Abstract
The Golgi apparatus (GA), a critical sub-cellular organelle, plays a pivotal role in numerous biological signaling pathways, including the post-translational modification of proteins and their secretion to various cellular destinations. Dysregulation of GA function is implicated in the development of several diseases, including cancer. As a result, detouring clinically approved drugs into the GA for an enhanced anti-cancer effect remained a major challenge. To address this, herein, we designed and synthesized NSAID-based conjugates incorporating a fluorophore (1,8-naphthalimide) and a Golgi-homing moiety (phenylsulfonamide). Screening these conjugates in cervical (HeLa) and colon (HCT-116) cancer cells identified a particularly promising candidate: the ibuprofen-1,8-naphthalimide-phenylsulfonamide conjugate (7a) which exhibited significant cytotoxicity against HCT-116 cells as well as in lung cancer (A549), colon carcinoma (Caco-2) and breast cancer (MCF7) cells. Interestingly, compound 7a self-assembled into nanoscale petal-like structures in water and efficiently homed into the GA as well as in the endoplasmic reticulum (ER) within 30 min to induce morphological damage to the GA. Compound 7a mediated GA damage increased the expression of Beclin and LC3-I/II proteins to induce autophagy which was further inhibited by chloroquine (CQ) and bafilomycin A1 (BFA) leading to remarkable HCT-116 cell death in combination with 7a. Moreover, compound 7a triggered apoptosis by downregulating anti-apoptotic Bcl-2 and Cas-3 as well as cleaving PARP proteins in HCT-116 cells, while demonstrating no toxicity towards non-cancerous human retinal pigment epithelial cells (RPE-1). Interestingly, compound 7a also reduced the size and growth of the HeLa 3D spheroids significantly after 72 h. This ibuprofen derivative (7a) holds promise as a valuable tool for illuminating the chemical biology of the GA in cancer cells and as a potential candidate for anti-cancer therapy.
We synthesized a small molecule library and identified an ibuprofen–1,8-naphthalimide–phenylsulfonamide conjugate, which self-assembled into nanopetals, homed into the Golgi apparatus to induce autophagy and apoptosis in cancer cells.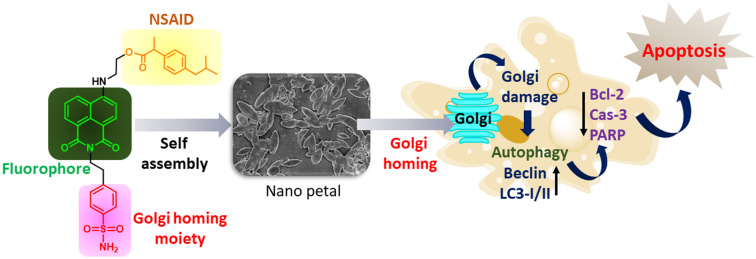
Introduction
The Golgi apparatus (GA) is a vital sub-cellular organelle that orchestrates key cellular processes, including the post-translational modification of proteins, protein sorting, transport, secretion, ion exchange, oxidative stress regulation, and apoptosis.1–6 Dysfunctions in the GA are strongly implicated in the progression of various diseases, including cancer.7–11 Hence, routing small molecule drugs into the GA to improve their anti-cancer effect emerged as a potential strategy for next-generation targeted cancer therapy.12–15
In this context, non-steroidal anti-inflammatory drugs (NSAIDs) have recently gained attention as a novel class of anti-cancer agents.16–21 Notably, some recent reports suggest that NSAIDs, particularly cyclooxygenase-2 (COX-2) inhibitors, can impair GA function.22–25 However, directing NSAIDs specifically to the GA to explore their therapeutic potential has been challenging due to the lack of effective Golgi-targeting moieties, leaving this area underexplored.26
To overcome this challenge, we designed and synthesized NSAID derivatives (ibuprofen, indomethacin V, meclofenamic acid, and naproxen) tagged with a fluorophore (1,8-naphthalimide) and a Golgi-homing moiety (phenylsulfonamide) using a concise synthetic strategy (Fig. 1a–c). Screening these compounds for cell viability in cancer cell lines (HeLa and HCT-116) identified ibuprofen–1,8-naphthalimide–phenylsulfonamide (7a), which exhibited remarkable cytotoxicity against HCT-116 colon cancer cells as well as in the lung cancer (A549), colon carcinoma (Caco-2) and breast cancer (MCF7) cells. Interestingly, 7a self-assembled into nanoscale petal-like structures in water, localized into the GA and ER within 30 min to damage the GA morphology followed by induction of autophagy which was inhibited by chloroquine (CQ) and bafilomycin A1 (BFA) to cause remarkable HCT-116 cell death in combination with 7a. Furthermore, compound 7a induced apoptosis by downregulating anti-apoptotic proteins Bcl-2 and Cas-3 as well as by cleavage of PARP in HCT-116 cells, while showing no toxicity in non-cancerous human retinal pigment epithelial (RPE-1) cells (Fig. 1d). Furthermore, compound 7a showed significant reduction in the growth of the 3D HeLa spheroids after 72 h. This novel ibuprofen derivative represents a promising chemical biology tool for elucidating the effects of NSAIDs on the Golgi apparatus and holds significant potential for the development of innovative anti-cancer therapeutics.
