ABSTRACT
Objective
To evaluate the association between maternal neutrophil‐to‐lymphocyte ratio (NLR) and delivery within 24 h in women with preterm premature rupture of membranes (PPROM).
Study Design and Setting
Retrospective cohort study in a single university‐affiliated tertiary medical centre.
Population
Women with PPROM at ≤ 36 + 6 weeks' gestation planning vaginal delivery. Exclusions included women who lacked complete blood count (CBC) data within the first 24 h from PPROM caesarean delivery.
Methods
Maternal demographic and clinical data, including age, gestational age, body mass index, parity and mode of conception, were collected. Women who delivered within 24 h of membrane rupture were compared to those who did not via univariate and multivariate Cox analyses.
Main Outcome Measure
Women who delivered within 24 h from rupture of membrane.
Results
Among 145 833 deliveries during the study period, 1498 women (0.9%) presented with PPROM. After exclusions, 371 women were included, with 173 (46.6%) delivering spontaneously within 24 h. Cox regression analysis identified NLR > 10 (HR = 1.60; 95% CI, 1.06–2.40; p = 0.025) as an independent risk factor for spontaneous delivery within 24 h.
Conclusion
Elevated maternal NLR is associated with delivery within 24 h in PPROM and may support clinical assessment for anticipating imminent preterm delivery, aiding in management decisions for this population.
Keywords: delivery, NLR, PPROM, risk factors
1. Introduction
Preterm premature rupture of membranes (PPROM) affects about 3% of pregnancies in the United States [1] and contributes to one‐third of preterm deliveries [2, 3]. It is linked to significant perinatal risks, including respiratory distress syndrome, neonatal sepsis, umbilical cord prolapse, placental abruption and foetal death, as well as maternal risks [4]. Timely interventions aimed at managing prematurity, such as transferring to a Level III hospital, administration of magnesium sulphate and corticosteroid therapy, can improve outcomes [5]. Therefore, anticipating spontaneous delivery within 24 h in this population is clinically significant.
Several studies have explored risk factors for spontaneous delivery within 24 h in women with PPROM. These included increased cervical dilation at admission, elevated leucocyte counts upon admission and the transition from clear to meconium‐stained or bloody amniotic fluid [6]. Additionally, short cervical length [7] and oligohydramnios [8] have been identified as independent predictors of delivery within 48 h.
In recent studies, the neutrophil‐to‐lymphocyte ratio (NLR) has emerged as an inflammatory biomarker associated with various diseases and complications. Obstetric research has investigated the correlation between this ratio and complications such as preeclampsia [9, 10], gestational diabetes mellitus [11], intrahepatic cholestasis [12] and the prediction of spontaneous preterm delivery [13]. Its role in spontaneous delivery following PPROM has not yet been thoroughly investigated.
Thus, we aimed to determine the association between maternal NLR and spontaneous delivery within 24 h from rupture of membranes in women with PPROM.
2. Methods
2.1. Study Population
We conducted a retrospective cohort study involving all women who delivered at a single University‐affiliated tertiary medical centre between 2011 and 2023.
Women admitted with PPROM at less than 37 + 0 weeks' gestation and who planned a vaginal delivery were included in the study. They were classified into two groups based on the timing of delivery following PPROM: those who delivered within 24 h (study group) and those who delivered after 24 h (control group). To minimise confounding and accurately assess the association between NLR at admission and the timing of delivery, we excluded women who lacked complete blood count (CBC) data within the first 24 h after PPROM or who underwent medically indicated caesarean delivery.
According to our protocol, women admitted with PPROM were treated with corticosteroids (if < 34 weeks of gestation), antibiotics and magnesium sulphate for foetal neuroprotection, as recommended by ACOG [14].
