Abstract
In adult myocardium, the heartbeat originates from the sequential activation of ionic currents in pacemaker cells of the sinoatrial node. Ca2+ release via the ryanodine receptor (RyR) modulates the rate at which these cells beat. In contrast, the mechanisms that regulate heart rate during early cardiac development are poorly understood. Embryonic stem (ES) cells can differentiate into spontaneously contracting myocytes whose beating rate increases with differentiation time. These cells thus offer an opportunity to determine the mechanisms that regulate heart rate during development. Here we show that the increase in heart rate with differentiation is markedly depressed in ES cell-derived cardiomyocytes with a functional knockout (KO) of the cardiac ryanodine receptor (RyR2). KO myocytes show a slowing of the rate of spontaneous diastolic depolarization and an absence of calcium sparks. The depressed rate of pacemaker potential can be mimicked in wild-type myocytes by ryanodine, and rescued in KO myocytes with herpes simplex virus (HSV)-1 amplicons containing full-length RyR2. We conclude that a functional RyR2 is crucial to the progressive increase in heart rate during differentiation of ES cell-derived cardiomyocytes, consistent with a mechanism that couples Ca2+ release via RyR before an action potential with activation of an inward current that accelerates membrane depolarization.
In adult heart, the heartbeat originates from the sequential activation/inactivation of ionic currents in pacemaker cells of the sinoatrial (SA) node. In pacemaker cells, calcium released from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyR) during diastolic depolarization and before an action potential (AP) activates the Na+/Ca2+ exchanger to produce an inward current that accelerates the rate of spontaneous membrane depolarization, resulting in a more rapid rate of contraction (1, 2). Ca2+ activation of the Na+/Ca2+ exchanger is therefore critical to the generation of the heartbeat, and RyR play a critical modulatory role for Ca2+ release in the automaticity of isolated pacemaker cells from adult animals (1, 2).
In primary heart tubes of developing chicken embryos and the outflow tract of early rat embryonic heart, a functional SR is lacking (3), and in mice, regulated calcium release via the RyR in early embryonic hearts is thought to be of little importance (4). The presumption is that sarcolemmal Ca2+ currents working with the Na+/Ca2+ exchanger play a predominant role in the regulation of cytosolic Ca2+ to drive the heart's contraction during early cardiac development, and that RyR2 is required primarily to participate in the maintenance of intracellular Ca2+ homeostasis. RyR2 expression in developing myocardium however precedes the time when augmented heart function is critical to the continued development of the embryo (5). Because an increase in heart rate is critical to enhanced cardiac output as embryonic development proceeds (6–8), an understanding of the mechanisms that regulate heart rate during early cardiac development is needed.
One approach to studying the mechanisms underlying beating rate is through exploitation of embryonic stem (ES) cell-derived cardiomyocytes (9, 10). Cardiomyocytes formed within embryoid bodies (EB) follow developmental stages similar to those found in the mouse embryo (11). Specifically, ES cell-derived cardiomyocytes express ion channels in a developmentally regulated pattern (12, 13) and show an increase in spontaneous beating rate with in vitro differentiation (12, 14). ES cell-derived cardiomyocytes also have ultrastructural, molecular, and electrophysiological characteristics typical of primary myocardial pacemaker-like cells that subsequently differentiate into atrial-, ventricular-, His/Purkinje-, and adult pacemaker-like cells (10, 15, 16). Importantly, ES cell-derived cardiomyocytes can be used to understand the role of essential proteins that cause embryonic lethality during early development (17).
Recently, it was reported that ES cell-derived cardiomyocytes have rhythmic beatings that occur in response to intracellular Ca2+ oscillations. These oscillations were thought to evoke small membrane depolarizations that could trigger Ca2+ channel-driven action potentials (14), but a specific role for the RyR was not addressed. We therefore hypothesized that calcium release via the cardiac ryanodine receptor (RyR2) might be essential for the increase in beating rate that occurs in early heart cells as development proceeds. To test this hypothesis, we have generated ES cells (Line R1) deficient in RyR2 and compared the differentiation and electrophysiological characteristics of cardiomyocytes derived from wild-type (wt) and RyR2 null ES cells.
Experimental Procedures
ES Cell Cultivation.
