chlorinated and iodinated samples had low signal/noise ratios, silk-I (S/N = 5.0) and silk-Cl (S/N = 14.2), presumably due to the low abundance of potential sites for halogenation on silk fibroin (∼5.3% tyrosine) and trace contamination of bromine ions due to the 9.3 M LiBr processing step needed to solubilize the fibers.
2.
XPS analysis of unmodified and halogenated silk fibroin films. Unmodified = unmodified silk, Cl = chlorinated silk, Br = brominated silk, and I = iodinated silk.
3.
High-resolution XPS plots of individual halogen peaks.
Secondary Structure Characterization of Halogenated Silk Fibroin
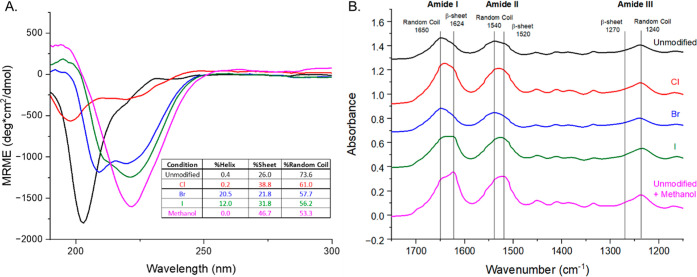
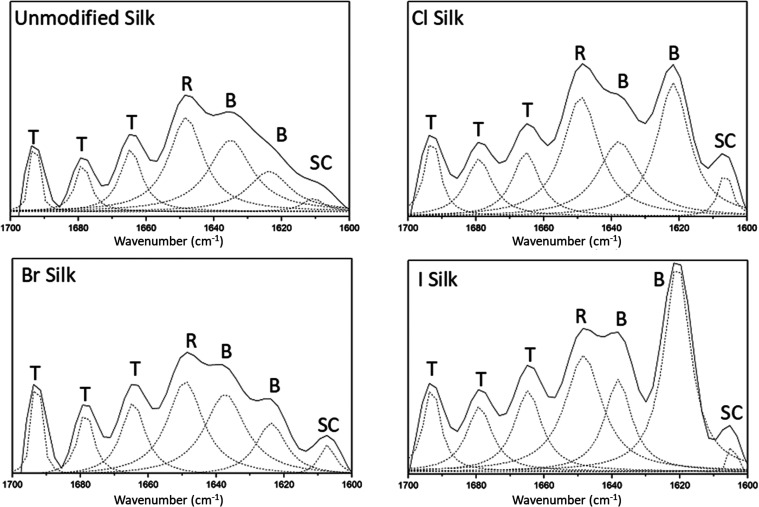
Multiple techniques were used to characterize the secondary structure of silk fibroin before and after halogenation in the polymeric region of the molecule. First, CD spectroscopy was performed to characterize the most abundant secondary structures present in solution (Figure A). The CD data show that unmodified silk adopts a primarily random coil conformation. With halogenation, however, a greater α-helical content is observed, which may be an intermediate for the crystalline structure shown in the methanol-treated unmodified sample. The addition of methanol to silk solutions is often used to induce β-sheet crystallites which are similar to what is formed in spun silk by the silkworm, B. mori. As shown by Ha et al., silk fibroin in halogenated solvents (HFIP, HFA) prefer a helical structure presumably due to the interaction between the solvents and the hydroxyl groups on various residues of the amorphous regions (serine and tyrosine) that stabilize this conformational change. Next, FTIR was used to further characterize the secondary structures of films. The Amide I band (1600 cm–1–1700 cm–1), which is assigned to the stretching of the CO on the peptide bond, clearly showed the unmodified silk fibroin film in a random coil conformation (Figure B). FTIR analysis of the Cl and I halogenated silk fibroin films indicated that the peptide backbone arranged into the β-sheet conformation. The same trend can be seen in the Amide II peak (1500 cm–1–1600 cm–1), which corresponds to the N–H bend of the in-plane amide group on the peptide bond. These changes were not observed in the Br-halogenated silk films. After deconvolution, the Amide I peak of unmodified silk revealed that a random coil (R) conformation was the major secondary structure with very little contribution from the side-chain residues which was that of tyrosine. However, in the chlorinated and iodinated samples, the presence of β-sheet structures increased at peak 1621 cm–1, representing an increased participation to an intermolecular hydrogen bonding network. For all halogenated samples, the contribution from the side-chain residues increased in comparison to the unmodified samples, which suggests that the halogens either contribute to β-crystallite formation or enhance stacking of tyrosine residues (Figure ). The incorporation of a halogen on tyrosine residues appears to reorient the interior noncovalent network within the silk molecule both in solution and as a film. This suggests that, through halogen modification, the protein must accommodate this change and transition to a favorable confirmation which for silk fibroin is a β-sheet. Similar changes are observed in other proteins that include halogenated tyrosines which appear to stabilize their structures.
