Abstract
The calcium-dependent phosphatase calcineurin and its downstream transcriptional effector nuclear factor of activated T cells (NFAT) are important regulators of inducible gene expression in multiple cell types. In T cells, calcineurin-NFAT signaling represents a critical event for mediating cellular activation and the immune response. The widely used immunosuppressant agents cyclosporin and FK506 are thought to antagonize the immune response by directly inhibiting calcineurin-NFAT signal transduction in lymphocytes. To unequivocally establish the importance of calcineurin signaling as a mediator of the immune response, we deleted the gene encoding the predominant calcineurin isoform expressed in lymphocytes, calcineurin Aβ (CnAβ). CnAβ−/− mice were viable as adults, but displayed defective T cell development characterized by fewer total CD3 cells and reduced CD4 and CD8 single positive cells. Total peripheral T cell numbers were significantly reduced in CnAβ−/− mice and were defective in proliferative capacity and IL-2 production in response to PMA/ionomycin and T cell receptor cross-linking. CnAβ−/− mice also were permissive to allogeneic tumor-cell transplantation in vivo, similar to cyclosporin-treated wild-type mice. A mechanism for the compromised immune response is suggested by the observation that CnAβ−/− T cells are defective in stimulation-induced NFATc1, NFATc2, and NFATc3 activation. These results establish a critical role for CnAβ signaling in regulating T cell development and activation in vivo.
Calcineurin is a calcium-activated Ser-Thr phosphatase composed of a catalytic A subunit (59–62 kDa) and a regulatory B subunit (19 kDa). Three catalytic genes (A subunit) have been identified in vertebrate species, of which CnAα and CnAβ are ubiquitously expressed, whereas CnAγ is restricted to the testis and brain (1–3). Calcineurin catalytic activity can be inhibited by the immunosuppressive agents cyclosporin A (CsA) and FK506 through complexes with cyclophilins and FK506-binding proteins, respectively (1–3). An important mechanism whereby calcineurin promotes T cell activation and cytokine gene induction is largely attributed to a family of transcriptional regulators referred to as nuclear factor of activated T cells (NFAT; ref. 4). Receptor-mediated stimuli or mitogens that produce sustained elevations in intracellular calcium concentration result in calmodulin saturation and the direct activation of calcineurin (1–3). Activated calcineurin then directly binds NFAT transcription factors located in the cytoplasm, resulting in their dephosphorylation and subsequent translocation into the nucleus. Once in the nucleus, NFAT functions as an important coinducer of cytokine gene expression (4). Five NFAT transcription factors have been identified, of which NFATc1–4 are regulated by calcineurin-mediated dephosphorylation (4, 5).
Genetic experiments in gene-targeted mice have provided important insight into the function of individual NFAT and calcineurin family members as mediators of lymphocyte activation (reviewed in ref. 6). Whereas a number of studies support a role for calcineurin as an important regulator of cellular activation, targeted disruption of the CnAα gene in the mouse did not reveal a striking defect in lymphocyte development or function (7). Specifically, whereas CnAα−/− mice were partially defective in responding to an in vivo sensitized-antigen challenge assay, they did not demonstrate obvious defects in lymphocyte development, proliferation in response to mitogen or T cell receptor cross-linking, or NFAT nuclear translocation, in contrast to the known actions of CsA (7). The modest immunologic phenotype of CnAα−/− mice is, however, consistent with quantitative immunoblotting experiments that reveal CnAβ, and not CnAα, as the predominant calcineurin catalytic isoform expressed in T and B cells (8). Although it is not disputed that CsA and FK506 mediate immunosuppression through calcineurin inhibition, each drug can have calcineurin-independent effects (9). Thus, it remains critical to determine a functional role for calcineurin in the regulation of T cell development and activation. In this regard, a genetic approach was used to examine the immune responsiveness of mice lacking the major calcineurin catalytic isoform expressed in lymphocytes, CnAβ.
Methods
Generation of CnAβ−/− Mice.
The CnAβ-targeting vector consisted of a 2.5-kb genomic fragment (5′ to exon 2—short arm), the neomycin resistance gene (neo), and a 4.0-kb genomic fragment (3′ to exon 2—long arm), which replaced the second exon encoding most of the catalytic domain (Fig. 1A). Two targeted embryonic stem (ES) cell clones (Sv129 strain) were obtained from a total of 400 and injected into C57BL/6 blastocysts generating chimeric mice that were bred against C57BL/6 mice to obtain germline heterozygous-targeted mice (both ES clones generated null mice with similar phenotypes).
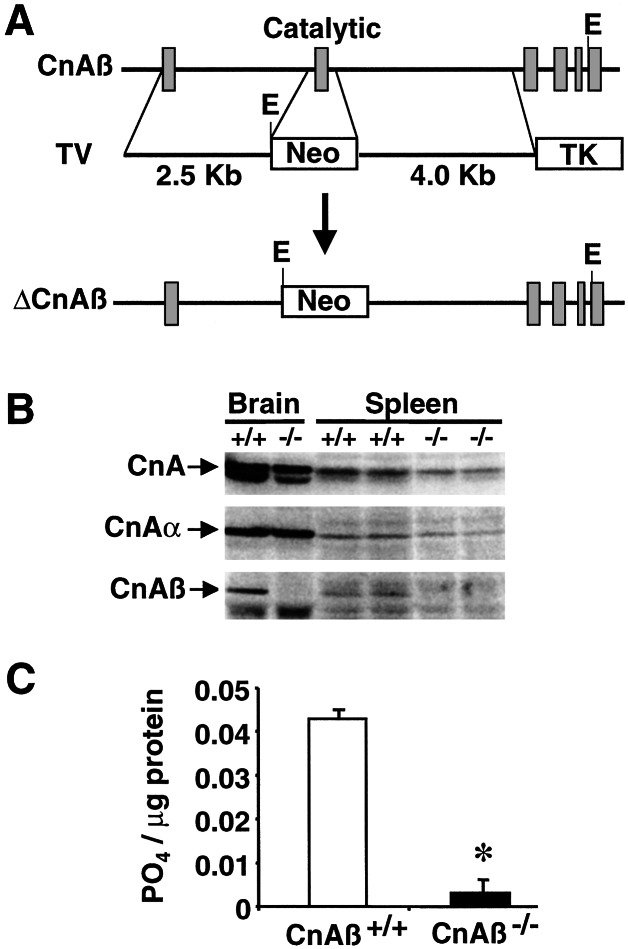
Figure 1.