2.2. Statistical Analysis
Univariate analyses were conducted to identify differences between the groups. Continuous variables were assessed using either the two‐tailed unpaired Student's t‐test or the Mann‐Whitney test for non‐normally distributed data, while categorical variables were analysed with the chi‐square test or Fisher's exact test, as appropriate. A multivariable logistic regression model and Cox regression analysis, controlling for variables that showed significant differences between the groups or had clinical relevance, were employed to assess the role of independent variables.
Data analysis was performed using SPSS software (version 21.0; SPSS Inc., Chicago, IL, USA), with a P‐value < 0.05 considered statistically significant.
2.3. Data Collection
All medical records were retrieved from computerised delivery room logbooks. The database included comprehensive demographic, obstetric and clinical data, such as maternal age, pre‐gestational body mass index (BMI), parity, gravidity, prior caesarean deliveries and gestational age at delivery. Additionally, data on the colour of the ruptured membranes (clear vs. non‐clear, including blood and meconium‐stained fluid), maternal fever (defined as a temperature ≥ 38°C) and uterine contractions at admission (indicating spontaneous onset of labour) were also collected. Antibiotic use was defined as either Group B Streptococcus (GBS) prophylaxis in cases of unknown GBS status or treatment for suspected clinical chorioamnionitis, which included maternal fever, uterine fundal tenderness, maternal tachycardia (> 100 bpm), foetal tachycardia (> 160 bpm) or purulent/foul‐smelling amniotic fluid.
NLR values, defined as the absolute neutrophil count divided by the absolute lymphocyte count, were obtained from laboratory tests performed upon patient admission and prior to the initiation of any medical treatment. An NLR > 10 corresponds to the upper quartile of our cohort.
The study was approved by the Institutional Review Board (IRB TLV‐0284‐08, 10 July 2024).
3. Results
During the study period, 145 833 deliveries were recorded at our centre, with 1498 (0.98%) women admitted with PPROM. After applying exclusion criteria, 371 women were eligible for analysis, of whom 173 (46.6%) experienced delivery within 24 h from PPROM (Figure 1).
FIGURE 1.
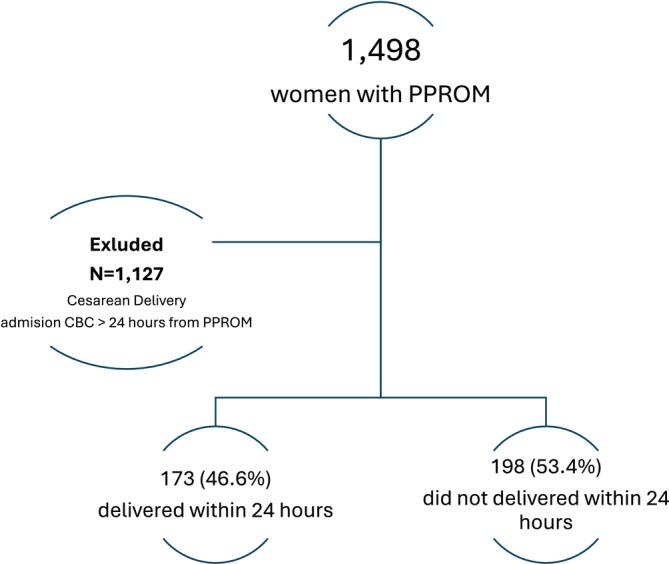
Study population.
The overall median time from PPROM to delivery was 27 h (IQR: 9–77 h). Among women who delivered within 24 h of PPROM, the median time to delivery was 9 h, compared to 72 h for those who delivered beyond 24 h [p < 0.001].
The demographic and obstetric characteristics of the study population are presented in Table 1. Women who delivered within 24 h of PPROM had a significantly lower gestational age at delivery compared to those who delivered later (34.5 weeks [IQR 26.8–36.2] vs. 35.2 weeks [IQR 33.4–36.3]; p = 0.013). The study group also had a higher incidence of multiple pregnancies (12.7% vs. 6.1%; p < 0.001). Additionally, symptomatic presentation at admission (76.9% vs. 56.4%; p < 0.001) and maternal fever (24.4% vs. 12.9%; p = 0.013) were more common among women who delivered within 24 h. In contrast, clear amniotic fluid was more frequently observed in the control group (99% vs. 89%; p < 0.001). No significant differences were found between the groups regarding maternal age, nulliparity, body mass index, history of caesarean delivery, mode of conception, gestational diabetes mellitus or antibiotic use.
