Summary
Background
Atorvastatin and rosuvastatin are the most widely used statins in patients with ischemic stroke or transient ischemic attack (TIA). However, evidence on their effectiveness and safety during actual use is scarce. This study aims to compare the effectiveness and safety of initiating atorvastatin versus rosuvastatin among patients with ischemic stroke or TIA.
Methods
This observational study was based on the Third China National Stroke Registry (CNSR-III), which recruited consecutive adult patients with ischemic stroke or TIA within 7 days from the onset of symptoms to enrollment from August 2015 to March 2018. This study identified 3322 adults aged ≥18 years who had a pre-stroke modified Rankin Scale (mRS) score of 0 and initiated atorvastatin or rosuvastatin on the day of onset. The primary outcome was the ideal outcome, as defined by a mRS score of 0, at 3 months. The secondary outcomes included the ideal outcome at discharge, at 6 months, and at 12 months, along with 12-month stroke recurrence, all-cause mortality, cardiovascular mortality, and major adverse cardiovascular events.
Findings
A total of 3322 eligible patients were identified, with 2605 initiating atorvastatin and 717 initiating rosuvastatin. The proportion of patients achieving an ideal outcome, as defined by a modified Rankin Scale of 0, was 44.63% in rosuvastatin initiators, significantly higher than 41.46% in atorvastatin initiators, with a relative rate of 1.12 (95% confidence interval 1.03, 1.22). Also, a greater percentage of rosuvastatin initiators attained the ideal outcome at discharge and at 6 months, compared with atorvastatin initiators. Regarding other secondary outcomes, no statistically significant difference was observed.
Interpretation
Compared with atorvastatin, rosuvastatin was associated with a potentially higher proportion of patients attaining a mRS score of 0 among patients with ischemic stroke or TIA who initiate atorvastatin or rosuvastatin, which was not yet sufficient to guide clinical practice. Further research is needed to validate these findings.
Funding
This work was supported by National Key Research and Development Program of China (2022YFC2502400, 2022YFC2502404), Beijing Natural Science Foundation Haidian original innovation joint fund (L222123), and Youth Innovation Fund of Beijing Neurosurgical Institute (2025 Reform and Development–Youth 15).
Keywords: Atorvastatin, Rosuvastatin, Ischemic stroke, Transient ischemic attack, Observational study
Research in context.
Evidence before this study
We searched PubMed from database inception to May 1, 2025, for relevant articles using the following search terms: “atorvastatin” AND “rosuvastatin” AND (“stroke” OR “cerebral infarction” OR “transient ischemic attack” OR “TIA”), without language restrictions. There were no randomized controlled trials or large-sample multicenter studies comparing the functional outcomes of atorvastatin and rosuvastatin among patients with ischemic stroke or transient ischemic attack (TIA). An observational study found that compared with atorvastatin, rosuvastatin was associated with a reduced risk of a 1-year composite endpoint (recurrent stroke, myocardial infarction, and all-cause mortality) among patients who experienced ischemic stroke onset within 7 days and were prescribed either atorvastatin or rosuvastatin at discharge. In summary, the comparative benefits and risks of atorvastatin and rosuvastatin remain ambiguous in the treatment of ischemic stroke and TIA. Consequently, the choice between these two statins often presents a challenging dilemma for both healthcare professionals and patients.
Added value of this study
This observational study included 3322 patients aged ≥18 years who had a pre-stroke modified Rankin Scale score of 0 and initiated atorvastatin or rosuvastatin on the day of onset, and all baseline characteristics were well balanced between atorvastatin initiators and rosuvastatin initiators. The proportion of patients achieving an ideal outcome at 3 months, as defined by a modified Rankin Scale of 0, was 44.63% in rosuvastatin initiators, significantly higher than 41.46% in atorvastatin initiators. Similar improvement in an ideal outcome was observed both at discharge and at 6 months.
Implications of all the available evidence
Among patients with ischemic stroke or TIA, rosuvastatin was potentially associated with higher proportion of patients achieving an ideal outcome compared with atorvastatin. Future studies, incorporating larger sample sizes and extended follow-up periods, are warranted to providing more evidence and gain a comprehensive understanding in the area, enhancing the precision therapy of statins in patients with ischemic stroke or TIA.
Introduction
The imposing burden of ischemic stroke and transient ischemic attack (TIA) continues to be a significant health concern, necessitating robust therapeutic and secondary prevention strategies to mitigate their devastating impacts.1 One of the core measures in managing the risk factors of these diseases lies in the strategic use of lipid-lowering therapy, as recommended by established clinical guidelines and demonstrated through clinical practice.2,3 Statins, specifically the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, have solidified their status as cornerstone agents in lipid-lowering therapy, owing to their potent cholesterol-reducing efficacy and plaque stabilization mechanisms.4 This therapeutic efficacy has been instrumental in markedly decreasing the occurrence of stroke.5 Since the publication of the results of 4S (Scandinavian Simvastatin Survival Study) in 1994, numerous randomized controlled clinical trials (RCTs) and subsequent meta-analyses have consistently demonstrated that statins significantly decrease the incidence of stroke and mortality from cardiovascular causes.6, 7, 8, 9
In clinical practice, atorvastatin and rosuvastatin have emerged as the most prevalent statin options, reflecting their widespread acceptance and utilization.10 A vast array of research has rigorously examined the role of them in the treatment paradigm of ischemic stroke and TIA, which have explored diverse aspects, including varying therapy intensities and combination regimens with other lipid modifiers.11, 12, 13, 14 Nonetheless, the comparative benefits and risks of atorvastatin and rosuvastatin in the treatment of ischemic stroke and TIA remain ambiguous, which stems from the absence of evidence, whether derived from authoritative guidelines or RCTs. Consequently, the choice between these two statins often presents a challenging dilemma for both healthcare professionals and patients. Concerning this matter, an observational study found that compared with atorvastatin, rosuvastatin was associated with a reduced risk of a 1-year composite endpoint (recurrent stroke, myocardial infarction, and all-cause mortality) among patients who experienced ischemic stroke onset within 7 days and were prescribed either atorvastatin or rosuvastatin at discharge.15 Also, current studies reported that rosuvastatin was associated with lower low-density lipoprotein cholesterol (LDL-C) levels in patients with coronary artery disease, lower all-cause mortality in participants older than 16 years, compared with atorvastatin.16,17 Moreover, it was reported that rosuvastatin might be more efficacious as compared with atorvastatin in reducing coronary atherosclerotic plaque volume.18 On the basis of comprehensive review and consideration, we hypothesize that rosuvastatin may be associated with increased effectiveness in enhancing functional outcomes and decreasing the incidence of stroke recurrence in patients with ischemic stroke or TIA, compared with atorvastatin.