4.
(A) CD spectra of unmodified silk fibroin without methanol (black), unmodified with methanol (pink), chlorinated (red), brominated (blue), and iodinated silk fibroin (green). (B) FTIR spectra of unmodified and halogenated silk fibroin.
5.
Deconvoluted Amide I peaks from unmodified and halogenated samples. T = beta turns, R = random coil, B = beta-sheet, and SC = side chain mostly from tyrosine.
Finally, Raman spectroscopy was used to probe the effects of halogenation on the silk fibroin secondary structure. From the Raman spectra, the peaks corresponding to the Fermi resonance phenomena of the ring breathing vibrations and out-of-plane ring-bending vibrations (853 cm–1 and 830 cm–1) can be compared in a relative intensity ratio (R Tyr = I 853/I 830) to estimate tyrosine’s hydrogen bonding activity as it pertains to the side chain’s −OH group. , In Figure S5, the unmodified silk fibroin showed a higher R Tyr value of 1.26. While in the chlorinated, brominated, and iodinated silks, the R Tyr was 1.13, 1.09, and 1.15, respectively. If the phenol ring is buried in the amorphous region, then –OH acts as a strong hydrogen bond donor, which is reflected in the decrease in intensity of the 853 cm–1 band. The decrease in R Tyr observed with the halogenated silk fibroins is consistent with this.
Material Properties of Halogenated Silk Fibroin
Halogenation was detected and shown to influence the secondary structure of silk fibroin to varying degrees. How halogenation impacts the material properties of silk fibroin was also explored. Contact angle measurements were performed to assess the hydrophilic or hydrophobic nature of halogenated silk fibroin by measuring the angle of the interface between a water droplet on a silk fibroin film surface (Figure S6). Table summarizes the results by showing that the angle changed significantly from unmodified (∼68°) to chlorinated (∼59°), brominated (∼36°), and iodinated (∼45°) silk fibroin films. The ANOVA results (Table S4) indicated a statistically significant difference between the groups (F(3,7) = 28.01, p < 0.001). The brominated silk fibroin sample had the lowest contact angle measurement which supports the R Tyr from the Raman spectroscopy data. This seems to suggest that bromination was either more efficient at hydrogen/halogen bonding or that the tyrosine –OH on the phenol ring was in the most optimal position for hydrogen bonding. Additional investigation is needed to confirm this phenomenon. We also observed that other methods of converting silk fibroin into a hydrogel material, such as enzymatic cross-linking through horseradish peroxidase (HRP) or sonication, were not effective when used on the halogenated proteins (Figure S7). The mechanism of HRP cross-linking involved the formation of dityrosine bonds, which was likely blocked by tyrosine halogenation. Sonication was not possible due to the increased stability caused by the present halogen. However, silk fibroin films treated with 30% ammonium sulfate, acetone, and methanol were able to crystallize and became water insoluble compared to films treated with water (Figure S8).
