Abstract
Shape-persistent conjugated macrocycles have attracted interest for their unique optoelectronic and self-assembly properties, but the syntheses to obtain these structures can be laborious. In this work, we describe the straightforward synthesis of a recently discovered class of macrocycle, the cyclo[n]furan, using Suzuki–Miyaura cross-coupling of a simple aromatic monomer. We demonstrate that the combination of hexyl 2-bromo-5-(boronic acid pinacol ester)furan-3-carboxylate with tris(dibenzylideneacetone)dipalladium(0), tri-tert-butylphosphonium tetrafluoroborate and cesium fluoride leads to cyclo[6]- and cyclo[7]furan esters in 45% yield (28% and 17%, respectively). Crude 1H NMR spectroscopy revealed that total conversion to macrocycles was 52 ± 6% over 3 runs, highlighting the robustness of this protocol for cyclofuran synthesis. The oligomerizations are rapid, and model compound studies suggest that a chain-growth mechanism may be operative. The hexyl-substituted cyclo[n]furan esters (n = 6 and 7) are separable via column chromatography. The unique optical and electronic features for each cycle can be partially explained by the size difference for the two systems, as well as the increased conformational flexibility for the larger, ester-functionalized cyclo[7]furan.
Introduction
Conjugated cyclic macromolecules comprised only of aromatic rings, have emerged as a novel class of organic electronic material. − Benzene, pyrrole and thiophene have been the most common building blocks to construct macrocyclic derivatives, particularly with no spacers. This includes the meta and para [n]cyclophenylenes ([n]CMPs or [n]CPPs), the cyclo[n]pyrroles (CnPs) , and the cyclo[n]thiophenes (CnTs). − The shape, structure and properties of the macrocycles are dependent on the building block, the number of repeat units, and the substitution pattern of the repeat unit (Figure ), which leads to questions regarding aromaticity, optical/redox properties, and solid-state organization. −
1.

A) Macrocycle families derived from either benzene, pyrrole or thiophene repeat units. − B) Recently reported cyclo[n]furans. −
Cyclo[n]furans (CnFs) have been less studied, which may be due to questions regarding the oxidative stability of long chain conjugated oligo- and polyfurans, − and the potential of furans to undergo cycloaddition chemistry. , Nevertheless, furan is a unique building block for the construction of macrocycles as demonstrated by Gidron and co-workers in a computational study in 2018. CnFs comprised of 6–8 repeat units were predicted to have the lowest ring strain, which results in planar or near planar macrocycles with all the furan repeat units adopting a syn conformation with respect to one another. This is markedly different than the analogous CnTs, where 11–15 repeat units leads to low strain energies. , Moreover, CnTs with 11–15 repeat units are still predicted to be distorted. The different internal angles for furans versus thiophenes, leads to the prediction of small, compact CnFs, and the high twist energies around the inter-ring bond in oligofurans, , promotes planar or near-planar structures.
To partially combat the oxidative instability of oligofurans, electron-withdrawing groups can be installed on the furan repeat unit, which was critical to the first reports on cyclo[n]furans. − Gidron and co-workers first reported on the cyclo[n]bifuran diimides in 2019, with recent follow-ups making different sized macrocyclic rings in 2021, and reducing the imide side groups of the bifuran in 2022 (Figure – Bottom). − Our group reported on the isolation of a hexyl-cyclo[6]furan ester (hex-C6FE) in 2021, starting from a regioregular ester-substituted furan dimer (Figure – Bottom), which was obtained from 3-furoic acid in 7% overall yield over the 8 synthetic steps. Alternating furan-acetylene and furan-thiophene macrocycles have also been prepared recently.
Herein, we describe a general approach for cyclo[n]furan ester (CnFE) synthesis, starting from a single furan monomer (Figure ). In addition, an odd-membered furan macrocycle has been isolated for the first time (hex-C7FE). The method enables rapid production of CnFEs from 3-furoic acid in a total of 4 synthetic steps (24% overall yield from the acid). The macrocyclization reaction is carried out like a polymerization reaction, with two key factors playing a role in obtaining >50% conversion to macrocycles. First, the ester side chain on the furan ring leads to an energetic preference for syn conformers as the chain length increases (Figure ). Second, the precise cross-coupling conditions (tri-tert-butylphosphine, cesium fluoride and palladium precursor) are also critical to the process. Model compound studies suggest that the reaction proceeds via a catalyst-transfer mechanism, − with preferential oxidative addition on the chain-end of the growing oligomer. Protocols to construct precise homocyclic compounds from a single aromatic repeat unit are rare, as conjugated macrocycles are often built via stepwise or iterative coupling. The potential to make precise conjugated macromolecules with specific lengths are highly desirable to control properties and self-assembly. For example, Hawker employed chromatographic separation , to create well-defined libraries of discrete conjugated linear oligomers and Seferos developed a monomer-by-monomer addition reaction to create oligo/polythiophenes of discrete length. ,
2.

Cyclization concept for a furan monomer using a Pd0 catalyst, enabling formation of hex-CnFE (where n = 5, 6, 7). Figure adapted from ref .
Results and Discussion
Synthesis
In prior synthetic efforts, we employed the commercially available G3-PdSPhos to cross-couple hexyl 2-bromo-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)furan-3-carboxylate (Br-Fu-Bpin) and observed 3 macrocyclic products which were difficult to separate (x = 0, 1, or 2 in Figure ). In addition, low conversion to the three macrocyclic species was observed in the initial study (Table , entry 1), prompting further investigation.
1. Optimization of Reaction Conditions for Macrocyclization.
| Entry | Catalyst | Base | Total macrocycle conv. | C5FE | C6FE | C7FE | BrFu |
|---|---|---|---|---|---|---|---|
| 1 , | G3PdSPhos | K3PO4 | 10 | 3 | 6 | 1 | 6 |
| 2 | G3PdSPhos | K3PO4 | 21 | 10 | 9 | 2 | – |
| 3 | G3PdSPhos | CsF | 11 | 1 | 9 | 1 | 22 |
| 4 | Pd2dba3 + SPhos | K3PO4 | 1 | 0 | 1 | – | 23 |
| 5 | Pd2dba3 + SPhos | CsF | 4 | 1 | 3 | 0 | 15 |
| 6 | Pd2dba3 + [(t-Bu)3PH]BF4 | CsF | 52 | – | 33 | 19 | – |
| 7 | Pd2dba3 + [(t-Bu)3PH]BF4 | K3PO4 | 4 | – | 3 | 1 | 1 |
| 8 | Pd2dba3 + PAd3 | CsF | 51 | – | 37 | 14 | – |
| 9 | Pd2dba3 + PAd3 | K3PO4 | 1 | – | 1 | 0 | 7 |
| 10 | Pd2dba3 + PCy3 | CsF | 12 | – | 10 | 2 | 4 |
| 11 | G3PdP(t-Bu)3 | K3PO4 | 13 | – | 9 | 4 | – |
Typical conditions: Br-Fu-Bpin monomer (0.125 mmol) was combined with catalyst (10 mol % Pd and 10 mol % ligand), 3 equiv of base, THF:H2O (5:1, 30 mM) and trimethoxybenzene (∼0.2 equiv) as an internal standard. Reactions carried out at 50 °C. Conversion was determined using 1H NMR spectroscopy after 1 h, and for experiments which lead to >5% conv, the reported values are an average over 3 runs. Concentration of oligomers/polymers (P3HEF) was not quantified.
