Abstract
Background.
Epidemiology and laboratory studies support the conclusion that air pollution is carcinogenic to humans. Chemically specific biomarkers are useful for determining human exposure to, and metabolism of, potentially toxic and carcinogenic components of air pollution, and distinguishing exposures due to air pollution from those resulting from tobacco smoke.
Methods.
Representative chemically specific biomarkers, quantified by mass spectrometry and related techniques, are discussed. The biomarkers are related to exposure to polycyclic aromatic hydrocarbons, volatile toxicants and carcinogens, oxidants, DNA damaging compounds, and metals found in polluted air.
Results.
Quantifiable chemically specific biomarkers potentially useful in studies of air pollution carcinogenesis include urinary 1-hydroxypyrene, metabolites of benzo[a]pyrene, phenanthrene, benzene, 1,3-butadiene, acrolein, and crotonaldehyde, as well as products of oxidative damage such as urinary 8-hydroxy-2’-deoxyguanosine, malondialdehyde, and F2-isoprostanes, certain DNA adducts, and metals. Tobacco-specific metabolites such as cotinine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol can be used to distinguish tobacco-related exposures from those resulting from air pollution.
Conclusions.
The following validated chemically specific biomarkers are currently the optimal ones for use in studies of air pollution and cancer: urinary 1-hydroxypyrene, phenanthrene metabolites, S-phenyl mercapturic acid, urinary or blood Cd, 8-hydroxydeoxyguanosine and F2-isoprostanes such as 8-iso-PGF2α. This suite of biomarkers will reliably establish exposure to carcinogenic polycyclic aromatic hydrocarbons, benzene and Cd, and will also provide critical information on oxidative damage and inflammation, both of which are important in carcinogenesis.
Keywords: Air pollution, carcinogenesis biomarkers, polycyclic aromatic hydrocarbons, volatile toxicants and carcinogens, oxidative damage, DNA adducts, metals
INTRODUCTION
The World Health Organization (WHO) has concluded that air pollution is a major environmental risk to health (https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health). More than 90% of the world’s population lives in locations where WHO air quality guidelines were unmet. WHO’s International Agency for Research on Cancer (IARC) has classified outdoor air pollution and particulate matter from outdoor air pollution as carcinogenic to humans based on sufficient evidence of carcinogenicity in humans and experimental animals and strong mechanistic evidence (1). Epidemiology studies and experiments with laboratory animals provide a consistent picture of the carcinogenicity of air pollution. The epidemiology studies in particular demonstrate an increased risk of lung cancer in cohort and case-control studies which included millions of people and thousands of lung cancer cases from Europe, North America, and Asia (1). Fine particulate matter (PM2.5) is widely used as an indicator pollutant in air pollution studies, and its concentrations range from less than 10 to more than 100 μm/m3 worldwide (1). In addition to particles, air pollution is a complex mixture of chemicals and toxicants, some of which are established carcinogens and are involved in cancer etiology due to air pollution. This review discusses quantifiable chemically specific biomarkers which can be used to assess human exposure to carcinogenic airborne pollutants including polycyclic aromatic hydrocarbons, volatile toxicants and carcinogens, oxidants, DNA damaging compounds, and metals. We also examine biomarkers that can be applied in epidemiological studies to distinguish exposures to air pollution versus tobacco smoke. While previous reviews have discussed the application of biomarkers to air pollution studies (2–7), ours is unique in focusing only on analytically validated chemically specific biomarkers.
Polycyclic Aromatic Hydrocarbons (PAH)
PAH are a group of pollutants generated from incomplete combustion of organic substances and are widespread in the environment. Humans are exposed to PAH from different sources, including occupational exposures, air pollution, cigarette smoke, food and water, soil and dust. A large body of evidence implicates PAH as significant causes of cancer in cigarette smokers and workers with occupational exposure (8). They may also play a potentially important role in the development of lung cancer among lifelong never smokers (9). For the general population, air pollution, cigarette smoke and food are the main exposure pathways. Concentrations of several typical PAH including pyrene, benzo[a]pyrene (BaP), and phenanthrene in cigarette smoke, indoor and outdoor air, and food are listed in Table 1 of the Supplementary Data. Urinary metabolites have been extensively used as biomarkers to assess human exposure to PAH. Metabolites derived from 3 PAH - pyrene, BaP, and phenanthrene - are discussed here; representative structures are illustrated in Figure 1.
Fig. 1.

Structures of main PAH metabolites discussed in this review.
Pyrene Metabolites
Pyrene is a relatively abundant and non-carcinogenic component of all PAH mixtures. Metabolism of pyrene leads to formation of 1-hydroxypyrene (1-OHP) which undergoes phase II metabolism to 1-OHP-glucuronide and sulfate. F.J. Jongeneelen et al. first proposed the use of 1-OHP as a biomarker to assess exposure to PAH in coal-tar treated patients and workers (10–12). After that, it was determined in hundreds of studies to assess human exposure to PAH. Many analytical procedures have been developed, including HPLC with fluorescence detection (HPLC-FLD) (13,14), gas chromatography-mass spectrometry (GC-MS) or GC-high resolution mass spectrometry (GC-HRMS) with silylation (15–17), and liquid chromatography-tandem mass spectrometry (LC-MS/MS) with or without derivatization (18–20). Table 1 summarizes some representative data from recent investigations of urinary 1-OHP.
Table 1.
Representative urinary 1-OHP concentrations in various subjects in recent studies.
| Subject categories | Country | 1-OHP level | Reference |
|---|---|---|---|
| Coke oven workers (N=122) | China | 35120 (19870, 61930) pg/mg creatinine [median (25th, 75th percentile)] | (21) |
| Office workers (N=137) | South Korea | 85.6 (2.09) pg/mg creatinine [GM(SD)] | (22) |
| Painting workers (N=82) | 587.9 (3.45) pg/mg creatinine | ||
| Charcoal workers (N=25) | Nigeria | 4280 ± 2450 (675–9320) pg/mg creatinine [mean (range)] | (23) |
| Charcoal workers (N=23) | 2550 ± 1250 (656 – 6230) pg/mg creatinine | ||
| Non-charcoal workers (N=20) | 617 ± 502 (19.3 – 792000) pg/mg creatinine | ||
| E-waste recycling workers (N=72) | Ghana | 1330 (780 – 2520) pg/mg creatinine [median (25th, 75th percentile)] | (24) |
| Controls (N=40) | 540 (290 – 800) pg/mg creatinine | ||
| Firefighters pre-fire (N=27) | Canada | 100 (10) pg/mg creatinine [GM (SE)] | (25) |
| Firefighters post-fire (N=31) | 270 (20) pg/mg creatinine | ||
| Controls (N=13) | 70 (10) pg/mg creatinine | ||
| E-cigarette users (N=28) | U.S. | 82.9 (56.7 – 120) pg/mL [GM (95% CI)] | (26) |
| Cigarette smokers (N=17) | U.S. | 192 (120 – 308) pg/mL | (27) |
| Cigarette smokers (N=165) | U.S. | 212 (175–255) pg/mL | (28) |
| Never tobacco users (N=58) | Iran | 412.0 (344.2 ~ 493.1) pg/mg creatinine [GM (95% CI)] | (29) |
| Exclusive cigarette smokers (N=33) | 636.0 (504.0 ~ 802.4) pg/mg creatinine | ||
| Exclusive waterpipe smokers (N=37) | 960.8 (725.1 ~ 1273.0) pg/mg creatinine | ||
| Exclusive nass smokers (N=30) | 441.9 (340.2 ~ 574.0) pg/mg creatinine | ||
| Cigarette smokers (N=885) | U.S. | 259 (240 ~ 280) pg/mg creatinine [GM (95% CI)] | (30) |
| Nonsmokers (N=1296) | 96.7 (90.8 ~ 103) pg/mg creatinine | ||
| Mexican Americans (N=450) | U.S. | 125 (117 ~ 133) pg/mL [GM (95% CI)] | (30) |
| Non-Hispanic blacks (N=578) | 182 (168 ~ 198) pg/mL | ||
| Non-Hispanic whites (N=979) | 128 (118 ~ 138) pg/mL | ||
| All Hispanics (N=698) | 129 (120 ~ 139) pg/mL | ||
| Asians (N=352) | 93.2 (83.6 ~ 104) pg/mL | ||
| General population (N=84) | China | 667 (378) pg/mL [mean (median)] | (31) |
| General population (N=23) | Vietnam | 641 (463) pg/mL | |
| General population (N=34) | Japan | 183 (75) pg/mL | |
| General population (N=38) | India | 699 (424) pg/mL | |
| General population (N=29) | Malaysia | 186 (65) pg/mL | |
| General population (N=60) | Korea | 167 (103) pg/mL | |
| General population (N=38) | Kuwait | 320 (220) pg/mL | |
| Children aged 7–13 years (N=1206) | China | 184 (212 ~ 237) pg/mL [GM (95% CI)] | (32) |
| Children aged 6–11 years (N=415) (2009–2010) | U.S. | 147 (125 ~ 174) pg/mL [GM (95% CI)] | (30) |
| Children aged 6–11 years (N=397) (2011–2012) | 130 (111 ~ 153) pg/mL | ||
| Children aged 6–11 years (N=399) (2013–2014) | 131 (118 ~ 145) pg/mL | ||
| Adults aged 20+ years (N=1911) (2009–2010) | 113 (103 ~ 122) pg/mL | ||
| Adults aged 20+ years (N=1703) (2011–2012) | 107 (96.2 ~ 119) pg/mL | ||
| Adults aged 20+ years (N=1802) (2013–2014) | 128 (120 ~ 137) pg/mL |
This biomarker has several notable strengths. It is the principal metabolite of pyrene, which is always found in PAH mixtures in reasonably high proportions and it has a high detection frequency in human urine (30). While pyrene itself is non-carcinogenic, levels of 1-OHP are representative of carcinogenic PAH exposure. Levels of 1-OHP were 2.5 to 6.9 times higher in various occupationally exposed workers than in controls, and consistently about twice (1.5–2.7) as great in cigarette smokers as in non-smokers (26,29,33). Levels in the urine of the general population from the U.S. and several Asian countries vary considerably and are likely to be strongly influenced by airborne pollutants (Table 1), although diet and genetic polymorphisms in metabolizing enzymes due to ethnic/racial differences may also contribute (30,31). In one ten-year study (2003–2014) in the U.S., children aged 6–11 years had higher urinary 1-OHP levels than adults aged 21+ years (30). Possible causes for this are the unique activity patterns and behavior of children, as well as physiological differences between them and adults. Exposure to air pollution and secondhand smoke have been associated with the development of childhood asthma, lower respiratory infections and leukemia (34–37).
All of the studies described above have measured 1-OHP after treatment of urine with deconjugating enzymes, β-glucuronidase and/or aryl-sulfatase, since 1-hydroxypyrene glucuronide conjugate (1-OHPG) was the major pyrene metabolite in human urine (38). Methods for direct measurement of 1-OHPG have been established without enzymatic hydrolysis using immune-affinity chromatography with synchronous fluorescence spectroscopy (IAC–SFS) (38,39), HPLC-FLD (40,41), and LC-MS/MS (42,43). Urinary concentrations of 1-OHPG were about 5.6 times higher in occupationally exposed workers (2.16 pmol/mL, mean value) than among workers (0.38 pmol/mL) with no or low exposure (44). Cigarette smokers had a 2–3 fold higher concentration of 1-OHPG compared with nonsmokers (44–46). Exposure to secondhand smoke and air pollution would also contribute to the urinary 1-OHPG in children (39,47).
BaP Metabolites
BaP is the prototypic carcinogenic and mutagenic PAH and is classified by IARC as carcinogenic to humans (8). BaP requires metabolic activation to exert its carcinogenic and other adverse effects. Phase I reactions of BaP are catalyzed primarily by CYP1A1 and CYP1B1 and produce a series of metabolites including phenols in the detoxification pathway, whereas epoxides, dihydrodiols, quinones and tetraols are produced in the bioactivation pathways, with BaP diol epoxide (BPDE) as a major ultimate carcinogenic metabolite which can attack DNA (Figure 2). These metabolites are mainly excreted in feces and are present in extremely low levels in urine. Thus, direct analysis of urinary BaP metabolites can be challenging. 3-HydroxyBaP (3-OHBaP) has been proposed as a good surrogate for assessing exposure to carcinogenic PAH, since its excretion, but not that of 1-OHP, correlated significantly with the levels of DNA adducts in humans. Methods for quantification of 3-OHBaP have been developed based on HPLC-FLD (14,48), gas chromatography-mass spectrometry (GC-MS) or GC- high resolution mass spectrometry (HRMS) with or without silylation (15,49,50), and LC-MS/MS with or without derivatization (20,51,52). Urinary levels of 3-OHBaP in some recent studies are summarized in Table 2 of the Supplementary Data. In general, urinary 3-OHBaP concentrations were 2 to 4 orders of magnitude lower than those of 1-OHP, not only because urinary metabolites account for a very low proportion of total BaP, but also due to the more complex metabolism of BaP. A biological limit value (0.45 nmol/mol creatinine), higher than most of the reported levels, was proposed by Michel et al based on the correlation between urinary 3-OHBaP and atmospheric BaP (53).
Fig. 2.

Metabolism of BaP and Phe to dihydrodiols, phenols, quinones and tetraols. P450, cytochrome P450; EH, epoxide hydrolase. AKR, aldo-keto reductases.
3-OHBaP represents a detoxification pathway of BaP metabolism. Conversely, urinary r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (trans, anti-BaPT), the major end product of the BaP bay region diol epoxide metabolic activation pathway, was proposed to be a more relevant biomarker linked to bioactivation and carcinogenicity (54–56). Methods for quantification of trans, anti-BaPT based on GC-negative ion chemical ionization (NICI)-MS/MS or GC-atmospheric pressure laser ionization (APLI)-MS have been described (54–60). For occupational exposure, mean trans, anti-BaPT was 0.4 ± 0.3 nmol/mol creatinine in coke-oven workers, ~ 10 times higher than that (0.03 ± 0.03 nmol/mol creatinine) in referents (p < 0.001) (61). An extremely high level of trans, anti-BaPT (17.2 ± 13.5 fmol/mL) was observed in creosote workers, ~ 29 and 66 times higher than that in smokers (0.587 ± 0.311 fmol/mL) and nonsmokers (0.26 ± 0.04 fmol/mL) without occupational exposure to PAH (55,62). The relationship of trans, anti-BaPT to exposure to air pollution has not been reported.
Phenanthrene Metabolites
Phenanthrene (Phe) is the simplest non-carcinogenic PAH with structural features (bay region) and enzymology profile similar to that of BaP (Figure 2). Thus, Phe metabolites may provide information on both exposure and metabolic activation.