1. Contact Angle Measurements of Halogenated Silks Compared to Unmodified Silk.
| sample | degrees (°) (n = 3) |
|---|---|
| unmodified silk | 68 ± 2 |
| chloro silk | 59 ± 0 |
| bromo silk | 36 ± 4 |
| iodo silk | 45 ± 7 |
Conclusion
Our study presents a novel method using enzyme-mediated halogenation for modulation of the structural biomaterial characteristics of silk fibroin, and this material fabrication process has the potential to be used for downstream applications. Although many strategies have been demonstrated to modify silk, ,, halogenation can be used as a more versatile method. Since the enzyme has a broad reactivity with multiple aromatic residues, as shown in Figure B, tyrosine is the most fit for this analysis in silk fibroin. The regular distribution of tyrosine within the amorphous region of the sequence allows halogenation throughout the molecule and offers a wide array of applications for the creation of novel function silk biomaterials. The quantity of enzymatic halogenation was difficult to determine due to large protein size of silk fibroin (417 kDa) and the existence of N-and C-terminal disulfide bonds that contribute to complexity of the molecule and corresponding low reactivity. While mass spectrometry was able to confirm halogenation on both, N-terminal and C-terminal ends, in the future, carbon–halogen coupling chemistries could be an excellent way to quantify the amount of halogenation for two reasons including: (1) Suzuki coupling is specific only for the carbon–halogen and (2) there is a catalog of boronic acids that are commercially available for this type of chemistry that can be employed while also determining silk’s amenability to these new groups. Halogenation was found to change many aspects of silk fibroin’s structure, with preferred α-helical secondary structure in solution and tendency to induce β-sheet conformation when dried, which has been seen in other modifying techniques. Raman spectroscopy data coupled with contact angle measurements show the increase in hydrophilicity of the silk material, which suggests that after halogenation the tyrosine ring tends to bury itself in the hydrophobic core to shield the hydrophobic halogens from the aqueous solution. This results in a stretching of the –OH from the phenyl ring, supporting higher availability for hydrogen bonding. This structuring might also explain the tendency of halogenated silks to show more α-helical formation, as determined by CD spectroscopy, and have more contribution from the tyrosine residues in the deconvoluted Amide I peak shown in the FTIR results. Additional work on controlling the amount of halogenation would be necessary if the desired outcome is to use this strategy specifically to design the material properties. However, this strategy can be used to couple more complex chemistries aimed at enhancing the functionality of silk fibroin paving the way for novel applications such as drug targeting/delivery, responsive biomaterials, and tissue engineering scaffolds. There is growing interest in surface modification of materials and since tyrosines have been identified to be on the outside of silk fibers, this approach could add additional functional chemistries to these biomaterials. This approach is ideal for adding additional chemistries to such materials.
Supplementary Material
Acknowledgments
The research reported in this publication has been cleared for public release under reference numbers AFRL-2025-2744. The views expressed are those of the authors and do not reflect the official guidance or position of the United States Government, the Department of Defense, the United States Air Force, or the United States Space Force.
Glossary
Abbreviations
- VHPO
vanadium-dependent haloperoxidase
- CD
circular dichroism
- FTIR-ATR
Fourier transform infrared-attenuated total reflectance
- HOX
hypohalous acid
- XPS
X-ray photoelectron spectroscopy
- NMR
Nuclear magnetic resonance
- VClPO
Vanadium chloroperoxidase
- PCR
polymerase chain reaction
- HPLC
high-performance liquid chromatography
- Q-TOF
quadrupole-time-of-flight
- HRP
horseradish peroxidase
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.5c03545.
Sequence alignment supplemental data (XLSX)
Gel electrophoresis of purified VClPO from C. inaequalis; schematic and results of the activity assay of the phenol red assay with VClPO; LC–MS QTOF data; Raman scattering spectra of the tyrosine region of silk fibroin for all samples; silk fibroin samples treated with HRP for hydrogel formation; and primer sequence for PCR of VClPO from C. inaequalis into pET15b vector (PDF)
⊥.
Department of Chemistry and Biochemistry, University of Maryland, College Park, Maryland 20742, United States
#.
Oscar N. Ruiz Biomaterials Branch, Photonic, Electronic & Soft Materials Division, Materials and Manufacturing Directorate, Air Force Research Laboratory, 2179 12th Street Wright-Patterson Air Force Base, Ohio, USA 45433-7131.
¶.
J.A.M. and A.L.C. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by the Materials and Manufacturing Directorate, Air Force Research Laboratory and in collaboration with AFRL/RQTF under agreement number FA8650-16-2-2605 and AFRL Sustainment Accelerator Fund (63199 AFRL STAF).
The authors declare no competing financial interest.