THF:H2O (30:1) was added.
7.7 mol % of the catalyst was added.
The products and side products that can arise with this cross-coupling reaction are shown in the top of Table which includes: formation of hex-CnFEs, formation of open chain oligomers/polymers (P3HEF), and loss of the boron group by protodeboronation (Br-Fu). , Formation of the three macrocyclic products (C5FE-C7FE) as well as the protodeboronated species were quantified by crude 1H NMR spectroscopy by examination of the aromatic region and comparing to an internal standard (Table ). Determining conversion to the open chain oligomers and polymers was difficult using this approach since P3HEF has a very broad aromatic signal (spans ∼700 Hz).
Initially, we noted that simply increasing the proportion of water in the cross-coupling reaction had a beneficial effect, nearly doubling the total conversion to 21% for hex-C5FE, hex-C6FE and hex-C7FE using G3PdSPhos (Table , entry 2), though effective separation of all 3 macrocycles using chromatography was still difficult. Extensive monomer protodeboronation was observed when G3-PdSPhos was paired with CsF as the base (Table , entry 3). The weaker base likely leads to inefficient catalyst activation, so we explored tris(dibenzylideneacetone)dipalladium (Pd2dba3) as a Pd precursor. Surprisingly, the combination of Pd2dba3, SPhos and a base did not result in significant quantities of CnFE’s formed (Table , entries 4–5).
Notably, the combination of tri-tert-butylphosphine − (P(t-Bu)3) and tri(1-adamantyl)phosphine (PAd3) with Pd2dba3 led to >50% conversion to hex-C6FE and hex-C7FE with CsF (Table , entries 6 and 8). A representative stack plot illustrating the conversion determined using 1H NMR spectroscopy is shown in Figure . Hex-C5FE formation is minimal if trialkylphosphine ligands are employed in the macrocyclization (facilitating the separation), and hex-C6FE is formed in higher proportion compared to hex-C7FE. Attempts to monitor reaction kinetics for the oligomerization were difficult as the Br-Fu-Bpin monomer was typically consumed within 2 min. The air-stable phosphonium precursor ([(t-Bu)3PH]BF4) was used in all instances to generate P(t-Bu)3 in situ, simply due to ease of handling and air stability as noted by Fu and co-workers previously. Since PAd3 has been reported to be an air-stable crystalline solid, this was used directly in that form. When using K3PO4 as the base, the monomer is still consumed rapidly, but CnFEs are formed in very small proportions (Table , entries 7, 9 and 11). These results highlight the critical importance of CsF as the base for cyclization.
3.
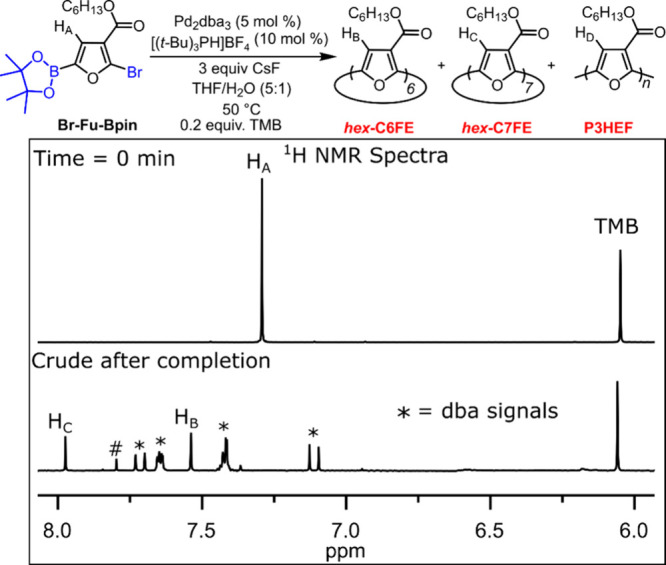
Stack plot of the crude 1H NMR spectra (500 MHz, CD2Cl2, 22 °C) collected for a macrocyclization experiment with Pd2dba3 (5 mol %), [(t-Bu)3PH]BF4 (10 mol %) and CsF (3 equiv). Conversion to macrocycle was determined by comparison with the aromatic signal from the 1,3,5-trimethoxybenzene (TMB) internal standard (0.2 equiv). Top corresponds to t = 0 min and monomer (HA). Bottom corresponds to t = 1 h after addition of catalyst, base and water. New signals for hex-C6FE (HB) and hex-C7FE (HC). The * signals correspond to the dba ligand from the catalyst and the # signal is unidentified.
Ananikov and co-workers have noted that Pd2dba3 decomposes over time, and that the degree of purity can vary depending on commercial sources. Our findings are consistent with that report, and macrocycle conversion dropped to ∼20% when utilizing a commercial sample of 40% pure Pd2dba3 (Figure S5). Given this, the precatalyst was purified via recrystallization in CHCl3 prior to use in macrocyclizations (Figure S4).
The fast reaction time and good yield for the cyclization suggested that this reaction may proceed by a different mechanism than the expected step-growth process. Chain-growth polycondensation of aromatics to form conjugated polymers has been hypothesized to proceed by association of the catalyst to the growing chain during the reaction via a π-bonding interaction between the metal and growing chain. − Direct evidence to illustrate that the metal coordinates to the growing aromatic chain is rare, while indirect evidence for this process has been commonly inferred from model reactions. − If aromatic dihalides are combined with a stoichiometric deficiency of a cross-coupling partner and only undergo exhaustive coupling to form trimers (with leftover starting material remaining in the reaction mixture), this suggests that the catalyst remains coordinated to the aromatic substrate after the first coupling and “walks” to the other halide for subsequent intramolecular activation. − A model reaction was examined to provide evidence for a chain-growth mechanism (Table S2), which we hypothesize contributes to the fast reaction rate and improved yield in this case.