Targeted disruption of the CnAβ gene. (A) Schematic representation of the CnAβ genomic locus, the targeting vector (TV), and the result of homologous recombination. (B) Western blot analysis of protein extracts from brain and spleen tissue with pan-calcineurin A catalytic subunit antisera, CnAβ-, or CnAα-specific antisera. (C) Calcineurin activity from splenocytes derived by measuring the dephosphorylation rate of a synthetic phosphopeptide substrate (RII peptide). *, P< 0.01 vs. CnAβ+/+.
Immunoblot Analysis.
Proteins were electrophoresed and transferred onto Hybond-P membranes (Amersham Pharmacia), blocked for 2 h at room temperature with Tris-buffered saline containing 0.1% Tween 20, 10 mM Tris (pH 7.5), 150 mM NaCl (TBST), and 5% nonfat milk and then incubated in antiserum overnight at 4°C. Immunoreactivity was detected by using the enhanced chemiluminescence system (ECL; Amersham Pharmacia). Immunoreactive bands on films were digitized and quantified for fluorescence with a Storm 860 PhosphoImager (Molecular Dynamics). The pan-calcineurin catalytic A subunit antibody was purchased from Chemicon; CnAα, CnAβ, NFATc1, NFATc2, and NFATc3 antisera were purchased from Santa Cruz Biotechnology. NFATc1 polyclonal antisera also was provided by Nancy Rice (National Cancer Institute, Frederick, MD). Antibody against the proto-oncogene Cbl p120 was used as a control for cytosolic protein extract integrity (antibody from Santa Cruz Biotechnology). For analysis of NFAT translocation, thymocytes were stimulated with 2.5 ng/ml phorbol 12-myristate 13-acetate (PMA) plus 500 ng/ml of ionomycin.
Calcineurin Phosphatase Activity.
Phosphatase activity was measured by using the calcineurin assay kit (Biomol, Plymouth Meeting, PA) according to the manufacturer's instructions. Spleen cells from either wild-type or CnAβ−/− mice were isolated, and calcineurin activity was measured as the dephosphorylation rate of a synthetic phosphopeptide substrate (RII peptide) in the presence or absence of EGTA. The amount of PO4 release was determined photometrically by using the Biomol Green reagent.
Preparation of Lymphocytes.
The thymus, spleen, and inguinal lymph nodes were isolated from either wild-type or CnAβ−/− mice, dissociated into single-cell suspensions, filtered through a nylon strainer (70 μm), centrifuged at 300 × g for 5 min, and resuspended in RPMI medium 1640. Red blood cells were removed from spleen cells by adding 10 ml of red blood cell lysing solution for 3 min at room temperature. After resuspensions, cells were washed and counted with a hemocytometer, centrifuged at 300 × g for 5 min, and resuspended in RPMI medium 1640 for a final concentration of 5 × 106 cells per ml.
Flow Cytometric Analyses.
Cells (0.5 × 106 cells per well) were placed in 96-well tissue-culture plates, centrifuged at 300 × g for 5 min at 4°C, and resuspended with 100 μl of PBS containing 2% BSA and 1–2 μl of PE- or FITC-labeled antibodies (PharMingen) per well. After 60 min of incubation at 4°C, cells were washed three times with PBS containing 0.1% BSA and filtered into fluorescence-activated cell sorter (FACS; Becton Dickinson) tubes for a final volume of 400 μl. Flow cytometry was performed on 10,000 cells in each assay.
Proliferation Assays.
Isolated splenocytes or enriched T cells (using B220 negative selection) were plated in triplicate at a density of 100,000 cells per well in a 96-well tissue-culture plates. Cells were incubated for 1 h at 37°C before the addition of stimulating agents. Cells then were stimulated with 2.5 ng/ml PMA plus 75 ng/ml of ionomycin or cultured for 48 h at 37°C on αCD3-precoated dishes. After the 48 h of stimulation, cultures were pulsed with 1 μCi (1 Ci = 37 GBq) per well [3H]thymidine for 6 h.
Cytokine Production Assays.
Splenocytes from either genotype were plated at a density of 1.0 × 106 cells per well and incubated with 5 μg/ml αCD3 antibody (or αCD3/αCD28 antibodies) or 2.5 ng/ml PMA plus 75 ng/ml of ionomycin for 48 h at 37°C. Supernatants were assayed for levels of IL-4, transforming growth factor (TGF)-β1, and IFN-γ by enzyme-linked immunosorbent assay according to the manufacturer's protocol (PharMingen). Serum also was collected for the detection of circulating TGF-β1 levels.
RNase Protection Assays.
RNA was obtained from 5 × 106 cells stimulated for 16 h, hybridized overnight to the 32P-labeled RNA probes, then treated with RNase, purified, and resolved on 6% polyacrylamide-Tris-urea gels according to the Riboquant multiprobe kit (PharMingen). The gels were exposed and quantitated in a PhosphorImager (Molecular Dynamics) using IMAGEQUANT software. Cytokine transcripts were identified by the length of the respective fragments, and intensities were normalized to L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) message intensity.