TABLE 1.
Demographic and obstetric characteristics for the study population.
| Delivery after 24 h, N = 198 | Delivery within 24 h, N = 173 | p | |
|---|---|---|---|
| Maternal age (mean, SD) | 33.26 (5.21) | 33.18 (4.38) | 0.43 |
| Gestational week at admission (median, IQR) | 35.2 (33.4, 36.3) | 34.5 (26.8, 36.2) | 0.013 |
| BMI (median, IQR) | 22.57 (20.2, 24.8) | 21.67 (19.8, 24.9) | 0.37 |
| Nulliparity n (%) | 123 (62.4) | 117 (67.6) | 0.32 |
| Previous CS, n (%) | 8 (4.1) | 5 (2.9) | 0.58 |
| IVF pregnancy, n (%) | 37 (19.8) | 21 (13) | 0.11 |
| Multiple pregnancy, n (%) | 12 (6.1) | 22 (12.7) | 0.03 |
| GDM, n (%) | 26 (13.1) | 22 (12.7) | 1 |
| Clear ROM, n (%) | 195 (99) | 154 (89) | < 0.001 |
| Antibiotic use, n (%) | 110 (55.6) | 80 (46.2) | 0.078 |
| Spontaneous onset of delivery, n (%) | 110 (56.4) | 130 (76.9) | < 0.001 |
| Maternal fever, n (%) | 24 (12.9) | 29 (24.4) | 0.013 |
| WBC K/μL (median, IQR), n (%) | 13.5 (10.5, 16.7) | 14.5 (11.3, 17.6) | 0.058 |
| Hb g/dL (median, IQR), n (%) | 11.6 (10.9, 12.3) | 11.8 (10.9, 12.6) | 0.064 |
| PLT K/μL (median, IQR), n(%) | 211 (176, 258) | 211 (172, 261) | 0.67 |
| NLR > 10, n (%) | 31 (15.7) | 50 (28.9) | 0.002 |
Abbreviations: BMI, Body Mass Index; GDM, gestational diabetes Miletus; IQR, interquartile range; NLR, Neutrophils lymphocytes ratio; SD, standard deviation; SPCS, status post caesarean section.
Women who delivered within 24 h of PPROM were significantly more likely to have an NLR > 10 compared to those who delivered later (28.9% vs. 15.7%; p = 0.002). NLR > 10 demonstrated a specificity of 84.3%, sensitivity of 28.9%, positive predictive value (PPV) of 61.7% and negative predictive value (NPV) of 57.6% for predicting delivery within 24 h.
Multivariate Cox regression analysis (Table 2) was performed to identify independent risk factors of delivery within 24 h following PPROM. After adjusting for variables found to be significant in the univariate analysis, the following factors remained independently associated with early delivery: neutrophil‐to‐lymphocyte ratio (NLR) > 10 (HR = 1.60; 95% CI, 1.06–2.40; p = 0.025), multiple gestation (HR = 1.92; 95% CI, 1.10–3.30; p = 0.020), gestational age < 34 weeks (HR = 1.65; 95% CI, 1.12–2.40), non‐clear amniotic fluid at presentation (HR = 4.47; 95% CI, 2.30–8.60) and spontaneous onset of labour (HR = 2.25; 95% CI, 1.40–3.50).
TABLE 2.