Ethyl-2,5-dibromofuran-3-carboxylate was combined with furan-2-boronic acid pinacol ester (FuBpin) in a 2:1 ratio with a small selection of catalysts (Table S2). For G3PdSPhos and G3PdP(t-Bu)3 complete consumption of the boronic ester was observed with 94% and 99% conversion to trimer, respectively (Table S2, entries 1–2). This suggests that the Pd P(t-Bu)3 may be slightly more efficient in the chain-growth process for the oligomerization of the furan monomer. We also carried out the same cross-coupling reaction with Pd2dba3 and the 3 different trialkylphosphines (Table S2, entries 3–5). As expected, both P(t-Bu)3 and PAd3 afforded >98% conversion to the furan trimer, in line with the good yields for macrocycle formation.
Spectroscopic Properties
The separation of hex-C6FE and hex-C7FE from P3HEF was accomplished using column chromatography (Supporting Information). Minor impurities attributed to the open chain oligomers were removed by washing the solid with acetonitrile, followed by recrystallization via solvent diffusion using CH2Cl2 and methanol (1:3). The purity of hex-C6FE and hex-C7FE was confirmed by NMR spectroscopy, MALDI-TOF mass spectrometry, and FTIR spectroscopy (Figure A-D) and the spectral data for hex-C6FE was consistent with the prior report. A sharp singlet corresponding to the aromatic proton for hex-C6FE is observed at 7.46 ppm (HD in blue, Figure A), while the aromatic signal for hex-C7FE appears ∼0.5 ppm further downfield at 7.99 ppm (HD in red, Figure A). It should be noted that the aromatic signal for hex-C6FE can range from δ ∼ 7.60–7.45 ppm depending on concentration in CDCl3, due to aggregation. The triplet signal corresponding to the methylene protons of the hexyl ester side chain appears at 4.28 ppm for hex-C7FE (HF in red, Figure A), 0.11 ppm downfield as compared to the same signal for hex-C6FE (4.17 ppm, HF in blue, Figure A). The other signals for the alkyl groups (0.89 ppm to 1.80 ppm) also exhibit slight downfield shifts in hex-C7FE compared to those in hex-C6FE.
4.
(A) Stack plot of the 1H NMR spectra (500 MHz, CDCl3, 22 °C) of hex-C6FE (Blue) and hex-C7FE (Red). (B) Stack plot of the 13C{1H} NMR spectra (126 MHz, CDCl3, 22 °C) of hex-C6FE (Blue) and hex-C7FE (Red). (C) MALDI-TOF mass spectra of hex-C6FE (Blue) and hex-C7FE (Red). (D) FT-IR (ATR) spectra of hex-C6FE (Blue) and hex-C7FE (Red). The NMR and MALDI-TOF data for hex-C6FE were previously reported, and are included for comparison.
The 13C{1H} NMR spectra of the two different CnFE macrocycles are similar, though some subtle key differences are observed. Specifically, the C2 and C5 carbons of the furan ring (labeled A and B in Figure B) are upfield by ∼1 ppm for hex-C7FE. Furthermore, while the C3 and C4 carbons of the hex-C6FE ring are nearly overlapping at 116.4 and 116.3 ppm, while these are much more distinct in hex-C7FE at 118.4 and 117.3 ppm (labeled C and D in Figure B).
The progressive downfield shift for the aromatic signal in the 1H NMR spectra of the hex-CnFEs is quite different than in the previously reported cyclo[n]thiophenes, where 1H NMR chemical shifts change minimally with larger ring size. To explore the chemical shift differences in more detail, density functional theory (DFT) calculations of NMR shielding constants were computed using the gauge independent atomic orbital (GIAO) method. The predicted spectra are shown in the Supporting Information (Figures S36 and S37). Geometry optimizations were completed on cyclo[6]- and cyclo[7]furans with and without side chains for comparison. Optimizations were carried out in all instances using the B3LYP-D3(BJ) functional and a 6-31G(d,p) basis set, with a continuum solvation model (IEFPCM) in CH2Cl2.
The parent 6- and 7-membered macrocycles (no alkyl or ester substituents) are denoted as C6F and C7F (structure shown in Figure S36). Macrocycles with methyl groups on each furan repeat unit are denoted as me-C6F and me-C7F (structure shown in Figure S36). Finally, macrocycles with the methyl ester groups on each furan are denoted as me-C6FE and me-C7FE (direct analogs of the synthesized hex-C6FE and hex-C7FE). The chemical shift of the aromatic signals are predicted to be the same for C6F and C7F (7.5 ppm) and the methyl-substituted derivatives me-C6F and me-C7F (7.3 ppm). In contrast, a large downfield chemical shift is predicted with the larger macrocycle if ester side chains are present on the furan rings (δ ∼ 9.5 and 10.5 ppm predicted for the aromatic signals of me-C6FE and me-C7FE, respectively). A shortened distance (and stronger interaction) between the carbonyl oxygen and adjacent aromatic furan ring proton (2.27 Å in me-C6FE to 2.10 Å in me-C7FE) was noted in the computed structures. This decreased distance is likely the main contributor to the observed downfield chemical shift. The NMR-GIAO calculations are also consistent with the changes observed in the 13C{1H} spectrum (Figure S37).
The major peak observed in MALDI-TOF mass spectrometry for each macrocycle is a close match to the expected mass: 1164.4 g/mol for hex-C6FE (blue) and 1358.5 g/mol for hex-C7FE (red) as shown in Figure C. The isotope patterns also match with expectation for both hex-C6FE and hex-C7FE (Figure C) further confirming the successful formation and isolation of the macrocycles. The FTIR spectra of hex-C7FE and hex-C6FE are similar, but the 7-membered macrocycle spectrum is clearly broadened, with slightly shifted absorption bands (Figure D). The CO stretching frequency in hex-C7FE is broader (∼1670 cm–1), slightly weaker, and merges with CC vibrational modes when compared to the CO stretch for hex-C6FE (1709 cm–1). Additionally, the C–O stretching differs in intensity and is broader in hex-C7FE. These spectral differences suggest variations in bond environments, likely influenced by ring strain and conformational effects. To further investigate these factors, we analyzed ring strain in both macrocycles and examined their flexibility using molecular dynamics simulations.