Tumor Assays.
J558L mouse plasmacytoma cells (BALB/c background) were cultured in RPMI 1640 medium supplemented with 10% (vol/vol) FBS, 2 mM L-glutamine, and antibiotics (10). Cells were harvested, washed three times in Dulbecco's PBS, and resuspended at a density of 5 × 106 cells per 0.2 ml and injected s.c. into the abdominal skin (shaved) of 6- to 8-week-old C57BL/6SV129 CnAβ−/−, C57BL/6SV129 wild-type, C57BL/6SV129 wild-type treated with 10 mg/kg/day of CsA, or syngeneic Balb/C mice. Mice that failed to develop tumors by 17 days were defined as “tumor free”. Mice were killed 17 days after implantation or when tumors exceeded 2.5 cm in diameter.
Statistical Analysis.
Statistical analyses between the experimental groups were performed by using a Student's t test or one-way ANOVA when comparing multiple groups. Data are reported as mean ± SEM. Values of P < 0.05 were considered significant.
Results
Gene targeting was performed in the mouse to investigate the function of the CnAβ as a potential regulator of lymphocyte development and/or function. Specifically, the exon encoding the catalytic domain of CnAβ was replaced with the neomycin gene in ES cells by homologous recombination (Fig. 1A). Heterozygous targeted ES cells were injected into C57BL/6 blastocysts to generate chimeric mice and subsequent heterozygous CnAβ targeted mice. Breeding of heterozygote mice produced CnAβ−/− mice at the predicted Mendelian frequency, which were overtly normal as adults.
To evaluate the effectiveness of the gene-targeting strategy, protein extracts were generated from both the brain and spleen and subjected to immunoblotting. Western blotting with cross-reactive calcineurin A subunit antisera demonstrated an ≈70% reduction in total protein content from CnAβ−/− splenocytes (Fig. 1B). Similar analysis using isoform-specific calcineurin A antisera demonstrated a complete absence of CnAβ protein without a compensatory change in CnAα levels (Fig. 1B). Total calcineurin phosphatase activity was also measured from splenocytes demonstrating a 9-fold reduction in enzymatic activity in null mice compared with wild-type strain matched control mice (n = 3; P < 0.01; Fig. 1C). Splenocytes were examined largely because calcineurin immunoreactivity was not detectable by Western blotting of protein extracts from thymocytes (data not shown).
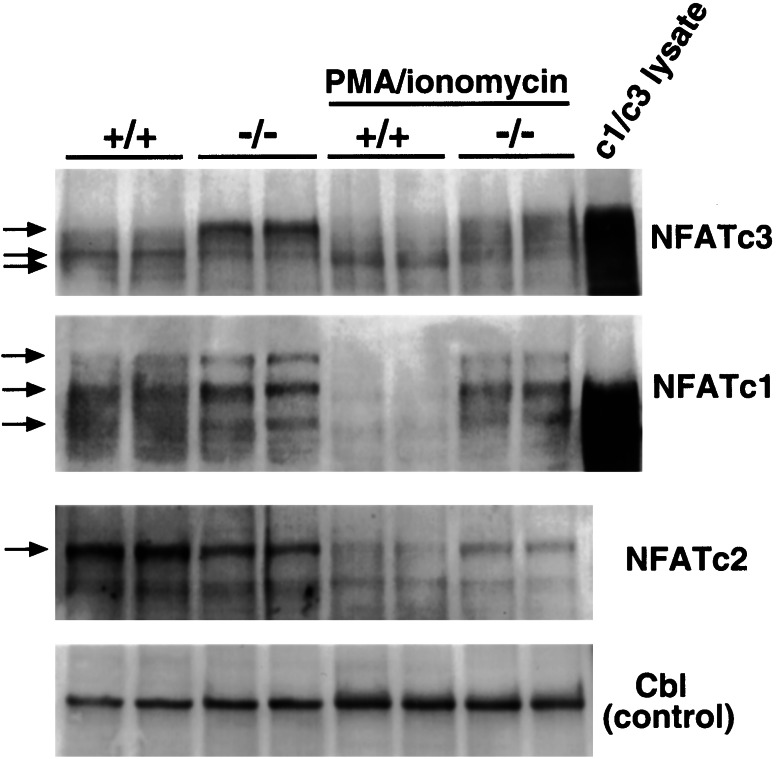
The family of NFAT transcriptional regulators directly translocate from the cytoplasm to the nucleus upon calcineurin-mediated dephosphorylation (4). To evaluate basal NFAT phosphorylation status and subcellular distribution in CnAβ−/− mice first, unstimulated thymocytes were isolated, and cytosolic protein extracts were generated for Western blot analysis. At baseline, the cytosolic fraction of NFATc1 and NFATc3 proteins demonstrated slower electrophoretic mobilities from CnAβ−/− thymocytes compared with wild-type thymocytes (arrows), suggesting a hyperphosphorylated state in the absence of CnAβ (Fig. 2). In contrast, NFATc2 protein migration did not vary between wild-type and null thymocytes at baseline (Fig. 2). However, PMA/ionomycin stimulation induced a significant reduction in NFATc1, NFATc2, and NFATc3 protein content within the cytoplasm of wild-type thymocytes, but this reduction was significantly attenuated from CnAβ−/− thymocytes (Fig. 2). In addition, the electrophoretic migration of NFATc3 was significantly altered (faster) in wild-type thymocytes compared with CnAβ−/− thymocytes (slower), suggesting increased phosphorylation of cytoplasmic NFATc3 in the absence of CnAβ (Fig. 2). Western blotting for the thymus-enriched cytoplasmic proto-oncogene Cbl (120 kDa) also was performed to control for nonspecific variations in protein levels within the assay (equivalent levels were observed; Fig. 2). The quality of the NFATc1 and NFATc3 Western blots and the number of discrete immunoreactive bands (corresponding to different phosphorylation states) are comparable to previous reports in the literature (11, 12). In addition, the identity of NFATc1 and NFATc3 was confirmed by Western blotting of Cos cell extracts transfected with each expression vector (Fig. 2). Collectively, these results indicate that the subcellular distribution and activation state of NFAT proteins is dramatically altered in CnAβ−/− lymphocytes.