As the main metabolites in the detoxification pathway, five phenanthrols - 1-OHPhe, 2-OHPhe, 3-OHPhe, 4-OHPhe and 9-OHPhe - have been identified in human urine by using GC-MS (63,64), HPLC-FLD (65), LC-MS/MS (66). Table 3, Supplementary Data summarizes some representative data from recent investigations of urinary phenanthrols. Similar to 1-OHP, levels of phenanthrols in the urine of the general population from the U.S. and several Asian countries also vary considerably, ~ 10 times lower in the U.S. population than those from China, which is likely due to air pollution (30,31). Kuang et al reported that levels of 1-, 2-, and 3-OHPhe were ~3–4 times higher in high exposure coke-oven workers than controls, while 9-OHPhe concentration was only ~1.6 times higher, and 4-OHPhe levels were almost the same and even lower in exposure group than the controls (64). Levels of 1-, 2-, 3- and 4-OHPhe in smokers were 1.7 ~2.9 times higher than in nonsmokers in the U.S. CDC’s National Health and Nutrition Examination Survey (NHANES) 2011–2012 with large sample size (33). And the ratio of 3- plus 4-OHPhe to 1- plus 2-OHPhe ([3-OHPhe+4-OHPhe] / [1-OHPhe+2-OHPhe]) increased from 0.39 to 0.57 with cigarette consumption (33), confirming the results obtained by Heudorf & Angerer and Jacob et al (67,68). This phenomenon is thought to be caused by the induction by cigarette smoke of P450 1A2, which plays an important role in 3,4-oxidation of Phe (69,70).
Phe dihydrodiols including Phe-1,2-diol, Phe-3,4-diol and Phe-9,10-diol were also introduced to assess the metabolic pathways of Phe. They have been quantified in human urine by GC-MS (71,72).
While trans, anti-BaPT is relevant to bioactivation and carcinogenicity, its concentration in human urine is so low that it cannot be used in large studies. Thus,, r-1, t-2,3, c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene (trans, anti-PheT) was proposed as a surrogate measure of carcinogenic PAH metabolic activation by the bay-region diol epoxide pathway (62,73). Urinary trans, anti-PheT levels have associated with lung cancer risk in smokers and nonsmokers in cohort studies, and a strong correlation between overall trans, anti-PheT and trans, anti-BaPT was observed in human urine (9,62,74). A simple high throughput method using one step 96-well format SPE extraction coupled with GC-NICI-MS/MS was developed for the analysis of trans, anti-PheT, allowing its measurement in large studies.
Phe quinones (PheQ) are components in airborne particulate matter (PM) and generate reactive oxygen species in a redox cycling process. PheQ can also form during P450s and aldo-keto reductases catalyzed metabolism of Phe. 9,10-PheQ and its glucuronide conjugate have been identified in human urine at a quite low level (39.7 ~ 128.2 nmol/mol creatinine), 85 ~ 275 times lower than total OHPhe, accounting for a small proportion (~1% or less) of Phe metabolites (63,64,75).
Individual susceptibility plays an important role in cancer development in humans exposed to chemical carcinogens, thus identification of high-risk individuals is critical in cancer prevention and therapy. Up to now, Phe is the only PAH which has a bay region and for which most kinds of metabolites can be conveniently analyzed. This provides an opportunity to closely mimic metabolic phenotyping of BaP resulting from bioactivation and detoxification pathways. A ratio of bioactivation metabolites to detoxification metabolites has been proposed to assess human individual sensitivity to carcinogenic PAH exposure. trans, anti-PheT/HOPhe ratios were significantly higher in smokers than in nonsmokers in a longitudinal study, showing that smoking induces the diol epoxide metabolic activation pathway of Phe (76).
Volatile Toxicants and Carcinogens
Benzene
Benzene is an established cause of acute myeloid leukemia and acute non-lymphocytic leukemia in humans (77). Cigarette smoking is a major source of human exposure to benzene. There are a number of occupational sources of exposure to benzene including in the petrochemical industry, coke oven industry, around gasoline service stations, in urban work settings, and in certain manufacturing industries. Sources of benzene exposure in the general population include areas with heavy traffic, near gasoline filling stations, and consumption of contaminated food and water (77).
While benzene itself in blood or urine and trans, trans-muconic acid in urine have been used as biomarkers of benzene exposure, they each have disadvantages. Benzene is volatile, so could be lost from blood or urine during collection, and blood collection is invasive. trans, trans-Muconic acid is a metabolite of both benzene and sorbic acid, thus detracting from its specificity as a benzene biomarker. The benzene metabolites phenol and catechol also have multiple dietary sources, so cannot be directly linked to benzene exposure (78,79). The most generally useful biomarker of benzene exposure is urinary S-phenylmercapturic acid (SPMA, Figure 3), which is formed by conjugation of the benzene metabolite benzene oxide with glutathione (78,79). The resulting intermediate spontaneously dehydrates to S-phenyl glutathione (SPG) and is processed metabolically by the mercapturic acid pathway (ɣ-glutamyl transpeptidase and cysteinyl glycinase followed by acetylation) to give SPMA. Some undehydrated product (e.g. 1-hydroxy-2-N-acetylcysteinyl)-1,2-dihydrobenzene) can also be formed from the intermediate during this process; treatment of the urine sample with acid converts this metabolite to SPMA. SPMA is determined by LC-MS/MS (80,81).
Figure 3.
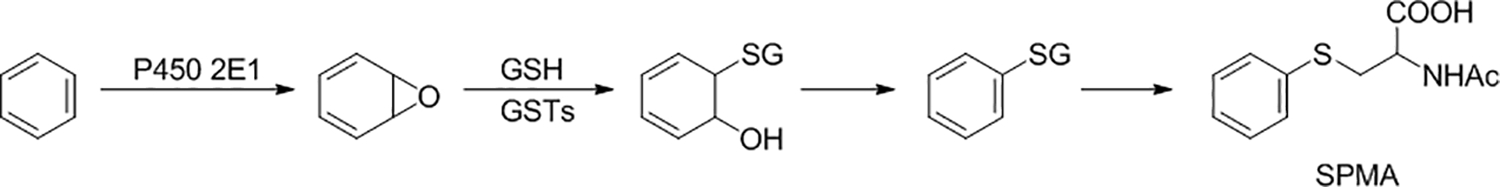
Metabolic formation of SPMA from benzene. Benzene is converted to benzene oxide, catalyzed mainly by cytochrome P450 2E1. Benzene oxide is detoxified by reaction with glutathione (GSH), catalyzed by glutathione transferases (GSTs). Dehydration of the resulting intermediate yields phenyl glutathione, which is metabolized by the mercapturic acid pathway to the urinary metabolite and biomarker SPMA. Some of the undehydrated intermediate from the initial reaction with glutathione may also be processed by the mercapturic acid pathway giving 1-hydroxy-2-N-acetylcysteinyl)-1,2-dihydrobenzene; acid treatment of the urine sample converts this to SPMA.
The highest levels of urinary SPMA are observed in cigarette smokers. In one study, levels of SPMA in cigarette smokers from 5 different ethnic groups ranged from 3.05 – 4.56 pmol/mg creatinine (about 1.4 μg per 24h) while another study found levels of 4.72 – 7.05 μg per 24h (81,82). Cessation of smoking resulted in an approximate 90% reduction of SPMA within 3 days to a level of about 0.4 pmol/mg creatinine, consistent with the major effect of cigarette smoking on SPMA levels (82,83). A study carried out in Qidong, China, a region with substantial air pollution, found higher levels of SPMA in urine of non-smokers (0.98 pmol/mg creatinine) than reported in non-smoking residents of Singapore (about 0.5 pmol/mg creatinine) (84–86). Similarly, urine samples collected from non-smokers in Shanghai, China from 1986–1989 when general environmental pollution was likely considerable, had about 1 pmol/mg creatinine SPMA (9). Some studies show elevated SPMA levels in occupationally exposed subjects such as taxi drivers, but the effects are less than those due to smoking or high air pollution (87–90). Genotype can have a substantial effect on SPMA levels in urine (91). In one study, GSTT1 deletion explained up to 31.6% of the variation in SPMA levels; thus determination of GST genotype is critical in studies using SPMA as a biomarker (81). GSTT1 null genotype theoretically signifies a higher risk for health effects upon benzene exposure since its detoxification will be decreased, yet the null genotype results in lower SPMA levels, which presents some difficulties in interpretation.
1,3-Butadiene
1,3-Butadiene is considered carcinogenic to humans by IARC; it causes cancer of the hematolymphatic organs (77,92). Cigarette smoking is the major source of exposure to 1,3-butadiene. Levels of 1,3-butadiene in outdoor air are typically relatively low, with a mean of about 0.1 μg/m3, although the levels can be somewhat higher in areas of urban industry and high road traffic (77).
The most useful and widely applied biomarkers of 1,3-butadiene exposure are the urinary metabolites 1-(N-acetyl-L-cysteine-S-yl)-2-hydroxybut-3-ene and 2-(N-acetyl-L-cysteine-S-yl)-1-hydroxybut-3-ene, collectively termed MHBMA, resulting from glutathione detoxification of 3,4-epoxy-1-butene, as illustrated in Figure 4. In one study of more than 1,000 smokers, MHBMA levels determined by LC-MS/MS averaged 4.8 ng/ml urine while another study reported a range of 3.75 – 5.69 μg/24h (82,93). Cessation of smoking resulted in a 77 – 90% reduction in MHBMA levels (82,83). As is the case with benzene-derived mercapturic acids, GSTT1 status has a strong effect on urinary MHBMA levels 14. Mercapturic acids of additional 1,3-butadiene epoxide metabolites have been analyzed by LC-MS/MS to demonstrate exposure to this carcinogen in industrial settings (94). LC-MS/MS analysis of MHBMA in non-smoking non-occupationally exposed subjects from the general population resulted in mainly values below the detection limit of the method. Hemoglobin adducts and DNA adducts of 1,3-butadiene have also been developed as biomarkers although not yet extensively used in human biomonitoring (95,96).
Figure 4.

Metabolic formation of MHBMA from 1,3-butadiene
Acrolein and Crotonaldehyde
Acrolein and crotonaldehyde are strong eye and respiratory tract toxicants causing irritation, inflammation, cell proliferation, and multiple other effects (97–99). Both compounds react directly with DNA forming adducts that may be involved in carcinogenesis although neither is a strong carcinogen (100–102). Acrolein and crotonaldehyde are also formed endogenously during lipid peroxidation and related processes (97–99).
The mercapturic acid metabolites 3-hydroxypropyl mercapturic acid (3-HPMA) and 3-hydroxy-1-methylpropyl mercapturic acid (HMPMA, Figure 5), determined by LC-MS/MS, are well-established biomarkers of acrolein and crotonaldehyde exposure (103). Cigarette smoking is the major source of exposure to acrolein and crotonaldehyde. In one study of more than 2200 cigarette smokers, median levels of 3-HPMA and HMPMA ranged from about 2000 to 3600 pmol/ml urine while another reported about 2000 – 6000 μg/24 h (82,104). When cigarette smokers stopped smoking, levels decreased by 70 – 85% (82,83). In the NHANES study, cigarette smokers and non-users had 1.089 mg/g creatinine and 0.219 mg/g creatinine levels of 3-HPMA, respectively, and 1.61 and 0.313 mg/g creatinine of HMPMA, respectively (105,106). No significant relationship was found between location (urban vs. rural) and levels of 3-HPMA and HMPMA in pregnant women in the National Children’s Health Study (107).
Figure 5.

Formation of 3-HPMA and HMPMA from acrolein (R = H) and crotonaldehyde (R = CH3)
Ethylene oxide and propylene oxide
Ethylene oxide is considered carcinogenic to humans by IARC based on sufficient evidence for carcinogenicity in laboratory animals and extensive mechanistic data including the induction of sister chromatid exchanges, chromosomal aberrations and micronucleus formation in the lymphocytes of exposed workers (92). Cigarette smoking is an important source of ethylene oxide exposure; levels of the hydroxyethyl valine adduct of ethylene oxide in hemoglobin, one of the most widely used biomarkers of ethylene oxide exposure, are increased upon exposure to cigarette smoke, and correlated with the number of cigarettes smoked (92). Urinary 2-hydroxyethyl mercapturic acid (HEMA), formed by glutathione conjugation of ethylene oxide followed by normal metabolic processing, is another established biomarker of ethylene oxide exposure. Exposure of hospital workers to ethylene oxide used in sterilization processes resulted in elevated levels of these biomarkers. Pregnant women who were cigarette smokers had significantly elevated levels of HEMA in the National Children’s Study (107). When smokers stopped smoking, their levels of HEMA decreased by 75% within 3 days (83). N7-(2-Hydroxyethyl)guanine, formed in DNA upon reaction with ethylene oxide, is an additional potential biomarker of ethylene oxide exposure, but is also formed endogenously as are the other biomarkers discussed here (92).
Propylene oxide is considered “possibly carcinogenic to humans” by IARC (108). Urinary 2-hydroxypropyl mercapturic acid is a biomarker of propylene oxide exposure (109). Similar to the other volatiles discussed here, cigarette smoking results in significant elevations of this biomarker, which decrease upon cessation (109). Air pollution has not been established as a source of elevated 2-hydroxypropyl mercapturic acid in urine (107).
Other Compounds and PM2.5
There is some human exposure to polychlorinated biphenyls (PCBs) even though their production has been banned for decades and their concentrations in the air are quite low. Hydroxylated PCBs in human urine or plasma are commonly used biomarkers (110–112). Oxy- and nitro-PAHs can be formed directly or indirectly by oxidation or nitration of PAH. Among them, 1-nitropyrene, 6-nitrochrysene, 3-nitrobenzanthrone, and anthraquinone have received considerable attention (113,114). Metabolites of 1-nitropyrene have been used to examine personal exposures (115,116).
PM2.5 refers to particulate matter with an aerodynamic diameter <2.5 μm, which can penetrate deeply into the human lung and even the bloodstream. PM2.5 is monitored in the atmosphere as a critical indicator of air pollutant dose, but doesn’t have a specific exposure biomarker. Rather, biomarkers of human expxosure to PM2.5 are mainly based on its bound chemicals, the concentrations of which may have strong positive correlations with PM2.5.
Oxidative damage products
Exposure to airborne pollutants from inhaling polluted urban air, cigarette smoke, and industrial and occupational toxicants is an established cause for cancer and a range of respiratory and cardiovascular diseases, among other health outcomes (117–119). Oxidative stress and inflammation are key mechanisms underlying such diseases. There are several possible pathways by which particles and chemicals in polluted air and cigarette smoke can trigger these processes: the inhaled particulate matter (PM) can cause inflammation in the lung, resulting in the release of various reactive oxygen species by macrophages; inflammatory processes can also be induced by the chemical toxicants and carcinogens present in the polluted air or deposited on the solid particles of PM; or oxidative stress can be induced via Fenton chemistry by transition metals present in PM (120,121). These and other mechanisms are likely to act together to produce persistent oxidative stress, leading to the formation of reactive species capable of oxidizing key macromolecules such as DNA and lipids, and inducing cellular damage (122–125). Products of these interactions can be measured in various biological samples and serve as biomarkers of oxidative stress in populations exposed to air pollution, cigarette smoke, and other inhaled toxic and carcinogenic agents (126–130). Urine is a widely used biological matrix for the analyses of many such biomarkers, including DNA adducts (131–137).
