Abstract
This study was to investigate the effects of Albiflorin (ALB) on oxidative stress and inflammation in diabetic retinopathy (DR) and explore its potential mechanism involving the Toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) signaling pathway. Human retinal microvascular endothelial cells (HRMECs) were treated with high glucose (HG) and ALB. Cell viability was assessed by MTT assay. Oxidative stress markers and inflammatory cytokines were measured by ELISA. TLR4/NF-κB pathway proteins were analyzed by Western blot. A streptozotocin (STZ)-induced diabetic rat model was established to examine retinal histological changes. Serum metabolic parameters, oxidative stress markers, and inflammatory cytokines were evaluated in the DR model and ALB intervention groups. Results showed that ALB improved HRMEC viability under HG induction and reduced oxidative stress and inflammation. ALB inhibited the TLR4/NF-κB pathway in HG-induced HRMECs. Overexpression of TLR4 partially reversed the protective effects of ALB. In diabetic rats, ALB ameliorated metabolic disorders, improved retinal histological structure, and reduced oxidative stress and inflammation. ALB also suppressed the TLR4/NF-κB signaling pathway in vivo. In conclusion, ALB improves DR by resolving oxidative stress and inflammation through inhibiting the TLR4/NF-κB signaling pathway. These findings suggest ALB as a potential therapeutic agent for DR.
Keywords: Albiflorin, diabetic retinopathy, TLR-4/NF-κB signaling, oxidative stress, inflammation
Introduction
Diabetic retinopathy (DR) is a common neurodegenerative and microvascular disorder in diabetes mellitus and a leading cause of vision loss in severe cases. It is caused by a series of physiological changes, including chronic hyperglycemia, elevated blood pressure, dyslipidemia, oxidative stress, inflammation, formation of advanced glycation end-products (AGEs), and altered blood flow in retinal vessels.1–3 DR affects a significant proportion of people with diabetes, with prevalence increasing with the duration of diabetes. Global estimates suggest that about one-third of people with diabetes have some degree of DR, and about one-tenth have vision-threatening DR.4–5 Current therapeutic approaches primarily focus on anti-vascular endothelial growth factor (VEGF) drugs, glucocorticoids, and laser surgery. However, these treatments can produce adverse renal effects, necessitating the development of novel therapeutic strategies for DR.6 While the fundamental mechanism of DR is known to involve retinal vascular damage, the underlying signaling pathway of oxidative stress and inflammation in DR remains to be fully elucidated.7–9
Albiflorin (ALB), a monoterpenoid glycoside extracted from the roots of Paeonia lactiflora, demonstrates anti-inflammatory, analgesic, immunomodulatory, neuroprotective, and antioxidant effects. It can effectively alleviate chronic inflammation and pain, regulate the immune system, protect nerve cells from damage, and demonstrate potential therapeutic effects in anti anxiety and anti depression.9 Previous studies have shown that ALB can suppress neuroinflammation and cell injury in middle cerebral artery occlusion (MCAO) model.10 Furthermore, ALB has been reported to inhibit high glucose-induced endothelial cells apoptosis and reactive oxygen species (ROS) generation.11 Notably, ALB has ameliorated diabetic encephalopathy in streptozotocin (STZ)-induced diabetic rats.12 However, the protective effect of ALB on DR has not been deciphered.
In systemic inflammation, the activation of the Toll-like receptor 4 (TLR4)/NF-κB signaling pathway induces the expression of inflammatory cytokines, such as TNF-α, interleukin-1β (IL-1β), and interleukin-6 (IL-6).15 Suppression of the TLR4/NF-κB signaling pathway to reduce inflammatory responses may be beneficial in preventing DR.16 Given the documented protective effects of ALB against inflammatory and oxidative damage in various diabetic complications,12 we postulated that ALB might similarly attenuate DR progression by mitigating inflammation and oxidative stress in retinal tissues. This study utilized both in vitro human retinal microvascular endothelial cell (HRMEC) and in vivo diabetic rat models to investigate the effects of ALB on DR. ALB reduced oxidative stress and inflammation in both models, primarily by inhibiting the TLR4/NF-κB signaling pathway. Our findings suggest ALB as a potential therapeutic agent for DR, warranting further investigation for clinical applications in treating diabetic complications.
