Abstract
Human colon carcinoma Caco-2 cell monolayers undergo conversion into cells that share morphological and functional features of M cells when allowed to interact with B lymphocytes. A lymphotropic (X4) HIV-1 strain crosses M cell monolayers and infects underlying CD4+ target cells. Transport requires both lactosyl cerebroside and CXCR4 receptors, which are expressed on the apical surface of Caco-2 and M cells. Antibodies specific for each receptor block transport. In contrast, a monotropic (R5) HIV-1 strain is unable to cross M cell monolayers and infect underlying monocytes, despite efficient transport of latex beads. Caco-2 and M cells do not express CCR5, but transfection of these cells with CCR5 cDNA restores transport of R5 virus, which demonstrates that HIV-1 transport across M cells is receptor-mediated. The follicle-associated epithelium covering human gut lymphoid follicles expresses CCR5, but not CXCR4, and lactosyl cerebroside, suggesting that HIV-1 infection may occur through M cells and enterocytes at these sites.
Transmission of HIV-1 infection during heterosexual or homosexual intercourse by the anorectal, cervicovaginal, foreskin, and urethral epithelia accounts for as much as 80% of AIDS (1). Infection can involve both free and cell-associated virus, because body secretions such as blood and semen contain free HIV-1 virions and HIV-1-infected lymphocytes (2). For successful sexual transmission, HIV-1 first has to cross the mucosal barrier of the intestinal or genital tracts to infect CD4+ T cells. In adult macaques, free or cell-associated simian immunodeficiency virus can cross an intact vaginal mucosa and cause an infection (3). Transcytosis of HIV-1 across simple epithelial cell monolayers (4) or by M cells of the follicle-associated epithelium (FAE) over intestinal or tonsil lymphoid follicles (5–7) has been proposed to mediate mucosal infection. Alternatively, the virus may cross an intact epithelium by way of HIV-1-infected lymphocytes, followed by infection of underlying target cells (8), or by way of intraepithelial dendritic cells (9, 10). Finally, and obviously, epithelial breaches frequently observed on genital mucosal surfaces still represent likely portals of entry (11).
In vitro studies showed that human intestinal cell lines that lack CD4 are infectable by HIV-1 (12). The glycosphingolipid galactosylceramide (GalCer or lactosyl cerebroside) that binds with high-affinity gp120 has been proposed to act as a CD4 surrogate HIV-1 receptor on various epithelial cell lines (13). On the basis of their cellular tropism, replication kinetics and ability to induce syncytia formation on infection, HIV-1 strains have been grouped into two categories, the nonsyncytium-inducing/monotropic or R5 strain and the syncytium-inducing/lymphotropic or X4 strain (14, 15). The identification of chemokine receptors as HIV-1 coreceptors has provided a molecular basis for the different tropism between X4 and R5 strains. CXCR4, the natural receptor for stromal cell-derived factor-1, binds both T cell-adapted and primary X4 strains (16). CCR5, which binds the RANTES, MIP-1α, and MIP-1β chemokines, serves as the main coreceptor for primary R5 strains (17, 18). Sequence differences on the third variable loop (V3) of the gp120 envelope glycoprotein are responsible for the selective tropism of X4 and R5 strains (19). Initial infection with HIV-1 is usually transmitted with R5 viruses (20). Several epithelial cell lines and intestinal crypts and villi express various chemokine receptors including CXCR4 and CCR5 (21).
To investigate the early steps of HIV-1 penetration through epithelial cells that mimic the rectal epithelium, we used Caco-2 cells, a human intestinal cell line that was shown to become infected by HIV-1 by way of GalCer and CXCR4 receptors (22). Caco-2 cells, when cocultured as monolayers with B lymphocytes, undergo phenotypic conversion into cells that share the features of M cells (23) and allow efficient transepithelial transport of bacteria, viruses, and inert particles (24). We show that free and cell-associated X4 (syncytium-inducing) or R5 (nonsyncytium-inducing) HIV-1 can infect and cross both Caco-2 cells and M cells provided the epithelial cells express galactosylceramide and the appropriate chemokine coreceptor. We also provide evidence that the follicle-associated epithelium of human Peyer's patches expresses GalCer and CCR5 receptors.
Methods
Transfection of Caco-2 Cells.
The coding region of CCR5 (1.1 kb) was cloned into the expression vector pCDNA3 (Invitrogen) by using the BamHI and XhoI sites. The plasmid was introduced into the Caco-2 cells by Lipofectin (Life Technologies, Paisley, Scotland), and transfectants were selected with Geneticin.
Isolation and Infection of Human CD4+ T Cells and Monocytes.