R1 ES cells were cultivated on primary mouse feeder layers as described (10). Differentiation of ES cells into cardiac cells was performed by a hanging drop technique (10). After 7 days in suspension, EBs were plated onto gelatin-coated (0.1%) tissue culture dishes, and the percentage of EBs containing beating cardiomyocytes was determined. Spontaneous beating frequencies were measured (32 ± 2°C) using a video-based camera system [Crescent Electronics (Salt Lake City) Video Edge Detector, VED-104, and a Panasonic (Secaucus, NJ) TV Camera, VW-1100A]. Rate data were analyzed in real-time by using a threshold-crossing criterion, and averaged every 5 s. Single cardiomyocytes were isolated from EB clusters by enzymatic dissociation with collagenase (10), and dissociated cells were plated on laminin/gelatin-coated glass coverslips.
Gene Targeting and Cellular Analyses.
A 129/Sv mouse genomic library (lambda DASH II, Stratagene) was screened (18) using a rat RyR2 cDNA clone and a 9.6-kb NotI-XhoI genomic fragment subcloned into pBS-SK (Stratagene). The final targeting construct consisted of (i) a phosphoglycerate kinase (pgk) promoter-driven neomycin phosphotransferaseR (neoR) cassette (1.8 kb) with a wt loxP sequence (104 bp) from pBS246 (GIBCO/BRL) inserted into the AatII site; (ii) a loxP sequence (115 bp) in the SpeI site; and (iii) a pgk-thymidine kinaseR gene (tkR) cassette (2.8 kb) (Fig. 1). The linearized (XhoI) targeting vector was electroporated (250 V and 500 μF) into ≈3.5 × 106 R1 ES cells. Two clones (R1-8 and -36), surviving G418 (300 μg/ml) and gancyclovir (2 μM) selection, were identified by Southern analysis to have appropriate targeting events (10). R1-36 ES cells were cotransfected with an expression vector containing Cre recombinase (pBS185, GIBCO/BRL) and a replacement vector containing a pgk-puromycin resistance cassette (pZ-PpacP) flanked by two wt loxP sites (Fig. 1). One RyR2+/− ES cell line, identified by Southern, was retargeted by repeating the steps above to produce RyR2−/− ES cell lines (seven independent clones).
Figure 1.
Generation and characteristics of RyR2-targeted ES cells. (A) Schematic representation of the RyR2 allele (WT), targeting vector (TV), targeted allele (TA), and deleted allele (KO) mediated by Cre Recombinase/loxP recombination. The targeting vector was constructed by introduction of two wt loxP sites, PGK-NeoR and PGK-tkR cassettes (RyR+/+(neo)) into the cloning vector. Site-specific deletion of the floxed exon, corresponding to amino acids 651–734 of human RyR2, was mediated by cotransfection of pBS185 and pZ-PpacP. Exons are indicated by black boxes. (B) Probes A and B, located outside of the targeting vector, were used for genomic Southern blot analysis after SpeI (S) or EcoRI (E) digestions. (C) RNA analysis of RyR2 mRNA expression. (Upper) Reverse transcription (RT)-PCR analysis of RyR2 mRNA in EBs at various developmental stages. ES, undifferentiated cells; 7 + 5, 7, 14, 21 (7+ days after plating); −C, no template negative control; +C, RNA from mouse heart. (Lower) Western blot analysis of RyR2 protein in undifferentiated and differentiated ES cells (8, 10, 12, 14, and 21 days after plating). RyR2+/+ (shown) and RyR2+/− myocytes expressed the monomeric protein, but RyR2 was not detected in the KO cells (not shown).
ES cells, EBs, and cardiomyocytes were used to isolate total RNA (19) or protein. PCR reactions for β-tubulin (317 bp; ref. 10) were performed in parallel to those for RyR2. A 330-bp product was amplified by RyR2-specific primers: GACGGCAGAAGCCACTCACCTGCG and CCTGCAGAGAAACTGACAACTGGA. Western blots were performed on 5% Tris⋅HCl gels (Bio-Rad) by using 5 μg of protein extract. After transfer to Immobion-P poly(vinylidene difluoride) (PVDF) membranes, RyR was detected using a mouse anti-RyR antibody (Affinity BioReagents, MA3-916), a secondary rabbit anti-mouse antibody and a tertiary goat anti-rabbit horseradish peroxidase antibody (Zymed). Signals were detected using Pierce Supersignal ECL substrate.
For immunofluorescence assays, isolated cells were fixed with methanol/acetone/H2O (50/30/20%). Tissues were rehydrated and blocked with 10% goat serum. Anti-troponin T (JLT-12) or anti-α-sarcomeric actin (5C5, Sigma) were used as primary antibodies with a goat anti-mouse IgG-FITC or IgM-FITC (Zymed) secondary antibody, respectively (10).
Herpes Simplex Virus (HSV)-1 Amplicon-Mediated Rescue.
