Abstract
Insulator DNAs and promoter competition regulate enhancer–promoter interactions within complex genetic loci. Here we provide evidence for a third mechanism: promoter-proximal tethering elements. The Scr-ftz region of the Antennapedia gene complex includes two known enhancers, AE1 and T1. AE1 selectively interacts with the ftz promoter to maintain pair-rule stripes of ftz expression during gastrulation and germ-band elongation. The T1 enhancer, located 3′ of the ftz gene and ≈25 kb 5′ of the Scr promoter, selectively activates Scr expression in the prothorax and posterior head segments. A variety of P element minigenes were examined in transgenic embryos to determine the basis for specific AE1-ftz and T1-Scr interactions. A 450-bp DNA fragment located ≈100 bp 5′ of the Scr transcription start site is essential for T1-Scr interactions and can mediate long-range activation of a ftz/lacZ reporter gene when placed 5′ of the ftz promoter. We suggest that the Scr450 fragment contains tethering elements that selectively recruit T1 to the Scr promoter. Tethering elements might regulate enhancer–promoter interactions at other complex genetic loci.
Previous studies have identified two different mechanisms for regulating enhancer–promoter interactions within complex genetic loci: promoter competition and insulator DNAs (for reviews, see refs. 1 and 2). In the case of competition, a shared enhancer selectively interacts with the strongest of linked promoters (3, 4). This preferred interaction sequesters the enhancer so it is unavailable for interacting with weaker promoters. Insulator DNAs selectively block the interaction of a distal enhancer with a target promoter when the insulator is positioned between the two (reviewed in ref. 5). This block does not interfere with the activation of proximal genes by the same enhancer. Here we present evidence for a third mechanism of regulating enhancer–promoter interactions: promoter-proximal tethering elements.
The Antennapedia gene complex (ANT-C) is one of the two major Hox gene clusters in the Drosophila genome (summarized in Fig. 1). It is ≈500 kb in length and contains nine homeobox genes, including five homeotic selector genes that pattern the head and thorax (6, 7). The Scr selector gene is expressed in the anterior compartment of the first thoracic segment, as well as portions of the labial and maxillary head segments. This complex pattern of Scr expression depends, at least in part, on a distal enhancer, T1, that is located ≈25 kb 5′ of the Scr promoter (8). T1 is located downstream of the pair-rule gene ftz, which exhibits a seven-stripe pattern of expression distinct from the Scr gene. The ftz pattern is regulated by an intergenic enhancer, AE1, located between the divergently transcribed Scr and ftz genes (9, 10). Specific T1-Scr and AE1-ftz interactions are essential for the normal patterning of the early embryo. Misexpression of either gene disrupts segmentation and causes embryonic lethality (11, 12).
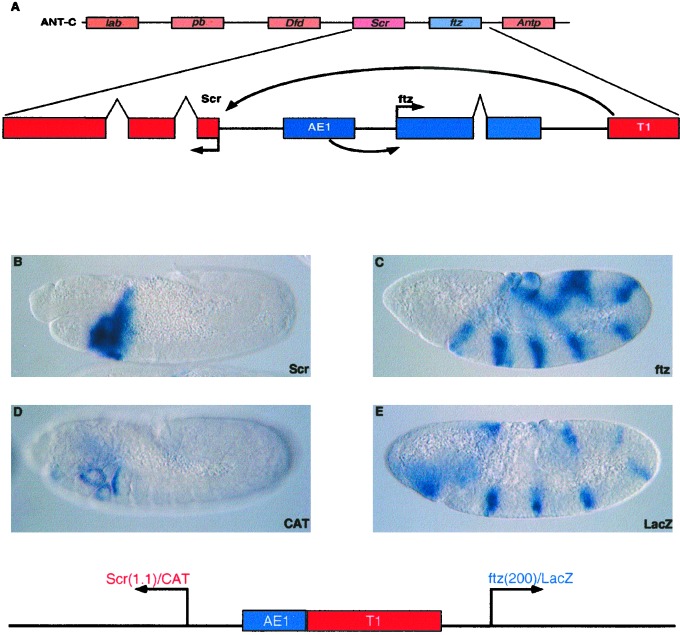
Figure 1.
