Executive summary
Coronary artery disease has long been understood through the paradigm of epicardial coronary artery obstruction, causing myocardial ischaemia (a mismatch between myocardial blood supply and demand). However, this model, which focuses on diagnosing and managing coronary artery disease based on ischaemia and cardiovascular events, is flawed. By the time ischaemia manifests, it is often too late for optimal intervention, limiting the effectiveness of treatment options. Despite decades of medical advances, coronary artery disease continues to be a leading cause of morbidity and mortality globally, highlighting the inadequacy of this traditional ischaemic-centric approach.
The central limitation of current approaches is the focus on the temporary solutions of restoring myocardial blood flow after obstruction, rather than tackling the underlying disease. Coronary artery disease, caused by atherosclerosis, often results in myocardial infarction through mechanisms that emerge earlier in the progression of disease. The focus of medical care has predominantly been on the recognition of symptoms and treatment of acute events, missing opportunities for early detection and prevention of disease. Billions of dollars in health-care funding continue to be spent on identifying and managing coronary ischaemia; yet, the dominant mechanisms for myocardial infarction are atherosclerotic plaque rupture or erosion and, to a lesser extent, erupted calcified nodules that can emerge at a much earlier stage of the disease.
This Commission advocates for a shift in the conceptual framework of coronary artery disease. We suggest reclassifying the condition as atherosclerotic coronary artery disease (ACAD), moving away from the traditional emphasis on ischaemia and acute cardiac events towards a more systematic understanding of atherosclerosis. This reframing will enable the identification and management of the disease much earlier in its course, potentially saving millions of lives worldwide.
Risk of ACAD develops over a lifetime, beginning in utero, progressing through childhood and adolescence, and continuing into older age. The early stages of disease, which involve the formation of atherosclerotic plaques, are often undetected. A major shift is needed from acute event-centred care to strategies focused on early diagnosis, prevention, and management of atherosclerosis. In this new framework, ACAD should be recognised across all stages, from the earliest signs of atheroma formation to the advanced stages of disease. Our goals should not just to be to manage symptoms and events but to prevent the disease from developing in the first place and, where possible, reverse its course.
Early detection and prevention of ACAD
The prevention of ACAD must begin with early detection and modification of risk factors. If behavioural and metabolic risk factors, such as smoking, hypertension, high cholesterol, and poor diet, were eliminated or controlled early in life, the global burden of ACAD could be dramatically reduced. Eliminating these risk factors by 2050 could decrease the rate of ACAD deaths by 82·1%, potentially saving 8·7 million lives annually. Public health initiatives should emphasise lifestyle changes and the management of metabolic disorders to prevent the onset of atherosclerosis.
Early detection and effective prevention remain a challenge. The implementation of screening strategies to identify individuals at risk of developing ACAD is crucial. Targeted screening programmes, integrated into health-care systems, can detect early signs of atherosclerosis and enable timely intervention. Such interventions, if applied early, have the potential to halt, delay, or even reverse the progress of the disease, reducing the risk of cardiovascular events in later life.
Addressing the global burden of ACAD
ACAD is not a uniform problem across the globe. Disparities exist between high-income countries and low-income to middle-income countries in terms of prevention, diagnosis, and treatment. These differences contribute to variations in health outcomes. Between 2022 and 2050, mortality rates from ACAD are forecasted to increase by 19·2% in lower-middle-income countries and 4·2% in upper-middle-income countries. This global disparity underscores the importance of equitable access to prevention, diagnosis, and treatment to reduce the global burden of ACAD.
Health-care systems must be designed to prioritise prevention and early detection of ACAD rather than simply treating advanced disease. This prioritisation requires a fundamental shift in the education and training of health-care providers, with a focus on the early stages of the disease and the integration of prevention strategies into clinical practice.
A comprehensive international approach to ACAD treatment requires increased research funding, the development of novel treatments, and investment in early detection methods. The development of new therapies to prevent, reverse, and eradicate atherosclerosis is crucial. Research funding must be increased to support these efforts, particularly in the development of transformative therapies and imaging technologies that can accurately assess disease progression at all stages.
Current research is insufficient and does not match the global burden of the disease. Research is not representative of diverse populations, often neglecting the specific needs of low-income and middle-income countries. More research is needed to ensure that clinical pathways for the prevention and treatment of ACAD are adaptable and effective across all health-care settings.
The reframing of coronary artery disease as ACAD represents a crucial shift in the way we approach the disease. By recognising ACAD as a lifelong condition, from early atherosclerotic plaques to advanced disease, and shifting priority towards early detection, prevention, and treatment, the potential to save millions of lives annually is substantial. Implementing these changes will require global collaboration, increased investment, and a commitment to equitable health-care delivery. Stakeholders should work together towards the stabilisation, reversal, and ultimate elimination of ACAD, reducing the global burden of this preventable disease.
Editorial note:
The Lancet Group takes a neutral position with respect to territorial claims in any published maps and institutional affiliations.
Introduction
The history of coronary artery disease and ischaemic heart disease has been intertwined with the evolution of technology, which has led to the currently used definitions, diagnostic methods, and treatments for these conditions. The earliest known description of symptoms resembling angina dates to ancient civilisations,1 and by the 18th and 19th centuries, autopsies showed evidence of coronary arterial narrowing and occlusion in individuals who had died from heart-related symptoms.2,3 In 1912, James B Herrick made the groundbreaking observation that a patient who died after reporting angina had coronary artery occlusion at autopsy.4 Advances in diagnostic techniques gave clinicians the electrocardiogram, enabling detection of myocardial ischaemia and infarction. In the 1950s, selective coronary angiography provided a direct means to visualise coronary artery stenosis. In 1961, the first successful coronary artery bypass graft surgery was performed, and in 1977, the first coronary angioplasty took place.5,6 These advances heralded the rapid expansion of methods to detect ischaemia and therapeutics to relieve coronary obstruction. As a result, coronary artery disease research has largely focused on the diagnosis and treatment of coronary obstruction and ischaemia.
From the 1990s, key developments in non-invasive coronary anatomical imaging have shifted the focus towards detection of the underlying pathology of atheroma. By the time obstructive coronary artery disease is detected, prevention is no longer possible, and fewer therapeutic options are available. Although revascularisation appears to be an attractive solution, it provides only a temporary fix in a limited anatomical location that cannot halt the progression of systemic atherosclerosis.
As disease definitions change in response to scientific advances, the way cardiovascular disease is characterised, prevented, and managed continues to improve.7,8 Ischaemic heart disease was the term traditionally used to denote obstructive coronary artery disease, and is still used in present medical coding to denote clinically manifested coronary artery disease (chronic coronary syndrome and acute coronary syndrome) and its associated consequences. However, ischaemia in the presence of an epicardial coronary artery stenosis refers to physiologically significant coronary artery disease and is a late manifestation of the disease. Atherosclerotic coronary artery disease (ACAD) is defined as the presence of atheroma in the wall of a coronary artery, which might be present at a very early and asymptomatic stage. We propose that the definition of coronary artery disease should be reframed away from ischaemia and the late stages of disease and focus instead towards the presence of atheroma. Directing attention instead on ACAD will lead to prioritisation of strategies for early risk factor detection and modification, as well as screening aimed at prevention, early diagnosis, regression, and cure of a systemic disease with repercussions beyond the heart.
Coronary atherosclerosis develops over time, but accumulation of plaque is not linear and can be punctuated by periods of accelerated progression or abrupt instability that either resolve spontaneously or lead to an acute clinical event. Guidelines have been developed to consider distinct entities of prevention and acute and chronic coronary syndromes.7–9 Although definitions of these coronary syndromes are appealing for diagnosis, their categorical nature does not reflect the biological continuum in which ACAD exists. In addition, the detection of subclinical ACAD by screening in the absence of symptoms does not fit into our current categories of acute or chronic coronary artery disease. Current definitions are limited to end-stage disease and restrain innovation in the prevention, diagnosis, treatment, and cure of ACAD and systemic atherosclerosis. Clear delineation between symptomatic and asymptomatic people with ACAD overstates the association between symptoms and severity of disease. These definitions and guidelines need to change from dichotomous categories and towards acknowledgment of the continuous spectrum of ACAD.
Globally, health-care spending costs for cardiovascular disease in 2021 were US$1·66 trillion dollars, and are projected to increase to $2·59 trillion by 2050, assuming no change in current trends. If all behavioural and metabolic risk factors were controlled and eliminated, 82·1% of atherosclerotic heart disease deaths could be prevented and 8·7 million lives could be saved by 2050 (figure 1).10,11 Moving the current focus away from the diagnosis and treatment of the clinical consequences of coronary atherosclerosis and towards the root cause is essential to enhance the potential to reduce the global number of cardiovascular events, the personal, societal, and economic burden of the disease, and to relegate acute coronary syndrome to events that are seen as a failure of prevention rather than a consequence of the disease. Each acute coronary syndrome event should lead to an investigation of what went wrong and what can be learned for future care. In the 1980s, AIDS-defining illnesses were seen as the inevitable consequence of HIV infection. In the present day, thanks to transformative investment in scientific discovery, HIV is treated as a chronic disease that is no longer life-limiting in most patients worldwide. Imagining an analogous outcome for coronary artery disease is possible, if attention is directed towards prevention and cure rather than diagnosis and intervention at the later stages of the disease.
Figure 1: Number of deaths from ACAD from 1990 to 2021 and projection to 2050 with and without elimination of metabolic and behavioural risk factors.

Raw data were acquired from the Institute for Health Metrics and Evaluation through the Global Burden of Disease Foresight and Results tools. Data from 1990 to 2021 are based on past observations.10 Data from 2022 to 2050 are forecasted.11 The forecasted data consists of two scenarios: a reference scenario and an improved behavioural and metabolic risks scenario (with 5-year delay). In both scenarios, the risk, demographic, and environmental factors were first forecasted until 2050 and then used to regress the mortality trends. Under the improved behavioural and metabolic risks scenario, the original study assumed that: (1) metabolic risks, high adult BMI, high systolic blood pressure, high LDL-cholesterol, and high fasting plasma glucose, are linearly eliminated by 2050; (2) exposure to non-optimal diet is eliminated by 2050; (3) the number of smokers reduces linearly until reaching zero in 2050; and (4) there are no new smokers after 2022.11 For each measurement, the mean value and the 95% uncertainty interval (from percentile 2·5–97·5; shown as shaded areas) were used. Data were visualised using the Matplotlib, Seaborn, and GeoPandas packages in Python programming. No imputation and alteration of the data was performed. ACAD=atherosclerotic coronary artery disease.
Scope and aims
With this Commission, we aim to shift the focus towards ACAD, to consider the risk factors and continuum of systemic atherosclerosis, and to move away from the historical attention on late sequelae of the disease in terms of obstructive epicardial coronary stenoses and ischaemia. We identify areas of need for future research with an overarching goal of reducing the global burden of ACAD and related morbidity and mortality. We will focus on the risk factors, prevention, diagnosis, and treatment of atherosclerosis, noting that ischaemic and acute coronary syndromes in the absence of atherosclerosis are beyond the scope of this Commission.
A clear and universally agreed upon definition of ACAD that identifies the distinct stages of pathology according to the severity of cardiac and extracardiac involvement, and that considers the effect of these stages on survival is urgently needed. We need to recognise that the evolution or progression of ACAD might not necessarily occur in a sequential, linear and predictable way, and understand the potential role of therapies in disease reversal. ACAD needs to be considered along a biological spectrum and a likely manifestation of systemic atherosclerosis. Conventional terms such as primary and secondary prevention might be unhelpful and their application to individuals with subclinical atherosclerotic disease detected by non-invasive or invasive imaging is undefined. A timepoint for the end of an acute coronary syndrome and subsequent stabilised chronic coronary syndrome is arbitrary, and the utility of these distinctions is uncertain and unlikely to be helpful in reducing the global burden and impact of the disease.
We will consider this Commission to have been successful if acute coronary syndrome events are seen as a failure of upstream preventive and curative care, and become avoidable and rare because of transformative advances in care.
Mechanisms of atherosclerotic coronary artery disease
The pathogenesis of ACAD is a complex interplay of endothelial dysfunction, lipid accumulation, inflammation, and vascular smooth muscle cell proliferation. Understanding the underlying pathogenesis of ACAD is indispensable for prevention. ACAD pathogenesis can be summarised into the following stages: (1) maladaptive endothelial function and intimal thickening associated with inflammation; (2) formation of fatty streaks marked by lipid-laden macrophages termed foam cells; (3) migration and proliferation of vascular smooth muscle cells, which contribute to plaque bulk through extracellular matrix components; (4) cellular necrosis and inflammation within the core of the developing plaque; and (5) gradual encroachment upon the luminal area, or potential rupture or erosion of the plaque, stimulating thrombus formation and impaired blood flow through the vessel.12
Endothelial cellular dysfunction, characterised by impaired vasodilation, increased permeability, and altered expression of adhesion molecules, is a pivotal initial step in atherogenesis.13,14 Another key feature of incipient coronary atherosclerosis is retention of apolipoprotein B (ie, LDLs, intermediate-density lipoproteins, and VLDLs) in the vessel wall, triggering an inflammatory cascade and recruitment of leukocytes, including macrophages.15 Macrophages, via macrophage scavenger receptors, engulf oxidised LDLs, leading to the formation of foam cells—a hallmark of early coronary atherosclerosis.13 Oxidised lipoproteins can lead to foam cell death, resulting in necrotic debris and cholesterol clefts within the lesion. Lipoproteins, particularly when oxidised or glycated, also stimulate cytokine production by endothelial and vascular smooth muscle cells. Immune-mediated responses further affect the arterial wall, with cytokines and adhesion molecules amplifying the recruitment of immune cells and further promoting atherosclerosis.16 Leukocytes tend to accumulate at the shoulder regions of plaques, where plaques merge with typical vessel architecture. This leukocyte clustering is thought to contribute to the increased vulnerability of the plaque region to rupture. Proliferation and migration of vascular smooth muscle cells further contribute to plaque formation and remodelling. Over time, atherosclerotic coronary artery plaques evolve into complex structures typically characterised by a fibrous cap and lipid core, often with calcification. Vascular smooth muscle cells migrate from the media into the neointima, proliferate, and produce extracellular matrix constituents, such as collagen, proteoglycans, and procalcifying signals. The extracellular matrix proteins often compose a substantial volume of the plaque and the fibrous cap, which might vary in thickness and stability. An interactive relationship exists between inflamed perivascular fat and plaque formation.17 Mature atherosclerotic plaque is composed of a fibrous cap consisting of smooth muscle cells and extracellular matrix proteins overlying a necrotic lipid core, which includes free cholesterol, foam cells, other leukocytes (such as T cells), and necrotic debris. Plaque factors associated with increased risk of rupture include a thin fibrous cap, a large lipid core, and an abundance of inflammatory cells concentrated at the shoulder regions of the plaque. Necrotic debris within the plaque and the presence of prothrombotic tissue factor increases the risk of thrombus formation and obstruction of blood flow.
Vascular remodelling involves the restructuring of cellular or non-cellular components of the wall and can occur in response to a variety of stimuli, such as hypertension. In atherosclerosis, remodelling often consists of compensatory enlargement of the vessel to preserve luminal area. The development of endothelial dysfunction, lipid accumulation, and inflammation are driven by traditional, non-traditional, and emerging risk factors. Traditional risk factors, such as dyslipidaemia and atherogenic lipoproteins, blood pressure, smoking, obesity, and diabetes, all lead to endothelial dysfunction, lipid accumulation, and inflammation.18 Non-traditional and emerging risk factors, such as hypertensive disorders of pregnancies in women, air pollution, stress, disturbed sleep, the microbiome, and related social determinants, are also causal in atherogenesis through endothelial dysfunction and inflammation.14
Clinical manifestations of ACAD, such as angina or acute coronary syndrome, arise from narrowing of the coronary artery and any combination of plaque instability, erosion, rupture, and thrombosis, resulting in myocardial perfusion supply–demand mismatch. Once partial or complete occlusion of the coronary artery occurs, myocardial ischaemia can lead to angina or myocardial infarction. Atherosclerosis is a non-linear process, in which even mild coronary plaques can rupture, leading to acute events. The association between angina and disease severity is also not linear and more symptoms are not always associated with an increased burden of atherosclerosis. Importantly, the hallmarks of ACAD development are often observed much earlier in life than typically expected, including precursor features in children and adolescents.19 Clinical and research efforts need to shift from detection and treatment of end-stage disease to detection of risk factors and early-stage ACAD earlier in both the life course and disease course (when prevention, regression, and cure are still achievable), and on optimisation of long-term outcomes.
Global burden of atherosclerotic coronary artery disease
Current outlook
Cardiovascular disease is the leading cause of death worldwide, with ACAD being the main contributor and the focus of the modelling within this Commission (figure 2).20 Data on the prevalence and incidence of ACAD are minimal. The Global Burden of Disease reports on ischaemic heart disease (of which ACAD constitutes the vast majority) and most of the available data come from high-income countries with established systems for data reporting and collection. Between 1990 and 2019, the global prevalence of ischaemic heart disease increased from 1811 per 100 000 population to 2549 per 100 000 population.21,22 As the disease burden from infectious diseases and malnutrition declines, shifts towards cardiovascular disease are being observed in low-income and middle-income countries (figures 3, 4).23
Figure 2: Projected change in number of ACAD deaths from 2021 to 2050.