Helper virus-free stocks of HyRGN (EGFP) and HSVRyR2 (full-length rabbit RyR2) amplicon vectors were prepared, and vector stocks purified and concentrated as described (20). Briefly, amplicon DNA was cotransfected with DNA from cosmid set C6Δ48Δa into 2–2 cells by using lipofectamine (GIBCO/BRL). Cosmid set C6Δ48Δa represents the HSV-1 genome in five overlapping clones that can form circular replication-competent virus genomes (21). Because the HSV-1 DNA cleavage/packaging signals (pac) are deleted from the cosmid set, reconstituted virus genomes are not packaged, but provide all of the helper functions required for the replication and packaging of cotransfected amplicon DNA.
Individual or small clusters of beating cardiomyocytes from collagenase-treated RyR2 knockout (KO) ES cells (day 7 + 6) were plated and the beating rate determined. Cells were infected with helper virus free HSV-1 amplicon virions (titer, 1.5 × 105; HyRGN or HSVRyR2), or treated with no virions for a period of 2 h before dilution by addition of cultivation medium. Media was changed after an overnight incubation.
Electrophysiological Measurements.
AP data were acquired on spontaneously beating isolated cardiomyocytes with an Axopatch-1D patch clamp amplifier (Axon Instruments, Foster City, CA), using a perforated patch-clamp technique to record spontaneous APs (2). The spontaneous cycle length (time intervals between successive APs), AP amplitude, AP duration at 50% repolarization, maximal diastolic potential, and the slope of diastolic depolarization were determined using a custom-written computer program.
Isolated cells were loaded with Fluo-4-acetoxymethyl ester (22 μM; Molecular Probes) at 25°C for 10 min. [Ca2+]i measurements were obtained with a confocal laser scanning microscope in line-scan mode (LSM 410, Zeiss), and were analyzed with idl software (Version 5.2, Research Systems, Boulder, CO) as described (2). Electrophysiological measurements were made at 36 ± 1°C and, where noted, in the presence of ryanodine or caffeine. Separately, isolated cells were loaded with 25 μM Indo-1 AM and 1% pluronic acid (Molecular Probes) for 10 min at room temperature. Calcium transients were measured with external pacing at room temperature as described (22).
Statistics.
Data are expressed as means ± SEM. Differences between beating rates with time were estimated by a repeated measures analysis. Other statistics were performed using a Tukey honestly significantly different test for unequal N′s, a Scheffé post hoc test for differences between specific time groups, or one-way ANOVA (statistica software, Version 5.1, StatSoft, Tulsa, OK). Significant differences between the two mean values were estimated by paired or unpaired Student's t test. P < 0.05 was considered significant.
Results
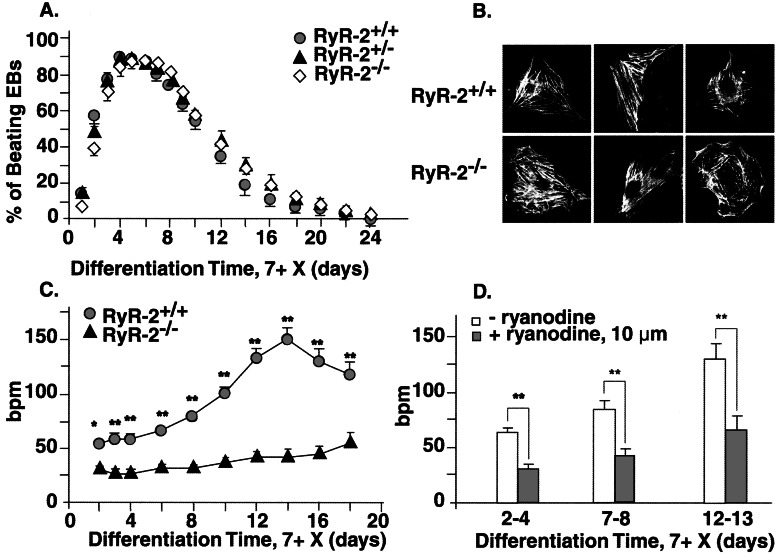
ES cells with targeted RyR2 genes led to a functional knockout (RyR−/−; Fig. 1). Undifferentiated RyR2+/+, RyR2+/−, and RyR2−/− ES cells had normal growth characteristics and could be differentiated into spontaneously beating cardiomyocytes that were visible ≤1 day after plating (Fig. 2A). Three to five days later, 80–100% of the EBs developed spontaneously beating cardiomyocytes, the number of which decreased with prolonged differentiation. Cardiomyocytes that no longer beat spontaneously within the EBs could be field stimulated and, when isolated, had cellular morphologies typical of atrial- and ventricular-like cells (Fig. 2B). The number of spontaneously beating cardiomyocytes and the number of beating areas within an individual EB were indistinguishable between wt and KO cells. The results indicate that RyR−/− ES cells readily differentiate in vitro to cardiomyocytes with a cellular morphology and a differentiation profile indistinguishable from wt cells.