To address the research gap and validate our hypothesis, we designed and executed an observational study, leveraging the data from the Third China National Stroke Registry (CNSR-III). The aim of this study is to provide novel and tailored insights for patients with ischemic stroke or TIA who initiate treatment with atorvastatin or rosuvastatin.
Methods
Study population and design
The source population for the observational study was the entire population of the CNSR-III, a nationwide clinical registry of ischemic stroke or TIA in China. The registry recruited consecutive adult patients with ischemic stroke or TIA within 7 days from the onset of symptoms to enrollment from August 2015 to March 2018. A total of 15,166 patients were eligible and had complete information at baseline. The comprehensive details have already been published elsewhere.19
Using the records from the CNSR-III, we performed an inpatients-based, new-user, retrospective post-hoc analysis to compare the outcomes among patients with ischemic stroke or TIA who initiated atorvastatin or rosuvastatin. We included adults aged ≥18 years who had a pre-stroke mRS score of 0 and initiated atorvastatin or rosuvastatin on the day of onset, determined by issuance of the pertinent medical prescription or order. Acute ischemic stroke was diagnosed according to the World Health Organization (WHO) criteria and confirmed by magnetic resonance imaging (MRI) or brain computed tomography (CT).2,20 Patients who had previously received lipid-lowering medications or with documented concurrent therapy with atorvastatin and rosuvastatin on the day of onset were excluded from the study. The determination of whether patients had a history of prior lipid–lowering medication use was ascertained through in–person interviews conducted with patients at the time of enrollment, complemented by a thorough review and verification of their medical records. All medication-related data (including drug type, treatment duration, drug names, and dosages) were prospectively recorded by trained research coordinators, and were systematically validated against official medical orders or prescription records. Eligible patients were retrospectively categorized into the atorvastatin group or the rosuvastatin group based on the statin they were initially prescribed prior to study inclusion. For each participant, the baseline time point was defined as the date when they met the inclusion and exclusion criteria and initiated treatment with either atorvastatin or rosuvastatin.
Ethical considerations
This study adheres to the EQUATOR Network’s STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines. The protocol of the CNSR-III study was approved by ethics committee at Beijing Tiantan Hospital (IRB approval number: KY2015-001-01) and all participating centers. All participants enrolled in the CNSR-III provided informed consent before enrollment. In the present study, necessity for informed consent was waived considering the retrospective design and the fact that the data were processed anonymously.
Outcomes
The primary outcome was the ideal outcome, as defined by a modified Rankin Scale (mRS) score of 0, at 3 months. The mRS score of 0 signifies complete recovery to the patients’ previous condition, with no symptoms.21 The design for a mRS score of 0 was informed by the hypothesis that a notable segment of our study population is anticipated to attain optimal outcomes, taking into account their predominantly low National Institutes of Health Stroke Scale (NIHSS) total score at admission, prior absence of lipid-lowering drug therapy, and prompt hospital admission within 24 h of disease onset. The secondary outcomes encompassed achieving a mRS score of 0 upon discharge, at the 6-month mark, and at the 12-month follow-up, along with the incidence of stroke recurrence, all-cause mortality, cardiovascular mortality, and major adverse cardiovascular events (MACE) within 12 months. MACE was defined as a composite endpoint that included cardiovascular death, non-fatal stroke, non-fatal myocardial infarction, and non-fatal heart failure.
The follow-up commenced on the day of onset (treatment-begun date), and was conducted at various time points, including at discharge, 3 months, 6 months, and 12 months post-onset. Upon discharge and at the 3-month mark, patients participated in face-to-face interviews, whereas at the 6-month and 12-month follow-ups, they were contacted by telephone by skilled research coordinators. During each follow-up assessment, the following information was gathered and documented: the compliance with medication therapy, mRS score, stroke recurrence (including the onset date, if applicable), mortality (including the date and cause, if applicable), myocardial infarction (including the onset date, if applicable), and heart failure (including the onset date, if applicable) (Fig. 1).
Fig. 1.
Study design of the observational study. MACE, major adverse cardiovascular events; mRS, modified Rankin Scale.
Baseline characteristics
Baseline characteristics included sociodemographic factors (age, and gender), physiological characteristics (body mass index [BMI]), onset date (treatment-begun date), medical centers classified by the four economic regions of China, admission diagnoses (ischemic stroke or TIA, and NIHSS total score), treatment received (intravenous thrombolysis, and endovascular therapy, including arterial thrombolysis, mechanical thrombectomy, and stent therapy), personal lifestyle (smoking, alcohol consumption, and physical activity), disease history (stroke, transient ischemic attack, lipid metabolism disorders, diabetes, hypertension, heart diseases, infection within two weeks, and migraine), medication history (antiplatelet, anticoagulant, antidiabetic, and antihypertensive agents), and laboratory data within 24-h baseline period (high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, alanine aminotransferase, aspartate aminotransferase, uric acid, fasting glucose, and estimated glomerular filtration rate derived through the Chronic Kidney Disease Epidemiology Collaboration 2021 creatinine-based equation). Disease history and medication history were recorded at enrollment, and comorbidities (lipid metabolism disorder, pathoglycemia, hypertension, coronary heart disease, atrial fibrillation, liver disease, and renal insufficiency) were assessed prior to statin initiation. Physical activity encompassed farming–related work, occupational tasks, household chores, transport-related physical activities, recreational exercises or sports. Pathoglycemia included impaired glucose tolerance, impaired fasting glucose, and diabetes. Lipid metabolism disorder manifested as a spectrum of conditions, including hypercholesterolemia, hypertriglyceridemia, and hypo-high-density lipoprotein cholesterolemia.
Statistical analysis
To address missing data for the mRS score at each follow-up time point, the last observation carried forward (LOCF) method was employed for handling. Regarding stroke recurrence, all-cause mortality, cardiovascular mortality, and MACE, no imputation was performed for missing values. Missing values for baseline characteristics were presented in eTable 1 in Supplement 1 and were handled using multiple imputation. We used descriptive statistics to characterize patient categories. Accordingly, baseline characteristics were presented as either the mean with standard deviations (SD) for normally distributed continuous variables or the median with interquartile range (IQR) for skewed continuous variables, while categorical variables were summarized by the number of patients with percentages. To assess whether the baseline characteristics were balanced, standardized differences between treatment groups (with <0.100 indicating negligible differences) were employed.