Cox regression assessing the risk factors for delivery within 24 h.
| HR (95% CI) | p | |
|---|---|---|
| Preterm delivery < 34 weeks of gestation | 1.65 (1.12–2.4) | 0.01 |
| Multiple pregnancy | 1.92 (1.1–3.3) | 0.02 |
| Non‐clear ROM | 4.47 (2.3–8.6) | < 0.001 |
| Spontaneous onset of delivery | 2.25 (1.4–3.5) | < 0.001 |
| Maternal fever | 1.46 (0.9–2.27) | 0.09 |
| NLR > 10 | 1.6 (1.06–2.4) | 0.025 |
Figure 2 presents the cumulative hazard function for time from PPROM to delivery, stratified by the NLR groups, indicating a higher cumulative hazard for delivery over time following PPROM in the study group. Moreover, the risk of delivery within any given time frame after PPROM is greater in this group. An ROC curve, derived from the aforementioned multivariable Cox regression analysis, was constructed to assess the risk of delivery within 24 h of PPROM based on an NLR > 10. This analysis yielded an area under the curve (AUC) of 0.71 (95% CI, 0.65–0.77), with p < 0.001 (Figure S1).
FIGURE 2.
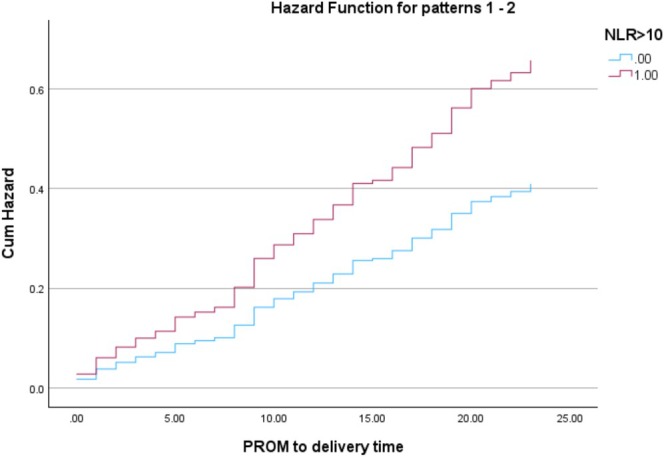
Cox proportional hazards model.
When stratifying the cumulative hazard function by multiple gestation (Figure S2), an NLR > 10 remained an independent risk factor for delivery within 24 h (HR = 1.68; 95% CI, 1.10–2.54; p = 0.014). Furthermore, an NLR > 10 was also independently associated with an increased risk of delivery within 36, 48, 60 and 72 h following PPROM, with hazard ratios of 1.56 (95% CI, 1.07–2.27), 1.60 (95% CI, 1.13–2.27), 1.71 (95% CI, 1.20–2.40) and 1.63 (95% CI, 1.17–2.30), respectively. The cumulative hazard functions stratified by time from PPROM to delivery are illustrated in Figure S3.
4. Discussion
4.1. Main Findings
In this study, we identified that NLR above 10 is an independent risk factor for delivery within 24 h in women with PPROM.
While previous studies have associated elevated NLR with adverse pregnancy outcomes, this study is the first to specifically examine the association between NLR and spontaneous delivery in the context of PPROM.
4.2. Strengths and Limitations
This retrospective cohort study has several limitations. First, the observational nature of the study may introduce selection bias, as the data were collected from a specific population of women with PPROM, potentially limiting the generalisability of our findings. Second, reliance on existing medical records may have led to incomplete data, particularly regarding confounding variables such as cervical length and cervical dilatation at admission, which could influence latency to delivery. Additionally, other inflammatory markers, such as C‐reactive protein, and pathological evidence of inflammation were not included in the analysis, as these data were not consistently available in the medical records. Furthermore, given the limited sensitivity and modest positive and negative predictive values observed, the poor predictive value of NLR as a standalone tool should be recognised.