Material and methods
Cells and cell culture
Human retinal microvascular endothelial cells (HRMECs) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in PM-002 media supplemented with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere.13 . To simulate a high glucose environment, HRMECs were exposed to 30 mM glucose (HG; Solarbio, Beijing, China) and then treated with different dose of Albiflorin (Beijing Ounaer Bioengineering Technology Co., Ltd) in deionized water.
MTT assay
HRMECs were seeded into 96-well plates and treated with various compounds for 24 hours. Subsequently, 10 μL of MTT reaction reagent was added to each well, and the cells were incubated for 4 hours at 37°C. The absorbance was measured at 450 nm using a BioTek microplate reader (BioTek Instruments, Winooski, VT, USA). Cell viability was calculated and expressed as a percentage relative to the untreated control group.
Western blot
Cells or tissues were lysed using RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA) containing protease inhibitors. Protein concentrations were determined using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Equal amounts of protein were separated by SDS-PAGE and transferred onto PVDF membranes. Membranes were blocked with bovine serum albumin (BSA) for 1 hour, then incubated overnight at 4°C with primary antibodies against TLR4, NF-κB, Lamin A, and β-actin (1:1000 dilution, Cell Signaling Technology, Danvers, MA, USA). After washing, membranes were incubated with appropriate HRP-conjugated secondary antibodies (1:5000, Cell Signaling Technology) for 1 hour at room temperature. Protein bands were visualized using a ChemiDoc Touch Imaging System (Bio-Rad Laboratories, Hercules, CA, USA) and quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Induction of diabetes retinal injury in rats
Male Sprague-Dawley rats were purchased from Zhejiang Laboratory Animal Center (Hangzhou, Zhejiang, China). All animal procedures were approved by the Animal Use and Care Ethics Committee of Beilun District People's Hospital. All experiments were performed in accordance with the relevant national and institutional guidelines and regulations. To establish the diabetic retinopathy (DR) rat model, animals initially received a single intraperitoneal injection of streptozotocin (STZ, 65 mg/kg in 50 mM citrate buffer; Sigma-Aldrich, St. Louis, MO, USA).14 Rats were randomly divided into four groups: 1) Sham (injected with equal amount of normal saline); 2) ALB 100 mg/kg (Sham group treated with ALB); 3) DR (STZ injection only); 4) DR + ALB: (STZ injection group treated with different doses of ALB (50, 100, or 200 mg/kg, oral administration)) for 6 weeks. The DR model rats were fed a high-fat diet (60% chow, 10% egg yolk powder, 10% lard, 1.5% cholesterol, and 0.1% sodium cholate), while the sham group received standard chow. Diabetes onset was confirmed by two consecutive random blood glucose measurements ≥16 mmol/L, taken every other day. At the end of experiment, blood was collected from the eyes and serum was obtained by centrifugation. All animals were euthanized by cervical dislocation, and the retinal tissues were collected for histological examination.
ELISA (Enzyme-Linked Immunosorbent Assay)
The levels of malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), inflammatory cytokines (TNF-α, IL-6, and IL-1β), blood glucose, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured using ELISA kits from Elabscience Biotechnology Co., Ltd. (Wuhan, Hubei, China). To account for variations in sample concentration, all ELISA data were normalized to the total protein content of each sample. Total protein concentration was determined using a Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA), and the results were expressed as units per milligram of protein.
Hematoxylin and Eosin (H&E) staining
Hematoxylin and Eosin staining was performed using the H&E Staining Kit (ab245880, Abcam, Cambridge, UK) following the manufacturer's instructions with some modifications. Retinal paraffin sections (5 μm thick) were deparaffinized in xylene (2 changes, 10 minutes each) and rehydrated through a graded ethanol series (100%, 95%, 70%, 50%, 5 minutes each), followed by a rinse in distilled water for 5 minutes. Sections were then stained with Mayer's hematoxylin solution for 5 minutes to visualize nuclei, followed by a rinse in running tap water for 5 minutes. Differentiation was performed by quickly dipping the slides 10 times in acid ethanol (1% HCl in 70% ethanol), followed by a brief rinse in tap water. Slides were then immersed in 0.2% ammonia water for 30 seconds to blue the nuclei, followed by a 5-minute wash in running tap water. Counterstaining was performed with eosin Y solution for 30 seconds to 1 minute. Finally, the sections were cleared in xylene (2 changes, 5 minutes each) and mounted with Permount mounting medium (Fisher Scientific, Hampton, NH, USA).