Figure 2.
Phenotypic characteristics of differentiated ES cell lines. (A) Beating profiles of cardiomyocytes/EB (%) were determined with time. (B) Immunostaining of RyR+/+ and RyR−/− cardiomyocytes showing sarcomeric structures. Cardiomyocytes had a number of cell morphologies that varied with time of in vitro differentiation, but specific morphological differences between wt- and KO-derived cells could not be demonstrated. (C) Spontaneous beating rates of cardiomyocytes in EBs. (D) Effect of ryanodine (10 μM, 30 min) on spontaneous beating rate in RyR2+/+. Ryanodine had no significant effect on RyR2−/− cells. *, P < 0.01; **, P < 0.001.
With in vitro differentiation of wt cardiomyocytes, spontaneous contractions rapidly increase (Fig. 2C), reaching an average maximum rate of ≈150 beats per minute (bpm) at 7 + 10 ± 4 days, although individual beating rates of >350 bpm were observed. In KO myocytes, the increase in beating rate was significantly decreased at each differentiation time point examined. Ryanodine (10 μM), a specific inhibitor of RyR2, did not affect the beating rates of RyR2−/− myocytes at any differentiation time. In RyR2+/+ cardiomyocytes, the beating rate was significantly (P < 0.001) decreased to the level of the KO cells by addition of ryanodine, which ultimately depleted SR Ca2+ content (Fig. 2D). Thapsigargin (1 μM, 30 min), a SR Ca2+ ATPase inhibitor, also depressed the beating rate of RyR+/+ cardiomyocytes by ≈25–40%, comparable to the decrease seen with ryanodine. Similar to ryanodine, thapsigargin did not affect (P > 0.05) the beating rate of RyR2−/− cells at any differentiation stage (data not shown). The results indicate that wt ES cell-derived cardiomyocytes have functional SR and some form of SR calcium release. Because thapsigargin had no effects on KO cells, RyR2 is the major, if not only, RyR-mediated calcium release mechanism present in the SR of these cells.
Membrane potentials were recorded in isolated and spontaneously beating wt and KO ES cell-derived cardiomyocytes (7 + 7 − 8). The maximum diastolic potential did not differ and RyR2−/− cardiomyocytes had a maximum upstroke velocity similar to that of wt cardiomyocytes (Fig. 3). The decrease in beating rate of KO cells was associated with an increase in the amplitude and duration and a decrease in the slope of diastolic depolarization of the AP. Fig. 3 shows typical APs recorded in individual RyR+/+ myocytes before and after ryanodine (10 μM). When ryanodine was added to wt cardiomyocytes, a 2-fold increase in AP duration and an approximately 3-fold decrease in the rates of diastolic depolarization were observed (Table 1). The AP amplitude also tended to increase following ryanodine. These data demonstrate that AP parameters in wt myocytes treated with ryanodine become similar to AP parameters in untreated RyR−/− myocytes.
Figure 3.
Action potential (AP) parameters and the effects of ryanodine. AP recordings in representative RyR+/+ (A) and RyR−/− (D) cardiomyocytes. Firing rate of RyR+/+ cardiomyocytes before (B, 71 bpm) and after (C, 26 bpm) ryanodine (Ry, 10 μM). The firing rate of RyR2 KO cardiomyocytes before (E) and after (F) exposure to ryanodine did not change (26 bpm).
Table 1.
AP parameters of ES cell-derived cardiomyocytes
| BR, bpm | APA, mV | APD50, ms | DD, mV/s | MDP, mV | |
|---|---|---|---|---|---|
| RyR2+/+ | 143.4 ± 26.7 | 79.7 ± 3.9 | 86.5 ± 15.7 | 30.7 ± 5.3 | −67.7 ± 4.8 |
| RyR2+/+ + Ry | 59.8 ± 21.3* | 85.3 ± 5.5 | 158.0 ± 22.6* | 8.5 ± 2.6** | −70.0 ± 4.7 |
| RyR2−/− | 37.6 ± 9.5** | 95.7 ± 3.1* | 173.3 ± 25.5* | 9.3 ± 2.5** | −72.6 ± 3.4 |
AP parameters in RyR+/+ (n = 8) and RyR−/− (n = 7) cardiomyocytes and in RyR+/+ (n = 4) cardiomyocytes after a challenge with ryanodine (Ry). BR, beating rate; APA, AP amplitude; APD50, AP duration at 50% of the amplitude; DD, rate of diastolic depolarization; MDP, maximum diastolic potential.