Summary of the Scr-ftz region of the ANT-C. (A) The diagram (Upper) shows the order of the five Hox-containing homeotic selector genes. The pair-rule gene ftz is also indicated. The second diagram presents an enlarged view of the Scr-ftz interval. The two genes are divergently transcribed, and the intron-exon organization of each gene is indicated. The AE1 enhancer is located in the intergenic region between Scr and ftz but selectively interacts with the ftz promoter to maintain the seven-stripe expression pattern in embryos undergoing germ-band elongation. Scr expression depends on the T1 enhancer, which is located 3′ of the ftz transcription unit. (B) Scr expression pattern in an 8- to 9-hr embryo undergoing germ-band retraction. Staining was visualized after hybridization with a digoxigenin-labeled Scr antisense RNA probe. Expression is detected in different tissues of parasegment 2, which includes the posterior-most regions of the head and the anterior compartment of the prothorax. (C) ftz expression pattern in a 4- to 5-hr elongating embryo. Staining is detected in seven stripes along the germ band. Staining was visualized after hybridization with a digoxigenin-labeled ftz antisense RNA probe. (D and E) Transgenic embryos were obtained from adults containing the P element transformation vector indicated in the diagram beneath the photomicrographs. Divergently transcribed CAT and lacZ reporter genes were placed under the control of the Scr and ftz promoter regions, respectively. The Scr promoter region is 1.1 kb in length and includes ≈555 bp 5′ of the transcription start site. The ftz promoter region is 200 bp and includes ≈100 bp of 5′ flanking sequence. The 430-bp AE1 enhancer and the 3.8-kb T1 enhancer were placed between the CAT and lacZ reporter genes. The leftward CAT gene is activated by the T1 enhancer in posterior head segments and the anterior compartment of the prothorax, similar to the endogenous Scr expression pattern (compare D with B). The lacZ reporter gene exhibits seven stripes of ftz expression, similar to endogenous ftz expression (compare E with C). CAT and lacZ expression patterns were visualized after hybridization with digoxigenin-labeled CAT or lacZ antisense RNA probes.
Previous studies have shown that AE1 prefers TATA-containing promoters (13). This observation suggests that AE1-ftz specificity is governed by promoter competition; the native ftz promoter is stronger than the Scr promoter. However, this type of simple competition mechanism cannot account for specific T1-Scr interactions. The distal T1 enhancer bypasses the strong proximal ftz promoter to activate the weak Scr promoter. Analysis of chimeric Scr-ftz promoter sequences identified a 450-bp DNA fragment that facilitates T1-Scr interactions. This fragment is located immediately 5′ of the Scr promoter and permits T1 to activate a ftz-lacZ reporter gene when placed 5′ of the ftz promoter. Scr450 does not foster AE1-ftz or AE1-Scr interactions. We propose that Scr450 contains “tethering” elements that selectively recruit the distal T1 enhancer. It is conceivable that tethering represents a common mechanism for regulating enhancer–promoter interactions within complex genetic loci.
Materials and Methods
P-Transformation Assays.
yw67 flies were used for all P-transformation assays. Fusion genes were introduced into the Drosophila germ line by using standard methods (14). Multiple transformants were generated for each construct, and at least three independent lines were examined by in situ hybridization. Embryos were collected, fixed, and hybridized with digoxigenin-labeled CAT and lacZ probes, as previously described (15, 16).
Preparation of Enhancers and Promoters.
The AE1 enhancer is located ≈2.5 kb upstream of the ftz transcription start site (10). This 430-bp fragment was isolated from genomic DNA by conventional PCR methods and cloned into the XbaI site of a p-Bluescript vector, modified with AscI sites flanking the polylinker. The T1 enhancer is located ≈4 kb downstream of the ftz coding region (8). This 3.8-kb fragment was isolated from the genome by PCR and cloned into the HindIII site of the AscI modified p-Bluescript vector.