The stained sections were examined under a light microscope (Olympus BX53, Olympus Corporation, Tokyo, Japan) equipped with a digital camera. Morphological changes in the ganglionic cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), and outer nuclear layer (ONL) were observed and photographed at 200x and 400x magnifications. Quantitative alterations in layer thickness and cell numbers were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). For each sample, at least five random fields were analyzed, and the average values were used for statistical analysis.
Statistical analysis
All experiments were independently performed in triplicate, and data are expressed as mean ± standard deviation (SD). GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Significance between experimental groups was determined using Student's t-test (for two groups) or one-way ANOVA (for three or more groups). A p-value < 0.05 was considered statistically significant.
Results
ALB inhibits high glucose (HG)-induced oxidative stress and inflammatory injury in HRMECs
We initially investigated the protective effects of ALB against high glucose-induced oxidative stress and inflammation in HRMECs. ALB exhibited no cytotoxicity to HRMECs within the concentration range of 0-80 μM, as determined by MTT assay (Figure 1A). Based on these results, we selected 5, 10, and 20 μM as working concentrations for subsequent experiments. In the HG-induced retinal injury cell model, 30 mM glucose significantly reduced cell viability to approximately 70% compared to the control group. ALB treatment, however, enhanced cell survival in a dose-dependent manner (Figure 1B).
Figure 1.
ALB mitigates high glucose-induced oxidative stress and inflammation in HRMECs. (A). Cell viability of HRMECs treated with varying concentrations of ALB (0-80 μM) for 24 hours, as determined by MTT assay. B. Cell viability of HRMECs exposed to normal glucose (5.5 mM), high glucose (30 mM), or high glucose with ALB treatment (5, 10, and 20 μM). C. Levels of oxidative stress markers (SOD, MDA, and CAT) in HRMECs under different treatment conditions, as measured by ELISA. D. Expression levels of inflammatory cytokines (TNF-α, IL-6, and IL-1β) in HRMECs, quantified by ELISA. ***p<0.001 compared to control group, #p<0.05, ##p<0.01, and ###p<0.001 compared to HG group.
Oxidative stress, characterized by an imbalance between oxidative free radicals and their elimination, is a crucial factor in HG-induced retinal injury.15 To assess cellular oxidative stress, we measured the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) using ELISA. HG treatment decreased SOD and CAT levels while increasing MDA content in HRMECs compared to the control group. Conversely, ALB administration increased SOD and CAT concentrations, with statistically significant changes observed at 4 and 20 μM. ALB also reduced MDA levels in a dose-dependent manner compared to the HG group (Figure 1C). Meanwhile, the levels of inflammatory cytokines TNF-α, IL-6, and IL-1β were markedly elevated upon HG treatment, and ALB attenuated their increases in a dose-dependently (Figure 1D). These findings collectively indicate that ALB can effectively mitigate HG-induced oxidative stress and inflammation in HRMECs.
ALB inhibits TLR4/NF-κB signaling pathway in HG-induced HRMECs
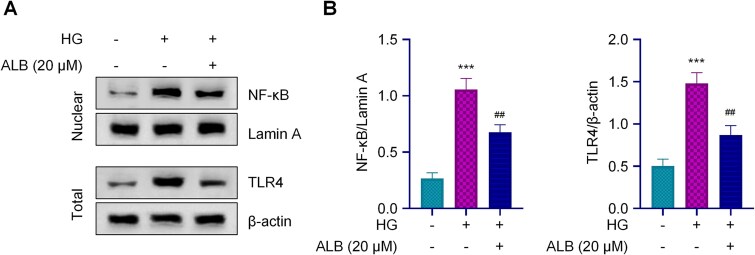
To elucidate the underlying mechanism of ALB's protective effect against high HG-induced retinal cell injury, we investigated the activities of the TLR4/NF-κB signaling pathway using Western blot analysis. In the HG group, the expression of NF-κB and TLR4 was significantly increased compared to the normal glucose group (Figure 2A). ALB treatment at 20 μM effectively suppressed both the nuclear expression of NF-κB and the total level of TLR4 in HRMECs exposed to high glucose. Statistical analysis of the Western blot results confirmed these observations, demonstrating a significant reduction in both NF-κB and TLR4 expression levels in ALB-treated cells compared to the HG group (Figure 2B). These results suggest that ALB's protective effects against HG-induced retinal cell injury are mediated, at least in part, through the inhibition of the TLR4/NF-κB signaling pathway.
Figure 2.
