Abstract
Tumor necrosis factor (TNF) is a central mediator in lethal shock and an interesting cytokine for anticancer therapy. To inhibit TNF-induced lethal shock, it is important to identify protective genes. Here we demonstrate that the SPRET/Ei mouse strain, derived from Mus spretus, exhibits an extremely dominant resistance to TNF-induced lethal inflammation. An interspecific backcross experiment revealed that the TNF hyporesponse is linked to loci on chromosomes 2, 6, and 11. Treatment of inoculated tumors with TNF and IFN-γ leads to regression and a highly reduced toxicity in (C57BL/6 × SPRET/Ei)F1 mice.
Tumor necrosis factor (TNF) is a pleiotropic cytokine mainly produced by macrophages after stimulation with bacterial or viral antigens (1). TNF acts by binding to two different receptors, namely p55 (TNF-RI) and p75 (TNF-RII), which are present on virtually all cell types (2). TNF-RI is regarded as the main signal-transducing receptor and has been shown to be essential in cytotoxicity (3). Although TNF-RII was originally considered to be a ligand-passing receptor involved in fine-tuning the TNF response (2), it has become clear that it plays a more important role in certain cell types. However, by using TNF-RII-deficient mice, TNF-RII has been shown to play a very marginal role in TNF-induced lethal shock (4).
Especially in combination with IFN, TNF is able to kill tumors efficiently (5). However, TNF is also a strong proinflammatory molecule. TNF stimulates the expression of cytokines and adhesion molecules both on leukocytes and endothelial cells (6, 7). As a result, TNF induces extravasation of WBCs (8). Injection of TNF into patients with cancer and experimental animals revealed the development, due to proinflammatory activities, of a systemic inflammatory response syndrome, leading to hypotension and liver failure (9). Consequently, the application of TNF as an antitumor drug is currently limited to locoregional treatments (10).
TNF plays a central role in several pathologies, especially in endotoxemia, and has been found in the serum of patients with septic shock (11). Treatment of mice or baboons with neutralizing antibodies protects against lethal endotoxemia induced either by lipopolysaccharide or Gram-negative bacteria (12, 13). Furthermore, injection of TNF or lipopolysaccharide into animals results in indistinguishable inflammatory and shock-like responses (13, 14). TNF has also been shown to be centrally involved in the development of arthritis, inflammatory bowel disease, and multiple sclerosis (15). To develop TNF as a systemically applicable antitumor drug and to apply new strategies in the treatment of TNF-mediated pathologies, such as rheumatoid arthritis or asthma, new targets or protective gene products are indispensable.
We studied the sensitivity of several mouse strains to TNF-induced lethal inflammatory shock and found that SPRET/Ei, an inbred strain derived from Mus spretus, is extremely resistant. We demonstrate that SPRET/Ei mice are completely protected against all pathological changes induced by TNF. On the basis of the evolutionary distance of ≈3 million years between M. spretus and Mus musculus (16), this protection provides an ideal basis on which to map the genes responsible for TNF hyporesponsiveness. We demonstrate, using an interspecific backcross between (B × S)F1 and C57BL/6JIco, that loci conferring resistance to TNF-induced lethality are found on chromosomes 2 and 6, and that a locus conferring sensitivity to TNF is present on chromosome 11. We also show that (B × S)F1 mice are resistant to the lethality induced by a combination therapy with TNF/IFN-γ, although tumor regression still occurs in (B × S)F1 mice. Thus, the cloning of genes responsible for TNF resistance of SPRET/Ei mice opens interesting perspectives for the systemic use of TNF in anticancer therapies.
Experimental Procedures
Mice.
The strains C57BL/6JIco (hereafter designated as C57BL/6 or B), BALB/cJIco, and C3H/HeOuJIco, originating from The Jackson Laboratory were purchased from Iffa Credo. SPRET/Ei (S), CAST/Ei, NOD/LtJ, FVB/NJ, LP/J, PL/J, CBA/J, SWR/J, and 129/SvJ strains were obtained from The Jackson Laboratory. The strain DBA/2CrlBr was purchased from Charles River Laboratories (Sulzfeld, Germany). F1 hybrid mice were generated in the Ghent laboratory by crossing parental strains (C57BL/6 × SPRET/Ei). F1 mice were obtained by crossing C57BL/6 female mice with SPRET/Ei males, and are denominated (B × S)F1. MAI/Pas and MBT/Pas mice originated from the Institut Pasteur (Paris). The mice were kept in individually ventilated cages in a conventional animal house and received food and water ad libitum. All mice were used at the age of 8 weeks and had a comparable bodyweight.