Computed Ring Strain
The geometry optimizations for me-C6FE and me-C7FE revealed the ester-functionalized 7-membered ring is slightly curved and likely to be more strained (Figure ). The open form of the 7-mer (me-L7FE) shows partial overlap of the furan end groups, while the 6-mer (me-L6FE) does not (Figure A). Indeed, the computed ring strain energy for me-C7FE is 14.9 kcal/mol while that of me-C6FE is 3.1 kcal/mol, suggesting decreased strain for the 6-mer. The ring strain energies were computed using a hyperhomodesmotic reaction equation (Figures S29 and S30). The side chain substituents also impact the computed strain energies for the macrocycles as shown in the Supporting Information (Figures S29–S34). For example, the computed strain for C6F (6.4 kcal/mol) and C7F (2.4 kcal/mol) suggests that formation of the 7-mer is more favorable without ester groups present (when compared to the all syn open-chain form). It should also be noted a range of approaches exist to compute strain, which will impact the final values.
5.
(A) Optimized structures of open furan ester oligomers from DFT calculations at the B3LYP-D3(BJ)/6-31G(d,p) level of theory with a continuum solvation model (IEFPCM) in CH2Cl2. (B) Optimized structures of cyclized furan ester oligomers, computed as noted in (A). (C) The dihedral angle (θ) between the planes formed by the me-C7FE (plane 1, red; plane 2, blue), calculated from the optimized structure. Side chains are omitted for better visualization.
Molecular Dynamics
We then considered whether the higher ring strain and curved geometry for hex-C7FE might impact the conformational flexibility of the structure. Molecular dynamics (MD) simulations were carried out using GFN2-xTB on me-C6FE and me-C7FE. − This semiempirical approach significantly reduces the computational cost of molecular simulations but retains essential quantum mechanical features, which makes it a useful tool for large molecular systems. The parametrization of GFN2-xTB employs global and element-specific parameters, which ensures a consistent description across different chemical environments without relying on specific pairwise parameters. This characteristic is particularly advantageous when analyzing molecular flexibility, as it allows for a comprehensive sampling of possible conformations without the need for highly customized parameter sets. The MD simulations were performed with an initial heating phase to bring the system to the desired temperature, followed by equilibration and production phases. During the production phase, the structures were sampled at regular intervals to capture the range of possible conformations.
Simulations were carried from 300 to 500 K with steps of 50K. Trajectories were obtained for 100 ps, and sampling was performed every 1 fs. The SHAKE algorithm, which constrains all bonds during simulation (no bond breaking), was turned off to capture C–H stretching vibrations. The dihedral angles of rotatable C–O bonds in the computed me-C6FE and me-C7FE structures (highlighted in Figure ) were extracted along the trajectory of the molecular dynamics’ simulation, to analyze flexibility. The changes in these torsional angles are periodic, which enabled a Fourier Transform of the peaks in the trajectory to obtain vibration related frequencies.
6.
Top left - dihedral angles of rotatable C–O bonds in me-C6FE (top) and me-C7FE. Top right - low vibrational frequencies of me-C6FE (blue) and me-C7FE (red) obtained from molecular dynamics. The black lines represent normal modes calculated by xTB from DFT optimized geometries. At the bottom of the scheme, pink vectors illustrate the 6 lowest normal modes calculated by xTB for both me-C6FE and me-C7FE.
Figure also illustrates the computed low-frequency vibrational modes of the me-C6FE (blue) and me-C7FE (red) macrocycles from molecular dynamics simulations, , alongside the normal modes calculated by xTB (black lines). These frequencies, found at the low-energy end of the vibrational spectrum, correspond to collective deformations of the macrocyclic framework, including torsional, bending, and puckering motions. The pink vectors shown on the structures (Figure ) illustrate the atomic displacements associated with the six lowest normal modes, as calculated by xTB. The presence of distinct peaks and their relative intensities reflect the inherent flexibility of each macrocycle, highlighting how ring size and topology influence the accessible conformational landscape.
The larger number of low-frequency modes observed for me-C7FE as compared to me-C6FE (from both molecular dynamics and xTB), is consistent with increased conformational freedom arising from its larger size. The six-membered system displays relatively well-defined low-frequency modes, suggesting a more restricted dynamic range of accessible conformations. The seven-membered ring supports a broader set of low-energy deformations with the additional peaks in the low-frequency region, indicating the presence of multiple modes associated with subtle ring distortions and torsion. As a result, the seven-membered macrocycle can more readily transition between various geometries with lower energy barriers.
Optical and Redox Properties
The UV–vis spectrum of hex-C7FE was collected and compared with hex-C6FE (Figure A-B). The λmax for hex-C7FE is red-shifted by 17 nm relative to hex-C6FE, which is expected due to the additional furan repeat unit. Time-dependent DFT (TD-DFT) calculations on me-C7FE (dotted red trace, Figure B) align with experimental data, predicting an intense S0 → S2/S3 transition at 373 nm, 25 nm red-shifted compared to TD-DFT results for me-C6FE. The computed S0 → S2 and S0 → S3 transitions occur at the same wavelength and oscillator strength for each macrocycle due to the double degeneracy of the higher-energy unoccupied molecular orbitals following the singly degenerate LUMO. The HOMO–LUMO transition for both me-C6FE and me-C7FE is predicted to have zero oscillator strength (f = 0). The symmetry-forbidden S 0→S 1 transition for hex-C7FE occurs at ∼510 nm, close to the predicted transition at 534 nm. Notably, the absorption profile of hex-C7FE lacks the distinct structure seen in hex-C6FE, which is consistent with the prediction that it is more conformationally flexible. The featureless absorption profile is similar to the alternating ester-functionalized furan-thiophene macrocycle as well as the cyclo[10]thiophene. , The decreased rigidity for hex-C7FE may also partially explain the slightly increased intensity for the formally forbidden HOMO–LUMO transition (∼510 nm). No significant luminescence was noted for either macrocycle in solution.
7.
Normalized absorption spectrum of hex-C6FE (A) and hex-C7FE (B) collected in CHCl3 (solid line) and predicted by TD-DFT (dotted line, CAM-B3LYP/6–31G(d,p) IEFPCM(CH2Cl2)). The calculated natural transition orbitals are shown in the inset. (C) Cyclic voltammograms of hex-C6FE (blue trace) and hex-C7FE (red trace) in degassed CH2Cl2 (0.63 mg/mL) using NBu4PF6 as the supporting electrolyte (0.07 M), with a scan rate of 100 mV/s. Voltammograms were referenced using Fc/Fc+ as an internal standard. Determination of the electrochemical band gap of hex-C7FE from the onsets of oxidation and reduction at +0.44 and −1.86 V, respectively, yields a value of E g = 2.30 eV. (D) Overlay of the cyclic voltammograms of hex-C7FE with varying scan rates in degassed CH2Cl2 (0.63 mg/mL) using NBu4PF6 as the supporting electrolyte (0.07 M).