ALB inhibits the TLR4/NF-κB signaling pathway in high glucose-induced HRMEC injury. A. A. Western blot analysis of TLR4 and nuclear NF-κB expression in HRMECs treated with normal glucose (5.5 mM), high glucose (30 mM), or HG + ALB (20 μM). B. Quantitative analysis of TLR4 and nuclear NF-κB protein levels. Lamin A and β-actin were used as internal controls for nuclear and total protein, respectively. ***p<0.001 compared to control group, and ##p<0.01 compared to HG group.
TLR4 overexpression attenuates ALB-mediated protection against HG-induced oxidative stress and inflammation in HRMECs
To further elucidate the regulatory effect of ALB on TLR4/NF-κB signaling in terms of ROS and inflammation, we overexpressed TLR4 by transfecting HRMECs with a TLR4-OE plasmid. HG enhanced TLR4 expression and nuclear NF-κB levels, while ALB significantly reduced these increases. Importantly, TLR4 overexpression partially reversed the ALB-induced reduction of both TLR4 and nuclear NF-κB (Figure 3A). We next examined the effect of TLR4 overexpression on ALB-mediated inhibition of ROS. HG treatment increased MDA levels and decreased SOD and CAT levels compared to the control. ALB administration mitigated these alterations in ROS markers. However, TLR4 overexpression reversed the suppressive effect of ALB on ROS by increasing MDA and decreasing SOD and CAT levels (Figure 3B). Furthermore, we observed a similar trend in the inflammatory response induced by HG. ALB treatment decreased the elevated concentrations of TNF-α, IL-6, and IL-1β caused by HG. TLR4 overexpression reversed this ALB-mediated reduction in inflammatory cytokines, resulting in higher levels of TNF-α, IL-6, and IL-1β compared to ALB-treated cells without TLR4 overexpression (Figure 3C). These results indicate that ALB inhibits HG-induced ROS production and inflammation in HRMECs by modulating the TLR4/NF-κB signaling pathway, and that TLR4 overexpression attenuates these protective effects of ALB.
Figure 3.
TLR4 overexpression attenuates ALB-mediated protection against high glucose-induced oxidative stress and inflammation in HRMECs. A. Western blot analysis of TLR4 and nuclear NF-κB expression in HRMECs under different treatment conditions, including TLR4 overexpression. B. Levels of oxidative stress markers (SOD, MDA, and CAT) in HRMECs under various treatment conditions, as measured by ELISA. C. Expression levels of inflammatory cytokines (TNF-α, IL-6, and IL-1β) in HRMECs under different treatments, quantified by ELISA. ***p<0.001 compared to control group, ##p<0.01 compared to HG group, and ^p<0.05; ^^p<0.01 and ^^^p<0.001 compared with ALB treatment group.
ALB ameliorates diabetes-induced metabolic disorders in a rat model of DR
To evaluate the protective effects of ALB against diabetes-induced retinal damage, we constructed an streptozotocin (STZ)-induced DR rat model and also included an intervention group with ALB treatment. Our results demonstrated that STZ-induced DR) group displayed significantly increased body weight compared to the sham group sis weeks after STZ injection. ALB treatment significantly reduced body weight in diabetic rats by approximately 15%, 25%, and 35% at doses of 25, 50, and 100 mg/kg, respectively (Figure 4A-B), suggesting that ALB mitigated STZ-induced weight gain. To further investigate the role of ALB in glucose and lipid metabolism, we analyzed serum levels of blood glucose, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) using ELISA. As shown in Figure 4C-F, diabetic rats exhibited markedly elevated levels of blood glucose, TC, TG, and LDL-C, while HDL-C levels decreased. ALB treatment alleviated the increase in blood glucose, TC, TG, and LDL-C levels in a dose-dependent manner. Notably, ALB (200 mg/kg) restored HDL-C levels to near-normal baseline values observed in the sham group, with no significant difference between the ALB (200 mg/kg) and sham groups. These findings indicate that ALB improves glucose and lipid metabolic disorders induced by diabetes.
Figure 4.
ALB ameliorates diabetes-induced metabolic disorders in a rat model of diabetic retinopathy. Rats were randomly divided into six groups and treated with different drugs for 6 weeks: (1) Sham group; (2) ALB 200 mg/kg group; (3) DR group; (4) DR+ALB 50mg/kg; (5) DR+ALB 100 mg/kg; (6) DR+ALB 200 mg/kg (n=6). A. Body weight changes in rats over 6 weeks of treatment. B. Blood glucose levels in rats over the 6-week treatment period. C-F: Serum levels of glucose and lipid metabolism indicators (TC, TG, LDL-C, and HDL-C) as measured by ELISA.***p<0.001 compared with sham group, and #p<0.05 and ##p<0.01 compared to DR group.