Urinary 8-hydroxy-2′-deoxyguanosine (8-OH-dG)
8-Hydroxy-2′-deoxyguanosine (8-OH-dG) is one of the most widely studied urinary DNA-derived biomarkers of oxidative stress. There are several potential mechanisms leading to the excretion of 8-OH-dG in urine, including the removal of the major oxidation adduct 8-oxo-dG from DNA by base excision repair, removal from the nucleotide pool by nucleotide phosphatases, and release from DNA after cell death (138–142). Some studies show that urinary 8-OH-dG correlates with oxidative stress and inflammation, and with the risk of cancer and cardiovascular diseases (135,143–147). A positive correlation between urinary 8-OH-dG and the levels of this biomarker in plasma and saliva has been also reported, supporting its relevance to the overall systemic oxidative status (148,149).
Enzyme-linked immunosorbent assay (ELISA) is one of the most frequently used methods for the analysis of 8-OH-dG in urine and other biological fluids; however, it suffers from a lack of sensitivity and selectivity when compared to mass-spectrometry based assays. Comparison of data obtained by the two methodologies shows much higher levels measured by ELISA than by LC -MS/MS, and a lack of correlation between the results of the two assays, calling into question the accuracy of published data generated by using ELISA (150).
Numerous MS-based assays have been reported for the analysis of 8-OH-dG in urine, including high-throughput techniques (151–154). Studies employing such assays generally show positive associations between exposure to air pollution and urinary 8-OH-dG. For instance, a study examining associations between indoor air quality and health in participants with chronic obstructive pulmonary disease (COPD) in Boston, USA, found a positive association between traffic-related black carbon exposure and urinary 8-OH-dG (155). The relationship between traffic-related air pollution and urinary 8-OH-dG and other biomarkers of oxidative stress has been also observed in studies outside the U.S. A study of traffic conductors in Taiwan showed that their urinary 8-OH-dG levels and the frequency of DNA strand breaks were significantly higher than in indoor office workers studied as controls (156). The same study showed a significant association of 8-OH-dG with urinary 1-OH-PG, which in turn was associated with the levels of PAH in airborne particulates. Other studies assessed changes in urinary 8-OH-dG in response to changes in exposure. In one study in a limited number of subjects traveling from Germany to China, higher levels of urinary 8-OH-dG were observed in these individuals after their trip to China than before the trip, consistent with the higher air pollution levels in China (157). A study conducted in Italy showed that levels of this biomarker in children increased in the evening compared to morning levels, most likely due to day-long exposures to industrial and urban air pollution (158). Increased levels of urinary 8-OH-dG have been reported in smokers, and a correlation between urinary total NNAL, a tobacco-specific biomarker, and 8-OH-dG was reported in individuals exposed to secondhand smoke (159–162). Studies that employed HPLC with electrochemical detection are also generally consistent with those based on LC-MS/MS measurements of 8-OH-dG. Such studies generally show increased levels of urinary 8-OH-dG associated with urban air pollution (163–165), exposures to smoke and fumes from cooking (166,167), and occupational toxic exposures (168).
Urinary malondialdehyde
Malondialdehyde is a major product of lipid peroxidation (169). It undergoes oxidative metabolism, and being highly reactive, can lead to DNA and protein adduct formation and is mutagenic (170–172). However, substantial levels of malondialdehyde are still present in the general circulation and excreted in urine, and it has been widely used as a biomarker of oxidative stress caused by air pollution, smoking, and occupational exposures, and has been associated with risk of cancer and atherosclerosis (147,173–175).
While an ELISA method is commonly used for malondialdehyde analysis, many studies employed HPLC with fluorescence detection for its analysis in biological samples, including urine, taking advantage of its reaction with thiobarbituric acid which produces an intensely colored chromogen fluorescent red adduct (176,177). Similar to urinary 8-OH-dG, such studies generally find positive associations between air pollution and urinary malondialdehyde levels. Thus, the Boston study of COPD patients showed a positive association between black carbon exposure and urinary malondialdehyde (155). A study of the impact of Beijing Olympic air pollution control measures showed significant decreases in urinary malondialdehyde during the Olympics, compared to the pre-Olympic period, and subsequent increases in the post-Olympic period, consistent with the trends in air pollution levels (178). In children, urinary malondialdehyde was associated with seasonal and regional variations in air pollution, as well as exposures to secondhand smoke (47,179). Associations with occupational exposures to metals also have been reported (180). However, the thiobarbituric acid-based assay is nonspecific, and MS-based assays are preferred for a more accurate and precise measurement of this biomarker (181). Studies that employed LC- or GC-MS/MS for the analysis of urinary malondialdehyde are less consistent in reporting on the association between its levels and air pollution. For example, the study of individuals traveling from Germany to China showed an increase in the levels of urinary malondialdehyde after the trip, similar to the results for 8-OH-dG (157). However, a randomized crossover intervention study of portable air filter use by Canadian healthy adults showed substantial reductions in indoor fine particle concentrations (60% reduction after 7 days of filter use), but no changes in urinary malondialdehyde levels (182).
Urinary 8-epi-PGF2α
F2-isoprostanes are prostaglandin (PG)-like compounds generated through oxidative stress-induced peroxidation of arachidonic acid (183,184). These compounds are mostly stable and robust indicators of oxidative stress present in all human tissues and fluids, and their levels have been related to a variety of chronic conditions including cardiovascular disease, diabetes, obesity, and neurodegenerative diseases (184). Among existing F2-isoprostanes, urinary 8-iso-PGF2α is a commonly used and accepted biomarker of oxidative damage (183,184).
Published studies reporting on the levels of F2-isoprostanes in various biological fluids, including urine, used analytical techniques such as ELISA, GC, and GC- or LC-MS/MS (185,186). Most environmental exposure studies used ELISA and found positive associations between air pollution and isoprostane levels in urine (187–190). Environmental exposure studies that employed LC-MS/MS for the analysis of urinary 8-iso-PGF2α are limited. The study that assessed oxidative stress markers in travelers returning to Germany after the trip to China showed that the LC-MS/MS-measured urinary 8-iso-PGF2α was higher after, than before, the trip to China, consistent with the observations for urinary 8-OH-dG and malondialdehye (157). However, a study in Peru that investigated the effect of interventions to prevent exposures from wood-burning stoves showed no difference in urinary 8-iso-PGF2α between the intervention and the control groups (166). Furthermore, a trend was observed towards negative association between this biomarker and personal PM2.5 exposure (r=−0.21, p=0.09) after controlling for the effect of proximity to the road.
Many studies consistently report the association of 8-iso-PGF2α with smoking. The levels of this biomarker were reported to be higher in smokers than in nonsmokers (191–195), associated with smoking intensity (196), and declining after smoking cessation (196,197). A recent study investigated the longitudinal stability of 8-iso-PGF2α over a period of 20 weeks in a large multi-site clinical trial (198). The intra-class correlation coefficient for urinary 8-iso-PGF2α was 0.51 (95% CI, 0.45, 0.57), indicating fair longitudinal stability of this biomarker in cigarette smokers. This is an important result because another recent study reported a significant association between prospectively measured 8-iso-PGF2α and lung cancer incidence in cigarette smokers (199).
DNA adducts
Many chemical toxicants and carcinogens present in polluted air and cigarette smoke are capable of forming DNA adducts, either directly (e.g., aldehydes) or following metabolic activation to reactive electrophiles (e.g., PAH). Furthermore, oxidative stress and inflammation also result in the formation of a wide range of DNA adducts. Such DNA modifications, if not repaired efficiently, can lead to miscoding and mutations in oncogenes and/or tumor suppressor genes, and therefore play a key role in carcinogenic outcomes associated with exposures to air pollution and cigarette smoke (200). Therefore, analysis of DNA adducts in exposed individuals offers a more relevant measure of harm and subsequent health risk caused by chemical constituents present in polluted air and cigarette smoke, as well as oxidative damage and inflammation triggered by such exposures.
Studies of DNA adduct formation in response to environmental exposures widely employed the 32P-postlabeling approach to measure “bulky” DNA adducts formed by a range of chemical carcinogens, particularly PAH (201,202). Such studies generally report increases in the levels of bulky adducts in response to air pollution and smoking (201,203–206). However, 32P-postlabeling lacks specificity, which undermines the identity and the accuracy of quantitation of the adducts measured by this technique. More selective and sensitive MS-based assays have been developed for the analysis of individual DNA adducts, and studies employing such assays will be discussed here.
DNA adducts derived from specific chemicals
PAH.
The most studied PAH-derived adduct is BPDE-N2-dG, which is formed by BaP (207–210). One approach to measure this adduct is to analyze trans, anti-BaPT released upon DNA hydrolysis (207). As an example, this method was used to show an association of BaP-DNA adduct levels in cord blood and in utero exposure to secondhand tobacco smoke with decreased fetal growth and reductions in cognitive development among children in the World Trade Center Cohort (211,212). However, the specificity of this method has been questioned, given that the frequency of BPDE-N2-dG detection in human samples by the more selective and sensitive MS-based assays is very low (213–216). Recently, an ultrasensitive LC-nanoelectrospray ionization (NSI)-high-resolution tandem mass spectrometry (HRMS/MS) method was developed for the analysis of BPDE-N2-dG and applied to the analysis of human lung samples obtained during surgery for lung cancer (217). In that study, smokers had approximately 3-times higher levels of BPDE-N2-dG in their lungs than nonsmokers, and the measured levels of the adduct were ~1000-fold lower than those measured in previous studies by the immunochemical technique (208). Another potential approach to the analysis of DNA damage caused by exposure to PAH is the analysis of depurinated adducted DNA bases in urine. For instance, a study of such urinary biomarkers analyzed by LC-MS/MS showed that levels of BaP-DNA bases were much higher in the urine of women exposed to coal smoke than in smokers’ urine, and that the two exposures produced very different urinary biomarker profiles (218).
Aldehydes.
Human exposure to airborne aldehydes can occur through exposures to automobile exhaust, heated cooking oil, cigarette smoke, and certain occupational exposures (219). While the presence of DNA adducts induced by acrolein, formaldehyde, acetalehyde, and crotonaldehyde in humans has been reported in several studies, application of such biomarkers in environmental exposure studies is relatively limited. Reaction of acrolein with DNA leads to the formation of 1,N2-propanodeoxyguanosine adducts α-OH-Acr-dG and γ-OH-Acr-dG, of which α-OH-Acr-dG has higher mutagenicity but γ-OH-Acr-dG is the predominant isomer detected in human DNA from leukocytes, liver, and lung (220,221). Studies that employed mass spectrometry-based techniques showed no significant difference in the levels of these adducts between smookers and nonsmokers (220,222). The most abundant product of the reaction of formaldehyde with DNA is N6-hydroxymethyldeoxyadenosine (HOMe-dA). Our group was the first to demonstrate the presence of this adduct in human leukocyte DNA, showing much higher levels in smokers (mean ± SD, 179 ± 205 fmol/μmol dAdo) compared to nonsmokers (15.5 ± 33.8 fmol/μmol dAdo; P < 0.001) (27). Results from another study showed a similar trend toward higher levels of adducts in smokers, however the difference was not statistically significant (222). The major acetaldehyde DNA adduct is N2-ethylidenedeoxyguanosine (N2-ethylidene-dG). In addition, similar to crotonaldehyde (Cro), acetaldehyde can form Cro-dG adducts. Both types of adducts can be measured in human tissues and leukocyte DNA by LC-MS/MS-based methods (223,224). Levels of N2-ethylidene-dG have been shown to decrease after smoking cessation (225); however, the difference in the levels in oral cells from smokers and nonsmokers did not reach statistical significance (222). On the other hand, analysis of Cro-dG in human saliva by LC-MS/MS in the same study showed clear differences between smokers and nonsmokers (222). Both adduct measurements have been used in air pollution studies. A study of the effect of Chinese-style wok cooking in nonsmoking Chinese women showed no difference in N2-ethylidene-dG between women who engaged in regular home cooking and controls, despite elevated exposures to a range of volatile toxicants (85). In another study, higher levels of urinary Cro-dG were observed in subjects exposed to urban air pollution compared to controls (p < 0.05) (226).
Other organic compounds.
Examples of other DNA damaging toxicants that are present in polluted air or cigarette smoke include aromatic amines, 1,3-butadiene, and acrylamide. The aromatic amine 4-aminobiphenyl (4-ABP) is a known bladder carcinogen in both animals and humans and forms the DNA adducts 4-ABP-C8-dG (major), 4-ABP-C8-dA, and 4-ABP-N2-dG (227,228). In contrast to the reported data generated by 32P-postlabeling methods, the frequency of detection of these adducts by LC-MS/MS in human tissues is relatively low (215,228–230). The most abundant adduct of 1,3-butadiene is 7-(2, 3, 4-trihydroxybut-1-yl) guanine (7-THBG). Levels of this adduct are not different between smokers and nonsmokers and do not change in smokers after smoking cessation; however, occupational exposure to 1,3-butadiene leads to higher levels of 7-THBG (231). A major DNA adduct derived from acrylamide exposure is 7-(2-carbamoyl-2-hydroxyethyl)guanine. Similar to 7-THBG findings, measurement of this adduct in human urine did not produce a statistically significant difference between smokers and nonsmokers, but increased levels were meausred in workers occupationally exposed to acrylamide (232,233).
DNA adducts derived from oxidative damage
A comprehensive review of oxidative stress-induced DNA damage was published recently (234). The most widely studied DNA adduct is 8-oxo-dG, which can be formed via direct oxidation of DNA by oxidizing agents present in polluted air and cigarette smoke. This highly mutagenic adduct was detected in leukocytes and various human tissues. Studies employing HPLC with electrochemical detection report positive associations between air pollution and 8-oxo-dG. For instance, a study of students living and studying in Copenhagen showed that personal exposure to PM2.5 was predictive of 8-oxo-dG levels in lymphocyte DNA, with an 11% increase in 8-oxodG per 10 g/m3 increase in PM2.5 exposure (163). In another study, the levels of this adduct were significantly higher in asbestos-exposed workers compared to the non-exposed control group (235). A subsequent study in the same population reported no association between 8-oxo-dG and a wide range of potential confounding factors, further supporting the role of asbestos exposure in elevated oxidative DNA damage in exposed workers (236). However, studies employing mass spectrometry-based methods for the investigation of the impact of air pollution or smoking on 8-oxo-dG in DNA (as opposed to urine or plasma) are relatively scarce. A study that investigated the effect of air pollution on 8-oxo-dG measured in human leukocytes by LC-MS/MS observed some associations between exposure and the adduct level; however the effect was different across the three locations included in the study (237).