For the outcome defined as a mRS score of 0 at each follow-up time points, the differences between two groups in the percentage of patients were analyzed by the log-binomial regression, with relative rates and corresponding 95% confidence intervals (CI) estimated. Regarding stroke recurrence, all-cause mortality, cardiovascular mortality, and MACE, the Cox proportional hazards model was utilized to estimate hazard ratios (HR) with 95% CI, and the Fine–Gray model was applied to account for competing events. Specifically, for the outcome of stroke recurrence, mortality was considered as a competing event. For cardiovascular death and MACE, cardiovascular mortality served as the competing event. In the log-binomial regression and Cox proportional hazards model, age, gender, BMI, NIHSS total score at admission, onset date, and the presence of concurrent lipid metabolism disorders were adjusted. The cumulative incidence of stroke recurrence, all-cause mortality, cardiovascular mortality, and MACE was captured using Kaplan–Meier curves among atorvastatin and rosuvastatin initiators. In order to evaluated the robustness of our primary and secondary outcomes to unmeasured confounders, an E value was calculated along with its 95% lower confidence interval (LCI) or upper confidence interval (UCI).22 All analyses were performed using SAS statistical software, version 9.4 (SAS Institute), and statistical significance was defined as two tailed P value of <0.05.
Subgroup and sensitivity analyses
Subgroup analyses were conducted across various categories, including age (categorized as < 60 years, and ≥60 years), gender (male, and female), diagnosis (ischemic stroke, and TIA), the presence or absence of concurrent lipid metabolism disorders, NIHSS total score (≤3, and >3), and daily medication dosage, based on which patients were divided into a low-dose group (atorvastatin ≤20 mg or rosuvastatin ≤10 mg) and a high-dose group (atorvastatin >20 mg or rosuvastatin >10 mg). Also, the P-value for interaction was reported. We conducted several sensitivity analyses to ensure the robustness of our findings. First, we conducted our analysis among patients who adhered to the treatment regimen, defined as receiving a continuous regimen of either atorvastatin or rosuvastatin (depending on their respective group assignment) up to the respective follow–up time points. Patient adherence to the treatment regimen was confirmed by documenting medication use through direct patient inquiry at each follow-up visit. Additionally, adherence was cross-verified using medical records and prescription records, when available. Second, we utilized stabilized inverse probability of treatment weighting (IPTW) to balance baseline characteristics. The propensity scores were estimated applying a multivariable logistic regression model, incorporating aforementioned sociodemographic factors, physiological characteristics, onset date (treatment-begun date), medical centers classified by the four economic regions of China, admission diagnoses, treatment received, personal lifestyle, disease history, medication history, and laboratory data at baseline. Thereafter, a stabilized weight for each patient was calculated based on the propensity scores, adjusting for the treatment prevalence. Third, we applied overlap weighting based on propensity scores to balance baseline characteristics. Fourth, for each follow-up timepoint, patients lost to follow-up were excluded from the analysis. Fifth, given the limitations and potential biases of the LOCF imputation approach, we employed multiple imputation to address missing mRS score.23 Sixth, concurrent coronary heart disease, hypertension history, and prior use of antidiabetic agents were additionally included in the log-binomial regression and Cox proportional hazards model, considering minor imbalances in baseline characteristics.
Role of funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had access to the dataset. AW and XM had the final responsibility for the decision to submit for publication.
Results
The observational study identified 3322 eligible patients from CNSR-Ⅲ: 2605 initiating atorvastatin and 717 initiating rosuvastatin, whose baseline characteristics were well balanced (standardized differences <0.100) (Fig. 2, Table 1). The mean age was 62 in atorvastatin initiators and 63 in rosuvastatin initiators. Regarding gender, 69.75% (1817/2605) of atorvastatin initiators and 69.74% (500/717) of rosuvastatin initiators were men. More than 50% of patients had a NIHSS total score of ≤3 upon admission. A total of 4 patients experienced symptomatic intracranial hemorrhage (sICH), with 3 cases in the atorvastatin group and 1 case in the rosuvastatin group. The median 3-month duration of follow-up was 94 days (eTable 2 in Supplement 1). At the 12-month follow-up, 47 patients were lost to follow-up, with 39 atorvastatin initiators and 8 rosuvastatin initiators.