CD4+ T cells were purified from peripheral blood mononuclear cells with the IsoCell Human Isolation kit (Pierce) and cultured in RPMI 1640 medium (GIBCO) supplemented with 10% FCS and 40 units/ml of human IL-2 (Seromed, Berlin). Cells (106) were activated for 3 days with 1 μg/ml of phytohemagglutinin and infected with 103.5 tissue culture 50% infective dose (TCID50)/ml X4 (39.28.G1 primary isolate from Ana-Maria de Roda, CLB, Amsterdam, The Netherlands) or R5 HIV-1 (19.34.C2 primary isolate, from H. Schuitemaker, CLB, Amsterdam). The infected CD4+ T cells were cultured for 7 days before use. Monocytes (106) isolated from erythrocyte and platelet-free peripheral blood mononuclear cells were plated in 24-well plates coated with 1% gelatin. Nonadherent T and B cells were removed 12 h later, and adherent monocytes were detached with PBS/5 mM EDTA. The monocyte population (>98% CD14+ CD3−, CD19− cells) were infected with 19.34.C2 R5 HIV-1 for 48 h and further cultured for 5 days in the absence of virus.
Coculture System.
The TC7 Caco-2 subclone (25, 26) (provided by Zweibaum, Villejuif, France) was cocultured (prior HIV-1 infection) as described (23). In brief, 106 Raji B cells (CCL-86 from American Type Culture Collection) were added to Caco-2 monolayers and cocultured for 4 days. The monolayer tightness and M cell transcytosis were assessed as reported (23). Infected CD4+ T cells labeled with 10 mM FITC dye (CellTracker Green CMFDA, Molecular Probes), were added apically and analyzed after 30 min, 1 h, or 2 h in the basal medium or the monolayers. Because no labeled cells were recovered from the lower compartment or detected in the monolayers, paracellular and apical to basolateral transport of HIV-1 infected cells can be ruled out.
Infection of Caco-2 Monolayers.
Noninfected CD4+ T cells or monocytes (2 × 105) were added to the lower Transwell chamber. The Caco-2 or M cell monolayers were apically exposed for 30 min, 1 h, or 2 h to free HIV-1 (X4: 39.28.G1 or R5: 19.34.C2, 103.5 TCID50/ml) or HIV-1 infected CD4+ T cells or monocytes (2 × 105/filter). The filters were removed and the target cells left in culture for 7 days. To determine whether X4 HIV-1 was reverse-transcribed in Caco-2 cells, monolayers were exposed for 30 min or 2 h to free HIV-1 (X4: 39.28.G1, 103.5 TCID50/ml) in the presence or the absence of 1 μg/ml of zidovudine (Roche, Basel). Pseudotyped viruses, carrying the envelope for X4 (HxB2) or R5 (ADA) HIV-1 or vesicular stomatitis virus (VSV) as positive control, and producing green fluorescent protein (GFP) on integration in the host genome (27), were also used (1.7 × 106 infectious particles per milliliter) to infect Caco-2 or transfected Caco-2/CCR5 monolayers on day 9. Viral production and calculation of viral infectivity was performed as described (27). Infected monolayers were cultured for 2, 24 h, 4 days, or 8 days postinfection. Filters were then fixed in 3% paraformaldehyde and mounted in CitiFluor (CitiFluor, London).
p24 ELISA and Detection of Viral HIV-1 DNA.
Apical medium (100 μl) was removed after HIV-1 addition, and basolateral medium (100 μl) was recovered (30 min to 7 days postinfection). p24 concentration was determined by ELISA (HIVAG-1 Monoclonal, Abbott). CD4+ T, monocyte read-out cells were recovered on day 7 postinfection and DNA was extracted from these cells and from Caco-2 or M cell monolayers by an overnight incubation at 57°C with 1.5 mM MgCl 2, 0.5% Tween 20, 0.5% Nonidet P-40, 12.5 μg of Proteinase K (Roche Molecular Biochemicals). DNA was amplified by PCR with SK38 and SK39 gag-specific primers (28). Monolayers infected with GFP-producing viruses were analyzed for GFP expression at 488 nm by confocal microscopy (Zeiss LSM410) along the x–y and x–z axes (0.2645 μm, 260 lines per section, interval of 0.2).
Detection of HIV-1 Receptors and Coreceptors.
Caco-2 monolayers (day 9) were fixed in 3% paraformaldehyde, treated for 15 min with 50 mM NH 4Cl-PBS, saturated with PBS-0.1% BSA, incubated with a 1/100 dilution of H8H9 anti-GalCer, 5 μg/ml anti-CXCR4 (12G5, R & D Systems) or 2 μg/ml anti-CCR5 (45549.111, R & D Systems) antibodies, followed by anti-mouse IgG FITC-coupled (ICN) and mounted in CitiFluor. Filters were examined by confocal microscopy (Zeiss LSM410). Analysis along the x–y and x–z axes (0.2645 μm, 260 lines per section, interval of 0.2) was performed. Frozen sections (6 μm) from snap-frozen (−180°C) unfixed biopsies of Peyer's patches from the distal ileum were incubated at 4°C with 2% goat and human sera and 1% BSA. After an overnight incubation at 4°C with anti-CXCR4 and anti-CCR5 or an isotype-matched IgG2a (control), at the same concentrations as above, the sections were fixed with 3% paraformaldehyde, further incubated with a biotinylated goat antibody anti-mouse Ig (PharMingen) and streptavidin-horseradish peroxidase (PharMingen) as described (23).
Inhibition Experiments.