Cyclic voltammetry studies of hex-C7FE were carried out in CH2Cl2 with Fc/Fc+ with NBu4PF6 as the supporting electrolyte (Figure C). Half-wave potentials are reported for the quasi-reversible oxidation and reduction while the inflection potential (E i) is reported to estimate the potential for the second oxidation. Quasi-reversible oxidations were observed at 0.49 V (E 1/2) and 0.91 V (E i) for hex-C7FE (0.56 and 1.09 V for hex-C6FE). Both oxidations are lower for hex-C7FE, reflecting the influence of the extra repeat unit. A single quasi-reversible reduction is observed for hex-C7FE (E 1/2 = −1.96 V), which is more cathodic than the first of two quasi-reversible reductions observed for hex-C6FE (E 1/2 = −1.89 V and −2.09 V). Given the additional current passed for reduction as compared to oxidation, it is possible that the reduction is a 2-electron process for hex-C7FE. Notably, the quasi-reversible reduction for hex-C7FE became irreversible at low scan rates (50 mV/s) and more reversible at faster scan rates (225 mV/s in Figure D). This irreversibility at lower scan rates suggests instability of the reduced species, which was also noted for P3HEF previously. Both macrocycles exhibit reasonable redox stability, as repeated cycling at the same scan rate produces essentially identical voltammograms.
Optimizations and frequency calculations to calculate ionization potentials (IP) and electron affinities (EA) for the first and second redox steps of me-C6FE and me-C7FE were carried out at the B3LYP-D3(BJ)/6-31G(d,p) levels using a continuum solvation model (IEFPCM) with CH2Cl2 as the solvent (Figure S35). The first oxidation and first reduction were used to estimate the computed bandgap (2.15 and 1.96 eV for me-C6FE and me-C7FE, respectively). The electrochemical bandgap (E g), estimated from oxidation and reduction onsets, was within 0.11 eV of the computed gap for hex-C6FE (2.26 eV). A larger deviation (0.34 eV) was noted for hex-C7FE (E g = 2.3 eV). The optical and electrochemical bandgaps are in a similar range for both macrocycles, but it should be noted that the optical gap is slightly lower for hex-C7FE while the electrochemical gap is slightly lower for hex-C6FE.
While the neutral form of me-C7FE is predicted to be slightly curved, the doubly oxidized and reduced forms are predicted to be planar as shown in Figure . Nucleus Independent Chemical Shift (NICS) was used to determine whether the macrocycle is globally aromatic upon double oxidation or reduction, similar to prior work. , The zz components of NICS were computed at 1 Å above the plane of the macrocycle and values are reported in ppm and shown in Figure (NICS(1) zz ). Both the doubly reduced and doubly oxidized have large negative NICS(1) zz values (−21.7 and −33.8), indicating a diatropic ring current and an aromatic structure. This prediction for me-C7FE is also consistent with the prior results for me-C6FE and the 8-membered ester-functionalized alternating thiophene-furan macrocycle. ,
8.
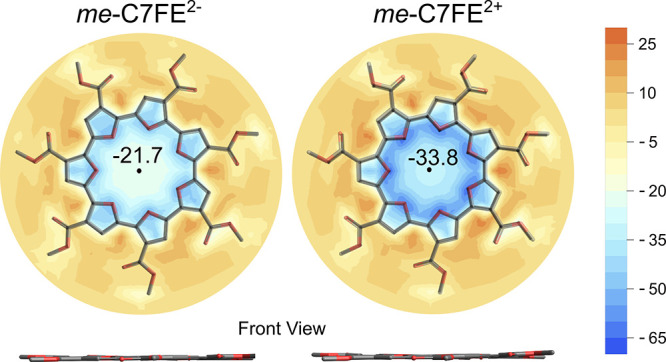
NICS(1) zz plots for me-C7FE in their dianion (left) and dication (right) state. The color scale bar indicates the degree of aromaticity or antiaromaticity, with more negative values representing a higher aromatic character and positive values representing greater antiaromatic character. Macrocycles are annotated with NICS values at the center of the cycle. Calculations were performed using the NMR-GIAO method with the B3LYP-D3(BJ) functional and 6-31G(d,p) basis set, employing a continuum solvation model (IEFPCM) in CH2Cl2. NICS values were obtained on a polar grid placed 1 Å above the macrocycle plane.
Conclusion
In this work, we have demonstrated that hexyl 2-bromo-5-(boronic acid pinacol ester)furan-3-carboxylate can be converted to a cyclo[n]furan (where n = 6 or 7) in good yield in the presence of Pd2dba3, P(t-Bu)3 and CsF. The rapid reaction kinetics, along with model studies, suggest that the process may proceed via a chain-growth mechanism. An odd-membered hexyl-substituted cyclo[7]furan ester was isolated for the first time, and the optical and redox properties of this structure were examined. Spectroscopic and computational studies revealed that the ester-functionalized cyclo[7]furan is more strained, slightly curved and more conformationally flexible than the 6-membered analog. Computational studies predict the 7-membered macrocycle is planar and globally aromatic in the doubly oxidized and reduced states, like its 6-membered counterpart. Altogether, the developed synthetic method offers straightforward access to cyclo[n]furans of different sizes, and future work will focus on synthetic modifications to the macrocycle, metal coordination and examination of molecular packing in the solid-state.
Supplementary Material
Acknowledgments
K.J.T.N. and T.K. are grateful to the NSF for support of this work (CHE-2109065 and CHE-2404367).
The data underlying this study are available in the published article and its .
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.5c00526.
Experimental procedures, NMR spectra, gas chromatograms (GC), MALDI-TOF mass spectra, absorbance spectra, cyclic voltammograms, computed energies, computed NMR spectra, computed orbital energies (PDF)
FAIR data, including the primary NMR FID files, for compounds hex-C6FE and hex-C7FE (ZIP)
Optimized geometries of computed structures in xyz format (ZIP)
The authors declare no competing financial interest.
