The authors report Decr2-deficient tumors as more resistant to ferroptosis, which diminishes the induction of polyunsaturated ether phospholipids. Facilitating tumor cell ferroptosis has the potential to improve immunotherapy efficacy.
Abstract
Immune checkpoint blockade therapies have transformed the landscape of cancer care, but durable clinical responses are achieved in only a subset of patients. To identify genes that can contribute to immunotherapy resistance, a genome-wide CRISPR screen was performed. Selection for mutants that are resistant to T cell–mediated killing identified the gene encoding Decr2, a peroxisomal 2,4-dienoyl-CoA reductase. We show that Decr2 in tumor cells participates in CD8+ T cell–mediated tumor cell killing and that Decr2 knockdown reduces the efficacy of anti–PD-L1 therapy in vivo. Knocking down Decr2 expression resulted in diminished ferroptosis that was associated with reduced induction of polyunsaturated ether phospholipids. Analysis of tumor RNA sequencing data from patients with melanoma revealed that upregulation of Decr2 was associated with anti–PD-1 efficacy, and patients with Decr2 gene deletions showed worse clinical outcomes. Our results identify Decr2 as a regulator of immunomediated tumor cell killing, with implications for improving immunotherapy efficacy.
Introduction
Immune checkpoint blockade has emerged as one of the most important therapeutic options for patients with a broad array of cancer types (1, 2). However, despite this substantial advancement in clinical care, the majority of patients receiving checkpoint blockade therapies do not achieve durable responses (3, 4). Therefore, it is critical to understand the mechanisms driving immunotherapy resistance. Data from our laboratory and from others have suggested that the lack of a T cell–inflamed tumor microenvironment (TME) represents a major mechanism of primary resistance to immunotherapies (5–12). However, not all patients with T cell–inflamed tumors develop a clinical response, and it has become clear that up to 25% of responding patients can ultimately develop progressive disease over time after initial benefit (13–15). These observations indicate that secondary resistance also can emerge, and new research efforts are being invested to identify and understand acquired resistance to immunotherapies, such as anti–PD-1.
CD8+ cytolytic T cells are key effectors of antitumor immunity because they are capable of directly killing transformed cells (16). Traditionally, cytolytic T cells have been thought to eliminate tumor cells by inducing cell death via the process of apoptosis (16, 17), either through the action of cytotoxic granules or through engagement of death receptors, such as Fas. However, recent evidence has suggested that T cell–mediated tumor cell killing might also occur through a distinct process called ferroptosis (18, 19). Ferroptosis is a mechanism of cell death distinct from apoptosis that is driven by excessive lipid peroxidation, through a mechanism dependent on iron, free radicals, and fatty acids (18). During ferroptosis, polyunsaturated fatty acids–phospholipids (PUFA-PL) are the most susceptible to peroxidation. The acyl-CoA synthetase long-chain family member 4 (ACSL4)–lysophosphatidylcholine acyltransferase 3 (LPCAT3) pathway has been implicated in the production of most PUFA-PLs during ferroptosis, and peroxisome-dependent PUFA ether PLs (PUFA-ePL) production constitutes a second pathway (20). Ferroptosis is countered by glutathione (GSH) synthesis, and glutathione peroxidase 4 (GPX4) is a central repressor of ferroptosis in cancer cells (21). Cystine, as one of the GSH synthesis substrates, is imported into cells by the transporters SLC7A11 and SLC3A2 (22). Recent work has implicated ferroptosis in T cell–mediated tumor control in mouse model systems. Ferroptosis was found to be induced in tumor cells by activated CD8+ T cells (19). Tumor cells engineered to lack ACSL4 were resistant to ferroptosis inducers in vitro (23–25) and showed diminished responsiveness to anti–PD-L1 therapy in vivo (19).
Genome-wide CRISPR screens have emerged as a valuable tool to identify critical tumor cell–expressed genes for cancer immunotherapy efficacy (26, 27). To identify genes essential for tumor cell killing by cytolytic T cells, we performed a genome-wide CRISPR–Cas9 screen to identify mutants that escaped the cytolytic activity of antigen-specific CD8+ T cells in vitro. Decr2, peroxisomal 2,4-dienoyl-CoA reductase, was identified by this screen. Decr2-deficient tumors were more resistant to antigen-specific CD8+ T-cell killing in vitro and responded poorly to anti–PD-L1 therapy in vivo. Knockdown (KD) of Decr2 resulted in diminished ferroptosis, triggered either by pharmacologic inducers or antigen-specific CD8+ T cells. Mechanistic studies revealed that Decr2 KD resulted in diminished induction of PUFA-ePLs, which was due to a reduced PUFA-ePL biosynthesis rate. An analysis of melanoma tumors from human patients revealed that upregulation of Decr2 was associated with anti–PD-1 efficacy, and Decr2 gene deletions were identified that were associated with worse clinical outcomes. Our results suggest that Decr2 is a regulator of immunomediated tumor cell death and strategies to facilitate tumor cell ferroptosis could improve immunotherapy efficacy.
Materials and Methods
Tumor cell lines
B16F10 (RRID: CVCL_0159; ATCC, 2014) cells were engineered to express DsRed fused in-frame with the model antigen SIYRYYGL, as previously described (28). The MC38 (RRID: CVCL_B288) cell line was generously provided by Dr. Yang-Xin Fu (UT Southwestern) in 2000. DsRed expression was routinely monitored as it can shift over time, and flow cytometric sorting was performed periodically to ensure uniform expression. HEK293T (RRID: CVCL_0063) cells were purchased from ATCC in 2016. The human diffuse large B-cell lymphoma cell line OCI-Ly8 (RRID: CVCL_8803) was provided by Dr. Justin Kline (University of Chicago) in 2024. B16.SIY and MC38 cells were maintained in complete DMEM Growth Media (Gibco, 11-995-073), 10% FBS (Atlanta Biologicals), 1% penicillin–streptomycin (Gibco, 15-140-122), and 1% 3-(N-morpholino) propanesulfonic acid buffer (Sigma-Aldrich, M1254). HEK293T cells were maintained in DMEM Growth Media (Gibco, 11-995-073) with 10% FBS (Atlanta Biologicals). OCI-Ly8 cells were maintained in RPMI-1640 (Gibco) containing 10% FBS (Atlanta Biologicals), 1× minimum essential medium (MEM) nonessential amino acid solution (Gibco, 11140050), 1 mmol/L sodium pyruvate (Gibco, 11360-070), 1% GlutaMAX (Gibco, 35050061), 1% N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES; Gibco, 15630080), and 0.1% β-2-mercaptoethanol (Gibco, 31350010). All the cell lines were authenticated via RNA sequencing (RNA-seq) or morphologic analysis and yearly confirmed to be negative for Mycoplasma contamination (R&D Systems, CUL001B). All cell lines were used in experiments within 10 passages after thawing.
Lentivirus production
To generate a genome-wide CRISPR guide RNA (gRNA) library lentivirus, 6 × 106 cells HEK293T (RRID: CVCL_0063) cells were seeded in a 10-cm dish 1 day before transfection. An hour before transfection, the complete RPMI medium was replaced by 10 mL of freshly made antibiotics-free medium. Cells were transfected with Lipofectamine 3000 according to the manufacturer’s instruction (Invitrogen). Briefly, 5 μg of pooled mouse gRNA plasmid library (RRID: Addgene_67988) was complexed with 3.75 μg psPAX2 (RRID: Addgene_12260), 2.5 μg pMD2.G (RRID: Addgene_12259), and 30 μL P3000 reagent in 750 μL Opti-MEM for each dish. About 30 μL Lipofectamine 3000 was diluted with 750 μL Opti-MEM and combined with a mixture of plasmids and P3000 reagent. The mixture was incubated for 10 minutes at room temperature and then added dropwise to the HEK293T (RRID: CVCL_0063) cells. The medium containing lentivirus was collected 48 hours after transfection.
Screening transduced tumor cells for resistance to T cell–mediated killing in vitro
B16.SIY cells were transfected by the Cas9 piggyBac vector (RRID: DGRC_1156) and selected by blasticidin to generate a stable Cas9 expression cell line. The B16.SIY Cas9 cell line was then transduced by freshly made genome-wide pooled gRNA library lentivirus (29). To cover the whole genome–wide library, 6 × 107 cells were transduced in two independent experiments, and transduction efficiency was about 30%. After transduction, B16.SIY cells were puromycin selected for 3 days and expanded for 5 days. For one screen, 5 × 107 cells were co-cultured with pre-activated 2C T cells at a 1:2 target:effector ratio overnight (16 hours). The T cells were removed, and the surviving B16.SIY cells were cultured to recover for 1 week. Two independent screens were performed. To evaluate single-guide RNA (sgRNA) enrichment, cells with and without T-cell co-culture were collected (along with an early timepoint after puromycin selection) and frozen. Genomic DNA was extracted from living cells by a two-step PCR amplification performed on genomic DNA using Q5 High-Fidelity DNA Polymerases (New England Biolabs). The first-step PCR (PCR1) included amplification of the region containing the sgRNA cassette using gRNA F1 (CTTGAAAGTATTTCGATTTC); gRNA R (ACTCGGTGCCACTTTTTCAAGT) primers, and the second-step PCR (PCR2) included amplification using uniquely barcoded Illumina adapter-containing primers F2: AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTCTTGAAAGTATTTCGATTTC; R2 index 2: CAAGCAGAAGACGGCATACGAGATACATCGGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTACTCGGTGCCACTTT (control sample amplification); and R2 index 4: CAAGCAGAAGACGGCATACGAGATTGGTCAGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTACTCGGTGCCACTTT (enriched sample amplification) to allow multiplexing of samples in a single MiSeq. About 100 μg of gDNA was used per sample, in 20 PCR1 reactions. For each PCR1 reaction, 5 μg of gDNA was used in a 50 μL reaction that was performed under cycling conditions: 95°C for 5 minutes; 18 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds; and 72°C for 3 minutes. PCR1 products for each sample were pooled and used for amplification with barcoded PCR2 primers. For each sample, we performed 10 PCR2 reactions using 5 μL of the pooled PCR1 product per PCR2 reaction. The pooled product was then gel-purified from a 1.5% E-Gel EX (Life Technologies) using the QIAquick Gel Extraction Kit (QIAGEN). Libraries were sequenced on a NovaSeq X.
The sequencing results were analyzed on MAGeCK; a one-sided test for enrichment or depletion of the sgRNAs and sgRNA rank aggregation was performed for each gene using MAGeCK [model-based analysis of genome-wide CRISPR–Cas9 knockout (KO)], with default parameter settings (30). Based on the MAGeCK output, a gene was considered to exhibit a significant enrichment/depletion of its sgRNA if P ≤ 0.05.
Cell line genetic manipulation
The Decr2 sgRNA expression vector was constructed as follows: D forward (caccAGGGAGGCACCGCTTTCCGGgt); D reverse (taaaacCCGGAAAGCGGTGCCTCCCT) 26-nt oligonucleotides were mixed at 10 mmol/L each in 10 mmol/L Tris-HCl (pH 8.0) and 5 mmol/L MgCl2 in a total volume of 100 μL. The mixture was incubated at 95°C for 5 minutes and cooled to room temperature. The duplex oligonucleotide was then cloned into the BbsI site of pKLV-U6sgRNA(BbsI)-PGKpuro2ABFP (RRID: Addgene_50946). For transient transfection of Cas9, a vector containing Cas9-GFP (Applied Biological Materials) was used. Vector transfection was performed using Lipofectamine 3000 reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. After expansion, the cells were sorted on GFP (Cas9) and blue fluorescent protein (sgRNA) expression. The sorted cells were cultured for more than 5 days. Single-cell sorting of tumor cells was performed using a BD FACSAria II Cell Sorter (BD Biosciences). Cells were sorted into single wells of a 96-well plate containing complete RPMI media [10% FBS, 0.01 M 3-(N-morpholino) propanesulfonic acid, and 100 U/mL penicillin/streptomycin]. Visible colonies from single cells occurred after approximately 2 weeks. After single-cell clones obtained confluence, they were transferred to a 24-well plate and maintained until all clones were expanded or until 18 clones from each single-cell sorting was obtained. After 1 to 2 days of expansion, an aliquot of each clone was tested for disruption of Decr2 through the sequence of Decr2 genomic DNA locus. The PCR primers used to amplify the Decr2 genomic DNA locus are Decr2 gF: TGGCAGGTACCCCAACAAAC; Decr2 gR: TCCAGAGGACATCCAGGGTA. Then, the PCR product was cloned (Zero Blunt PCR Cloning Kit from Thermo Fisher Scientific) and sequenced.