Anti-CXCR4 (12G5, 5 μg/ml), anti-GalCer (H8H9, 1/200) or isotype-matched control antibodies were added apically on Caco-2 monolayers for 1 h at 4°C, followed by exposure for 1 h at 37°C to 1.7 × 106 infectious particles of HxB2-env pseudotyped GFP-producing X4 HIV-1. Monolayers were then washed, left in culture for 8 days, fixed in 3% paraformaldehyde, and assessed by confocal microscopy for GFP expression.
Results
X4, but Not R5, HIV-1 Enters, Crosses and Infects Caco-2 Cell Monolayers and Underlying Target Lymphocytes.
To determine whether Caco-2 cells were able to transport HIV-1, the cells were cultured for 9 days on Transwell filters until they formed tight monolayers with electrical transepithelial resistance of ≈300 Ω·cm2. Then the monolayers were exposed to the same TCID50 dose of either cell-free X4 or R5 primary HIV-1 isolates, or to cell-associated virus (X4-infected CD4+ T cells or R5-infected monocytes). As soon as 30 min after apical exposure to free or cell-associated X4 HIV-1, viral DNA was clearly detected in the Caco-2. This detection, however, did not result from de novo reverse transcription, because the HIV-1 amplicon was detected in the presence of 1 μg/ml zidovudine (Fig. 1e). HIV-1 DNA was also detected in the CD4+ T cells present in the lower chamber underneath the Caco-2 monolayers (Fig. 1a). To detect DNA, the target CD4+ T cells were further cultivated for 7 days after exposure of the monolayers to the virus, allowing HIV-1 to replicate within them. In contrast, the Caco-2 cells and the monocytes in the lower chamber could not be infected with free or cell-associated R5 HIV-1 (Fig. 1b). Higher viral doses tested did not restore susceptibility of the Caco-2 cells or monocytes to R5 virus infection (data not shown). This finding was supported further by the observation that Caco-2 monolayers were infected with X4 but not R5 HIV-1 env-pseudotyped viruses that induce GFP expression upon integration in the host genome. Low levels of GFP protein, carried by the virions, could be detected 2 h after viral exposure (data not shown). Maximum level of X4 HIV-1/GFP expression was observed 8 days postinfection (Fig. 2a), a time course comparable to VSV/GFP that served as a positive control (Fig. 2c). Monolayers exposed to R5 HIV-1/GFP remained negative for GFP expression (Fig. 2b).
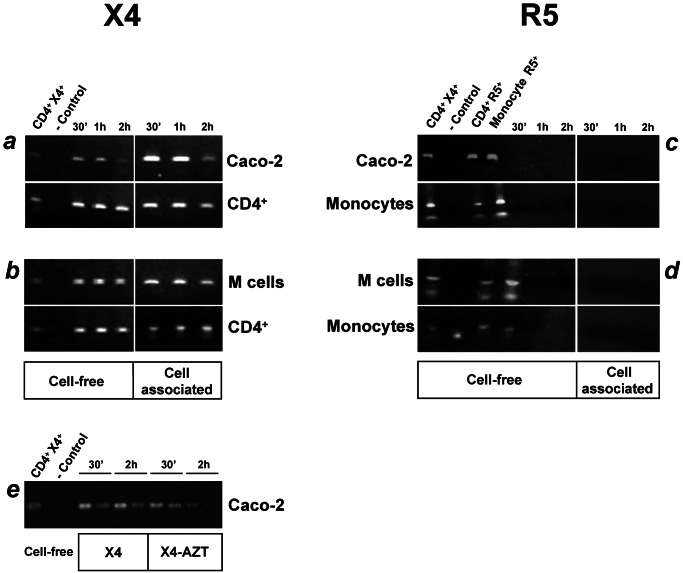
Figure 1.
X4 but not R5 HIV-1 infects and crosses Caco-2 or M cell monolayers. PCR analysis, with gag-specific primers, of DNA extracted from Caco-2 or M cell monolayers at day 9 and from CD4+ T cells (X4 targets) or monocytes (R5 targets) in the lower chamber of the Transwell devices. Target cells have been further cultivated for 7 days after exposure of the monolayers to HIV-1. An HIV-1 gag amplicon (115 bp) was detected in epithelial cells, whether or not converted, and in target CD4+ T cells as soon as 30 min after apical addition of X4 HIV-1 (a and b). No transcripts were detected in the Caco-2 or M cells and the target monocytes when exposed to R5 HIV-1 strains (c and d). The X4 HIV-1 amplicon observed in Caco-2 cells was not the result of de novo reverse transcription, because the virus was detected in the presence of 1 μg/ml zidovudine (e). A similar TCID50 for X4 and R5 HIV-1 was applied. CD4+ T cells infected with X4 or R5 HIV-1 and monocytes infected with R5 HIV-1 served as positive control. The first two lanes in the right panel and the four lanes in the left panel are controls. One representative result of four independent experiments is shown.