Figure 2.
Defective NFAT activation in CnAβ−/− thymocytes. Western blot analysis of protein extracts isolated from the cytosol of unstimulated or PMA/ionomycin-stimulated thymocytes taken from either wild-type or CnAβ−/− mice. The arrows on the left illustrate the change in NFAT migration due to phosphorylation status of each NFAT isoform. Controls include Cos cell protein extracts from NFATc1 or NFATc3 transfections (lanes at right) as well as Western blotting for a nonmodulated cytosolic protein, Cbl, to monitor for extract integrity and equivalent loading.
Defects in cellular activation often compromise T cell development and maturation. Flow cytometric analysis was performed to analyze the composition and distribution of T and B cells in the thymus, spleen, and lymph nodes of CnAβ−/− mice. Although total CD19 positive (B cells) cells from the spleen and lymph nodes did not vary, CnAβ−/− mice demonstrated a significant reduction in CD3 positive (T cells) cells in the periphery compared with control mice (Fig. 3A). Furthermore, thymocytes from CnAβ−/− mice showed a significant reduction in both CD4 and CD8 single-positive cells (Fig. 3B). Quantitation of total thymocytes from three independent experiments showed that CnAβ−/− CD4 and CD8 single-positive thymocyte populations were reduced by 75.1 and 64.6%, respectively, compared with strain- and age-matched control mice (P < 0.01; Fig. 3 B and C). Consistent with these observed defects in T cell development and maturation, CnAβ−/− mice also were characterized by a 21% decrease in thymal cellularity at 4–8 weeks of age (data not shown). However, FACS analysis of camptothecin-induced apoptosis levels in CD4 and CD8 single-positive thymocytes, or CD4/CD8 double-positive or double-negative thymocytes did not vary between wild-type and CnAβ−/− mice (data not shown; see Discussion).
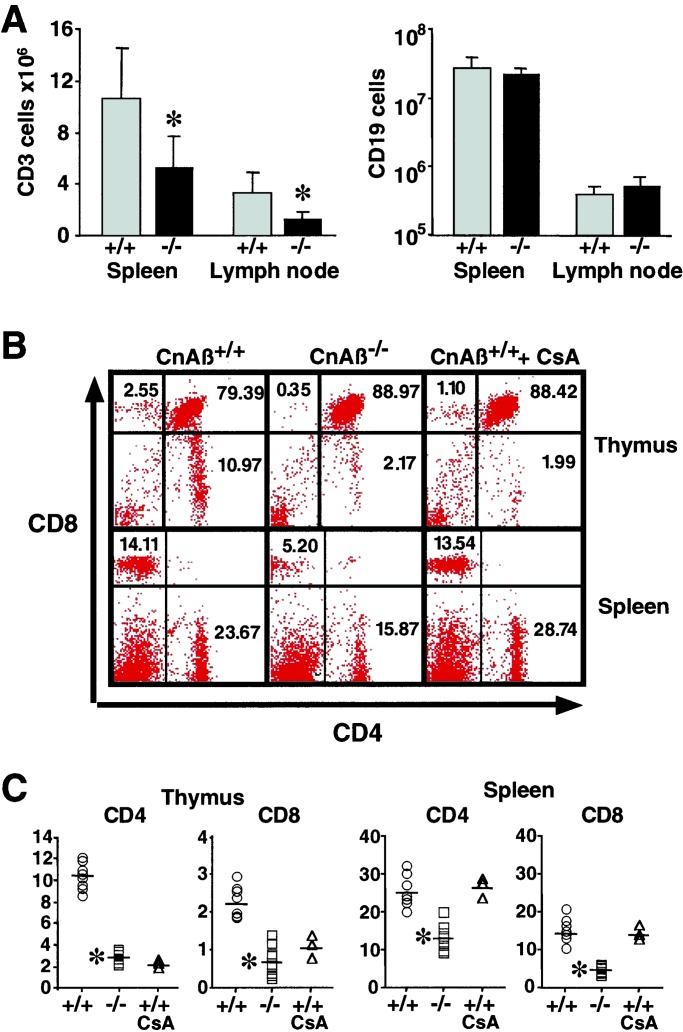
Figure 3.
Impaired T cell development in CnAβ−/− mice. (A) Total number of CD3 positive cells (T cells) and CD19 positive cells (B cells) isolated from spleen and lymph nodes were quantified by flow cytometric analysis (four independent experiments). (B) A representative FACS experiment of thymocytes (Top) or splenocytes (Bottom) from wild-type, CnAβ−/−, or wild-type mice treated with CsA were stained with FITC-CD4 and PE-CD8 antibodies. (C) Percentage of CD4- and CD8-positive cells summarized from the FACS data. (*, P < 0.01 vs. wild type).