Agents.
Recombinant murine TNF and IFN-γ were expressed in Escherichia coli and purified in the Ghent laboratory. TNF had a specific activity of 7.8 × 107 units/mg, the protein concentration being 1.1 mg/ml. The TNF preparation contained <10 endotoxin units/mg protein, as determined in a Limulus amebocyte lysate assay. IFN-γ had a specific activity of 1.16 × 107 units/mg. D-Galactosamine (GalN) and actinomycin D (ActD) were purchased from Sigma.
Injections, Blood Collections, and Measurement of Body Temperature.
TNF was diluted in endotoxin-free PBS immediately before injection. All injections were given i.v. or i.p. in a total volume of 250 μl. Most mice were injected i.v., but inbred strains derived from mice recently caught in nature were injected i.p. because of their wild behavior. C57BL/6 mice were regarded as a reference and were therefore injected both i.v. and i.p. Blood was collected by cardiac puncture under avertin anesthesia; it was allowed to clot for 1 h at 37°C, and for another hour at 4°C. Serum was prepared and stored at −20°C. Rectal body temperatures were measured with an electronic thermometer (Comarks, Littlehampton, U.K.).
Tissue Sections.
Mice were anesthetized with avertin. Bowel, liver, lung, thymus, and spleen were removed and fixed overnight in 4% paraformaldehyde. Tissues were dehydrated and embedded in paraffin, to be cut in slices of 4 μm. Sections were stained with hematoxylin and eosin by use of standard methods.
Determination of IL-6 and TNF in the Serum.
IL-6 was determined by using an IL-6-dependent 7TD1 hybridoma cell line (17). In the presence of serially diluted serum or mouse IL-6 as a standard, 7,000 cells per well were cultured in 96-well plates. After 3 days of culture, the number of cells was determined by using the hexosaminidase colorimetric method, which has a detection limit of 1 pg/ml. TNF was measured by using the sensitive cell line WEHI 164 cl 13 (18). In brief, 50,000 cells per well were cultured with 1 μg/ml ActD in the presence of diluted serum samples in flat-bottom, 96-well microtiter plates. After 18 h of incubation, the number of surviving cells was determined with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (19, 20). Several recombinant TNF preparations were included as standards, the detection limit being ≈0.5 pg/ml.
NOx Determination in Serum Samples.
The NOx concentration in the serum, being the sum of the stable NO metabolites nitrate and nitrite, was determined as described (21). Thirty microliters of nitrite and nitrate standards (added to pooled normal mouse serum) or of samples was transferred to V-shaped 96-well microtiter plates. Pseudomonas oleovorans bacteria were quickly thawed and diluted in TC100 medium up to a final concentration of 5 × 109 colony-forming units per ml. Thirty microliters of this bacterial suspension was added to samples and to the nitrate standard, and incubated for 2 h at 37°C. TC100 medium (30 μl) was added to the nitrite standard. Then, the plates were centrifuged at 1,300 × g for 5 min to remove the bacterial pellet. Forty microliters of supernatant was transferred to other V-shaped 96-well microtiter plates, to which 80 μl of Griess reagent was added, namely, one part of 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride in water and one part of 1% sulfanilamide in 5% phosphoric acid. After mixing, 80 μl of 10% trichloroacetic acid was added to every well, and the plate was centrifuged at 1,300 × g for 15 min to remove the protein precipitate. Finally, 120 μl of supernatant was transferred to flat-bottom, 96-well microtiter plates; the absorbance was determined at 546 nm (test wavelength) and 620 nm (background).
Alanyltransferase (ALT) Determination.
Serum ALT was measured with an ALT kit from Sigma.
Mapping of TNF Resistance Genes.
To map the genes responsible for the TNF resistance of SPRET/Ei, an interspecific backcross between female (B × S)F1 mice and male C57BL/6 mice (BSB backcross) was performed. Backcross mice were injected with 150 μg TNF at the age of 8 weeks. A genome scan was conducted by using 69 microsatellite markers evenly spread across the genome. Primer sequences were obtained from the Massachusetts Institute of Technology (Cambridge) at http://carbon.wi.mit.edu:8000/cgi-bin/mouse/gmap search? database = mouserelease; primers were custom made by Life Technologies (Paisley, U.K.). PCR were performed on 100 ng of genomic tail DNA. Survival data and genotyping data were analyzed by using MAP MANAGER QTX VERSION B13 (22).
Isolation of Mouse Embryonic Fibroblasts (MEFs) and Test of Cytotoxic Response to TNF/ActD.