ALB ameliorates retinal pathology and reduces oxidative stress and inflammation in a rat model of DR
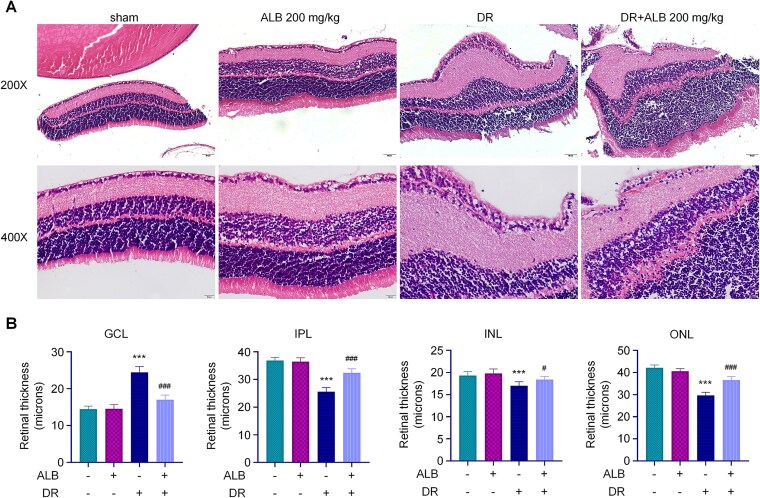
Next, we investigated the effects of ALB on retinal pathological changes, oxidative stress, and inflammation in the DR rat model, as well as its potential regulatory mechanism. Histological examination revealed normal retinal structure in the sham group, with distinct ganglionic cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), and outer nuclear layer (ONL). In DR tissues, ganglion cells appeared swollen with shrunken nuclei and reduced cell numbers. ALB treatment alone did not induce pathological changes compared to the sham group. The recovery group (DR+ALB) showed improved retinal tissue structure, with only minor spaces around nuclei (Figure 5A). Quantitative analysis of retinal layers demonstrated increased GCL thickness and decreased IPL, INL, and ONL thickness in DR compared to sham retinal tissues. ALB treatment reversed these pathological changes, decreasing GCL thickness while gradually increasing IPL, INL, and ONL thickness (Figure 5B), indicating suppression of diabetes-induced retinal injury.
Figure 5.
ALB improves retinal histopathology in diabetic rats. A. Representative H&E stained images of retinal sections from different treatment groups. Scale bar = 200 μm. B. Quantitative analysis of retinal layer thickness (GCL, IPL, INL, and ONL) across treatment groups. ***p<0.001 compared to sham group, and #p<0.05 and ##p<0.01 compared with DR group.
To elucidate the underlying mechanism of ALB treatment in diabetic retinal injury, we assessed oxidative stress markers and inflammatory cytokines. Diabetic conditions elevated MDA levels and reduced SOD and CAT levels compared to the sham group. ALB treatment increased SOD and CAT concentrations while decreasing MDA activity, thus mitigating oxidative stress in DR rat retinas (Figure 6A). Additionally, DR rats exhibited elevated levels of inflammatory cytokines TNF-α, IL-6, and IL-1β, which were significantly reduced by ALB treatment (Figure 6B). Western blot analysis of TLR4/NF-κB pathway proteins revealed increased expression of TLR4 and nuclear NF-κB in DR compared to the sham group. ALB treatment (100 mg/kg) notably inhibited these inflammatory markers, with approximately 30% reduction in both TLR4 and NF-κB levels (Figure 6C). These results indicate that ALB alleviates DR by inhibiting oxidative stress and inflammation through modulation of the TLR4/NF-κB signaling pathway.
Figure 6.
ALB reduces oxidative stress and inflammation by modulating the TLR4/NF-κB signaling pathway in diabetic rat retinas. A. Levels of oxidative stress markers (SOD, MDA, and CAT) in rat retinas, as measured by ELISA. B. Expression levels of inflammatory cytokines (TNF-α, IL-6, and IL-1β) in rat retinas, quantified by ELISA. C. Western blot analysis and quantification of TLR4 and nuclear NF-κB protein levels in rat retinas. ***p<0.001 compared to sham group, and ###p<0.001 compared to DR group.