For generating the Decr2 short hairpin RNA (shRNA) KD mouse cell lines, control shRNA (SHC002) and Decr2 shRNAs (TRCN0000042100 and TRCN0000042101) were purchased from Sigma-Aldrich. TRCN0000042100 target sequence: GCTTTGTCTTTCAATGCCTTTG that had a KD efficiency of about 85% was used for most of the studies. TRCN0000042101 target sequence: CCAGGACAAAGTGGCCTTTAT.
For generating the Decr2 shRNA KD Ly8 (RRID: CVCL_8803) cell lines: control shRNA (plasmid ID: VB010000-0005mme), shRNA1 (VB230310-1354faw), and shRNA2 (VB230613-1547jkx) were purchased from VectorBuilder. The cells were stably transduced by shRNA lentivirus produced from HEK293T (RRID: CVCL_0063) cells and selected under puromycin for 4 days. The KD efficiency was confirmed by using qRT-PCR or Western blotting.
For generating the B16.SIY Decr2 overexpression cell line, control vector (VB200312-7528gkx) and Decr2 overexpression vector that contains a puromycin marker (VB190926-1134nsv) were purchased from VectorBuilder. B16.SIY cells were stably transduced with a lentivirus expressing Decr2. The overexpression of Decr2 was confirmed by using qRT-PCR and Western blotting.
qRT-PCR
Total RNA was extracted from sorted cell populations using the RNeasy Micro Kit (QIAGEN) following the manufacturer’s protocol. For qRT-PCR, cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s protocol. Decr2 qF (ATATCCGTGTCAACAGCCTG) and Decr2 qR (CTGTGAGCGATCTCTGTCTTG) were used for detecting mouse Decr2 expression level. Decr1 qF (ACCAAGGAGGAGTGGGATATAA) and Decr1 qR (CTGTAGCACTCTAGTCCCTAACT) are qPCR primers used for detecting the Decr1 expression level. Aifm2 qF (GAGTACATCAAGGTGGAGACAG) and Aifm2 qR (TCGTTCACTTTCAGAGCACC) were used for detecting Aifm2. Acsl4 qF (TTGGCTACTTACCTTTGGCTC) and Acsl4 qR (AATCACCCTTGCTTCCCTTC) were used for detecting Acsl4. Lpcat3 qF (GACATACCAGGGAAGATGCC) and Lpcat3 qR (CCAGAAAGGGCGGTTATCATAG) were used for detecting Lpcat3. Gpx4 qF (GCAATGAGGCAAAACTGACG) and Gpx4 qR (CTTGATTACTTCCTGGCTCCTG) were used for detecting Gpx4. Slc7a11 qF (TCTTCGATACAAACGCCCAG) and Slc7a11 qR (TGATAAGAAAACCGACCCCG) were used for detecting Slc7a11. Reactions were run on a StepOnePlus Real-Time PCR System (Applied Biosystems, 4376600) in MicroAmp Fast Optical 96-Well Reaction Plates (Applied Biosystems, 434907). Each sample was run in three duplicate wells, and the cycle threshold (Ct) of those wells was averaged before expression levels were calculated as follows: ΔCt = Ct GAPDH − Ct gene of interest; expression level = 2ΔCt.
Western blotting
Cells were harvested and lysed in RIPA buffer, and proteins were separated on SDS polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore). The membranes were blocked using 5% nonfat milk, then immunoblotted with primary antibodies at 4°C overnight. After being washed three times with TBS with Tween 20, membranes were incubated for 1 hour with appropriate secondary antibodies at room temperature. Vinculin (ab129002) was used as the loading control. Membranes were visualized using ImageQuant LAS 4000 (General Electric Company). Anti–human DECR2 (ab153849) was used to detect Ly8 DECR2 expression. Anti–mouse Decr2 (ARP43567_P050 from Aviva Systems Biology) was used to detect B16.SIY DECR2 expression. Anti–mouse Slc7a11 (ab175186) and anti–mouse GPX4 (ab125066) were used to detect SLC7A11 and GPX4 separately.
2C T-cell isolation and co-culture with SIY+ tumor cells
2C/Rag−/− mouse splenocytes were isolated and homogenized. CD8+ T cells were purified using EasySep Mouse Naïve CD8+ T Cell Isolation Kit (STEMCELL Technologies). 2C/Rag−/− CD8+ T cells (5 × 105 cell/mL) were seeded in a nontissue culture–treated 24-well plate coated with 5 g/mL of goat anti-hamster antibody. To activate the CD8+ T cells, 1 μg/mL CD3 antibody (RRID: AB_2892846), 1 μg/mL CD28 antibody (RRID: AB_321560), and 50 U/mL hIL2 (Thermo Fisher Scientific) were added to complete RPMI medium [complete RPMI medium contains: RPMI, 10% FBS, 100 U/mL penicillin–streptomycin (Thermo Fisher Scientific, cat. #15140122), and 50 μmol/L β-mercaptoethanol]. The cells were incubated at 37°C for 3 days.
For the co-culture of 2C cells and SIY+ tumor cells, B16.SIY or MC38.SIY cells were seeded overnight; 2C CD8+ T cells were then added into the culture at different ratios. All the cells were harvested by using trypsinization and analyzed using FlowJo software (version 10.6.1 or 10.10.0, TreeStar).
Chimeric antigen receptor T-cell killing
The CD19-BBz chimeric antigen receptor (CAR) was cloned into the pHR (RRID: Addgene_205438) lentiviral vector as described previously; GFP was fused at the C-terminal of the CAR protein to track its expression, following a previously reported method (31). Cryopreserved peripheral blood mononuclear cells purchased from STEMCELL Technologies (cat. #70025) were thawed and activated on day 0 with Dynabeads Human T-Activator CD3/CD28 (Thermo Fisher Scientific, 11131D) at a 1:1 bead:cell ratio in T-cell medium, which is TexMACS GMP medium (Miltenyi Biotec, 170-076-307) supplemented with 10% FBS and 5 ng/mL recombinant human IL7 and IL15 (PeproTech, 200-07 and 200-15). Lentiviral transduction was conducted on day 1 with multiplicity of infection = 5. T cells were cultured with fresh medium every 2 to 3 days and normalized to 0.5e6 cells/mL. T cells were harvested or cryopreserved on day 10 or day 14 for functional assays.
The human diffuse large B-cell lymphoma cell line OCI-Ly8 was transduced with a lentivirus encoding tTomato and Luciferase (Addgene 48688), and tTomato-positive cells were sorted by using FACS for functional assays.
Mice
C57BL/6 mice were purchased from Taconic Biosciences (RRID: MGI:2164831) or The Jackson Laboratory (RRID: IMSR_JAX:000664). Rag2–/– (NCI) mice were bred in-house under specific pathogen–free conditions. 2C T-cell receptor (TCR) Tg × Rag2–/– mice were bred at our facility. The 2C TCR mice were crossed with Rag2–/– mice. The F1 generation of mice (2C+ Rag2+/–) were crossed to get 2C+ Rag2–/– mice.
Tumor inoculation and treatment
For tumor inoculation, 2 × 106 B16.SIY or MC38 tumor cells were subcutaneously injected into C57BL/6 mice (both male and female mice were used). Tumor area was measured three times per week and calculated using width × length. All mice were maintained according to the NIH animal care guidelines and used under Institutional Animal Care and Use Committee–approved protocols. All in vivo mouse experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Chicago.
For antibody treatments, mice received 100 μg of anti–PD-L1 (RRID: AB_2927503) intraperitoneally three times after tumor engraftment. For CD8+ T-cell depletion, mice received 200 μg of anti-CD8 (Bio X Cell, clone YTS 169.4) days before tumor engraftment and once every 7 days thereafter.
Hematoxylin and eosin staining
The tumors from each group were soaked in 4% formaldehyde solution, dehydrated through graded alcohols, and embedded in paraffin wax. The 0.5-μm-thick paraffin sections were then cut into slices from these paraffin-embedded tissue blocks. The tissue sections were deparaffinized by immersing in xylene and rehydrated. The slices were stained using hematoxylin and eosin.
Tumor-infiltrating lymphocyte and tumor cell isolation
Tumors were harvested from mice at the indicated timepoints. For tumor-infiltrating lymphocyte (TIL) isolation, tumors were digested using an enzyme mix containing Collagenase (1 mg/mL; Sigma-Aldrich, C5138), DNase (200 μg/mL; Sigma-Aldrich, D5025), and hyaluronidase (100 μg/mL; Sigma-Aldrich, H6254) for 30 minutes at 37°C while rotating. The tumor suspension was filtered through a 50-μm filter and washed with PBS.
Flow cytometry and antibodies
For the live cell analysis, cells were harvested by trypsinization, washed with PBS, and stained using FACS buffer (2% FBS, 2 mmol/L EDTA, and 0.001% NaN3). Anti-CD45 (Invitrogen, 11-0454-82) and Fixable Viability Cell Dye 506 or ef780 (eBioscience) were added for 20 minutes for staining. For tumor-infiltrating immune cells isolated from tumor-bearing mice, cells were first stained using H-2Kb/SIY-pentamer (PE-labeled; ProImmune) for 20 minutes at room temperature, followed by staining with the remaining antibodies for 20 minutes on ice. Antibodies against the following molecules were used: Thy1.2 (RRID: AB_1272237) or CD3 (RRID: AB_493696), Ly6C (RRID: AB_2565852), CD11b (RRID: AB_10896949), CD19 (RRID: AB_2562136), Ly6G (RRID: AB_2871000), I-A/I-E (RRID: AB_493528), CD11c (RRID: AB_492850), NK1.1 (RRID: AB_830870), F4/80 (RRID: AB_914344), CD45 (RRID: AB_10829797 or AB_11218871), CD8α (RRID: AB_2563056 or AB_1834433), and CD4 (RRID: AB_11219396 or AB_2819769). Fixable Viability Dye 506, eF780 (eBioscience) or Zombie NIR Fixable Viability Kit (BioLegend) was used for live/dead discrimination.
For intracellular staining, isolated tumor-infiltrating cells were first stained by using surface makers (CD45, CD8, CD4, Thy1.2, or CD3ε and Fixable Viability Dye) and then fixed using freshly prepared Fix/Perm solution (BD Biosciences) at room temperature for 1 hour. Fixed cells were washed using Perm/Wash Buffer (BD Biosciences) and stained using anti-Foxp3 (FJK-16s) or Ki67 (SolA15) for 30 minutes. After staining, cells were washed and fixed in 2% formaldehyde. All flow cytometric analyses were conducted on an LSRFortessa (BD Biosciences) and analyzed using FlowJo software (version 10.6.1 or 10.10.0, TreeStar; RRID: SCR_008520).
ELISpot
Enzyme-linked ImmunoSpot (ELISpot) plates were coated using anti–mouse IFN-γ Ab and stored overnight at 4°C. Plates were then washed and blocked using DMEM supplemented with 10% FCS for 2 hours at room temperature. Splenocytes from individual tumor-challenged mice were harvested on day 7 after tumor injection and plated in triplicate at 1 × 106 cells/well. Stimulation was performed using SIY peptide (80 nmol/L). Stimulation with media alone or with phorbol 12-myristate 13-acetate (50 ng/mL) and ionomycin (500 nmol/L) served as the negative and positive controls, respectively. Plates were stored at 37°C in an 8% CO2 incubator overnight, washed, and coated with a detection antibody for 2 hours at room temperature. Plates were again washed and coated with avidin–peroxidase for 1 hour at room temperature, then washed and developed by the addition of 3-amino-9-ethylcarbazole substrate. Developed plates were dried overnight, read using an ImmunoSpot Series III Analyzer, and analyzed using ImmunoSpot software for analyzing ELISpot assays (RRID: SCR_011082).
IFN-γ, TNF-α, and IL2 detection
A multiplex assay for cytokines was performed using LEGENDplex MU Th Cytokine Panel (12-plex) w/VbP V03 from the BioLegend (cat. #741044), by following the kit instructions.