, P < 0.05;
, P < 0.01 vs. RyR2+/+ values.
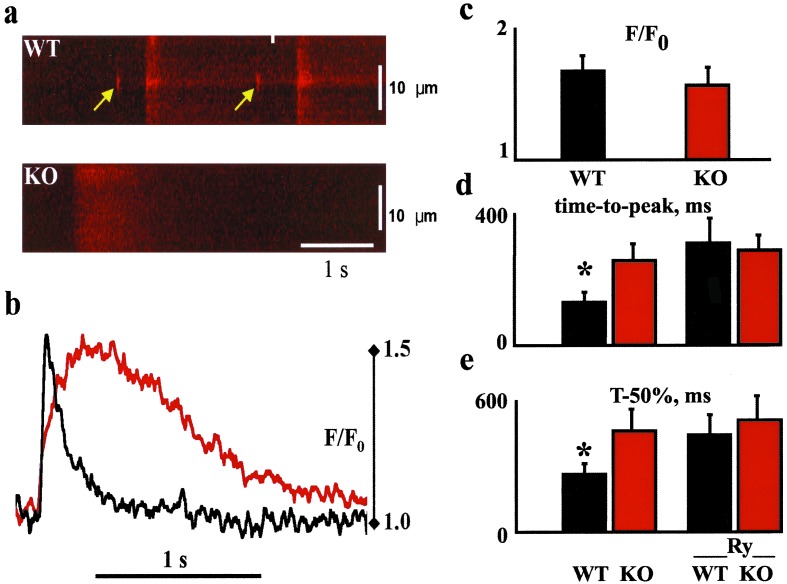
Intracellular Ca2+ release in individual isolated cardiomyocytes at day 7 + 7 − 8 was investigated using Fluo-4 as a Ca2+ indicator. RyR2+/+ cardiomyocytes exhibited frequent areas of spontaneous local Ca2+ release (Ca2+ sparks; refs. 12 and 23), which were not detected in RyR2−/− cardiomyocytes (Fig. 4a). The calcium transients differed markedly in spontaneously beating cells in that the time-to-peak of the Ca2+ transient and its duration at 50% of the maximum amplitude were prolonged in KO cells. The amplitude of spontaneous Ca2+ transients did not differ between these KO and wt cardiomyocytes (Fig. 4 b and c). The source of Ca2+ influx in KO or wt cells treated with ryanodine could occur via reverse mode Na+/Ca2+ exchanger following the rapid upstroke of the AP, sarcolemmal L-type calcium channels, or possibly from IP3-sensitive stores. Exposure of wt cardiomyocytes to caffeine (10 mM), an activator of Ca2+-induced Ca2+ release from RyR2, caused a brisk, large Ca2+ transient in RyR2+/+ myocytes but no response in RyR2−/− cells. To compare the kinetics of the Ca2+ transient in wt and KO cells before and following ryanodine, additional cells were loaded with Indo-1 AM and externally paced at 0.5 Hz. Ryanodine had no effect in KO cells; however, in wt cells, ryanodine reduced the velocity of the Ca2+ transient upstroke and prolonged the time-to-peak and time-to-relaxation (Fig. 4 d and e). In the presence of ryanodine, the kinetics of the Ca2+ transient were indistinguishable in paced wt and KO cells.
Figure 4.
Representative [Ca2+]i images. (a) Line-scan images of Fluo-4-loaded wt and RyR2 KO cardiomyocytes; the arrows point to local Ca2+ releases (i.e., Ca2+ sparks) preceding two spontaneous global Ca2+ transients. In KO cardiac cells no sparks were detected. (b) Examples of line traces from wt (black) and KO (red) cells. Both traces had similar Ca2+ signal amplitudes (F/F0); however, the time course of the Ca2+ transients markedly differed. (c) Amplitude (F/F0) of spontaneous contractions for wt (n = 10) and KO cells (n = 12). (d and e) Kinetics of the Ca2+ transient in wt and KO cells loaded with Indo-1 AM and externally paced at 0.5 Hz before and following ryanodine (Ry); time-to-peak, the time to the maximum point of the signal; T-50%, the time to 50% decay of the maximum amplitude. *, P < 0.05.