Fig. 2.
Flowchart of the study.
Table 1.
Baseline characteristics of atorvastatin initiators and rosuvastatin initiators.
| Variables | Atorvastatin (n = 2605) | Rosuvastatin (n = 717) | Standardized difference | P value |
|---|---|---|---|---|
| Age (years, mean [SD]) | 62.17 (11.22) | 62.66 (10.59) | 0.045 | 0.30 |
| Men (no. [%]) | 1817 (69.75%) | 500 (69.74%) | <0.001 | 0.99 |
| BMI (mean [SD]) | 24.62 (3.33) | 24.76 (3.31) | 0.042 | 0.33 |
| Diagnosis (no. [%]) | ||||
| Ischemic stroke | 2341 (89.87%) | 641 (89.40%) | 0.015 | 0.72 |
| Transient ischemic attack | 264 (10.13%) | 76 (10.60%) | ||
| NIHSS total score | ||||
| Mean (SD) | 4.20 (4.20) | 4.23 (4.13) | 0.007 | 0.88 |
| ≤3 (no. [%]) | 1402 (53.82%) | 379 (52.86%) | 0.019 | 0.65 |
| Treatment (no. [%]) | ||||
| Intravenous thrombolysis | 538 (20.65%) | 138 (19.25%) | 0.035 | 0.41 |
| Endovascular therapy | 22 (0.84%) | 6 (0.84%) | <0.001 | 0.98 |
| Comorbidities (no. [%]) | ||||
| Lipid metabolism disorders | 902 (34.63%) | 216 (30.13%) | 0.096 | 0.024 |
| Pathoglycemia | 735 (28.21%) | 185 (25.80%) | 0.054 | 0.20 |
| Hypertension | 1840 (70.63%) | 505 (70.43%) | 0.004 | 0.92 |
| Coronary heart disease | 405 (15.55%) | 93 (12.97%) | 0.073 | 0.087 |
| Atrial fibrillation | 202 (7.75%) | 60 (8.37%) | 0.023 | 0.59 |
| Liver disease | 143 (5.49%) | 34 (4.74%) | 0.034 | 0.43 |
| Renal insufficiency | 25 (0.96%) | 5 (0.70%) | 0.029 | 0.87 |
| Personal lifestyle (no. [%]) | ||||
| Smoking | 1267 (48.64%) | 369 (51.46%) | 0.057 | 0.18 |
| Alcohol consumption | 1217 (46.72%) | 319 (44.49%) | 0.045 | 0.29 |
| Physical activity | 1567 (60.15%) | 422 (58.86%) | 0.026 | 0.53 |
| Disease history (no. [%]) | ||||
| Stroke | 385 (14.78%) | 97 (13.53%) | 0.036 | 0.40 |
| Transient ischemic attack | 74 (2.84%) | 14 (1.95%) | 0.058 | 0.19 |
| Lipid metabolism disorders | 129 (4.95%) | 45 (6.28%) | 0.058 | 0.16 |
| Diabetes | 503 (19.31%) | 129 (17.99%) | 0.034 | 0.43 |
| Hypertension | 1554 (59.65%) | 403 (56.21%) | 0.070 | 0.10 |
| Heart diseases | 347 (13.32%) | 95 (13.25%) | 0.002 | 0.96 |
| Infection within two weeks | 67 (2.57%) | 23 (3.21%) | 0.038 | 0.41 |
| Migraine | 36 (1.38%) | 13 (1.81%) | 0.034 | 0.61 |
| Medication history (no. [%]) | ||||
| Antiplatelet agents | 214 (8.21%) | 55 (7.67%) | 0.020 | 0.64 |
| Anticoagulant agents | 22 (0.84%) | 3 (0.42%) | 0.054 | 0.24 |
| Antidiabetic agents | 405 (15.55%) | 92 (12.83%) | 0.078 | 0.071 |
| Antihypertensive agents | 1096 (42.07%) | 290 (40.45%) | 0.033 | 0.43 |
| Laboratory data (mean [SD]) | ||||
| HDL-C (mmol/L) | 1.14 (0.30) | 1.13 (0.30) | 0.033 | 0.39 |
| LDL-C (mmol/L) | 2.60 (0.96) | 2.51 (0.97) | 0.093 | 0.12 |
| Triglycerides (mmol/L) | 1.59 (1.10) | 1.57 (0.85) | 0.020 | 0.62 |
| Alanine aminotransferase (U/L) | 21.40 (15.50) | 22.35 (17.37) | 0.058 | 0.17 |
| Aspartate aminotransferase (U/L) | 20.98 (9.95) | 21.41 (10.09) | 0.043 | 0.31 |
| eGFR (mL/min/1.73m2) | 95.83 (22.80) | 96.14 (24.61) | 0.013 | 0.75 |
| Uric acid (μmol/L) | 304.98 (90.11) | 309.23 (93.05) | 0.046 | 0.27 |
| Fasting glucose (mmol/L) | 6.50 (2.66) | 6.48 (2.81) | 0.007 | 0.87 |
| Calendar year of onset (no. [%]) | ||||
| 2015 | 554 (21.27%) | 133 (18.55%) | Reference | 0.013 |
| 2016 | 874 (33.55%) | 286 (39.89%) | 0.148 | |
| 2017 | 1154 (44.30%) | 290 (40.45%) | 0.021 | |
| 2018 | 23 (0.88%) | 8 (1.12%) | 0.079 | |
| Medical centers classified by the four economic regions of China (no. [%]) | ||||
| Eastern region | 1150 (44.15%) | 320 (44.63%) | Reference | 0.18 |
| Central region | 812 (31.17%) | 197 (27.48%) | 0.020 | |
| Western region | 381 (14.63%) | 117 (16.32%) | 0.024 | |
| Northeastern region | 262 (10.06%) | 83 (11.58%) | 0.045 | |
BMI, body mass index; eGFR, estimated Glomerular Filtration Rate; mRS, modified Rankin scale; NIHSS, National Institute of Health stroke scale; SD, standard deviation.
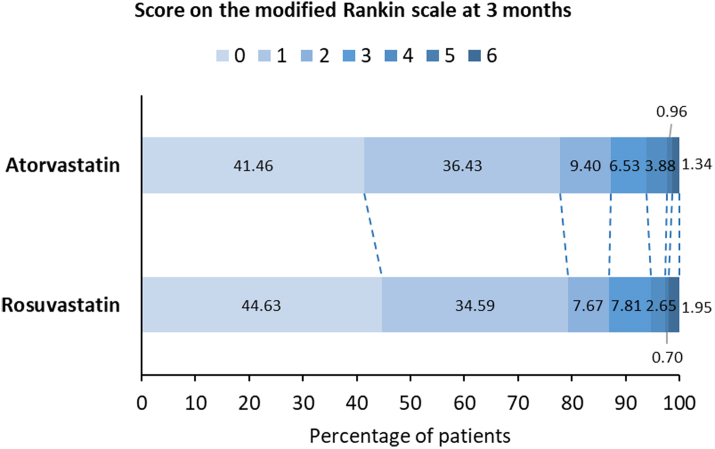
At the 3-month follow-up, 41.46% (1080/2605) of atorvastatin initiators and 44.63% (320/717) of rosuvastatin initiators attained the ideal outcome (mRS score of 0), with a relative rate of 1.12 (95% CI 1.03, 1.22) and an E value (95% LCI) of 1.49 (1.21) (Table 2, Fig. 3). In the subgroup of patients with a NIHSS total score ≤3 at admission, the relative rate was 1.17 (95% CI 1.07, 1.28) and the E value (95% LCI) was 1.62 (1.34), comparing rosuvastatin initiators with atorvastatin initiators (Fig. 4, eTable 3 in Supplement 1). Sensitivity analyses validated the robustness of the findings (Table 3, eTables 5–9 in Supplement 1).
Table 2.
Primary and secondary outcomes of atorvastatin initiators and rosuvastatin initiators.