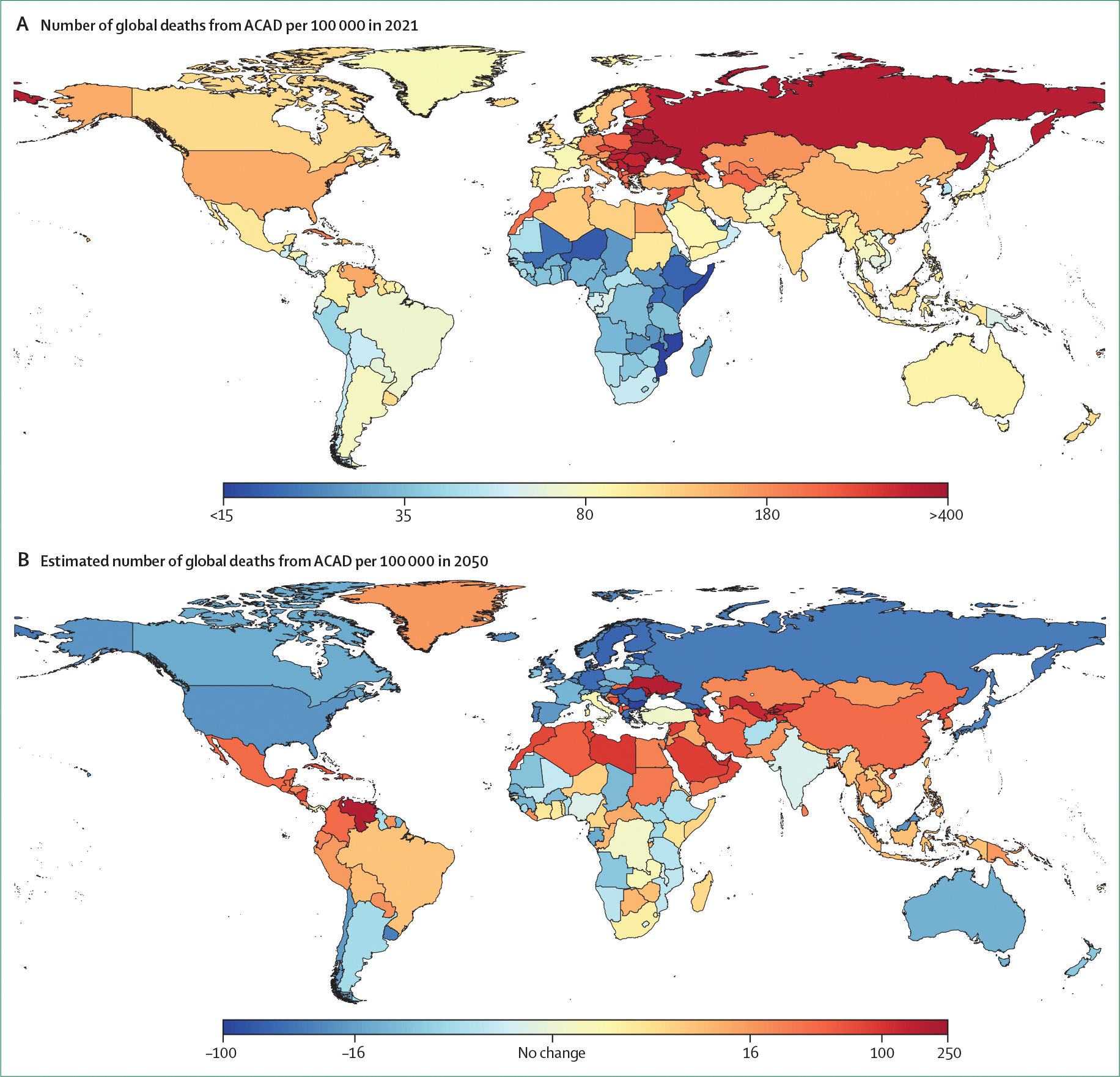
(A) Number of global deaths from ACAD per 100 000 population in 2021. (B) Projected change in global deaths from ACAD per 100 000 population from 2021 to 2050. Global Burden of Disease estimates for ischemic heart disease were used to construct choropleth maps. ACAD=atherosclerotic coronary artery disease.
Figure 3: Absolute number of deaths from ACAD by income and geographical region, current and projected to 2050.
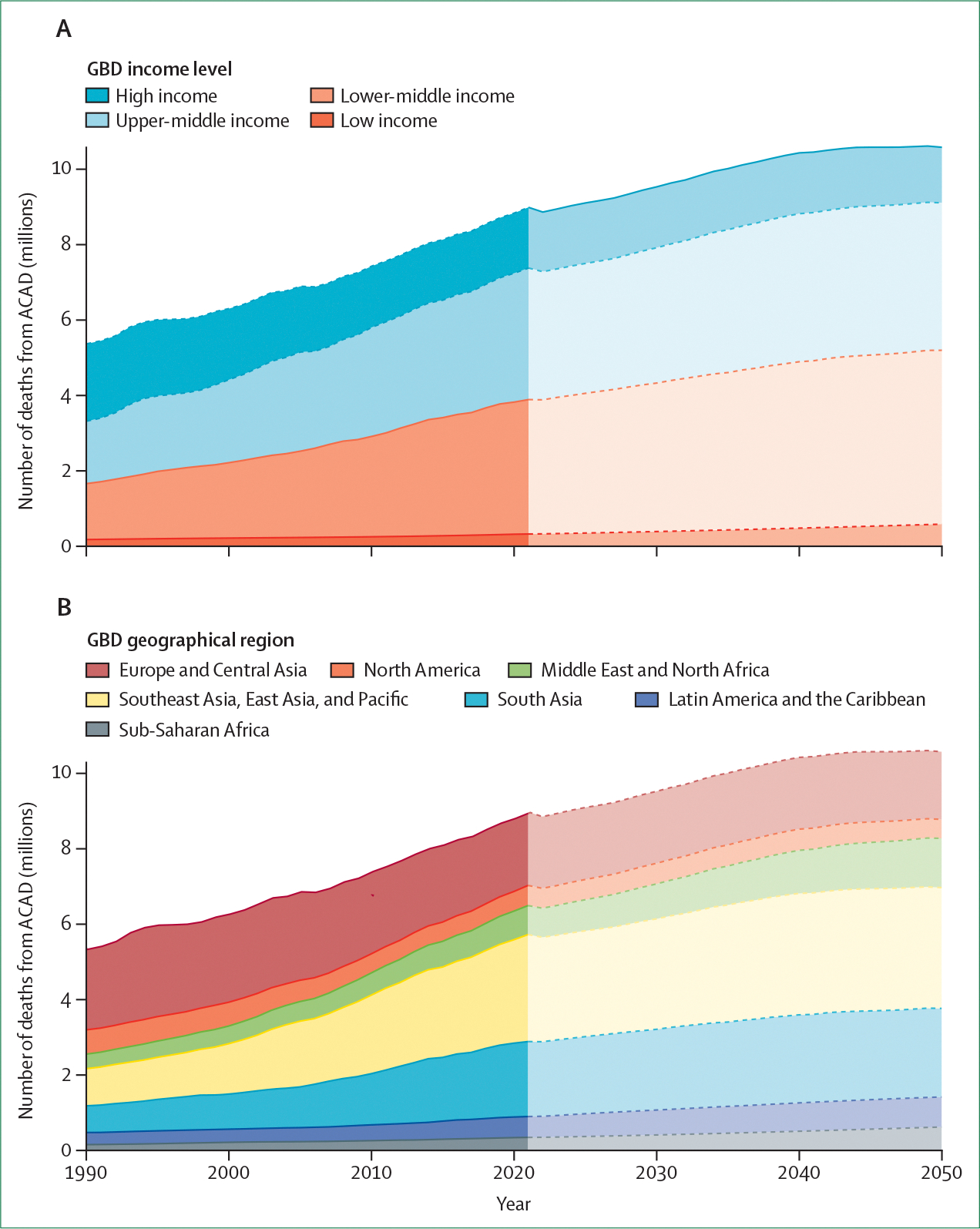
(A) Deaths by income level. (B) Deaths by geographical region. Estimates are from the GBD model and definitions. ACAD=atherosclerotic coronary artery disease. GBD=Global Burden of Disease.
Figure 4: Number of deaths from ACAD per 100 000 population by income and geographicalregion, current and projected to 2050.

(A) Deaths by income level. (B) Deaths by geographical region. Data are from GBD estimates. ACAD=atherosclerotic coronary artery disease. GBD=Global Burden of Disease.
In 2019, the highest rates of ischaemic heart disease were in eastern Europe (eg, Ukraine, Poland, and Russia). The next most affected countries were upper-middle-income countries and lower-middle-income countries (including northern Africa, the Middle East, and Asia), followed by high-income countries (Europe, USA, Canada, and Australia). Rates of ischaemic heart disease are rising in China.24 The lowest rates of ischaemic heart disease were seen in the high-income countries of Japan and South Korea. However, differences in the prevalence of risk factors across these countries are complex. Both Japan and South Korea have diets characterised by low caloric intake, low red meat intake, and high fish consumption.25 Rates of obesity, a crucial risk factor underlying both hypertension and diabetes, are low in both Japan (4·5%) and South Korea (5·9%).26 Conversely, tobacco use in both nations remains moderately high, at 16·7% in Japan and 20·8% in South Korea.27
Understanding of the differences in the prevalence and incidence of ACAD is hindered by variations in data collection, differing availability of national resources to accurately categorise cause of death, inconsistency in primary hospitalisation discharge diagnoses, and absence of a globally representative cohort or cross-sectional studies on the prevalence of common diseases. Furthermore, existing data sources might undersample specific populations, for example, women, Indigenous people, people from rural or remote areas, and other underserved population subgroups, complicating cross-national comparisons.
Age-adjusted mortality from cardiovascular disease has progressively decreased since the 1990s globally, although prevalence has largely plateaued.22 In the past 30 years, age-standardised rates of some major risk factors, such as tobacco use, decreased substantially. However, during the same period, the prevalence of other risk factors such as obesity, dyslipidaemia, diabetes, and exposure to air pollution continued to rise, offsetting the benefits of reduction in other risk factors.26,28,29 Although ACAD is increasingly seen in older populations within high-income countries, individuals presenting with ACAD in low-income countries tend to be younger and many are affected by ST-elevation myocardial infarction, which is particularly noteworthy because such cardiovascular events during the most economically productive period of adulthood in low-income settings is particularly damaging to both the individual and the country.30 The prevalence of suboptimal diet and low rates of physical activity are also increasing with the modernisation of societies. As a result of this shift in risk factors towards increased rates of obesity, diabetes, and sedentary behaviour, the global ACAD-related death rate is predicted to double by 2050 (figure 5).28 Although these data suggest rates might not increase in sub-Saharan Africa, under-recognition and limited quantity and quality of data might underestimate both current prevalence and future incidence. When coupled with population growth and ageing, there will be an inevitable rise in the absolute numbers of people with coronary artery disease globally. All countries, particularly low-income and middle-income, are at risk of societal and economic instability and conflict that can disrupt health structures and reverse progress made against preventable diseases, with substantial disparity between urban and rural areas.
Figure 5: Projected changes in absolute numbers of deaths from ACAD by geographical regions from 2021 to 2050.

Dark-coloured circles indicate number of deaths in 2021 from ACAD. Light-coloured circles indicate the projected change over time to 2050. Population size in each geographical region is represented by size of circle. Data from Global Burden of Disease models. ACAD=atherosclerotic coronary artery disease.
Data collection and reporting standards
Accurate monitoring of disease is essential to achieve health equity and improve population health.31 Globally, poor data standardisation is a major impediment to understanding and reducing the rates of ACAD. The cataloguing of accurate diagnostic information and treatment varies greatly across countries and regions, and certification errors in cause of death are common and occur worldwide.32
Helping nations and communities across the economic spectrum to develop more robust data systems to monitor ACAD is crucial to reversing the current projected trends and narrowing the observed disparities in health-care outcomes. The projected growth of preventable death from ACAD globally will jeopardise gains in life expectancy and place strain on health-care systems worldwide. Standardised data points and wider use of electronic health records and clinical registries would allow comparisons and facilitate exchange of data within and between countries. Data gathering needs to be inclusive, particularly with respect to inclusion of women and under-represented groups, which can differ between countries.
Further work is needed to improve understanding of the global burden of ACAD. This work requires addressing the current variability of data collection, data quality, availability of national resources to accurately code cause of death, and inconsistency in primary hospitalisation and discharge diagnoses. The current accepted method of disease coding involves using WHO’s ICD, which categorises diagnoses into acute ischaemic and chronic ischaemic heart disease—coding that perpetuates the focus on end-stage disease. Crucially, the condition of interest needs to be coronary atherosclerosis rather than myocardial ischemia. The proposed ICD-11 codes offer some improvement from ICD-10, with the inclusion of coronary atherosclerosis of native arteries, bypass grafts, or unspecified origin. However, the diagnostic codes are still limited by a focus on the reporting of ischaemic heart disease, under the assumption that the disease is predominantly due to coronary atherosclerosis. Reframing of ICD coding to mandate reporting of coronary atherosclerosis at stages that precede ischaemia and making a distinction between atherosclerotic and non-atherosclerotic causes of ischaemic heart disease would improve international reporting standards of ACAD and help to refocus direct attention towards atheroma and away from ischaemia. To facilitate this change, we propose updates to ICD-11 coding (table 1).
Table 1:
Current classification of ischaemic heart disease and proposed updates to promote global reporting of atherosclerotic coronary artery disease
| ICD-11 code | Definition | Proposed update | |
|---|---|---|---|
|
| |||
| Acute ischaemic heart disease | BA41 | Acute myocardial infarction is described as ST elevation myocardial infarction (BA41.0) and non-ST elevation myocardial infarction (BA41.1) | Include mechanism of myocardial infarction in coding to distinguish between atherosclerotic and non-atherosclerotic causes |
| Chronic ischaemic heart disease | BA52 | Coronary atherosclerosis of native arteries (BA52.0), bypass grafts (BA52.1 and BA52.2), or unspecified (BA5Z); subcodes for severity focused on single vessel (XS2V) versus multiple vessel (XS8U) disease; codes used to associate with angina and myocardial infarction | Recode to a new umbrella term of atherosclerotic coronary artery disease, focused on coronary artery atheroma; include codes that identify the stages of disease from early atheroma through to extensive atherosclerosis |
Risk factors
ACAD develops over the life course, with initial features of atherosclerosis evident as early as the first decade of life.19 Many factors are known to increase the risk of ACAD and can be broadly categorised as behavioural, metabolic, environmental, genetic, or related to other comorbidities (figure 6; panel 1). Although several traditional risk factors for ACAD are well recognised, the emphasis has moved towards novel, emerging, and undiscovered risk factors, which require further investigation across diverse populations and age groups.33
Figure 6: Contribution of leading risk factors to global ACAD deaths between 1990 and 2021.

Data are from Global Burden of Disease models. ACAD=atherosclerotic coronary artery disease. Dietary risk is defined as a diet low in fruits, vegetables, legumes, whole grains, fibre, nuts and seeds, seafood omega-3 fatty acids, calcium, polyunsaturated acids, and milk, as well as a diet high in red meat, processed meat, sugar-sweetened beverages, sodium, and trans fatty acids.
Panel 1: Addressing risk factors for atherosclerotic coronary artery disease.
Obesity, sedentary behaviour, poor diet, hypertension, and diabetes constitute an epidemic of risk factors that begin in early life with risk exposure even in utero. These risk factors are particularly relevant in high-income and middle-income countries and are also becoming more prevalent in low-income countries. Screening for risk factors for atherosclerosis should therefore begin earlier in life
Control of traditional risk factors continues to be crucial in reducing the burden of atherosclerotic coronary artery disease (ACAD). Global ACAD risk factor data do not capture important regional and intracountry and intercountry differences that require personalised approaches to mitigate individual ACAD risk
Identification of novel ACAD risk factors, encompassing not only biological but also social and technological factors, will provide further opportunities to improve health outcomes and to reduce disparities within and between regions
Multi-measure and multi-territory longitudinal studies are required to understand the relationship between risk factors and health outcomes from ACAD over time
The landscape of ACAD risk factors is expected to evolve considerably as demographics, ecology, lifestyle, and environment changes and technological advancements unfold. Global disparity in risk factors is a crucial public health issue that reflects the uneven distribution across different regions, populations, and socioeconomic groups. This disparity is influenced by a complex interplay of genetic, environmental, and behavioural factors, as well as factors related to health care. Understanding these differences is essential to developing targeted interventions to reduce the global burden of ACAD. The division between traditional and novel risk factors is arbitrary and dependent on the timing of discovery, rather than conveying importance or utility. Novel risk factors have, at times, been called risk enhancers, although overwhelming evidence shows that they independently increase ACAD risk.