Despite these limitations, our study has notable strengths. To the best of our knowledge, this is the first study to identify maternal NLR as a novel risk factor for delivery within 24 h following PPROM, contributing valuable insights to the existing literature. Moreover, patients without available CBC data within the first 24 h of admission were excluded in order to minimise confounding and to reduce the potential impact of factors such as corticosteroid and antibiotic treatment on inflammatory markers. In addition, we employed multivariate Cox regression analysis to control various confounding variables, enhancing the robustness of our findings.
4.3. Our Findings in the Context of Other Observations
A key novel finding of our study is the independent association between elevated NLR and spontaneous delivery within 24 h of PPROM. NLR, a marker of potential systemic inflammation, has been linked to adverse outcomes across various medical conditions, including cardiovascular disease and sepsis [15]. Elevated NLR has been associated with preeclampsia, gestational diabetes and preterm labour [16, 17]. Our results align with the findings of Lee et al., who identified maternal NLR above 7.75 as an independent risk factor for acute histologic chorioamnionitis in cases of spontaneous preterm birth [18], a condition known to precipitate spontaneous delivery [19, 20]. Notably, even after adjusting for other indirect markers of inflammation (such as leukocytosis, thrombocytosis, antibiotic use and maternal fever), as well as conducting sub‐analysis by multiple gestation and time to delivery, NLR remained an independent risk factor for early delivery. This emphasises the observed association between NLR and early delivery within our preterm study population.
Several studies have demonstrated that lower gestational age at the time of PPROM is associated with a shorter latency period to delivery. For instance, Point et al. reported that lower gestational age at PPROM was significantly associated with a reduced latency interval [8]. These findings are consistent with our results. In addition, inflammation and infection, whether infection‐induced or sterile, are known to play key roles in the pathogenesis of preterm birth and PPROM [6]. Elevated levels of inflammatory markers in the amniotic fluid, such as interleukin‐6 and matrix metalloproteinase‐8, have been linked to imminent preterm delivery and adverse neonatal outcomes [21, 22]. These mechanisms provide support for the observed association between elevated NLR and early delivery in our study population.
Multiple pregnancies are well‐established risk factors for preterm and extremely preterm birth [23]. Our study also identified multiple gestations as a risk factor for delivery within 24 h following PPROM. These findings are consistent with previous studies, which demonstrated that multiple pregnancies are associated with shorter latency periods after PPROM compared to singleton pregnancies [24, 25].
In our study, the presence of non‐clear amniotic fluid was significantly associated with delivery within 24 h of PPROM. Previous studies have shown that meconium‐stained amniotic fluid in the context of PPROM is linked to higher rates of adverse perinatal outcomes, including an increased incidence of chorioamnionitis and placental abruption [26, 27], both of which are established contributors to a shortened latency period. These findings may account for the association observed in our cohort.
4.4. Clinical Implications
The ability to predict imminent preterm birth, particularly in the context of PPROM, is critical, as timely preventive management can significantly improve neonatal outcomes. Several interventions have proven essential in such cases. Corticosteroid administration, for example has been shown to reduce the incidence of neonatal respiratory complications, with the timing of administration playing a pivotal role in its efficacy [28]. Therefore, precise forecasting of delivery timing is vital to ensure optimal corticosteroid delivery. Furthermore, magnesium sulphate serves as a neuroprotective agent with the potential to improve outcomes for preterm neonates. Similarly, effective prediction of labour onset and the appropriate timing of magnesium administration are crucial for maximising its therapeutic benefits [29]. Moreover, delivering high‐risk infants at a high‐level neonatal intensive care unit (NICU) is associated with improved neonatal outcomes [30]. Therefore, accurately predicting the timing of delivery can facilitate decision‐making regarding the transfer of women to a tertiary care facility.
Various studies have aimed to develop predictive models utilising specialised tests, such as fibronectin assays, biomarkers and ultrasound measurements [31, 32]. In contrast, our study presents a simple and widely accessible tool: maternal NLR. We believe that this measure can assist physicians in decision‐making for patients with PPROM, ultimately optimising neonatal outcomes. However, given the limited predictive values, NLR should not be used in isolation to guide clinical decision‐making.