Developmental defects also were identified in T cells isolated from the spleens of CnAβ−/− mice (Fig. 3 B and C). In four independent experiments, the number of mature CD4 and CD8 single-positive splenocytes was reduced from 25 ± 4% to 13 ± 3% and 15 ± 3% to 4.9 ± 1.2% in CnAβ−/− vs. wild-type control mice, respectively (P < 0.01). Similar reductions in T cell populations were observed in the thymus of wild-type mice treated with CsA, although 10 days of CsA was not sufficient to reduce mature T cell populations in the spleen (Fig. 3 B and C). Collectively, FACS analysis demonstrated defective lymphocyte development and maturation in CnAβ−/− mice.
Activation of the T cell receptor stimulates a wide array of intracellular signaling pathways that regulate, in part, cellular proliferation and clonal expansion. To determine whether CnAβ is an important intracellular mediator of lymphocyte activation and subsequent proliferation, splenocytes from null mice were stimulated with either αCD3-activating antibodies or PMA/ionomycin. Total splenocytes isolated from CnAβ−/− mice demonstrated a significant reduction in [3H]thymidine incorporation and IL-4 and IFNγ cytokine production in response to T cell receptor cross-linking/αCD28 and PMA/ionomycin (data not shown). Although analysis of total splenocyte proliferation and cytokine production represents a physiologic analysis of the immune response, it is also influenced by total T cell numbers, which are significantly reduced in CnAβ−/− mice. Given this consideration, T cells were purified (enriched) and normalized from the spleens of CnAβ−/− mice and control mice and subsequently analyzed for proliferative capacity. The data show a significant reduction in proliferation induced by CD3 cross-linking or PMA/ionomycin in CnAβ−/− T cells respectively, suggesting an autonomous defect in cellular activation in the absence of CnAβ (Fig. 4A). In three independent experiments, examination of IL-2 cytokine gene expression from purified T cells using a ribonuclease protection assay, which is a critical regulator of cellular activation and proliferation, demonstrated a significant reduction in PMA/ionomycin-induced IL-2 expression (Fig. 4B). These results demonstrate defective lymphocyte activation in CnAβ−/− mice in response to a calcium-dependent signal and in response to T cell receptor activation.
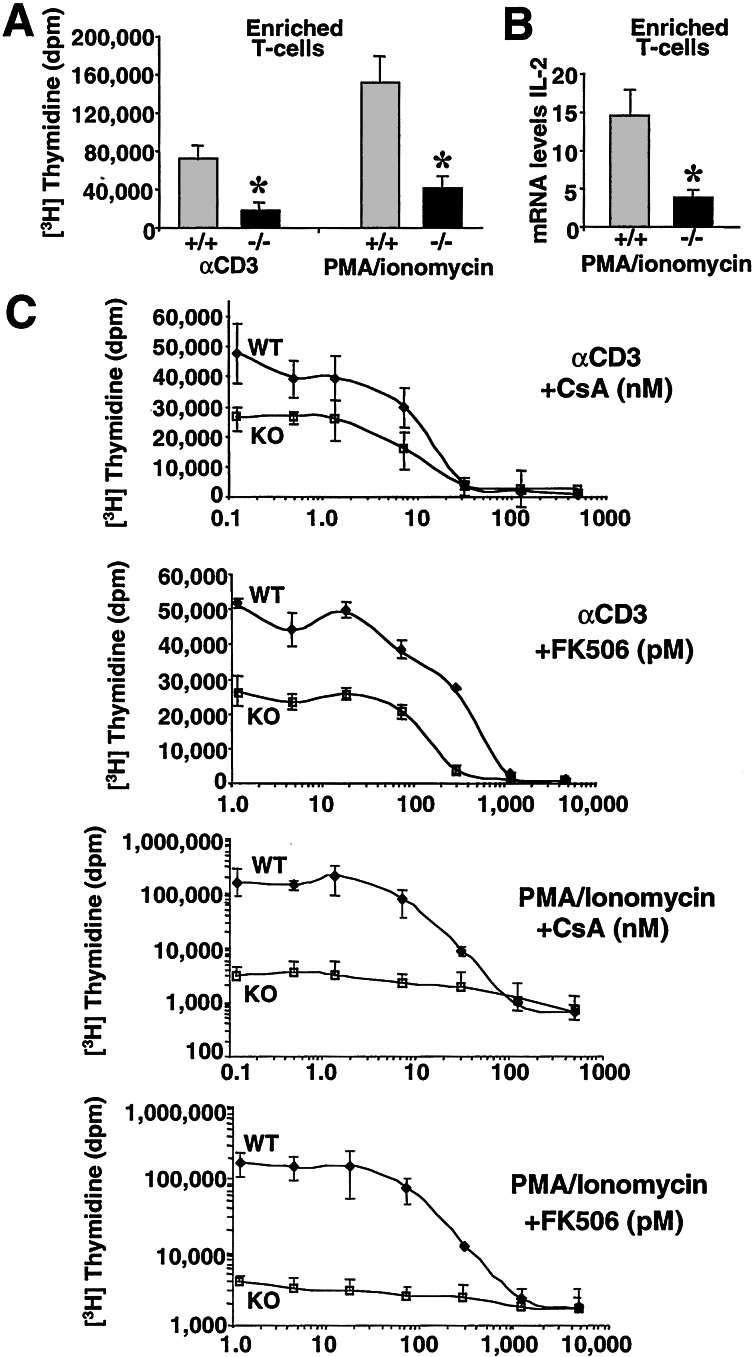
Figure 4.