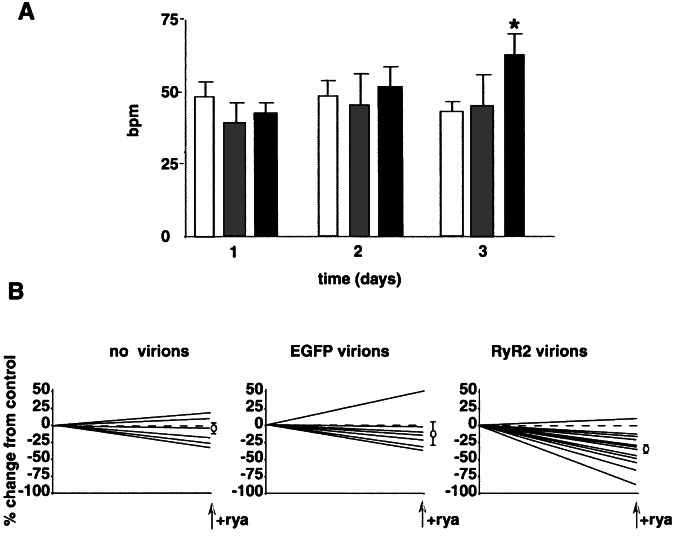
To ensure that the rate differences were specifically due to the functional KO of RyR2 and not to other genetic or epigenetic abnormalities associated with the production of these cells, experiments were designed to rescue the null phenotype by using HSV-1 amplicon virions containing either EGFP or full-length RyR2 cDNA. Four days after HSV-1 amplicon infections, the maximum number of cells infected with the EGFP-containing virions was ≈37%. Two days after infection with the RyR2-containing virions, many cells died; however, some surviving cells showed steady increases in the beating rate (42 ± 4 bpm vs. 62 ± 7 bpm, P < 0.05; Fig. 5A). Neither the noninfected cells nor the EGFP-virion infected cells showed signs of cytotoxicity or any increases in beating rate, which is what would be expected when using this virion system. Cell death in the RyR2 virion group was therefore not due to virus infection, but most likely can be attributed to overexpression of RyR2, and possible calcium overload, which is well known to lead to cell death. As in wt cells, ryanodine markedly inhibited the spontaneous rates of contraction of rescued KO cells (P < 0.005). No significant effect of ryanodine could be demonstrated on noninfected or EGFP-infected KO cells (P > 0.05; Fig. 5B). Rescue of KO cells by RyR2-containing virions establishes that RyR2 is critical for SR Ca2+ release in ES cell-derived cardiomyocytes, and that its function is essential for regulation of the intrinsic beating rate.
Figure 5.
Rescue of RyR−/− cardiomyocytes with helper virus-free HSV-1 amplicons containing full-length rabbit RyR2 cDNA constructs. Individual beating areas were identified and monitored for up to 4 days after infection. (A) Change in beating rate plotted against time postinfection for no virion (open bar). EGFP vivion (gray), and RyR2 virion (black). (B) Effects of treating infected cells with ryanodine (10 μM) on days 3 and 4 postinfection. Measurements were made before and 2 h after exposure to drug. Data are expressed as a percent change from the initial measured rate, and mean percentage changes are shown to the right of each graph. The no virion control (mean change, −7.5 ± 7.9) and EGFP virion treated (−10.1 ± 17.1) cells displayed a negligible response to ryanodine. The RyR2 virion-treated cells had a significant reduction in beating rate (−34.0 ± 6.4). *, P < 0.05 vs. day-1 values.
Discussion
In the adult heart, RyR-mediated Ca2+ release has a role in governing not only the strength of contraction but also the beating rate. The coupling of electrical excitation of the heart to the production of contraction (EC coupling) involves the interaction of a number of cellular proteins to regulate cytosolic calcium. In adult ventricles, Ca2+ enters cardiomyocytes following the AP upstroke through depolarization-activated Ca2+ channels. Entry Ca2+ triggers Ca2+ release from SR through RyRs, which serves as the major SR calcium release channel to mediate a rapid rise of cytosolic free Ca2+ in heart (24, 25). The combination of Ca2+ influx and release increases the free cytosolic Ca2+ concentration, permitting Ca2+ binding to the troponin complex and activation of the contractile machinery. For relaxation to occur, primarily four proteins remove cytosolic Ca2+: the SR Ca2+-ATPase, sarcolemmal Na+/Ca2+ exchanger, sarcolemmal Ca2+-ATPase, or the mitochondrial Ca2+ uniport (26). In contrast to adult ventricular cells, RyR Ca2+ release in adult sinoatrial node cells precedes the AP and is thought to modulate the slope of diastolic depolarization.