| Outcome measures | Atorvastatin (n = 2605) | Rosuvastatin (n = 717) |
|---|---|---|
| Primary outcomes | ||
| mRS score of 0 at 3 months | ||
| No of events | 1080 | 320 |
| Event rate (%) | 41.46% | 44.63% |
| Absolute rate difference | Reference | 3.17 |
| Relative rate (95% CI) | Reference | 1.12 (1.03, 1.22) |
| P value | Reference | 0.0076 |
| E value (95% LCI) | Reference | 1.49 (1.21) |
| Secondary outcomes | ||
| mRS score of 0 at discharge | ||
| No of events | 856 | 276 |
| Event rate (%) | 32.86% | 38.49% |
| Absolute rate difference | Reference | 5.63 |
| Relative rate (95% CI) | Reference | 1.20 (1.08, 1.32) |
| P value | Reference | 0.0003 |
| E value (95% LCI) | Reference | 1.69 (1.37) |
| mRS score of 0 at 6 months | ||
| No of events | 1171 | 357 |
| Event rate (%) | 44.95% | 49.79% |
| Absolute rate difference | Reference | 4.84 |
| Relative rate (95% CI) | Reference | 1.10 (1.01, 1.19) |
| P value | Reference | 0.016 |
| E value (95% LCI) | Reference | 1.43 (1.11) |
| mRS score of 0 at 12 months | ||
| No of events | 1242 | 362 |
| Event rate (%) | 47.68% | 50.49% |
| Absolute rate difference | Reference | 2.81 |
| Relative rate (95% CI) | Reference | 1.07 (0.99, 1.15) |
| P value | Reference | 0.084 |
| E value (95% LCI) | Reference | 1.34 (1.00) |
| Stroke recurrence in 12 months | ||
| No of events | 112 | 34 |
| Event rate (%) | 4.30% | 4.74% |
| Median (P25, P75) follow-up days | 367 (363, 375) | 368 (361, 380) |
| Absolute rate difference | Reference | 0.44 |
| Hazard ratio (95% CI) | Reference | 1.08 (0.74, 1.59) |
| P value | Reference | 0.68 |
| E value (95% LCI) | Reference | 1.37 (1.00) |
| All-cause mortality in 12 months | ||
| No of events | 91 | 27 |
| Event rate (%) | 3.49% | 3.77% |
| Median (P25, P75) follow-up days | 368 (364, 376) | 369 (364, 382) |
| Absolute rate difference | Reference | 0.28 |
| Hazard ratio (95% CI) | Reference | 1.07 (0.70, 1.65) |
| P value | Reference | 0.76 |
| E value (95% LCI) | Reference | 1.34 (1.00) |
| Cardiovascular mortality in 12 months | ||
| No of events | 68 | 20 |
| Event rate (%) | 2.61% | 2.79% |
| Median (P25, P75) follow-up days | 368 (364, 376) | 369 (364, 382) |
| Absolute rate difference | Reference | 0.18 |
| Hazard ratio (95% CI) | Reference | 1.04 (0.63, 1.73) |
| P value | Reference | 0.87 |
| E value (95% LCI) | Reference | 1.24 (1.00) |
| MACE in 12 months | ||
| No of events | 188 | 55 |
| Event rate (%) | 7.22% | 7.67% |
| Median (P25, P75) follow-up days | 367 (363, 375) | 368 (361, 380) |
| Absolute rate difference | Reference | 0.45 |
| Hazard ratio (95% CI) | Reference | 1.04 (0.77, 1.41) |
| P value | Reference | 0.79 |
| E value (95% LCI) | Reference | 1.24 (1.00) |
CI, confidence interval; LCI, lower confidence interval; MACE, major adverse cardiovascular events; mRS, modified Rankin Scale; NNT, number needed to treat; P25, 25th percentile; P75, 75th percentile.
Fig. 3.
Distribution of scores on the modified Rankin scale of atorvastatin initiators and rosuvastatin initiators at 3 months.
Fig. 4.
Subgroups analyses for primary outcome of atorvastatin initiators and rosuvastatin initiators. Values are no. with event/total no. (%) unless stated otherwise. CI, confidence intervals; High-dose, atorvastatin >20 mg or rosuvastatin >10 mg; Low-dose, atorvastatin ≤20 mg or rosuvastatin ≤10 mg.
Table 3.
Primary and secondary outcomes among patients who adhered to the treatment regimen.
| Outcome measures | Atorvastatin | Rosuvastatin |
|---|---|---|
| Primary outcomes | ||
| mRS score of 0 at 3 months | ||
| No of total patients | 1890 | 458 |
| No of events | 789 | 205 |
| Event rate (%) | 41.75% | 44.76% |
| Absolute rate difference | Reference | 3.01 |
| Relative rate (95% CI) | Reference | 1.12 (1.01, 1.24) |
| P value | Reference | 0.028 |
| E value (95% LCI) | Reference | 1.49 (1.11) |
| Secondary outcomes | ||
| mRS score of 0 at discharge | ||
| No of total patients | 2581 | 713 |
| No of events | 848 | 276 |
| Event rate (%) | 32.86% | 38.71% |
| Absolute rate difference | Reference | 5.85 |
| Relative rate (95% CI) | Reference | 1.20 (1.09, 1.23) |
| P value | Reference | 0.0002 |
| E value (95% LCI) | Reference | 1.69 (1.40) |
| mRS score of 0 at 6 months | ||
| No of total patients | 1657 | 379 |
| No of events | 756 | 201 |
| Event rate (%) | 45.62% | 53.03% |
| Absolute rate difference | Reference | 7.41 |
| Relative rate (95% CI) | Reference | 1.18 (1.06, 1.29) |
| P value | Reference | 0.0010 |
| E value (95% LCI) | Reference | 1.64 (1.31) |
| mRS score of 0 at 12 months | ||
| No of total patients | 1401 | 289 |
| No of events | 692 | 161 |
| Event rate (%) | 49.39% | 55.71% |
| Absolute rate difference | Reference | 6.32 |
| Relative rate (95% CI) | Reference | 1.16 (1.03, 1.28) |
| P value | Reference | 0.0072 |
| E value (95% LCI) | Reference | 1.59 (1.21) |
| Stroke recurrence in 12 months | ||
| No of total patients | 1401 | 289 |
| No of events | 62 | 13 |
| Event rate (%) | 4.43% | 4.50% |
| Median (P25, P75) follow-up days | 367 (364, 376) | 369 (365, 380) |
| Absolute rate difference | Reference | 0.57 |
| Hazard ratio (95% CI) | Reference | 0.98 (0.54, 1.78) |
| P value | Reference | 0.95 |
| E value (95% UCI) | Reference | 1.16 (1.00) |
| All-cause mortality in 12 months | ||
| No of total patients | 1401 | 289 |
| No of events | 6 | 1 |
| Event rate (%) | 0.43% | 0.35% |
| Median (P25, P75) follow-up days | 368 (365, 377) | 370 (365, 382) |
| Absolute rate difference | Reference | 0.08 |
| Hazard ratio (95% CI) | Reference | 0.98 (0.12, 8.28) |
| P value | Reference | 0.98 |
| E value (95% UCI) | Reference | 1.16 (1.00) |
| Cardiovascular mortality in 12 months | ||
| No of total patients | 1401 | 289 |
| No of events | 3 | 0 |
| Event rate (%) | 0.21% | 0.00% |
| Median (P25, P75) follow-up days | 368 (365, 377) | 370 (365, 382) |
| Absolute rate difference | Reference | 0.21 |
| Hazard ratio (95% CI) | Reference | NA |
| P value | Reference | NA |
| E value (95% CI) | Reference | NA |
| MACE in 12 months | ||
| No of total patients | 1401 | 289 |
| No of events | 68 | 13 |
| Event rate (%) | 4.85% | 4.50% |
| Median (P25, P75) follow-up days | 367 (364, 376) | 369 (365, 380) |
| Absolute rate difference | Reference | 0.35 |
| Hazard ratio (95% CI) | Reference | 0.90 (0.50, 1.62) |
| P value | Reference | 0.71 |
| E value (95% UCI) | Reference | 1.46 (1.00) |
CI, confidence interval; LCI lower confidence interval; MACE, major adverse cardiovascular events; mRS, modified Rankin Scale; NA, not applicable; NNT, number needed to treat; P25, 25th percentile; P75, 75th percentile; UCI, upper confidence interval.