The initial Scr promoter used in this study is 1.1 kb in length and includes 555 bp of 5′ flanking sequence and 590 bp of 3′ sequence (17). Scr promoter deletions were generated by PCR. Two hundred twenty-five base pairs of 5′ flanking sequence was removed from the 1.1-kb promoter to generate Scr900. Another 225 bp of 5′ sequence was deleted to generate Scr700. The minimal core Scr promoter is just 80 bp in length and extends from −36 to +38. The Scr downstream promoter element (DPE) is located from +28 to +33 (GCACGT) and is a 6-for-6 match to the DPE consensus: (A/G/T)(C/G)(A/T)(C/T)(A/C/G)(C/T) (18). The mutagenized Scr chimera containing a TATA box was created by using a mutagenic oligonucleotide that converts the sequence TGATGCTCA (−31 to −23) to GTATAAAAG. To replace the Scr DPE with the corresponding sequence from ftz, a mutagenic oligonucleotide was used that changed GCACGT to ACATCG.
The ftz promoter used in Fig. 1 is ≈200 bp in length and extends from −107 to +91 (19). Subsequent experiments used a smaller ftz promoter that extends 5 bp upstream of the TATA box (TATATA) to 5 bp downstream of the DPE (ACATCG). For the ftz chimera lacking a TATA element, a mutagenic oligonucleotide was used to replace the sequence TATATA to GATGCT. Likewise, a mutagenic primer was used to replace the ftz DPE with the corresponding sequence from Scr. For the ftzScr450 chimera, a hybrid primer (5′-GCCTTACTTGCTCGTACTCGCTTTGCTATATATGCAGGATCTGCCG-3′) was used to fuse the Scr450 tethering element (−555 to −102 upstream of Scr +1) to the minimal ftz core promoter.
Construction of P Element Transposons.
The CAT/lacZ P-transformation vector that was used for all of the experiments presented in this study is a modification of pCasPer, which contains divergently transcribed white and lacZ reporter genes (14). It was modified by insertion of a CAT reporter gene between white and lacZ (13).
Promoters were isolated as AscI-BamHI fragments and cloned into a unique BamHI site located at the 5′ end of either the CAT or lacZ coding sequence present in p-Bluescript vectors. The CAT fusion genes were subsequently isolated as AscI-NotI fragments and used to replace the AscI-NotI CAT fragment in the pCasPer vector. The lacZ fusion genes were isolated as AscI-XbaI fragments and used to replace the AscI-XbaI lacZ fragment in the pCasPer vector. The AE1 and T1 enhancers were isolated as an AscI fragment and cloned into the unique AscI site located between the divergently transcribed reporter genes.
For the long-range tethering constructs, a 1.6-kb spacer from bacteriophage λ was isolated as an AscI fragment and cloned into the unique AscI site in pCasPer. The T1 enhancer was modified with flanking SbfI sites and cloned into the unique PstI site located downstream of the lacZ reporter gene.
Results and Discussion
The organization and expression of the Scr-ftz region of the ANT-C is summarized in Fig. 1. The Scr and ftz genes are divergently transcribed, and the two promoters are separated by a ≈15-kb intergenic region that contains the AE1 enhancer. AE1 selectively interacts with the ftz promoter and does not regulate Scr (10, 20). One of the major enhancers regulating Scr expression, T1, is located 3′ of the ftz transcription unit and maps ≈25 kb upstream of the Scr promoter (8). Scr expression is first detected at the onset of gastrulation and persists in the posterior head segments and prothorax during germ-band elongation, retraction, and segmentation (Fig. 1B). In contrast, ftz expression is detected before the completion of cellularization and exhibits seven stripes of expression during gastrulation and germ-band elongation (Fig. 1C). These distinct patterns of expression depend on selective AE1-ftz and T1-Scr enhancer–promoter interactions (Fig. 1A).