References
- Iyoda M., Yamakawa J., Rahman M. J.. Conjugated Macrocycles: Concepts and Applications. Angew. Chem., Int. Ed. 2011;50:10522–10553. doi: 10.1002/anie.201006198. [DOI] [PubMed] [Google Scholar]
- Hirst E. S., Jasti R.. Bending Benzene: Syntheses of [n]Cycloparaphenylenes. J. Org. Chem. 2012;77:10473–10478. doi: 10.1021/jo302186h. [DOI] [PubMed] [Google Scholar]
- Iyoda M., Shimizu H.. Multifunctional π-expanded oligothiophene macrocycles. Chem. Soc. Rev. 2015;44:6411–6424. doi: 10.1039/C5CS00388A. [DOI] [PubMed] [Google Scholar]
- Leonhardt E. J., Jasti R.. Emerging applications of carbon nanohoops. Nat. Rev. Chem. 2019;3:672–686. doi: 10.1038/s41570-019-0140-0. [DOI] [Google Scholar]
- Staab H. A., Binnig F.. Synthese und eigenschaften von hexa-m-phenylen. Tetrahedron Lett. 1964;5:319–321. doi: 10.1016/0040-4039(64)80020-4. [DOI] [Google Scholar]
- Jasti R., Bhattacharjee J., Neaton J. B., Bertozzi C. R.. Synthesis, Characterization, and Theory of [9]-, [12]-, and [18]Cycloparaphenylene: Carbon Nanohoop Structures. J. Am. Chem. Soc. 2008;130:17646–17647. doi: 10.1021/ja807126u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel D., Lynch V., Sessler J. L.. Cyclo[8]pyrrole: A Simple-to-Make Expanded Porphyrin with No Meso Bridges. Angew. Chem., Int. Ed. 2002;41:1422–1425. doi: 10.1002/1521-3773(20020415)41:8<1422::AID-ANIE1422>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Köhler T., Seidel D., Lynch V., Arp F. O., Ou Z., Kadish K. M., Sessler J. L.. Formation and properties of Cyclo[6]pyrrole and Cyclo[7]pyrrole. J. Am. Chem. Soc. 2003;125:6872–6873. doi: 10.1021/ja035089y. [DOI] [PubMed] [Google Scholar]
- Krömer J., Rios-Carreras I., Fuhrmann G., Musch C., Wunderlin M., Debaerdemaeker T., Mena-Osteritz E., Bäuerle P.. Synthesis of the first fully α-conjugated macrocyclic oligothiophenes: Cyclo[n]thiophenes with tunable cavities in the nanometer regime. Angew. Chem., Int. Ed. 2000;39:3481–3486. doi: 10.1002/1521-3773(20001002)39:19<3481::AID-ANIE3481>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G., Debaerdemaeker T., Bäuerle P.. C-C bond formation through oxidatively induced elimination of platinum complexes - A novel approach towards conjugated macrocycles. Chem. Commun. 2003:948–949. doi: 10.1039/b300542a. [DOI] [PubMed] [Google Scholar]
- Zhang F., Götz G., Winkler H. D. F., Schalley C. A., Bäuerle P.. Giant Cyclo[n]thiophenes with Extended π Conjugation. Angew. Chem., Int. Ed. 2009;48:6632–6635. doi: 10.1002/anie.200900101. [DOI] [PubMed] [Google Scholar]
- Muñoz-Castro A.. Local and global aromaticity in a molecular carbon nanobelt: insights from magnetic response properties in neutral and charged species. Phys. Chem. Chem. Phys. 2018;20:3433–3437. doi: 10.1039/C7CP08323H. [DOI] [PubMed] [Google Scholar]
- Toriumi N., Muranaka A., Kayahara E., Yamago S., Uchiyama M.. In-Plane Aromaticity in Cycloparaphenylene Dications: A Magnetic Circular Dichroism and Theoretical Study. J. Am. Chem. Soc. 2015;137:82–85. doi: 10.1021/ja511320f. [DOI] [PubMed] [Google Scholar]
- Kayahara E., Kouyama T., Kato T., Takaya H., Yasuda N., Yamago S.. Isolation and Characterization of the Cycloparaphenylene Radical Cation and Dication. Angew. Chem., Int. Ed. 2013;52:13722–13726. doi: 10.1002/anie.201306881. [DOI] [PubMed] [Google Scholar]
- Zhang F., Götz G., Mena-Osteritz E., Weil M., Sarkar B., Kaim W., Bäuerle P.. Molecular and electronic structure of cyclo[10]thiophene in various oxidation states: polaron pair vs. bipolaron. Chem. Sci. 2011;2:781–784. doi: 10.1039/c0sc00560f. [DOI] [Google Scholar]
- Mena-Osteritz E., Zhang F., Götz G., Reineker P., Bäuerle P.. Optical properties of fully conjugated cyclo[n]thiophenes - An experimental and theoretical approach. Beilstein J. Nanotechnol. 2011;2:720–726. doi: 10.3762/bjnano.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisula W., Kastler M., Yang C., Enkelmann V., Müllen K.. Columnar Mesophase Formation of Cyclohexa-m-phenylene-Based Macrocycles. Chem.Asian J. 2007;2:51–56. doi: 10.1002/asia.200600338. [DOI] [PubMed] [Google Scholar]
- Zade S. S., Bendikov M.. Cyclic oligothiophenes: Novel organic materials and models for polythiophene. A theoretical study. J. Org. Chem. 2006;71:2972–2981. doi: 10.1021/jo0525229. [DOI] [PubMed] [Google Scholar]
- Mulay S. V., Dishi O., Fang Y., Niazi M. R., Shimon L. J. W., Perepichka D. F., Gidron O.. A macrocyclic oligofuran: synthesis, solid state structure and electronic properties. Chem. Sci. 2019;10:8527–8532. doi: 10.1039/C9SC03247A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishi O., Malakar P., Shimon L. J. W., Ruhman S., Gidron O.. Ring Size Determines the Conformation, Global Aromaticity and Photophysical Properties of Macrocyclic Oligofurans. Chem.Eur. J. 2021;27:17794–17801. doi: 10.1002/chem.202103536. [DOI] [PubMed] [Google Scholar]
- Dishi O., Rahav Y., Carmieli R., Gidron O.. A Macrocyclic Furan with Accessible Oxidation States: Switching Between Aromatic and Antiaromatic Global Ring Currents. Chem.Eur. J. 2022;28:e202202082. doi: 10.1002/chem.202202082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni A. J., Kawakami M., Tristram-Nagle S. A., Yaron D., Kowalewski T., Noonan K. J. T.. Design, synthesis, and properties of a six-membered oligofuran macrocycle. Org. Chem. Front. 2021;8:1775–1782. doi: 10.1039/D1QO00084E. [DOI] [Google Scholar]