Fig. 1. (a) Design of Golgi targeting fluorescent NSAIDs. (b) Chemical structures of the NSAIDs used in this study. (c) Synthetic scheme of the Golgi targeting NSAIDs. (d) Mechanism of action of self-assembled 7a for Golgi targeting in cancer cells.
Results and discussion
To evaluate the effect of the NSAIDs on the GA, we designed the Golgi targeting NSAID derivatives having NSAIDs conjugated with a fluorophore and a Golgi homing moiety (Fig. 1a). We have chosen ibuprofen (6a), indomethacin V (6b), meclofenamic acid (6c) and naproxen (6d) (Fig. 1b) as NSAIDs due to their anti-cancer activities. As the fluorescence probe and Golgi homing moiety, we have chosen 1,8-naphthalimide and phenylsulfonamide, respectively.27,28 Recently, we developed 2,6-dihydroxybenzylhydrazone tagged with phenylsulfonamide as a GA imaging agent, which lacked a cancer therapy probe.29 However, herein, we introduced a fluorescence probe (1,8-naphthalimide), NSAIDs and a GA homing moiety in a single molecule to improve theranostic applications. To synthesize the Golgi targeting NSAIDs, 4-bromo-1,8-naphthalic anhydride (1) was reacted with 4-(2-aminoethyl) benzenesulfonamide (2) in the presence of acetic acid to obtain the 4-bromo-1,8-naphthalimide conjugate of benzenesulfonamide (3) in 72% yield (Fig. 1c). Compound 3 was further reacted with 2-aminoethan-1-ol (4) to afford a 1,8-naphthalimide–benzenesulfonamide–ethanolamine conjugate (5) in 87% yield.
Finally, compound 5 was separately conjugated with ibuprofen (6a), indomethacin V (6b), meclofenamic acid (6c) and naproxen (6d) in the presence of EDC/DMAP as a coupling agent to obtain NSAID–1,8-naphthalimide–benzenesulfonamide conjugates (7a–7d) (Fig. S1†). All the intermediates and the final products were characterized by 1H, 13C NMR and HR-MS spectroscopy (Fig. S2–S19†). We further characterized 7a–7d by UV-vis spectroscopy which showed absorbance at λmax = 431.5–440 nm in DMSO (Fig. S20a†). We also assessed compounds 7a–7d by fluorescence spectroscopy in DMSO and water for bioimaging. Compounds 7a–7d showed fluorescence emission at λem = 523–526 nm and 541 nm in DMSO and water, respectively (Fig. S20b–d†). All the Golgi targeting NSAIDs showed an 86 nm Stokes shift which is ideal for bioimaging.
To evaluate the effect of the Golgi targeting NSAIDs on cancer cells, we treated cervical (HeLa) and colon (HCT-116) cancer cells with 7a–7d in a dose dependent manner for 24 h followed by measuring the cell viability by MTT assay. It was interesting to find that compounds 7a and 7d showed IC50 = 8.16 and 3.6 μM, respectively (Fig. 2a), in HeLa cells, whereas compounds 7b and 7c showed negligible HeLa cell killing. On the other hand, in HCT-116 cells, compounds 7a and 7d demonstrated IC50 = 1.74 and 5.8 μM, respectively, along with IC50 > 10 μM for both 7d and 7c (Fig. 2b). From these MTT assays, it was clear that compound 7a was most effective in killing HCT-116 cells.
Fig. 2. Cell viability of Golgi targeting NSAIDs in (a) HeLa and (b) HCT-116 cells at 24 h post incubation. (c–f) Dose dependent cell viability assay of compound 7a in A549, MDA-MB-231, Caco-2 and MCF-7 cells, respectively, at 24 h post-incubation.