To identify the cis-regulatory elements responsible for this specificity, the Scr and ftz promoter regions were attached to divergently transcribed CAT and lacZ reporter genes (Fig. 1 D and E). The Scr promoter region is 1.1 kb in length and includes ≈555 bp of 5′ flanking sequence. The ftz promoter region includes just 200 bp and contains 105 bp of 5′ flanking sequence. The AE1 and T1 enhancers were placed between the two reporter genes. The T1 enhancer fails to activate the proximal ftz-lacZ gene but directs strong CAT staining in the maxillary and labial head segments, as well as the anterior compartment of the prothorax (Fig. 1D). In contrast, although closer to the leftward Scr-CAT gene, AE1 specifically interacts with the rightward ftz-lacZ gene to direct seven stripes of lacZ expression in embryos undergoing germ-band elongation (Fig. 1E). The CAT and lacZ staining patterns mimic the endogenous Scr and ftz expression patterns (Fig. 1 D and E; compare with Fig. 1 B and C), indicating that the Scr and ftz promoter sequences are sufficient to reproduce authentic regulatory specificity in the Scr-ftz region.
Core promoter sequences were altered to determine whether T1-Scr and AE1-ftz specificity depends on TFIID recognition elements, such as TATA and the DPE. The native Scr and ftz promoters are quite distinct. ftz contains a strong TATA element and a 5-of-6 match to the DPE consensus sequence. Conversely, Scr lacks TATA but contains a 6-of-6 match to the DPE consensus (18, 21). Chimeric Scr-ftz promoters were created to investigate the role of TFIID recognition elements. Core promoter elements from ftz were replaced with the corresponding regions from Scr. Similarly, constructs were built replacing the Scr core elements with those from ftz. However, none altered AE1-ftz and T1-Scr specificity (data not shown). These results are in contrast to previous studies where TFIID recognition elements direct enhancer targeting (13, 22) and suggest specificity is mediated by elements outside of the core promoter.
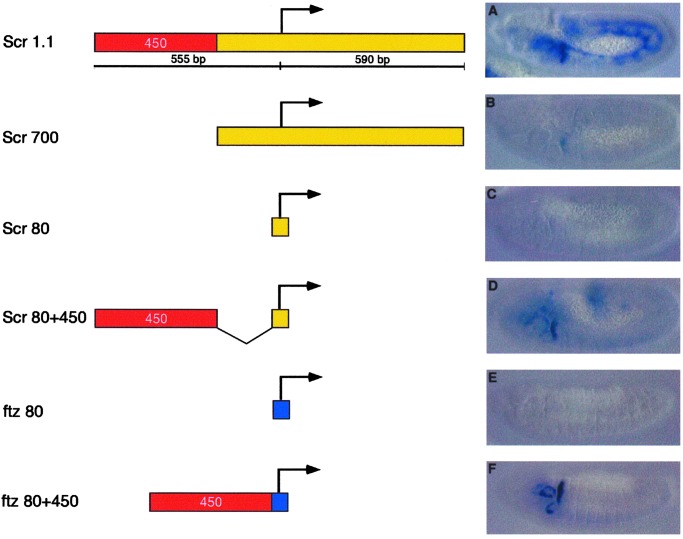
A series of truncated Scr promoter sequences were analyzed in an effort to identify the region(s) responsible for activation by T1 (Fig. 2). The most minimal Scr promoter identified that exhibited normal activation by T1 is just 530 bp in length. It includes a core 80-bp Scr promoter and a 450-bp DNA fragment from the 5′ flanking region (Scr80 + 450; see Fig. 2D). Only weak staining is obtained with the Scr700 promoter sequence (Fig. 2B), which lacks the 5′ 450-bp DNA fragment (“Scr450”), whereas a minimal core promoter (“Scr80”) exhibits no T1 expression (Fig. 2C).
Figure 2.
Identification of promoter-proximal tethering elements. The T1 enhancer was placed 5′ of a series of truncated Scr-lacZ fusion genes. The “full-length” Scr promoter region is 1.1 kb in length and includes 555 bp of 5′ flanking sequence (A). Truncations that remove a 450-bp fragment exhibit diminished levels of expression (B and C). This 450-bp fragment is sufficient to stimulate expression from the minimal Scr core promoter (Scr80 + 450; D). Inserting this fragment immediately upstream of the heterologous ftz promoter is sufficient to drive strong T1 expression (ftz80 + 450; F) on an otherwise unresponsive promoter (ftz80; E).