Age
Age is a powerful risk factor for ACAD, generally considered to be non-modifiable; therefore, age tends not to be adequately studied and discussed, which means that important potential therapeutic targets might have been so far overlooked. The deleterious effects of ageing have been linked to specific maladaptive processes such as genome and epigenome instability, telomere shortening, dysregulated nutrient sensing, mitochondrial dysfunction, stem-cell exhaustion, and cellular senescence.34 This dissociation of biological from chronological ageing suggests that the ageing process might be modifiable; therefore, the associated damaging processes might be modifiable also.35 Perhaps more importantly, ageing is a measure of the duration of risk factor exposure. With enormous demographic shifts predicted, such as the number of people age 65 years or older projected to reach 2·2 billion by the late 2070s— surpassing the number of children (under age 18 years)—any age-related variance in risk factor profile (eg, hypertension) or absence thereof is amplified in the therapeutic effect of interventions.36 Given the growth of risk factors in the current young adult age group (age 18–24 years), the duration of their exposure to hypertension will be substantial by the time they reach age 65 years. For example, in certain regions, such as Zimbabwe, prevalence of hypertension is as high as 32% of young adults aged 18–24 years, with potential for long duration of exposure.37,38
Specific regions and countries are going through marked demographic shifts that are masked within the overall global numbers. For example, the proportion of older people aged 65 years or older is predicted to double between 2024 and 2054 from 17% to 33%, particularly in the group of countries that have already peaked in size, such as China, South Korea, and Hong Kong.36 This shift has considerable implications for access to health care, disease burden, and focus on preventive interventions in low-income countries. As clinical trials in ACAD have often excluded older people and have not been reflective of real-world data,39 more research is needed to establish the modifiability of age-related risk.
Sex
Biological females live longer and develop ACAD later in life compared with biological males, and there is an interaction between sex and country-specific socioeconomic status.40,41 However, these findings should not obscure the fact that ACAD is now the leading cause of death of biological females globally. ACAD risk factors specific to biological females include premature menopause, polycystic ovary syndrome, gestational diabetes, and hypertensive disorders of pregnancy.41 These factors require specific consideration and investigation of strategies for prevention and management to adequately mitigate risk of atherosclerosis.
Hormonal changes, particularly involving sex hormones, also influence the risk of ACAD. Oestrogen concentrations decline in females who are postmenopausal.41 Oestrogen has protective effects on the cardiovascular system, including vasodilation, anti-inflammatory properties, and favourable lipid profiles. Testosterone concentrations in males also have a role, with both low and excessively high concentrations being linked to increased risk of ACAD.42
Family history, genetics, and genomics
Having a closely related family member with premature ACAD is associated with a higher individual risk. Part of this association is related to rare monogenic conditions, such as familial hypercholesterolaemia; however, ACAD is more commonly associated with polygenic clustering, and is most likely to have myriad genomic and potentially epigenomic variations. Use of such information requires adequate study in diverse populations due to regional genetic and genomic variation.43 In addition, the definition of a family history of ACAD is inconsistent across studies and often inadequately reported. In the context of heterogeneous definitions, prevention guidelines highlight that family history is only incrementally predictive of ACAD risk over other risk factors.9 Conversely, smoking can increase risk of ACAD by more than five-times in older individuals.9 Emerging new approaches might be able to isolate heritable risk and improve the utility of risk estimates in clinical practice.
Smoking
Tobacco initiates and accelerates progression of ACAD and associated deaths (figure 7).44,45 The precise mechanisms are not fully understood but have been linked to nicotine, carbon monoxide, oxidant gases, and other potentially toxic components of tobacco smoke (including second hand smoke). These components, in turn, appear to negatively interact with established pathophysiological mechanisms known to initiate or accelerate atherosclerosis, such as platelet activation, endothelial dysfunction, and upregulated inflammatory pathways.
Figure 7: Leading risk factor contributions to ACAD DALYs by income level for 2021.

Data are from Global Burden of Disease models. ACAD=atherosclerotic coronary artery disease. DALYs=daily-adjusted life-years.
Although tobacco use prevalence over the past two decades has declined globally from one-third to one-quarter of adults (with similar reductions seen in males and females, although males are five-times more likely to be smokers than females),27 the effects of tobacco smoke on ACAD events will remain substantial due to the delay between exposure and events (figure 8). Tobacco use accelerates the development of ACAD and increases the risk of associated events. The risk of myocardial infarction for someone who smokes a light amount (less than 20 lifetime pack-years of smoking) returns to the same risk as someone who never smoked approximately 5 years after cessation, while it takes 15 years for those who smoked heavily (20 or more pack-years) to return to the same risk as someone who never smoked.46
Figure 8: Global prevalence of leading risk factors contributing to all causes of death between 2021 and projected to 2050.
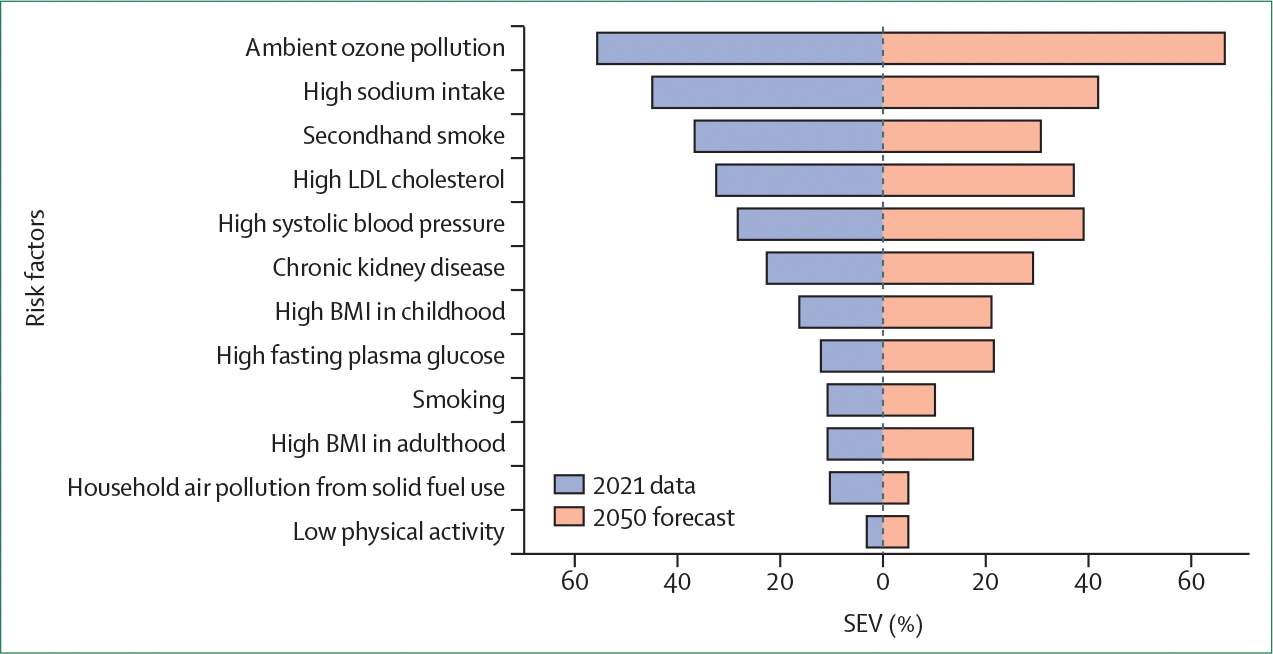
SEV is the ratio between the weighted average of the excess risks among individuals in the global population and the maximum excess risk. Data are from Global Burden of Disease models. This metric includes both the prevalence of the exposure and the extent to which such exposure would affect the disease. SEV is 0% when everyone in the population has no excess risk and 100% when everyone is at maximum risk. For a dichotomous risk factor, SEV is equivalent to the prevalence. SEV=summary exposure value.
Despite population growth, absolute numbers of tobacco users have remained relatively stable at 1·3 billion people globally, with the age-standardised tobacco use prevalence declining, on average, in all WHO regions.27 While overall tobacco smoking prevalence has decreased, the slowest decline is occurring in the Western Pacific, African, and Eastern Mediterranean WHO regions27 with increases projected in Africa and Eastern Mediterranean regions by 2030.47 In north Africa, smoking is the most prevalent modifiable risk factor for ACAD based on local registry studies.48
Sex differences are narrowest in the Americas and Europe, where the male-to-female ratio is only 2 to 1, which is appreciably different from the demographic situation in the Western Pacific (where the ratio is 17 to 1).27 Continued reductions in smoking prevalence are anticipated, caused by regulatory measures and the increase of alternative nicotine delivery systems.47 However, the long-term cardiovascular effects of these alternatives, such as e-cigarettes, remain uncertain. The growth in the use of these products, which are predominantly used by adolescents (age 10–17 years) and young adults (age 18–24 years) is of particular concern given their relatively recent emergence and consequent scarcity of longitudinal outcome studies. Public health efforts must continue to focus on reducing tobacco use globally to decrease ACAD risk.
Obesity
Obesity is a spectrum of atypical or excessive fat accumulation that presents a risk to health, is relapsing and progressive in nature, and requires continuous effort to control or improve.49 Overall, 2·5 billion adults (43%) globally in 2022 were overweight or obese. This percentage varies considerably by region, from 31% in the WHO South-East Asia and African regions to 67% in the Americas region.26 Global prevalence of overweight and obesity has more than doubled in 30 years, from 16% in 1990 to 43% in 2022, with one of eight adults currently having obesity. The number of children with obesity has quadrupled globally in the same period, with 160 million (8%) children now with obesity.26 Although the increase in obesity rates is particularly problematic in high-income countries, the shift in such demographics in other areas is just as troubling. In Africa, the number of children with obesity has increased by approximately 20% since 2000.26 Almost half of all children aged younger than 5 years with obesity currently live in Asia.26
Obesity is strongly associated with a higher risk of ACAD prevalence and death (figure 7).50 Previous concepts of the obesity paradox (ie, lower cardiovascular risk with higher BMI) are largely dispelled with the nuanced understanding that BMI is a suboptimal measure of obesity, which is more closely related to visceral fat content rather than weight.51 Furthermore, so-called metabolically healthy people with obesity have a higher risk of metabolic syndrome over time and of incident ACAD than metabolically healthy people without obesity.52
The limitations of BMI are now well recognised,51 although familiarity, cost, resources, and measurement accuracy are reasons that BMI continues to be widely used instead of waist circumference, waist–hip ratio, waist–height ratio, bioimpedance, and dual energy x-ray absorptiometry. The most useful measure for risk assessment of ACAD and whether the associations are robust across age, sex, and ethnicity is not yet clear.
The obesity–ACAD connection is underscored by countless physiological and metabolic disturbances that derive from obesity, which collectively promote the development and progression of ACAD, largely through adverse effects on other risk modifiers, although Mendelian randomisation studies suggest there might be direct effects.53 Pathophysiological mechanisms almost ubiquitous with obesity include atherogenic dyslipidaemia (such as increased triglycerides and decreased high LDL cholesterol), insulin resistance, raised blood pressure (through activation of renin–angiotensin–aldosterone and sympathetic nervous systems), and chronic inflammation (as visceral fat secretes proinflammatory and elevated atherogenic cytokines such as TNF and IL-6).54 All of these mechanisms further contribute to enhanced endothelial dysfunction, which is directly affected by adipokines55 and is a key early event in the development of atherosclerosis.
Given the global rise in obesity prevalence, addressing this modifiable risk factor is paramount to preventing and eliminating ACAD. Effective management will substantially mitigate the risk of ACAD initiation, progression, and events.
Raised blood pressure and hypertension
Raised blood pressure exerts both mechanical and neurohormonal stress on the coronary arterial wall to perturb endothelial homoeostasis, leading to initiation and acceleration of coronary atherosclerosis.56 Reducing blood pressure is an extremely effective method to reduce risk of ACAD. For every 10 mm Hg reduction in blood pressure, the risk of ACAD is reduced by 17%, irrespective of starting blood pressure.57 Raised blood pressure remains the most important risk factor for ACAD in terms of mortality and disability-adjusted life-years (figure 7).
Despite more than 70 years of randomised clinical trial evidence, proven cheap therapies, and affordable diagnostics, global hypertension control rates are abysmal.58 Current hypertension prevalence is approximately 1·5 billion59 and is projected to remain unchanged until 2040. However, this projection masks important regional variations, such as marked increases in hypertension in various countries such as Pakistan, Croatia, Trinidad and Tobago, Chad, Uganda, and Niger.60
Insulin resistance, hyperglycaemia, diabetes, and metabolic steatotic liver disease
500 million people globally have diabetes, and this number is predicted to triple to 1·3 billion by 2050, when almost half of all countries will have a prevalence of 10% or higher (caused largely by obesity).29 Diabetes is more prevalent in particular ethnicities and regions, such as in south Asia.26 Oceania, for example, has a six-times higher age-standardised diabetes prevalence compared with east Africa.29 Diabetes, insulin resistance, and hyperglycaemia lead to ACAD through distinct biochemical pathways that directly or indirectly damage the coronary vasculature and induce coronary inflammation.61 The association of hyperglycaemia with coronary atherosclerosis is continuous and has been observed well below the diagnostic thresholds for diabetes or prediabetes.62 Insulin resistance, diabetes, and obesity are further associated with metabolic dysfunction-associated steatotic liver disease, increasing in prevalence and risk of ACAD.
Dyslipidaemia
Dyslipidaemia is a well known risk factor for ACAD63 and is caused by monogenic and polygenic risk, diet, and comorbidities (such as diabetes and obesity). Hyperlipidaemia has received intense focus as a modifiable risk factor because of increased availability of drugs that alter blood lipid concentrations and reduce risk of ACAD, ACAD-related events, and deaths. LDL cholesterol accumulates in the vascular wall, causing endothelial dysfunction and promoting leukocyte influx, whereby macrophages internalise oxidised LDLs and become proinflammatory with feed-forward effects on endothelial health and oxidation of LDLs, accumulation of more inflammatory leukocyte in the subendothelial space, and generation of atherosclerotic plaque.64
Dyslipidaemia, characterised by elevated concentrations of LDL cholesterol and low concentrations of HDL cholesterol, will continue to be a crucial risk factor for ACAD. Elevated cholesterol has often been thought of as a problem for high-income countries eating a so-called Western diet. Rapid changes in dietary patterns that render this notion a fallacy have already happened.65 Over the past 40 years, no change in total or non-HDL cholesterol globally was noted. Cholesterol concentrations were highest in northern Europe in 1980; however, the concentrations are now highest in east Asia and Western Pacific regions.66
Blood lipids other than LDL and HDL cholesterol are also important. Lipoprotein(a) consists of an LDL-like particle and a specific protein called apolipoprotein(a), which distinguishes it from other lipoproteins. Elevated concentrations of lipoprotein(a) are genetically determined and vary widely, with individuals of African descent having higher values, and concentrations increasing by approximately 25% for women after menopause.67 This association is notable because the relationship between lipoprotein(a) and ACAD is established.68
Chronic kidney disease
Chronic kidney disease is a major and independent risk factor for ACAD (figure 7).69 The relationship between chronic kidney disease and ACAD is complex and multifaceted, involving a combination of traditional cardiovascular risk factors, which are over-represented in the chronic kidney disease patient population, and non-traditional risk factors unique to chronic kidney disease. This relationship is bidirectional because cardiovascular disease also contributes to the progression of chronic kidney disease. Myocardial infarction and other cardiovascular events can lead to decreased renal perfusion, further impairing kidney function—a cycle in which chronic kidney disease and ACAD exacerbate each other, leading to further adverse clinical outcomes. The progression of chronic kidney disease also exacerbates the risk of ACAD due to the interplay of metabolic, vascular, and inflammatory processes that affect the cardiovascular system. Both declining glomerular filtration rate (<60 mL/min per 1·73 m2) and increased albuminuria are independent risk factors for ACAD.70,71
In individuals with chronic kidney disease, altered mineral metabolism, chronic inflammation, oxidative stress, and endothelial dysfunction are enhanced, leading to increased ACAD risk. In particular, chronic kidney disease disrupts calcium and phosphate homoeostasis (via elevated parathyroid hormone and fibroblast growth factor-23) leading to coronary calcification, a key contributor to ACAD.69 Inflammation and oxidative stress are also heightened in chronic kidney disease, contributing to endothelial dysfunction and accelerated atherosclerosis, thereby exacerbating the risk of ACAD.
The complex relationship between chronic kidney disease and ACAD highlights the need for early intervention, comprehensive management of both renal and cardiovascular health, and lifestyle modifications to reduce the burden of these inter-related conditions.
Novel risk factors: refinement and opportunities
Inflammation
Chronic inflammation has a crucial role in the pathogenesis of ACAD. Beyond the acute inflammatory responses seen in myocardial infarction, low-grade chronic inflammation is now recognised as a distinct risk factor. Elevated concentrations of high sensitivity C-reactive protein, an acute-phase reactant, have been linked to increased risk of ACAD. Similarly, IL-6 and TNF are cytokines involved in systemic inflammation and have been implicated in atherosclerosis.
Moreover, immune system dysregulation, including autoimmunity, has been garnering increased attention. Immune cells contribute to the progression and evolving vulnerability of atherosclerotic plaques.64 Autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, are associated with higher incidences of ACAD, suggesting that autoimmune-mediated vascular inflammation might contribute to atherosclerosis. Inflammatory indices have been added to particular risk calculators (eg, QRISK-3) and some anti-inflammatory therapeutics appear to reduce ACAD outcomes in secondary prevention,72 although these therapies have not been tested in primary prevention. Beyond blood biomarkers, imaging of perivascular fat attenuation indices is now a well established marker of inflammation and is highly predictive of major adverse cardiovascular events.73,74
Hyperhomocysteinaemia
Hyperhomocysteinaemia has been identified as a potential independent risk factor for coronary atherosclerosis, primarily due to its association with endothelial dysfunction and oxidative stress.75 However, this link has been challenged by several high-quality randomised clinical trials and meta-analyses, which have shown that lowering homocysteine concentrations with folic acid and B vitamins does not reduce the risk of cardiovascular events in a northern European population.76 Despite these findings, emerging evidence suggests a stronger association between hyperhomocysteinaemia and coronary artery disease in Asian and African populations, highlighting the need for further research in diverse populations.77,78
Microbiome
The human gut microbiota, comprising trillions of microorganisms, has emerged as a novel factor influencing ACAD risk.79,80 Dysbiosis, or the imbalance of gut microbiota, can lead to the production of proatherogenic metabolites, such as trimethylamine N-oxide. Elevated trimethylamine N-oxide concentrations have been associated with increased risk of ACAD by promoting cholesterol deposition in arterial walls and enhancing inflammatory responses. Measurement of the microbiome is not straightforward or necessarily reproducible. Most studies linking microbiome to atherosclerosis have been conducted in high-income countries with diets less relevant to other regions. Leveraging this information for ACAD benefit might require the testing of probiotics to alter microbiome taxa without the need for test–retest conditions.