5. Conclusions
In conclusion, this study identifies an NLR > 10 as an independent factor associated with delivery within 24 h in women with PPROM. These findings suggest that NLR may represent an adjunctive biomarker reflecting inflammatory involvement in the timing of labour, rather than a standalone predictive tool. Future prospective studies are warranted to confirm these associations, explore the underlying mechanisms and investigate potential interventions to reduce preterm delivery and associated maternal and neonatal complications in this population.
Author Contributions
Daniel Gabbai and Emmanuel Attali contributed equally, conducted the literature search and drafted the manuscript. Itamar Gilboa, Anat Lavie and Yariv Yogev helped conceptualise the study design and edited and revised the manuscript. All authors revised the article for important intellectual content and approved the final version submitted for publication.
Ethics Statement
The trial was conducted in accordance with the Declaration of Helsinki (2000) for human studies (IRB number TLV‐0284‐08).
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Figure S1. ROC curve.
Figure S2. Cox proportional hazards model by multiple pregnancy.
Figure S3. Cox proportional hazards model by delivery time.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The data that support the findings of this study are not publicly available due to institutional privacy policies and the use of patient medical records. De‐identified data may be available from the corresponding author upon reasonable request and with permission from the institutional review board.
References
- 1. ACOG Practice Bulletin No. 188 Summary: Prelabor Rupture of Membranes,” Obstetrics & Gynecology 131, no. 1 (2018): 187–189. [DOI] [PubMed] [Google Scholar]
- 2. Practice Bulletin No. 160 Summary: Premature Rupture of Membranes,” Obstetrics and Gynecology 127, no. 1 (2016): 192–194. [DOI] [PubMed] [Google Scholar]
- 3. Helmer H., “Continuing Challenges in Treating Preterm Labour: Preterm Prelabour Rupture of the Membranes,” BJOG: An International Journal of Obstetrics and Gynaecology 113, no. Suppl 3 (2006): 111–112. [DOI] [PubMed] [Google Scholar]
- 4. Linehan L. A., Walsh J., Morris A., et al., “Neonatal and Maternal Outcomes Following Midtrimester Preterm Premature Rupture of the Membranes: A Retrospective Cohort Study,” BMC Pregnancy and Childbirth 16 (2016): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Practice Bulletin No. 159 Summary: Management of Preterm Labour,” Obstetrics and Gynecology 127, no. 1 (2016): 190–191. [DOI] [PubMed] [Google Scholar]
- 6. Simhan H. N. and Canavan T. P., “Preterm Premature Rupture of Membranes: Diagnosis, Evaluation and Management Strategies,” BJOG: An International Journal of Obstetrics and Gynaecology 112, no. Suppl 1 (2005): 32–37. [DOI] [PubMed] [Google Scholar]
- 7. Rouzaire M., Corvaisier M., Roumeau V., et al., “Predictors of Short Latency Period Exceeding 48 h After Preterm Premature Rupture of Membranes,” Journal of Clinical Medicine 10, no. 1 (2021): 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Point F., Ghesquiere L., Drumez E., et al., “Risk Factors Associated With Shortened Latency Before Delivery in Outpatients Managed for Preterm Prelabor Rupture of Membranes,” Acta Obstetricia et Gynecologica Scandinavica 101, no. 1 (2022): 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qiang T., Ding X., Ling J., and Fei M., “Is Platelet to Lymphocyte Ratio Predictive of Preeclampsia? A Systematic Review and Meta‐Analysis,” Journal of Obstetrics and Gynaecology 43, no. 2 (2023): 2286319. [DOI] [PubMed] [Google Scholar]