Impaired lymphocyte proliferation in CnAβ−/− mice. (A) Enriched splenic T cells stimulated with αCD3 antibodies or PMA and ionomycin for 48 h show reduced proliferation. All proliferation assays were performed in triplicate within a single experiment, and similar results were observed in multiple experiments (*, P < 0.01 vs. wild type). (B) IL-2 gene expression from enriched CnAβ−/− or wild-type splenocyte T cells was assessed by RNase protection assay after stimulation with PMA/ionomycin for 16 h and expressed relative to GAPDH (n = 3; *, P < 0.01 vs. wild type). (C) Enriched T cell proliferation levels from wild-type (WT) and CnAβ−/− (KO) mice stimulated with either αCD3 or PMA/ionomycin in the presence of increasing amounts of CsA or FK506. The assay background was approximately 300 dpm, whereas PMA/ionomycin stimulation induced approximately 4,000 dpm in CnAβ−/− lymphocytes (because of the scale of the graph, lower levels of proliferation are not obvious). The results were derived from three wild-type and three CnAβ−/− mice. Similar results were observed in three independent experiments.
To examine the contribution of CnAβ vs. CnAα in mediating cellular activation, enriched T cells from CnAβ−/− and wild-type mice were treated with CsA and FK506. Similar to the data presented in Fig. 4A, proliferation stimulated by CD3 antibody or PMA/ionomycin was significantly reduced in enriched T cells isolated from CnAβ−/− mice (Fig. 4C). Furthermore, PMA/ionomycin- and CD3-induced proliferation of CnAβ−/− lymphocytes was only partially affected by CsA and FK506 (Fig. 4C). However, the observation that CnAβ−/− T cell remained partially sensitive to CsA and FK506 inhibition suggests a residual role for CnAα. Nearly identical CsA and FK506 inhibitory dose-response relationships were observed in CnAα−/− immune cells (7). For example, both CnAα- (7) and CnAβ-null (Fig. 4C) thymocytes showed a reduction in the IC50 to FK506, suggesting greater sensitivity to calcineurin inhibitory agents. Taken together, these observations suggest that CnAα and CnAβ can partially compensate for one another in mediating residual T cell activation, but that both are required for proper cellular activation.
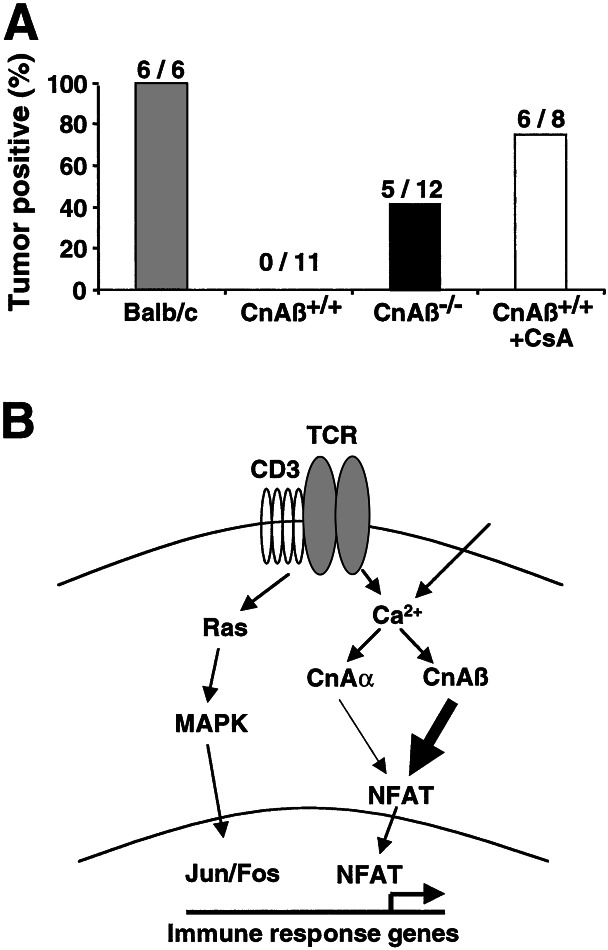
CsA and FK506 are used to prevent rejection of transplanted allografts in humans. If these agents inhibit the immune response primarily through blockade of CnAβ, then null mice should be largely permissive to allograft acceptance. Accordingly, J558L mouse plasmacytoma tumor cells (BALB/c derived; ref. 10) were injected s.c. into syngeneic BALB/c mice, allogeneic C57BL/6 CnAβ−/− mice, or allogeneic C57BL/6 wild-type strain-matched control mice. The results of these experiments show that 42% of CnAβ−/− mice developed allogeneic tumors between 10–17 days (5/12), whereas no tumor growth was observed in C57BL/6 control mice (0/11). Additional controls demonstrated that 100% of syngeneic BALB/c mice (6/6) and 75% of CsA-treated C57BL/6 wild-type mice developed tumors (6/8) (Fig. 5A). These data demonstrate that CnAβ−/− mice have impaired allograft rejection, comparable to the effects observed with CsA administration.
Figure 5.
Impaired allogeneic tumor cell rejection in CnAβ−/− mice. (A) J558L mouse plasmacytoma cells (BALB/c background) were injected s.c. into BALB/c, wild-type, CnAβ−/−, and CsA-treated wild-type mice so that allogeneic tumor growth could be scored 10–17 days later. (B) Model for T cell activation supports a critical role for the CnAβ gene as the major calcium-dependent transducer of lymphocyte activation, when compared with CnAα. Calcineurin activation then promotes NFATc1/c2/c3 dephosphorylation, which promotes their translocation into the nucleus, where NFAT factors interact with other response pathways to orchestrate the immune response.