Physical activity
Physical inactivity and prolonged sedentary behaviour are well recognised risk factors for ACAD.81 Minimal physical activity can lead to obesity, insulin resistance, hypertension, and dyslipidaemia, all of which contribute to ACAD. Regular physical activity has been shown to improve endothelial function, reduce inflammation, and enhance lipid profiles.82 Adjunctive resistance training is further associated with fewer ACAD events and can also aid in balance and offset age-related declines in activities of daily living. Physical rehabilitation after myocardial infarction is well recognised as lifesaving, although large-scale exercise studies in primary prevention of ACAD have not been conducted.
Diet
Diet and nutrition are a cornerstone of ACAD prevention, although the optimal dietary pattern for ACAD health is yet to be defined.9 While most guidelines recommend elements or complete versions of the Dietary Approaches to Stop Hypertension, Mediterranean, or plant-based diets, the effects on the cardiovascular system of novel diets, such as palaeolithic and ketogenic, as well as intermittent fasting, are not yet fully known. The most studied dietary modification for cardiovascular health is reduction of sodium intake, largely thought to work by lowering blood pressure, which has proven benefits on vascular mortality and in reducing rates of acute coronary syndrome.83
Stress
Psychosocial factors, including chronic stress, depression, and anxiety, have been linked to ACAD.84 Chronic stress triggers the release of stress hormones like cortisol and epinephrine, which can lead to increased heart rate, hypertension, and endothelial dysfunction. Additionally, stress can lead to unhealthy behaviours such as poor diet, smoking, and physical inactivity, further exacerbating ACAD risk.
Sleep and obstructive sleep apnoea syndrome
Short and long sleep durations have been associated with increased risk of cardiovascular disease, particularly in individuals with high risk of ACAD.85 Obstructive sleep apnoea syndrome is increasingly recognised as a crucial independent risk factor for cardiovascular diseases, particularly hypertension, atrial fibrillation, heart failure, coronary atherosclerosis, and stroke. The intermittent hypoxia and sleep fragmentation inherent in obstructive sleep apnoea syndrome contribute to systemic inflammation, oxidative stress, and sympathetic nervous system activation, which accelerate the development and progression of atherosclerotic lesions in coronary arteries.86
Cancer
A previous diagnosis of cancer is associated with an increased risk of atherosclerosis. In survivors of childhood cancer (≥5-year survival from a cancer that was diagnosed before age 21 years), there is evidence of an increased future risk of ACAD-related mortality.87 The relationship between cancer and ACAD risk is complex and likely mediated by factors specific to the patient, disease, and treatment, including an increased prevalence of other modifiable risk factors for atherosclerosis and cardiotoxic effects of particular cancer treatments (eg, mediastinal radiation). Beyond this link, evidence to conclude that a previous diagnosis of cancer is an independent risk factor for ACAD is insufficient. However, given improvements in prognosis of many cancers, there are reasonable grounds for increased recognition, heightened surveillance, and early lifestyle and risk factor modification in long-term (≥5 years) cancer survivors. There is plausible evidence of shared mechanisms of atherogenesis and malignancy, centred mainly around atypical cell proliferation,88 offering the intriguing possibility of therapeutic targets that could reduce the two leading causes of global mortality.
Social determinants of health
Social determinants of health pervade all known risk factors. Access to high-quality education, the quality of the neighbourhood and environment people reside in, the support from social networks and communities, availability of quality health care, and financial stability all affect an individual’s ability to reduce their risk of ACAD. Low socioeconomic status is also associated with higher levels of stress and poor mental health, which contribute to ACAD. In Brazil, a prospective cross-sectional cohort study in a single region showed that higher rates of cardiovascular risk factors were present in Indigenous communities with a higher rate of urbanisation (based on geographical location, proximity and contact with cities, maintenance of traditional culture; and influence of the city on the group’s dynamics) compared with those with lower urbanisation, suggesting that living in cities might have a negative effect on ACAD risk.89
In many high-income countries, there are advanced health-care systems, comprehensive public health initiatives, and greater access to medical care. However, even within these countries, substantial disparities between different socioeconomic groups exist. For instance, in the USA, widely documented epidemiological analyses show that Black African American and Hispanic populations have higher rates of obesity, hypertension, and diabetes than other population subgroups. This association has been proposed to be, in part, due to inadequate access to high-quality health care and societal biases. However, even within these demographic subclassifications, there is heterogeneity with respect to the prevalence of cardiovascular risk factors. For example, within the Hispanic population in the USA, analyses of the National Health Interview Survey data by country and area of origin (Mexico, Puerto Rico, central America, South America, Cuba, and Dominican Republic) showed that the prevalence of raised blood pressure and diabetes varied by country of origin. US citizens from Puerto Rico and Dominican Republic had an almost two-fold increase of raised blood pressure prevalence of 24% and 22%, respectively, than US citizens from Mexico, with 13% prevalence.90 Research of health disparities should include representation from all Hispanic subgroups. Furthermore, the effects on health resulting from shifting immigration patterns worldwide has yet to be fully realised.
Education plays an important role in health behaviours and outcomes. Higher levels of education are associated with healthier lifestyles and better access to health care. Promoting education and health literacy can empower individuals to make informed health decisions, ultimately reducing the risk of ACAD. Investments in education and health literacy programmes are crucial for improved long-term outcomes in ACAD. In low-income countries, promotion of healthy lifestyles (eg, school programmes) is crucially absent. One of the barriers to advocacy of healthy lifestyles is the absence of belief of policy makers in their cost-effectiveness and ability to improve long-term cardiovascular outcomes.
Socioeconomic factors, access to health care, education, and digital poverty have crucial roles in cardiovascular health. Policy interventions aimed at reducing these disparities will be essential to combat ACAD in the future. These interventions include improving access to preventive care, addressing social determinants of health, and ensuring equitable distribution of health-care resources.
Over the next few decades, the integration of wearable devices (eg, smart watches), digital health platforms, and the application of artificial intelligence (AI) and machine learning could enhance our ability to monitor cardiovascular risk factors, predict future events, and personalise therapies. The application of technological advances has the potential to improve access and equity of care for patients with potential ACAD. Telemedicine gained prominence during the COVID-19 pandemic and is likely to become a mainstay in health-care delivery. This shift could improve access to cardiovascular care, especially in remote and underserved areas, potentially reducing the burden of ACAD through improved management of risk factors.
ACAD is a leading cause of life expectancy differences noted in epidemiological studies between Indigenous and non-Indigenous populations in the USA, Canada, and Australia.91 Both cardiometabolic (obesity, diabetes, dyslipidaemia, and hypertension) and lifestyle (smoking and sedentariness) risk factors are invariably reported as being of higher prevalence in Indigenous populations than in non-Indigenous populations in Australia, New Zealand, and the USA.92 Most of the literature on the health of Indigenous populations is descriptive and does not propose or implement potential solutions.93
Both people in south Asian countries (eg, Bangladesh, India, Pakistan, and Sri Lanka) and in the south Asian diaspora are observed to have a high prevalence of ACAD and, in particular, premature ACAD (usually defined as onset of cardiovascular events 10 years earlier than standard norms).94 Analysis of the UK Biobank cohort suggests that this prevalence is not explainable by existing cardiovascular risk factors used to derive cohort risk equations (eg, QRISK-3 or pooled cohort equations),95 suggesting an interplay of social determinants (eg, economic, nutritional, sociocultural, and environmental factors) and biological risk factors, including genomic and epigenomic differences. For example, for cardiovascular mortality, poor education and grip strength were observed in the global PURE epidemiological survey to have the highest population-attributable risk fraction compared with other traditional modifiable risk factors in south Asians.96 Addressing the modifiable risk factors and improving the socioeconomic status of the population are crucial to reduce the burden of these diseases. Given the high prevalence of ACAD and related premature mortality, the conceptual move from ischaemia to atheroma as a focus for prevention and diagnosis would be predicted to make further improvements with respect to disability-adjusted life-years and other related health metrics.
How best to use information on social determinants of health to refine risk estimation or management for individuals remains unknown. However, tackling barriers to access screening and diagnosis of ACAD are key to improving clinical outcomes across diverse populations. Systematic identification and coding are also essential to improving practice.
Global disparity in cardiovascular risk factors is a crucial public health issue that reflects the uneven distribution of heart disease risk across different regions, populations, and socioeconomic groups. This disparity is influenced by a complex interplay of genetic, environmental, and behavioural factors, as well as access to health care. Understanding these differences is essential to the development of targeted interventions to reduce ACAD globally. Effective global public health policies and interventions, such as smoking cessation programmes, obesity prevention campaigns, reduced dietary salt, and efforts to reduce air pollution will be fundamental in addressing the multifaceted risk factors for ACAD. However, the implementation of such policies faces numerous challenges. Limited financial resources, competing health priorities, and political instability often hinder the development and enforcement of effective public health strategies. Collaboration between governments, health-care providers, and communities will be key to the success of these initiatives. Additionally, public health policies must be adaptable to emerging challenges across diverse settings and informed by the latest scientific evidence. Through a combination of scientific innovation, technological advancement, and equitable health-care policies, the future burden of ACAD could be considerably mitigated.
Climate and pollution
Climate change can increase ACAD risk through extreme weather events, heat stress, the spread of infectious diseases, migration, and urbanisation. These factors can exacerbate existing cardiovascular conditions and contribute to new cases of ACAD.97 Adaptation and mitigation strategies will be necessary to address the health effects of climate change. Public health systems must be strengthened to respond to climate-related health challenges, and global efforts to reduce greenhouse gas emissions must be intensified.
Environmental pollutant exposure (air pollutants, fine particulate matter, and heavy metals) also contributes to the development of ACAD.98 Air pollution is a growing concern for cardiovascular health due to the magnitude of the exposed population (figure 8), and predictions of increased population at risk by 2050, particularly for ground-level ozone. Exposure to fine particulate matter (PM2·5) and ground-level ozone is linked to increased ACAD risk through diverse mechanisms such as oxidative stress leading to increased inflammation; loss of bioavailable nitric oxide leading to endothelial dysfunction; enhanced sympathetic signalling leading to raised blood pressure; and increased platelet activation.99 Microplastic and nanoplastic exposures might also be associated with increased risk of ACAD.100 Industrialisation and urbanisation in low-income countries are likely to exacerbate this issue. Efforts to mitigate pollution through cleaner technologies, stricter regulations, and global cooperation will be essential. Additionally, research into the cardiovascular effects of other environmental pollutants, such as noise and light, is needed, and appropriate mitigation strategies are required.
Future research on risk factors
Interdisciplinary research that integrates genetics, epigenetics, environmental science, and social sciences will be crucial for understanding the complex interactions between various risk factors for ACAD across the life course. Collaborative research efforts that bridge these disciplines could lead to more holistic and effective prevention and treatment strategies. Funding and support for interdisciplinary research initiatives will be important for advancing the field. Longitudinal studies that track individuals over extended periods will be essential in understanding the long-term effects of novel risk factors on ACAD. These studies can provide insights into how risk factors interact over time and inform the development of comprehensive prevention strategies. Investment in large-scale, multicentre longitudinal studies enrolling and validating across diverse populations will be crucial to advance understanding of ACAD.
Prevention
ACAD is an acquired and preventable condition. The purpose of prevention is to avoid or delay development of the disease, halt progression of existing disease, and avert acute events related to established disease (panel 2). Large-scale, multinational, case-control, and prospective cohort studies, such as the INTERHEART study, suggest that 70–80% of coronary events are attributable to a small cluster of modifiable risk factors.101 Preventive strategies for ACAD are impactful throughout the lifespan but might be more effective if instituted earlier.102 Preventive measures include overlapping health and education strategies, screening and diagnostic detection approaches, and diverse treatment targets. Current guidance considers the categories of primary and secondary prevention. Moving towards consideration of ACAD along the continuum of disease and early prevention at the primordial stage has the potential to deliver on the promise of earlier prevention and eradication of the disease.
Panel 2: Atherosclerotic coronary artery disease prevention targets.
Recognise and prioritise the role of primordial prevention of atherosclerotic coronary artery disease (ACAD)
Research the effect of screening for risk factors and starting preventive strategies earlier in life to reduce lifelong risk of ACAD
Understand the trajectory of ACAD risk factors and the initiation of preventive strategies in childhood and adolescence within populations with increased genetic and environmental risk
Establish early detection strategies using diverse tools that measure disease activity or directly visualise atherosclerosis
Develop tools incorporating multimodal data computed by appropriate predictive models, including using artificial intelligence, with a focus on early detection and longitudinal assessment of risk
Further evaluate the effectiveness of long-acting therapies, vaccines, polypills, and novel therapies on cardiovascular disease risk factors, patient adherence, and ACAD outcomes
Evaluate genetic testing and genomic-based therapies in preventing ACAD
Repurpose proven therapeutics into different populations (eg, GLP-1 receptor agonists for prevention of ACAD beyond individuals with diabetes and obesity)
In addition, clearer preventive strategies throughout the life course need to be defined. Individuals at higher baseline risk of clinical events will tend to have greater absolute reductions in risk with preventive interventions. However, more upstream preventive strategies might have more lifetime cumulative effect. Proactive primordial and primary preventive strategies might render prevention in later life less necessary. Conversely, prevention at age 65 years and older, when there is more immediate risk of ACAD-related events, might improve absolute risk reduction. A pragmatic focus on near-term risk, such as with the 10-year pooled risk calculator, obscures this exchange. However, evaluating comparative preventive strategies across the lifespan is difficult to study within prospective trials.
Major advancements in genomics are expected by 2050, which will provide deeper insights into the predisposition to ACAD. Precision medicine approaches and use of genetic information could lead to targeted and effective prevention and treatment strategies. Understanding the complex interactions between genomic factors and environmental influences will be crucial to the development of personalised interventions.
Epigenetic changes, influenced by environmental factors and lifestyle, can alter gene expression and contribute to ACAD risk. Knowledge of these modifications could lead to novel interventions aimed at reversing harmful epigenetic changes. This field is still in the early stages of development, but is promising for future ACAD management. Research into the epigenetic effects of diet, exercise, stress, and exposure to toxins will be integral to comprehensive prevention strategies.
Primordial prevention
Primordial prevention of ACAD refers to prevention of the development of risk factors (figure 9), usually achieved through lifestyle interventions. Such measures might begin at conception and continue through the lifespan. Those who reach midlife with optimal levels of all physiological risk factors and who do not smoke have very low (≤8%) remaining lifetime risk of major ACAD-related events.102 Moreover, once a risk factor develops, even with restoration of optimal levels, cardiovascular risk remains higher than in people whose risk levels were always optimal.102 For this reason, primordial prevention is an opportunity to greatly reduce the burden of ACAD.102
Figure 9: Opportunities and strategies to prevent atherosclerotic coronary artery disease over the life course.

Early life defined as age 0–3 years. Young life defined as age 4–17 years. Adult life defined as age 18 years and older. ACAD=atherosclerotic coronary artery disease.
Prenatal exposures are being increasingly recognised to predispose to increased risk of ACAD in later life.103 Gestational diabetes, hypertensive disorders, and tobacco or drug exposure are associated with development of adverse metabolic risk factors, vascular dysfunction, and worsening cardiovascular outcomes for offspring in later life.104 Maternal dyslipidaemia is associated with a higher incidence of atherosclerotic lesions during the fetal period and faster progression of these lesions in childhood (age 1–13 years), even in normocholesterolemic offspring.105 However, disentangling the contribution of concomitant social determinants, environmental exposures (eg, pollution or infection), and genetic factors that also predispose to ACAD is challenging. Moreover, the most effective strategies to control risk factors during pregnancy remain poorly understood. A comprehensive baseline assessment of ACAD risk during childhood years is much needed.
Pregnancy and childhood are major opportunities for early identification and modification of future risk of ACAD and have potential for primordial prevention by introducing lifelong healthy habits. Pregnancies complicated by diabetes or hypertension have a higher prevalence in underserved populations,106 which might reflect social determinants of health versus underlying genetic factors. However, high-income and middle-income countries currently report growing trends in childhood obesity and type 2 diabetes, largely caused by shifts in lifestyle and food consumption.26 To date, randomised trials of early childhood interventions show only modest ability to reduce rates of metabolic syndrome, hypertension, and dyslipidaemia through education and counselling, and policy-based models identify this period as high value to reduce rates of adult death and disability.107 Attention has been given to dietary factors, but the quality of contemporary research on nutrition epidemiology remains poor. The prevention and control of hypertension, obesity, diabetes, tobacco and drug use, and dyslipidaemia are high-yield targets for reducing ACAD burden. However, how to best implement these large public health interventions, evaluate their effect, and attenuate the increasing prevalence of cardiovascular risk factors is poorly understood and therefore ideally suited to hybrid studies of effectiveness and implementation.