In addition to interaction of oxidizing agents with DNA, processes such as lipid peroxidation can lead to DNA adduct formation. As an example, 4-hydroxy-2-nonenal, a product of lipid peroxidation, can form the promutagenic adducts 1,N6-etheno-2-deoxyadenosine (εdA), and 3,N4-etheno-2′-deoxycytidine (εdC). A study by LC-ESI-MS/MS showed that levels of εdA and εdC in urine of workers exposed to diesel engine exhaust were higher than in the control group, while there was no significant contribution of smoking to the levels of these adducts (238). Another product of lipid peroxidation - malondialdehyde - reacts with DNA to form 3-(2-deoxy-β-d-erythro-pentafuranosyl)pyrimido[1,2-α]purin-10(3H)-one deoxyguanosine (M1dG) (239). Several studies demonstrated that increased M1dG formation is associated with occupational and environmental exposures to air pollution, smoking, increased cancer risk, and tumor progression (240–244). However, most of these studies used the 32P-postlabeling technique, and new studies using MS based assays are needed. A recently developed LC-NSI-HRMS/MS method for the analysis of M1dG in human leukocyte DNA showed no difference in the adduct levels between smokers and nonsmokers (245).
Metals
Particles in polluted air and cigarette smoke can contain metals and metalloids on their surface, including those capable of inducing oxidative stress either directly or through the Fenton reaction (121,246,247). Mechanistic and laboratory animal studies show that such exposures can induce inflammatory processes in lung tissues (247–249). Multiple studies of environmental and occupational exposures to metals investigated the association of such exposures with oxidative stress and inflammation. Metal exposures are typically measured by their analysis in urine or blood by either inductively coupled plasma MS (ICP-MS) or atomic absorption spectroscopy (AAS) methods (250–252). For example, Hu et al. studied the effect of occupational exposure to As on oxidative stress in semiconductor workers by analyzing levels of urinary inorganic arsenic (iAs3+, iAs5+), monomethylarsonic acid, and dimethylarsinic acid by HPLC with flow injection AAS, and urinary 8-OH-dG by LC-MS/MS (250). Their results showed elevated levels of these biomarkers in exposed workers compared to controls, and an association between urinary 8-OH-dG and As biomarkers. The impact of As exposure on inflammatory processes was also observed in copper smelters in Poland (253). Studies of non-ferrous metal smelter workers who were occupationally exposed to Pb and/or Cd showed an association between the levels of these metals in various biological matrices and α-glutathione-S-transferase, a biomarker of proximal tubular injury in nephrotoxicity (254,255). Another study of the effects of As, Cd, Cr, Ni, and Pb exposure in male coke-oven workers in China showed an association between urinary levels of such metals and biomarkers of oxidative stress, as well as an effect of interaction between metal and PAH exposure on oxidative markers (256).
Environmental exposures to metals have also been widely investigated. A cohort panel study involving 97 elderly subjects living in the Los Angeles metropolitan area showed significant positive associations between biomarkers of airway oxidative stress and inflammation and traffic pollution-related metal exposures (257). Metallothionein levels in blood were used as a biomarker of exposure to metals in local fishermen in Abu Qir Bay, Egypt (258). The study showed high levels of metallothionein in fishermen’s blood, and its association with the levels of Cd, Cu, Pb, Cr, and Zn. Metabolomics approaches were also employed to investigate the impact of environmental metal exposures on health outcomes. A study of highway commuters applied LC-MS high-resolution metabolomics to analyze the relationship between in-vehicle particulate exposures to Al, Pb, and Fe and metabolomic profiles as well as targeted inflammatory biomarker levels (259). The measured exposures and changes in inflammatory biomarkers were associated with metabolomics-identified molecular alterations that reflected changes in arachidonic acid, leukotrienes, and tryptophan metabolism, suggesting that traffic-associated exposures to metals may induce a broad inflammatory response. Another metabolomics study also showed that increased environmental metal exposure was associated with various oxidative stress-related effects, such as antioxidant depletion, increased lipid peroxidation, and changes in mitochondrial lipid metabolism (260). Other studies are also supportive of the role of environmental metal exposures in inflammatory processes and the associated health outcomes, including cardiovascular and neurotoxic effects (261–263).
Childhood exposures to metals were also studied. The effect of exposure to As and Cd and urinary 8-OH-dG was shown in environmentally exposed pregnant women in rural Bangladesh, indicating that such exposures may play a role in birth outcomes (252). A study of Cr exposure in households adjacent to Cr waste sites in New Jersey showed a statistically significant relationship between log-transformed urine Cr concentration and Cr dust concentration, and age-stratified analyses revealed that exposures in young children accounted for much of the relationship in the entire population (264). Dust levels of Pb were associated with blood biomarker levels in infants near the Tar Creek Superfund Site (Oklahoma, USA) (265). Deciduous teeth also have been used for the investigation of in-utero and early childhood exposures to Pb, suggesting airborne pollution as the primary source and exposure pathway (266). Analysis of Pb and Cd in blood of preschool children living in the vicinity of industrially contaminated sites in Poland showed higher levels of these biomarkers in children living closer to the sources of exposure as well as in those living with mothers who smoke at home (267). A targeted study of the effect of secondhand smoke exposure on metal uptake in children analyzed urinary levels of 23 trace elements (Li, Be, B, Ti, V, Cr, Mn, Co, Ni, Cu, Zn, Ga, Rb, Sr, Cd, Sn, Sb, Te, Cs, Tl, Pb, Bi, U) in a sample of Italian schoolchildren, showing an independent association between smoke exposures at home and urinary levels of Li, Ti, V, Co, Ga and Sr (251). A study in Sweden also showed a positive association between urinary Cd and Pb and the levels of urinary cotinine, a biomarker of nicotine intake, in secondhand smoke-exposed children (268).
Distinguishing Exposures from Cigarette Smoking versus Air Pollution
A particularly important question is how to use biomarkers to separate the potential health impacts of cigarette smoking from those of ambient air pollution in epidemiological studies. Cigarette smoke is frequently the major source of exposure to carcinogenic combustion products such as PAH, benzene, and metals which are also important constituents of polluted air. Furthermore, cigarette smoke is an established cause of oxidative damage. In this section, we discuss several well established biomarkers – cotinine, total nicotine equivalents, anatabine, anabasine, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) – which are specifically related to cigarette smoking and related practices and can be used to assess the role of smoking in toxicant and carcinogen exposure.
Cotinine
Nicotine is the critical constituent of tobacco products that is responsible for their addictive propertie (269). Nicotine is not a particularly useful biomarker due to its relatively short half-life of approximately 2h. Cotinine is the major metabolite of nicotine and a useful biomarker because of its relatively long half-life (8 – 30h). Cotinine can be quantified in a number of readily available biological fluids including blood, saliva, and urine. Large studies have investigated levels of cotinine that distinguish cigarette smokers from non-smokers who may be exposed to secondhand smoke, usually in indoor environments. For example, the NHANES study from 1990–2004 concluded that a serum cotinine level of 3 ng/ml distinguished cigarette smokers from non-smokers, while a study in the United Kingdom determined a level of 12 ng/ml in saliva (270,271). A free cotinine level of about 30 ng/ml urine distinguishes smokers from non-smokers.
There are other sources of nicotine exposure that need to be considered. These include the use of nicotine replacement products and e-cigarettes, which both deliver substantial amounts of nicotine resulting in cotinine levels similar to those observed in cigarette smokers. However, these products do not deliver PAH or most volatiles in significant amounts.
Total nicotine equivalents
Total nicotine equivalents, determined in urine by liquid chromatography-tandem mass spectrometry (LC-MS/MS), encompasses nicotine, cotinine, and 3’-hydroxycotinine plus their glucuronides, and in some cases nicotine-N-oxide and other minor metabolites as well (272). Total nicotine equivalents is considered the gold standard for determining nicotine dose because all major metabolites are quantified. For the purpose of distinguishing exposure to cigarette smoke versus air pollution, it does not have any significant advantages over cotinine, and has similar limitations such as use of other nicotine containing products.
Anatabine and anabasine
Anatabine and anabasine are minor tobacco alkaloids which are present in all tobacco products but not in nicotine replacement products. Therefore, analysis of these compounds in urine by LC-MS/MS can confirm tobacco product use. Mean levels of anatabine, anabasine, and total nicotine equivalents in the urine of 827 smokers were 39.9 pmol/ml, 52.8 pmol/ml, and 56.4 nmol/ml, respectively. Levels of anatabine and anabasine correlated with those of total nicotine equivalent (273).
NNAL
NNAL is a metabolite of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), which is found exclusively in tobacco products, not in nicotine replacement products or electronic cigarette vapor (274). NNAL and its glucuronides, termed total NNAL, are major metabolites of NNK in all vertebrates including humans. Total NNAL is most commonly measured in urine by LC-MS/MS and can be used to distinguish tobacco use or secondhand smoke exposure from environmental pollution because of its absolute specificity to tobacco products. Another advantage of NNAL as a biomarker is its relatively long urinary half-life of 10–45 days. Secondhand smoke exposure typically results in urinary NNAL levels of 0.03 – 0.08 pmol/ml (274).
CONCLUSIONS
We have presented a summary of the most important and widely used chemically specific biomarkers which can be applied in molecular epidemiology studies of air pollution and cancer. We have focused on biomarkers which can be determined by state of the art analytical chemistry methods, mostly using mass spectrometry. All methods discussed here have been widely applied and are fully validated. We conclude that the following chemically specific biomarkers are currently the optimal ones for use in studies of air pollution and cancer: urinary 1-OHP, phenanthrene metabolites, SPMA, urinary or blood Cd, 8-OH-dG and 8-iso-PGF2α. This suite of biomarkers will reliably establish exposure to carcinogenic PAH, benzene and Cd, and will also provide critical information on oxidative damage and inflammation, both of which are important in carcinogenesis. Furthermore, we strongly recommend analysis of the same samples for cotinine or NNAL in order to reliably assess the contribution of tobacco use.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Cooperative Agreement 1U2CES026533 from the National Institute of Environmental Health Sciences and Grant No. CA-81301 from the National Cancer Institute.
REFERENCES
- 1.Loomis D, Grosse Y, Lauby-Secretan B, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013; 14(13): 1262–3. [DOI] [PubMed] [Google Scholar]
- 2.Castano-Vinyals G, D’Errico A, Malats N, et al. Biomarkers of exposure to polycyclic aromatic hydrocarbons from environmental air pollution. Occup Environ Med. 2004; 61(4): e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vineis P, Husgafvel-Pursiainen K. Air pollution and cancer: biomarker studies in human populations. Carcinogenesis. 2005; 26(11): 1846–55. [DOI] [PubMed] [Google Scholar]
- 4.Demetriou CA, Raaschou-Nielsen O, Loft S, et al. Biomarkers of ambient air pollution and lung cancer: a systematic review. Occup Environ Med. 2012; 69(9): 619–27. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira BF, Chacra AP, Frauches TS, et al. A curated review of recent literature of biomarkers used for assessing air pollution exposures and effects in humans. J Toxicol Environ Health B Crit Rev. 2014; 17(7–8): 369–410. [DOI] [PubMed] [Google Scholar]
- 6.Demetriou CA, Vineis P. Carcinogenicity of ambient air pollution: use of biomarkers, lessons learnt and future directions. J Thorac Dis. 2015; 7(1): 67–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahadin MS, Ab Mutalib NS, Latif MT, et al. Challenges and future direction of molecular research in air pollution-related lung cancers. Lung Cancer. 2018; 118: 69–75. [DOI] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, v 92. Lyon, FR: IARC; 2010. p. 35–818. [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan JM, Butler LM, Gao YT, et al. Urinary metabolites of a polycyclic aromatic hydrocarbon and volatile organic compounds in relation to lung cancer development in lifelong never smokers in the Shanghai Cohort Study. Carcinogenesis. 2014; 35(2): 339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jongeneelen FJ, Anzion RBM, Leijdekkers CM, et al. 1-Hydroxypyrene in human-urine after exposure to coal-tar and a coal-tar derived product. Int Arch Occ Env Hea. 1985; 57(1): 47–55. [DOI] [PubMed] [Google Scholar]
- 11.Jongeneelen FJ, Bos RP, Anzion RBM, et al. Biological monitoring of polycyclic aromatic-hydrocarbons - metabolites in urine. Scand J Work Env Hea. 1986; 12(2): 137–43. [DOI] [PubMed] [Google Scholar]
- 12.Jongeneelen FJ, Anzion RBM, Scheepers PTJ, et al. 1-Hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic-hydrocarbons in several work environments. Ann Occup Hyg. 1988; 32(1): 35–43. [DOI] [PubMed] [Google Scholar]
- 13.Chetiyanukornkul T, Toriba A, Kizu R, et al. Determination of 1-hydroxypyrene in human urine by high-performance liquid chromatography with fluorescence detection using a deuterated internal standard. J Chromatogr A. 2002; 961(1): 107–12. [DOI] [PubMed] [Google Scholar]
- 14.Barbeau D, Maitre A, Marques M. Highly sensitive routine method for urinary 3-hydroxybenzo[a]pyrene quantitation using liquid chromatography-fluorescence detection and automated off-line solid phase extraction. Analyst. 2011; 136(6): 1183–91. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Romanoff LC, Trinidad DA, et al. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal Chem. 2006; 78(16): 5744–51. [DOI] [PubMed] [Google Scholar]