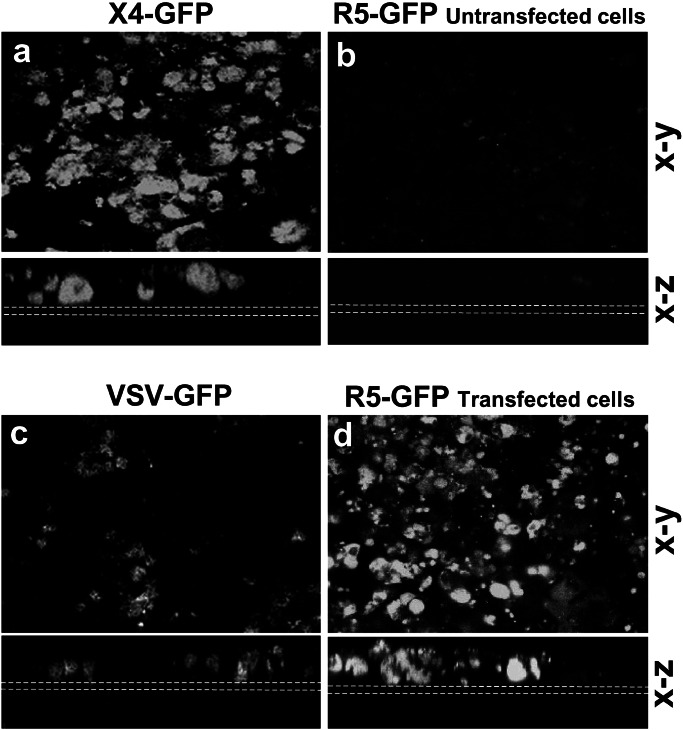
Figure 2.
Chemokine receptors specify HIV-1 strain specificity of infection. Confocal microscopy (x–y and x–z axes) of untransfected Caco-2 monolayers infected at 9 days with HxB2 X4 (a), ADA R5 HIV-1 (b), or VSV as a positive control (c). On integration in the host cell, the virus produces the GFP protein. On day 8 postinfection the filters were examined for GFP expression. Caco-2 infected with X4 (a) and with VSV (c) were GFP-positive, whereas monolayers infected with R5 remained negative (b). In contrast CCR5-transfected Caco-2 monolayers when exposed to R5 HIV-1 were positive for GFP expression (d), indicating that R5 successfully infected the CCR5-transfected Caco-2 cells. Dotted white lines indicate the position of the filters. (Bar = 20 μm.)
Our data indicate that free or cell-associated X4, but not R5 HIV-1, can enter, cross, and later infect Caco-2 cell monolayers. The rapid infection of target CD4+ T cells in the lower chamber observed already 30 min after inoculation of HIV-1 in the upper chamber could result from transcytosis of HIV-1 particles across epithelial cells by either a receptor-mediated mechanism or by bulk uptake.
M Cells Do Not Transport R5 HIV-1.
To test whether M cell-mediated transcytosis could overcome the block of R5 HIV-1 uptake and transport and the subsequent infection of underlying target monocytes, we converted the Caco-2 cells into M cells. M cells are specialized epithelial cells that transport inert particles and numerous microorganisms across the epithelium that covers mucosa-associated lymphoid tissue (24). Caco-2 cells that formed tight monolayers after 5 days in culture were cocultivated with Raji B lymphocytes for 4 days, which resulted in conversion into M cells that efficiently transport bacteria and latex beads (23). Fluorescent latex beads followed by X4 or R5 HIV-1 were added to the apical medium of M cell monolayers and transcytosis of the beads, and infection of the target CD4+ T cells or monocytes was monitored in the lower chamber. X4 HIV-1 efficiently entered the converted M cell monolayers and infected the underlying target CD4+ T cells (Figs. 1b and 3a). Despite efficient transcytosis of the beads across M cell monolayers, monocytes remained uninfected by R5 HIV-1 (Fig. 1d), indicating that bulk uptake transcytosis is not sufficient for transepithelial infection by HIV-1 (Fig. 3a). After apical infection of Caco-2 or M cell monolayers with X4 or R5 HIV-1 the concentration of p24 antigen was measured in the serosal medium. High levels of p24 were recovered when both Caco-2 and M cell monolayers were exposed to X4 but not to R5 HIV-1 (Fig. 3b), further indicating that R5 virus is not transported by bulk uptake transcytosis. These results indicate that Caco-2 cells and M cells behave similarly and present no difference when exposed to HIV-1. Bulk uptake of HIV-1 by M cells does not lead to infection of those cells or transmission of infectious virus to the serosal side, suggesting that receptor-mediated transcytosis is required for viral transport across M cells.