References
- Altman G. H., Diaz F., Jakuba C., Calabro T., Horan R. L., Chen J., Lu H., Richmond J., Kaplan D. L.. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. doi: 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- Meinel L., Betz O., Fajardo R., Hofmann S., Nazarian A., Cory E., Hilbe M., McCool J., Langer R., Vunjak-Novakovic G.. et al. Silk based biomaterials to heal critical sized femur defects. Bone. 2006;39(4):922–931. doi: 10.1016/j.bone.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Shi Z., Cai X., Zhang S., Corder S. G., Li X., Zhang Y., Zhang G., Chen L., Liu M.. et al. The Use of Functionalized Silk Fibroin Films as a Platform for Optical Diffraction-Based Sensing Applications. Adv. Mater. 2017;29(15):1605471. doi: 10.1002/adma.201605471. [DOI] [PubMed] [Google Scholar]
- Heslot H.. Artificial fibrous proteins: A review. Biochimie. 1998;80(1):19–31. doi: 10.1016/S0300-9084(98)80053-9. [DOI] [PubMed] [Google Scholar]
- Zhou C.-Z., Confalonieri F., Jacquet M., Perasso R., Li Z.-G., Janin J.. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins: Struct., Funct., Bioinf. 2001;44(2):119–122. doi: 10.1002/prot.1078. [DOI] [PubMed] [Google Scholar]
- Chen J., Venkatesan H., Hu J.. Chemically Modified Silk Proteins. Adv. Eng. Mater. 2018;20(7):1700961. doi: 10.1002/adem.201700961. [DOI] [Google Scholar]
- Gotoh Y., Tsukada M., Minoura N.. Chemical modification of silk fibroin with cyanuric chloride-activated poly(ethylene glycol): Analyses of reaction site by proton NMR spectroscopy and conformation of the conjugates. Bioconjugate Chem. 1993;4(6):554–559. doi: 10.1021/bc00024a020. [DOI] [PubMed] [Google Scholar]
- Murphy A. R., Kaplan D. L.. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009;19(36):6443–6450. doi: 10.1039/b905802h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A. R., John P. S., Kaplan D. L.. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials. 2008;29(19):2829–2838. doi: 10.1016/j.biomaterials.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T., Suita K., Kameda T., Afonin S., Ulrich A. S.. Structural role of tyrosine in Bombyx mori silk fibroin, studied by solid-state NMR and molecular mechanics on a model peptide prepared as silk I and II. Magn. Reson. Chem. 2004;42(2):258–266. doi: 10.1002/mrc.1337. [DOI] [PubMed] [Google Scholar]
- Partlow B. P., Hanna C. W., Rnjak-Kovacina J., Moreau J. E., Applegate M. B., Burke K. A., Marelli B., Mitropoulos A. N., Omenetto F. G., Kaplan D. L.. Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater. 2014;24(29):4615–4624. doi: 10.1002/adfm.201400526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernie Liu T.-N., M’Timkulu T., Geigert J., Wolf B., Neidleman S. L., Silva D., Hunter-Cevera J. C.. Isolation and characterization of a novel nonheme chloroperoxidase. Biochem. Biophys. Res. Commun. 1987;142(2):329–333. doi: 10.1016/0006-291X(87)90277-4. [DOI] [PubMed] [Google Scholar]
- Littlechild J.. Haloperoxidases and their role in biotransformation reactions. Curr. Opin. Chem. Biol. 1999;3(1):28–34. doi: 10.1016/S1367-5931(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Barnett P., Kruitbosch D. L., Hemrika W., Dekker H. L., Wever R.. The regulation of the vanadium chloroperoxidase from Curvularia inaequalis. Biochim. Biophys. Acta. 1997;1352(1):73–84. doi: 10.1016/S0167-4781(96)00238-2. [DOI] [PubMed] [Google Scholar]
- Van Schijndel J. W. P. M., Barnett P., Roelse J., Vollenbroek E. G. M., Wever R.. The Stability and Steady-State Kinetics of Vanadium Chloroperoxidase from the Fungus Curvularia Inaequalis. Eur. J. Biochem. 1994;225(1):151–157. doi: 10.1111/j.1432-1033.1994.00151.x. [DOI] [PubMed] [Google Scholar]