- 16.Campo L, Rossella F, Fustinoni S. Development of a gas chromatography/mass spectrometry method to quantify several urinary monohydroxy metabolites of polycyclic aromatic hydrocarbons in occupationally exposed subjects. J Chromatogr B. 2008; 875(2): 531–40. [DOI] [PubMed] [Google Scholar]
- 17.Jiang J, Ip HSS, Zhou JQ, et al. Supported-liquid phase extraction in combination with isotope-dilution gas chromatography triple quadrupole tandem mass spectrometry for high-throughput quantitative analysis of polycyclic aromatic hydrocarbon metabolites in urine. Environ Pollut. 2019; 248: 304–11. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Zhang JF, Zhang L, et al. Selective detection of monohydroxy metabolites of polycyclic aromatic hydrocarbons in urine using liquid chromatography/triple quadrupole tandem mass spectrometry. Rapid Commun Mass Sp. 2004; 18(19): 2299–308. [DOI] [PubMed] [Google Scholar]
- 19.Fan RF, Wang DL, Ramage R, et al. Fast and simultaneous determination of urinary 8-hydroxy-2 ‘-deoxyguanosine and ten monohydroxylated polycyclic aromatic hydrocarbons by liquid chromatography/tandem mass spectrometry. Chem Res Toxicol. 2012; 25(2): 491–9. [DOI] [PubMed] [Google Scholar]
- 20.Luo K, Gao Q, Hu JY. Derivatization method for sensitive determination of 3-hydroxybenzo[a]pyrene in human urine by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2015; 1379: 51–5. [DOI] [PubMed] [Google Scholar]
- 21.Deng QF, Dai XY, Feng W, et al. Co-exposure to metals and polycyclic aromatic hydrocarbons, microRNA expression, and early health damage in coke oven workers. Environment International. 2019; 122: 369–80. [DOI] [PubMed] [Google Scholar]
- 22.Kho Y, Lee EH, Chae H, et al. 1-Hydroxypyrene and oxidative stress marker levels among painting workers and office workers at shipyard. Int Arch Occ Env Hea. 2015; 88(3): 297–303. [DOI] [PubMed] [Google Scholar]
- 23.Olujimi OO, Ogunseye OO, Oladiran KO, et al. Preliminary Investigation into Urinary 1-Hydroxypyrene as a Biomarker for Polycyclic Aromatic Hydrocarbons exposure among Charcoal Workers in Ogun and Oyo States, Nigeria. Saf Health Work-Kr. 2018; 9(4): 416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldt T, Fobil JN, Wittsiepe J, et al. High levels of PAH-metabolites in urine of e-waste recycling workers from Agbogbloshie, Ghana. Science of the Total Environment. 2014; 466: 369–76. [DOI] [PubMed] [Google Scholar]
- 25.Keir JLA, Akhtar US, Matschke DMJ, et al. Elevated exposures to polycyclic aromatic hydrocarbons and other organic mutagens in ottawa firefighters participating in emergency, on-shift fire suppression. Environ Sci Technol. 2017; 51(21): 12745–55. [DOI] [PubMed] [Google Scholar]
- 26.Hecht SS, Carmella SG, Kotandeniya D, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015; 17(6): 704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Cheng G, Balbo S, et al. Clear differences in levels of formaldehyde-DNA adduct in leukocytes of smokers and non-smokers. Cancer Res. 2009; 69: 7170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010; 105(2): 343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etemadi A, Poustchi H, Chang CM, et al. Urinary biomarkers of carcinogenic exposure among cigarette, waterpipe, and smokeless tobacco users and never users of tobacco in the Golestan Cohort Study. Cancer Epidem Biomar. 2019; 28(2): 337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services CDC. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019, Vol. 1. 2019. [Google Scholar]
- 31.Guo Y, Senthilkumar K, Alomirah H, et al. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technol. 2013; 47(6): 2932–8. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Liu Q, Ostbye T, et al. Levels and risk factors for urinary metabolites of polycyclic aromatic hydrocarbons in children living in Chongqing, China. Sci Total Environ. 2017; 598: 553–61. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services CDC. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019, Vol. 2. 2019. [Google Scholar]
- 34.Alotaibi R, Bechle M, Marshall JD, et al. Traffic related air pollution and the burden of childhood asthma in the contiguous United States in 2000 and 2010. Environ Int. 2019; 127: 858–67. [DOI] [PubMed] [Google Scholar]
- 35.Oberg M, Jaakkola MS, Woodward A, et al. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011; 377(9760): 139–46. [DOI] [PubMed] [Google Scholar]
- 36.Filippini T, Hatch EE, Rothman KJ, et al. Association between outdoor air pollution and childhood leukemia: A systematic review and dose response meta-analysis. Environ Health Persp. 2019; 127(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metayer C, Zhang LP, Wiemels JL, et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidem Biomar. 2013; 22(9): 1600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strickland PT, Kang D, Bowman ED, et al. Identification of 1-hydroxypyrene glucuronide as a major pyrene metabolite in human urine by synchronous fluorescence spectroscopy and gas-chromatography mass-spectrometry. Carcinogenesis. 1994; 15(3): 483–7. [DOI] [PubMed] [Google Scholar]
- 39.Peters KO, Williams DL, Abubaker S, et al. Predictors of polycyclic aromatic hydrocarbon exposure and internal dose in inner city Baltimore children. J Expo Sci Env Epid. 2017; 27(3): 290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh R, Tucek M, Maxa K, et al. A rapid and simple method for the analysis of 1-hydroxypyrene glucuronide: a potential biomarker for polycyclic aromatic hydrocarbon exposure. Carcinogenesis. 1995; 16(12): 2909–15. [DOI] [PubMed] [Google Scholar]
- 41.Yang MQ, Wang Y, Ren JW, et al. A Rapid and sensitive method of determination of 1-hydroxypyrene glucuronide in urine by UPLC-FLD. Chromatographia. 2019; 82(5): 835–42. [Google Scholar]
- 42.Kakimoto K, Toriba A, Ohno T, et al. Direct measurement of the glucuronide conjugate of 1-hydroxypyrene in human urine by using liquid chromatography with tandem mass spectrometry. J Chromatogr B. 2008; 867(2): 259–63. [DOI] [PubMed] [Google Scholar]
- 43.Li MX, Wang Q, Zhu J, et al. A simple analytical method of determining 1-hydroxypyrene glucuronide in human urine by isotope dilution with ultra performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2017; 409(6): 1513–8. [DOI] [PubMed] [Google Scholar]
- 44.Kang DH, Rothman N, Cho SH, et al. Association of exposure to polycyclic aromatic-hydrocarbons (estimated from job category) with concentration of 1-hydroxypyrene glucuronide in urine from workers at a steel plant. Occupational and Environmental Medicine. 1995; 52(9): 593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sithisarankul P, Vineis P, Kang D, et al. Association of 1-hydroxypyrene-glucuronide in human urine with cigarette smoking and broiled or roasted meat consumption. Biomarkers. 1997; 2(4): 217–21. [DOI] [PubMed] [Google Scholar]
- 46.Fagundes RB, Abnet CC, Strickland PT, et al. Higher urine 1-hydroxy pyrene glucuronide (1-OHPG) is associated with tobacco smoke exposure and drinking mate in healthy subjects from Rio Grande do Sul, Brazil. Bmc Cancer. 2006; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon HS, Lee KM, Lee KH, et al. Polycyclic aromatic hydrocarbon (1-OHPG and 2-naphthol) and oxidative stress (malondialdehyde) biomarkers in urine among Korean adults and children. Int J Hyg Envir Heal. 2012; 215(4): 458–64. [DOI] [PubMed] [Google Scholar]
- 48.Lafontaine M, Champmartin C, Simon P, et al. 3-hydroxybenzo[a]pyrene in the urine of smokers and non-smokers. Toxicol Lett. 2006; 162(2–3): 181–5. [DOI] [PubMed] [Google Scholar]
- 49.Woudneh MB, Benskin JP, Grace R, et al. Quantitative determination of hydroxy polycylic aromatic hydrocarbons as a biomarker of exposure to carcinogenic polycyclic aromatic hydrocarbons. J Chromatogr A. 2016; 1454: 93–100. [DOI] [PubMed] [Google Scholar]
- 50.Richter-Brockmann S, Dettbarn G, Jessel S, et al. Ultra-high sensitive analysis of 3-hydroxybenzo[a]pyrene in human urine using GC-APLI-MS. J Chromatogr B. 2019; 1118: 187–93. [DOI] [PubMed] [Google Scholar]
- 51.Hu H, Liu BZ, Yang J, et al. Sensitive determination of trace urinary 3-hydroxybenzo[a]pyrene using ionic liquids-based dispersive liquid-liquid microextraction followed by chemical derivatization and high performance liquid chromatography-high resolution tandem mass spectrometry. J Chromatogr B. 2016; 1027: 200–6. [DOI] [PubMed] [Google Scholar]
- 52.Raponi F, Bauleo L, Ancona C, et al. Quantification of 1-hydroxypyrene, 1-and 2-hydroxynaphthalene, 3-hydroxybenzo[a]pyrene and 6-hydroxynitropyrene by HPLC-MS/MS in human urine as exposure biomarkers for environmental and occupational surveys. Biomarkers. 2017; 22(6): 575–83. [DOI] [PubMed] [Google Scholar]
- 53.Lafontaine M, Gendre C, Delsaut P, et al. Urinary 3-hydroxybenzo[A]pyrene as a biomarker of exposure to polycyclic aromatic hydrocarbons: An approach for determining a biological limit value. Polycycl Aromat Comp. 2004; 24(4–5): 441–50. [Google Scholar]
- 54.Hecht SS, Carmella SG, Villalta PW, et al. Analysis of phenanthrene and benzo[a]pyrene tetraol enantiomers in human urine: relevance to the bay region diol epoxide hypothesis of benzo[a]pyrene carcinogenesis and to biomarker studies. Chem Res Toxicol. 2010; 23(5): 900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong Y, Carmella SG, Hochalter JB, et al. Analysis of r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in human urine: a biomarker for directly assessing carcinogenic polycyclic aromatic hydrocarbon exposure plus metabolic activation. Chem Res Toxicol. 2011; 24(1): 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hecht SS, Hochalter JB. Quantitation of enantiomers of r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]-pyrene in human urine: evidence supporting metabolic activation of benzo[a]pyrene via the bay region diol epoxide. Mutagenesis. 2014; 29(5): 351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simpson CD, Wu MT, Christiani DC, et al. Determination of r-7,t-8,9,c-10-tetrahydroxy-7,8,9, 10-tetrahydrobenzo[a]pyrene in human urine by gas chromatography/negative ion chemical ionization/mass spectrometry. Chem Res Toxicol. 2000; 13(4): 271–80. [DOI] [PubMed] [Google Scholar]
- 58.Hilton DC, Trinidad DA, Hubbard K, et al. Measurement of urinary Benzo[a]pyrene tetrols and their relationship to other polycyclic aromatic hydrocarbon metabolites and cotinine in humans. Chemosphere. 2017; 189: 365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barbeau D, Lutier S, Choisnard L, et al. Urinary trans-anti-7,8,9,10-tetrahydroxy-7,8,9,10-tetrahydrobenzo(a)pyrene as the most relevant biomarker for assessing carcinogenic polycyclic aromatic hydrocarbons exposure. Environment International. 2018; 112: 147–55. [DOI] [PubMed] [Google Scholar]
- 60.Richter-Brockmann S, Dettbarn G, Jessel S, et al. GC-APLI-MS as a powerful tool for the analysis of BaP-tetraol in human urine. J Chromatogr B. 2018; 1100: 1–5. [DOI] [PubMed] [Google Scholar]
- 61.Wu MT, Simpson CD, Christiani DC, et al. Relationship of exposure to coke-oven emissions and urinary metabolites of benzo(a)pyrene and pyrene in coke-oven workers. Cancer Epidem Biomar. 2002; 11(3): 311–4. [PubMed] [Google Scholar]
- 62.Hochalter JB, Zhong Y, Han SM, et al. Quantitation of a minor enantiomer of phenanthrene tetraol in human urine: Correlations with levels of overall phenanthrene tetraol, benzo[a]pyrene tetraol, and 1-hydroxypyrene. Chem Res Toxicol. 2011; 24(2): 262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carmella SG, Chen M, Yagi H, et al. Analysis of phenanthrols in human urine by gas chromatography-mass spectrometry: Potential use in carcinogen metabolite phenotyping. Cancer Epidem Biomar. 2004; 13(12): 2167–74. [PubMed] [Google Scholar]
- 64.Kuang D, Zhang WZ, Deng QF, et al. Dose-response relationships of polycyclic aromatic hydrocarbons exposure and oxidative damage to DNA and lipid in coke oven workers. Environ Sci Technol. 2013; 47(13): 7446–56. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Zhang WB, Dong YL, et al. Quantification of several monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine by high-performance liquid chromatography with fluorescence detection. Anal Bioanal Chem. 2005; 383(5): 804–9. [DOI] [PubMed] [Google Scholar]
- 66.Wang YS, Meng L, Pittman EN, et al. Quantification of urinary mono-hydroxylated metabolites of polycyclic aromatic hydrocarbons by on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2017; 409(4): 931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jacob J, Grimmer G, Dettbarn G. Profile of urinary phenanthrene metabolites in smokers and non-smokers. Biomarkers. 1999; 4(5): 319–27. [DOI] [PubMed] [Google Scholar]
- 68.Heudorf U, Angerer J. Urinary monohydroxylated phenanthrenes and hydroxypyrene - the effects of smoking habits and changes induced by smoking on monooxygenase-mediated metabolism. Int Arch Occ Env Hea. 2001; 74(3): 177–83. [DOI] [PubMed] [Google Scholar]
- 69.Jacob J, Doehmer J, Grimmer G, et al. Metabolism of phenanthrene, benz[a]anthracene, benzo[a]pyrene, chrysene and benzo[c]phenanthrene by eight cDNA-expressed human and rat cytochromes P450. Polycycl Aromat Comp. 1996; 10(1–4): 1–9. [Google Scholar]
- 70.Schober W, Pusch G, Oeder S, et al. Metabolic activation of phenanthrene by human and mouse cytochromes P450 and pharmacokinetics in CYP1A2 knockout mice. Chem-Biol Interact. 2010; 183(1): 57–66. [DOI] [PubMed] [Google Scholar]
- 71.Seidel A, Spickenheuer A, Straif K, et al. New biomarkers of occupational exposure to polycyclic aromatic hydrocarbons. J Toxicol Env Heal A. 2008; 71(11–12): 734–45. [DOI] [PubMed] [Google Scholar]
- 72.Lotz A, Pesch B, Dettbarn G, et al. Metabolites of the PAH diol epoxide pathway and other urinary biomarkers of phenanthrene and pyrene in workers with and without exposure to bitumen fumes. Int Arch Occ Env Hea. 2016; 89(8): 1251–67. [DOI] [PubMed] [Google Scholar]
- 73.Hecht SS, Chen ML, Yagi H, et al. r-1,t-2,3,c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: A potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidem Biomar. 2003; 12(12): 1501–8. [PubMed] [Google Scholar]
- 74.Yuan JM, Gao YT, Murphy SE, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011; 71(21): 6749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asahi M, Kawai M, Toyama T, et al. Identification and Quantification of in Vivo Metabolites of 9,10-Phenanthrenequinone in Human Urine Associated with Producing Reactive Oxygen Species. Chem Res Toxicol. 2014; 27(1): 76–85. [DOI] [PubMed] [Google Scholar]