Primary prevention
Currently, primary prevention is defined as the detection and treatment of modifiable risk factors to prevent ACAD or related complications. Primary prevention is directed at the adult population without known cardiovascular disease, but with an adverse risk factor profile that places them at moderate-to-high risk of acute atherosclerotic events in the next 5–10 years or the remainder of their lifetime. There are multiple unresolved questions regarding who should be screened for ACAD risk factors and the appropriate timing and frequency of repeated screening, given that risk levels over the lifespan are dynamic. Identifying effective primary prevention therapies is resource-intensive and clinical trials require large sample sizes and long-term follow-up. Alternative strategies aimed at detection of the course of disease progression or activity have not been defined and might provide alternative trial designs using surrogate outcomes of serial assessment.
Risk prediction tools
Assessment of ACAD risk is the foundation of primary prevention.9 However, the accuracy of current cardiovascular risk prediction scores in the young, older people, women, and individuals of diverse race and ethnicity who have been under-represented in population research is unknown.9,41,108,109 Because age is the most important predictor of cardiovascular disease risk, many risk scores are not used in individuals aged younger than 50 years and do not estimate lifetime risk.108 Many of these models were originally created and validated in small populations mostly consisting of White men aged 30–62 years from high-income countries, and therefore more research is needed to enhance accuracy across different races and ethnicities. Older predictive scores overestimate risk in contemporary populations, likely the result of better preventive therapies that are more broadly used in primary prevention populations.109 In addition, traditional risk prediction scores have variable inclusion of novel or emerging risk factors, such as physical activity, inflammation, and sleep disturbance. Data-driven techniques based on AI and machine learning trained on very large datasets that capture a wide range of types of information might have promise in improving risk prediction models by incorporating increased numbers of traditional and non-traditional risk factors.110 The incorporation of data on novel risk factors, available biomarkers, and socioeconomic factors with use of AI might enable risk prediction models to be used at a young age and enable lifetime risk prediction. However, although machine learning algorithms have been seen to outperform traditional risk scores in ACAD risk prediction, the incremental value is modest.111 Longitudinal studies are needed to validate such risk scores across populations and regions worldwide, and implementation research is needed to examine how AI models can be used for primary prevention. In the Mediterranean region, where several national cardiology societies are sister societies of the European Society of Cardiology, cardiovascular risk assessment was made possible by including these populations in widely used risk prediction scores, such as SCORE2 and SCORE2-Diabetes. The advent of smartphone risk calculators might improve access to cardiovascular risk assessment and prescription of preventive medications such as statins, ezetimibe, SGLT2 inhibitors, and GLP-1 receptor agonists.
The use of polygenic risk scores in risk prediction models has the potential to improve precision, with the aim of facilitating earlier and targeted preventive measures.112,113 Polygenic risk scores are the weighted sum of the risk conferred by multiple disease-associated single nucleotide variants across the genome. The polygenic basis of the development of cardiometabolic diseases is supported by the fact that monogenic risk variants, which are rare but confer a high risk of disease (eg, familial hypercholesterolaemia), only account for a small proportion of heritable cardiovascular disease risk in familial aggregation studies (ie, many families do not have monogenic variants and still have cardiovascular disease).112,113 Common genetic variations, present in at least 1% of the population, contribute considerably to risk.114 The polygenic basis of cardiometabolic diseases has been confirmed by genome-wide association studies. Further study into the complementary role of polygenic risk scores in risk prediction is needed.9 Identification of biological processes such as clonal haematopoiesis of indeterminate potential might also yield important future personalised measures of risk.115
Future directions for traditional risk scores and polygenic risk scores include the concept of enhanced phenotyping using a patient’s extensive medical record from birth onward, with support from large language models. Such an approach could consider risk-enhancing factors such as inflammatory conditions, pregnancy-related complications, early menopause, and social determinants. Given the availability of electronic health records (including social determinants of health, albeit with varied degrees of completeness), there is a need for research that aggregates available health data in prediction of ACAD risk.
Primary prevention strategies
The greatest missed opportunity for prevention of ACAD is effective implementation of primary prevention strategies that are known to be clinically effective. The target is to reach normal blood pressure range and blood glucose concentrations, reduce tobacco use, and treat dyslipidaemia. Several primary prevention strategies are grounded in high-level evidence from randomised controlled trials among high-risk populations, although many are not contemporary. Pooled data from randomised controlled trials on the use of antihypertensives and lipid-lowering medications provide evidence for the effect of medical therapies to reduce incident ACAD in populations at risk.116,117 Modelling studies suggest that from a cost perspective, treating all populations with a 10-year ACAD risk of 2·5% with a generic statin medication is cost-effective and reduces population-wide cardiovascular events.118 Trials evaluating aspirin have found little or no benefit in older people or people with hypertension, hyperlipidaemia, and diabetes for primary prevention and are no longer endorsed by society guidelines for this indication.9 This gap between trial-proven efficacy strategies and real-world implementation remains a major hurdle to control of risk factors across larger clinical population subsets.
Diet and lifestyle modifications are recommended by multiple international guidelines for prevention of cardiovascular disease based on substantial data linking poor diet with ACAD outcomes.9 Modelling based on data from the Global Burden of Disease study suggests that high-quality diet (ie, high intake of fruits, vegetables, nuts, legumes, wholegrains, and seafood, with low intake of red or processed meat, sugar-sweetened beverages, trans fatty acids, and sodium) has the potential to reduce death from multiple causes, and especially from coronary artery disease.119 However, few randomised controlled studies of dietary interventions for prevention have been published. The dietary intervention with the most evidence of effects on ACAD is the Mediterranean diet, but associated data are limited by issues of study design and methods.120,121 Over the next 50 years, promoting healthy eating habits and ensuring access to nutritious foods will be crucial. Public health campaigns, policy interventions, and community-based programmes can encourage dietary changes that reduce ACAD risk. Advances in nutrition science, personalised nutrition, and food technology might also offer new opportunities for improving cardiovascular health. More randomised trials assessing diet are a high priority for research given the effect of diet on multiple cardiovascular risk factors such as obesity, diabetes, and hypertension.
The interventions that are most likely to produce substantial reductions in smoking rates are population strategies such as taxation and legislative reform. Nowadays, over 70 countries have some form of smoking restriction, and several countries plan for generational restriction on tobacco.122 Pharmacotherapy for smoking cessation has not been shown to substantially decrease risk of ACAD, but can be used in combination with medical care supplemented by behavioural support.123 Clinical trials of lifestyle and nutritional interventions need to be rigorous in design and attempt to answer questions and hypotheses based on promising observational research.
Advances in understanding the biological pathways linking stress to ACAD could lead to better prevention and treatment strategies. However, addressing the societal changes needed to reduce stress levels, such as improving work–life balance and social support systems, is a complex and multifaceted undertaking. Urbanisation and the shift towards more sedentary jobs have reduced physical activity levels globally. High-income countries attempted to reduce this shift by providing better infrastructure for promoting physical activity, such as parks and recreational facilities, but many low-income and middle-income countries still do not have enough safe spaces for exercise. Public health measures to increase physical activity will need to be designed with local gender, culture, climate, and built environment issues at the forefront of these decisions.
The global obesity epidemic is expected to increase, posing a substantial risk to programmes in addressing ACAD (figure 8), with more than half of the global population predicted to have obesity by 2035.124 Addressing obesity is crucial in the prevention and management of ACAD. Strategies include lifestyle modifications (eg, behavioural therapy, dietary changes, and physical activity), pharmacotherapy, and interventional procedures. Advanced pharmacotherapies and interventional procedures proven to reduce general cardiovascular risk have limited availability, even in high-income countries. Urbanisation, sedentary jobs, and the increasing availability and normalisation of daily consumption of high-calorie, ultra-processed, low-nutrient foods contribute to rising obesity rates. Public health initiatives, policies, and education aimed at promoting physical activity and healthy eating will be crucial in combating these trends globally. Advances in behavioural interventions, weight management programmes, and increased access to pharmacological and interventional treatments might offer additional tools to address the risk of obesity-associated ACAD. Cultural and gender-related differences in attitudes to physical activity cannot be overlooked if such interventions are to work in different global settings. Studies suggest that GLP-1 receptor agonists as an obesity prevention strategy reduce cardiovascular events related to ACAD.125 There is indirect evidence for beneficial effects of naltrexone–bupropion on cardiovascular mortality.126 Other weight loss drugs show no evidence for effects on ACAD or have uncertain evidence for effects on risk factors other than diabetes.127,128 Recently developed weight loss drugs (eg, tirzepatide and retatrutide) have been proposed to decrease bodyweight by 20% or more, with multiple mechanistic avenues currently being pursued in the development of effective weight loss drugs.129,130 Expanded use of these medications to patients at lower risk with pre-diabetes should be a priority for future clinical trials. However, for these agents, issues remain in relation to sustainability of weight loss, cost-effectiveness, and the need for concomitant dietary changes (including increased protein intake) coupled with aerobic exercise and strength training.
Non-pharmacological obesity treatment (with or without total diet replacement or bariatric surgery) is not supported by evidence from randomised studies for effects on morbidity other than diabetes,128 although non-randomised data suggest an increase in life expectancy after bariatric surgery in a propensity-matched control cohort.131
Advances in health-care systems are anticipated to improve hypertension management. For instance, provision of blood pressure management and salt substitution by non-medical workers has shown to reduce blood pressure and major adverse cardiovascular events, including myocardial infarction.83,132 Innovations in decision support systems133 or use of remote monitoring could aid in better management and early detection, potentially further reducing the prevalence of hypertension-related ACAD. However, disparities in health-care access, particularly in low-income and middle-income countries, might stymie these benefits. In these countries, other issues pertaining to hypertension management include the absence of unified national screening programmes and few standardised management protocols. Access to hypertension medications is unequal between regions and dependent on socioeconomic factors. Public policies related to salt intake restriction might help to substantially improve blood pressure control.
Appropriate management of diabetes, especially using modern therapies such as SGLT2 inhibitors, appears to have a specific effect on reducing ACAD outcomes.134 Advances in diabetes management, such as artificial pancreas systems, continuous glucose monitors, and personalised medicine, might mitigate some of the risks. However, ensuring widespread adoption and equitable access to these technologies remains a challenge. Modifications towards healthy lifestyles have an important role in risk factor control, including programmes for aerobic exercise and strength training. Addressing social determinants of health and implementing comprehensive public health interventions will be essential in reducing the burden of diabetes-related ACAD.
Specific consideration should be given to the elevation of cardiovascular risk due to familial hypercholesterolaemia. Important questions and uncertainty remain about appropriateness, timing, and cutoffs for population-based screening as an alternative to cascade screening following case detection, which is reflected in global heterogeneity in public health approaches to familial hypercholesterolaemia. Beyond identification, equally broad questions remain about preventive strategies incorporating new technologies, including serial atherosclerotic imaging to assess plaque progression and regression. Only 3% of patients with familial hypercholesterolaemia reach target LDL cholesterol concentrations,135 highlighting the need for innovative interventions. Passive immunotherapy using monoclonal antibodies, targeting PCSK9 for example, are known to provide robust and long-term LDL cholesterol reduction, although these treatments require frequent repeat parenteral dosing.136 Active immunotherapy, such as vaccination, promises long-term neutralisation of disease-specific interactions (eg, PCSK9)137 or other atherogenic moieties (eg, ANGPTL3),138 although whether the technologies can avoid activation of the destructive autoreactive T-cell response yet maintain the breakdown of B-cell tolerance will require further preclinical work and careful evaluation in phase 1 trials.
Beyond the well established causal association between LDL cholesterol and risk of ACAD, there is growing interest in the role of other lipid metabolites as markers of risk and potential therapeutic targets.139 For example, lipoprotein(a), concentrations of which are genetically determined, has possible proinflammatory and prothrombotic effects that increase the risk of ACAD. Traditional lipid-reducing therapies, such as statins, are not effective in reducing lipoprotein(a). However, novel treatments, including antisense oligonucleotides and RNA interference therapies, are being developed to specifically reduce lipoprotein(a) concentrations. Whether all individuals at risk of ACAD should have lipoprotein(a) measured at least once in their lifetime for risk refinement is debated and could further contribute to health-care inequities if not managed.
The emerging importance of lipoprotein(a) highlights the need for a sophisticated approach to the evaluation of lipid profiles beyond the existing reliance on total cholesterol, LDL and HDL cholesterol, triglycerides to identify markers of risk and, where causation is identified, the potential for novel therapeutic targets.
The advent of genome-altering technology holds promise for people with genetically driven ACAD risk. CRISPR-based technology allows targeted gene base editing of disease-specific mutations in vivo and in the organ of interest, with the potential to achieve complete or near-complete prevention of disease. Natural experiments in patients with loss of function mutations in PCSK9 suggest that moderate lifelong reduction of LDL cholesterol is associated with a considerably reduced risk of ACAD.140 So-called one-and-done treatment with a liver-directed PCSK9-targeted mRNA-derived base editor to selectively edit the PCSK9 gene to a non-functional form can reduce LDL cholesterol in non-human primates by 70% after a single infusion at 15 months of follow-up.141 First-in-human studies are underway, but how long-term safety data for off-target effects can be evaluated and extrapolated for lifelong genetic editing, potentially for people at extreme longitudinal risk from early age, is ethically and scientifically complex to contemplate. Furthermore, whether there is patient acceptance of genetic manipulation or whether these types of technologies will finally obviate the adherence gap is still to be ascertained.
Secondary prevention
Patients with established ACAD have a high risk of subsequent events, including myocardial infarction, stroke, peripheral vascular disease, and death.6,142 Patients with current symptoms of ACAD, those who have had acute coronary syndrome, and those who have undergone coronary revascularisation qualify for prevention. Secondary prevention is defined as strategies aimed at preventing or delaying the onset of clinical manifestations of ACAD or reducing recurrent events in individuals with established ACAD. However, if acute coronary syndrome starts to be considered as a so-called never event and a failure of intervention, this terminology will become redundant and a misnomer. Programmes that focus on early risk stratification with advanced imaging or other laboratory markers and initiation of treatment to stop the progression of disease process are highly recommended for all. Secondary prevention interventions have well established benefits in improving survival, restoring quality of life, maintaining or improving functional capacity, and preventing further acute manifestations of ACAD.
Evidence-based interventions such as lifestyle modification, smoking cessation, control of lipid and glucose concentrations, control of blood pressure, and antiplatelet agents are of proven benefit to improve outcomes, with varying degrees of evidence for each intervention (figure 1).7–9,142
Women and men are believed to derive similar benefits from secondary prevention therapies. However, when ACAD is diagnosed, women are less likely to receive risk-reducing recommendations,33,41,190,191 and have been under-represented in prevention and revascularisation trials, which restricts the generalisability of results in practice.8,41 Therefore, the perceived absence of evidence perpetuates the underuse of established therapies.39 This perception is compounded in cross-sectionality of ethnic diversity and sex; for example, national mortality database statistics in Australia show that Aboriginal and Torres Strait Islander women have approximately double the rate of ACAD-related death than non-Indigenous Australian women.192
Improved understanding of the biological, societal, and behavioural differences driving these observations could provide insights on risk factor reduction that could improve care for both men and women. People who are transgender and gender diverse are at increased risk of ACAD events compared with people who are cisgender.193 This difference is likely multifactorial in origin and strategies to mitigate this risk, as well as clarification of contribution to risk by gender-affirming hormone use, are needed.
Implementation of preventive measures
Implementation of prevention measures known to reduce morbidity and mortality from ACAD is limited by long-term patient adherence,9 which ranges from 50% for primary prevention to 66% for secondary prevention.194 Poor medication adherence accounts for an estimated 9% of people with ACAD in Europe.195 Research on the use of polypills (ie, single solid dose formulations containing two or more medications targeting different pathophysiological mechanisms) and long-acting preventive therapies for ACAD risk factors (eg, injectables, depot injection, and antihypertensive or lipid-lowering therapies) could change the current situation for ACAD treatment and offer a more durable implementation of therapies.196 Strategies that enhance implementation across the lifespan are needed. Mobile phone-delivered interventions or wearable devices for medication adherence have yielded inconsistent results.197 The low quality of these studies creates uncertainty regarding the effectiveness and safety of such interventions, highlighting the need for further research.198 Likewise, in some low-income countries (eg, in the north African region), due to shortages in qualified medical staff, therapeutic education after acute coronary syndrome is often dispensed by the practising cardiologist or the intensivist in the cardiac care unit.
Cardiac rehabilitation is a comprehensive and multidisciplinary intervention that encompasses exercise training, counselling, education, risk factor modification, nutritional guidance, and vocational and psychosocial support.9 Prevention and rehabilitation programmes have been shown to reduce cardiovascular hospitalisations, myocardial infarction, cardiovascular mortality, and all-cause mortality after an ACAD event or myocardial revascularisation,199 and are cost-effective.200 Despite the proven benefits, rates of referral, participation, and implementation remain low, especially among women, and are not often available in low-income countries.201 Cost, intrapersonal, interpersonal, clinical, logistical, health system, and cardiac rehabilitation-related barriers have all been associated with non-participation and dropout from such programmes.202 Therefore, designing and evaluating programmes that address these factors to increase uptake is essential. A text message-based prevention programme that delivered semi-personalised text messages four times per week with advice, motivation, and information to improve diet, increase physical activity, and encourage smoking cessation (if applicable) resulted in modest improvements in LDL cholesterol concentrations and improvement in other cardiovascular disease risk factors compared with usual care.203 Mobile health delivery using smartphones for cardiac rehabilitation and heart failure management is feasible, with high rates of engagement, acceptance, usage, and adherence. This strategy was as effective as traditional centre-based cardiac rehabilitation programmes, with statistically significant improvements in quality of life.204 More research is needed to explore the widespread uptake of delivery of care via smartphones and novel wearable devices to remote and rural areas and to those with minimal financial means and cognitive impairment.