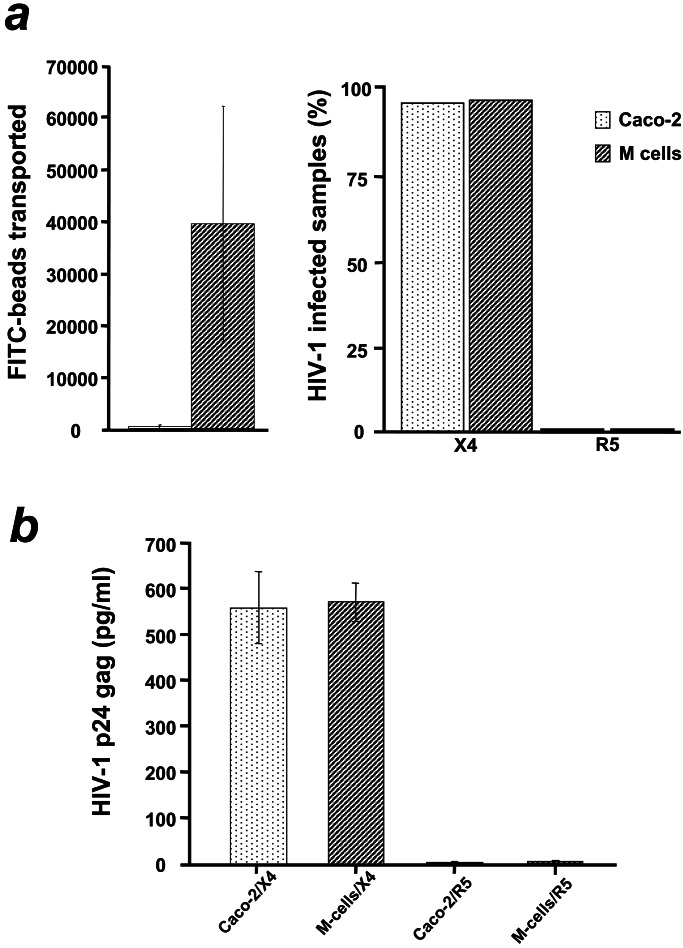
Figure 3.
Receptor-mediated transcytosis accounts for early HIV-1 penetration of the epithelial monolayers. (a Left) FITC-coupled beads added on the apical medium of both Caco-2 and M cell monolayers were transported within 1 h across converted but not across normal Caco-2 monolayers. Bars represent the average number of beads measured by cytofluorometry in the medium of the lower chamber from four independent experiments. Monolayers with low transepithelial resistance (TER), high 3H-inulin diffusion or high number of beads transported after the first 15 min of incubation were discarded. (a Right) The same monolayers used to monitor transcytosis were exposed apically to X4 or R5 HIV-1 and the underlying target CD4+ T cells or monocytes were recovered and tested for infection by PCR. No infection of target monocytes (0% infected samples) was observed in converted monolayers despite efficient transport of fluorescent beads. In contrast CD4+ T cells under normal monolayers that were totally impermeable to latex beads were infected. Mean values of four independent experiments (12 monolayers per experiment) expressed as percentage of infected samples (pool of mononuclear target cells underneath converted or not Caco-2 monolayers) with HIV-1. (b) HIV-1 p24 gag antigen was captured by ELISA in the basolateral medium, as soon as 30 min after apical infection of normal (n = 12) or converted Caco-2 (n = 12) monolayers, only when X4 but not R5 HIV-1 was added. Bars represent the average p24 gag concentration in picograms per milliliter in two independent experiments. Equal concentration of X4 and R5 HIV-1 was added apically. The maximum threshold of the detection system is 600 pg/ml; 10 pg of p24 correspond to 105 particles.
Caco-2 Cells Express GalCer and CXCR4 but Not CCR5 on Their Apical Cell Surface.
To determine whether transcytosis was receptor-mediated, we examined which HIV-1-specific receptors or coreceptors were expressed on the apical surface of Caco-2 cells. GalCer, a CD4 surrogate HIV-1 receptor but not CD4 (data not shown), and CXCR4, the chemokine receptor specific for X4 HIV-1 strains, but not CCR5, the chemokine receptor specific for R5 strains, were expressed at the luminal cell surface of Caco-2 cells (Fig. 4 a–c). Cell surface expression of these receptors was further confirmed by fluorescence-activated cell sorter analysis (data not shown). Thus, susceptibility of Caco-2 cells to infection by X4 but not R5 strains correlates with the presence of GalCer and CXCR4.
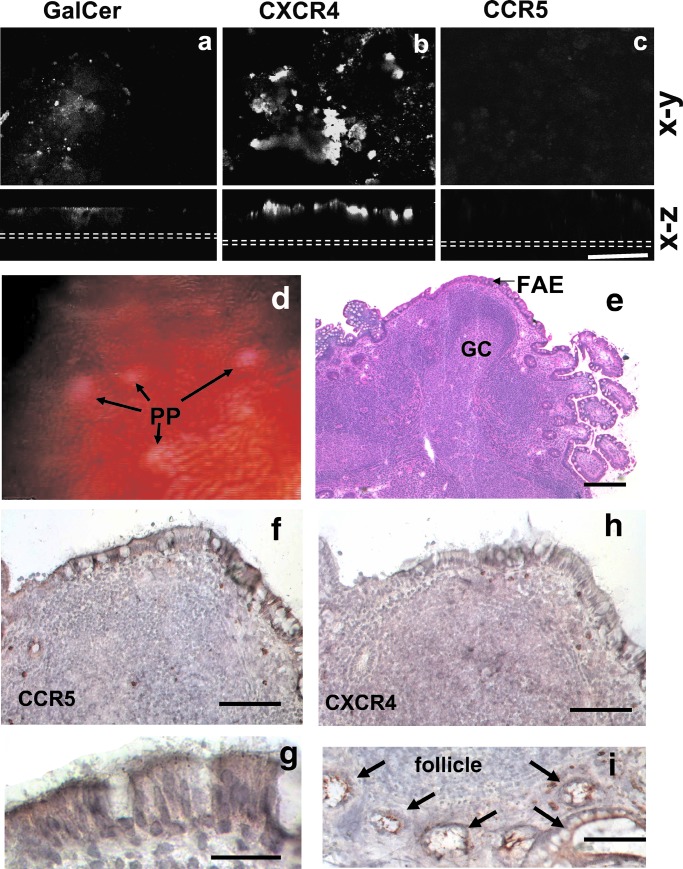
Figure 4.