- Glenis S., Benz M., LeGoff E., Schindler J. L., Kannewurf C. R., Kanatzidis M. G.. Polyfuran: a new synthetic approach and electronic properties. J. Am. Chem. Soc. 1993;115:12519–12525. doi: 10.1021/ja00079a035. [DOI] [Google Scholar]
- Politis J. K., Nemes J. C., Curtis M. D.. Synthesis and Characterization of Regiorandom and Regioregular Poly(3-octylfuran) J. Am. Chem. Soc. 2001;123:2537–2547. doi: 10.1021/ja003588i. [DOI] [PubMed] [Google Scholar]
- Varni A. J., Fortney A., Baker M. A., Worch J. C., Qiu Y., Yaron D., Bernhard S., Noonan K. J. T., Kowalewski T.. Photostable Helical Polyfurans. J. Am. Chem. Soc. 2019;141:8858–8867. doi: 10.1021/jacs.9b01567. [DOI] [PubMed] [Google Scholar]
- Kappe C. O., Murphree S. S., Padwa A.. Synthetic applications of furan Diels-Alder chemistry. Tetrahedron. 1997;53:14179–14233. doi: 10.1016/S0040-4020(97)00747-3. [DOI] [Google Scholar]
- Gandini A.. The furan/maleimide Diels-Alder reaction: A versatile click-unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013;38:1–29. doi: 10.1016/j.progpolymsci.2012.04.002. [DOI] [Google Scholar]
- Dishi O., Gidron O.. Macrocyclic Oligofurans: A Computational Study. J. Org. Chem. 2018;83:3119–3125. doi: 10.1021/acs.joc.7b03030. [DOI] [PubMed] [Google Scholar]
- Sharma S., Bendikov M.. α-Oligofurans: A Computational Study. Chem.Eur. J. 2013;19:13127–13139. doi: 10.1002/chem.201300257. [DOI] [PubMed] [Google Scholar]
- Sharma S., Zamoshchik N., Bendikov M.. Polyfurans: A Computational Study. Isr. J. Chem. 2014;54:712–722. doi: 10.1002/ijch.201400008. [DOI] [Google Scholar]
- Rahav Y., Rajagopal S. K., Dishi O., Bogoslavsky B., Gidron O.. Alternating behavior in furan-acetylene macrocycles reveals the size-dependency of Hückel’s rule in neutral molecules. Commun. Chem. 2023;6:100. doi: 10.1038/s42004-023-00902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Sharma D., Varni A. J., Tristram-Nagle S., Yaron D., Kowalewski T., Noonan K. J. T.. Design, Synthesis and Aromaticity of an Alternating Cyclo[4]Thiophene[4]Furan. Chem.Eur. J. 2023;29:e202300477. doi: 10.1002/chem.202300477. [DOI] [PubMed] [Google Scholar]
- Yokozawa T., Yokoyama A.. Chain-Growth Condensation Polymerization for the Synthesis of Well-Defined Condensation Polymers and π-Conjugated Polymers. Chem. Rev. 2009;109:5595–5619. doi: 10.1021/cr900041c. [DOI] [PubMed] [Google Scholar]
- Bryan Z. J., McNeil A. J.. Conjugated Polymer Synthesis via Catalyst-Transfer Polycondensation (CTP): Mechanism, Scope, and Applications. Macromolecules. 2013;46:8395–8405. doi: 10.1021/ma401314x. [DOI] [Google Scholar]
- Yokozawa T., Ohta Y.. Transformation of Step-Growth Polymerization into Living Chain-Growth Polymerization. Chem. Rev. 2016;116:1950–1968. doi: 10.1021/acs.chemrev.5b00393. [DOI] [PubMed] [Google Scholar]
- Lawrence J., Goto E., Ren J. M., McDearmon B., Kim D. S., Ochiai Y., Clark P. G., Laitar D., Higashihara T., Hawker C. J.. A Versatile and Efficient Strategy to Discrete Conjugated Oligomers. J. Am. Chem. Soc. 2017;139:13735–13739. doi: 10.1021/jacs.7b05299. [DOI] [PubMed] [Google Scholar]
- Murphy E. A., Zhang C., Bates C. M., Hawker C. J.. Chromatographic Separation: A Versatile Strategy to Prepare Discrete and Well-Defined Polymer Libraries. Acc. Chem. Res. 2024;57:1202–1213. doi: 10.1021/acs.accounts.4c00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown G. R., Ye S., Cheng S., Seferos D. S.. Homogenous Synthesis of Monodisperse High Oligomers of 3-Hexylthiophene by Temperature Cycling. J. Am. Chem. Soc. 2019;141:17053–17056. doi: 10.1021/jacs.9b08240. [DOI] [PubMed] [Google Scholar]
- Xu H., Ye S., Zhao R., Seferos D. S.. Homogeneous Synthesis of Monodisperse Sequence-Defined Conjugated Oligomers by Temperature Cycling. Angew.Chem. Int. Ed. 2022;61:e202210340. doi: 10.1002/anie.202210340. [DOI] [PubMed] [Google Scholar]
- Bruno N. C., Tudge M. T., Buchwald S. L.. Design and preparation of new palladium precatalysts for C-C and C-N cross-coupling reactions. Chem. Sci. 2013;4:916–920. doi: 10.1039/C2SC20903A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P. A., Leach A. G., Campbell A. D., Lloyd-Jones G. C.. Protodeboronation of Heteroaromatic, Vinyl, and Cyclopropyl Boronic Acids: pH-Rate Profiles, Autocatalysis, and Disproportionation. J. Am. Chem. Soc. 2016;138:9145–9157. doi: 10.1021/jacs.6b03283. [DOI] [PubMed] [Google Scholar]
- Cox P. A., Reid M., Leach A. G., Campbell A. D., King E. J., Lloyd-Jones G. C.. Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From Concerted Proton Transfer to Liberation of a Transient Aryl Anion. J. Am. Chem. Soc. 2017;139:13156–13165. doi: 10.1021/jacs.7b07444. [DOI] [PubMed] [Google Scholar]
- Littke A. F., Fu G. C.. A Convenient and General Method for Pd-Catalyzed Suzuki Cross-Couplings of Aryl Chlorides and Arylboronic Acids. Angew.Chem. Int. Ed. 1998;37:3387–3388. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3387::AID-ANIE3387>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Littke A. F., Dai C., Fu G. C.. Versatile Catalysts for the Suzuki Cross-Coupling of Arylboronic Acids with Aryl and Vinyl Halides and Triflates under Mild Conditions. J. Am. Chem. Soc. 2000;122:4020–4028. doi: 10.1021/ja0002058. [DOI] [Google Scholar]