- 76.Hecht SS, Chen MA, Yoder A, et al. Longitudinal study of urinary phenanthrene metabolite ratios: Effect of smoking on the diol epoxide pathway. Cancer Epidem Biomar. 2005; 14(12): 2969–74. [DOI] [PubMed] [Google Scholar]
- 77.International Agency for Research on Cancer. A Review of Human Carcinogens: Chemical Agents and Related Occupations. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, v 100F. Lyon, FR: IARC; 2012. p. 249–94. [PMC free article] [PubMed] [Google Scholar]
- 78.Hoet P, De Smedt E, Ferrari M, et al. Evaluation of urinary biomarkers of exposure to benzene: correlation with blood benzene and influence of confounding factors. Int Arch Occup Environ Health. 2009; 82(8): 985–95. [DOI] [PubMed] [Google Scholar]
- 79.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002; 23: 907–22. [DOI] [PubMed] [Google Scholar]
- 80.Medeiros AM, Bird MG, Witz G. Potential biomarkers of benzene exposure. J Toxicol Environ Health. 1997; 51(6): 519–39. [DOI] [PubMed] [Google Scholar]
- 81.Haiman CA, Patel YM, Stram DO, et al. Benzene uptake and glutathione S-transferase T1 status as determinants of S-phenylmercapturic acid in cigarette smokers in the Multiethnic Cohort. PLoS One. 2016; 11(3): e0150641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tricker AR, Stewart AJ, Leroy CM, et al. Reduced exposure evaluation of an Electrically Heated Cigarette Smoking System. Part 3: Eight-day randomized clinical trial in the UK. Regul Toxicol Pharmacol. 2012; 64(2 Suppl): S35–44. [DOI] [PubMed] [Google Scholar]
- 83.Carmella SG, Chen M, Han S, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009; 22(4): 734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kensler TW, Ng D, Carmella SG, et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis. 2012; 33(1): 101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hecht SS, Seow A, Wang M, et al. Elevated levels of volatile organic carcinogen and toxicant biomarkers in Chinese women who regularly cook at home. Cancer Epidemiol Biomarkers Prev. 2010; 19(5): 1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hecht SS, Koh WP, Wang R, et al. Elevated levels of mercapturic acids of acrolein and crotonaldehyde in the urine of Chinese women in Singapore who regularly cook at home. PLoS One. 2015; 10(3): e0120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Angelini S, Kumar R, Bermejo JL, et al. Exposure to low environmental levels of benzene: evaluation of micronucleus frequencies and S-phenylmercapturic acid excretion in relation to polymorphisms in genes encoding metabolic enzymes. Mutat Res. 2011; 719(1–2): 7–13. [DOI] [PubMed] [Google Scholar]
- 88.Manini P, De Palma G, Andreoli R, et al. Environmental and biological monitoring of benzene exposure in a cohort of Italian taxi drivers. Toxicol Lett. 2006; 167(2): 142–51. [DOI] [PubMed] [Google Scholar]
- 89.Fracasso ME, Doria D, Bartolucci GB, et al. Low air levels of benzene: correlation between biomarkers of exposure and genotoxic effects. Toxicol Lett. 2010; 192(1): 22–8. [DOI] [PubMed] [Google Scholar]
- 90.Manini P, De Palma G, Andreoli R, et al. Occupational exposure to low levels of benzene: Biomarkers of exposure and nucleic acid oxidation and their modulation by polymorphic xenobiotic metabolizing enzymes. Toxicol Lett. 2010; 193(3): 229–35. [DOI] [PubMed] [Google Scholar]
- 91.Dougherty D, Garte S, Barchowsky A, et al. NQO1, MPO, CYP2E1, GSTT1 and GSTM1 polymorphisms and biological effects of benzene exposure--a literature review. Toxicol Lett. 2008; 182(1–3): 7–17. [DOI] [PubMed] [Google Scholar]
- 92.International Agency for Research on Cancer. 1,3-Butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol 97. Lyon, FR: IARC; 2008. p. 45–309. [PMC free article] [PubMed] [Google Scholar]
- 93.Boldry EJ, Patel YM, Kotapati S, et al. Genetic determinants of 1,3-butadiene metabolism and detoxification in three populations of smokers with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev. 2017; 26(7): 1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kotapati S, Esades A, Matter B, et al. High throughput HPLC-ESI-MS/MS methodology for mercapturic acid metabolites of 1,3-butadiene: Biomarkers of exposure and bioactivation. Chem Biol Interact. 2015; 241: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sangaraju D, Villalta PW, Wickramaratne S, et al. NanoLC/ESI+ HRMS3 quantitation of DNA adducts induced by 1,3-butadiene. J Am Soc Mass Spectrom. 2014; 25(7): 1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Il’yasova D, McCarthy BJ, Erdal S, et al. Human exposure to selected animal neurocarcinogens: a biomarker-based assessment and implications for brain tumor epidemiology. J Toxicol Environ Health B Crit Rev. 2009; 12(3): 175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008; 52(1): 7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beauchamp RO Jr., Andjelkovich DA, Kligerman AD, et al. A critical review of the literature of acrolein toxicity. CRC Critical Reviews in Toxicology. 14 ed. Clevelan: CRC Press Inc.; 1985. p. 309–80. [DOI] [PubMed] [Google Scholar]
- 99.Moghe A, Ghare S, Lamoreau B, et al. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015; 143(2): 242–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Minko IG, Kozekov ID, Harris TM, et al. Chemistry and biology of DNA containing 1,N2-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem Res Toxicol. 2009; 22(5): 759–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.International Agency for Research on Cancer. Acrolein. IARC Monogr Eval Carcinog Risks Hum. 63. Lyon, FR: IARC; 1995. p. 337–72. [PMC free article] [PubMed] [Google Scholar]
- 102.Chung FL, Tanaka T, Hecht SS. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986; 46: 1285–9. [PubMed] [Google Scholar]
- 103.Carmella SG, Chen M, Zarth A, et al. High throughput liquid chromatography-tandem mass spectrometry assay for mercapturic acids of acrolein and crotonaldehyde in cigarette smokers’ urine. J Chromatog B. 2013; 935: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park SL, Carmella SG, Chen M, et al. Mercpaturic acids derived from the toxicants acrolein and crotonaldehyde in the urine of cigarettes smokers from five ethnic groups with differing risks for lung cancer. PLoS One. 2015; 10: e0124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alwis KU, deCastro BR, Morrow JC, et al. Acrolein exposure in U.S. tobacco smokers and non-tobacco users: NHANES 2005–2006. Environ Health Perspect. 2015; 123(12): 1302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bagchi P, Geldner N, deCastro BR, et al. Crotonaldehyde exposure in U.S. tobacco smokers and nonsmokers: NHANES 2005–2006 and 2011–2012. Environ Res. 2018; 163: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boyle EB, Viet SM, Wright DJ, et al. Assessment of exposure to VOCs among pregnant women in the national children’s study. Int J Environ Res Public Health. 2016; 13(4): 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.International Agency for Research on Cancer. Some Industrial Chemicals. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. 60. Lyon, FR: IARC; 1994. p. 181–213. [Google Scholar]
- 109.Zarth A, Carmella SG, Le CT, et al. Effect of cigarette smoking on urinary 2-hydroxypropylmercapturic acid, a metabolite of propylene oxide. J Chromatog B. 2014; 953–954: 126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marek RF, Thorne PS, Wang K, et al. PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities (vol 47, pg 3353, 2013). Environ Sci Technol. 2013; 47(16): 9555–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Quinete N, Kraus T, Belov VN, et al. Fast determination of hydroxylated polychlorinated biphenyls in human plasma by online solid phase extraction coupled to liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2015; 888: 94–102. [DOI] [PubMed] [Google Scholar]
- 112.Quinete N, Esser A, Kraus T, et al. Determination of hydroxylated polychlorinated biphenyls (OH-PCBs) in human urine in a highly occupationally exposed German cohort: New prospects for urinary biomarkers of PCB exposure. Environment International. 2016; 97: 171–9. [DOI] [PubMed] [Google Scholar]
- 113.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some chemicals present in industrial and consumer products, food and drinking-water. IARC Monogr Eval Carcinog Risks Hum. 2013; 101: 9–549. [PMC free article] [PubMed] [Google Scholar]
- 114.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Diesel and gasoline engine exhausts and some nitroarenes.. IARC Monogr Eval Carcinog Risks Hum. 1052014. p. 9–699. [PMC free article] [PubMed] [Google Scholar]
- 115.Toriba A, Kitaoka H, Dills RL, et al. Identification and quantification of 1-nitropyrene metabolites in human urine as a proposed biomarker for exposure to diesel exhaust. Chem Res Toxicol. 2007; 20(7): 999–1007. [DOI] [PubMed] [Google Scholar]
- 116.Miller-Schulze JP, Paulsen M, Kameda T, et al. Evaluation of urinary metabolites of 1-nitropyrene as biomarkers for exposure to diesel exhaust in taxi drivers of Shenyang, China. J Expo Sci Environ Epidemiol. 2013; 23(2): 170–5. [DOI] [PubMed] [Google Scholar]
- 117.Pope CA 3rd, Turner MC, Burnett RT, et al. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015; 116(1): 108–15. [DOI] [PubMed] [Google Scholar]
- 118.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009; 374(9691): 733–43. [DOI] [PubMed] [Google Scholar]
- 119.Pope CA 3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002; 287(9): 1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li XY, Gilmour PS, Donaldson K, et al. In vivo and in vitro proinflammatory effects of particulate air pollution (PM10). Environ Health Perspect. 1997; 105 Suppl 5: 1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han JY, Takeshita K, Utsumi H. Noninvasive detection of hydroxyl radical generation in lung by diesel exhaust particles. Free Radic Biol Med. 2001; 30(5): 516–25. [DOI] [PubMed] [Google Scholar]
- 122.Autrup H, Daneshvar B, Dragsted LO, et al. Biomarkers for exposure to ambient air pollution - Comparison of carcinogen-DNA adduct levels with other exposure markers and markers for oxidative stress. Environ Health Perspect. 1999; 107(3): 233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Betteridge DJ. What is oxidative stress? Metabolism. 2000; 49(2 Suppl 1): 3–8. [DOI] [PubMed] [Google Scholar]
- 124.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006; 8(9–10): 1865–79. [DOI] [PubMed] [Google Scholar]
- 125.Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003; 60(8): 612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barregard L, Sallsten G, Andersson L, et al. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med. 2008; 65(5): 319–24. [DOI] [PubMed] [Google Scholar]
- 127.Ceylan E, Kocyigit A, Gencer M, et al. Increased DNA damage in patients with chronic obstructive pulmonary disease who had once smoked or been exposed to biomass. Respir Med. 2006; 100(7): 1270–6. [DOI] [PubMed] [Google Scholar]
- 128.Neophytou AM, Hart JE, Cavallari JM, et al. Traffic-related exposures and biomarkers of systemic inflammation, endothelial activation and oxidative stress: a panel study in the US trucking industry. Environ Health. 2013; 12: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Romieu I, Barraza-Villarreal A, Escamilla-Nunez C, et al. Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma. J Allergy Clin Immunol. 2008; 121(4): 903–9 e6. [DOI] [PubMed] [Google Scholar]
- 130.Zhang J, Zhu T, Kipen H, et al. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res Rep Health Eff Inst. 2013; (174): 5–174. [PMC free article] [PubMed] [Google Scholar]
- 131.Meerang M, Nair J, Sirankapracha P, et al. Increased urinary 1,N6-ethenodeoxyadenosine and 3,N4-ethenodeoxycytidine excretion in thalassemia patients: markers for lipid peroxidation-induced DNA damage. Free Radic Biol Med. 2008; 44(10): 1863–8. [DOI] [PubMed] [Google Scholar]
- 132.Dechakhamphu S, Pinlaor S, Sitthithaworn P, et al. Lipid peroxidation and etheno DNA adducts in white blood cells of liver fluke-infected patients: protection by plasma alpha-tocopherol and praziquantel. Cancer Epidemiol Biomarkers Prev. 2010; 19(1): 310–8. [DOI] [PubMed] [Google Scholar]
- 133.Nair J, Srivatanakul P, Haas C, et al. High urinary excretion of lipid peroxidation-derived DNA damage in patients with cancer-prone liver diseases. Mutat Res. 2010; 683(1–2): 23–8. [DOI] [PubMed] [Google Scholar]
- 134.Cooke MS, Henderson PT, Evans MD. Sources of extracellular, oxidatively-modified DNA lesions: implications for their measurement in urine. J Clin Biochem Nutr. 2009; 45(3): 255–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.European Standards Committee on Urinary Lesion Analysis, Evans MD, Olinski R, et al. Toward consensus in the analysis of urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine as a noninvasive biomarker of oxidative stress. FASEB J. 2010; 24(4): 1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen HJ, Kao CF. Effect of gender and cigarette smoking on urinary excretion of etheno DNA adducts in humans measured by isotope dilution gas chromatography/mass spectrometry. Toxicol Lett. 2007; 169(1): 72–81. [DOI] [PubMed] [Google Scholar]
- 137.Hillestrom PR, Covas MI, Poulsen HE. Effect of dietary virgin olive oil on urinary excretion of etheno-DNA adducts. Free Radic Biol Med. 2006; 41(7): 1133–8. [DOI] [PubMed] [Google Scholar]
- 138.Lonkar P, Dedon PC. Reactive species and DNA damage in chronic inflammation: Reconciling chemical mechanisms and biological fates. International Journal of Cancer. 2011; 128(9): 1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paz-Elizur T, Sevilya Z, Leitner-Dagan Y, et al. DNA repair of oxidative DNA damage in human carcinogenesis: potential application for cancer risk assessment and prevention. Cancer Lett. 2008; 266(1): 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maynard S, Schurman SH, Harboe C, et al. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009; 30(1): 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Friedman JI, Stivers JT. Detection of damaged DNA bases by DNA glycosylase enzymes. Biochemistry. 2010; 49(24): 4957–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007; 447(7147): 941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chuang HC, Juan HT, Chang CN, et al. Cardiopulmonary toxicity of pulmonary exposure to occupationally relevant zinc oxide nanoparticles. Nanotoxicology. 2014; 8(6): 593–604. [DOI] [PubMed] [Google Scholar]
- 144.Cooke MS, Lunec J, Evans MD. Progress in the analysis of urinary oxidative DNA damage. Free Radic Biol Med. 2002; 33(12): 1601–14. [DOI] [PubMed] [Google Scholar]