MEFs were isolated from 18-days-postcoitum embryos, cultured in RPMI medium 1640 (supplemented with 10% FCS, penicillin, streptomycin, gentamycin, and L-glutamine) and seeded in culture flasks. To determine the sensitivity to TNF in combination with ActD, cells were trypsinized and seeded in 96-well plates at 25,000 cells per well. TNF was added to the cells to a final concentration of 2,000 units/ml; ActD was added to a final concentration of 1 μg/ml. After 18 h of culture, the surviving cells were stained with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Isolation of Peritoneal Macrophages and Induction of IL-6.
Thioglycolate-elicited peritoneal macrophages were seeded on 24-well plates at 50,000 cells per well in RPMI medium 1640 containing 10% FCS, penicillin, streptomycin, gentamycin, β-mercaptoethanol, and L-glutamine. TNF was added to the cultures to a final concentration of 2,000 units/ml; 24 h later, supernatant was harvested and IL-6 was measured by using a 7TD1 bioassay.
Antitumor Experiments.
(B × S)F1 and C57BL/6 mice were inoculated s.c. in the hind limb with 5 × 105 B16BL/6 melanoma cells (23) on day 0. On day 10, paralesional treatment with 20 μg of TNF in combination with 10,000 units of IFN-γ was started. Mice were treated daily for 10 days. Lethality and tumor size index were scored daily. The tumor size index is expressed as a × b, a being the larger diameter of the tumor and b the largest diameter perpendicular to a.
Statistical Analysis.
Data of final lethality were analyzed by using a χ2 test. Survival curves (Kaplan–Meier plots) were compared with a log-rank test. All other data were analyzed by using a one-tailed Student's t test.
Results
SPRET/Ei Mice Are Resistant to Increasing TNF Doses.
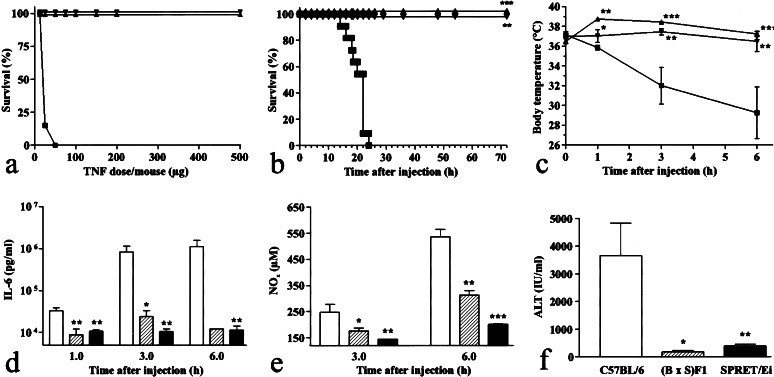
Several mouse strains were tested for their lethal response to TNF. Groups of female mice (n ≥ 6) were injected with increasing amounts of TNF. Most strains have an LD100 of ≈12.5–25 μg of TNF (C57BL/6, C3H/HeOuJ, LP/J, and PL/J). Compared with the commonly used laboratory strain C57BL/6, the strains BALB/cJIco, CAST/Ei, and SWR/J were slightly more sensitive to the lethal effect of TNF. The strains FVB/NJ, MBT/Pas, DBA/2CrlBR, CBA/J, NOD/LtJ, and MAI/Pas displayed a moderate resistance, the LD100 being 50–100 μg per mouse. Of all strains tested, SPRET/Ei is the most resistant with an LD100 of >500 μg of TNF (Fig. 1a). Among the C57BL/6 mice, 85% died from a dose of 25 μg and 100% from 50 μg. SPRET/Ei mice were completely resistant to all doses of TNF injected (up to 500 μg per mouse). The resistance of SPRET/Ei is a dominant trait because (B × S)F1 mice seemed to be as resistant to TNF as SPRET/Ei, at least in the applied dose range of maximally 500 μg per mouse.
Figure 1.
Response of C57BL/6, (B × S)F1, and SPRET/Ei mice to TNF. (a) Lethal response of C57BL/6 (■), (B × S)F1 (▾), and SPRET/Ei (▴) mice to increasing doses of TNF injected i.p. Lethality was monitored for 1 week (no further deaths occurred). n = 8, except for 150 μg and 200 μg where n = 2. (b) Kinetics of lethal response after injection of 150 μg TNF. ■, C57BL/6 (n = 24); ▾, (B × S)F1 (n = 13); ▴, SPRET/Ei (n = 7). (c) Induction of hypothermia after injection of 150 μg of TNF. ▪, C57BL/6 (n = 4); ▾, (B × S)F1 (n = 4); ▴, SPRET/Ei (n = 5). (d–f) IL-6, NOx, and ALT concentrations after injection of 150 μg of TNF. White bars, C57BL/6 mice (n = 5); hatched bars, (B × S)F1 mice (n = 4); black bars, SPRET/Ei mice (n = 5). *, P < 0 05, **, P < 0.01; ***, P < 0.0001 (compared with C57BL/6 mice).