To assess the efficacy of compound 7a in other cancer cells, we further treated A549 (lung), MDA-MB-231 (metastatic triple negative breast), Caco-2 (colon adenocarcinoma) and MCF-7 (breast) cancer cells with compound 7a in a dose-dependent manner for 24 h followed by MTT assay. The cell viability assay exhibited that compound 7a showed effective cancer cell killing with IC50 = 2, 10.26, 1.7 and 0.5 μM in A549, MDA-MB-231, Caco-2 and MCF-7 cells, respectively (Fig. 2c–f). To further compare the efficacy of compound 7a against a standard chemotherapeutic drug, we treated HCT-116 and HeLa cells with cisplatin, as an anti-cancer drug, in a dose dependent manner for 24 h and evaluated the cell viability. The MTT assay demonstrated that cisplatin induced much less HCT-116 and HeLa cell killing ability compared to compound 7a with IC50 = 18 and >20 μM, respectively (Fig. S21†), which is in accordance with the reported data.30,31
We further estimated the effect of 7a in non-cancerous human retinal pigment epithelial cells (RPE-1) in a dose dependent manner at 24 h incubation. The MTT assay revealed that 7a showed marginal RPE-1 cell killing even at 10 μM concentration (Fig. S22†). Hence, we proceeded further to evaluate the Golgi targeting of 7a in HCT-116 cells.
Due to the amphiphilic nature, we evaluated the self-assembly of compound 7a in water. To our surprise, compound 7a showed a 125.4 nm hydrodynamic diameter with a −8.5 mV zeta potential measured by dynamic light scattering (DLS) (Fig. 3a). Furthermore, we visualized the size, shape and morphology of the self-assembled structure of 7a in water by field emission scanning electron microscopy (FESEM) and atomic force microscopy (AFM). The FESEM images revealed that compound 7a self-assembled into nanoscale petal like structures at 2 μM concentration (Fig. 3b and S23†). The petal like morphology was also confirmed by the AFM images (Fig. 2c and d and S24†). DLS, FESEM and AFM confirmed that compound 7a self-assembled into sub 200 nm sized nanoscale petal like structures in water, which can be efficiently localized into the tumor tissues through the enhanced permeability and retention (EPR) effect in the in vivo models to increase the anti-cancer efficacy with less toxic side effects in the healthy tissues.32
Fig. 3. (a) Hydrodynamic diameter and zeta potential of compound 7a self-assembled in water measured by dynamic light scattering (DLS). (b) FESEM image of the nanopetals formed by the self-assembly of 7a in water. (c and d) AFM images of the nanopetals formed by the self-assembly of 7a in water.
We hypothesized that Golgi targeting NSAIDs would home into the sub-cellular GA in cancer cells. To validate our hypothesis, we treated HCT-116 cells with compound 7a in a time dependent manner for 30 min, 1 h, 3 h, 6 h and 24 h followed by staining the cells with GolgiTracker Red dye. The live cells were then visualized under confocal microscopy. The confocal fluorescence images of the HCT-116 cells revealed that compound 7a (green) homed into the GA (red) to produce merged yellow fluorescence signals. The efficiency of homing of compound 7a was measured by Pearson's correlation coefficients (PCCs) to be 0.77, 0.74, 0.78, 0.71 and 0.76 at 30 min, 1 h, 3 h, 6 h and 24 h, respectively (Fig. 4 and S25†).
Fig. 4. Confocal microscopy images of the HCT-116 cells treated with compound 7a for 30 min, 1 h, 3 h, 6 h and 24 h followed by staining the Golgi apparatus with GolgiTracker Red dye. Scale bar = 10 μm.
To validate the effectiveness of phenylsulfonamide as a GA homing moiety, we synthesized a control molecule (10) without phenylsulfonamide and an NSAID in two steps (Fig. S26†). 4-Bromo-1,8-naphthalic anhydride (1) was reacted with aniline (8) in ethanol under refluxing conditions to obtain the 4-bromo-1,8-napththalimide conjugate of aniline (9) in 76% yield, which was further reacted with 2-aminoethan-1-ol (4) to afford a 1,8-naphthalimide–aniline–ethanolamine conjugate (10) in 68% yield. Compounds 9 and 10 were characterized by 1H, 13C NMR spectroscopy and HR-MS (Fig. S27–S32†). We further characterized compound 10 by UV-vis and fluorescence spectroscopy which exhibited absorbance at λmax = 442 nm in water and DMSO as well as fluorescence emission at λem = 555 nm in water (Fig. S33†), suitable for sub-cellular fluorescence imaging.