Expression of HIV-1 receptors and coreceptors on Caco-2 monolayers and human ileal Peyer's patches. Confocal microscopy (x–y or x–z axes) of Caco-2 monolayers on day 9 labeled with human anti-GalCer (a), anti-CXCR4 (b), or anti-CCR5 (c) antibodies. GalCer (a) or CXCR4 (b) are expressed on the apical surface of the monolayers. CCR5 is absent (b). Dotted white lines indicate the position of the filters. (Bar = 50 μm.) Endoscopic view (d) of ileal Peyer's patches (PP) (white-yellow) were biopsied (square) and processed as described in Methods. (e) Low magnification of a Peyer's patch frozen section with typical follicles and their germinal centers (GC). The follicles are overlaid by the follicle-associated epithelium that is enriched in M cells (hematoxylin/eosin). (Bar = 100 μm.) (f) CCR5 labeling of the follicle-associated epithelium (Bar = 50 μm) showing labeling of the apical membrane of the enterocytes, better seen in g. (Bar = 20 μm.) (h) CXCR4 labeling of a serial section of (f). The FAE remains unlabeled, but as seen in (i), crypts (arrows) around the lymphoid follicle show strong apical labeling as already reported for colon crypts (21). (Bar = 50 μm.)
Transfection of Caco-2 Cells with CCR5 cDNA Restores Susceptibility to R5 HIV-1.
The resistance to R5 HIV-1 infection correlated with the lack of CCR5 expression in the Caco-2 cell line that we used. Consequently, expressing CCR5 in Caco-2 cells would be expected to restore susceptibility to infection. Caco-2 cells transfected with CCR5 cDNA were grown to confluence and infected with either R5- or X4-GFP-producing HIV-1. These CCR5-transfected Caco-2 cells expressed levels of CCR5 similar to that of CXCR4, as tested by fluorescence-activated cell sorter analysis (data not shown). R5 HIV-1 successfully infected the CCR5-transfected Caco-2 cells (Fig. 2d), whereas nontransfected cells remained resistant to R5 infection (Fig. 2b).
X4 and GalCer-Specific Antibodies Prevent Infection of Caco2 Cell Monolayers and Underlying Target Cells.
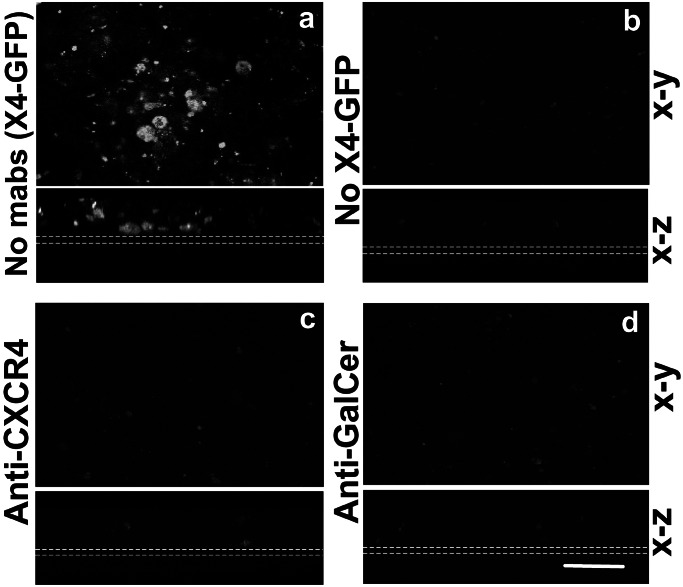
To assess the relative contribution of the chemokine receptors and GalCer, we incubated Caco-2 monolayers at day 9 with neutralizing antibodies against CD4 (data not shown), CXCR4, GalCer, or isotype-matched control antibodies and infected them with GFP-producing X4 HIV-1. The monolayers were assessed for GFP expression 8 days later. In the absence of neutralizing antibodies, Caco-2 monolayers were infected with X4 HIV-1 (Fig. 5a). In contrast, both anti-CXCR4 (Fig. 5c) and anti-GalCer (Fig. 5d) antibodies prevented infection, indicating that both receptors are required for productive infection. In addition, infection of underlying CD4+ T cells by a primary X4 HIV-1 isolate was also inhibited when Caco-2 cell monolayers were incubated with anti-CXCR4 or anti-GalCer neutralizing antibodies (data not shown).