Discussion
DR is a common neurodegenerative and microvascular disorder caused by a series of physiological changes associated with diabetes mellitus.1–3 DR is characterized by the loss of retinal pericytes and abnormal angiogenesis. The abnormal angiogenesis manifests as retinal microvascular damage, which is a primary cause of vision loss in DR patients, particularly in older individuals.17–18 To date, the underlying mechanisms of DR are not fully elucidated. Current treatments include laser photocoagulation, anti-VEGF therapy, and intravitreal corticosteroids19; however, these interventions are associated with potential adverse effects. Therefore, there is an urgent need to identify novel therapeutic agents that specifically target retinal microvascular damage in DR.
STZ-induced diabetes in animal models mimics many aspects of human diabetes.15,16 Diabetic conditions induce recurrent acute inflammation and cumulative alterations that lead to retinal damage.20 The retina is particularly vulnerable to oxidative stress due to its high oxygen consumption, glucose oxidation rate, and abundance of polyunsaturated fatty acids.4,6,21 In our study, STZ was used to establish a DR model, and oxidative stress levels were measured using ELISA. Elevated concentrations of oxidative stress markers were confirmed in the diabetic retina. As a consequence of increased oxidative stress, microvascular dysfunction and cellular damage occur, including injury to retinal microvascular endothelial cells.22 Furthermore, the overabundance of reactive oxygen species and the occurrence of oxidative stress contribute to higher rates of dyslipidemia and inflammation.23,24
ALB is a monoterpenoid glycoside extracted from the roots of Paeonia lactiflora, exhibiting anti-inflammatory and antioxidant properties in many diseases, such as ulcerative colitis and cerebral ischemia-reperfusion injury.25,26 Besides, ALB has multiple pharmacological effects such as analgesic, immunomodulatory, neuroprotective effects.27–28 It can effectively alleviate regulate the immune system, protect nerve cells from damage, and demonstrate potential therapeutic effects in anti anxiety and anti depression. Consistently, our study confirmed that ALB modulated oxidative stress indicators, including MDA, SOD, and CAT levels, in high glucose-induced cell model and STZ-induced rat model of DR, suggesting antioxidant effects of ALB. Additionally, ALB inhibited the secretion of pro-inflammatory cytokines TNF-α, IL-6, and IL-1β. These findings are consistent with the previously reported anti-inflammation and antioxidant effect of ALB in diabetic rat model.29 Our findings further advance the understanding of underlying mechanism contributing to ALB-mediated anti-inflammatory and antioxidant effect by demonstrating the inhibition on TLR4/NF-κB axis.
Toll-like receptors (TLRs) play a crucial role in innate immunity. Among them, Toll-like receptor 4 (TLR4) has been implicated in inflammatory responses across various pathological conditions, including lipopolysaccharide (LPS)-induced injury and ischemia-reperfusion injury.30 TLR4 activation triggers the overexpression of pro-inflammatory cytokines and the generation of reactive oxygen species. The NF-κB pathway, a key mediator of inflammatory responses, regulates numerous downstream proteins involved in diverse cellular processes.31 Recent studies have highlighted the therapeutic potential of modulating the TLR4/NF-κB axis in diabetic renal injury.32 Therefore, we detected whether ALB impacted TLR4/NF-κB pathway, as a promising therapeutic strategy in diabetic complications, including retinopathy. Our results demonstrated the ALB's protective effects in diabetic retinopathy by TLR4/NF-κB pathway.
To conclude, our demonstrates that ALB effectively ameliorates diabetic DR by mitigating oxidative stress and inflammation through inhibition of the TLR4/NF-κB signaling pathway. In both in vitro and in vivo models, ALB reduced oxidative stress markers, decreased pro-inflammatory cytokine levels, and improved retinal histopathology. These findings highlight the potential of ALB as a therapeutic agent for DR, offering a novel approach to address the underlying pathological mechanisms of this condition. Future research should focus on optimizing ALB delivery methods, investigating potential synergistic effects with current DR treatments, and conducting clinical trials to validate ALB's efficacy and safety in human patients with diabetic retinopathy.
Acknowledgement
Not applicable.
Contributor Information
Liuyi Xie, Department of Ophthalmology, Beilun District People's Hospital, Ningbo, Zhejiang Province, 315826, China.