We further evaluated the GA homing ability of control compound 10 without having the phenylsulfonamide moiety in HCT-116 cells. We incubated HCT-116 cells with compound 10 in a time dependent manner for 30 min, 1 h and 3 h, followed by staining the GA with GolgiTracker Red dye and visualized the cells under confocal microscopy. To our satisfaction, we hardly visualized any sub-cellular localization of compound 10 inside the cells as well as into GA even after 3 h (Fig. S34†). Confocal microscopy confirmed that the phenylsulfonamide moiety is extremely necessary for sub-cellular GA homing.
Moreover, GA and endoplasmic reticulum (ER) cross-talk with each other to transport proteins and lipids. As a result, it was expected that after localizing into the GA, compound 7a will also be transported into the ER.33–36 Hence, we treated HCT-116 cells with compound 7a for 30 min, 1 h and 3 h followed by staining the cells with ERTracker Red dye. Interestingly, the confocal images revealed that compound 7a also homed into the ER with PCC = 0.92, 0.85 and 0.88, respectively, at 30 min, 1 h and 3 h (Fig. 5).37 We further evaluated the sub-cellular localization of compound 7a in other organelles like lysosomes and mitochondria. We treated HCT-116 cells with compound 7a for 1 h and stained the lysosome and mitochondria with LysoTracker Red and MitoTracker Red dyes, respectively, followed by visualizing the live cells under confocal microscopy. The confocal images demonstrated that compound 7a marginally localized into the lysosome and mitochondria with PCC = 0.62 and 0.63, respectively (Fig. S35†). These confocal microscopy images and quantification exhibited that compound 7a efficiently homed into the GA and ER within 30 min and retained in the GA for 24 h with marginal homing into lysosomes and mitochondria.
Fig. 5. Confocal microscopy images of the HCT-116 cells treated with compound 7a for 30 min, 1 h and 3 h followed by staining the ER with ERTracker Red dye. Scale bar = 10 μm.
After localizing into the GA, we anticipated that compound 7a would damage the GA.38 To evaluate the GA damage, we treated HCT-116 cells with compound 7a for 24 h, stained the GA with GolgiTracker Red dye and visualized the cells under confocal microscopy. Interestingly, we observed a compact flattened and slightly elongated morphology of the GA in the control cells, which were completely lost and exhibited punctate structures in the compound 7a treated cells (Fig. 6a and S36†). Moreover, in the control cells, the GA was found to be mainly localized around the nucleus. However, in the compound 7a treated cells, the fragmented GA was dispersed in cytosol. Furthermore, under GA damage, the Golgi-mediated protein transport through a microtubule will also be hampered.39 Hence, to visualize the microtubule impairment, we treated HCT-116 cells with compound 7a for 24 h, stained the cells with Tubulin Tracker Deep Red dye and observed the microtubule network under confocal microscopy. Confocal microscopy revealed elongated filamentous microtubule morphology in the control cells. However, in the compound 7a treated cells, the filamentous structures of the microtubule were remarkably disrupted as well as reduced in numbers along with the increase in truncated and condensed structures (Fig. 6b and S37†). These confocal images confirmed that compound 7a impaired the GA morphology along with the damage in the microtubule network because of GA stress.
Fig. 6. Confocal images of HCT-116 cells after treatment with compound 7a for 24 h followed by staining the cells with (a) GolgiTracker Red and (b) Tubulin Tracker Deep Red dye, respectively. Scale bar = 10 μm.
After GA damage, we anticipated that the cells induce autophagy.40,41 To evaluate autophagy, we treated HCT-116 cells with compound 7a for 24 h and the cellular proteins were harvested for gel electrophoresis. The western blot analysis revealed that compound 7a increased the expression of Beclin and LC3-I/II proteins as autophagy markers by 2 folds and 16 folds, respectively, compared to the control cells (Fig. 7a and b, and S38a and b†), which confirmed that 7a-mediated GA damage induced autophagy. To further confirm autophagy induction, we treated HCT-116 cells with an autophagy inhibitor chloroquine (CQ) (12.5 μM) and bafilomycin A1 (BFA) (25 nM) in combination with dose dependent treatment with compound 7a and estimated the cell viability by MTT assay. To our surprise, the CQ and BFA combination treatment with 7a caused a remarkable increase in HCT-116 cell death with IC50 = 0.19 μM and 0.1 μM, respectively, compared to only 7a treatment (Fig. 7c and d). We also checked the cytotoxicity of CQ in HCT-116 cells at 12.5 μM for 24 h which showed that CQ was not at all toxic to the HCT-116 cells (Fig. S39†). Gel electrophoresis and cell viability assay clearly confirmed that compound 7a induced autophagy in HCT-116 cells as a result of GA damage.