Figure 5.
GalCer and chemokine coreceptors are required for HIV-1 infection of Caco-2 cells. Confocal microscopy (x–y and x–z axes) of Caco-2 monolayers on day 9 incubated for 1 h with HxB2 X4 GFP-producing HIV-1 and isotype-matched control (a), anti-CXCR4 (c), or anti-GalCer (d) antibodies. Non-infected monolayers served as negative control (b). Infection was inhibited with anti-CXCR4 (c) or anti-GalCer (d) antibodies as reflected by the absence of GFP fluorescence when compared with control antibodies (a). Dotted white lines indicate the position of the filters. (Bar = 20 μm.)
CCR5 and GalCer, but Not CXCR4, Are Expressed on the Luminal Side of Human FAE Enterocytes and M Cells.
To see whether our in vitro observations could apply to the situation in the human digestive tract, we analyzed the distribution of the HIV-1 receptors mediating uptake and transport of HIV-1 in the follicle-associated epithelium over human gut mucosa-associated lymphoid tissue. GalCer was found associated with the brush border of the FAE enterocytes and the luminal surface of M cells (data not shown). In contract to Caco-2 cells, CCR5 but not CXCR4 (Fig. 4 d and e) was detected on the luminal sites of all FAE enterocytes (Fig. 4h), but not on the enterocytes of adjacent villi. The two chemokine receptors, however, were expressed in the crypts of the small intestine, as already reported in the colon (21).
Discussion
In this article, we provide evidence that chemokine receptors, together with GalCer, a glycolipid expressed on the luminal surface of enterocytes, mediate not only HIV-1 infection of intestinal cells, but also transepithelial transport of infectious virions. Infection of human intestinal epithelial cell lines by HIV-1 has been shown to require GalCer and X4 receptors (22). We demonstrate that the absence of infection by R5 HIV-1 strains is due to the lack of CCR5 receptors in Caco-2 cells and that transfection of the cells with cDNA encoding the chemokine receptor restores susceptibility to monotropic R5 strains. It is surprising that conversion of Caco-2 cells into M cells that efficiently transport latex beads does not allow infection of underlying monocytes, which demonstrates that transcytosis of HIV-1 across M cells is receptor-mediated and requires more than one receptor. Thus, chemokine receptors together with GalCer act synergistically and determine the specificity of the viral tropism for epithelial cells, indicating that the genetic background of the epithelial cells is crucial for HIV-1 penetration.
Contact between human epithelial cell lines and HIV-1-infected mononuclear cells results in the rapid budding of HIV-1 virions toward the epithelium followed by viral uptake into endosomes (8) and infection of the epithelial cells (22). Successful infection of cultured epithelial cells is usually more efficient with cell-associated virus released upon contact between infected lymphocytes and epithelial cells (29), than with free virus (30–32). Here we present evidence that infection by both free and cell-associated viruses is comparable, provided the epithelial cells express GalCer on their cell surface, and one of the two chemokine coreceptors. GalCer on epithelial cells, in a way similar to CD4 on mononuclear cells, probably induces the conformational changes on gp120 required for the binding of the virus to CCR5 or CXCR4 and the subsequent fusion with the cell membrane, which leads to infection or transcytosis. Two distinct events could occur when HIV-1 binds on the apical surface of epithelial cells: fusion and infection of the epithelial cells or transcytosis of viral particles with the subsequent release of virions at the basolateral side of the epithelial cells. This possibility implies that receptor and coreceptor interactions with HIV-1 envelope glycoproteins are necessary but not sufficient to specify a fusion event between the viral and the epithelial cell membrane and that some virions can be released as infectious particles after transport across an intact epithelial cell layer.
We also provide evidence that the enterocytes of the follicle-associated epithelium over human organized mucosa-associated lymphoid tissue, including Peyer's patches, the appendix, and the single lymphoid follicles found in the colon and the rectum, express CCR5 receptors and GalCer making them an ideal target for monotropic HIV-1 strains. The follicle-associated epithelium is unique in that it contains M cells and few goblet cells (24). M cells lack the typical brush border made of closely packed microvilli and instead have variable microvilli or microfolds interspersed with large plasma membrane subdomains that are exposed to the lumen. M cells also lack the apical thick filamentous brush-border glycocalyx, a thick (400–500 nm) layer of membrane-anchored glycoproteins that seems to form a continuous unstirred layer on the enterocyte surface. Thus, viruses such as HIV-1 can easily gain access to the cell surface of M cells that is free of the structures restricting diffusion of particles and microbes elsewhere in the gut epithelium. In humans, the rectum contains the highest number of follicles per unit area. Because it is particularly enriched in M cells, the rectoanal epithelium represents a potential site for infection by HIV-1 through an intact epithelial layer.
In conclusion, we report that transcytosis of HIV-1 across M cells and enterocytes is receptor-mediated. This process may account for infection across an intact rectal mucosal surface, because the epithelium that covers mucosal lymphoid follicles in the human gut expresses galactosyl cerebroside and the CCR5 coreceptor, found, in our in vitro system, to mediate transepithelial transport of R5 HIV-1.