Response of C57BL/6, (B × S)F1, and SPRET/Ei Mice to TNF.
To study the resistance of SPRET/Ei mice in more detail, a dose of 150 μg of TNF was given i.p., which is lethal to C57BL/6 but not to SPRET/Ei mice. A dose of 150 μg of TNF caused 100% lethality in C57BL/6 mice (n = 24) (Fig. 1b). Deaths occurred between 15 and 24 h after the challenge. In contrast, none of the SPRET/Ei mice (n = 7; P < 0.0001) and (B × S)F1 mice (n = 13; P < 0.0001) succumbed to the TNF challenge.
After injection of TNF, a dramatic decrease in body temperature was observed in C57BL/6 mice. Within 6 h, the body temperature dropped from normal to less than 30°C. SPRET/Ei and (B × S)F1 mice were significantly protected against TNF-induced hypothermia (Fig. 1c). On the contrary, SPRET/Ei mice developed an initial febrile response (P < 0.0001 compared with t0), which normalized within 6 h after the treatment (B × S). F1 mice did not respond.
Injection of TNF induces the appearance of high concentrations of IL-6 in the serum (24). One, three, and six hours after injection of TNF, mice were bled and IL-6 was measured in the serum. TNF was able to induce high amounts of IL-6 in C57BL/6 as well as in SPRET/Ei and (B × S)F1 mice. However, the serum concentrations in SPRET/Ei and F1 mice remained significantly lower than in C57BL/6 mice, especially at later time points (Fig. 1d). No IL-6 could be detected in noninjected controls.
TNF is also known to induce NO (25), a potent vasodilator. The NO concentration in the serum inversely correlates with the mean arterial blood pressure (26). Three and six hours after the challenge with TNF, serum was collected and NOx was measured. At both time points, significantly less induction of NOx was observed in SPRET/Ei and (B × S)F1 mice than in C57BL/6 mice (Fig. 1e). F1 mice had an intermediate response compared with their parental strains. No NO could be detected in noninjected controls.
TNF induces necrosis of hepatocytes (9). A measure for this damage is the appearance of liver-specific enzymes in the serum, such as ALT. Six hours after the TNF challenge, mice were bled and ALT was measured. Significantly less ALT was found in the serum of SPRET/Ei and (B × S)F1 mice than in C57BL/6 mice (Fig. 1f). ALT levels in noninjected controls were below the detection limit of 50 units/liter.
Injection of TNF leads to hemorrhage and necrosis of the bowel, which contributes to lethality (14). Six hours after TNF injection, the jejunum was sampled and tissue sections were stained with hematoxylin/eosin. TNF induced severe tissue destruction both in C57BL/6 and (B × S)F1 mice: the morphology of the epithelium was destroyed, crypts were lost, and severe erosion was observed. In SPRET/Ei mice, however, no such signs of tissue destruction were observed and the morphology of the bowel seemed normal (not shown). To exclude altered clearing of the TNF injected as a reason for resistance of SPRET/Ei mice, the TNF concentration was measured 3 and 6 h after injection of 150 μg of TNF (later sampling was impossible because of insufficient blood pressure in C57BL/6 mice). Significant differences in serum TNF concentrations were not observed (not shown).
TNF Resistance of SPRET/Ei Mice Is Linked to Chromosomes 2, 6, and 11.
To map the genes conferring resistance to TNF in SPRET/Ei mice, an interspecific backcross was set up. Female (B × S)F1 mice were crossed back to C57BL/6JIco males. The offspring, BSB backcross mice, were injected at the age of 8 weeks with 150 μg of TNF, a dose at least 3× LD100 for C57BL/6JIco, but nonlethal for SPRET/Ei mice. Of 178 mice tested, 52 survived the challenge, which is significantly different from what would be expected from a simple Mendelian inheritance pattern, predicting 89 surviving mice (P < 0.0001). Thus, TNF resistance is a complex trait, controlled by multiple loci.