Our results with KO ES cells and wt cells treated with ryanodine show that RyR2 is critical to the modulation of beating rate. We interpret our results to indicate that the primary purpose of local Ca2+ elevations during diastolic depolarization before an AP is to activate the Na+/Ca2+ exchanger, a protein expressed abundantly in early rodent heart and early ES cell-derived cardiomyocytes (27, 28). The increased inward Na+/Ca2+ exchanger current would accelerate the rate of spontaneous membrane depolarization before an AP (2). These results suggest that RyR2 and Na+/Ca2+ exchanger act as partners to regulate heart rate during development. Although loss of RyR2 decreases the beating rate in ES cell-derived cardiomyocytes, loss of the NCX1 gene is phenotypically more pronounced and leads to a complete cessation of spontaneous beating in heart (29). The Na+/Ca2+ exchanger is therefore required for beating and proper cardiac development, but the action of the RyR2 is essential to modulate the beating rate. In vivo, loss of RyR2 and, consequently, loss of Na+/Ca2+ exchanger activation should therefore result in a slower heart rate, reducing the cardiac output and thus reducing blood perfusion.
The present results not only show the importance of functional RyR2 to the modulation of beating rate, but also to the Ca2+ release following the AP. Takeshima et al. (4) suggested that RyRs do not regulate calcium release in early embryonic hearts, and that embryonic lethality of RyR2 knockout mice could be attributed to cardiomyocyte dysfunction resulting from abnormal calcium homeostasis. This finding was based on speculation that RyR2 is required as a major Ca2+ leak channel but not as a functional calcium release channel. The presence of calcium sparks and the results with thapsigargin and ryanodine indicate that wt ES cell-derived cardiomyocytes have functional SR and some form of SR calcium release. In RyR2−/− cardiomyocytes, calcium transients are also markedly prolonged, and although the peaks are similar to those from wt cells, the peak is achieved only at a very slow (unphysiologic) rate. These findings are consistent with previous observations in vitro (12). The in vivo results of Moorman et al. (3), who demonstrated the presence of a functional SR in the atrial, but not out-flow regions of the embryonic rat heart, together with the in vitro results provided here suggest that SR dependence of the atrial region and the effects of RyR2 on beating rate and calcium transients might account for the embryonic lethality seen in RyR2 KO mice.
Our in vitro data therefore support four major conclusions. First, RyRs are functional calcium release channels in early wt ES cell-derived cardiomyocytes. Second, the absence of Ca2+ sparks in the KO cells and the fact that the observed characteristics of KO cells are largely indistinguishable from those observed in wt ES cell-derived cardiomyocytes in the presence of ryanodine demonstrates that SR calcium release in wt cardiomyocytes is almost exclusively due to calcium release through RyR2, and not any other RyR isoforms. Third, RyR2 expression is essential to increase the rate of beating in ES cell-derived cardiomyocytes, and as such, would be expected to regulate the rate of beating in developing myocardium. Fourth, the slowing of the rate of spontaneous diastolic depolarization in KO myocytes is consistent with a mechanism that couples preaction potential SR calcium release to activation of an inward current, which in adult pacemaker cells is the Na+/Ca2+ exchanger (2). RyR2, therefore, is not necessary for the in vitro differentiation of ES cells to early pacemaker-like and specialized cardiomyocytes, but RyR2-mediated calcium release during development is critical to the modulation of beating rate of cardiomyocytes and for a physiologic Ca2+ transient following excitation.
Acknowledgments
We thank Dan Riordon and Lydia O'Neill for technical assistance.