Research focused on developing vaccines to mitigate the progression of atherosclerosis and its risk factors or even curing ACAD is an optimistic approach but one that should be prioritised and not regarded as unrealistic.205
Early ACAD detection: screening strategies
A refocus from risk prediction to early detection of coronary atherosclerosis might improve our ability to individualise treatment and prevent cardiovascular events based on the key questions on who, when, and how to screen for ACAD (figure 1; panel 3).
Panel 3: Screening strategies for atherosclerotic coronary artery disease (ACAD).
Develop and test strategies for early atherosclerotic coronary artery disease (ACAD) detection through screening asymptomatic individuals
Focus on imaging to detect plaque burden and morphology, with consideration of cost-effectiveness and safety (ie, minimising radiation exposure)
Conduct research assessing long-term outcomes of early ACAD detection strategies and strengthen evidence for surrogate endpoints, such as the halting or regressing of atherosclerosis
For primary prevention, screening currently relies on risk prediction, measuring traditional risk factors integrated into a risk score upon which treatment decisions are made.9 Existing risk prediction scores are mostly derived from outdated cohorts and might not be applicable in contemporary populations, resulting in large and diverse populations that are poorly risk stratified.206 There is a need to reassess the potential value of screening with a strategy to detect ACAD. Screening strategies would ideally identify the disease early in the disease course and target individuals earlier in life, given the rising prevalence of cardiovascular disease risk factors among adults aged 18–24 years. Detection of ACAD could lead to early treatment and implementation of lifestyle-modifying behaviours to avoid progression of coronary atheroma.
The range of permutations of who, how, and when to screen for ACAD is broad, and failure of a single approach does not mean that the concept cannot succeed. In people with undiagnosed coronary atherosclerosis, diagnostic tools that detect disease at an early stage, or even precursor features before an acute coronary syndrome event, are necessary. Research using this diagnostic approach will be limited by potentially low diagnostic yield and prevalence of outcomes. For screening, defining improved outcomes at earlier ACAD stages might require adaptations from our traditional clinical endpoints to include atherosclerosis-related endpoints to measure halting of disease progression or inducing regression. Alternatively, long follow-up periods would be required to show reductions in major adverse cardiovascular outcomes. Importantly, the potential benefits of early ACAD detection must be balanced against cost and safety issues, particularly as screening is rolled out to asymptomatic individuals.
CT imaging for screening and detection of ACAD
CT imaging, including CT coronary artery calcium (CAC) scoring, has been well studied as a tool to screen for ACAD in asymptomatic individuals.207 Studies have shown208–210 that CAC scoring can enhance risk stratification, and the presence of coronary calcification has been associated with a higher risk of myocardial infarction, stroke, and cardiac death. Programmes providing CAC scoring at low or no cost to patients have been shown to increase access for women and men of diverse backgrounds and incomes.211 Moreover, incorporation of CAC scoring has improved risk factor control compared with standard risk factor assessment alone.212 However, larger randomised trial data have failed to show the efficacy of CAC scoring in reducing cardiovascular events or improving survival. One screening trial using CT CAC scoring of Danish men aged 65–74 years did not reduce all-cause death when CAC and ankle brachial index testing were combined with risk factor assessment and compared with a control group.213 Detection of early ACAD would ideally focus on non-calcified plaque or lipid pools; therefore, CAC might not be the ideal tool for early detection, with poor utility in individuals aged younger than 40 years.
Coronary CT angiography (CCTA) provides a more comprehensive assessment of the burden of both calcified and non-calcified plaque, along with measurement of vascular fat inflammation.73,214 10-year follow-up from the SCOT-HEART trial showed that CCTA-guided care improved death rates from coronary heart disease and non-fatal myocardial infarction when compared with standard care in patients with stable angina.215 This finding did not appear to be related to further revascularisation, with similar rates of invasive angiography and coronary revascularisation in the CCTA group and standard care group. More preventive therapies (ie, statins and aspirin) were initiated in patients in the CCTA group, which persisted for over 10 years. Such a strategy in asymptomatic individuals might be useful for the detection of atherosclerosis, guide appropriate preventive therapies, and potentially increase patient motivation to implement healthy lifestyle changes. By contrast, individuals without atherosclerosis could have preventive medicines deferred, with due consideration to their lifetime or genetic ACAD risk. Given our limited knowledge regarding the onset of atherosclerosis and details of progressive disease patterns, cautiously supporting concepts of reducing risk remains important. However, deferred treatment might be a consideration, with near-term re-evaluation of risk.
Technological advances in CCTA have the potential to increase its utility and value in the management of coronary atheroma. Integration of the assessment of novel imaging biomarkers, such as perivascular fat, might enhance cardiac risk prediction and restratification to personalise the application of preventive strategies.74 The additional benefit of integration of imaging-derived physiology is debatable and requires further study.216
Widespread global utilisation of CCTA must be built around an approach focused on technological advancements, cost containment, low radiation exposure, repeatability of imaging measures, and accuracy across diverse populations. Although CCTA is available in some low-income countries where it is commonly used as a non-invasive tool to rule out ACAD in tertiary care centres (eg, Morocco, Algeria, and Tunisia), CCTA is not readily available in other region, such as sub-Saharan Africa. Although the use of CCTA has grown tremendously worldwide, the implementation of high-quality imaging, which is a requirement for accurate plaque measurement and low-dose radiation practices, remains unknown. Alternative low-cost imaging approaches (eg, ultrasound) also require good image quality with high spatial resolution across varied body habitus.
Future directions in ACAD imaging: incorporation of data from routine imaging
Widespread collection of CAC could be achieved using measurements from imaging performed for other indications, such as AI-based image segmentation of routine chest CTs performed for lung screening or other indications; however, this approach would capture a high-risk population and might not ideally focus on early ACAD detection.217 Breast calcification has also been reported to correlate with CAC and provides prognostic information,218 yet is uncommonly reported on final mammography reports. Quantifying the catchment of individuals at risk based on new technology would support understanding of the potential benefits of identifying unique patient populations. For individuals identified by novel technology, systems should be in place for collection and reporting of these data and providing care guidance for preventive therapy and lifestyle changes.
Diagnosis of ACAD in symptomatic individuals
Across the ACAD continuum, we define an optimal diagnostic strategy as one that links detectable abnormalities to a comprehensive preventive and treatment strategy, which includes lifestyle changes and preventive therapies that aim to slow progression of atherosclerosis and prevent acute coronary syndrome. Across clinical settings, core diagnostic strategies for ACAD will need to adjust according to the clinical scenario, local and regional practices, and diverse populations (panel 4). In people with acute coronary syndrome, particularly myocardial infarction with coronary occlusion, early diagnosis is essential to facilitate reperfusion and reduce mortality. Prehospital and hospital health-care systems vary widely across countries, with considerable disparities observed even within the same country. Rapid advances in diagnostic methods have the potential to exacerbate health inequities and the importance of developing cost-effective and translatable approaches is paramount. AI and digital health to allow virtual emergency and electrocardiogram review might assist quicker access to reperfusion therapies.219 Given the wide variability in prehospital care worldwide and rapidly evolving technologies, we envision that wearables, mobile phone technology, telemedicine, and other approaches might help to diagnose acute coronary syndrome promptly and accurately to foster more timely care. Point-of-care testing of cardiac biomarkers220 has the potential to be performed by a much wider group of health-care providers to facilitate early diagnosis, rapid transfer, and care, and might be useful in resource-limited settings. These technologies would ideally reduce disparities by increasing access, delivery, and cost-effectiveness of acute cardiovascular care. However, research is needed to specifically target rural and remote areas with fewer health-care resources to reduce disparities in access, cost, and adverse outcomes.220,221
Panel 4: Steps to improve diagnosis of atherosclerotic coronary artery disease.
Develop strategies to foster broader access to effective diagnostic strategies for atherosclerotic coronary artery disease in resource-limited settings, including access to timely care for acute and high-risk coronary artery disease
Develop accurate diagnostic tools to detect biological processes and mechanisms (eg, plaque burden and morphology or inflammation) to predict future risk, with consideration of cost-effectiveness and safety
In the assessment of symptomatic patients with suspected or established ACAD, stress testing remains a dominant means of assessing for ischaemia. Yet the goal of diagnosing demand ischaemia focuses again on end-stage ACAD (ie, angina, left ventricular dysfunction, and myocardial ischaemia) rather than in the detection of ACAD itself.222 Widespread use of stress testing to diagnose coronary disease has, in turn, been reinforced by traditional diagnostic pathways that lead to invasive coronary angiography, resulting in the lumen being imaged with a focus on stenosis severity, not coronary atheroma burden. Despite a long-standing diagnostic focus on obstructive disease, landmark trials have not shown survival benefit with revascularisation in stable coronary artery disease,223,224 and have shown that the total burden of coronary atheroma is associated with adverse events, rather than the presence of obstructive lesions.225,226 Furthermore, the historic focus on stress testing, ischaemia, and epicardial stenoses means that the broader population with early atherosclerosis is usually missed.227 A crucial step forward will be linked to detection of atheroma itself, as well as understanding the underlying dynamic biological states of atherosclerosis and atherothrombosis. Further understanding can be achieved through diagnostic strategies that include CCTA and invasive imaging methods (eg intravascular ultrasound and optical coherence tomography) to diagnose atheroma presence, burden, and high-risk features (eg, thin cap fibroatheroma and inflammation) that correlate with adverse events.228–231 By linking diagnostic tools to the biology of plaque stability, there is potential to move beyond diagnosis to monitoring and guiding the management of people with coronary atheroma. However, such diagnostic approaches will have to overcome challenges, such as balancing imaging quality with reductions in radiation exposure, reducing the time for offline computational analyses, and the inherent risk of complications with invasive diagnostic pathways.
In addition to a shift in the diagnostic focus towards atheroma, future diagnostic strategies could incorporate other patient characteristics, such as genetic susceptibility232 and use of AI-based algorithms to estimate acute coronary syndrome probability for specific features.233 These data could be integrated with temporal information across the life course, including characterisation of symptoms and other health status measures, to reveal patterns of instability and features that lead to acute events. Once the diagnostic focus changes to detection of coronary atheroma, the overarching challenge then becomes understanding how the extent and type of ACAD can lead to better management strategies.
Therapeutics for ACAD
Key therapeutic targets
Historically, therapeutics for ACAD have had two major objectives: first, to reduce the risk of future events and improve survival, and second, to reduce symptoms and improve quality of life. The most successful therapies to increase lifespan in ACAD have been small-molecule drugs targeting the molecular substrates of atherosclerosis and its risk factors. In the coming decades, approaches using robotics, digital interventions, biological, genetic, and cell-based therapeutics have the potential to enter and transform clinical practice.
We believe that future progress in therapeutics should have three aims. First, to improve the universal implementation of underused therapies that are known to be effective. Second, to develop more intensive targetted treatment of coronary atheroma. Third, to ensure that these strategies are globally applicable. These pathways offer the hope of eradication of ACAD in our lifetime (panel 5).
Panel 5: Targeting atheroma before the end phenotype of ischaemia is established.
Examine whether, and how, some people benefit more from existing and new treatments and the efficacy of personalisation
Develop new treatments that could reverse, cure, or eradicate atherosclerosis
Identify broadly applicable and locally effective strategies to increase uptake of, and adherence to, proven treatments
Reassess benefits of established treatments to identify obsolete therapies and adapt therapies to new indications
Pharmacological interventions
Lipid-lowering agents
Overwhelming evidence139 supports a causative role of LDL cholesterol in ACAD. Reduction of LDL cholesterol with high-potency statin treatment is effective in prevention of complications of ACAD.117 Although all drugs have possible side-effects, most muscular adverse effects with statins are nocebo in nature—a fact not well known by the public and many health-care professionals.234,235 Key unresolved research topics include how statins can be provided to all who would benefit, how to reduce public mistrust and hesitancy, side-effects, and nocebo effects, and how to improve long-term adherence.236
Newer mechanisms to reduce LDL cholesterol and earlier interventions could potentially eradicate ACAD. Further reduction of LDL cholesterol to concentrations not achievable with statins alone through monoclonal antibodies or RNA-based oligonucleotide therapeutics targeting PCSK9 has additional benefits,237,238 with evidence of changes in plaques into a more quiescent phenotype.239 Because LDL-lowering follows targets set by guidelines (with different guidelines recommending different target concentrations),6,8,142 research is needed to ascertain whether LDL-lowering should be refocused towards so-called the lower the better or the earlier the better approaches, either universally or for particular groups.
Antihypertensive drugs
Blood pressure reduction is associated with a reduction in the risk of major cardiovascular events by approximately 10% per 5 mm Hg achieved, irrespective of baseline blood pressure.240 However, many knowledge and implementation gaps in this area remain. For example, although early potent lipid-lowering after myocardial infarction reduces risk, similar studies of blood pressure lowering soon after myocardial infarction have not been performed. Concerns of a possible J-shaped risk curve (with excess blood pressure-lowering associated with higher risk) need to be resolved to understand whether optimum blood pressure targets can be identified or if risk reduction lies on a continuum and the-lower-the-better approach is appropriate.
Even more important than finding the optimal treatment threshold is identifying people with high blood pressure in the first place. Blood pressure measurement is variable, heterogeneous, or absent across patient groups and geographical locations, often driven by significant clinical inequalities and treatment inertia.241 The burden of hypertension in young adults is large, and risk for women with past hypertensive disorders of pregnancy and their children is particularly high.38 Social determinants of health factors integrated within an electronic medical record across the lifespan could be used to target young individuals at risk. Further research is needed to identify the best strategies to find individuals with high blood pressure at both an individual and population level, and on how to assess and define who benefits most from treatment, and how to increase implementation of, and adherence to, treatment.
Antiplatelet and antithrombotic drugs
Many acute coronary syndrome events are ultimately triggered by platelet activation and aggregation (triggered by ruptured plaque). In primary prevention, a net benefit with aspirin has not been seen.9,242 The benefits of aspirin and P2Y12 inhibitors are well evidenced among people with established ACAD,6,8,142 with further research needed to define ideal treatment duration and combination that reduces ischaemic risk without being offset by increased bleeding events. The addition of novel anticoagulants such as factor XI inhibitors to conventional antiplatelet therapy is currently being studied.243
A definitive answer to the question of whether additional antiplatelet or anticoagulant treatment is superior in specific contexts requires head-to-head comparisons.244,245 Development of more nuanced strategies to identify both high thrombotic risk and high bleeding risk is also needed to individualise strategies. Although aspirin has been the mainstay of antiplatelet treatment, whether it should remain so warrants further evaluation; nevertheless, definitive trials might never be performed.246 Furthermore, with expanded use of CCTA, there is a melding of features deemed sufficient for coronary artery disease diagnosis, including a focus on both atherosclerotic plaque and obstructive stenosis that results in confusion on strategies for primary antiplatelet prevention, as universal treatment does not appear to be effective.247 These evolutions in diagnosis mean that targeting residual risk through antiplatelet and anticoagulant agents in ACAD will require new trials with large effect sizes and substantial costs to be clinically meaningful and cost-effective.
Glucose-lowering agents
GLP-1 receptor agonists and SGLT2 inhibitors have shown remarkable cross-profile cardiovascular risk reduction. GLP-1 receptor agonists reduce rates of cardiovascular death, non-fatal myocardial infarction, and stroke in people who have obesity or overweight with pre-existing cardiovascular disease.125 SGLT2 inhibitors improve cardiovascular outcomes in patients with heart failure, type 2 diabetes at high cardiovascular risk, and chronic kidney disease, but have not been shown to have benefit in patients early after acute myocardial infarction.248 The rising prevalence of obesity and diabetes makes research on the most effective ways to treat these conditions, and the benefits of such treatment, a high priority. However, long-term adherence, durable weight loss, and effectiveness of bariatric surgery compared with (or in addition to) these new drugs remain unclear.
Anti-inflammatory drugs
Inflammation is central to development of atherosclerosis.249,250 The presence of C-reactive protein is a marker of increased ACAD risk.251 Strategies to target inflammatory mechanisms upstream of C-reactive protein, such as IL-1β, through use of monoclonal antibodies or colchicine (which has several anti-inflammatory actions), or via anti-IL-6 mechanisms, have shown incremental benefit on ACAD outcomes.72,252,253 However, not all anti-inflammatory strategies show benefit,254,255 and hampering inflammatory responses is likely to increase the risk of infection.72 Further research on the role of anti-inflammatories, possibly targeted to aspects of plaque, perivascular fat characteristics, or patient endotype (ie, subcategorisation of patients with ACAD by demographic, clinical, genomic, proteomic, metabolomic, or imaging markers) is warranted. Novel imaging applications to target inflammatory fat depots, such as in the pericoronary adipose tissue,73 might prove useful for targeted anti-inflammatory treatment in selected patients.
Antianginal medications
The use of many antianginal medications is supported by placebo-controlled evidence.256,257 However, most evidence on antianginal drugs is old, usually derived from small numbers in non-diverse participants, and using endpoints that might not reflect health measures that are meaningful to patients (such as time to ST depression on exercise). Whether adding a second antianginal drug alone or in combination with a first-line medication is superior in improving symptoms and quality of life in people with symptomatic ACAD remains unclear.