A genome scan on 167 backcross animals was performed (11 DNA samples were lost). Survival data and genotyping data were introduced into MAP MANAGER QTX B13. Linkage analysis was performed by using the “QT LINKS” of the program. A lethal response was defined as 1 and a surviving response as 20. Likelihood ratio statistic (LRS) thresholds corresponding to suggestive linkage (genome-wide type I error probability of 0.63) and significant linkage (genome-wide type I error probability of 0.05) were determined empirically by using a permutation test. The phenotype data were randomly permutated 5,000 times among the progeny. An LRS value was calculated for each permutated set at regular intervals throughout the genome, after which the maximal LRS value was recorded. Values at the appropriate percentile points of the empirical distribution were used as threshold values to establish significance. Suggestive linkage corresponded to an LRS value of 6.5, and significant linkage to an LRS value of 12.8. These criteria are somewhat less stringent than those determined previously, the LRS value corresponding to significant linkage being set at 15 (27). By using the empirically determined criteria for significance, loci on chromosomes 6 and 11 were found to show significant linkage to TNF response. A locus on chromosome 2 showed suggestive linkage and a locus on chromosome 7 was approaching the suggestive level (Table 1). To obtain a more precise location of putative quantitative trait loci (QTL), a simple-interval-mapping analysis was performed. Such an analysis evaluates the association between the phenotype and the expected contribution of hypothetical QTL at multiple analysis points between each pair of adjacent marker loci. The analysis point yielding the most significant association may be regarded as the location of putative QTL (22). Simple-interval-mapping analysis with MAP MANAGER QTX B13 also showed linkage to chromosomes 2, 6, and 11 (Fig. 2). However, with the current data, these localizations are rather poor, as can be deduced from the corresponding 95% confidence intervals (Table 1).
Table 1.
Markers showing linkage to TNF hyporesponsiveness
| Locus | Position* | N† | LRS | %‡ | P value | 95% CI§ | Effect¶ |
|---|---|---|---|---|---|---|---|
| D2MIT32 | 11 | 164 | 9.4 | 5 | 0.00217 | 66 | + |
| D2MIT417 | 15 | 162 | 9.9 | 5 | 0.00164 | 67 | + |
| D2MIT367 | 26 | 161 | 5.7 | 3 | 0.01723 | 112 | + |
| D6MIT132 | 32 | 166 | 6.2 | 3 | 0.01248 | 108 | + |
| D6MIT103 | 45 | 157 | 9.5 | 5 | 0.00208 | 69 | + |
| D6MIT104 | 45 | 155 | 18.8 | 10 | 0.00001 | 35 | + |
| D6MIT150 | 51 | 144 | 16.5 | 9 | 0.00005 | 42 | + |
| D6MIT194 | 51 | 167 | 16.1 | 9 | 0.00006 | 36 | + |
| D7MIT222 | 38 | 158 | 6.7 | 3 | 0.00965 | 114 | − |
| D11MIT364 | 40 | 158 | 6.6 | 3 | 0.01008 | 114 | − |
| D11MIT333 | 70 | 157 | 15.3 | 9 | 0.00009 | 38 | − |
Expressed in centimorgans according to http://www.informatics.jax.org.
Population size.
Observed variance attributable to locus.
Confidence interval according to ref. 38.
+, resistance gene; −, sensitivity gene.
Figure 2.
Simple-interval-mapping analysis of backcross data. *, Suggestive level (genome-wide type I error probability of 0.63); **, significant level (genome-wide type I error probability of 0.05).
The effect of individual QTL on the overall variance is rather small, ranging from 5 to 10% (Table 1). The cumulative effect of several detected QTL accounts for ≈25% of the total variance, which might mean that either epistatic interactions between detected QTL or some additional QTL were missed. With the current data, one might expect that QTL accounting for less than 6.3% of the observed variance are not detected as significant.
MEFs Derived from M. spretus Are Sensitive to TNF/ActD-Induced Cell Death.
To test the cytotoxicity mediated by TNF-RI, MEFs from C57BL/6, (B × S)F1, and M. spretus mice were isolated and their sensitivity to TNF in combination with ActD was compared. MEFs from both C57BL/6 and (B × S)F1 mice were equally sensitive to the cytotoxic effects of TNF. MEFs from M. spretus, on the other hand, were ≈25 times more sensitive (Fig. 3a). Consequently, M. spretus still has a functional TNF-RI, capable of inducing cell death after binding of TNF in MEFs, at least at the 18-day postcoitum stage.
Figure 3.