Single-cell RNA sequencing analysis
The tumors were isolated from tumor-bearing mice and digested. CD45+ live cells were sorted using the Big Foot sorting machine (Thermo Fisher Scientific). Single-cell partitioning and library creation were performed by the University of Chicago Genomics Core using a chromium controller from 10× Genomics by following the “Chromium Single Cell 3′ Reagent Kits v3” user guide and associated reagents. A total of 10,000 cells per sample were targeted for encapsulation. Libraries were sequenced in PE100 format of an SP-100 flow cell of an Illumina NovaSeq X instrument. Each group contained three tumor samples. We processed each sample individually using the count command from 10× Genomics Cell Ranger (v6.1.2) against the mouse mm10-2020 reference. We then carried out downstream quality control and data integration using the R package scRICA (https://github.com/yan-cri/scRICA/tree/main). scRICA automates a standard Seurat v4 (32) integration workflow and was run with filters to remove any genes expressed in fewer than three cells, as well as any cells with <500 features or >20% mtRNA content. Putative doublets were removed using the tool DoubletDecon (default parameters; ref. 33), and the integration was implemented with a standard Seurat workflow, using 3,000 variable features to find anchors and by using the Reciprocal Principal Component Analysis method.
After QC and data integration, we identified clusters using FindClusters, retaining the results at resolution 0.8 for annotation. We then found the top markers defining each cluster using FindMarkers; more specifically, B cells: Cd19+, Cd79a+, and Ms4a1+; CD8 T cells: Cd3+ (d, e, or g); and Cd8a+—cell phase was determined using the CellCycleScoring function from the Seurat package with genes associated with G2–M- and S-phases taken from a resource in this tutorial (https://hbctraining.github.io/scRNA-seq/lessons/cell_cycle_scoring.html)—CD4 T cells: Cd3+ and Cd4+; regulatory T cells (Treg): Cd4 T cells with Foxp3 expression; and NK T cells: Nkg7+ and Cd8−. These marker combinations were used to identify and classify the respective immune cell populations. A significant fraction of the cluster expressed Klrb1c; NK cells: Nkg7+ and Cd3−; macrophage: Itgam+ and Adgre1+; dendritic cells (DC): merged smaller clusters that were plasmacytoid DCs (Siglech+), cDC1s (Xcr1+ or Clec9a+), and likely cDC2s (Sirpa+ Adgre1−); and others: the remaining clusters.
Immunofluorescence staining and confocal image analysis
B16 (RRID: CVCL_0159) cells were transfected with pLV-Decr2-Flag (VectorBuilder, cat. #VB210111-1234naf). B16 (TKG, cat. #TKG 0144, RRID: CVCL_F936) control cells and cells expressing Decr2-Flag were seeded in Corning BioCoat Poly-D-Lysine four-well culture slide (cat. #354577) overnight (2 × 104) before staining. The cells were fixed using 4% paraformaldehyde in PBS pH 7.4 for 10 minutes at room temperature. The fixed cells were washed one time with PBS before being permeabilized with 0.1% Triton X-100 in PBS for 10 minutes. Permeabilized cells were washed again one time with PBS for 5 minutes and blocked using 1% BSA in PBS with Tween 20 for 30 minutes at room temperature. The cells were incubated with rabbit anti-Flag polyclonal antibody (RRID: AB_2572291 ) at 4°C overnight. Then, they were washed using PBS three times and stained using the Alexa Fluor 488–conjugated donkey anti–rabbit IgG secondary antibody (RRID: AB_2866495) for 1 hour at room temperature. The stained cells were washed in PBS three times and blocked using 1% BSA in PBS with Tween 20 for 1 hour at room temperature. The blocking solution was replaced with goat anti–mouse catalase (Novus Biologicals, AF3398-SP), rat anti–mouse Lamp1 (Thermo Fisher Scientific, cat. #14-1071-82) or mouse anti–mouse Atp5a (Abcam, ab14748) and left to incubate overnight at 4°C. After incubation, the cells were washed again with PBS three times. Then, the cells were stained using donkey anti–goat IgG (H + L) cross-adsorbed secondary antibody, Alexa Fluor 647 (RRID: AB_2535864 ), donkey anti–rat IgG (H + L) highly cross-adsorbed secondary antibody, Alexa Fluor 647 (RRID: AB_2910635), or donkey anti–mouse IgG (H + L) highly cross-adsorbed secondary antibody, Alexa Fluor 647 (RRID: AB_162542) for 1 hour at room temperature. The stained cells were counterstained with 4',6-diamidino-2-phenylindole (DAPI) and mounted using ProLong Gold (Invitrogen). Immunofluorescence (IF) staining cells were imaged by using confocal SP5 (objective lenses ×40). The images were analyzed using Fuji software.
ELISA
For IFN-γ assays, CD8+ T cells were purified from 2C/Rag mice, isolated using a CD8 T-cell isolation kit (Miltenyi Biotec), and co-cultured with 2 × 104 B16.SIY tumor cell in a 24-well plate with T-cell media (RPMI-1640 + 10% FBS + 1% 2-mercaptoethanol + 1% penicillin/streptomycin). The co-cultured cells were incubated at 37°C for 48 hours. The supernatant was collected for subsequent ELISA for IFN-γ (Invitrogen, cat. #KMC4021) and analyzed on a plate reader.
Lipid peroxidation assessed by BODIPY-C11 and Liperfluo staining
B16.SIY cells (105 cells/well) were seeded in a six-well plate 1 day before the experiment, and then the cells were exposed to 105 pre-activated 2C T cells. To analyze the tumor lipid peroxidation ex vivo, tumors isolated from B6 mice were disrupted into a single-cell suspension and stained. For Liperfluo (Thermo Fisher Scientific) staining, cells were stained using Liperfluo (5 μmol/L) in PBS for 30 minutes at 37°C. Cells were washed two times by PBS and analyzed immediately using a flow cytometer.
To perform BODIPY-C11 (Thermo Fisher Scientific) staining, B16.OVA cells with or without treatment (pre-activated OT1 T cells or RSL3) were trypsinized and washed with PBS. The cells were resuspended in 500 μL PBS containing 1 μmol/L BODIPY 581/591 C11 and incubated for 30 minutes at 37°C in a cell culture incubator. Cells were washed two times with PBS and resuspended in 500 μL FACS buffer, then analyzed immediately using a flow cytometer. MC38 (RRID: CVCL_B288) cells were isolated from the tumor-bearing mice and resuspended in 1 mL PBS containing 1 μmol/L BODIPY 581/591 C11 and incubated for 30 minutes at 37°C in a tissue culture incubator. Cells were washed two times with PBS and resuspended in 500 μL FACS buffer, then analyzed immediately using a flow cytometer. For BODIPY 581/591 C11 staining, the signals from both nonoxidized C11 (Texas Red channel) and oxidized C11 (FITC channel) were recorded. The ratio of the mean fluorescence intensities of FITC to the mean fluorescence intensities of Texas Red was calculated for each sample.
Lactate dehydrogenase assay
Lactate dehydrogenase activity in supernatants was measured using the LDH Assay Kit (Cytotoxicity) by following the manufacturer’s protocol (Abcam, ab65393).
GSH/oxidized glutathione determination
Tumor cells were treated with Erastin (10 μmol/L) or DMSO for 24 hours. Cells were collected and counted. Then, 100 μL of lysis buffer was added to 105 cells. The GSH levels and oxidized glutathione (GSSG) levels were determined using a GSH/GSSG Ratio Detection Assay Kit II (Fluorometric–Green; Abcam, ab205811).
Mass spectrometry
The control shRNA or Decr2 KD B16.SIY cells were cultured with DMSO or RSL3 for 24 hours. Another set of cells were cultured with medium or pre-activated 2C CD8+ T cells for 12 hours. Then, the cells were washed three times using PBS and trypsinized. The cells were collected quickly and frozen to −80°C immediately. Each of the conditions and samples had four replicates. The samples were then shipped to Metabolon, Inc., in a box filled with dry ice. The samples were prepared using the automated MicroLab STAR system from Hamilton Company. Several recovery standards were added prior to the first step in the extraction process for QC purposes. To remove protein, small molecules bound to protein were dissociated or trapped in the precipitated protein matrix, and to recover chemically diverse metabolites, proteins were precipitated using methanol with vigorous shaking for 2 minutes (Glen Mills GenoGrinder 2000) followed by centrifugation. The resulting extract was divided into four fractions: two for analysis by two separate reverse-phase/ultra-performance liquid chromatography–tandem mass spectrometry (UPLC/MS-MS) methods with positive ion mode electrospray ionization (ESI); one for analysis by reverse-phase UPLC/MS-MS with negative ion mode ESI; and one for analysis by hydrophilic interaction liquid chromatography (HILIC)–UPLC/MS-MS with negative ion mode ESI. All methods used a Waters ACQUITY UPLC and a Thermo Fisher Scientific Q-Exactive high-resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. The sample extract was dried and then reconstituted in solvents compatible to each of the four methods. Each reconstitution solvent contained a series of standards at fixed concentrations to ensure injection and chromatographic consistency. One aliquot was analyzed using acidic positive ion conditions, chromatographically optimized for more hydrophilic compounds. In this method, the extract was gradient eluted from a C18 column (Waters UPLC BEH C18, 2.1 × 100 mm, 1.7 μm) using water and methanol, containing 0.05% perfluoropentanoic acid and 0.1% formic acid. Another aliquot was also analyzed using acidic positive ion conditions; however, it was chromatographically optimized for more hydrophobic compounds. In this method, the extract was gradient eluted from the same aforementioned C18 column using methanol, acetonitrile, water, 0.05% perfluoropentanoic acid, and 0.01% formic acid and was operated at an overall higher organic content. Another aliquot was analyzed using basic negative ion optimized conditions using a separate dedicated C18 column. The basic extracts were gradient eluted from the column using methanol and water, but with 6.5 mmol/L ammonium bicarbonate at pH 8. The fourth aliquot was analyzed via negative ionization after elution from a HILIC column (Waters UPLC BEH Amide column, 2.1 × 150 mm, 1.7 μm) using a gradient consisting of water and acetonitrile with 10 mmol/L ammonium formate, pH 10.8. The mass spectromety (MS) analysis alternated between MS and data-dependent (dd) multistage tandem mass spectrometry scans using dynamic exclusion. The scan range varied slighted between methods but covered 70 to 1,000 m/z. Biochemical components were identified on the basis of retention time/index, mass-to-charge ratio (m/z), and chromatographic data (MS-MS spectral data) by comparison with the Metabolon reference library. Peaks were quantified using the AUC. After normalization to Bradford protein concentration (cells), log transformation, and imputation of missing values (if any) with the minimum observed value for each compound, ANOVA contrasts were used to identify biochemicals that differed significantly between experimental groups. Analysis using the two-way ANOVA identified biochemicals exhibiting significant interaction and main effects for experimental parameters of genotype and treatment.
Plasmalogen biosynthesis assay
Cells were seeded in a six-well plate at a density of 2 × 105 cells per plate media containing stable isotope tracer D9-choline (3 mg/L, DLM-549-PK from Cambridge Isotope Laboratories) and 12 mmol/L μmol/L of D4-ethanolamine (DLM-552-PK from Cambridge Isotope Laboratories) supplemented with 10% dialyzed FBS, followed by additional 24 hours of growth. The plates were trypsinized, briefly washed using PBS, and an equal number of cells transferred to the tubes. An ice-cold mixture of 300 μL of methanol + 900 μL of methyl-tert-butyl ether was added to the cell pellet tubes to extract lipids. The tubes were sonicated for 3 minutes in ice-cold water bath, and vortexed for 15 minutes at 4°C and 2,000 rpm using a ThermoMixer. Phase separation was induced by adding 300 μL of ice-cold water, followed by sonication for 3 minutes, then vortexed for 15 minutes and centrifuged at 21,000g at 4°C for 15 minutes. The upper organic layer (400 μL) was dried down using the GeneVac EZ-2.4.0 Elite Evaporator System and stored at −80°C. On the analysis day, the dried lipid extract from the samples was resuspended in 80 μL of 3/2/1 isopropanol/acetonitrile/water at room temperature.
Lipids were separated on the Thermo Fisher Scientific Accucore C30 (2.1 × 150 mm, 2.6 μm) column connected to a Vanquish Horizon UHPLC System and IQ-X Tribrid mass spectrometers. The column temperature, injection volume, and flow rate were 45°C, 2 μL, and 0.26 mL/minute, respectively. The mobile phase A was 60/40 acetonitrile/water, 10 mmol/L ammonium formate+0.1% formic acid, and the mobile phase B was 89.1/9.9/0.99 isopropanol/acetonitrile/water, 10 mmol/L ammonium formate+0.1% formic acid. The chromatographic gradient was 0 minute: 30% B, 2.00 minutes: 43% B, 2.1 minutes: 55% B, 12.00 minutes: 65% B, 18.00 minutes: 85% B, 20.00 minutes: 100% B, 25.00 minutes: 100% B, 25.1 minutes: 30% B, and 30.00 minutes: 30% B.