The effect of therapies intended to reduce symptoms and improve quality of life should be tested on the endpoints that are meaningful to contemporary patients. Inclusion of patient-reported outcome measures and digital technologies able to frequently and longitudinally capture quality-of-life metrics alongside other meaningful aspects of health (such as physical activity) in clinical studies and routine health care could improve ability to understand the benefits of these therapies. Efforts to better personalise therapies to identify those likely to benefit could provide greater clinical benefit and reduce the need for more expensive and high-risk revascularisation. Current pharmacological approaches for the management of ACAD, alongside challenges and potential research needs, are summarised in table 2.
Table 2:
Current pharmacological treatments, challenges, and research needs for atherosclerotic coronary artery disease
| Specific drugs | Challenges | Future research goals | |
|---|---|---|---|
|
| |||
| Lipid-lowering agents | Statins, ezetimibe, bempedoic acid, and PCSK9 inhibitors (eg, evolocumab, alirocumab, inclisiran) | Poor implementation or up-titration in eligible people; public mistrust and suspicion regarding benefits and side-effects; low adherence rates; unclear optimal timing, duration, or indications for deprescribing | Investigate the ideal therapeutic goals according to risk groups; develop strategies to build public trust and understanding; develop strategies to reduce nocebo and side-effects |
| Blood-pressure lowering agents | ACE-I, ARB, calcium-channel blockers, β blockers, diuretics | Large proportion of population globally at risk with undiagnosed or untreated hypertension; little evidence investigating low blood pressure targets in acute coronary syndrome | Establish treatment targets for blood pressure in the early phase after acute coronary syndrome; define strategies to improve detection of hypertension on a broad scale |
| Antianginals | Nitrates (short-acting for acute angina or long-acting for angina prophylaxis), β blockers; calcium-channel blockers (non-dihydropyridine agents such as verapamil or diltiazem), dihydropyridine agents (eg, long-acting nifedipine and amlodipine), ivabradine; nicorandil, ranolazine, trimetazidine | Previous evidence of efficacy often based on surrogate and non-patient centred outcomes; variable treatment response; clinically significant side-effects; unclear efficacy of single therapy versus combined therapy; scarce evidence on strategies to personalise treatment | Develop new approaches to targeted antianginal medication and strategies based on endotype of disease and patient characteristics |
| Antiplatelets and antithrombotics | Antiplatelets and antithrombotics (administered for pretreatment, peri-interventional, postinterventional, and maintenance treatment); low-dose aspirin, oral and intravenous P2Y12 inhibitors (eg, Ticagrelor, prasugrel and cangrelor), glycoprotein IIb-IIIa antagonists (eg abciximab and tirofiban), direct oral anticoagulants (eg, rivaroxaban), factor XIa inhibitors | High risk of bleeding; variation in treatment response for some agents; uncertainty around which patient groups benefit most from which treatment combination or duration | Individualise choice and duration of dual vs single antiplatelet therapy according to ischaemic and bleeding risk; individualise platelet function and genetic polymorphisms; role of using antithrombin (eg, factor XIa inhibitor) in addition to antiplatelet therapy after acute coronary syndrome |
| β blockers | Cardio-selective β blockers (eg, carvedilol, bisoprolol, and extended-release metoprolol), non-selective (eg, propanolol) | Contemporary trials show limited benefit in patient groups without impaired LVEF; side-effect profile (eg, fatigue, bradycardia, hypotension, and worsening heart failure when decompensated) | Ascertain improvement in quality of life, exercise, and angina in stable patients with ACAD; identify which subgroups benefit after myocardial infarction or stable ACAD, with preserved LVEF; identify ways to reduce side-effects |
| RAAS inhibitors | ACE-I, ARB, sacubitril–valsartan | ACE-I and ARB commonly used after myocardial infarction and in coronary artery disease management; limited data in context of evolving contemporary medical therapy; minimal outcome data in preserved LVEF after myocardial infarction; no additional improvement in cardiovascular outcomes with new agents (sacubitril–valsartan) vs ACE-I | Investigate whether RAAS blockers reduce cardiovascular events in patients with coronary artery disease and typical left ventricular function; investigate benefit in context of contemporary coronary artery disease therapy |
| Glucose-lowering agents | SGLT2 inhibitors, GLP-1 receptor agonists | Polypharmacy with associated challenges in adherence | Identify further indications for use in different groups |
| Anti-inflammatory agents | Colchicine, allopurinol IL-6 inhibitors, monoclonal antibody IL-1β (canakinumab) | Minimal data on risk reduction; side-effect profile and infection risk | Measure outcome data; quantify frequency and define side-effect profile |
ACAD=atherosclerotic coronary artery disease. ACE-I=angiotensin converting enzyme inhibitor. ARB=angiotensin II receptor blockers. LVEF=left ventricular ejection fraction. RAAS=renin-angiotensin–aldosterone system.
Surgical and device interventions
Whether revascularisation reduces future risk and improves prognosis, and in which subgroups, remains intensely debated. Although outdated registries suggested a survival benefit of coronary artery bypass graft surgery (CABG) over medical therapy in patients with multivessel disease,258 left main coronary artery lesions,259,260 and left ventricular dysfunction,261 most of this evidence preceded contemporary, intensive secondary prevention and current optimal medical treatment, making benefits in the current era uncertain. Since 2007, randomised controlled trials have shown that revascularisation with percutaneous coronary intervention (PCI) does not reduce mortality or other major adverse cardiovascular events when compared to optimal medical therapy alone in patients with stable coronary artery disease with preserved left ventricular ejection fraction and without left main coronary artery disease.222,223,262–264 These findings were consistent across subgroups including those with diabetes, multivessel disease, moderate-to-severe ischaemia, or when PCI was guided by fractional flow reserve. Data on outcomes from surgical registries are quasi-absent and many practitioners prefer PCI over CABG in patients with diabetes or with severe left ventricular dysfunction because CABG is associated with greater procedural risk and morbidity.265
Revascularisation has an updisputed central role in improving outcomes and mortality in acute coronary syndrome. In non-ST elevation myocardial infarction, a routine invasive approach reduces the composite endpoints of death, recurrent myocardial infarction, and rehospitalisation for ischaemia.266 In ST-elevation myocardial infarction, primary PCI reduces mortality, myocardial infarction, stroke, and bleeding when compared with thrombolysis.267 Complete revascularisation with multivessel PCI reduces cardiovascular death or reinfarction in acute coronary syndrome without cardiogenic shock;268 however, the anatomical or physiological definition of complete revascularisation itself is unclear. The role and optimal timing of PCI in different groups (such as cardiogenic shock, out-of-hospital arrest, or bleeding) remains incompletely defined. Recognising that optimal therapies might vary between regions and health-care systems, there is still a need to establish the best possible strategies, such as pharmacoinvasive strategies with thrombolytics and immediate primary PCI in regions where rapid primary PCI is not available.269
Crucial gaps in evidence exist for specific populations at high risk of ACAD and require focused research (table 3). Advances in invasive and non-invasive imaging and molecular approaches to measure genomic, proteomic, metabolomic, and other plaque or clinical features might allow a better understanding of when revascularisation is appropriate to reduce future risk.
Table 3:
Challenges and opportunities for future research in revascularisation for specific subgroups
| Challenges | Future research goals | ||
|---|---|---|---|
|
| |||
| Patients with diabetes | Diffuse and extensive coronary artery disease; high mortality and repeat revascularisation rates | Ascertain the possible synergy between drugs and medical devices in diabetes | |
| Older patients | Comorbidities; increased risk of bleeding complications and periprocedural events (for both surgical and percutaneous revascularisation); complex lesions (eg, bifurcations, calcifications, and tortuosity) | Perform more studies of revascularisation specifically recruiting older people and inclusive of patients who are frail and at high risk, with multiple comorbidities | |
| Patients with chronic kidney disease | High rates of complex and calcified plaques; increased risk of acute kidney injury and bleeding | Perform research on acute kidney injury minimisation strategies; improved tools for management of calcified plaques; increase recruitment of people with kidney disease into clinical trials | |
| Patients with left ventricular dysfunction | Role of percutaneous coronary intervention remains uncertain; uncertainties exist regarding the most suitable method to assess myocardial viability and whether myocardial viability can predict outcomes with revascularisation | Perform randomised studies with standardised viability assessment and quantification; develop new tools to assess viability; perform studies assessing the role of percutaneous coronary intervention with physiology and imaging guidance, studies defining the optimal role of ventricular support devices during revascularisation, and studies defining the prognostic importance of complete anatomical or functional revascularisation | |
Symptoms and quality of life
Data on the effect of revascularisation on symptoms and quality of life comes largely from unblinded clinical trials and clinical experience.270,271 However, the overall effect of therapy on a subjective endpoint, such as symptoms, is composed of both the true physical effect and the placebo component.272 Interventional procedures increase the placebo effect273 resulting in unblinded effect sizes that are often far larger than in blinded studies.274 The first placebo-controlled blinded trial found the efficacy of PCI for improving symptoms was far smaller than expected.275,276
There is a paucity of data on the benefit of CABG for symptom relief. Data from the unblinded ISCHEMIA trial showed angina reduction with CABG or PCI when compared with a conservative approach.224 In the absence of a clear reduction in mortality and myocardial infarction rates with PCI, guidelines recommend PCI primarily for symptom relief.277 Importantly, many patients remain symptomatic when taking at least two antianginal medications. However, the placebo-controlled ORBITA-2 trial showed that PCI was most effective as an antianginal monotherapy.278 There is a possibility that guidelines suggesting that PCI is only offered to those with symptoms refractory to optimal antianginal medication select those with the least to gain. Many patients remain symptomatic after PCI, therefore more research is needed on how to target revascularisation to those who are most likely to benefit and to investigate causes and treatments for patients with post-PCI angina. Importantly, symptom characteristics themselves might help to stratify who is most likely to benefit from PCI.279,280
Cardiac symptoms can be difficult to evaluate, especially because they are assessed in various health-care settings by numerous health-care professionals. Each time a patient is asked to report their symptoms, the nature of questioning can influence their answers. Symptom reporting is highly dependent on multple factors on multiple factors including culture, language, physical functioning, socioeconomic status, and environment. There are substantial limitations in the tools used to evaluate symptoms in clinical practice and clinical research. Interpatient and intrapatient variability, as well as recall and reporting bias, are some of the major limitations of the current patient-reported outcome measures used to evaluate symptoms.281–283 Novel tools for the evaluation of angina might address some of these confines.284 Data are needed to investigate and improve these tools because the efficacy of an antianginal therapy can only be adequately evaluated when the best methods to predictably and reproducibly study symptoms are understood.
Other therapies are being evaluated to reduce angina and improve quality of life. In addition to drug-eluting stents, drug-coated balloons, and bioresorbable scaffolds,285,286 devices to reduce coronary sinus blood flow287,288 have shown promise in placebo-controlled angina improvement. Enhanced external counter pulsation,289 extracorporeal shockwave myocardial revascularisation,290 transmyocardial laser revascularisation,291 and cell therapies292,293 have all been tested for the treatment of angina, but the benefits are uncertain, and there are challenges such as poor availability, time investment, costs, and logistical difficulties. These therapies must be evaluated rigorously with placebo-control to test angina relief before widely entering clinical practice.
Failure of ischaemia-guided approaches
Ischaemia-guided approaches are inadequate, with no clear role established for revascularisation in improving prognosis in stable coronary artery disease. This should lead to a change in the framework of how such invasive and costly procedures, with associated procedural short-term and long-term risk, are used. Similarly true for non-invasive stress testing for detection of ischaemia, the benefit of revascularisation in acute coronary syndrome, but not stable disease, shows that plaque phenotype rather than anatomy and degree of stenosis can identify groups that benefit, and the substrate of the culprit plaque (eg, rupture vs erosion) might be related to prognosis with potential therapeutic implications.294,295
Targeted therapeutics for atherosclerotic plaque
Coronary atheroma burden is a stronger predictor of adverse cardiovascular outcomes than the presence of ischaemic coronary stenoses.225,226 Non-obstructive atherosclerotic lesions can destabilise and lead to acute coronary syndrome despite optimal medical therapy or revascularisation of culprit and non-culprit clinically significant lesions.228,229,296,297 Thus, therapeutic interventions targeted at obstructive ischaemia-producing lesions, rather than the overall atherosclerotic burden, can leave vulnerable and biologically active plaque untreated. Advancements in non-invasive and intravascular imaging have allowed the identification of high-risk plaque features, such as a thin fibrous cap and a bulky necrotic core, which are associated with increased rates of acute coronary syndrome despite being functionally insignificant.230 Patients undergoing CCTA are decidedly at a lower risk than patients undergoing invasive coronary angiography and this lower risk might hinder the ability of CCTA to predict future acute coronary syndromes.231,233 Furthermore, CT imaging of atherosclerotic plaque might be difficult in secondary prevention, such as in patients with previous revascularisation in which coronary metallic stents can impede plaque quantification using CCTA.226 Moreover, current understanding of the mechanisms of atherosclerosis progression, including variable timing across patient populations of varied risk levels, remains poor and must be improved to halt or regress ACAD.
The evaluation of high-risk plaque might be most important in patients with multivessel coronary artery disease presenting with an acute coronary syndrome. In this population, the PROSPECT study using intravascular ultrasound showed that major adverse cardiovascular events at follow-up could occur in both infarct-related and non-infarct-related arteries.296 High-risk characteristics, such as a plaque burden of 70% or more, minimal lumen area 4·0 mm2 or less, or thin-cap fibroatheroma, were predictors of potential plaque destabilisation. Similarly, the CLIMA study (using optical coherence tomography)297 and the PROSPECT II study (using a combination of intravascular ultrasound and near-infrared spectroscopy)298 substantiated the value of these techniques in identifying high-risk plaques. These studies underscore the predictive value of imaging features such as lipid-rich plaques and large plaque burden in assessing future risk. Therapies such as high-intensity statins, PCSK9 inhibitors, and anti-inflammatory agents can reduce plaque burden and stabilise high-risk plaques, offering a chance for intensive plaque-centric management strategies, rather than ischaemic-centric.299 Theoretically, PCI might seal and stabilise vulnerable plaques, potentially reducing the risk of acute coronary events. PCI also offers advantages such as no systemic side-effects (beyond dual antiplatelet therapy-related bleeding), reduced procedure duration, and concentrated costs rather than prolonged expenditure over time. However, the effect of PCI remains confined to the treated lesion, and there is a risk of stent-related complications. Preventive PCI has been studied and centres around the criteria used to diagnose and treat vulnerable plaque.300 Future work is needed to establish a clinical outcome benefit that outweighs the risks of an invasive procedure, and further studies should aim to identify the optimal intervention technique among drug-eluting stents, drug-eluting balloons, and bioresorbable vascular scaffold.301 Although intravascular imaging can identify high-risk features with a very high negative predictive value (96–100%) for adverse events up to 5 years, only a small percentage (4–25%) of these high-risk plaques results in acute events,302 restricting the usefulness of imaging-guided interventions for such lesions. Additionally, this approach requires three-vessel invasive intravascular imaging for screening of high-risk atherosclerotic segments and does not capture the dynamic natural history of ACAD or the changes in activity and quiescence of atherosclerosis over the lifespan. Such an approach would need to ideally combine plaque characteristics with patient characteristics to identify at-risk lesions in patients who are also at heightened risk.
Implementation of therapeutics
Available treatments for coronary artery disease are more numerous than many health-care systems can afford, and this fact will inevitably affect most, if not all, nations as more new treatments are added. Identifying both broadly applicable and locally effective strategies to increase uptake of, and adherence to, new treatments is necessary. Decades of successful discovery mean the focus should shift towards implementing what is already known to be effective and maximising efficient use of existing therapies. The greatest immediate value will be gained by understanding how to better implement treatments that are known to be effective by identification of implementation strategies that increase use of proven therapies for ACAD, and designing better trials to test our ability to increase the healthy lifespan of people with ACAD. Attention should be targeted in parallel to new drug and device discovery.
Strategies to increase the use of and adherence to treatment that could work across different regions would be of much value. Geographically relevant implementation research, including active patient engagement, should be prioritised alongside matched governmental and health-care system policies co-formulated at the same scale and pace. For high-income countries, these strategies might be a matter of prioritisation. For low-income and middle-income countries, considerable additional investment and the influence of non-governmental organisations (eg, WHO) might be required to enact change.
Clinical trials must include diverse participants from different groups and countries. The use of existing treatments consumes a huge amount of health-care resources; yet, the available data, particularly in acute coronary syndrome treatment, comes predominantly from White males of an average age of 62 years, with under-representation of the elderly, females, and non-White participants.39 As a result, whether such treatments are equally effective for these other groups remains unclear.39 These historically understudied groups tend to have more comorbidities and associated symptoms, are diagnosed later, and have suboptimal patterns of medical therapy use.29,190,191,303 Underrepresentation in revascularisation and therapeutics trials of these groups must be addressed to ensure that therapies are used in the most effective way in groups that are proven to benefit.
Patients with ACAD have been recruited to randomised controlled trials in low-income and middle-income countries; however, hindrances to conducting such trials include reduced access to guideline-directed pharmacological and invasive therapies, minimal personnel (including academic staff to conduct research), ill-equipped facilities, or insufficient willingness of patients to participate in medical research. As a result, most randomised controlled trials are conducted in high-income countries and yield results that are not always generalisable. Geographical differences in treatment effects should be considered during the decision-making process and be a subject of dedicated research.