In vitro response to TNF. (a) Sensitivity of MEFs from C57BL/6, (B × S)F1, and M. spretus. The absorbance of untreated cultures was regarded as 100%. (b) IL-6 induction on primary macrophages. White bars, C57BL/6; hatched bars, (B × S)F1; black bars, M. spretus. ***, P < 0.0001 compared with C57BL/6 mice. Data are representative of three independent experiments.
SPRET/Ei Mice Are Not Resistant to TNF/GalN.
To substantiate that TNF-RI-mediated cell death in SPRET/Ei mice is intact, the lethal response to TNF in combination with GalN was investigated. TNF-RI induces lethal hepatitis in mice after injection of TNF or TNF + GalN, a hepatotoxin that specifically inhibits transcription in hepatocytes (28) and sensitizes mice to TNF-induced lethality by a 100 factor (29, 30). C57BL/6 and SPRET/Ei mice were given a dose of 0.5 μg of TNF in combination with 20 mg of GalN. SPRET/Ei mice (8 of 12 deaths) were not significantly protected compared with C57BL/6 mice (15 of 16 deaths; P = 0.0641).
Primary Macrophages from SPRET/Ei and (B × S)F1 Mice Produce Significantly Less IL-6 than Macrophages from C57BL/6 Mice After Stimulation with TNF.
Binding of TNF to TNF-RI leads to cell death, but also to gene induction, mainly by activation of the transcription factor NF-κB (2). To test whether gene induction by TNF-RI was intact, macrophages from C57BL/6, SPRET/Ei, and (B × S)F1 mice were stimulated with 2,000 units/ml TNF; the IL-6 concentration in the supernatant was measured 24 h later. Macrophages isolated from (B × S)F1 and SPRET/Ei mice produced significantly less IL-6 in response to TNF than macrophages isolated from C57BL/6 mice (Fig. 3b).
Tumor-Bearing (B × S)F1 Mice Are Resistant to TNF/IFN-γ-Induced Lethality.
The antitumor activity of TNF in TNF-resistant (B × S)F1 mice was investigated with the model B16BL/6 melanoma. Tumor-bearing mice were treated daily with 20 μg of TNF in combination with 10,000 units of IFN-γ, a dose 2- to 4-fold higher than the one usually used (5, 31). Two independent experiments were performed (Fig. 4). Treatment of tumor-bearing C57BL/6 mice resulted in a total regression of the tumor (Fig. 4 c and d), but also in 100% lethality within 6 days from the start of the treatment (Fig. 4 a and b). B16BL/6 tumors grew equally well in (B × S)F1 and C57BL/6 mice. Treatment of tumor-bearing (B × S)F1 mice also resulted in a nearly complete regression (Fig. 4 c and d), but the toxic treatment resulted either in no lethality (Fig. 4a) or in only 30% lethality (Fig. 4b). The difference might be explained by the use of another batch of IFN-γ in the second experiment.
Figure 4.
Survival and tumor size index of B16BL/6 melanoma-bearing mice after a combined therapy with TNF and IFN-γ. ■ and white bars, C57BL/6 mice; ▾ and black bars, (B × S)F1 mice. **, P < 0.01; ***, P < 0.0001.
Discussion
To render TNF a safer and generally applicable antitumor drug and to inhibit those pathologies in which TNF plays a central role, it is essential to identify and to understand the mechanism of action of protective genes. The identification of such genes depends on two conditions: first, sensitive and resistant parental mouse strains must be identified; second, such parental strains have to display a broad genetic variation. We screened many inbred strains of mice by using TNF, and evaluated the LD100. The SPRET/Ei strain, compared with C57BL/6, seemed to be extremely resistant, namely by at least one log unit. SPRET/Ei is an inbred mouse strain, derived from the western Mediterranean short-tailed species M. spretus, a species that diverged from M. musculus ≈3 million years ago (32). Because of the long period of evolutionary divergence, the genetic variation between strains belonging to the M. musculus species (C57BL/6) and SPRET/Ei, belonging to the M. spretus species, is enormous. More than 90% of all known microsatellites are polymorphic between M. spretus and any of the commonly used laboratory strains (belonging to M. musculus) (32).
In this report we demonstrate that SPRET/Ei is extremely resistant to TNF-induced lethal shock, which is a dominant trait. We show that all TNF-induced changes in metabolic parameters do not occur in SPRET/Ei mice, or at a much reduced level: induction of hypothermia, IL-6, NO, and serum ALT, as well as necrosis of the epithelial layer of the small intestine. Induction of IL-6 by TNF occurs in SPRET/Ei and F1 mice, but remains ≈2 log units lower compared with C57BL/6.