- 145.Cooke MS, Evans MD, Dove R, et al. DNA repair is responsible for the presence of oxidatively damaged DNA lesions in urine. Mutat Res. 2005; 574(1–2): 58–66. [DOI] [PubMed] [Google Scholar]
- 146.Tagesson C, Kallberg M, Klintenberg C, et al. Determination of urinary 8-hydroxydeoxyguanosine by automated coupled-column high performance liquid chromatography: a powerful technique for assaying in vivo oxidative DNA damage in cancer patients. Eur J Cancer. 1995; 31A(6): 934–40. [DOI] [PubMed] [Google Scholar]
- 147.Tagesson C, Kallberg M, Wingren G. Urinary malondialdehyde and 8-hydroxydeoxyguanosine as potential markers of oxidative stress in industrial art glass workers. Int Arch Occup Environ Health. 1996; 69(1): 5–13. [DOI] [PubMed] [Google Scholar]
- 148.Wang CC, Chen WL, Lin CM, et al. The relationship between plasma and urinary 8-hydroxy-2-deoxyguanosine biomarkers measured by liquid chromatography tandem mass spectrometry. Environ Sci Pollut Res Int. 2016; 23(17): 17496–502. [DOI] [PubMed] [Google Scholar]
- 149.Hu CW, Huang YJ, Li YJ, et al. Correlation between concentrations of 8-oxo-7,8-dihydro-2’-deoxyguanosine in urine, plasma and saliva measured by on-line solid-phase extraction LC-MS/MS. Clin Chim Acta. 2010; 411(17–18): 1218–22. [DOI] [PubMed] [Google Scholar]
- 150.Cooke MS, Singh R, Hall GK, et al. Evaluation of enzyme-linked immunosorbent assay and liquid chromatography-tandem mass spectrometry methodology for the analysis of 8-oxo-7,8-dihydro-2’-deoxyguanosine in saliva and urine. Free Radic Biol Med. 2006; 41(12): 1829–36. [DOI] [PubMed] [Google Scholar]
- 151.Rodriguez-Gonzalo E, Herrero-Herrero L, Garcia-Gomez D. Development, validation and application of a fast analytical methodology for the simultaneous determination of DNA- and RNA-derived urinary nucleosides by liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2016; 1019: 132–9. [DOI] [PubMed] [Google Scholar]
- 152.Kataoka H, Mizuno K, Oda E, et al. Determination of the oxidative stress biomarker urinary 8-hydroxy-2’-deoxyguanosine by automated on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2016; 1019: 140–6. [DOI] [PubMed] [Google Scholar]
- 153.Rota C, Cristoni S, Trenti T, et al. A serially coupled stationary phase method for the determination of urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine by liquid chromatography ion trap tandem mass spectrometry. Redox Biol. 2013; 1: 492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hu CW, Wang CJ, Chang LW, et al. Clinical-scale high-throughput analysis of urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine by isotope-dilution liquid chromatography-tandem mass spectrometry with on-line solid-phase extraction. Clin Chem. 2006; 52(7): 1381–8. [DOI] [PubMed] [Google Scholar]
- 155.Grady ST, Koutrakis P, Hart JE, et al. Indoor black carbon of outdoor origin and oxidative stress biomarkers in patients with chronic obstructive pulmonary disease. Environ Int. 2018; 115: 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang HB, Lai CH, Chen GW, et al. Traffic-related air pollution and DNA damage: a longitudinal study in Taiwanese traffic conductors. PLoS One. 2012; 7(5): e37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu X, Lintelmann J, Klingbeil S, et al. Determination of air pollution-related biomarkers of exposure in urine of travellers between Germany and China using liquid chromatographic and liquid chromatographic-mass spectrometric methods: a pilot study. Biomarkers. 2017; 22(6): 525–36. [DOI] [PubMed] [Google Scholar]
- 158.Andreoli R, Spatari G, Pigini D, et al. Urinary biomarkers of exposure and of oxidative damage in children exposed to low airborne concentrations of benzene. Environ Res. 2015; 142: 264–72. [DOI] [PubMed] [Google Scholar]
- 159.Besaratinia A, Van Schooten FJ, Schilderman PA, et al. A multi-biomarker approach to study the effects of smoking on oxidative DNA damage and repair and antioxidative defense mechanisms. Carcinogenesis. 2001; 22(3): 395–401. [DOI] [PubMed] [Google Scholar]
- 160.Chiang HC, Huang YK, Chen PF, et al. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone is correlated with 8-hydroxy-2’-deoxyguanosine in humans after exposure to environmental tobacco smoke. Sci Total Environ. 2012; 414: 134–9. [DOI] [PubMed] [Google Scholar]
- 161.Chao MR, Cooke MS, Kuo CY, et al. Children are particularly vulnerable to environmental tobacco smoke exposure: Evidence from biomarkers of tobacco-specific nitrosamines, and oxidative stress. Environ Int. 2018; 120: 238–45. [DOI] [PubMed] [Google Scholar]
- 162.Chen CY, Jhou YT, Lee HL, et al. Simultaneous, rapid, and sensitive quantification of 8-hydroxy-2’-deoxyguanosine and cotinine in human urine by on-line solid-phase extraction LC-MS/MS: correlation with tobacco exposure biomarkers NNAL. Anal Bioanal Chem. 2016; 408(23): 6295–306. [DOI] [PubMed] [Google Scholar]
- 163.Sorensen M, Autrup H, Hertel O, et al. Personal exposure to PM2.5 and biomarkers of DNA damage. Cancer Epidemiol Biomarkers Prev. 2003; 12(3): 191–6. [PubMed] [Google Scholar]
- 164.Altemose B, Robson MG, Kipen HM, et al. Association of air pollution sources and aldehydes with biomarkers of blood coagulation, pulmonary inflammation, and systemic oxidative stress. J Expo Sci Environ Epidemiol. 2017; 27(3): 244–50. [DOI] [PubMed] [Google Scholar]
- 165.Buthbumrung N, Mahidol C, Navasumrit P, et al. Oxidative DNA damage and influence of genetic polymorphisms among urban and rural schoolchildren exposed to benzene. Chem Biol Interact. 2008; 172(3): 185–94. [DOI] [PubMed] [Google Scholar]
- 166.Commodore AA, Zhang JJ, Chang Y, et al. Concentrations of urinary 8-hydroxy-2’-deoxyguanosine and 8-isoprostane in women exposed to woodsmoke in a cookstove intervention study in San Marcos, Peru. Environ Int. 2013; 60: 112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ke Y, Cheng J, Zhang Z, et al. Increased levels of oxidative DNA damage attributable to cooking-oil fumes exposure among cooks. Inhal Toxicol. 2009; 21(8): 682–7. [DOI] [PubMed] [Google Scholar]
- 168.Tagesson C, Chabiuk D, Axelson O, et al. Increased urinary excretion of the oxidative DNA adduct, 8-hydroxydeoxyguanosine, as a possible early indicator of occupational cancer hazards in the asbestos, rubber, and azo-dye industries. Pol J Occup Med Environ Health. 1993; 6(4): 357–68. [PubMed] [Google Scholar]
- 169.McCord JM. Human disease, free radicals, and the oxidant/antioxidant balance. Clin Biochem. 1993; 26(5): 351–7. [DOI] [PubMed] [Google Scholar]
- 170.Esterbauer H, Eckl P, Ortner A. Possible mutagens derived from lipids and lipid precursors. Mutat Res. 1990; 238(3): 223–33. [DOI] [PubMed] [Google Scholar]
- 171.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology & Medicine. 1991; 11: 81–128. [DOI] [PubMed] [Google Scholar]
- 172.Barrera G, Pizzimenti S, Daga M, et al. Lipid peroxidation-derived aldehydes, 4-hydroxynonenal and malondialdehyde in aging-related disorders. Antioxidants (Basel). 2018; 7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Delfino RJ, Staimer N, Vaziri ND. Air pollution and circulating biomarkers of oxidative stress. Air Qual Atmos Health. 2011; 4(1): 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lykkesfeldt J Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta. 2007; 380(1–2): 50–8. [DOI] [PubMed] [Google Scholar]
- 175.Shamberger RJ, Andreone TL, Willis CE. Antioxidants and cancer. IV. Initiating activity of malonaldehyde as a carcinogen. J Natl Cancer Inst. 1974; 53(6): 1771–3. [PubMed] [Google Scholar]
- 176.Pryor WA. On the detection of lipid hydroperoxides in biological samples. Free Radic Biol Med. 1989; 7(2): 177–8. [DOI] [PubMed] [Google Scholar]
- 177.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990; 186: 407–21. [DOI] [PubMed] [Google Scholar]
- 178.Gong J, Zhu T, Kipen H, et al. Malondialdehyde in exhaled breath condensate and urine as a biomarker of air pollution induced oxidative stress. J Expo Sci Environ Epidemiol. 2013; 23(3): 322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bae S, Pan XC, Kim SY, et al. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ Health Perspect. 2010; 118(4): 579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pan CH, Jeng HA, Lai CH. Biomarkers of oxidative stress in electroplating workers exposed to hexavalent chromium. J Expo Sci Environ Epidemiol. 2018; 28(1): 76–83. [DOI] [PubMed] [Google Scholar]
- 181.Giera M, Lingeman H, Niessen WM. Recent advancements in the LC- and GC-based analysis of malondialdehyde (MDA): A brief overview. Chromatographia. 2012; 75(9–10): 433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Allen RW, Carlsten C, Karlen B, et al. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med. 2011; 183(9): 1222–30. [DOI] [PubMed] [Google Scholar]
- 183.Morrow JD, Hill KE, Burk RF, et al. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA. 1990; 87: 9383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J Biol Chem. 2008; 283(23): 15533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mori TA, Puddey IB, Burke V, et al. Effect of omega 3 fatty acids on oxidative stress in humans: GC-MS measurement of urinary F2-isoprostane excretion. Redox Rep. 2000; 5(1): 45–6. [DOI] [PubMed] [Google Scholar]
- 186.Walter MF, Blumberg JB, Dolnikowski GG, et al. Streamlined F2-isoprostane analysis in plasma and urine with high-performance liquid chromatography and gas chromatography/mass spectroscopy. Anal Biochem. 2000; 280(1): 73–9. [DOI] [PubMed] [Google Scholar]
- 187.Bono R, Tassinari R, Bellisario V, et al. Urban air and tobacco smoke as conditions that increase the risk of oxidative stress and respiratory response in youth. Environ Res. 2015; 137: 141–6. [DOI] [PubMed] [Google Scholar]
- 188.Li W, Wilker EH, Dorans KS, et al. Short-term exposure to air pollution and biomarkers of oxidative stress: The Framingham heart study. J Am Heart Assoc. 2016; 5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ambroz A, Vlkova V, Rossner P Jr., et al. Impact of air pollution on oxidative DNA damage and lipid peroxidation in mothers and their newborns. Int J Hyg Environ Health. 2016; 219(6): 545–56. [DOI] [PubMed] [Google Scholar]
- 190.Romanazzi V, Pirro V, Bellisario V, et al. 15-F(2)t isoprostane as biomarker of oxidative stress induced by tobacco smoke and occupational exposure to formaldehyde in workers of plastic laminates. Sci Total Environ. 2013; 442: 20–5. [DOI] [PubMed] [Google Scholar]
- 191.Yan W, Byrd GD, Ogden MW. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J Lipid Res. 2007; 48(7): 1607–17. [DOI] [PubMed] [Google Scholar]
- 192.Hecht SS, Yuan JM, Hatsukami D. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010; 23: 1001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Roethig HJ, Munjal S, Feng S, et al. Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine Tob Res. 2009; 11(10): 1216–25. [DOI] [PubMed] [Google Scholar]
- 194.Frost-Pineda K, Liang Q, Liu J, et al. Biomarkers of potential harm among adult smokers and nonsmokers in the total exposure study. Nicotine Tob Res. 2011; 13(3): 182–93. [DOI] [PubMed] [Google Scholar]
- 195.Taylor AW, Bruno RS, Traber MG. Women and smokers have elevated urinary F(2)-isoprostane metabolites: a novel extraction and LC-MS methodology. Lipids. 2008; 43(10): 925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.King CC, Piper ME, Gepner AD, et al. Longitudinal impact of smoking and smoking cessation on inflammatory markers of cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2017; 37(2): 374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pilz H, Oguogho A, Chehne F, et al. Quitting cigarette smoking results in a fast improvement of in vivo oxidation injury (determined via plasma, serum and urinary isoprostane). Thromb Res. 2000; 99(3): 209–21. [DOI] [PubMed] [Google Scholar]
- 198.Carmella SG, Heskin AK, Tang MK, et al. Longitudinal stability in cigarette smokers of urinary eicosanoid biomarkers of oxidative damage and inflammation. PLoS One. 2019; 14(4): e0215853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yuan JM, Carmella SG, Wang R, et al. Relationship of the oxidative damage biomarker 8-epi-prostaglandin F2alpha to risk of lung cancer development in the Shanghai Cohort Study. Carcinogenesis. 2018; 39(7): 948–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Poirier MC. Linking DNA adduct formation and human cancer risk in chemical carcinogenesis. Environ Mol Mutagen. 2016; 57(7): 499–507. [DOI] [PubMed] [Google Scholar]
- 201.Phillips DH, Venitt S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int J Cancer. 2012; 131(12): 2733–53. [DOI] [PubMed] [Google Scholar]
- 202.Munnia A, Giese RW, Polvani S, et al. Bulky DNA adducts, tobacco smoking, genetic susceptibility, and lung cancer risk. Adv Clin Chem. 2017; 81: 231–77. [DOI] [PubMed] [Google Scholar]
- 203.Rossner P Jr., Svecova V, Schmuczerova J, et al. Analysis of biomarkers in a Czech population exposed to heavy air pollution. Part I: bulky DNA adducts. Mutagenesis. 2013; 28(1): 89–95. [DOI] [PubMed] [Google Scholar]
- 204.Ayi-Fanou L, Avogbe PH, Fayomi B, et al. DNA-adducts in subjects exposed to urban air pollution by benzene and polycyclic aromatic hydrocarbons (PAHs) in Cotonou, Benin. Environ Toxicol. 2011; 26(1): 93–102. [DOI] [PubMed] [Google Scholar]
- 205.Taioli E, Sram RJ, Binkova B, et al. Biomarkers of exposure to carcinogenic PAHs and their relationship with environmental factors. Mutat Res. 2007; 620(1–2): 16–21. [DOI] [PubMed] [Google Scholar]
- 206.Ruchirawa M, Mahidol C, Tangjarukij C, et al. Exposure to genotoxins present in ambient air in Bangkok, Thailand--particle associated polycyclic aromatic hydrocarbons and biomarkers. Sci Total Environ. 2002; 287(1–2): 121–32. [DOI] [PubMed] [Google Scholar]
- 207.Pavanello S, Favretto D, Brugnone F, et al. HPLC/fluorescence determination of anti-BPDE-DNA adducts in mononuclear white blood cells from PAH-exposed humans. Carcinogenesis. 1999; 20(3): 431–5. [DOI] [PubMed] [Google Scholar]
- 208.Jin Y, Xu P, Liu X, et al. Cigarette smoking, BPDE-DNA adducts, and aberrant promoter methylations of tumor suppressor genes (TSGs) in NSCLC from Chinese population. Cancer Invest. 2016; 34(4): 173–80. [DOI] [PubMed] [Google Scholar]
- 209.Naidoo RN, Makwela MH, Chuturgoon A, et al. Petrol exposure and DNA integrity of peripheral lymphocytes. Int Arch Occup Environ Health. 2016; 89(5): 785–92. [DOI] [PubMed] [Google Scholar]