Fig. 7. (a and b) Expression of Beclin and LC3-I/II as markers of autophagy in HCT-116 cells after treatment with compound 7a for 24 h followed by western blot analysis. *** p < 0.0001, the p value is calculated using two-way ANOVA. (c and d) Viability of HCT-116 cells after treatment with autophagy inhibitor chloroquine (CQ) and bafilomycin A1 (BFA) in combination with 7a in a dose dependent manner for 24 h measured by MTT assay. (e–g) Expression of Bcl-2, Cas-3, PARP and cleaved PARP as apoptosis markers in HCT-116 cells after treatment with compound 7a for 24 h followed by western blot analysis. *** p < 0.0001, the p value is calculated using two-way ANOVA.
After the induction of autophagy, compound 7a would trigger apoptosis. We treated HCT-116 cells with compound 7a for 24 h followed by harvesting the sub-cellular proteins and performed the gel electrophoresis to evaluate the expression of anti-apoptotic Bcl-2, Cas-3, PARP and cleaved PARP as apoptotic markers. From the western blot analysis, it was observed that compound 7a reduced the expression of Bcl-2 and Cas-3 by 1.2 folds and 1.9 folds, respectively, compared to the non-treated control cells (Fig. 7e and f, and S38c and d†). Moreover, compound 7a reduced the expression of full-length PARP by 2.4 folds and increased the expression of cleaved PARP by 2.1 folds compared to the control cells (Fig. 7g and S38e†). From gel electrophoresis, it was confirmed that compound 7a- mediated Golgi impairment led to apoptosis by reducing the expression of Bcl-2, Cas-3 and PARP along with the increase in the expression of cleaved PARP.
To mimic the tumor environment better, we evaluated the effect of compound 7a on the HeLa 3d spheroid model. We treated the HeLa 3D spheroids with compound 7a for 72 h and visualized the spheroid size and growth by optical microscopy which revealed that compound 7a reduced the growth of the spheroids significantly compared to the non-treated control spheroids (Fig. 8a). Moreover, we calculated the area of the spheroids using the phase contrast images, which exhibited that compound 7a significantly inhibited the growth of the spheroids (Fig. 8b). HeLa 3D spheroid assay clearly confirmed that compound 7a has potential to be further explored for GA targeted anti-cancer therapy in in vivo models.
Fig. 8. HeLa 3D spheroid growth inhibition study. (a) Phase contrast images of the spheroids treated with compound 7a for 72 h. Scale bar = 500 μm. (b) Graph showing the area of spheroids depicting the growth of the spheroids in the absence and presence of compound 7a when treated to spheroids for 72 h. Unpaired Student's t test was performed for statistical analysis, where p value < 0.01 (n = 5).