A greater percentage of patients in the rosuvastatin group attained a mRS score of 0 at discharge (relative rate 1.20 [95% CI 1.08, 1.32], and E value [95% LCI] 1.69 [1.37]) and at 6 months (relative rate 1.10 [95% CI 1.01, 1.19], and E value [95% LCI] 1.43 [1.11]) as compared with those in the atorvastatin group (Table 2, eFig. 1 in Supplement 1). Sensitivity analyses among patients who adhered to the treatment regimen revealed the association of a mRS score of 0 with rosuvastatin over atorvastatin at discharge, as well as at the 6-month and 12-month follow-up points (Table 3). Moreover, in sensitivity analyses after IPTW, excluding missing follow-up data, with multiple imputation employed for missing data, and adjusting for concurrent coronary heart disease, hypertension history, prior use of antidiabetic agents additionally, rosuvastatin were associated with a mRS score of 0 at discharge and 6 months when compared with atorvastatin (eTables 5–9 in Supplement 1).
Regarding stroke recurrence, all analyses reported statistically insignificant difference between two groups. A total of 112 atorvastatin initiators and 34 rosuvastatin initiators suffered from recurrent strokes, yielding a hazard ratio of 1.08 (95% CI 0.74, 1.59) (Table 2, eFig. 2 in Supplement 1). Sensitivity analysis based on the patients who adhered to the treatment regimen, after IPTW, after overlap weighting, with missing follow-up data deleted, with multiple imputation employed for missing mRS data, and adjusting for concurrent coronary heart disease, hypertension history, prior use of antidiabetic agents additionally resulted in a hazard ratio of 0.98 (95% CI 0.54, 1.78), 1.12 (95% CI 0.77, 1.62), 1.18 (95% CI 0.66, 2.10), 1.08 (95% CI 0.74, 1.59), 1.08 (95% CI 0.74, 1.59), and 1.09 (95% CI 0.74, 1.59), respectively (Table 3, eTables 5–9 in Supplement 1).
For all-cause mortality, no statistically significant difference was demonstrated between two groups. A total of 118 death cases were reported, including 91 from atorvastatin group and 27 from rosuvastatin group, with a hazard ratio of 1.07 (95% CI 0.70, 1.65) (Table 2, eFig. 3 in Supplement 1). Cardiovascular mortality was reported in 68 atorvastatin initiators and 20 rosuvastatin initiators, corresponding to a hazard ratio of 1.04 (95% CI 0.63, 1.73) (Table 2, eFig. 4 in Supplement 1). Six sensitivity analyses all reported statistically insignificant results (Table 3, eTables 5–9 in Supplement 1).
A total of 188 atorvastatin initiators and 55 rosuvastatin initiators experienced MACE, and the main analysis reported a hazard ratio of 1.04 (95% CI 0.77, 1.41) (Table 2, eFig. 5 in Supplement 1). When conducting analysis among patients who adhered to the treatment regimen, after IPTW, after overlap weighting, excluding missing follow-up data, with multiple imputation employed for missing mRS data, and adjusting for three additional covariates, the hazard ratios were 0.90 (95% CI 0.50, 1.62), 1.07 (95% CI 0.80, 1.43), 1.06 (95% CI 0.68, 1.65), 1.04 (95% CI 0.77, 1.40), 1.04 (95% CI 0.77, 1.41), and 1.05 (95% CI 0.77, 1.41), respectively (Table 3, eTables 5–9 in Supplement 1).
Discussion
This observational study based on a national registry compared the effectiveness and safety of atorvastatin versus rosuvastatin in patients with ischemic stroke or TIA, focusing on patients who initiated atorvastatin or rosuvastatin therapy on the day of onset. The analyses indicated a potential association of rosuvastatin over atorvastatin with an ideal outcome (mRS score of 0) at discharge, 3 months and 6 months. Regarding stroke recurrence, all-cause mortality, cardiovascular mortality, and MACE, no notable disparities emerged between two groups.
In our study, it seems that rosuvastatin initiators were associated with improved functional outcomes compared with atorvastatin initiators, which validated our hypothesis regarding the improvement of functional outcomes. The functional outcome assessed by mRS, which reflects the overall recovery status of stroke patients, is the most commonly used outcome measure in stroke trials. An mRS score of 0 indicates complete recovery to the patients’ pre-stroke condition, characterized by the absence of any symptoms, and holds significant clinical importance.21 The available studies provide some initial indications that align with our findings. As for plaque stabilization, Thondapu et al. investigated coronary plaque response to treatment with rosuvastatin and atorvastatin, and found that the rosuvastatin group showed more rapid and robust plaque stabilization, and regression of plaque volume compared to the atorvastatin group.24 The METEOR-China study revealed that rosuvastatin significantly reduced the progression of carotid intima-media thickness over 2 years in Chinese patients with subclinical atherosclerosis, compared with placebo.11 Moreover, Panes et al. compared the effects of atorvastatin and rosuvastatin on platelet cholesterol and tissue factor mass and activity in hypercholesterolemic patients and revealed that rosuvastatin, but not atorvastatin treatment, normalized the membrane cholesterol, tissue factor protein and tissue factor-dependent procoagulant activity, possibly unveiling a new pleiotropic effect of rosuvastatin.25 Findings from the INSPIRES trial indicated that in comparison to a delayed intensive statin regimen, immediate initiation of intensive statin therapy did not result in a statistically significant reduction in the 90-day risk of stroke recurrence. Nevertheless, it was associated with a substantial improvement in functional outcomes.26 It is possible that targeted statin therapy has the potential of neuroprotective effects, contributing to better functional prognoses. Although existing research has explored the anti-inflammatory role of statins, a comprehensive and conclusive comparison between atorvastatin and rosuvastatin concerning their anti-inflammatory capabilities remains an unresolved issue.27 In a word, our findings necessitate further verification in extensive clinical studies, while the underpinning mechanisms await more fundamental research for clarification.