MS1 parameters were spray voltage: 3,500 V for positive ionization and 2,500 V for negative ionization modes; sheath gas: 40; auxiliary gas: 10; sweep gas: 1; ion transfer tube temperature: 300°C; vaporizer temperature: 350°C; Orbitrap resolution: 120 K; scan range (m/z): 200 to 1,500; RF lens (%): 60; automatic gain control target: 50%; and a maxIT of 100 ms. Quadrupole isolation and internal calibration using Easy IC were enabled. Lipids were identified by performing MS2 and MS3 experiments in the Orbitrap mass analyzer. A comprehensive dd–higher-energy collisional dissociation (HCD) MS2 experiment with conditional CID MS2 and MS3 data-acquisition strategy was applied for the in-depth characterization of lipid species. Lipid fragmentation was obtained by first MS1 data acquisition in full-scan mass range (200–1,500) followed by the dd-MS2 with the normalized stepped HCD collision energy (%) at 25, 30, 35, OT-30K resolution, and maxIT of 54 ms. If the HCD fragmentation had the fragment ion 184.0733 m/z (phosphocholine head group), then the same ions were subjected to dd-MS2 collision-induced dissociation (CID; fixed collision energy: 32%; activation time: 10 ms; activation Q: 0.25, 30K resolution; 100% normalized automatic gain control target; and maxIT of 54 ms) or MS3 CID scans (fixed collision energy: 35% and activation Q: 0.25) were triggered on top three most intense ions that lost neutral fatty acid plus ammonia (only for triacylglycerol). A total of six injections were made to generate fragmentation data using the AcquireX workflow on the unlabeled pooled QC samples.
The Thermo Fisher Scientific LipidSearch 5.0 was used to generate the list of identified lipids (precursor tolerance ± 3 ppm, production tolerance ±5.0 ppm, and product threshold 1.0). The phospholipids were identified using either grade “A” or grade “B.” The identified lipids with their formulae and retention times were supplied to the Stable Isotope Labeling workflow (label element: D, max. exchange: 9, and source efficiency: 98), a part of Compound Discoverer software 3.3 to quantify labeling percentages.
IFN-γ–induced B16.SIY tumor cell lipidomic analysis
For IFN-γ–stimulated lipid analysis, B16.SIY cells were cultured with 10 ng/mL IFN-γ or medium for 48 hours, and cells were washed using PBS three times and trypsinized. The cells were collected quickly and frozen to −80°C immediately. Each of the conditions and samples had four replicates. The lipid extraction and analysis methods were conducted using the same procedures outlined in the “Plasmalogen biosynthesis assay” section mentioned earlier.
Human melanoma mRNA and copy-number alternation analysis
Raw RNA-seq data from samples of patients with advanced melanoma on pretreatment and on-treatment with nivolumab were downloaded from the Gene Expression Omnibus series GSE91061. Patients had clinical outcome data marked as progressive disease (18), stable disease (SD, 15), partial response (6), or complete response (CR, 3; ref. 34). Samples were filtered by a minimum of 10,000,000 reads, and genes were filtered using the following cutoffs: a minimum of 15 samples were required to express a given gene, with a minimum count per million of 1. Normalization was done using the “calcNormFactors” function, using the trimmed mean of M-values method. The count data were transformed using “limma-voom” and fold change for Decr2 was calculated between matched on-treatment and pretreatment samples. Violin plots of fold changes were created using the clinical outcome data response categories, and the Mann–Whitney test was performed to assess significance. The experiments were performed in a double-blind fashion.
Decr2 mRNA expression levels, copy-number alterations, and overall survival time of patients with melanoma who underwent treatment with ipilimumab were acquired from cBioPortal (immunogenomic studies, melanoma).
Human tissue IF staining
An IF protocol was developed using antibodies specific to DECR2 (Abcam, ab153849, 1/50 dilution) and SOX10 (R&D Systems, MAB2864, 1/200 dilution) on formalin-fixed, paraffin-embedded tissue sections. Antigen retrieval was performed using Akoya Biosciences antigen retrieval buffer with low pH (pH 6; cat. #AR600250ML) or high pH (pH 9; cat. #AR900250ML). Each section was subjected to two rounds of antibody staining; each round consisting of blocking (Akoya Biosciences, cat. #ARD1001EA), primary antibody incubation, horseradish peroxidase–conjugated secondary antibodies (Akoya Biosciences, cat. #ARH1001EA) incubation, tyramide signal amplification visualization using fluorophores Opal 520 (Akoya Biosciences, cat. #FP1487001KT) and Opal 690 (Akoya Biosciences, cat. #FP1488001KT) diluted in 1× Plus Amplification Diluents (Akoya Biosciences, cat. #FP1135), and pressure cooker treatment. Tissue sections were then incubated using DAPI solution (Akoya Biosciences, cat. #FP1490) for 5 minutes at room temperature and mounted in ProLong Diamond Antifade Mountant (Invitrogen, cat. #P36961). Scanning of the slides was performed using the Vectra Polaris Imaging Station and Phenochart software, version 1.1.0 (Akoya Biosciences). A supervised machine learning algorithm within inForm software, version 2.3 (Akoya Biosciences), which assigned trained phenotypes and Cartesian coordinates to cells, was used to perform image analysis and cell phenotyping. The experiments were performed in a double-blind fashion.
Data availability
Source data for all figures are provided with the article online. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Single-cell RNA-seq processed and raw data were deposited to NCBI Gene Expression Omnibus database. The accession number is GSE284699. Constructs and cell lines generated in this study will be made available upon request, but a completed Material Transfer Agreement may be required if there is potential for commercial application.
Further information and request for resources and reagents should be directed to and will be fulfilled by the corresponding author Dr. Thomas F. Gajewski (tgajewsk@medicine.bsd.uchicago.edu).
Results
Genome-wide CRISPR screen identified Decr2 as an important gene in tumor cell sensitivity to T-cell killing
To identify tumor cell–intrinsic genes essential for antigen-specific CD8+ T-cell killing, we used the B16.SIY melanoma cell line that expressed the model antigen SIY (SIYRYYGL) co-expressed with DsRed, which can be recognized by 2C CD8+ TCR transgenic (Tg) T cells. B16.SIY cells were transduced and selected to stably express a Cas9 gene and then transduced with a genome-scale CRISPR gRNA library. The library consists of 87,897 sgRNA sequences targeting 19,150 mouse protein-coding genes. Transduced cells were cultured for 8 days to provide time for gene disruption and then co-cultured with pre-activated 2C CD8+ T cells at a 2:1 effector:target ratio for 16 hours, which we had previously shown results in around 99% killing of wild-type (WT) B16.SIY cells (35). By performing deep sequencing (Fig. 1A), we examined the sgRNA library representation in tumor cells with or without T-cell co-incubation by using MAGeCK analysis (Supplementary Table S1). From the sequence of enriched gRNAs of the resistant cells, we observed that Jak1 was enriched (Fig. 1B), consistent with previous reports (26, 35). The remaining top five candidate genes were studied in confirmatory assays by individual gene targeting, and Decr2-targeted B16.SIY cells (Supplementary Fig. S1A–S1C) were confirmed to be more resistant to effector CD8+ T-cell killing in vitro (Fig. 1C) than were control cells. To confirm this phenotype with an independent technique, we generated Decr2 KD B16.SIY cells using a lentiviral shRNA approach. The KD efficiency was confirmed by using qRT-PCR and Western blotting (Supplementary Fig. S1D and S1C). Decr2 KD B16.SIY cells also showed relative resistance to 2C CD8+ T-cell killing compared with control shRNA B16.SIY cells (Fig. 1D), arguing against an off-target effect of CRISPR. We confirmed that the loss of Decr2 conferred resistance to cytotoxic T-cell killing by using an in vitro competition assay, showing that Decr2 KD tumor cells selectively grew out of a mixture with WT cells when antigen-specific CD8+ T cells were present (Supplementary Fig. S1E and S1F). Furthermore, we generated a Decr2 KD MC38.SIY cell line to examine an independent cellular context, confirmed by qRT-PCR (Supplementary Fig. S1G). Decr2 KD MC38.SIY cells also showed relative resistance to CD8+ T-cell killing in vitro compared with control shRNA infected MC38.SIY cells (Fig. 1E). To examine if the killing resistant phenotype was generalizable beyond the SIY antigen, we generated Decr2 KD B16.OVA cells and co-cultured with pre-activated OT1 CD8+ T cells that are specific for the OVA peptide. Two different shRNAs targeting Decr2 yielded a greater number of alive cells than control shRNA-infected B16.OVA cells (Supplementary Fig. S1HC). Thus, the ability of Decr2 downregulation to cause tumor cell resistance to cytotoxic CD8+ T-cell killing was not dependent on the SIY antigen. We also examined the role of tumor-expressed DECR2 in a CAR T-cell model, using OCI-LY8 lymphoma cells that are recognized by human CD19-directed CAR T cells. DECR2 KD in LY8 cells also generated relative resistance to CAR T-cell killing in vitro (Supplementary Fig. S2A and S2B). Therefore, the increased resistance of DECR2 KD tumor cells to T-cell killing can occur with CAR recognition of CD19, extending beyond class I/peptide recognition by TCRs. Together, these results demonstrate that tumor cell expression of Decr2 is required for optimal killing by CD8+ T cells in vitro.
Figure 1.
Identification of Decr2 gene as being critical for effector CD8+ T-cell killing through a genome-wide CRISPR screen. A, Diagram of genome-wide CRISPR screen. B, Enrichment score for the abundance of the top five most enriched sgRNAs for every gene after 2C CD8+ T-cell killing. C–E, Relative live cell percentage of tumor cells co-cultured with activated CD8+ T cells in vitro. Tumor cells expressing SIY peptide were co-cultured with pre-activated 2C transgenic CD8+ T cells, which specifically target SIY antigen for 16 hours at an effector:target ratio of 1. T cells were removed, and tumor cells were recovered for 48 hours in fresh medium. Live cell survival (%) was calculated from control cells unexposed to T-cell selection. C, Live cell percentage of B16.SIY control cells or the Decr2 KO clone. D, Live cell percentage of B16.SIY cells expressing control shRNA or Decr2 shRNAs. E, Live cell percentage of MC38.SIY cells expressing control shRNA or Decr2 shRNA. Data are combined from two independent experiments. Data are mean ± SD; statistical significance was determined using the two-tailed t test. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
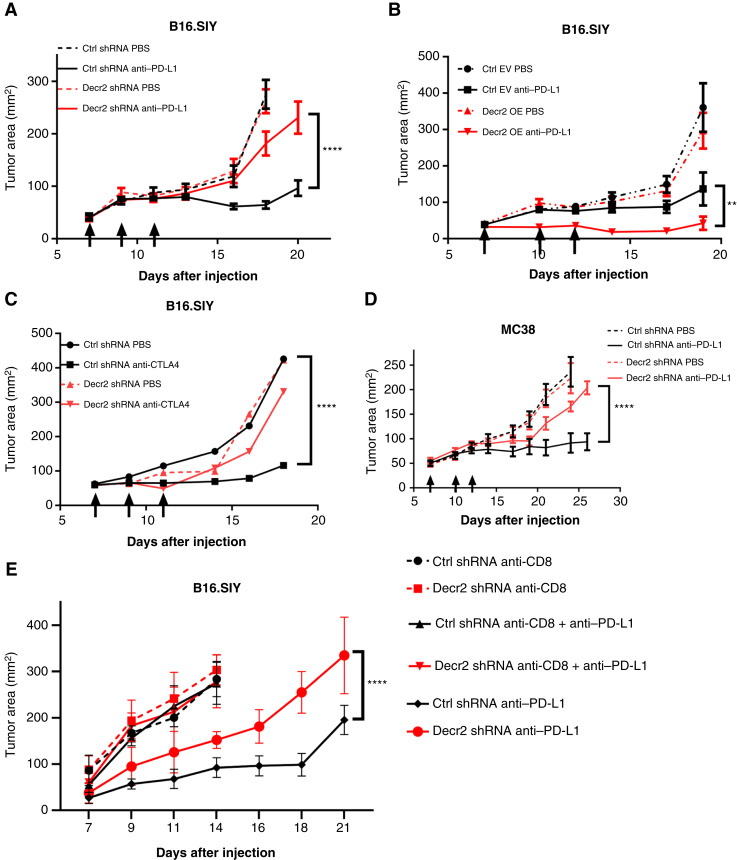
Decr2 expression levels modulate tumor sensitivity to anti–PD-L1 treatment in vivo
To examine the role of tumor cell Decr2 expression on tumor growth and antitumor immunity in vivo, we implanted Decr2 KD or shRNA control-infected B16.SIY tumor cells subcutaneously into C57BL/6 mice, and anti–PD-L1 treatment was performed on days 7, 9, and 11. Consistent with the relative resistance to effector T-cell killing in vitro, Decr2 KD B16.SIY tumors were largely resistant to anti–PD-L1 therapy in vivo (Fig. 2A; Supplementary Fig. S2C). As a complementary approach, we generated tumor cells that overexpressed Decr2 (Supplementary Fig. S3A and S3B), which were subcutaneously implanted in vivo compared with empty vector B16.SIY tumors. Although there was no difference in baseline tumor growth between the control and Decr2-overexpressing tumors, anti–PD-L1 treatment showed greater efficacy, with more than half (5/9) of Decr2-overexpressing B16.SIY tumors being completely rejected after anti–PD-L1 treatment (Fig. 2B; Supplementary Fig. S3C). Decr2 overexpression tumors showed increased sensitivity to anti–PD-L1 therapy, whether using the less-immunogenic B16F10 model (Supplementary Fig. S3D) or during treatment initiation at a later timepoint (Supplementary Fig. S3E). This enhanced responsiveness is unlikely to be due to spontaneous tumor necrosis or morphological changes, as hematoxylin and eosin staining of Decr2 overexpression tumors did not reveal any significant alterations (Supplementary Fig. S3F and S3G). To test whether Decr2 modulates the effects of other checkpoint blockade therapies, we examined the growth of Decr2 KD B16.SIY tumors treated with anti-CTLA4. We found that Decr2 KD B16.SIY tumors were less responsive to anti-CTLA4 treatment than were control tumors treated with the same therapy (Fig. 2C).