Abbreviations
- RyR
ryanodine receptor
- ES
embryonic stem
- EB
embryoid body
- KO
knockout
- wt
wild type
- HSV
herpes simplex virus
- SR
sarcoplasmic reticulum
- AP
action potential
- bpm
beats per minute
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Huser J, Blatter L A, Lipsius S L. J Physiol (London) 2000;524:415–422. doi: 10.1111/j.1469-7793.2000.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdanov K Y, Vinogradova T M, Lakatta E G. Circ Res. 2001;88:1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- 3.Moorman A F, Schumacher C A, de Boer P A, Hagoort J, Bezstarosti K, van den Hoff M J, Wagenaar G T, Lamers J M, Wuytack F, Christoffels V M, Fiolet J W. Dev Biol. 2000;223:279–290. doi: 10.1006/dbio.2000.9752. [DOI] [PubMed] [Google Scholar]
- 4.Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. EMBO J. 1998;17:3309–3316. doi: 10.1093/emboj/17.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorza L, Vettore S, Tessaro A, Sorrentino V, Vitadello M. J Mol Cell Cardiol. 1997;29:1023–1036. doi: 10.1006/jmcc.1996.0346. [DOI] [PubMed] [Google Scholar]
- 6.Patten B M. Phys Rev. 1949;29:31–47. doi: 10.1152/physrev.1949.29.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Keller B B, MacLennan M J, Tinney J P, Yoshigi M. Circ Res. 1996;79:247–255. doi: 10.1161/01.res.79.2.247. [DOI] [PubMed] [Google Scholar]
- 8.Moorman A F, de Jong F, Denyn M M, Lamers W H. Circ Res. 1998;82:629–644. doi: 10.1161/01.res.82.6.629. [DOI] [PubMed] [Google Scholar]
- 9.Metzger J M, Samuelson L C, Rust E M, Westfall M V. Trends Cardiovasc Med. 1997;7:63–68. doi: 10.1016/S1050-1738(96)00138-7. [DOI] [PubMed] [Google Scholar]
- 10.Wobus A M, Guan K, Yang H-T, Boheler K R. Methods Mol Biol (Totowa, NJ) 2002;185:127–156. doi: 10.1385/1-59259-241-4:127. [DOI] [PubMed] [Google Scholar]
- 11.Hescheler J, Fleischmann B K, Lentini S, Maltsev V A, Rohwedel J, Wobus A M, Addicks K. Cardiovasc Res. 1997;36:149–162. doi: 10.1016/s0008-6363(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 12.Sauer H, Theben T, Hescheler J, Lindner M, Brandt M C, Wartenberg M. Am J Physiol. 2001;281:H411–H421. doi: 10.1152/ajpheart.2001.281.1.H411. [DOI] [PubMed] [Google Scholar]
- 13.Wobus A M, Rohwedel J, Maltsev V, Hescheler J. Ann NY Acad Sci. 1995;752:460–469. doi: 10.1111/j.1749-6632.1995.tb17456.x. [DOI] [PubMed] [Google Scholar]
- 14.Viatchenko-Karpinski S, Fleischmann B K, Liu Q, Sauer H, Gryshchenko O, Ji G J, Hescheler J. Proc Natl Acad Sci USA. 1999;96:8259–8264. doi: 10.1073/pnas.96.14.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maltsev V A, Wobus A M, Rohwedel J, Bader M, Hescheler J. Circ Res. 1994;75:233–244. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- 16.Maltsev V A, Rohwedel J, Hescheler J, Wobus A M. Mech Dev. 1993;44:41–50. doi: 10.1016/0925-4773(93)90015-p. [DOI] [PubMed] [Google Scholar]
- 17.Fassler R, Rohwedel J, Maltsev V, Bloch W, Lentini S, Guan K, Gullberg D, Hescheler J, Addicks K, Wobus A M. J Cell Sci. 1996;109:2989–2999. doi: 10.1242/jcs.109.13.2989. [DOI] [PubMed] [Google Scholar]
- 18.Martin X J, Schwartz K, Boheler K R. J Mol Cell Cardiol. 1995;27:589–597. doi: 10.1016/s0022-2828(08)80053-3. [DOI] [PubMed] [Google Scholar]
- 19.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 20.Fraefel C, Song S, Lim F, Lang P, Yu L, Wang Y, Wild P, Geller A I. J Virol. 1996;70:7190–7197. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham C, Davison A J. Virology. 1993;197:116–124. doi: 10.1006/viro.1993.1572. [DOI] [PubMed] [Google Scholar]
- 22.Spurgeon H A, Stern M D, Baartz G, Raffaeli S, Hansford R G, Talo A, Lakatta E G, Capogrossi M C. Am J Physiol. 1990;258:H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H, Cannell M B, Lederer W J. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 24.Marks A R. Am J Physiol. 1997;272:H597–H605. doi: 10.1152/ajpheart.1997.272.2.H597. [DOI] [PubMed] [Google Scholar]
- 25.Bers D M, Perez-Reyes E. Cardiovasc Res. 1999;42:339–360. doi: 10.1016/s0008-6363(99)00038-3. [DOI] [PubMed] [Google Scholar]
- 26.Bers D M. Nature (London) 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 27.Koban M U, Moorman A F, Holtz J, Yacoub M H, Boheler K R. Cardiovasc Res. 1998;37:405–423. doi: 10.1016/s0008-6363(97)00276-9. [DOI] [PubMed] [Google Scholar]
- 28.Koushik S V, Bundy J, Conway S J. Mech Dev. 1999;88:119–122. doi: 10.1016/s0925-4773(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 29.Koushik S V, Wang J, Rogers R, Moskophidis D, Lambert N A, Creazzo T L, Conway S J. FASEB J. 2001;15:1209–1211. doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]