To determine whether Scr450 is sufficient to recruit the T1 enhancer to a heterologous promoter, Scr450 was placed upstream of a core ftz promoter that includes just 5 bp 5′ of TATA and extends 5 bp downstream of the DPE. The T1 enhancer activates this promoter and directs strong expression of the rightward ftz-lacZ reporter gene within the prothorax (Fig. 2F; compare with Fig. 2E). This observation suggests that Scr450 can selectively recruit the T1 enhancer to an adjacent promoter. AE1-ftz interactions are not augmented by the presence of Scr450 (see below).
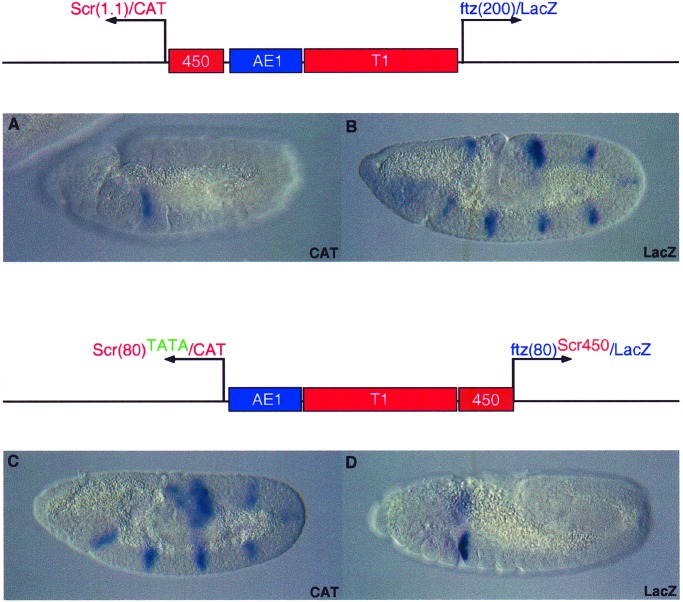
To determine whether a regulatory swap in the activities of the AE1 and T1 enhancers can be achieved, Scr450 was removed from the Scr promoter, and the core elements were modified within Scr80 to include a consensus TATA element. The T1 enhancer no longer activates this promoter. Instead, it is efficiently activated by AE1 (Fig. 3C). The leftward Scr-CAT gene exhibits seven pair-rule stripes of expression, and the rightward ftz-lacZ gene, which contains Scr450, is expressed in the prothorax and posterior head segments (Fig. 3D). As mentioned earlier, the addition of TATA alone is not sufficient for AE1 activation of Scr. In this particular configuration of enhancers, T1 is located closer to the ftz promoter than AE1. When T1 is removed from this construct, AE1 activation of ScrTATA is greatly diminished, suggesting that the T1 enhancer might possess slight enhancer blocking activity (data not shown). This blocking activity, coupled with the strengthening of Scr by inserting an optimal TATA element, permits AE1 to activate the Scr/CAT reporter gene. This result reinforces the idea that AE1 works through promoter competition (13). The enhancer blocking activity of T1 serves to weaken the ftz promoter, whereas adding TATA makes Scr stronger. The regulatory swap is nearly complete. T1 specificity is inverted; only expression from ftz/lacZ is observed. The modifications above, however, do not permit a complete swap in AE1 activity, as both promoters are activated by AE1 (data not shown).
Figure 3.
Swapping regulatory specificity in the Scr-ftz region. Transgenic embryos express the P element minigenes indicated above the photomicrographs. The AE1 enhancer selectively activates the rightward ftz/lacZ reporter gene, and T1 activates the leftward Scr/CAT gene (A and B; also see Fig. 1 D and E). Regulatory specificity is reversed on modification of the Scr and ftz promoters (C and D). The Scr450 tethering fragment was removed from the 5′ region of Scr and attached to the minimal ftz80 promoter. In addition, the minimal Scr promoter was modified to include an optimal TATA element. The AE1 enhancer now strongly activates the leftward Scr promoter, and induces seven stripes of CAT expression along the germ band. Conversely, the T1 enhancer selectively interacts with the rightward ftz/lacZ fusion gene and activates lacZ expression in the prothorax and weakly in posterior regions of the head. AE1 expression of Scr/CAT depends on the presence of T1 between AE1 and ftz/lacZ. Removal of T1 results in greatly diminished Scr/CAT staining.