Conclusions
In conclusion, we have designed and synthesized NSAIDs (ibuprofen, indomethacin V, meclofenamic acid and naproxen) tagged with a 1,8-naphthalimide fluorophore and bezenesulfonamide as a Golgi homing moiety. These NSAID derivatives were screened against HeLa and HCT-116 cancer cells to identify ibuprofen-1,8-naphthalimide-benezesulfonamide (7a) which self-assembled into nano-petal like structures in water. Compound 7a efficiently homed into the GA within 30 min, retained there for 24 h and damaged GA followed by microtubule network impairment leading to induction of autophagy which was further inhibited by chloroquine and bafilomycin A1 in combination with 7a causing remarkable HCT-116 cells death. Furthermore, compound 7a triggered apoptosis by reducing the expression of anti-apoptotic Bcl-2 and Cas-3 along with cleaving PARP leading to remarkable HCT-116 colon cancer cell killing without showing any toxicity to the non-cancerous RPE-1 cells. Moreover, compound 7a reduced the size and growth of the HeLa 3D spheroids after 72 h as a mimic of in vivo model. We anticipate this ibuprofen-derivative could be a potential tool to explore chemical biology of Golgi towards novel anti-cancer therapy.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
SB acknowledges IIT Gandhinagar, Gujarat Council on Science and Technology (GUJCOST/STI/R&D/2020-21/1302) and the Science and Engineering Research Board (CRG/2020/001127) for financial support. Aditi and TM thank IIT Gandhinagar for doctoral fellowship.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5md00215j
Data availability
The data supporting this article have been included as part of the ESI.†
References
- Tang D. Wang Y. Trends Cell Biol. 2013;23:296–304. doi: 10.1016/j.tcb.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J. Warren G. Annu. Rev. Cell Dev. Biol. 2002;18:379–420. doi: 10.1146/annurev.cellbio.18.030602.133733. [DOI] [PubMed] [Google Scholar]
- Wang Y. Seemann J. Cold Spring Harbor Perspect. Biol. 2011;3:a005330–a005330. doi: 10.1101/cshperspect.a005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Palade G. E. Trends Cell Biol. 1998;8:2–10. doi: 10.1016/S0962-8924(97)01187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh B. J. Howell K. E. Nat. Rev. Mol. Cell Biol. 2002;3:789–795. doi: 10.1038/nrm933. [DOI] [PubMed] [Google Scholar]
- Liu J. Huang Y. Li T. Jiang Z. Zeng L. Hu Z. Int. J. Mol. Med. 2021;47:38. doi: 10.3892/ijmm.2021.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman M. D. Rahajeng J. Field S. J. Cancer Res. 2015;75:624–627. doi: 10.1158/0008-5472.CAN-14-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. W. Zhu J. Wu X. X. Tu T. Huang J. Q. Chen G. Z. Liang L. Y. Zhou C. H. Xu X. Gong L. Y. Cell Death Dis. 2021;12:976. doi: 10.1038/s41419-021-04265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj R. Warner A. N. Fradette J. F. Gibbons D. L. Cells. 2022;11:1484. doi: 10.3390/cells11091484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj R. Kundu S. T. Grzeskowiak C. L. Fradette J. J. Scott K. L. Creighton C. J. Gibbons D. L. Oncogene. 2020;39:5979–5994. doi: 10.1038/s41388-020-01410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. Malvi P. Parajuli K. R. Janostiak R. Bugide S. Cai G. Zhu L. J. Green M. R. Wajapeyee N. Proc. Natl. Acad. Sci. U. S. A. 2020;117:12341–12351. doi: 10.1073/pnas.2005156117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C. Wu M. Guo Z. Zhang R. Wang Z. Peng X. Dong J. Sun X. Zhang Z. Xiao P. ACS Nano. 2023;17:24972–24987. doi: 10.1021/acsnano.3c07183. [DOI] [PubMed] [Google Scholar]
- Liang B.-B. Liu Q. Liu B. Yao H.-G. He J. Tang C.-F. Peng K. Su X.-X. Zheng Y. Ding J.-Y. Shen J. Cao Q. Mao Z.-W. Angew. Chem., Int. Ed. 2023;62:e202312170. doi: 10.1002/anie.202312170. [DOI] [PubMed] [Google Scholar]
- Tan W. Zhang Q. Wang J. Yi M. He H. Xu B. Angew. Chem., Int. Ed. 2021;60:12796–12801. doi: 10.1002/anie.202102601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. Xu J. Ya N. Deng L. Gan Q. Liu J. Zeng Y. ACS Appl. Nano Mater. 2024;7:7520–7532. [Google Scholar]