Figure 2.
Decr2 expression levels modulate tumor sensitivity to checkpoint blockade immunotherapy in vivo. A–C, 2 × 106 B16.SIY tumors cells were injected subcutaneously in C57BL/6 mice with or without checkpoint blockade immunotherapy treatment. Arrows indicate the days on which the mice received antibody therapy. A, Tumor area of B16.SIY control tumors (black) or Decr2 KD tumors (red) with or without anti–PD-L1 treatment. Data are mean ± SEM; n ≥ 7 mice per group; data are combined from two independent experiments. B, Tumor area of B16.SIY transduced with an empty vector (EV, black color) or Decr2 overexpression (OE) tumors (red color) with or without anti–PD-L1 treatment. Data are mean ± SEM; n ≥ 7 mice per group; data are combined from two independent experiments. C, Tumor area of B16.SIY control tumors (black) or Decr2 KD (red) tumors with or without anti-CTLA4 treatment. Data are mean ± SEM; n = 5 mice per group. D, Tumor area of MC38 control tumors (black) or Decr2 KD tumors (red) with or without anti–PD-L1 treatment. n ≥ 7 mice per group. E, Control (black) or Decr2 KD (red) B16.SIY tumor growth with or without anti–PD-L1 treatment in CD8+ T cell–depleted mice. Anti–CD8 antibody was intraperitoneally injected weekly, and anti–PD-L1 treatment was on days 7, 9, and 11. The control or Decr2 KD tumor growth treated with anti–PD-L1 in WT mice served as control groups. Data are mean ± SEM; n = 5 mice per group. P values determined using two-way ANOVA. **, P < 0.01; ****, P < 0.0001. Ctrl, control.
To determine if the anti–PD-L1–resistant phenotype upon Decr2 KD was generalizable beyond the B16.SIY tumor model, we generated Decr2 shRNA or control shRNA-infected MC38 tumor cell lines (Supplementary Fig. S3H) and monitored tumor growth in vivo. Control shRNA-infected MC38 tumor cells responded to anti–PD-L1 treatment as expected; however, Decr2 shRNA-infected MC38 tumors showed minimal response (Fig. 2D). To examine whether the growth difference between WT and Decr2 KD tumors was immune dependent, we subcutaneously implanted Decr2 shRNA- or control shRNA-infected B16.SIY tumor cells into CD8+ T cell–depleted mice (Supplementary Fig. S3I) and monitored tumor growth over time. The tumor growth difference when treated with anti–PD-L1 was lost in mice in which CD8+ T cells were antibody depleted (Fig. 2E), demonstrating a requirement for adaptive immunity.
Taken together, these data indicate that reduced expression of Decr2 in tumor cells is sufficient to mediate resistance to anti–PD-L1 and anti-CTLA4 therapy in vivo.
Decr2 KD tumors show defects in the final stages of antitumor immunity within the TME in vivo
To begin understanding the immunologic mechanism by which Decr2 KD tumors are rendered refractory to anti–PD-L1 therapy, the induction phase and the effector phase of the antitumor T-cell response were investigated. To investigate the priming phase, spleens were harvested on day 7 after tumor implantation and IFN-γ ELISpot was performed in response to SIY peptide stimulation. No significant difference was detected between mice bearing Decr2 shRNA-transduced B16.SIY tumors versus control shRNA tumors (Fig. 3A). There was no change in the percentage of SIY+ and CD69+CD8+ T cells among the total CD8+ T cells in the tumor-draining lymph nodes between the Decr2 KD and control tumors on day 7 (Supplementary Fig. S4A–S4C). There were no differences in IFN-γ, TNF-α, and IL2 production after 24 hours of SIY stimulation of tumor-draining lymph nodes between the groups (Supplementary Fig. S4D–S4F). These data indicate that the Decr2 KD tumors retained the capability to prime T-cell responses successfully. To thoroughly investigate the TME, we conducted single-cell RNA-seq analysis of CD45+ cells sorted from day 14 tumors. We found no differences in the baseline cell-type composition between control and Decr2 KD tumors (Supplementary Fig. S5A and S5B). No differences were observed in the proportion of major immune cell subsets, and there were only four genes differentially expressed by immune cells between control and Decr2 KD tumors (Supplementary Fig. S5C). Flow cytometric analysis of tumor-infiltrating CD45+ cells confirmed that there was no change in baseline infiltration of immune cells between tumors, including NK T cells, NK cells, DCs, and macrophages (Supplementary Figs. S6 and S7A–S7D).
Figure 3.
Decr2 KD B16.SIY tumors fail to show intratumoral expansion of SIY-specific CD8+ T cells after anti–PD-L1 treatment. A, The numbers of T cells secreting IFN-γ in response to SIY are shown. Splenocytes were isolated from B6 mice 7 days after B16.SIY tumor implantation. For detection of IFN-γ release, 106 splenocytes were stimulated with SIY peptide. Data are mean ± SD; n ≥ 9 mice per group; data combined from two independent experiments. B, The number of SIY antigen-specific CD8+ T-cell infiltration in control (black) or Decr2 KD (red) B16.SIY tumors on day 14 after tumor implantation. Data are mean ± SD; n ≥ 9 mice per group; data combined from two independent experiments. C, The number of alive B16.SIY tumors in B6 mice treated with or without anti–PD-L1. Data are mean ± SD; n ≥ 9 mice per group; data combined from two independent experiments. D and E, Tumor area of control (D) or Decr2 KD (E) B16.SIY tumors in WT B6 or Prf1 KO mice treated with anti–PD-L1 or PBS. Arrows indicate on which days mice received antibody therapy. Data are mean ± SEM; n = 5 mice per group. F, Tumor area of control or Decr2 overexpression B16.SIY tumors in Prf1 KO mice treated with anti–PD-L1 or PBS. Arrows indicate on which days mice received antibody therapy. Data are mean ± SEM; n = 5 mice per group. P values determined using the two-tailed t test (B, C) or two-way ANOVA (D, F). *, P < 0.05; **, P < 0.01; ****, P < 0.0001; ns, not significant. OE, overexpression.
To evaluate CD8+ T-cell expansion within the TME after anti–PD-L1 treatment, Decr2 KD or control B16.SIY tumors were implanted in vivo, and tumors were harvested at day 14 to generate cell suspensions for flow cytometric analysis with anti-CD8 and SIY peptide-Kb pentamers (Supplementary Fig. S8). A cohort of mice was also treated with anti–PD-L1 Ab to evaluate the intratumoral expansion of specific T cells that normally occurs after PD-1/PD-L1 blockade. Without treatment, both control and Decr2 KD tumors showed comparable accumulation of SIY-specific CD8+ T cells within the TME (Fig. 3B; Supplementary Fig. S7E), arguing that the initial recruitment of effector T cells was intact. However, with anti–PD-L1 treatment, antigen-specific CD8+ T cells expanded within control tumors, yet failed to expand within Decr2 KD tumors (Fig. 3B). We also measured the total number of Tregs (Thy1.2+ CD4+ Foxp3+) in these tumors. As expected, there was a significant increase in the number of FoxP3+ Treg numbers in control tumors after anti–PD-L1 treatment compared with nontreatment. However, this increase was not seen in Decr2 KD tumors (Supplementary Fig. S7F). The increase of FoxP3+ Tregs in WT conditions is correlated with an increased number of CD8+ T cells after anti–PD-L1 treatment, and we have reported previously that CD8+ TIL produce CCL22 that contributes to Treg recruitment (6). A similar failure of anti–PD-L1 to increase tumor-infiltrating CD8+ T-cell number was observed with Decr2 KD MC38 tumor cells (Supplementary Fig. S7H). To confirm that the increase in CD8+ TIL normally seen after anti–PD-L1 treatment was associated with proliferation, Ki67 staining was performed. The percentage of Ki67+CD8+ T cells in the TME did not increase in Decr2 KD tumors after anti–PD-L1 treatment compared with control tumors, indicating that the failed increase of tumor-infiltrating CD8+ T cells was due to less proliferation of CD8+ T cells (Supplementary Fig. S7G). In parallel with analyzing the immune infiltrate, the proportion of live tumor cells was interrogated after anti–PD-L1 treatment. As expected, with control B16.SIY tumors, fewer live tumor cells were detected after anti–PD-L1 treatment, consistent with the therapeutic effect observed on tumor growth rate. By contrast, no reduction of live tumor cells was observed with Decr2 KD B16.SIY cells (Fig. 3C), consistent with the lack of tumor cell death. Similar results were observed in the MC38 tumor model (Supplementary Fig. S7I). Taken together, the lack of intratumoral CD8+ T-cell expansion after anti–PD-L1 treatment in the context of Decr2 KD tumors suggests that initial tumor cell death itself may contribute to re-invigorated CD8+ T-cell expansion within the TME.
The perforin/granzyme pathway has traditionally been implicated as a major mechanism of T cell–mediated tumor cell killing, particularly in vitro (16). However, previous work in other model systems has indicated that T cell–based immunotherapy can occur normally in the absence of perforin in vivo (36). To examine the relative contribution of host perforin versus the Decr2-dependent cell death mechanism, perforin-deficient mice were used. B16.SIY tumors still responded to anti–PD-L1 therapy in perforin-deficient mice (Fig. 3D). Implanting Decr2 KD tumors into perforin-deficient mice did not lead to any further erosion of therapeutic efficacy (Fig. 3E). Overexpression of Decr2 in B16.SIY tumors instilled them to be more sensitive to anti–PD-L1 therapy than control B16.SIY in perforin-deficient mice (Fig. 3F). These data indicate that the perforin/granzyme pathway is not mandatory for the efficacy of PD-1/PD-L1 blockade therapy in vivo and that, in these model systems, the Decr2-dependent mechanism of tumor cell death may be dominant.
DECR2 localizes to the peroxisome and regulates tumor cell ferroptosis
To begin investigating the cell biological mechanism by which Decr2 KD tumors are resistant to T-cell killing, we examined the surface expression of MHC class I and PD-L1 by using flow cytometry. Previous CRISPR screens had identified molecules that regulated cell surface expression of these functionally important cell surface ligands (26, 27, 35). After stimulation with IFN-γ, MHC class I and PD-L1 were both upregulated normally in Decr2 KD tumors (Supplementary Fig. S9A and S9B), ruling out perturbed expression of these molecules as a resistance mechanism. To examine if antigen presentation in Decr2 KD tumors was affected, we co-cultured naïve 2C CD8+ T cells with Decr2 KD or control B16.SIY cells for 48 hours and measured IFN-γ production in the medium by using ELISA. There was no reduction in IFN-γ production by T cells in response to Decr2 KD compared with control tumor cells (Supplementary Fig. S9D). As an additional approach, we used staining with an mAb that recognizes the SIINFEKL peptide from ovalbumin bound to H-2Kb in either WT or Decr2 KD B16.OVA cells. Analysis of this peptide/MHC complex by using flow cytometry was comparable in WT versus Decr2 KD B16.OVA cells, indicating that antigen processing and presentation on the tumor surface was not affected by the loss of Decr2 (Supplementary Fig. S9C).