Discussion
Calcineurin has been identified as the target of the immunosuppressive drugs CsA and FK506 (13–15). The phenotypic analyses of CnAβ−/− mice demonstrated a defective immune response that was similar to CsA treatment. Indeed, CsA administration in vivo reduces CD4 and CD8 single-positive cells, reduces cytokine levels, and compromises T cell proliferation (16–18). Quantitative immunoblotting (8) and the results presented in this report demonstrate that CnAβ is the predominant isoform of calcineurin expressed in lymphocytes. Consistent with these observations, functional comparison between CnAα and CnAβ gene-targeted mice suggests a more critical role for CnAβ in lymphocyte development and function (Fig. 5B). For example, T cell populations were not reduced in CnAα−/− mice, nor was splenocyte or lymph node cell proliferation reduced in response to PMA/ionomycin, ConA, or αCD3 antibody stimulation (7). More importantly, CnAα−/− mice did not demonstrate altered NFAT nuclear translocation (7). In contrast, CnAβ−/− mice presented with a significant reduction in T cell development and activation in response to PMA/ionomycin and T cell receptor stimulation.
Despite the prominent role played by CnAβ, T cells from these null mice still demonstrated partial proliferative and cytokine responsiveness, suggesting a role for CnAα as well as other intracellular signaling pathways. Indeed, enriched T cells from CnAβ −/− mice demonstrated both CsA- and FK506-sensitive proliferation in response to CD3 receptor cross-linking and PMA/ionomycin stimulation. Although these observations suggest that CnAα can partially compensate for the loss of CnAβ in mediating lymphocyte proliferation, it is likely that other signaling pathways also influenced T cell activation in our assays. T cell receptor activation, as well as diverse T cell mitogens, recruit a large array of necessary intracellular signaling mediators such as mitogen-activated protein kinases family members, tyrosine kinases (Fyn-T, Lck, Syk, and Zap70) NFκB, Jak/Stat, and PKCθ (19–23). That the T cell receptor regulates multiple intracellular signaling pathways explains why αCD3-stimulated proliferation is slightly more resistant to CsA and FK506 treatment compared with PMA/ionomycin (Fig. 4C). As a final consideration, CsA treatment was slightly more effective in preventing rejection of the allogeneic J558L plasmacytoma cells compared with untreated CnAβ−/− mice, despite the fact that transient CsA treatment did not reduce peripheral T cell numbers. By comparison, CnAβ−/− mice had both a reduction in peripheral T cell counts, as well as a partial defect in cellular activation. These results either suggest that CnAα plays a significant compensatory role in the absence of CnAβ in mediating the allogeneic immune response, or that CsA also can reduce T cell activation, in part, through calcineurin-independent mechanisms (see below).
Immunological phenotypes also have been described in NFATc1-, NFATc2-, and NFATc3-null mice, providing additional evidence that calcineurin–NFAT signaling regulates the immune response (11, 24–28). Targeted disruption of the NFATc3 gene showed a defect in selection of thymocytes with a concomitant decrease in peripheral T cells, which most closely resembles the phenotype of CnAβ−/− mice (24). NFATc2-null mice demonstrated a more complex phenotype characterized by lymphocyte hyperproliferation, altered cytokine expression (both up- and down-regulation of select cytokines), and eosinophilia, suggesting a more negative regulatory role for NFATc2 (25–27). However, the combined disruption of both NFATc1 and NFATc2 significantly impaired effector functions such as Th cytokine production and cytolytic activity, suggesting an important role for NFATc1 in regulating T cell function (25).
The observed decrease in mature CD3 lymphocytes in the periphery of CnAβ−/− mice suggested either a developmental defect in lymphocyte maturation in the thymus or that mature lymphocytes are more susceptible to apoptosis. To distinguish between these two possibilities, total CD3-positive thymocytes (both double-positive and double-negative) were assayed for apoptosis at baseline or after apoptotic stimulation from wildtype and CnAβ−/− mice. No difference was observed in Annexin V-positive cell number or 7AAD permeable cell number after FACS analysis in multiple experiments (data not shown). In addition, no difference was observed between single-positive CD4 or CD8 lymphocytes and susceptibility to apoptosis at baseline or after stimulation (data not shown). Finally, thymus cellularity was reduced by 21% in CnAβ−/− mice. These data suggest that the T cell deficiency that characterizes the CnAβ−/− mouse results from defective cellular maturation and/or selection. CsA and FK506 were previously shown to inhibit positive selection of thymocytes in vivo, which was also associated with a reduction in mature T cell number in the periphery (29–31). Consistent with these reports, transgenic mice expressing an activated calcineurin protein in lymphocytes had enhanced efficiency of positive selection in the thymus and were more sensitive to signals through their T cell receptors (32).
CsA also has been shown to induce TGFβ1, a cytokine with immuno-modulatory qualities in diverse cells and tissues (33, 34). CnAβ−/− mice showed no difference in serum levels of TGFβ1 or in mRNA levels of TGFβ1 from the spleen and thymus, compared with wild-type strain-matched control mice (data not shown). In conjunction with the prominent defect in T cell function in CnAβ−/− mice, this result supports the hypothesis that calcineurin inhibition is the primary mechanism whereby CsA mediates immunosuppression. However, our data do not exclude a secondary role for CsA in attenuating T cell activation through calcineurin-independent pathways. Despite this consideration, the results of the present study suggest that CnAβ is a more selective target for immunosuppressive drug design, which might circumvent toxicity issues related to global calcineurin inhibition currently associated with CsA and FK506.
Acknowledgments
We thank Elizabeth Setser and Leon J. De Windt for technical assistance, Dr. Marsha Wills-Karp for evaluating the manuscript, and the Center for Environmental Genetics for knock-out mouse production. This work was supported by the National Institutes of Health (to J.D.M. and M.E.R) and the Pew Charitable Trusts (J.D.M). O.F.B and E.B.B. were supported by PostDoctoral Fellowships from the National Institutes of Health and American Heart Association, respectively.