- De Groot D. J. Le T. K. Regeling A. De Jong S. De Vries E. G. Br. J. Cancer. 2007;97:1077–1083. doi: 10.1038/sj.bjc.6604010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittoni M. A. Carbone D. P. Harris R. E. Mol. Clin. Oncol. 2017;6:917–920. doi: 10.3892/mco.2017.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. E. Beebe-Donk J. Doss H. Doss B. D. Oncol. Rep. 2005;13:559–583. [PubMed] [Google Scholar]
- Blackstock A. W. Herndon II J. E. Paskett E. D. Perry M. C. Graziano S. L. Muscato J. J. Kosty M. P. Akerley W. L. Holland J. Fleishman S. Green M. R. J. Natl. Cancer Inst. 2002;94:284–290. doi: 10.1093/jnci/94.4.284. [DOI] [PubMed] [Google Scholar]
- Guo Y. C. Chang C. M. Hsu W. L. Chiu S. J. Tsai Y. T. Chou Y. H. Hou M. F. Wang J. Y. Lee M. H. Tsai K. L. Chang W. C. Mol. 2013;18:6584–6596. doi: 10.3390/molecules18066584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akrami H. Aminzadeh S. Fallahi H. Tumor Biol. 2015;36:3237–3243. doi: 10.1007/s13277-014-2952-3. [DOI] [PubMed] [Google Scholar]
- Gurram B. Li M. Fan J. Wang J. Peng X. Front. Chem. Sci. Eng. 2020;14:41–52. [Google Scholar]
- Xiao P. Ma K. Kang M. Huang L. Wu Q. Song N. Ge J. Li D. Dong J. Wang L. Wang D. Tang B. Z. Chem. Sci. 2021;12:13949–13957. doi: 10.1039/d1sc03932f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Fan J. Wang J. Zhang S. Dou B. Peng X. J. Am. Chem. Soc. 2013;135:11663–11669. doi: 10.1021/ja4056905. [DOI] [PubMed] [Google Scholar]
- Yuan C. Smith W. L. J. Biol. Chem. 2015;290:5606–5620. doi: 10.1074/jbc.M114.632463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R.-Y. Xing L. Cui P.-F. Qiao J.-B. He Y.-J. Chang X. Zhou T.-J. Jin Q.-R. Jiang H.-L. Xiao Y. Biomater. Sci. 2018;6:2144. doi: 10.1039/c8bm00381e. [DOI] [PubMed] [Google Scholar]
- Wang H. Zhang X. Xiu T. Wang H. Li P. Tang B. Coord. Chem. Rev. 2024;502:215618. [Google Scholar]
- Xu S. Yan K.-C. Xu Z.-H. Wang Y. James T. D. Chem. Soc. Rev. 2024;53:7590–7631. doi: 10.1039/d3cs00171g. [DOI] [PubMed] [Google Scholar]
- Aditi Sanyam. Ingle J. Das B. Mondal A. Basu S. ChemBioChem. 2024;25:e202400507. [Google Scholar]
- Saber M. M. Al-mahallawi A. M. Nassar N. N. Stork B. Shouman S. A. BMC Cancer. 2018;18:822. doi: 10.1186/s12885-018-4727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becit M. Dilsiz S. A. Başaran N. Istanbul J. Pharm. 2020;50:202–210. [Google Scholar]
- Matsumura Y. Maeda H. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- Tan W. Zhang Q. Quiñones-Frías M. C. Hsu A. Y. Zhang Y. Rodal A. Hong P. Luo H. R. Xu B. J. Am. Chem. Soc. 2022;144:6709–6713. doi: 10.1021/jacs.2c02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L. Stamnes M. Ravazzola M. Amherdt M. Perrelet A. Söllner T. H. Rothman J. E. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Spang A. Cold Spring Harbor Perspect. Biol. 2013;5:a013391. doi: 10.1101/cshperspect.a013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodkowic D. Skommer J. McGuinness D. Hillier C. Darzynkiewicz Z. Leuk. Res. 2009;33:1440–1447. doi: 10.1016/j.leukres.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P. Mishra T. Sanyam Mondal A. Basu S. ACS Appl. Bio Mater. 2025;8:1524–1532. doi: 10.1021/acsabm.4c01722. [DOI] [PubMed] [Google Scholar]
- Liang B. Liu Q. Liu B. Yao H. He J. Tang C. Peng K. Su X. Zheng Y. Ding J. Shen J. Cao Q. Mao Z. Angew. Chem. 2023;62:e202312170. doi: 10.1002/anie.202312170. [DOI] [PubMed] [Google Scholar]
- Wu J. de Heus C. Liu Q. Bouchet B. P. Noordstra I. Jiang K. Hua S. Martin M. Yang C. Grigoriev I. Katrukha E. A. Altelaar A. F. M. Hoogenraad C. C. Qi R. Z. Klumperman J. Akhmanova A. Dev. Cell. 2016;39:44–60. doi: 10.1016/j.devcel.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Chen Y. Wu Y. Tian X. Shao G. Lin Q. Sun A. Cell Biosci. 2024;14:130. doi: 10.1186/s13578-024-01311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. Yang W. Y. IUBMB Life. 2022;74:361–370. doi: 10.1002/iub.2611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been included as part of the ESI.†