DECR2 is predicted to localize to the peroxisome, a membrane-bound oxidative organelle, which has been reported to contribute to ferroptosis. Ferroptosis is a cell death mechanism distinct from apoptosis, which occurs through a process dependent on iron and is mediated through lipid peroxidation (18). Peroxisomes have been reported to be involved in the biosynthesis of plasmalogens for lipid peroxidation, which leads to ferroptosis, and Decr2 had previously been implicated in intracellular lipid metabolism. Ferroptosis is countered by the glutathione pathway, which itself is driven by cysteine uptake via SLC7A11 and SLC3A2 (22). To examine the intracellular localization of DECR2, we generated an epitope-tagged version for immunofluorescence staining and confocal microscopy in B16 melanoma cells. The degree of colocalization of fluorescence signal originating from DECR2 and lysosomes (LAMP1), mitochondria (ATP5A), or peroxisome (catalase) was quantified by calculating Manders’ distinct colocalization coefficients M1 and M2 for the confocal images. The Manders’ coefficients M1 and M2 revealed a significantly greater colocalization of DECR2 and the peroxisome marker catalase in B16 tumor cells than LAMP1 and ATP5A. These data indicate that DECR2 localizes within peroxisomes (Supplementary Fig. S10A and S10B). Because peroxisomes were reported to contribute to ferroptosis (20), we then hypothesized that Decr2 KD tumor cells might be resistant to ferroptosis, perhaps through defective lipid peroxidation. We began by examining the susceptibility of Decr2 KD tumor cells to RSL3, Erastin, and ML210—pharmacologic agents defined to kill cells through ferroptosis (21). A dose titration of each of these drugs revealed that Decr2 KD B16.SIY cells were relatively less susceptible to death in response to ferroptosis inducers than control cells (Fig. 4A–C; Supplementary Fig. S11A, S11C, and S11D). Reintroducing Decr2 expression into Decr2 KD cells restored their sensitivity to RSL3 (Supplementary Fig. S11B). Similar results were seen with Decr2 KD MC38 cells (Supplementary Fig. S11G and S11H). In addition, death induced by RSL3 was rescued by ferrostatin-1 (Fer-1, a ferroptosis inhibitor; Fig. 4D) and deferoxamine (Supplementary Fig. S11E), supporting the mechanism of ferroptosis.
Figure 4.
Decr2 KD tumor cells are relatively resistant to ferroptosis inducers. A, B16.SIY cells expressing control or Decr2 shRNA were treated with RSL3 for 24 hours at various concentrations. The percentage of live cells was measured using Fixable Viability Dye staining. Data are from one representative study from two independent experiments. Data are mean ± SD. B, The percentage of live cells of B16.SIY tumors expressing control shRNA or Decr2 shRNA treated with different concentrations of Erastin for 24 hours. Data are from one representative study from two independent experiments. Data are mean ± SD. C, The percentage of live cells of B16.SIY tumors expressing control shRNA or Decr2 shRNA treated with different concentrations of ML210 for 24 hours. Data are from one representative study from two independent experiments. Data are mean ± SD. D, Viability of B16.SIY cells cultured with RSL3 (0.5 μmol/L) ± Fer-1 (2 μmol/L). Data are from one representative study from two independent experiments. Data are mean ± SD. E, Relative lipid ROS of B16.SIY tumor cells expressing control shRNA or Decr2 shRNA co-cultured with or without pre-activated 2C CD8+ T cells for 12 hours. Relative lipid ROS was measured using Liperfluo; the mean fluorescence intensity (MFI) is shown; n = 3 biological replicates. Data are from one representative study from two independent experiments. Data are mean ± SD. F, Relative lipid ROS of tumor cells expressing control shRNA or Decr2 shRNA ex vivo treated with or without anti–PD-L1 on day 14. n = 5 mice per group; data are from one representative study from two independent experiments. Data are mean ± SD. G, Volcano plots showing the changes in phospholipidome in the medium (left) or with RSL3 treatment (right): B16.SIY cells expressing control shRNA or Decr2 shRNA (n = 4). P value was determined using two-way ANOVA. H, The relative percentage of live cells in B16.SIY cells pretreated with AGPS-IN-2i for 24 hours and then treated with RSL3 (0.2 μmol/L). I, Plasmalogen levels with PUFA at sn-2 position in control-infected or Decr2 KD B16.SIY cells after RSL3 stimulation for 24 hours. Data are mean ± SD; P value was determined using the t test. J, Plasmalogen biosynthesis detection. B16.SIY cells were treated with DMSO or RSL3, and signals are normalized to the percent of the total of each respective class. Data are mean ± SD. P values determined using two-way ANOVA (A, B, C, F, G), the t test (D, H, I, J), and the two-tailed t test (E). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant.
To determine whether Decr2 regulated lipid peroxidation during T cell–mediated tumor cell death, Decr2 KD versus control B16.SIY cells were co-cultured with active 2C CD8+ T cells for 12 hours, and the levels of lipid reactive oxygen species (ROS) were quantified as relative fluorescence intensity using Liperfluo staining, which indicates lipid peroxidation levels. With control B16.SIY cells, lipid ROS were increased about twofold upon exposure to activated CD8+ T cells compared with culture medium control, consistent with ferroptosis being a component of T cell–mediated killing. By contrast, lipid ROS were significantly reduced in Decr2 KD B16.SIY cells (Fig. 4E). Lipid ROS were also examined in B16.OVA model co-cultured with pre-activated OT1 CD8+ T cells, and BODIPY-C11 assay was used to detect lipid ROS. Decr2 KD B16.OVA cells showed less lipid ROS induction when co-cultured with CD8+ T cells than control cells (Supplementary Fig. S11F). To determine whether a similar defect in lipid ROS induction would be observed in vivo, B16.SIY tumor cells were implanted subcutaneously into mice and anti–PD-L1 therapy was administered as reported previously, then tumors were harvested and analyzed by using flow cytometry with Liperfluo (Supplementary Fig. S12A). With control tumors, lipid ROS were upregulated in response to anti–PD-L1 treatment, but this increase failed to occur in Decr2 KD tumors (Fig. 4F). Similar results of failed induction of lipid ROS were observed in the MC38 model (Supplementary Figs. S11I and S12B).
To investigate the mechanism by which Decr2 regulates ferroptosis sensitivity, we examined alteration in cellular lipids. Because DECR2 was mainly localized in peroxisomes, which participate in the biosynthesis of ether-linked glycerolipids of two subtypes: [1-O-alkyl-(R1CH2CH2OCH2R2) ) and 1-O-alkenyl-glycerophospholipids (the latter known as plasmalogens, R1CH=CHOCH2R2)], it was hypothesized that Decr2 KD might result in decreased levels of plasmalogens. Plasmalogens contain a vinyl–ether and an ester bond at the sn-1 and sn-2 positions of the PUFA chain. Modification at the sn-2 position has been reported to be critical for ferroptosis sensitization (Supplementary Fig. S13A). We tested this idea by performing global mass spectrometry comparing Decr2 KD versus control tumor cells with the pharmacologic inducer of ferroptosis RSL3 or with activated CD8+ T cells. Without any treatment, Decr2 KD significantly reduced the levels of PUFA-ePLs (Fig. 4G). After treatment with RSL3 or 2C+ CD8 T cells, Decr2 KD B16.SIY tumors showed significantly reduced PUFA-ePLs, largely PUFA-plasmalogens (Fig. 4G and H; Supplementary Fig. S13B and S13C). To investigate if the plasmalogen amount level decrease in Decr2 KD tumors was due to reduced synthesis rate, we added stable isotope tracer D4-ethanolamine and D9-choline chloride to the cell culture medium and monitored lipid synthesis by using liquid chromatography/mass spectrometry. After RSL3 treatment, the biosynthetic rate of ethanolamine plasmalogen was increased in RSL3-treated B16.SIY tumor cells compared with cells treated with DMSO (Supplementary Fig. S14A). Most ethanolamine-containing glycerophospholipid synthesis rates were decreased in Decr2 KD tumor cells (Supplementary Fig. S14A–S14C). The ether-linked phospholipids synthesis rate (most of them are PUFA-ePLs) also was significantly decreased in the Decr2 KD treatment group compared with the control treatment group (Supplementary Fig. S14A and S14B). There was no difference of phosphatidylcholine biosynthesis rate between Decr2 KD tumor cells and control cells (Supplementary Fig. S14D). To test whether ether-linked phospholipid synthesis affected ferroptosis, the ether-linked phospholipid synthesis inhibitor AGPS-IN-2i was used to treat B16.SIY cells. B16.SIY cells pretreated for 24 hours with AGPS-IN-2i showed increased cell viability upon subsequent treatment with RSL3 (Fig. 4H). A previous report has shown that arachidonoyl (AA) is one of the main substrates of lipid peroxidation in ferroptosis (37). Consistent with this, the AA–plasmalogen amount was decreased in Decr2 KD tumor cells, and the biosynthesis rate was also significantly lower in response to RSL3 treatment (Fig. 4I and J).
We also examined glutathione content in Decr2 KD B16.SIY cells. The GSH levels in Decr2 KD B16.SIY cells were reduced to a similar level as that of control cells after Erastin treatment (Supplementary Fig. S15A). The GSSG level and GSH/GSSG ratio both were reduced to the similar level as between Decr2 KD and control cells when treated with Erastin (Supplementary Fig. S15B and S15C). In addition, knocking down of Decr2 in B16.SIY cells did not affect the expression of the related gene, Decr1 (Supplementary Fig. S15D). The expression of other ferroptosis-related genes was unaffected in Decr2 KD tumors, with or without anti–PD-L1 treatment, compared with controls (Supplementary Fig. S15E–S15I). Together, these results indicate that Decr2 KD tumor cells have reduced levels of plasmalogens due to a decreased rate of biosynthesis, rendering the cells less susceptible to ferroptosis.
Antigen-specific CD8+ T cells induce tumor-cell ferroptosis through IFN-γ signaling
We next asked by what cellular mechanism CD8+ T cells could induce Decr2-dependent lipid oxidation and ferroptosis. This cross-talk could be cell contact dependent, similar to cell death induced by the release of granzyme-containing cytotoxic granules or be mediated by soluble factors. To distinguish between these possibilities, we used a transwell culture system with 0.4-μm pores, and the effector and target cells were added either on the same side or to opposite sides of the membrane (Fig. 5A). We found that pre-activated antigen-specific cytotoxic CD8+ T cells co-cultured with B16.SIY tumors increased lipid ROS in tumor cells when they were on the same side of the transwell membrane, and also induced similar levels of lipid ROS in B16.SIY cells when cultured on the other side of the transwell membrane. These results suggest that T cell–induced tumor cell ferroptosis is mediated through soluble factors. Because of the importance of IFN-γ signaling on tumor cells for antitumor immunity (38, 39), we exposed tumor cells to IFN-γ and found a similar induction of lipid ROS (Fig. 5B). To examine if IFN-γ was necessary to induce tumor cell ferroptosis by T cells, we added an anti–IFN-γ Ab to the CD8+ T-cell co-culture and measured tumor cell lipid ROS. Tumor cell lipid ROS was reduced to baseline levels with IFN-γ blockade (Fig. 5C). CD8+ T cells secrete IFN-γ when the TCR recognizes cognate antigen, so as a comparison, we used pre-activated OT1 CD8+ T cells, which recognize the distinct antigen OVA. The pre-activated CD8+ T cells were washed two times using medium and incubated in fresh medium for 6 hours to eliminate any baseline IFN-γ secreted prior to being mixed with the tumor cells. As expected, no target cell killing or lipid ROS induction was detected when B16.SIY cells were cultured with activated OT1 cells (Supplementary Fig. S16A; Fig. 5D). IFN-γR KO B16.SIY cells were also tested and showed greater survival when co-cultured with 2C CD8+ T cells, and no induction of lipid ROS was detected (Supplementary Fig. S16B; Fig. 5E). These collective data indicate that IFN-γ is a major mediator of the ferroptosis component of T cell–mediated tumor cell killing.