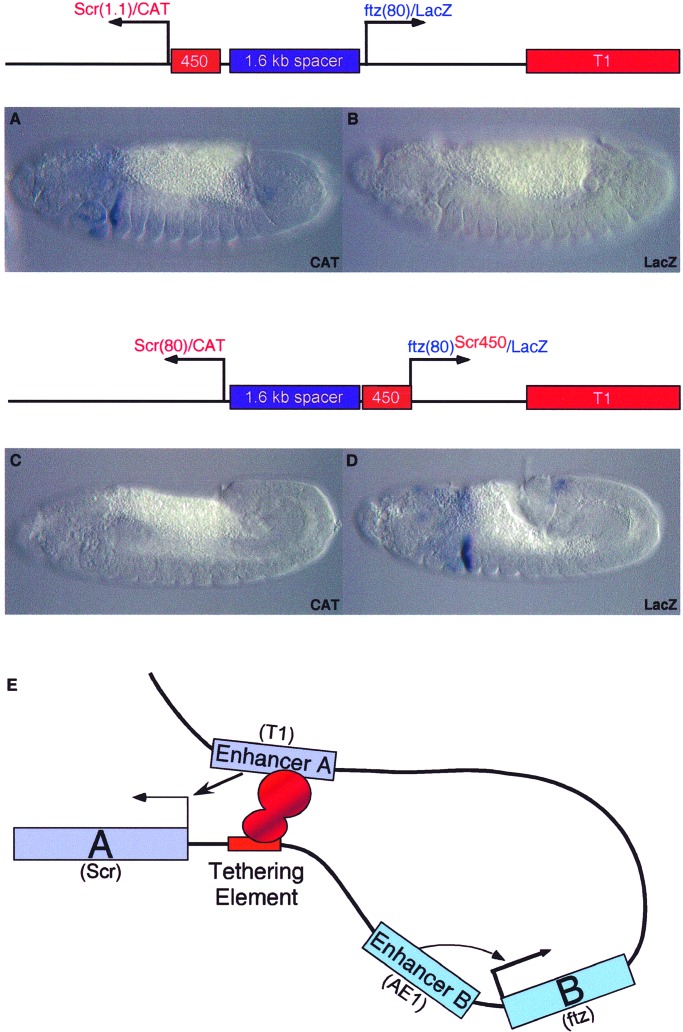
As discussed earlier, the T1 enhancer is located ≈25 kb 5′ of the Scr promoter. Additional experiments were done to determine whether Scr450 can recruit T1 over long distances to different target promoters (Fig. 4). T1 was placed downstream of divergently transcribed CAT and lacZ reporter genes that contain minimal Scr and ftz core promoters, respectively. The two promoters were separated by a 1.6-kb spacer DNA from bacteriophage λ. The T1 enhancer fails to activate either the distal CAT gene or the proximal ftz-lacZ gene (Fig. 4 B and C). In this configuration, T1 maps ≈6 kb 5′ of the Scr-CAT reporter gene. Modification of the distal reporter gene to include the Scr450-bp fragment results in strong activation of Scr-CAT within posterior head segments and the prothorax (Fig. 4A). The proximal ftz-lacZ gene remains silent (Fig. 4B). However, insertion of Scr450 5′ of the ftz-lacZ reporter gene results in the selective activation of lacZ expression in the prothorax (Fig. 4D). Preliminary results show that long-range tethering can be achieved with the Scr80 + 450 promoter, similar to that seen for Scr1.1 (data not shown). These results suggest that Scr450 is sufficient to recruit the distal T1 enhancer to a target promoter.
Figure 4.