Acknowledgments
We are grateful to Estelle Säuberli for excellent technical assistance in immunocytochemistry. We thank Lucy Hathaway for critical reading of the manuscript. This work was supported by Grants 31-47296.96 and 31-56936-99 from the Swiss National Science Foundation and by the Swiss League Against Cancer (SKL 635-2-1998).
Abbreviations
- VSV
vesicular stomatitis virus
- GFP
green fluorescent protein
- GalCer
glycosphingolipid galactosylceramide
- FAE
follicle-associated epithelium
- TCID50
tissue culture 50% infective dose
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Quinn T C. Curr Opin Immunol. 1989;1:502–512. doi: 10.1016/0952-7915(88)90034-9. [DOI] [PubMed] [Google Scholar]
- 2.Wolff H, Anderson D J. Fertil Steril. 1988;49:497–504. [PubMed] [Google Scholar]
- 3.Baba T W, Trichel A M, An L, Liska V, Martin L N, Murphey-Corb M, Ruprecht R M. Science. 1996;272:1486–1489. doi: 10.1126/science.272.5267.1486. [DOI] [PubMed] [Google Scholar]
- 4.Bomsel M. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 5.Amerongen H M, Weltzin R A, Farnet C M, Michetti P, Haseltine W A, Neutra M R. J Acquired Immune Defic Syndr. 1991;4:760–765. [PubMed] [Google Scholar]
- 6.Ruprecht R M. In: Retroviruses of Human A.I.D.S. and Related Animal Diseases. Girard M, Valette L, editors. Lyon, France: 8e Colloque des Cent Gardes 1993, Fondation Mérieux; 1994. pp. 99–106. [Google Scholar]
- 7.Stahl-Hennig C, Steinman R M, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K, Grobschupff G, Raschdorff B, Hunsmann G, Racz P. Science. 1999;285:1261–1265. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- 8.Phillips D M, Bourinbaiar A S. Virology. 1992;186:261–273. doi: 10.1016/0042-6822(92)90080-9. [DOI] [PubMed] [Google Scholar]
- 9.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. J Exp Med. 1996;183:215–25. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J J, Gardner M B, Miller C J. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreiss J K, Hopkins S G. J Infect Dis. 1992;168:1404–1408. doi: 10.1093/infdis/168.6.1404. [DOI] [PubMed] [Google Scholar]
- 12.Fantini J, Yahi N, Chermann J C. Proc Natl Acad Sci USA. 1991;88:9297–9301. doi: 10.1073/pnas.88.20.9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yahi N, Baghdiguian S, Moreau H, Fantini J. J Virol. 1992;66:4848–4854. doi: 10.1128/jvi.66.8.4848-4854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tersmette M, de Goede R E, Al B J, Winkel N, Gruters R A, Cuypers H T, Huisman H G, Miedema F. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng-Mayer C, Seto D, Tateno M, Levy J A. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 17.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L J, Mackay C R, Larosa G, Newman W, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 18.Deng H K, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Dimarzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien W A, Koyanagi Y, Namazie A, Zhao J Q, Diagne A, Idler K, Zack J A, Chen I S Y. Nature (London) 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 20.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 21.Dwinell M B, Eckmann L, Leopard J D, Varki N M, Kagnoff M F. Gastroenterology. 1999;117:359–367. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- 22.Delezay O, Koch N, Yahi N, Hammache D, Tourres C, Tamalet C, Fantini J. AIDS. 1997;11:1311–1318. doi: 10.1097/00002030-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Kernéis S, Bogdanova A, Kraehenbuhl J P, Pringault E. Science. 1997;277:948–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 24.Kraehenbuhl J P, Neutra M R. Annu Rev Cell Dev Biol. 2000;16:301–332. doi: 10.1146/annurev.cellbio.16.1.301. [DOI] [PubMed] [Google Scholar]
- 25.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Hafen K, Fogh J, Zweibaum A. Biol Cell. 1983;47:323–330. [Google Scholar]
- 26.Carriere V, Lesuffleur T, Barbat A, Rousset M, Dussaulx E, Costet P, De W I, Beaune P, Zweibaum A. FEBS Lett. 1994;355:247–250. doi: 10.1016/0014-5793(94)01199-0. [DOI] [PubMed] [Google Scholar]
- 27.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 28.Ou C, Kwok Y S, Mitchell S W, Mack D H, Sninsky J J, Krebs J W, Feorino P, Warfield D, Schochetman G. Science. 1988;239:295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- 29.Bourinbaiar A S, Phillips D M. J Acquired Immune Defic Syndr. 1991;4:56–63. [PubMed] [Google Scholar]
- 30.Adachi A, Koenig S, Gendelman H E, Daugherty D, Gattoni-Celli S, Fauci A S, Martin M A. J Virol. 1987;61:209–213. doi: 10.1128/jvi.61.1.209-213.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kage A, Shoolian E, Rokos K, Ozel M, Nuck R, Reutter W, Kottgen E, Pauli G. J Virol. 1998;72:4231–4236. doi: 10.1128/jvi.72.5.4231-4236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, Desgranges C. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]