Figure 5.
Ferroptosis can be induced by activated CD8+ T cells through IFN-γ. A, Experimental design. B, Relative fold change of lipid ROS in B16.SIY cells under different culture conditions. C, Relative fold change of lipid ROS in B16.SIY cells after blocking IFN-γ protein secreted by activated 2C CD8+ T cells. D, Relative lipid ROS of B16.SIY tumor cells co-cultured with or without pre-activated OT1 CD8+ T cells for 12 hours. Relative lipid ROS was measured by using Liperfluo; the mean fluorescence intensity (MFI) is shown; n = 3 biological replicates. Data are from one representative study from two independent experiments. E, Relative lipid ROS of IFN-γR2 KO or control B16.SIY tumor cells co-cultured with or without pre-activated 2C CD8+ T cells for 12 hours. Relative lipid ROS was measured by using Liperfluo; MFI is shown; n = 3 biological replicates. Data are from one representative study with two independent experiments. F, Relative Decr2 expression level of B16.SIY cells after being co-cultured with activated 2C CD8+ T cells or IFN-γ. G, Relative lipid ROS of B16.SIY tumor cells with or without IFN-γ treatment; MFI is shown; n = 3 biological replicates. H, The percentage of live cells in B16.SIY tumors expressing control shRNA, Decr2 shRNA, or Decr2 overexpression after IFN-γ treatment. Data are mean ± SD. P values determined using the two-tailed t test. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant.
We then investigated the mechanism by which IFN-γ might promote Decr2-dependent generation of oxidized lipids and examined Decr2 mRNA expression. We found that pre-activated 2C TCR Tg T cells induced Decr2 mRNA upregulation in B16.SIY tumor cells after a 12-hour co-culture period, which was also seen when tumor cells were treated with IFN-γ alone (Fig. 5F). IFN-γ–induced lipid ROS production did not occur in Decr2 KD cells (Fig. 5G), which showed increased cell viability (Fig. 5H). By contrast, cells overexpressing Decr2 had reduced viability compared with controls when treated with IFN-γ (Fig. 5H). Previous studies have shown that ACSL4 expression is also induced by IFN-γ treatment (25), and the increase in Decr2 expression after IFN-γ treatment may suggest a distinct pathway for IFN-γ–induced ferroptosis. IFN-γ treatment also altered overall lipid metabolism in B16.SIY cells. The total amount of PE-plasmalogens, which are influenced by Decr2, was significantly increased after IFN-γ treatment (Supplementary Fig. S16C). These results suggest that at least one mechanism by which IFN-γ promotes increased lipid ROS and ferroptosis is through upregulated expression of Decr2 itself.
Clinical and therapeutic relevance of Decr2
Because our murine studies indicated that decreased tumor expression of Decr2 could mediate relative resistance to anti–PD-L1 therapy, we investigated whether Decr2 mRNA levels were associated with anti–PD-1 efficacy in humans by analysis of a bulk tumor RNA-seq data set from pretreatment and on-treatment with nivolumab of patients with melanoma (34). The baseline expression of Decr2 was comparable between patients of anti–PD-1 CR, and progressive disease. However, by examining posttreatment biopsies with pretreatment samples, we found that patients with CR had significantly increased Decr2 mRNA expression in posttreatment biopsies compared with pretreatment biopsies, whereas patients who failed to achieve a clinical response did not (Fig. 6A).
Figure 6.
Decr2 expression correlates with patient outcomes, and Slc7a11 is a candidate therapeutic target. A, Fold changes of Decr2 in matched pretherapy and on-therapy samples from patients with melanoma. B, Relationship between overall survival and mRNA expression level of Decr2 in human patients treated with ipilimumab. C,Decr2 deletion (deep and shallow) and Decr2 copy-number gain association with overall survival in response to ipilimumab. D, IF staining of human melanoma tissue sections. DECR2 (green), SOX10 (red), and DAPI (blue). Scale bar, 50 μm. E, Percentage of Decr2+Sox10+ cells and relative intensity of DECR2 expression in tissues of patients with melanoma: pretreatment or on-treatment with anti–PD-1. F, KD of Slc7a11 in Decr2-deficient tumor cells restores anti–PD-L1 efficacy. Control shRNA–infected tumors (in black), Decr2 single KD tumors (in red, labeled as Decr2 KD), and Decr2 Slc7a11 double KD (in blue, labeled as Decr2 Slc7a11 DKD). n ≥ 9 mice per group. G, The end timepoint of tumor area with or without anti–PD-L1 treatment. Control shRNA–infected tumors (in black), Decr2 single KD tumors (in red, labeled as Decr2 KD), Decr2 Slc7a11 double KD (in blue, labeled as Decr2 Slc7a11 DKD). P values are determined using the Mann–Whitney test (A), the two-tailed t test (C, E, G), and two-way ANOVA (F). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant. CR, complete responder; PD, progressive disease; PR, partial responder; SD, stable disease.
We also analyzed another study of human patients with melanoma treated with CTLA4 blockade (40). Decr2 mRNA levels in posttreatment biopsies were strongly positively correlated with overall survival time (Fig. 6B). We also examined genomic deletion data and identified a subset of patients with melanoma with Decr2 gene deletion. Patients with a genomic deep or shallow deletion of gene Decr2 had significantly shorter survival times than those with a gain of Decr2, upon treatment with ipilimumab (Fig. 6C).
To extend these data, we examined the DECR2 protein levels by using IHC in pretreatment versus posttreatment biopsy samples of patients having CR upon anti–PD-1 treatment versus PD. DECR2 protein expression within melanoma tumor cells (SOX10+) was higher at baseline in the setting of a subsequent CR than in the PD setting. After anti–PD-1 treatment, the CR tumor tissues showed a further increase in Decr2+ SOX10+ cell number and DECR2 expression level, which did not occur in the setting of PD (Fig. 6D and E). These results indicate that increased tumor expression of DECR2 is associated with clinical benefit to anti–PD-1 therapy in patients.
Because Decr2 is a positive regulator of ferroptosis, it would not be a straightforward therapeutic target in cancer, as specific transduction of tumor cells to overexpress Decr2in vivo would be challenging to achieve. However, in as much as cell death by ferroptosis is counter-regulated by the glutathione pathway, interventions that block glutathione generation might favor ferroptosis (Fig. 7). Our genome-wide CRISPR screen showed that Slc7a11 KO B16.SIY cells were more sensitive to CD8+ T-cell killing, supporting this hypothesis (Supplementary Fig. S17A and S17B). We therefore postulated that reducing expression of Slc7a11 in Decr2-deficient tumor cells might restore anti–PD-L1 efficacy (Fig. 7). To this end, we generated Slc7a11 and Decr2 double-KD B16-SIY cells. KD of SLC7A11 also resulted in GPX4 downregulation (Supplementary Fig. S17C). Slc7a11 and Decr2 double-KD B16.SIY cells were implanted in vivo, and mice were treated with anti–PD-L1 Ab as before. Without anti–PD-L1 treatment, each of the KD tumor cell lines grew comparably in vivo. Upon treatment with anti–PD-L1, control tumors responded normally, and Decr2 KD tumors showed resistance, as expected. However, if Slc7a11 was additionally knocked down in Decr2 KD tumor cells, anti–PD-L1 efficacy was restored (Fig. 6F and G). These results suggest that inhibition of Slc7a11 could represent a viable therapeutic strategy to support improved immune-mediated tumor control by favoring ferroptosis.
Figure 7.
Working model of how Decr2 facilitates ferroptosis in tumor cells.
Discussion
We used a loss-of-function genetic screen in vitro to identify genes capable of modulating tumor cell death in response to CD8+ effector T cells, to identify candidate resistance mechanisms to T cell–based immunotherapies. This screen identified Decr2, a molecule involved in lipid metabolism. Deletion or KD of Decr2 in tumor cells rendered them relatively resistant to antigen-specific CD8+ T-cell killing in vitro, as well as to anti–PD-L1 treatment in vivo.
Decr2 was originally identified as a fatty acid metabolism gene (41, 42). It participates in the degradation of unsaturated fatty enoyl-CoA esters having double bonds in both even- and odd-numbered positions. We found that KD of Decr2 did not significantly change tumor cell viability and growth. This could imply that DECR2 is not a rate-limiting enzyme in basal lipid metabolism but rather that it could serve an alternative role (41). Unlike the related enzyme DECR1, DECR2 was predicted to localize to the peroxisome, which we confirmed by using confocal microscopy. Localization to the peroxisome suggests that DECR2 is not only involved in unsaturated long-chain fatty acid β-oxidation but also in biosynthesis (41, 43). Peroxisomes have been implicated in the process of ferroptosis through the biosynthesis of plasmalogens for lipid peroxidation (20). Our mass spectrometry data determined that Decr2 KD B16.SIY cells had decreased levels of plasmalogen–PUFA levels, in response to either RSL3 stimulation or activated CD8+ T cells, in alignment with the parallel observation that Decr2 KD tumor cells were resistant to ferroptosis. KD of Decr2 tumor cells also resulted in a reduced synthesis rate of ether–phospholipids. Because DECR2 is an auxiliary enzyme in β-oxidation, it is unlikely to directly participate in the synthesis of ether–phospholipids. The decrease in ether–phospholipids in the Decr2 KD tumor cells may be attributed to a reduced amount of acetyl-CoA generated from peroxisomal β-oxidation, which has been linked to the synthesis of ether lipids (44). It has been reported that ferroptosis can be induced by CD8+ T cells both in vivo and in vitro. Previous studies have demonstrated that IFN-γ secreted by CD8+ T cells is a key factor in inducing ferroptosis by decreasing cysteine uptake and altering the lipid profile of tumor cells through the upregulation of Acsl4 gene expression (19, 25). In our B16.SIY tumor cell model, Decr2 gene expression was also upregulated after IFN-γ treatment, and IFN-γ alone could change the lipid pattern. This may indicate that except Acsl4, there might be other ferroptosis-related genes that contribute to IFN-γ–induced ferroptosis. In addition to Decr2, our genome-wide screen also identified the genes Zeb1, Lpcat3, and Agpat6, which are also implicated in the pathway. In addition, Slc7a11, CD44, and Acsl3 were identified as candidates with the potential to increase tumor sensitivity to CD8+ T-cell killing (see Supplementary Table S1). These results are consistent with previous studies, although further work will be necessary to understand their role in cancer model systems (19).
The mechanism by which effector T cells actually kill tumor cells in vivo has been incompletely understood. In vitro, the two established mechanisms are via granule exocytosis and the perforin/granzyme pathway, and the death receptor pathway, most commonly via FasL engagement of Fas on target cells (45). Both of these mechanisms occur rapidly and induce target cell apoptosis. However, it is noteworthy that in a melanoma genetically engineered mouse model that included Cre-inducible expression of a neoantigen, adoptively transferred effector CD8+ TCR Tg T cells rapidly infiltrated tumors and had continuous tumor cell contact and yet did not achieve tumor cell killing until day 5 (46). These prolonged kinetics suggest a tumor cell killing mechanism that is more involved than rapid apoptosis through granule exocytosis. In addition, adoptively transferred T cells from perforin−/− or FasL-deficient mice can still cause tumor regression in vivo (36). Taken along with our current results, it seems likely that tumor cell ferroptosis plays an important role in immune-mediated tumor cell killing in vivo, either alone or in concert with other cell death mechanisms.
To further explore this connection, we examined how tumor cell ferroptosis influences immune responses within the TME. Our findings suggest that ferroptotic cell death can actively shape antitumor immunity. Expansion of CD8+ T cells within the TME after anti–PD-L1 treatment in vivo failed to occur when tumor cells were Decr2 deficient. These data argue that tumor cell death by ferroptosis might feed back to positively reinforce immune system activation. This could be because ferroptosis-mediated cell death is immunogenic or because the onset of tumor cell death might result in diminished inhibitory processes that enable TIL expansion. Consistent with our mouse models, human patients with cancer responding to anti–PD-1 showed upregulated expression of Decr2. Higher Decr2 expression after anti-CTLA4 treatment was correlated with longer survival. Testing of Decr2 expression during immunotherapy could be an attractive biomarker to evaluate. Patients with tumor genomic Decr2 deletion may be candidates for alternative therapies or combination treatments, whereas those with high expression might benefit from sustained immune checkpoint inhibition. Because ferroptosis-induced tumor cell death may enhance the effectiveness of checkpoint blockade immunotherapy, strategies to promote tumor cell ferroptosis should be explored in combination with these therapies. However, given that CD8+ T cells are also sensitive to ferroptosis inducers, tumor specificity would have to be pursued. It is also attractive to hypothesize that engineered T cells rendered to be Decr2 deficient might have increased survival in the solid TME.