Scr tethering elements mediate long-range enhancer–promoter interactions. Transgenic embryos express the Pelement minigenes indicated in the diagrams. In all cases, the T1 enhancer was placed 3′ of the lacZ reporter gene. Minimal Scr and ftz promoters are not activated by T1, which now maps ≈5 kb from the ftz promoter and more than 6 kb from Scr. The 3′ T1 enhancer activates the distal Scr/CAT reporter gene when 5′ tethering elements are included in the Scr promoter region (A). The proximal ftz/lacZ gene remains silent (B). Removal of the 5′ tethering elements from the Scr promoter region causes a loss in CAT expression (C). However, when the Scr450 fragment is placed 5′ of the ftz promoter, the 3′ T1 enhancer now activates the proximal lacZ reporter gene (D). E summarizes the model for enhancer tethering, whereby promoter-proximal elements selectively recruit a specific distal enhancer (Enhancer A, T1). Perhaps regulatory proteins bound to both the distal enhancer and the proximal tethering elements form homomeric complexes that stabilize enhancer-promoter loops. Enhancer B (AE1) is regulated through promoter competition, selectively interacting with the stronger promoter.
We propose that the promoter-proximal Scr450 DNA fragment contains tethering elements that specifically recruit the distal T1 enhancer to the Scr promoter but do not influence the activities of other enhancers located in the ANT-C, such as AE1 (summarized in Fig. 4E). It is conceivable that specific T1-Scr450 interactions depend on the homotypic association of common proteins bound to both the enhancer and promoter-proximal DNA. A similar mechanism might be used by other complex loci. For example, GATA and other transcription factors are known to bind within the distal locus-control region (LCR) as well as promoter-proximal regions of globin genes (23, 24). Perhaps GATA–GATA interactions facilitate long-range interactions between enhancers contained within the LCR and globin promoters.
Previous tissue culture assays have identified a number of regulatory proteins that bind to promoter-proximal DNA sequences located just 5′ of the core promoter. Some of these proteins are unable to function over long distances, but instead they stimulate transcription only when bound near the core promoter (25–27). We suggest that these proteins might not function as “classical” activators, which recruit transcription complexes (28). Instead, they might augment transcription indirectly by functioning as tethers for distal enhancers. In this regard, we note that the promoter-proximal protein Sp1 can mediate the formation of DNA loops via homotypic interactions when bound to both distal and promoter-proximal binding sites (29).
Tethering elements represent a flexible and specific mechanism for regulating enhancer–promoter interactions in complex genetic loci. For example, an insulator DNA located between AE1 and the Scr promoter would block both the heterologous AE1 enhancer as well as the cognate T1 enhancer. It was recently proposed that core promoters can possess distinct regulatory activities, whereby DPE-containing promoters interact with some enhancers, while TATA-containing promoters interact with others (22, 30). However, this mechanism might not play a critical role in regulating AE1-ftz and T1-Scr interactions. Genetic studies are consistent with the possibility that tethering elements are used by other Hox loci, including the Drosophila Abd-B locus (31).
Acknowledgments
We thank Robert Drewell for comments on the manuscript and Sumio Ohtsuki for technical assistance and advice. This work was funded by a grant from the National Institutes of Health (GM 34431).