Regarding stroke recurrence, all-cause mortality, cardiovascular mortality, and MACE, no statistically significant difference was found between the two groups. The possible reasons for these negative results may encompass the following two aspects: First, the patients’ conditions were relatively mild in our study, over 50% of them exhibiting a NIHSS score of 3 or lower upon admission. Consequently, the incidence rates of stroke recurrence and MACE were comparatively low, let alone mortality. This situation necessitates a larger sample size to potentially detect any differences between the two groups. In contrast, an observational study of more than 43,000 patients with acute ischemic stroke reported that rosuvastatin was associated with reducing the risk of the 1-year composite of recurrent stroke, myocardial infarction, and all-cause mortality by 11% and all-cause mortality by 19%, compared with atorvastatin.15 Second, with a follow–up duration of just one year in our study, an extended follow–up period might be necessary to identify potential disparities between the two groups. By contrast, an active comparator cohort study using target trial emulation reported lower incidence of 6-year all-cause mortality in rosuvastatin group than atorvastatin, where the event rates were also notably higher than those observed in our study.17 However, a secondary analysis of the LODESTAR trial reported that rosuvastatin and atorvastatin showed comparable efficacy for the composite outcome of all cause death, myocardial infarction, stroke, or any coronary revascularization at three years in adults with coronary artery disease.16 To summarize, there is no definitive conclusion regarding the differences between atorvastatin and rosuvastatin concerning stroke recurrence, all–cause mortality, cardiovascular mortality, and MACE in patients with ischemic stroke or TIA. Further studies, utilizing an expanded sample size and follow-up period, are needed to delve deeper into the disparities observed between atorvastatin and rosuvastatin.
The subgroup analyses indicated that, among patients with a NIHSS total score ≤3 at admission, treatment with rosuvastatin was associated with a significantly higher proportion of patients achieving a mRS score of 0 at 3 months. In our study, more than 50% of patients presented with a NIHSS score of ≤3 at admission. Given the findings from subgroup analyses, caution is warranted when generalizing our results to populations with moderate or severe stroke. However, more evidence pertaining to this is scarce at present. In the future, well–designed RCTs may be carried out to validate the potential differences in efficacy and safety between atorvastatin and rosuvastatin when administered to patients experiencing mild stroke. To date, we can observe that the dosing and timing of statin administration in the realm of stroke has garnered significant attention.28, 29, 30 There is a pressing need for additional research in the statin strategy for patients with ischemic stroke or TIA to inform individualized medication regimen.28
In this observational study, several strengths presented themselves. First, derived from a national registry-based investigation, the data possesses robust representativeness, reflecting the therapeutic landscape for patients suffering from ischemic stroke and TIA in China.19 Second, the research question possesses practical significance, providing guiding insights for clinical practice through a direct comparison of treatment outcomes between atorvastatin and rosuvastatin. Third, the baseline characteristics of atorvastatin group and rosuvastatin group were well-balanced, minimizing confounding bias. Fourth, the design of our study has been meticulously crafted to minimize the influence of constant time bias on the outcomes. By specifically including patients who commenced their medication regimen on the day of onset, which also aligns with the best clinical practice as recommended by current guidelines, we have ensured that medication administration, and follow-up assessments commenced concurrently on the same day, aligning seamlessly with the initiation of patient follow-up in the registry study.26,31 Fifth, we performed extensive subgroup and sensitivity analyses to ensure consistency in the results of the study.
Nonetheless, this study is characterized by several limitations that necessitate thorough consideration. First, given the observational nature of our study, our analysis only identified a potential association between rosuvastatin treatment and patient outcomes, and it is challenging to establish causality. Although our sensitivity analyses systematically evaluated the robustness and reliability of findings across diverse scenarios and assumptions, the possibility of unmeasured confounding should not be ignored. However, we employed the E value to evaluated the robustness of our outcomes to unmeasured confounders. Using the primary outcome in our main analysis as an illustrative case, an E value (95% LCI) of 1.49 (1.21) indicated the minimum magnitude of association that an unmeasured confounder would require with both rosuvastatin (compared with atorvastatin) and a mRS score of 0 to completely account for the association in our study. Thus, our findings appear to exhibit a notable degree of robustness against unmeasured confounding factors that could potentially challenge the validity of the conclusions. However, this phenomenon may, to a certain extent, be attributed to the limited number of studies that have directly compared atorvastatin and rosuvastatin, particularly within the context of stroke research. In a word, future RCTs are needed to validate our findings. Second, due to constraints imposed by utilization rates, the scope of our research questions was specifically confined to two most commonly used statins, excluding other statin medications, which necessarily constrained the extent of our conclusions. Third, owing to the inherent characteristics of real–world data, an imbalance in sample size between the two treatment groups was observed in this study. Fourth, due to the scarcity of data on adverse drug reactions associated with atorvastatin and rosuvastatin, we failed to compare outcomes on this aspect in our study. Fifth, given that this study was undertaken in the Chinese population, careful consideration must be given when generalizing the findings to those of other nations and ethnicities. Despite these limitations, the overall findings in the study outcomes remain discernible. Moving forward, future studies are warranted to providing more evidence and gain a comprehensive understanding in the area.
In conclusion, our study using real-world data indicated a potential association of rosuvastatin over atorvastatin with an ideal outcome (mRS score of 0) in patients with ischemic stroke or TIA who initiate treatment with atorvastatin or rosuvastatin. No notable disparities emerged between them regarding stroke recurrence, all-cause mortality, cardiovascular mortality, or MACE. Further RCTs are necessary to validate these findings, advancing the precision therapy of statins in patients with ischemic stroke or TIA.
Contributors
Jianhua Zhao and Xinya Li contributed equally to this work. Conceptualisation: Anxin Wang, Xia Meng, Jianhua Zhao, Xinya Li; Data curation: Anxin Wang, Xia Meng, Jianhua Zhao, Xinya Li; Formal analysis: Jianhua Zhao, Xinya Li, Xue Xia, Xue Tian, Qin Xu; Funding acquisition: Xia Meng, Anxin Wang, Xue Xia; Investigation: Jianhua Zhao, Xinya Li, Xue Xia, Xiaoli Zhang, Ruobing Tian; Methodology: Jianhua Zhao, Xinya Li, Xue Tian; Project administration: Anxin Wang, Xia Meng; Resources: Anxin Wang, Xia Meng; Software: Xinya Li; Supervision: Anxin Wang, Xia Meng; Validation: all authors; Visualisation: Jianhua Zhao, Xinya Li, Xue Xia, Xue Tian; Writing–original draft: Jianhua Zhao, Xinya Li; Writing–review & editing: all authors. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication.
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of interests
All authors declared no competing interests for this work.
Acknowledgements
This work was supported by National Key Research and Development Program of China (2022YFC2502400, 2022YFC2502404), Beijing Natural Science Foundation Haidian original innovation joint fund (L222123), and Youth Innovation Fund of Beijing Neurosurgical Institute (2025 Reform and Development–Youth 15). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. We thank all the investigators at each site, as well as all participants.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2025.103381.
Contributor Information
Xia Meng, Email: mengxia@ncrcnd.org.cn.
Anxin Wang, Email: wanganxin@bjtth.org.