- 10. Kang Q., Li W., Yu N., et al., “Predictive Role of Neutrophil‐To‐Lymphocyte Ratio in Preeclampsia: A Meta‐Analysis Including 3982 Patients,” Pregnancy Hypertens 20 (2020): 111–118. [DOI] [PubMed] [Google Scholar]
- 11. Sargın M. A., Yassa M., Taymur B. D., Celik A., Ergun E., and Tug N., “Neutrophil‐To‐Lymphocyte and Platelet‐To‐Lymphocyte Ratios: Are They Useful for Predicting Gestational Diabetes Mellitus During Pregnancy?,” Therapeutics and Clinical Risk Management 12 (2016): 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirbas A., Biberoglu E., Daglar K., et al., “Neutrophil‐To‐Lymphocyte Ratio as a Diagnostic Marker of Intrahepatic Cholestasis of Pregnancy,” European Journal of Obstetrics, Gynecology, and Reproductive Biology 180 (2014): 12–15. [DOI] [PubMed] [Google Scholar]
- 13. Vakili S., Torabinavid P., Tabrizi R., Shojazadeh A., Asadi N., and Hessami K., “The Association of Inflammatory Biomarker of Neutrophil‐To‐Lymphocyte Ratio With Spontaneous Preterm Delivery: A Systematic Review and Meta‐Analysis,” Mediators of Inflammation 2021 (2021): 6668381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Committee on Practice Bulletins‐Obstetrics , “ACOG Practice Bulletin No. 188: Prelabor Rupture of Membranes,” Obstetrics and Gynecology 131, no. 1 (2018): e1–e14. [DOI] [PubMed] [Google Scholar]
- 15. Christoforaki V., Zafeiriou Z., Daskalakis G., Katasos T., and Siristatidis C., “First Trimester Neutrophil to Lymphocyte Ratio (NLR) and Pregnancy Outcome,” Journal of Obstetrics and Gynaecology 40, no. 1 (2020): 59–64. [DOI] [PubMed] [Google Scholar]
- 16. Aslan M. M., Yeler M. T., Yuvacı H. U., Cerci I. A., Cevrioğlu A. S., and Ozden S., “Can the Neutrophil‐To‐Lymphocyte Ratio (NLR) Predicts Fetal Loss in Preeclampsia With Severe Features?,” Pregnancy Hypertens 22 (2020): 14–16. [DOI] [PubMed] [Google Scholar]
- 17. Yuce E., “Neutrophil‐To‐Lymphocyte Ratio (NLR) and Platelet‐To‐Lymphocyte Ratio (PLR) can Predict Spontaneous Preterm Birth?,” Journal of Inflammation Research 16 (2023): 2423–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee J. H., Park C. W., Moon K. C., Park J. S., and Jun J. K., “Neutrophil to Lymphocyte Ratio in Maternal Blood: A Clue to Suspect Amnionitis,” Journal of Clinical Medicine 10, no. 12 (2021): 2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryu H. K., Moon J. H., Heo H. J., Kim J. W., and Kim Y. H., “Maternal c‐Reactive Protein and Oxidative Stress Markers as Predictors of Delivery Latency in Patients Experiencing Preterm Premature Rupture of Membranes,” International Journal of Gynaecology and Obstetrics 136, no. 2 (2017): 145–150. [DOI] [PubMed] [Google Scholar]
- 20. Kim H. J., Lee K. N., Park K. H., Choi B. Y., Cho I., and Lee M. J., “Characterization of Inflammation/Immune‐, Acute Phase‐, Extracellular Matrix‐, Adhesion‐, and Serine Protease‐Related Proteins in the Amniotic Fluid of Women With Early Preterm Prelabor Rupture of Membranes,” American Journal of Reproductive Immunology 92, no. 2 (2024): e13913. [DOI] [PubMed] [Google Scholar]