Taking advantage of the genetic variation between SPRET/Ei and C57BL/6, we set up a backcross to map the TNF resistance genes. This experiment showed that the TNF response is linked to loci situated on chromosomes 6 and 11. Suggestive linkage is found with loci on chromosome 2. The loci on chromosomes 6 and 2 seem to be protective, whereas the locus on chromosome 11 seems to have a sensitizing effect. Because of the current resolution of the mapping experiment it is impossible to discuss in depth potential candidate genes located on these chromosomes. As clear from the rather large 95% confidence intervals obtained, any discussion of candidate genes is speculative. However, the tnfrsf1a gene, coding for TNF-RI, is located on distal chromosome 6 (33). A candidate gene on proximal chromosome 2 would be traf2 (34), coding for TNF receptor-associated factor 2, which is an important signaling molecule involved in activating NF-κB after TNF induction, the p38 mitogen-activated protein kinase pathway, and the Jun N-terminal kinase/stress-activated protein kinase pathway (35).
To investigate the role of a putatively defective TNF-RI in SPRET/Ei mice, we performed an in vitro study by using MEFs and primary macrophages. MEFs derived from (B × S)F1 and M. spretus seemed to be fully responsive to the cytotoxic combination of TNF and ActD. M. spretus-derived cells were even more sensitive than C57BL/6 cells to TNF/ActD-induced cell death, which agrees with observations with MEFs derived from mice deficient in TNF receptor-associated factor 2 (36). Furthermore, both SPRET/Ei and (B × S)F1 mice were not protected against TNF/GalN-induced lethal hepatitis, which strictly depends on TNF-RI. Hence we conclude that the pathway leading from TNF-RI to cell death is still functional in SPRET/Ei mice.
Primary macrophages from SPRET/Ei and (B × S)F1 mice produced significantly less IL-6 in response to TNF than macrophages from C57BL/6 mice. Such reduced IL-6 levels are in line with the reduced IL-6 serum levels found after injection of TNF in SPRET/Ei and (B × S)F1 mice. Therefore, we assume that the pathway leading to gene induction (IL-6) is defective in SPRET/Ei mice. In this respect, it should be noted that certain mutations in the membrane-proximal region of the intracellular part of TNF-RI have been shown to abrogate gene induction (NO synthase), but not to affect induction of cytotoxicity (37).
We also demonstrate that injected TNF is cleared as fast in SPRET/Ei mice as in C57BL/6 mice. SPRET/Ei mice are shown to be extremely resistant against TNF-induced lethal shock; moreover, this resistance seems to be dominant and to be linked to major loci on chromosomes 2, 6, and 11. To keep the TNF resistance of M. spretus and (B × S)F1 mice clinically relevant, a combination with IFN-γ is shown to be very promising, even at high doses.
Acknowledgments
This work was supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (Grant G023698N) and the Interuniversitaire Attractiepolen. J.S. and B.W. are fellows with the Vlaams Instituut voor de Bevordering van het Wetenschappelijk-technologisch Onderzoek in de Industrie.
Abbreviations
- ActD
actinomycin D
- ALT
alanyltransferase
- GalN
d-galactosamine
- LRS
likelihood ratio statistic
- MEF
mouse embryonic fibroblast
- QTL
quantitative trait loci
- TNF
tumor necrosis factor
- TNF-RI
p55 TNF receptor
- TNF-RII
p75 TNF receptor
References
- 1.Beyaert R, Fiers W. In: Cytokines. Mire-Sluis A R, Thorpe R, editors. San Diego: Academic; 1998. pp. 335–360. [Google Scholar]
- 2.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 3.Van Ostade X, Vandenabeele P, Everaerdt B, Loetscher H, Gentz R, Brockhaus M, Lesslauer W, Tavernier J, Brouckaert P, Fiers W. Nature (London) 1993;361:266–269. doi: 10.1038/361266a0. [DOI] [PubMed] [Google Scholar]
- 4.Erickson S L, de Sauvage F J, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan K C, Schreiber R D, Goeddel D V, Moore M W. Nature (London) 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- 5.Brouckaert P G G, Leroux-Roels G G, Guisez Y, Tavernier J, Fiers W. Int J Cancer. 1986;38:763–769. doi: 10.1002/ijc.2910380521. [DOI] [PubMed] [Google Scholar]