Our results identify tumor cell–expressed Decr2 as a regulator of tumor cell death in response to CD8+ effector T cells. Pharmacologic strategies to facilitate tumor cell ferroptosis could have therapeutic use.
Supplementary Material
Supplementary Figure S1. Decr2 allele and expression in transplantable tumor cell models and Decr2 knockdown tumor cells were resistant to CD8+ T cell killing in vitro.
Supplementary Figure S2. Decr2 knockdown OCI-Ly8 tumor cells were resistant to CD19-CAR T cell killing in vitro and Decr2 knockdown B16.SIY tumors exhibit resistance to anti-PD-L1 therapy in vivo.
Supplementary Figure S3. Decr2 modulates tumor sensitivity to anti-PD-L1 therapy in vivo.
Supplementary Figure S4. CD8+ T cell activation and cytokine secretion in tumor-draining lymph nodes.
Supplementary Figure S5. Single-cell transcriptomic profiling of CD45+ cells isolated from control versus Decr2 KD tumors of B6 mice.
Supplementary Figure S6. Gating strategy for Flow cytometry analysis of B16.SIY tumor microenvironment isolated from B6 mice.
Supplementary Figure S7. Tumor microenvironment analysis.
Supplementary Figure S8. Gating strategy for Flow cytometry analysis of B16.SIY tumor microenvironment isolated from B6 mice.
Supplementary Figure S9. MHC class I H2Kb, PD-L1 expression, and antigen presentation by tumor cells.
Supplementary Figure S10. Subcellular localization of Decr2.
Supplementary Figure S11. Decr2 knockdown tumors are resistant to ferroptosis.
Supplementary Figure S12. The gating strategy for flow cytometry analysis of lipid reactive oxygen species (ROS).
Supplementary Figure S13. Decreased polyunsaturated ether phospholipids levels in Decr2 knock-down tumor cells.
Supplementary Figure S14. Ethanolamine-containing glycerophospholipid and PC synthesis rate.
Supplementary Figure S15. Decr2 does not affect reduced glutathione (GSH), oxidized glutathione (GSSG), GSH/GSSG ratio, or expression of other ferroptosis genes.
Supplementary Figure S16. IFN-γ signaling is involved with T cell killing and reprogrammed lipids
Supplementary Figure S17. Genome-wide CRISPR reveals Slc7a11 gene was significantly depleted in B16.SIY cells co-cultured with 2C CD8+ T cells and Decr2 Slc7a11 double knockdown B16.SIY cells were generated.
Table S1: MAGeCK analysis of CRISPR screen results
Acknowledgments
We are grateful to the Flow Cytometry Core Facility, Genomics Core Facility, Integrated Light Microscopy Core Facility, and Human Tissue Resource Center at the University of Chicago, which are supported by a Cancer Center Support Grant (P30CA014599). We would like to acknowledge Dr. Vytas Bindokas for his assistance with immunofluorescence staining images. We thank Dr. Alexander Muir and Patick Jonker for lipid synthesis experiment advice. We thank the Human Tissue Resource Center at the University of Chicago for processing and staining the mouse tumor tissues. We thank the University of Chicago Medicine Comprehensive Cancer Center for assistance with stable isotope tracing experiments (RRID: SCR_022932). This work was supported by R35 CA210098 and a sponsored research agreement from Bristol Myers Squibb.
Footnotes
Note: Supplementary data for this article are available at Cancer Immunology Research Online (http://cancerimmunolres.aacrjournals.org/).
Authors’ Disclosures
H. Shah reports grants from the NCI during the conduct of the study. J. Trujillo reports grants from the NCI and Harold Amos Medical Faculty Development Program (AMFDP) of the Robert Wood Johnson Foundation during the conduct of the study. T.F. Gajewski reports grants from Bristol Myers Squibb and Merck, grants and personal fees from Pyxis, and personal fees from Allogene Therapeutics, Bicara Therapeutics, CatalYm, Samyang Biopharma, and Zai Lab outside the submitted work. No disclosures were reported by the other authors.
Authors’ Contributions
S. Li: Conceptualization, data curation, formal analysis, validation, investigation, writing–original draft, project administration, writing–review and editing. J.W. Shapiro: Formal analysis, writing–review and editing. H. Shah: Project administration, writing–review and editing. E.F. Higgs: Formal analysis, writing–review and editing. L. Xie: Resources, writing–review and editing. Y. Li: Writing–review and editing. Y. Zha: Formal analysis, writing–review and editing. J. Trujillo: Formal analysis, writing–review and editing. A. Cabanov: Writing–review and editing. T.A. Jones: Writing–review and editing. B. Flood: Writing–review and editing. K. Hatogai: Writing–review and editing. R. Tonea: Writing–review and editing. J. Kline: Resources, writing–review and editing. T.F. Gajewski: Conceptualization, data curation, supervision, funding acquisition, investigation, writing–original draft, writing–review and editing.
References
- 1. Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med 2016;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 2016;13:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gajewski TF. Improved melanoma survival at last! Ipilimumab and a paradigm shift for immunotherapy. Pigment Cell Melanoma Res 2010;23:580–1. [DOI] [PubMed] [Google Scholar]
- 4. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015;523:231–5. [DOI] [PubMed] [Google Scholar]
- 6. Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013;5:200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J 2010;16:399–403. [DOI] [PubMed] [Google Scholar]
- 8. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol 2013;25:268–76. [DOI] [PubMed] [Google Scholar]
- 10. Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 2009;69:3077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012;61:1019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016;315:1600–9. [DOI] [PubMed] [Google Scholar]
- 14. Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016;375:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, et al. Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 2016;167:397–404.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol 2002;2:401–9. [DOI] [PubMed] [Google Scholar]
- 17. Golstein P, Griffiths GM. An early history of T cell-mediated cytotoxicity. Nat Rev Immunol 2018;18:527–35. [DOI] [PubMed] [Google Scholar]
- 18. Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol 2021;18:280–96. [DOI] [PubMed] [Google Scholar]
- 19. Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019;569:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 2020;585:603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014;156:317–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021;12:599–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 2017;13:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophysical Res Commun 2016;478:1338–43. [DOI] [PubMed] [Google Scholar]
- 25. Liao P, Wang W, Wang W, Kryczek I, Li X, Bian Y, et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022;40:365–78.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, et al. Identification of essential genes for cancer immunotherapy. Nature 2017;548:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan D, Kobayashi A, Jiang P, Ferrari de Andrade L, Tay RE, Luoma AM, et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science 2018;359:770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams JB, Horton BL, Zheng Y, Duan Y, Powell JD, Gajewski TF. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J Exp Med 2017;214:381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera MDC, Yusa K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol 2014;32:267–73. [DOI] [PubMed] [Google Scholar]
- 30. Li W, Xu H, Xiao T, Cong L, Love MI, Zhang F, et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol 2014;15:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu Y, Cao G, Chen X, Huang X, Asby N, Ankenbruck N, et al. Antigen-multimers: specific, sensitive, precise, and multifunctional high-avidity CAR-staining reagents. Matter 2021;4:3917–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao Y, Fu L, Wu J, Peng Q, Nie Q, Zhang J, et al. Integrated analysis of multimodal single-cell data with structural similarity. Nucleic Acids Res 2022;50:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DePasquale EAK, Schnell DJ, Van Camp PJ, Valiente-Alandí Í, Blaxall BC, Grimes HL, et al. DoubletDecon: deconvoluting doublets from single-cell RNA-sequencing data. Cell Rep 2019;29:1718–27.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 2017;171:934–49.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams JB, Li S, Higgs EF, Cabanov A, Wang X, Huang H, et al. Tumor heterogeneity and clonal cooperation influence the immune selection of IFN-gamma-signaling mutant cancer cells. Nat Commun 2020;11:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kline J, Zhang L, Battaglia L, Cohen KS, Gajewski TF. Cellular and molecular requirements for rejection of B16 melanoma in the setting of regulatory T cell depletion and homeostatic proliferation. J Immunol 2012;188:2630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 2017;13:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grasso CS, Tsoi J, Onyshchenko M, Abril-Rodriguez G, Ross-Macdonald P, Wind-Rotolo M, et al. Conserved interferon-gamma signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer Cell 2021;39:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A 1998;95:7556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. De Nys K, Meyhi E, Mannaerts GP, Fransen M, Van Veldhoven PP. Characterisation of human peroxisomal 2,4-dienoyl-CoA reductase. Biochim Biophys Acta 2001;1533:66–72. [DOI] [PubMed] [Google Scholar]
- 42. Geisbrecht BV, Liang X, Morrell JC, Schulz H, Gould SJ. The mouse gene PDCR encodes a peroxisomal delta(2), delta(4)-dienoyl-CoA reductase. J Biol Chem 1999;274:25814–20. [DOI] [PubMed] [Google Scholar]
- 43. Cherfaoui M, Durand D, Bonnet M, Cassar-Malek I, Bauchart D, Thomas A, et al. Expression of enzymes and transcription factors involved in n-3 long chain PUFA biosynthesis in limousin bull tissues. Lipids 2012;47:391–401. [DOI] [PubMed] [Google Scholar]
- 44. Hayashi H, Oohashi M. Incorporation of acetyl-CoA generated from peroxisomal beta-oxidation into ethanolamine plasmalogen of rat liver. Biochim Biophys Acta 1995;1254:319–25. [DOI] [PubMed] [Google Scholar]
- 45. Groscurth P, Filgueira L. Killing mechanisms of cytotoxic T lymphocytes. News Physiol Sci 1998;13:17–21. [DOI] [PubMed] [Google Scholar]
- 46. Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 2017;31:711–23.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Decr2 allele and expression in transplantable tumor cell models and Decr2 knockdown tumor cells were resistant to CD8+ T cell killing in vitro.
Supplementary Figure S2. Decr2 knockdown OCI-Ly8 tumor cells were resistant to CD19-CAR T cell killing in vitro and Decr2 knockdown B16.SIY tumors exhibit resistance to anti-PD-L1 therapy in vivo.
Supplementary Figure S3. Decr2 modulates tumor sensitivity to anti-PD-L1 therapy in vivo.
Supplementary Figure S4. CD8+ T cell activation and cytokine secretion in tumor-draining lymph nodes.
Supplementary Figure S5. Single-cell transcriptomic profiling of CD45+ cells isolated from control versus Decr2 KD tumors of B6 mice.
Supplementary Figure S6. Gating strategy for Flow cytometry analysis of B16.SIY tumor microenvironment isolated from B6 mice.
Supplementary Figure S7. Tumor microenvironment analysis.
Supplementary Figure S8. Gating strategy for Flow cytometry analysis of B16.SIY tumor microenvironment isolated from B6 mice.
Supplementary Figure S9. MHC class I H2Kb, PD-L1 expression, and antigen presentation by tumor cells.
Supplementary Figure S10. Subcellular localization of Decr2.
Supplementary Figure S11. Decr2 knockdown tumors are resistant to ferroptosis.
Supplementary Figure S12. The gating strategy for flow cytometry analysis of lipid reactive oxygen species (ROS).
Supplementary Figure S13. Decreased polyunsaturated ether phospholipids levels in Decr2 knock-down tumor cells.
Supplementary Figure S14. Ethanolamine-containing glycerophospholipid and PC synthesis rate.
Supplementary Figure S15. Decr2 does not affect reduced glutathione (GSH), oxidized glutathione (GSSG), GSH/GSSG ratio, or expression of other ferroptosis genes.
Supplementary Figure S16. IFN-γ signaling is involved with T cell killing and reprogrammed lipids
Supplementary Figure S17. Genome-wide CRISPR reveals Slc7a11 gene was significantly depleted in B16.SIY cells co-cultured with 2C CD8+ T cells and Decr2 Slc7a11 double knockdown B16.SIY cells were generated.
Table S1: MAGeCK analysis of CRISPR screen results
Data Availability Statement
Source data for all figures are provided with the article online. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Single-cell RNA-seq processed and raw data were deposited to NCBI Gene Expression Omnibus database. The accession number is GSE284699. Constructs and cell lines generated in this study will be made available upon request, but a completed Material Transfer Agreement may be required if there is potential for commercial application.
Further information and request for resources and reagents should be directed to and will be fulfilled by the corresponding author Dr. Thomas F. Gajewski (tgajewsk@medicine.bsd.uchicago.edu).