- Smith D. R. M., Grüschow S., Goss R. J. M.. Scope and potential of halogenases in biosynthetic applications. Curr. Opin. Chem. Biol. 2013;17(2):276–283. doi: 10.1016/j.cbpa.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Wischang D., Brücher O., Hartung J.. Bromoperoxidases and functional enzyme mimics as catalysts for oxidative brominationA sustainable synthetic approach. Coord. Chem. Rev. 2011;255(19):2204–2217. doi: 10.1016/j.ccr.2011.04.003. [DOI] [Google Scholar]
- Rockwood D. N., Preda R. C., Yücel T., Wang X., Lovett M. L., Kaplan D. L.. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011;6(10):1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J. J., Fernández-Fueyo E., Li J., Guo Z., Renirie R., Wever R., Hollmann F.. Halofunctionalization of alkenes by vanadium chloroperoxidase from Curvularia inaequalis. Chem. Commun. 2017;53(46):6207–6210. doi: 10.1039/C7CC03368K. [DOI] [PubMed] [Google Scholar]
- Piotto M., Saudek V., Sklenář V.. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR. 1992;2(6):661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- Micsonai A., Wien F., Kernya L., Lee Y.-H., Goto Y., Réfrégiers M., Kardos J.. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 2015;112(24):E3095–E3103. doi: 10.1073/pnas.1500851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Kaplan D., Cebe P.. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules. 2006;39(18):6161–6170. doi: 10.1021/ma0610109. [DOI] [Google Scholar]
- Krimm, S. ; Bandekar, J. . Vibrational Spectroscopy and Conformation of Peptides, Polypeptides, and Proteins. In Advances in Protein Chemistry, Anfinsen, C. B. , Edsall, J. T. , Richards, F. M. , Eds.; Vol. 38; Academic Press, 1986; pp 181–364. [DOI] [PubMed] [Google Scholar]
- Ha S.-W., Asakura T., Kishore R.. Distinctive Influence of Two Hexafluoro Solvents on the Structural Stabilization of Bombyx m ori Silk Fibroin Protein and Its Derived Peptides: 13 C NMR and CD Studies. Biomacromolecules. 2006;7:18–23. doi: 10.1021/bm050783m. [DOI] [PubMed] [Google Scholar]
- Barth A.. Infrared spectroscopy of proteins. Biochim. Biophys. Acta, Rev. Bioenerg. 2007;1767(9):1073–1101. doi: 10.1016/j.bbabio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Chen X., Shao Z., Marinkovic N. S., Miller L. M., Zhou P., Chance M. R.. Conformation transition kinetics of regenerated Bombyx mori silk fibroin membrane monitored by time-resolved FTIR spectroscopy. Biophys. Chem. 2001;89(1):25–34. doi: 10.1016/S0301-4622(00)00213-1. [DOI] [PubMed] [Google Scholar]
- Ohtake K., Yamaguchi A., Mukai T., Kashimura H., Hirano N., Haruki M., Kohashi S., Yamagishi K., Murayama K., Tomabechi Y.. et al. Protein stabilization utilizing a redefined codon. Sci. Rep. 2015;5(1):9762. doi: 10.1038/srep09762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti P., Freddi G., Bertoluzza A., Kasai N., Tsukada M.. Raman spectroscopic studies of silk fibroin from Bombyx mori. J. Raman Spectrosc. 1998;29(4):297–304. doi: 10.1002/(SICI)1097-4555(199804)29:4<297::AID-JRS240>3.0.CO;2-G. [DOI] [Google Scholar]
- Siamwiza M. N., Lord R. C., Chen M. C., Takamatsu T., Harada I., Matsuura H., Shimanouchi T.. Interpretation of the doublet at 850 and 830 cm-1 in the Raman spectra of tyrosyl residues in proteins and certain model compounds. Biochemistry. 1975;14:4870–4876. doi: 10.1021/bi00693a014. [DOI] [PubMed] [Google Scholar]
- Johansson Seechurn C. C. C., Kitching M. O., Colacot T. J., Snieckus V.. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem., Int. Ed. 2012;51(21):5062–5085. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- Sahoo J. K., Hasturk O., Falcucci T., Kaplan D. L.. Silk chemistry and biomedical material designs. Nat. Rev. Chem. 2023;7(5):302–318. doi: 10.1038/s41570-023-00486-x. [DOI] [PubMed] [Google Scholar]
- Shao J., Liu J., Zheng J., Carr C. M.. X-ray photoelectron spectroscopic study of silk fibroin surface. Polym. Int. 2002;51(12):1479–1483. doi: 10.1002/pi.1092. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.