- 6.Bevilacqua M P, Pober J S, Majeau G R, Fiers W, Cotran R S, Gimbrone M A. Proc Natl Acad Sci USA. 1986;83:4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pober J S, Bevilacqua M P, Mendrick D L, Lapierre L A, Fiers W, Gimbrone M A. J Immunol. 1986;136:1680–1687. [PubMed] [Google Scholar]
- 8.Pober J S, Lapierre L A, Stolpen A H, Brock T A, Springer T A, Fiers W, Bevilacqua M P, Mendrick D L, Gimbrone M A. J Immunol. 1987;138:3319–3324. [PubMed] [Google Scholar]
- 9.Alexander R B, Rosenberg S A. In: Biologic Therapy of Cancer. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1991. pp. 378–392. [Google Scholar]
- 10.Liénard D, Ewalenko P, Delmotte J-J, Renard N, Lejeune F. J Clin Oncol. 1992;10:52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- 11.Waage A, Espevik T, Lamvik J. Scand J Immunol. 1986;24:739–743. doi: 10.1111/j.1365-3083.1986.tb02194.x. [DOI] [PubMed] [Google Scholar]
- 12.Beutler B, Milsark I W, Cerami A C. Science. 1985;229:869–871. [PubMed] [Google Scholar]
- 13.Tracey K J, Fong Y, Hesse D G, Manogue K R, Lee A T, Kuo G C, Lowry S F, Cerami A. Nature (London) 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 14.Tracey K J, Beutler B, Lowry S F, Merryweather J, Wolpe S, Milsark I W, Hariri R J, Fahey T J, III, Zentella A, Albert J D, et al. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 15.Vassalli P. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 16.Bonhomme F, Guénet J-L. In: Genetic Variants and Strains of the Laboratory Mouse. Lyon M F, Searle A G, editors. Oxford: Oxford Univ. Press; 1989. pp. 649–662. [Google Scholar]
- 17.Van Snick J, Cayphas S, Vink A, Uyttenhove C, Coulie P G, Rubira M R, Simpson R J. Proc Natl Acad Sci USA. 1986;83:9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espevik T, Nissen-Meyer J. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Niks M, Otto M. J Immunol Methods. 1990;130:149–151. doi: 10.1016/0022-1759(90)90309-j. [DOI] [PubMed] [Google Scholar]
- 21.Granger D L, Hibbs J B, Jr, Broadnax L M. J Immunol. 1991;146:1294–1302. [PubMed] [Google Scholar]
- 22.Manly K F, Cudmore R H, Jr, Meer J M. Mamm Genome. 2001;12:930–932. doi: 10.1007/s00335-001-1016-3. [DOI] [PubMed] [Google Scholar]
- 23.Hart I R. Am J Pathol. 1979;97:587–600. [PMC free article] [PubMed] [Google Scholar]
- 24.Libert C, Brouckaert P, Shaw A, Fiers W. Eur J Immunol. 1990;20:691–694. doi: 10.1002/eji.1830200333. [DOI] [PubMed] [Google Scholar]
- 25.Geng Y, Lotz M. J Cell Biol. 1995;129:1651–1657. doi: 10.1083/jcb.129.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Julou-Schaeffer G, Gray G A, Fleming I, Schott C, Parratt J R, Stoclet J C. Am J Physiol. 1990;259:H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- 27.Lander E, Kruglyak L. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 28.Decker K, Keppler D. Rev Physiol Biochem Pharmacol. 1974;71:77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- 29.Freudenberg M A, Galanos C. Infect Immun. 1991;59:2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel P, Köntgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Nature (London) 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 31.Cauwels A, Van Molle W, Janssen B, Everaerdt B, Huang P, Fiers W, Brouckaert P. Immunity. 2000;13:223–231. doi: 10.1016/s1074-7613(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 32.Silver L M. Mouse Genetics: Concepts and Applications. New York: Oxford Univ. Press; 1995. [Google Scholar]
- 33.Takao S, Mykytyn K, Jacob C O. Immunogenetics. 1993;37:199–203. doi: 10.1007/BF00191885. [DOI] [PubMed] [Google Scholar]
- 34.Nakano H, Shindo M, Yamada K, Yoshida M C, Santee S M, Ware C F, Jenkins N A, Gilbert D J, Yagita H, Copeland N C, Okumura K. Genomics. 1997;42:26–32. doi: 10.1006/geno.1997.4697. [DOI] [PubMed] [Google Scholar]
- 35.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodgett J R, Balsano C, Levrero M. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 36.Yeh W C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J L, Ferrick D, Hum B, Iscove N, et al. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 37.Tartaglia L A, Ayres T M, Wong G H, Goeddel D V. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 38.Darvasi A, Soller M. Behav Genet. 1997;27:125–132. doi: 10.1023/a:1025685324830. [DOI] [PubMed] [Google Scholar]