Yingjun Wang, Department of Ophthalmology, Beilun District People's Hospital, Ningbo, Zhejiang Province, 315826, China.
Yudan Gong, Department of Ophthalmology, Beilun District People's Hospital, Ningbo, Zhejiang Province, 315826, China.
Authors contributions
YD Gong mainly participated in literature search, study design, writing and critical revision,prepared Figure 1–3. LY Xie and YJ Wang mainly participated in data collection, data analysis and data interpretation, prepared Figure 4–6. All authors read and approved the final manuscript.
Funding
Not applicable.
Disclosure of conflict of interest
The authors declared that there was no conflict of interest associated with the manuscript.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Beilun District People's Hospital. All experiments were performed in accordance with the relevant national and institutional guidelines and regulations.
Consent for publication
Not applicable
References
- 1. Sen S et al. Comparative proteomics of proliferative diabetic retinopathy in people with Type 2 diabetes highlights the role of inflammation, visual transduction, and extracellular matrix pathways. Indian J Ophthalmol. 2023:71:3069–3079. 10.4103/IJO.IJO_276_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013:2013:343560. 10.1155/2013/343560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodríguez ML, Pérez S, Mena-Mollá S, Desco MC, Ortega ÁL. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid Med Cell Longev. 2019:2019:1–18. 10.1155/2019/4940825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang Q et al. Diabetic Retinopathy: New Treatment Approaches Targeting Redox and Immune Mechanisms. Antioxidants (Basel). 2024:13:594. 10.3390/antiox13050594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fung TH, Patel B, Wilmot EG, Amoaku WM. Diabetic retinopathy for the non-ophthalmologist. Clin Med (Lond). 2022;22(2):112–116. doi: 10.7861/clinmed.2021-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Safi SZ, Qvist R, Kumar S, Batumalaie K, Ismail IS. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed Res Int. 2014:2014:801269. 10.1155/2014/801269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Böhm EW et al. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023:68:102967. 10.1016/j.redox.2023.102967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang Q, Yang C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020:37:101799. 10.1016/j.redox.2020.101799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang R, Yang Y. Albiflorin attenuates high glucose-induced endothelial apoptosis via suppressing PARP1/NF-kappaB signaling pathway. Inflamm Res. 2023;72(1):159–169. doi: 10.1007/s00011-022-01666-z. [DOI] [PubMed] [Google Scholar]
- 10. Ou Z, Li P, Wu L, Wu Y, Qin L, Fang L, Xu H, Pei K, Chen J. Albiflorin alleviates neuroinflammation of rats after MCAO via PGK1/Nrf2/HO-1 signaling pathway.Int Immunopharmacol. 2024. 137;137:112439. doi: 10.1016/j.intimp.2024.112439. [DOI] [PubMed] [Google Scholar]
- 11. Yang R, Yang Y. Albiflorin attenuates high glucose-induced endothelial apoptosis via suppressing PARP1/NF-kappaB signaling pathway. Inflamm Res. 2023;72(1):159–169. doi: 10.1007/s00011-022-01666-z. [DOI] [PubMed] [Google Scholar]
- 12. Ma X, Song M, Yan Y, Ren G, Hou J, Qin G, Wang W, Li Z. Albiflorin alleviates cognitive dysfunction in STZ-induced rats. Aging (Albany NY). 2021;13(14):18287–18297. doi: 10.18632/aging.203274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu L, Lu Q, Chen W, Li J, Li C, Zheng Z. Vitamin D(3) Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway.J Diabetes Res. 2018. Feb 2018;2018:1, 11, 10.1155/2018/8193523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu SH et al. Paeoniflorin Suppressed High Glucose-Induced Retinal Microglia MMP-9 Expression and Inflammatory Response via Inhibition of TLR4/NF-kappaB Pathway Through Upregulation of SOCS3 in Diabetic Retinopathy. Inflammation. 2017:40:1475–1486. 10.1007/s10753-017-0571-z [DOI] [PubMed] [Google Scholar]