- 21. Tong M., Smith A. H., and Abrahams V. M., “Activated Neutrophils Propagate Fetal Membrane Inflammation and Weakening Through ERK and Neutrophil Extracellular Trap‐Induced TLR‐9 Signaling,” Journal of Immunology 206, no. 5 (2021): 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen L. M., Aronoff D. M., and Eastman A. J., “Matrix Metalloproteinases in Preterm Prelabor Rupture of Membranes in the Setting of Chorioamnionitis: A Scoping Review,” American Journal of Reproductive Immunology 89, no. 1 (2023): e13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tingleff T., Räisänen S., Vikanes Å., et al., “Different Pathways for Preterm Birth Between Singleton and Twin Pregnancies: A Population‐Based Registry Study of 481 176 Nulliparous Women,” BJOG: An International Journal of Obstetrics and Gynaecology 130, no. 4 (2023): 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fishel Bartal M., Ugwu L. G., Grobman W. A., et al., “Outcomes in Twins Compared With Singletons Subsequent to Preterm Prelabor Rupture of Membranes,” Obstetrics and Gynecology 138, no. 5 (2021): 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kibel M., Barrett J., Tward C., Pittini A., Kahn M., and Melamed N., “The Natural History of Preterm Premature Rupture of Membranes in Twin Pregnancies,” Journal of Maternal‐Fetal & Neonatal Medicine 30, no. 15 (2017): 1829–1835. [DOI] [PubMed] [Google Scholar]
- 26. Wertheimer A., Shemer A., Hadar E., Berezowsky A., Wiznitzer A., and Krispin E., “The Effect of Meconium‐Stained Amniotic Fluid on Perinatal Outcome in Pregnancies Complicated by Preterm Premature Rupture of Membranes,” Archives of Gynecology and Obstetrics 301, no. 5 (2020): 1181–1187. [DOI] [PubMed] [Google Scholar]
- 27. Attali E., Kern G., Reicher L., et al., “Early Preterm Meconium Stained Amniotic Fluid Is an Independent Risk Factor for Peripartum Maternal Bacteremia,” European Journal of Obstetrics, Gynecology, and Reproductive Biology 258 (2021): 75–79. [DOI] [PubMed] [Google Scholar]
- 28. Walters A. G. B., Lin L., Crowther C. A., Gamble G. D., Dalziel S. R., and Harding J. E., “Erratum to ‘Betamethasone for Preterm Birth: Auckland Steroid Trial Full Results and New Insights 50 Years on’ [The Journal of Pediatrics 255(2023):80–88],” Journal of Pediatrics 263 (2023): 113669. [DOI] [PubMed] [Google Scholar]
- 29. Shepherd E. S., Goldsmith S., Doyle L. W., et al., “Magnesium Sulfate Before Preterm Birth for Neuroprotection: An Updated Cochrane Systematic Review,” Obstetrics and Gynecology 144, no. 2 (2024): 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pyykönen A., Gissler M., Jakobsson M., Petäjä J., and Tapper A. M., “Determining Obstetric Patient Safety Indicators: The Differences in Neonatal Outcome Measures Between Different‐Sized Delivery Units,” BJOG: An International Journal of Obstetrics and Gynaecology 121, no. 4 (2014): 430–437. [DOI] [PubMed] [Google Scholar]
- 31. Lucaroni F., Morciano L., Rizzo G., et al., “Biomarkers for Predicting Spontaneous Preterm Birth: An Umbrella Systematic Review,” Journal of Maternal‐Fetal & Neonatal Medicine 31, no. 6 (2018): 726–734. [DOI] [PubMed] [Google Scholar]
- 32. Bruijn M. M. C., Kamphuis E. I., Hoesli I. M., et al., “The Predictive Value of Quantitative Fibronectin Testing in Combination With Cervical Length Measurement in Symptomatic Women,” American Journal of Obstetrics and Gynecology 215, no. 6 (2016): 793.e1–793.e8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ROC curve.
Figure S2. Cox proportional hazards model by multiple pregnancy.
Figure S3. Cox proportional hazards model by delivery time.
Data Availability Statement
The data that support the findings of this study are not publicly available due to institutional privacy policies and the use of patient medical records. De‐identified data may be available from the corresponding author upon reasonable request and with permission from the institutional review board.