Abbreviation
- DPE
downstream promoter element
References
- 1.Dorsett D. Curr Opin Genet Dev. 1999;9:505–514. doi: 10.1016/s0959-437x(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 2.Blackwood E M, Kadonaga J T. Science. 1998;281:61–63. doi: 10.1126/science.281.5373.60. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe J, Nonchev S, Gould A, Whiting J, Krumlauf R. EMBO J. 1998;17:1788–1798. doi: 10.1093/emboj/17.6.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley K P, Engel J D. Genes Dev. 1992;6:730–744. doi: 10.1101/gad.6.5.730. [DOI] [PubMed] [Google Scholar]
- 5.West A G, Gaszner M, Felsenfeld G. Genes Dev. 2002;16:271–288. doi: 10.1101/gad.954702. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman T C, Seeger M A, Olsen G. Adv Genet. 1990;27:309–362. doi: 10.1016/s0065-2660(08)60029-2. [DOI] [PubMed] [Google Scholar]
- 7.Adams M D, Celniker S E, Holt R A, Evans C A, Gocayne J D, Amanatides P G, Scherer S E, Li P W, Hoskins R A, Galle R F, et al. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 8.Gindhart J G, Jr, King A N, Kaufman T C. Genetics. 1995;139:781–795. doi: 10.1093/genetics/139.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pick L, Schier A, Affolter M, Schmidt-Glenewinkel T, Gehring W J. Genes Dev. 1990;4:1224–1239. doi: 10.1101/gad.4.7.1224. [DOI] [PubMed] [Google Scholar]
- 10.Schier A F, Gehring W J. Nature (London) 1992;356:804–807. doi: 10.1038/356804a0. [DOI] [PubMed] [Google Scholar]
- 11.Gibson G, Schier A, LeMotte P, Gehring W J. Cell. 1990;62:1087–1103. doi: 10.1016/0092-8674(90)90386-s. [DOI] [PubMed] [Google Scholar]
- 12.Struhl G. Nature (London) 1985;318:677–680. doi: 10.1038/318677a0. [DOI] [PubMed] [Google Scholar]
- 13.Ohtsuki S, Levine M, Cai H N. Genes Dev. 1998;12:547–556. doi: 10.1101/gad.12.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Small S, Blair A, Levine M. EMBO J. 1992;11:4047–4057. doi: 10.1002/j.1460-2075.1992.tb05498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tautz D, Pfeifle C. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Kosman D, Ip Y T, Levine M. Genes Dev. 1991;5:1881–1891. doi: 10.1101/gad.5.10.1881. [DOI] [PubMed] [Google Scholar]
- 17.LeMotte P K, Kuroiwa A, Fessler L I, Gehring W J. EMBO J. 1989;8:219–227. doi: 10.1002/j.1460-2075.1989.tb03367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutach A K, Kadonaga J T. Mol Cell Biol. 2000;20:4754–4764. doi: 10.1128/mcb.20.13.4754-4764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dearolf C R, Topol J, Parker C S. Genes Dev. 1989;3:384–398. doi: 10.1101/gad.3.3.384. [DOI] [PubMed] [Google Scholar]
- 20.Gorman M J, Kaufman T C. Genetics. 1995;140:557–572. doi: 10.1093/genetics/140.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke T W, Kadonaga J T. Genes Dev. 1997;11:3020–3031. doi: 10.1101/gad.11.22.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler J E, Kadonaga J T. Genes Dev. 2001;15:2515–2519. doi: 10.1101/gad.924301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Q, Dean A. Mol Cell Biol. 1993;13:911–917. doi: 10.1128/mcb.13.2.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gourdon G, Morle F, Roche J, Tourneur N, Joulain V, Godet J. Acta Haematol. 1992;87:136–144. doi: 10.1159/000204740. [DOI] [PubMed] [Google Scholar]
- 25.Chen J H, Wright C D. Oncogene. 1993;8:3375–3383. [PubMed] [Google Scholar]
- 26.Di Lisi R, Millino C, Calabria E, Altruda F, Schiaffino S, Ausoni S. J Biol Chem. 1998;273:25371–25380. doi: 10.1074/jbc.273.39.25371. [DOI] [PubMed] [Google Scholar]
- 27.Strom A C, Forsberg M, Lillhager P, Westin G. Nucleic Acids Res. 1996;24:1981–1986. doi: 10.1093/nar/24.11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemon B, Tjian R. Genes Dev. 2000;14:2551–2569. doi: 10.1101/gad.831000. [DOI] [PubMed] [Google Scholar]
- 29.Mastrangelo I A, Courey A J, Wall J S, Jackson S P, Hough P V. Proc Natl Acad Sci USA. 1991;88:5670–5674. doi: 10.1073/pnas.88.13.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smale S T. Genes Dev. 2001;15:2503–2508. doi: 10.1101/gad.937701. [DOI] [PubMed] [Google Scholar]
- 31.Sipos L, Mihaly J, Karch F, Schedl P, Gausz J, Gyurkovics H. Genetics. 1998;149:1031–1050. doi: 10.1093/genetics/149.2.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]