- 15. Qu Q et al. Sustained therapeutic effect of an anti-inflammatory peptide encapsulated in nanoparticles on ocular vascular leakage in diabetic retinopathy. Front Cell Dev Biol. 2022:10:10. 10.3389/fcell.2022.1049678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu K et al. Capsaicin ameliorates diabetic retinopathy by inhibiting poldip2-induced oxidative stress. Redox Biol. 2022:56:102460. 10.1016/j.redox.2022.102460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaštelan S, Orešković I, Bišćan F, Kaštelan H, Gverović Antunica A.Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem Med (Zagreb). 2020;30(3):030502. doi: 10.11613/BM.2020.030502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poprac P et al. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol Sci. 2017:38:592–607. 10.1016/j.tips.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 19. Fu C et al. Apigenin inhibits angiogenesis in retinal microvascular endothelial cells through regulating of the miR-140-5p/HDAC3-mediated PTEN/PI3K/AKT pathway. BMC Ophthalmol. 2023:23:302. 10.1186/s12886-023-03046-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Del Cura Mar P, Carballes MJC, Sastre-Ibanez M. Risk of renal damage associated with intravitreal anti-VEGF therapy for diabetic macular edema in routine clinical practice. Indian J Ophthalmol. 2023:71:3091–3094. 10.4103/IJO.IJO_44_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007:2007:43603. 10.1155/2007/43603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rossino MG et al. Oxidative Stress Induces a VEGF Autocrine Loop in the Retina: Relevance for Diabetic Retinopathy. Cells. 2020:9:1452. 10.3390/cells9061452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ceriello A, dello Russo P, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes. 1996:45:471–477. 10.2337/diab.45.4.471 [DOI] [PubMed] [Google Scholar]
- 24. Li J et al. High triglyceride levels increase the risk of diabetic microvascular complications: a cross-sectional study. Lipids Health Dis. 2023:22:109. 10.1186/s12944-023-01873-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng X, Wan J, Tan G. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in diabetic retinopathy. Front Immunol. 2023:14:1151185. 10.3389/fimmu.2023.1151185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang L, Xu J, Yin S, Wang Q, Jia Z, Wen T. Albiflorin Attenuates Neuroinflammation and Improves Functional Recovery After Spinal Cord Injury Through Regulating LSD1-Mediated Microglial Activation and Ferroptosis. Inflammation. 2024. Aug;47(4):1313–1327, 10.1007/s10753-024-01978-8. [DOI] [PubMed] [Google Scholar]
- 26. Kim JH, Kim M, Hong S, Kim EY, Lee H, Jung HS, Sohn Y. Albiflorin Promotes Osteoblast Differentiation and Healing of Rat Femoral Fractures Through Enhancing BMP-2/Smad and Wnt/beta-Catenin Signaling.Front Pharmacol. 2021. Jul 12;12:690113, 10.3389/fphar.2021.690113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fang P, Wang Y, Sun F, Lin H, Zhang X. Effects of albiflorin on oxidative stress and inflammatory responses in rats with acute spinal cord injury. Immun Inflamm Dis. 2023. Sep;11(9):e1015, 10.1002/iid3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li X, Hou R, Qin X, Wu Y, Wu X, Tian J, Gao X, Du G, Zhou Y. Synergistic neuroprotective effect of saikosaponin A and albiflorin on corticosterone-induced apoptosis in PC12 cells via regulation of metabolic disorders and neuroinflammation. Mol Biol Rep. 2022. Sep;49(9):8801–8813. doi: 10.1007/s11033-022-07730-5. Epub 2022 Aug 24. [DOI] [PubMed] [Google Scholar]
- 29. Zhou X, Fouda S, Zeng XY, Li D, Zhang K, Xu J, Ye JM.Characterization of the Therapeutic Profile of Albiflorin for the Metabolic Syndrome. Front Pharmacol. 2019. Oct 10;10:1151, 10.3389/fphar.2019.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen H, Zhang RQ, Wei XG, Ren XM, Gao XQ. Mechanism of TLR-4/NF-kappaB pathway in myocardial ischemia reperfusion injury of mouse. Asian Pac J Trop Med. 2016:9:503–507. 10.1016/j.apjtm.2016.03.021 [DOI] [PubMed] [Google Scholar]
- 31. Hong H et al. Hydnocarpin D attenuates lipopolysaccharide-induced acute lung injury via MAPK/NF-kappaB and Keap1/Nrf2/HO-1 pathway. Phytomedicine. 2022:101:154143. 10.1016/j.phymed.2022.154143 [DOI] [PubMed] [Google Scholar]
- 32. Ghaiad HR, Ali SO, Al-Mokaddem AK, Abdelmonem M. Regulation of PKC/TLR-4/NF-kB signaling by sulbutiamine improves diabetic nephropathy in rats. Chem Biol Interact. 2023:381:110544. 10.1016/j.cbi.2023.110544 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.