- Lou S., Fu G. C.. Palladium/Tris(tert-butyl)phosphine-Catalyzed Suzuki Cross- Couplings in the Presence of Water. Adv. Synth. Catal. 2010;352:2081–2084. doi: 10.1002/adsc.201000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Ren P., Carrow B. P.. Tri(1-adamantyl)phosphine: Expanding the Boundary of Electron-Releasing Character Available to Organophosphorus Compounds. J. Am. Chem. Soc. 2016;138:6392–6395. doi: 10.1021/jacs.6b03215. [DOI] [PubMed] [Google Scholar]
- Netherton M. R., Fu G. C.. Air-Stable Trialkylphosphonium Salts: Simple, Practical, and Versatile Replacements for Air-Sensitive Trialkylphosphines. Applications in Stoichiometric and Catalytic Processes. Org. Lett. 2001;3:4295–4298. doi: 10.1021/ol016971g. [DOI] [PubMed] [Google Scholar]
- Zalesskiy S. S., Ananikov V. P.. Pd2(dba)3 as a Precursor of Soluble Metal Complexes and Nanoparticles: Determination of Palladium Active Species for Catalysis and Synthesis. Organometallics. 2012;31:2302–2309. doi: 10.1021/om201217r. [DOI] [Google Scholar]
- He W., Patrick B. O., Kennepohl P.. Identifying the missing link in catalyst transfer polymerization. Nat. Commun. 2018;9:3866. doi: 10.1038/s41467-018-06324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita H., Kamigawara T., Miyazaki S., Shimada R., Katoh T., Ohta Y., Yokozawa T.. Intramolecular Palladium Catalyst Transfer on Benzoheterodiazoles as Acceptor Monomers and Discovery of Catalyst Transfer Inhibitors. Chem.Eur. J. 2023;29:e202301242. doi: 10.1002/chem.202301242. [DOI] [PubMed] [Google Scholar]
- Tokita Y., Katoh M., Ohta Y., Yokozawa T.. Mechanistic Investigation of Catalyst-Transfer Suzuki-Miyaura Condensation Polymerization of Thiophene-Pyridine Biaryl Monomers with the Aid of Model Reactions. Chem.Eur. J. 2016;22:17436–17444. doi: 10.1002/chem.201603581. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Tatsuta G., Sugie A., Mori A.. Studies on the preference of multiple coupling in the introduction of thiophene ring into poly-halogenated aromatic compounds with nickel NHC catalyst. Tetrahedron Lett. 2013;54:1976–1979. doi: 10.1016/j.tetlet.2013.01.127. [DOI] [Google Scholar]
- Larrosa I., Somoza C., Banquy A., Goldup S. M.. Two Flavors of PEPPSI-IPr: Activation and Diffusion Control in a Single NHC-Ligated Pd Catalyst? Org. Lett. 2011;13:146–149. doi: 10.1021/ol1027283. [DOI] [PubMed] [Google Scholar]
- Dong C.-G., Hu Q.-S.. Preferential oxidative addition in palladium(0)-catalyzed Suzuki cross-coupling reactions of dihaloarenes with arylboronic acids. J. Am. Chem. Soc. 2005;127:10006–10007. doi: 10.1021/ja052547p. [DOI] [PubMed] [Google Scholar]
- Weber S. K., Galbrecht F., Scherf U.. Preferential Oxidative Addition in Suzuki Cross-Coupling Reactions Across One Fluorene Unit. Org. Lett. 2006;8:4039–4041. doi: 10.1021/ol061476b. [DOI] [PubMed] [Google Scholar]
- Beryozkina T., Senkovskyy V., Kaul E., Kiriy A.. Kumada Catalyst-Transfer Polycondensation of Thiophene-Based Oligomers: Robustness of a Chain-Growth Mechanism. Macromolecules. 2008;41:7817–7823. doi: 10.1021/ma801660x. [DOI] [Google Scholar]
- Grimme S., Ehrlich S., Goerigk L.. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011;32:1456–1465. doi: 10.1002/jcc.21759. [DOI] [PubMed] [Google Scholar]
- Wheeler S. E., Houk K. N., Schleyer P. R., Allen W. D.. A Hierarchy of Homodesmotic Reactions for Thermochemistry. J. Am. Chem. Soc. 2009;131:2547–2560. doi: 10.1021/ja805843n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimme S., Bannwarth C., Shushkov P.. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All spd-Block Elements (Z = 1–86) J. Chem. Theory Comput. 2017;13:1989–2009. doi: 10.1021/acs.jctc.7b00118. [DOI] [PubMed] [Google Scholar]
- Bannwarth C., Ehlert S., Grimme S.. GFN2-xTBAn Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019;15:1652–1671. doi: 10.1021/acs.jctc.8b01176. [DOI] [PubMed] [Google Scholar]
- Bannwarth C., Caldeweyher E., Ehlert S., Hansen A., Pracht P., Seibert J., Spicher S., Grimme S.. Extended tight-binding quantum chemistry methods. WIREs Comput. Mol. Sci. 2021;11:e1493. doi: 10.1002/wcms.1493. [DOI] [Google Scholar]
- Elber R., Ruymgaart A. P., Hess B.. SHAKE parallelization. Eur. Phys. J. Spec. Top. 2011;200:211–223. doi: 10.1140/epjst/e2011-01525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias G., Baer M. D.. Generalized Normal Coordinates for the Vibrational Analysis of Molecular Dynamics Simulations. J. Chem. Theory Comput. 2011;7:2028–2039. doi: 10.1021/ct2001304. [DOI] [PubMed] [Google Scholar]
- Thomas M., Brehm M., Fligg R., Vöhringer P., Kirchner B.. Computing vibrational spectra from ab initio molecular dynamics. Phys. Chem. Chem. Phys. 2013;15:6608–6622. doi: 10.1039/c3cp44302g. [DOI] [PubMed] [Google Scholar]
- Espinoza E. M., Clark J. A., Soliman J., Derr J. B., Morales M., Vullev V. I.. Practical Aspects of Cyclic Voltammetry: How to Estimate Reduction Potentials When Irreversibility Prevails. J. Electrochem. Soc. 2019;166:H3175–H3187. doi: 10.1149/2.0241905jes. [DOI] [Google Scholar]
- Connelly N. G., Geiger W. E.. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996;96:877–910. doi: 10.1021/cr940053x. [DOI] [PubMed] [Google Scholar]
- Chen Z., Wannere C. S., Corminboeuf C., Puchta R., Schleyer P. R.. Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chem. Rev. 2005;105:3842–3888. doi: 10.1021/cr030088+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its .