Appendix A. Supplementary data
References
- 1.Hilkens N.A., Casolla B., Leung T.W., de Leeuw F.E. Stroke. Lancet. 2024;403(10446):2820–2836. doi: 10.1016/S0140-6736(24)00642-1. [DOI] [PubMed] [Google Scholar]
- 2.Mendelson S.J., Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review. JAMA. 2021;325(11):1088–1098. doi: 10.1001/jama.2020.26867. [DOI] [PubMed] [Google Scholar]
- 3.Walter K. What is acute ischemic stroke? JAMA. 2022;327(9):885. doi: 10.1001/jama.2022.1420. [DOI] [PubMed] [Google Scholar]
- 4.Ugusman A., Hisam N.S.N., Othman N.S., et al. Pharmacological interventions for intraplaque neovascularization in atherosclerosis. Pharmacol Ther. 2024;261 doi: 10.1016/j.pharmthera.2024.108685. [DOI] [PubMed] [Google Scholar]
- 5.Newman C.B., Preiss D., Tobert J.A., et al. Statin safety and associated adverse events: a scientific statement from the American heart association. Arterioscler Thromb Vasc Biol. 2019;39(2):e38–e81. doi: 10.1161/ATV.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 6.Baigent C., Keech A., Kearney P.M., et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 7.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 8.Yang W.Y., Li Y.F., Wang Z.R., et al. Combined therapy of intensive statin plus intravenous rt-PA in acute ischemic stroke: the INSPIRE randomized clinical trial. J Neurol. 2021;268(7):2560–2569. doi: 10.1007/s00415-020-10388-3. [DOI] [PubMed] [Google Scholar]
- 9.Baigent C., Blackwell L., Emberson J., et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paparodis R.D., Bantouna D., Livadas S., Angelopoulos N. Statin therapy in primary and secondary cardiovascular disease prevention. Curr Atheroscler Rep. 2024;27(1):21. doi: 10.1007/s11883-024-01265-9. [DOI] [PubMed] [Google Scholar]
- 11.Zheng H., Li H., Wang Y., et al. Rosuvastatin slows progression of carotid intima-media thickness: the METEOR-China randomized controlled study. Stroke. 2022;53(10):3004–3013. doi: 10.1161/STROKEAHA.120.031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura M., Fukukawa T., Kitagawa K., et al. Ten-year standardization of lipids and high-sensitivity C-reactive protein in a randomized controlled trial to assess the effects of statins on secondary stroke prevention: Japan Statin Treatment against Recurrent Stroke. Ann Clin Biochem. 2018;55(1):128–135. doi: 10.1177/0004563217693651. [DOI] [PubMed] [Google Scholar]
- 13.Montaner J., Bustamante A., García-Matas S., et al. Combination of thrombolysis and statins in acute stroke is safe: results of the STARS randomized trial (stroke treatment with acute reperfusion and Simvastatin) Stroke. 2016;47(11):2870–2873. doi: 10.1161/STROKEAHA.116.014600. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura S., Uchida K., Daimon T., Takashima R., Kimura K., Morimoto T. Randomized controlled trial of early versus delayed statin therapy in patients with acute ischemic stroke: ASSORT trial (administration of statin on acute ischemic stroke patient) Stroke. 2017;48(11):3057–3063. doi: 10.1161/STROKEAHA.117.017623. [DOI] [PubMed] [Google Scholar]
- 15.Kim J.T., Lee J.S., Kim H., et al. Comparative effectiveness of rosuvastatin versus atorvastatin in acute ischemic stroke treatment. J Am Heart Assoc. 2025;14(3) doi: 10.1161/JAHA.124.038080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y.J., Hong S.J., Kang W.C., et al. Rosuvastatin versus atorvastatin treatment in adults with coronary artery disease: secondary analysis of the randomised LODESTAR trial. BMJ. 2023;383 doi: 10.1136/bmj-2023-075837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou S., Chen R., Liu J., et al. Comparative effectiveness and safety of atorvastatin versus rosuvastatin : a multi-database cohort study. Ann Intern Med. 2024;177(12):1641–1651. doi: 10.7326/M24-0178. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A., Shariff M., Doshi R. Impact of rosuvastatin versus atorvastatin on coronary atherosclerotic plaque volume - a systematic review and meta-analysis with trial sequential analysis of randomized control trials. Eur J Prev Cardiol. 2020;27(19):2138–2141. doi: 10.1177/2047487319868035. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Jing J., Meng X., et al. The Third China National Stroke Registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline atient characteristics. Stroke Vasc Neurol. 2019;4(3):158–164. doi: 10.1136/svn-2019-000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. 1989;20(10):1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 21.Saver J.L., Chaisinanunkul N., Campbell B.C.V., et al. Standardized nomenclature for modified Rankin scale global disability outcomes: consensus recommendations from stroke therapy academic industry roundtable XI. Stroke. 2021;52(9):3054–3062. doi: 10.1161/STROKEAHA.121.034480. [DOI] [PubMed] [Google Scholar]
- 22.VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 23.Lachin J.M. Fallacies of last observation carried forward analyses. Clin Trials. 2016;13(2):161–168. doi: 10.1177/1740774515602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thondapu V., Kurihara O., Yonetsu T., et al. Comparison of rosuvastatin versus atorvastatin for coronary plaque stabilization. Am J Cardiol. 2019;123(10):1565–1571. doi: 10.1016/j.amjcard.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Panes O., González C., Hidalgo P., et al. Platelet tissue factor activity and membrane cholesterol are increased in hypercholesterolemia and normalized by rosuvastatin, but not by atorvastatin. Atherosclerosis. 2017;257:164–171. doi: 10.1016/j.atherosclerosis.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y., Jiang L., Pan Y., et al. Immediate- or delayed-intensive statin in acute cerebral ischemia: the INSPIRES randomized clinical trial. JAMA Neurol. 2024;81(7):741–751. doi: 10.1001/jamaneurol.2024.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taqueti V.R., Ridker P.M. Lipid-lowering and anti-inflammatory benefits of statin therapy: more than meets the plaque. Circ Cardiovasc Imaging. 2017;10(7) doi: 10.1161/CIRCIMAGING.117.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kytö V., Åivo J., Ruuskanen J.O. Intensity of statin therapy after ischaemic stroke and long-term outcomes: a nationwide cohort study. Stroke Vasc Neurol. 2025;10(1):142–145. doi: 10.1136/svn-2024-003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amarenco P., Bogousslavsky J., Callahan A., 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 30.Kleindorfer D.O., Towfighi A., Chaturvedi S., et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke. 2021;52(7):e364–e467. doi: 10.1161/STR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 31.Kelly A.G., Holloway R.G. Guideline: the AHA/ASA made 217 recommendations for early management of acute ischemic stroke in adults. Ann Intern Med. 2018;168(12) doi: 10.7326/ACPJC-2018-168-12-063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.