Abbreviations
- CsA
cyclosporin A
- NFAT
nuclear factor of activated T cells
- PMA
phorbol 12-myristate 13-acetate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rusnak F, Mertz P. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 2.Crabtree G R. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 3.Klee C B, Ren H, Wang X. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 4.Rao A, Luo C, Hogan P G. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 5.Lopéz-Rodríguez C, Aramburu J, Rakeman A S, Rao A. Proc Natl Acad Sci USA. 1999;96:7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiani A, Rao A, Aramburu J. Immunity. 2000;12:359–372. doi: 10.1016/s1074-7613(00)80188-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B W, Zimmer G, Chen J, Ladd D, Li E, Alt F W, Wiederrecht G, Cryan J, O'Neill E A, Seidman C E, et al. J Exp Med. 1996;183:413–420. doi: 10.1084/jem.183.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, Xiong F, Kong S, Ogawa T, Kobayashi M, Liu J O. Mol Immunol. 1997;34:663–669. doi: 10.1016/s0161-5890(97)00054-0. [DOI] [PubMed] [Google Scholar]
- 9.Halloran P F, Kung L, Noujaim J. Transplant Proc. 1998;30:2167–2170. doi: 10.1016/s0041-1345(98)00577-6. [DOI] [PubMed] [Google Scholar]
- 10.Tepper R I, Pattengale P K, Leder P. Cell. 1989;57:503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- 11.Ranger A M, Hodge M R, Gravallese E M, Oukka M, Davidson L, Alt F W, de la Brousse F C, Hoey T, Grusby M, Glimcher L H. Immunity. 1998;8:125–134. doi: 10.1016/s1074-7613(00)80465-3. [DOI] [PubMed] [Google Scholar]
- 12.Aramburu J, Yaffe M B, Lopez-Rodriguez C, Cantley L C, Hogan P G, Rao A. Nature (London) 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Farmer J D, Lane W S, Friedman J, Weissman I, Schreiber S L. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 14.Swanson S K, Born T, Zydowsky L D, Cho H, Chang H Y, Walsh C T, Rusnak F. Proc Natl Acad Sci USA. 1992;89:3741–3745. doi: 10.1073/pnas.89.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clipstone N A, Crabtree G R. Nature (London) 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 16.Kasaian M T, Biron C A. J Immunol. 1990;144:299–306. [PubMed] [Google Scholar]
- 17.Brooks E G, Wirt D P, Klimpel G R, Vaidya S, Goldblum R M. Clin Immunol Immunopathol. 1993;67:224–231. doi: 10.1006/clin.1993.1069. [DOI] [PubMed] [Google Scholar]
- 18.Umland S P, Shah H, Jakway J P, Shortall J, Razac S, Garlisi C G, Falcone A, Kung T T, Stelts D, Hegde V, et al. Am J Respir Cell Mol Biol. 1999;20:481–492. doi: 10.1165/ajrcmb.20.3.3266. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Arendt C W, Ellmeier W, Schaeffer E M, Sunshine M J, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg P L, Littman D R. Nature (London) 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 20.Dong C, Yang D D, Tournier C, Whitmarsh A J, Xu J, Davis R J, Flavell R A. Nature (London) 2000;405:91–94. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 21.Pages G, Guerin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouyssegur J. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 22.Kuo C T, Leiden J M. Annu Rev Immunol. 1999;17:149–187. doi: 10.1146/annurev.immunol.17.1.149. [DOI] [PubMed] [Google Scholar]
- 23.Chu D H, Morita C T, Weiss A. Immunol Rev. 1998;165:167–180. doi: 10.1111/j.1600-065x.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 24.Oukka M, Ho I C, de la Brousse F C, Hoey T, Grusby M J, Glimcher L H. Immunity. 1998;9:295–304. doi: 10.1016/s1074-7613(00)80612-3. [DOI] [PubMed] [Google Scholar]
- 25.Peng S L, Gerth A J, Ranger A M, Glimcher L H. Immunity. 2001;14:13–20. doi: 10.1016/s1074-7613(01)00085-1. [DOI] [PubMed] [Google Scholar]
- 26.Hodge M R, Ranger A M, Charles de la Brousse F, Hoey T, Grusby M J, Glimcher L H. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 27.Xanthoudakis S, Viola J P, Shaw K T, Luo C, Wallace J D, Bozza P T, Luk D C, Curran T, Rao A. Science. 1996;272:892–895. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
- 28.Viola J P, Kiani A, Bozza P T, Rao A. Blood. 1998;91:2223–2230. [PubMed] [Google Scholar]
- 29.Hollander G A, Fruman D A, Bierer B E, Burakoff S J. Transplantation. 1994;58:1037–1043. doi: 10.1097/00007890-199411150-00011. [DOI] [PubMed] [Google Scholar]
- 30.Gao E K, Lo D, Cheney R, Kanagawa O, Sprent J. Nature (London) 1988;336:176–179. doi: 10.1038/336176a0. [DOI] [PubMed] [Google Scholar]
- 31.Urdahl K B, Pardoll D M, Jenkins M K. J Immunol. 1994;152:2853–2859. [PubMed] [Google Scholar]
- 32.Hayden-Martinez K, Kane L P, Hedrick S M. J Immunol. 2000;165:3713–3721. doi: 10.4049/jimmunol.165.7.3713. [DOI] [PubMed] [Google Scholar]
- 33.Khanna A K, Cairns V R, Becker C G, Hosenpud J D. Transplantation. 1999;67:882–889. doi: 10.1097/00007890-199903270-00016. [DOI] [PubMed] [Google Scholar]
- 34.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Nature (London) 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]