- 210.Rundle A, Richards C, Neslund-Dudas C, et al. Neighborhood socioeconomic status modifies the association between individual smoking status and PAH-DNA adduct levels in prostate tissue. Environ Mol Mutagen. 2012; 53(5): 384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Perera FP, Tang D, Rauh V, et al. Relationships among polycyclic aromatic hydrocarbon-DNA adducts, proximity to the World Trade Center, and effects on fetal growth. Environ Health Perspect. 2005; 113(8): 1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Perera FP, Tang D, Rauh V, et al. Relationship between polycyclic aromatic hydrocarbon-DNA adducts, environmental tobacco smoke, and child development in the World Trade Center cohort. Environ Health Perspect. 2007; 115(10): 1497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ma B, Stepanov I, Hecht SS. Recent studies on DNA adducts resulting from human exposure to tobacco smoke. Toxics. 2019; 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bessette EE, Goodenough AK, Langouet S, et al. Screening for DNA adducts by data-dependent constant neutral loss-triple stage mass spectrometry with a linear quadrupole ion trap mass spectrometer. Anal Chem. 2009; 81(2): 809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bessette EE, Spivack SD, Goodenough AK, et al. Identification of carcinogen DNA adducts in human saliva by linear quadrupole ion trap/multistage tandem mass spectrometry. Chem Res Toxicol. 2010; 23(7): 1234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Monien BH, Schumacher F, Herrmann K, et al. Simultaneous detection of multiple DNA adducts in human lung samples by isotope-dilution UPLC-MS/MS. Anal Chem. 2015; 87(1): 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Villalta PW, Hochalter JB, Hecht SS. Ultrasensitive high-resolution mass spectrometric analysis of a DNA adduct of the carcinogen benzo[a]pyrene in human lung. Anal Chem. 2017; 89(23): 12735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Casale GP, Singhal M, Bhattacharya S, et al. Detection and quantification of depurinated benzo[a]pyrene-adducted DNA bases in the urine of cigarette smokers and women exposed to household coal smoke. Chem Res Toxicol. 2001; 14(2): 192–201. [DOI] [PubMed] [Google Scholar]
- 219.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012; 100(Pt E): 1–538. [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang S, Balbo S, Wang M, et al. Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chem Res Toxicol. 2011; 24(1): 119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chung FL, Wu MY, Basudan A, et al. Regioselective formation of acrolein-derived cyclic 1,N(2)-propanodeoxyguanosine adducts mediated by amino acids, proteins, and cell lysates. Chem Res Toxicol. 2012; 25(9): 1921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li X, Liu L, Wang H, et al. Simultaneous analysis of six aldehyde-DNA adducts in salivary DNA of nonsmokers and smokers using stable isotope dilution liquid chromatography electrospray ionization-tandem mass spectrometry. J Chromatogr B. 2017; 1060: 451–9. [DOI] [PubMed] [Google Scholar]
- 223.Hatsukami DK, Le CT, Zhang Y, et al. Toxicant exposure in cigarette reducers vs. light smokers. Cancer Epidemiol Biomarkers Prev. 2006; 15: 2355–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang M, Yu N, Chen L, et al. Identification of an acetaldehyde adduct in human liver DNA and quantitation as N2-ethyldeoxyguanosine. Chem Res Toxicol. 2006; 19: 319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chen L, Wang M, Villalta PW, et al. Quantitation of an acetaldehyde adduct in human leukocyte DNA and the effect of smoking cessation. Chem Res Toxicol. 2007; 20: 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Garcia CC, Freitas FP, Sanchez AB, et al. Elevated alpha-methyl-gamma-hydroxy-1,N2-propano-2’-deoxyguanosine levels in urinary samples from individuals exposed to urban air pollution. Chem Res Toxicol. 2013; 26(11): 1602–4. [DOI] [PubMed] [Google Scholar]
- 227.IARC Monographs Working Group on the Evaluation of Carcinogenic Risks to Humans. Some aromatic amines, organic dyes, and related exposures. IARC Monogr Eval Carcinog Risks Hum. 2010; 99: 1–658. [PMC free article] [PubMed] [Google Scholar]
- 228.Guo J, Villalta PW, Weight CJ, et al. Targeted and untargeted detection of DNA adducts of aromatic amine carcinogens in human bladder by ultra-performance liquid chromatography-high-resolution mass spectrometry. Chem Res Toxicol. 2018; 31(12): 1382–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xiao S, Guo J, Yun BH, et al. Biomonitoring DNA Adducts of Cooked Meat Carcinogens in Human Prostate by Nano Liquid Chromatography-High Resolution Tandem Mass Spectrometry: Identification of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine DNA Adduct. Anal Chem. 2016; 88(24): 12508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gu D, Turesky RJ, Tao Y, et al. DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 4-aminobiphenyl are infrequently detected in human mammary tissue by liquid chromatography/tandem mass spectrometry. Carcinogenesis. 2012; 33(1): 124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sangaraju D, Villalta P, Goggin M, et al. Capillary HPLC-accurate mass MS/MS quantitation of N7-(2,3,4-trihydroxybut-1-yl)-guanine adducts of 1,3-butadiene in human leukocyte DNA. Chem Res Toxicol. 2013; 26(10): 1486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Huang CC, Wu CF, Shih WC, et al. Potential association of urinary N7-(2-carbamoyl-2-hydroxyethyl) guanine with dietary acrylamide intake of smokers and nonsmokers. Chem Res Toxicol. 2015; 28(1): 43–50. [DOI] [PubMed] [Google Scholar]
- 233.Huang YF, Huang CJ, Lu CA, et al. Feasibility of using urinary N7-(2-carbamoyl-2-hydroxyethyl) Guanine as a biomarker for acrylamide exposed workers. J Expo Sci Environ Epidemiol. 2018; 28(6): 589–98. [DOI] [PubMed] [Google Scholar]
- 234.Yu Y, Cui Y, Niedernhofer LJ, et al. Occurrence, biological consequences, and human health relevance of oxidative stress-induced DNA damage. Chem Res Toxicol. 2016; 29(12): 2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Marczynski B, Rozynek P, Kraus T, et al. Levels of 8-hydroxy-2’-deoxyguanosine in DNA of white blood cells from workers highly exposed to asbestos in Germany. Mutat Res. 2000; 468(2): 195–202. [DOI] [PubMed] [Google Scholar]
- 236.Marczynski B, Kraus T, Rozynek P, et al. Association between 8-hydroxy-2’-deoxyguanosine levels in DNA of workers highly exposed to asbestos and their clinical data, occupational and non-occupational confounding factors, and cancer. Mutat Res. 2000; 468(2): 203–12. [DOI] [PubMed] [Google Scholar]
- 237.Singh R, Kaur B, Kalina I, et al. Effects of environmental air pollution on endogenous oxidative DNA damage in humans. Mutation Research. 2007; 620(1–2): 71–82. [DOI] [PubMed] [Google Scholar]
- 238.Bin P, Shen M, Li H, et al. Increased levels of urinary biomarkers of lipid peroxidation products among workers occupationally exposed to diesel engine exhaust. Free Radic Res. 2016; 50(8): 820–30. [DOI] [PubMed] [Google Scholar]
- 239.Marnett LJ. Lipid peroxidation - DNA damage by malondialdehyde. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis. 1999; 424(1–2): 83–95. [DOI] [PubMed] [Google Scholar]
- 240.Peluso M, Srivatanakul P, Munnia A, et al. Malondialdehyde-deoxyguanosine adducts among workers of a Thai industrial estate and nearby residents. Environ Health Persp. 2010; 118(1): 55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bono R, Romanazzi V, Munnia A, et al. Malondialdehyde-deoxyguanosine adduct formation in workers of pathology wards. The role of air formaldehyde exposure. Chem Res Toxicol. 2010; 23(8): 1342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: A review of published adduct types and levels in humans. Free Radical Biology & Medicine. 2007; 43: 1109–20. [DOI] [PubMed] [Google Scholar]
- 243.Bono R, Munnia A, Romanazzi V, et al. Formaldehyde-induced toxicity in the nasal epithelia of workers of a plastic laminate plant. Toxicol Res (Camb). 2016; 5(3): 752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Saieva C, Peluso M, Palli D, et al. Dietary and lifestyle determinants of malondialdehyde DNA adducts in a representative sample of the Florence City population. Mutagenesis. 2016; 31(4): 475–80. [DOI] [PubMed] [Google Scholar]
- 245.Ma B, Villalta P, Balbo S, et al. Analysis of a malondialdehyde-deoxyguanosine adduct in human leukocyte DNA by liquid chromatography nanoelectrospray-high-resolution tandem mass spectrometry. Chem Res Toxicol. 2014; 27: 1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gurgueira SA, Lawrence J, Coull B, et al. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002; 110(8): 749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ghio AJ, Richards JH, Carter JD, et al. Accumulation of iron in the rat lung after tracheal instillation of diesel particles. Toxicol Pathol. 2000; 28(4): 619–27. [DOI] [PubMed] [Google Scholar]
- 248.Ghio AJ, Kennedy TP, Stonehuerner J, et al. Iron regulates xanthine oxidase activity in the lung. Am J Physiol Lung Cell Mol Physiol. 2002; 283(3): L563–72. [DOI] [PubMed] [Google Scholar]
- 249.Dye JA, Adler KB, Richards JH, et al. Role of soluble metals in oil fly ash-induced airway epithelial injury and cytokine gene expression. Am J Physiol. 1999; 277(3): L498–510. [DOI] [PubMed] [Google Scholar]
- 250.Hu CW, Pan CH, Huang YL, et al. Effects of arsenic exposure among semiconductor workers: a cautionary note on urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine. Free Radic Biol Med. 2006; 40(7): 1273–8. [DOI] [PubMed] [Google Scholar]
- 251.Protano C, Astolfi ML, Canepari S, et al. Urinary levels of trace elements among primary school-aged children from Italy: The contribution of smoking habits of family members. Sci Total Environ. 2016; 557–558: 378–85. [DOI] [PubMed] [Google Scholar]
- 252.Engstrom KS, Vahter M, Johansson G, et al. Chronic exposure to cadmium and arsenic strongly influences concentrations of 8-oxo-7,8-dihydro-2’-deoxyguanosine in urine. Free Radic Biol Med. 2010; 48(9): 1211–7. [DOI] [PubMed] [Google Scholar]
- 253.Halatek T, Sinczuk-Walczak H, Janasik B, et al. Health effects and arsenic species in urine of copper smelter workers. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2014; 49(7): 787–97. [DOI] [PubMed] [Google Scholar]
- 254.Garcon G, Leleu B, Zerimech F, et al. Biologic markers of oxidative stress and nephrotoxicity as studied in biomonitoring of adverse effects of occupational exposure to lead and cadmium. J Occup Environ Med. 2004; 46(11): 1180–6. [DOI] [PubMed] [Google Scholar]
- 255.Garcon G, Leleu B, Marez T, et al. Biomonitoring of the adverse effects induced by the chronic exposure to lead and cadmium on kidney function: usefulness of alpha-glutathione S-transferase. Sci Total Environ. 2007; 377(2–3): 165–72. [DOI] [PubMed] [Google Scholar]
- 256.Wang T, Feng W, Kuang D, et al. The effects of heavy metals and their interactions with polycyclic aromatic hydrocarbons on the oxidative stress among coke-oven workers. Environ Res. 2015; 140: 405–13. [DOI] [PubMed] [Google Scholar]
- 257.Zhang X, Staimer N, Gillen DL, et al. Associations of oxidative stress and inflammatory biomarkers with chemically-characterized air pollutant exposures in an elderly cohort. Environ Res. 2016; 150: 306–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Saad AA, El-Sikaily A, Kassem H. Metallothionein and glutathione content as biomarkers of metal pollution in mussels and local fishermen in Abu Qir Bay, Egypt. J Health Pollut. 2016; 6(12): 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ladva CN, Golan R, Liang D, et al. Particulate metal exposures induce plasma metabolome changes in a commuter panel study. PLoS One. 2018; 13(9): e0203468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang Z, Xu X, He B, et al. The impact of chronic environmental metal and benzene exposure on human urinary metabolome among Chinese children and the elderly population. Ecotoxicol Environ Saf. 2019; 169: 232–9. [DOI] [PubMed] [Google Scholar]
- 261.Wu S, Deng F, Wei H, et al. Chemical constituents of ambient particulate air pollution and biomarkers of inflammation, coagulation and homocysteine in healthy adults: a prospective panel study. Part Fibre Toxicol. 2012; 9: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bowler RM, Harris M, Gocheva V, et al. Anxiety affecting parkinsonian outcome and motor efficiency in adults of an Ohio community with environmental airborne manganese exposure. Int J Hyg Environ Health. 2012; 215(3): 393–405. [DOI] [PubMed] [Google Scholar]
- 263.Wang JP, Maddalena R, Zheng B, et al. Arsenicosis status and urinary malondialdehyde (MDA) in people exposed to arsenic contaminated-coal in China. Environ Int. 2009; 35(3): 502–6. [DOI] [PubMed] [Google Scholar]
- 264.Stern AH, Fagliano JA, Savrin JE, et al. The association of chromium in household dust with urinary chromium in residences adjacent to chromate production waste sites. Environ Health Perspect. 1998; 106(12): 833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zota AR, Riederer AM, Ettinger AS, et al. Associations between metals in residential environmental media and exposure biomarkers over time in infants living near a mining-impacted site. J Expo Sci Environ Epidemiol. 2016; 26(5): 510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Shepherd TJ, Dirks W, Roberts NM, et al. Tracing fetal and childhood exposure to lead using isotope analysis of deciduous teeth. Environ Res. 2016; 146: 145–53. [DOI] [PubMed] [Google Scholar]
- 267.Kowalska M, Kulka E, Jarosz W, et al. The determinants of lead and cadmium blood levels for preschool children from industrially contaminated sites in Poland. Int J Occup Med Environ Health. 2018; 31(3): 351–9. [DOI] [PubMed] [Google Scholar]
- 268.Willers S, Gerhardsson L, Lundh T. Environmental tobacco smoke (ETS) exposure in children with asthma-relation between lead and cadmium, and cotinine concentrations in urine. Respir Med. 2005; 99(12): 1521–7. [DOI] [PubMed] [Google Scholar]
- 269.Hukkanen J, Jacob P III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005; 57(1): 79–115. [DOI] [PubMed] [Google Scholar]
- 270.Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009; 169(2): 236–48. [DOI] [PubMed] [Google Scholar]
- 271.Jarvis MJ, Fidler J, Mindell J, et al. Assessing smoking status in children, adolescents and adults: cotinine cut-points revisited. Addiction. 2008; 103(9): 1553–61. [DOI] [PubMed] [Google Scholar]
- 272.Murphy SE, Park SS, Thompson EF, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014; 35(11): 2526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.von Weymarn LB, Thomson NM, Donny EC, et al. Quantitation of the minor tobacco alkaloids nornicotine, anatabine, and anabasine in smokers’ urine by high throughput liquid chromatography-mass spectrometry. Chem Res Toxicol. 2016; 29(3): 390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hecht SS, Stepanov I, Carmella SG. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc Chem Res. 2016; 49(1): 106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