Examining whether and how some people benefit more from existing and new treatments is also of paramount importance. Decades of progress have led to a wide range of pharmacological and interventional options to address the symptomatic and prognostic effects of ACAD. Such therapeutics have necessarily been applied to broad populations both in clinical studies and routine practice. The success of such approaches is clear, but less evidence exists to guide more precise targeting of existing (or new) therapeutics to those who are most likely to benefit and those who are least likely to have negative consequences. Together, advances in imaging, omics, data science, and AI might allow for the identification of different disease endotypes.
Future interventions
The innovations with the greatest potential to reverse, cure, or eradicate ACAD might not have been discovered yet. The rapid growth of basic science and technological advancements in recent decades is astounding, could not have been predicted, and allows us to ask the question: which new discovery will change the way we prevent, treat, and cure ACAD?
With increasing technological efficiency in resourced settings, the possibility of biologically or individually targeted therapies to reverse or prevent ACAD is becoming realistic. Opportunities include detailed phenotypic characterisation of developing disease, providing information that might be harnessed to personalise therapy with a goal of deflecting the disease course. Characterisation of individual cellular, subcellular, or extracellular characteristics might also assist in determining optimised or personalised treatment (including polypill) combinations. Opportunities exist for development of proteomic-determined and genomic-determined therapies; therapies that use gene therapy or editing; RNA technologies; and cell-based therapies for the prevention, reversal, and cure of ACAD. Regenerative and nanotechnologies to include cell, tissue, and organoid engineering, as well as development and application of novel extracellular matrices, might be applied in the future as both preventive and reparative therapies. Importantly, implementation of novel therapies should be considered at different life stages, from prenatal to late-life interventions. All therapies must be accompanied by attendant emphasis on ethical considerations, short-term and long-term safety, and outcomes.
Additionally, innovative digital interventions, therapeutics, and strategies to influence individual behaviours to reduce the risk of ACAD require ongoing research. Developments in robotics might extend care delivery and improve clinical outcomes. Innovative transformational discovery research requires sizeable financial and human resource investment from sectors such as governmental, philanthropic, or industry. Translation of basic observations to translational therapies is possible when researchers, funders, and stakeholders align.
Drug re-evaluation and repurposing
More therapeutic options exist now than at any time in history, and more expensive strategies are in development. This constant innovation raises the question of when the therapeutic effect of existing therapies should be re-examined, in what trial design (eg, routine registries, randomised controlled trials, or pragmatic studies), and of how to disinvest in treatments that are no longer appropriate. For example, β blockers have been widely used after myocardial infarction and for angina for 50 years; however, their benefit is uncertain in the era of reperfusion without left ventricular dysfunction and is being re-evaluated in several randomised controlled trials (eg, NCT03278509, NCT03646357, NCT03778554, NCT03596385, and NCT03498066).
There are substantial barriers to such re-evaluation of existing therapies, particularly the cost and the ethical challenge of conducting a study in which some groups do not receive a therapy considered beneficial. A framework is needed to establish the circumstances in which re-evaluation of an existing treatment is warranted. This framework should consider aspects such as strength of previous evidence, previous size of benefit, size of population affected, cost of existing treatment, and scientific rationale for why previous benefits can no longer be assumed.
We hope in the coming decades that this Commission will encourage research to better understand how to provide therapies reliably so that the event reductions from trials can be replicated in clinical practice across all regions and populations. We also seek to build on advances in the identification of mechanistic pathways, ultimately leading to prenatal and primordial strategies that could prevent, reverse, and potentially eradicate ACAD. We are fortunate as patients, clinicians, and researchers to live in an era that follows more than five decades of high-quality and high-volume cardiovascular therapeutic innovation and trials. When effective risk reduction strategies are implemented in a rigorous randomised controlled trial setting, residual major adverse event risk after myocardial infarction can be reduced to an astonishingly low residual risk of approximately 3% per annum, and mortality to 1% per annum.238 However, that progress raises the bar for new preventive therapies. In a value-based framework, new therapies are judged based on incremental absolute magnitude of benefit relative to cost. Because absolute risk after myocardial infarction is already low, additional relative reduction in risk of new prevention interventions will have to be increasingly high in trials to produce absolute reductions in risk that are large enough to have favourable benefit–cost ratios and improve outcomes.304
Additionally, despite low adverse event rates after myocardial infarction in trials, both short-term and medium-term risk of recurrent ischaemic events remains high in real-world practice.305 This discrepancy further highlights the need for development and validation strategies to implement what is established in a trial setting as effective. This type of research, focused on inclusive implementation strategies, can coexist with other types of investigation, translating the identification of new cardiometabolic and lipid targets into validated upstream preventive strategies that could improve on optimised, well delivered current therapies. This combination of investigative approaches would allow both short-term improvement in outcomes to trial mortality and recurrent event estimates while pursuing the attainable goal of eradicating ACAD.
Precision medicine strategies
The value of precision medicine stems from the existence of heterogeneity in treatment effects (ie, patient-by-treatment interactions, meaning that some people respond to treatment better than others) of a magnitude that justifies personalisation of care.306 Some monogenic traits provide a clear case for genetic approaches to precision medicine. For ACAD, a relevant example is familial hypercholesterolaemia, for which genetic testing to guide therapeutic choices is recommended by expert panels. For example, PCSK9 inhibition may be targeted to patients with gain-of-function mutations who have the most to gain from the therapy.307
For complex chronic traits, as are most risk factors for ACAD, the situation is quite different. Establishing heterogeneity of treatment effect typically requires separating interperson from intraperson variance in treatment response in the outcome, which necessitates study designs that can separate treatment effects from time-period effects, and continuous outcome variables that can be measured repeatedly. Proper trial designs to identify the potential of precision medicine in treating complex traits have very rarely been used.
Hypertension would provide an optimal scenario for precision medicine, with several different first-line drug classes having similar preventive effect on a group level, but with unknown heterogeneity of treatment effect. This question was addressed using a novel randomised, double-blind, repeated crossover trial design,308 which is uniquely suited to explore heterogeneity of treatment effect because it allows both adequate determination of intraperson variance in the outcome and can separate treatment effects from time-period effects. Substantial heterogeneity of treatment effect was observed, indicating a potential for personalised monotherapy for hypertension,308 raising hopes of further use of this trial design.
If patient-by-treatment interactions cannot be studied, some information can be gained from studies of subgroup-by-treatment interactions. The most useful interaction analyses are usually those with subgroups based on absolute risk of the outcome, and such interactions will always be present on either the multiplicative scale, additive scale, or both.116 With similar effects of preventive drugs across different levels of absolute risk, the most important predictor of absolute treatment benefit will be absolute risk (as shown for blood pressure-lowering and lipid-lowering treatment)116 and risk-based preventive treatment—hence a key precision medicine strategy.
To substantially impact ACAD, more research is needed to find the right uses for precision medicine. A genetic test that can motivate adoption of a specific (new and costly) drug is often more popular than a genetic test that discourages use of a specific (old and cheap) drug. Even for clear cases, such as the well known actionable pharmacogenetic interactions for clopidogrel (CYP2C19; poor or intermediate metabolisers have reduced clopidogrel effect)309 or statins (SLCO1B1; poor function leads to increased risk of statin-induced myopathy),310 the enthusiasm for genetic testing is minimal. Steering precision medicine research to the most essential applications from a population-effect perspective is key.
Research and implementation
Research challenges and targets
Shifting care from late stages towards earlier stages of ACAD will require strategic alterations in core elements of effective research programmes to attain the ultimate goal of reducing the worldwide burden of disease. Evidence is unfolding regarding overlapping mechanisms involved in ACAD and related diseases, such that the emerging cadre of researchers will need to be varied and include those unaccustomed to the new focus on early ACAD detection and treatment. Medical training across research disciplines (eg, nephrology, neurology, and endocrinology) will be essential to facilitate understanding of the effect of age and comorbidity on progression of ACAD.
Research approaches
Comprehensive ACAD research strategies should incorporate not only randomised controlled trials but also document real-world implementation. Major hurdles, such as ensuring site capacity for diverse recruitment and full adherence to study protocols, will need to be overcome as the focus shifts towards early ACAD strategies. Use of standardised data points for ACAD research across clinical trials and registries will facilitate validation of major findings and potential pooling of data to define the heterogeneity of treatment effects.
Elements of pragmatism across ACAD research might improve clinical applicability and reduce research inefficiency. Countries such as Sweden, Denmark, and Norway have achieved notable success with national cardiovascular registries.33,311 By seamlessly linking patient data across health-care sectors, these registries enable efficient monitoring and systematic follow-up over extended periods, supporting research that improves patient care, making them a model of effective and sustainable research.
A focus on intervention for younger individuals (eg, age 18–39 years) will necessitate long-term follow-up from randomised trials or registries to enhance linkage to major adverse cardiac outcomes over time, as well as progression of atherosclerosis. Similarly, after imaging of ACAD, long-term follow-up will be necessary to study prognostic patterns of major adverse cardiac events. In a strategy of early ACAD detection, radiologists and other cardiovascular imaging professionals might be required to formulate a pathway of imaging-guided care. Varied ACAD mechanisms exist that might be targeted, and each unique or combined diagnostic and therapeutic approach will necessitate not only comprehensive and tailored protocols, but also follow-up designed to formulate precise links to the long-term consequences of ACAD. Safety assessments will be a crucial component of early intervention focusing on avoidance of inappropriate follow-up testing and intervention, as well as procedural complications outside of guideline-directed care.
ACAD research should aid in the development of individualised care and formulate comprehensive and lifelong strategies of care to optimise patient health throughout stages of atherosclerosis. Such approaches require reducing the inefficient silos of health care, which are detrimental to the optimisation of patient health and wellbeing. New diagnostic approaches will require seamless integration within a management strategy, enhancing adherence and surveillance of progressive ACAD and systemic atherosclerotic conditions and complications. Research strategies must not only focus on near-term changes in management but also devise adherence strategies to optimise long-term effectiveness.
Endpoint criteria and safety endpoints
Detecting early ACAD requires varied trial designs with substantially long-term follow-up or incorporation of surrogate (or intermediate) endpoints. Increased trial duration is required to establish links with major adverse cardiac events. Although the use of combined endpoints can reduce the required sample size, component endpoints should be chosen carefully to target progressive mechanistic states of ACAD and safety issues that are failures within the strategy of care.
Additionally, therapies exerting effects across ACAD mechanisms might require incorporation of various blood or imaging biomarkers to target adiposity, inflammatory measures, or plaque subgroups (notably non-calcified plaque burden), and can be used for surveillance. Tracking surrogate endpoints, such as cholesterol concentrations or blood pressure levels, are already established to show risk factor control. Atherosclerosis is a progressive disease for which novel interventions focused on halting or regressing ACAD could be supported by serial imaging techniques. Any surrogate endpoint must have an established causal relationship with future major adverse cardiac events.
Safety endpoints should be thoroughly integrated and statistically powered within trials. Many novel therapies have considerable side-effects that detract from long-term adherence. Drug development should focus on clinical care approaches to offset negative side-effects. Research studies should include sufficient resources for tracking of any safety related endpoint.
Global uptake of evidence-based therapies for ACAD is inadequate, and long-term medication adherence is worse. Suboptimal adherence is responsible for approximately 10% of major adverse cardiovascular events.194,195 Patients with ACAD are anticipated to require extended or lifelong treatment, which can be undesirable to the patient and therefore contribute to low long-term adherence. Strategies must be developed to augment adherence, especially over an extended duration of treatment.
Democratisation and inclusion
A foundational principle to reducing the population burden of ACAD is diverse representation across key subsets of women and men, inclusive of demographic or socioeconomic factors, within research. Deliberate efforts to remove barriers to clinical trial enrolment are needed to improve diversity (panel 6). Any therapeutic or diagnostic test should ensure enrolment of a racially and ethnically diverse population that is representative of clinical and population cohorts. Therapeutic trials targeting a specific or varied ACAD mechanisms might improve enrolment by enriching eligibility criteria with high-risk blood or imaging biomarkers.
Panel 6: Improving diversity of participants in clinical trials.
Barriers to representation
Difficulty in accessing study sites
Familial or caregiver responsibilities
Cultural or language barriers, screen failure, and not being considered for screening
Socioeconomic barriers
Participant concerns about safety
Limited inclusion criteria
Poor data collection on relevant non-cardiac history
Strategies to improve representation
Establish research sites in locations where potential participants already receive health care; provide language assistance for individuals with limited language proficiency; make reasonable modifications for persons with disabilities; and use remote (telephone or online) recruitment processes and study designs
Reduce burdens due to trial and study participation (eg, number or frequency of study-related procedures or visits, use of local laboratory and imaging, and telehealth)
Develop a diverse pool of investigators and staff, sustained community engagement, and screening protocols that promote unbiased screening; and promote communication adjusted to patient needs, enhancing transparency and trust
Improve access by providing assistance with issues such as transportation
Ensure prevalence-adjusted representation of individuals in cardiovascular clinical trials across relevant age or other categories
Consider important previous factors from early life (eg, pre-eclampsia, endometriosis, or polycystic ovary syndrome)
Effective implementation strategies
Given the transformative approach proposed for early ACAD detection and treatment, implementation strategies will be a key aspect of research. Optimisation of low-cost established strategies must be compared with any proposed novel and generally more expensive health-care options. Locally relevant implementation research should be prioritised—what works in one country or area might not work in another. Alongside this geographical consideration, coordinated governmental and health-care system policies guided by quality evidence and population needs must be coformulated for action on a large scale and in a timely way (figure 12).
Figure 12: Recommended actions to lower mortality and morbidity from atherosclerotic coronary artery disease.

Optimising low-cost care, resource efficiency, and cost-effectiveness
Numerous preventive therapies are under development or have been approved within the past 5 years with sizable costs that are unaffordable to many patient populations and across low-income and middle-income countries. Any novel therapy or diagnostic tool must have a proven value linked to improve population health that is balanced by any heightened cost and must also be evaluated in relation to low-cost options and show an acceptable value–cost ratio. Alternative low-cost strategies should be the comparator in resource-constricted settings. Further development of polypill strategies might improve outcomes199 and should continue to be validated across diverse settings.
Artificial intelligence
Many recent clinical advances in automated diagnoses, electronic health record phenotyping, symptom evaluation and tracking, and focused operational efficiencies are based on AI technology. Additional machine learning and AI tools might be able to improve cardiovascular workforce efficiency by offloading administrative tasks related to scheduling, clinical documentation, and billing. Within the field of screening and diagnosis, tools might be developed for earlier detection and triage of ACAD, from subclinical to catastrophic acute events. There are many other promising areas where AI might prove useful, such as in image quantification, which might allow for combined cancer and ACAD screening. Deep learning algorithms for image quantification might also allow for measurement of surrounding tissues (ie, adipose depots) or use novel radiomic signals for tissue phenotyping.
A wide range of potential machine learning or AI solutions might improve the reliability and diagnostic accuracy of current clinician-based interpretation and decision making. Performance of machine learning or other AI predictive models will need to be inclusive of diverse risk markers and require extensive external validation. Model performance using machine learning algorithms has not been reported to improve performance relative to traditional statistical methods.312 Importantly, the safety of AI-based solutions that triage, automate, or integrate ACAD parameters must be rigorously tested with ongoing surveillance and reporting of poor performance that might place patients at heightened risk. Public dissemination of AI tools using large language models for deep learning algorithms that can interact and provide reasonable answers to a vast array of questions are key to dissemination of effective ACAD detection and treatment strategies.
AI development should focus on providing low-cost solutions for efficient and effective care of patients at risk of ACAD. For example, tracking of outcomes might be achieved with minimal expense through smartphones (which are affordable in many low-income countries) and also wearable devices to enhance the efficiency of ACAD research.313,314 In some cases, implementation of AI technology might be easier than anticipated across resource-constricted settings, and benefit from technology or corporate partnership. Although AI has transformative potential in ACAD, many important unresolved questions remain in terms of accuracy in external validation, cost, and transparency, especially with regards to missing elements within predictive models. Human involvement should form a key component of any research strategy employing AI-based techniques.
Funding
Any new method of early ACAD intervention must be accompanied with sufficient financial support that ensures success and optimised population risk reduction. Research funding for cardiovascular disease does not match the worldwide burden of disease, and funding from governmental organisations tends to be insufficient and unduly difficult to access. The projected and increasing prevalence of risk factors315 and concomitant ACAD event risk will require nimble responses from funding organisations that are appropriate to meet the needs of patient populations at risk. We hope that this Commission will be foundational in supporting a new generation of research targeting early ACAD detection and treatment. Treating later stage ACAD has not altered the patient cohort at risk. An interventional pathway for early ACAD allows the envision of care that will have greater effect in reducing the burden of future acute coronary syndrome, heart failure, sudden cardiac death, or the need for symptom-based medical or surgical intervention. Funding should be allocated to meet the potential for sudden transformation of the burden of ACAD and improving the health and wellbeing of populations at risk around the world.
Health-care systems
For this Commission to meet its aim of reducing the global burden of ACAD, attention should be turned to the systems by which health care is delivered. Health-care services research is focused on improving the delivery and receipt of evidence-based care (panel 7). Training and provision of health care is biased towards the diagnosis and treatment of the end-stages of the disease, rather than prevention and early detection. Failures to implement effective therapy for atherosclerosis are well described in high-income counties, such as the USA, where approximately one-third of patients recommended for lipid-lowering therapy do not receive it; this inequity of access to treatment is even greater in patients from poorer backgrounds.316 In New Zealand, less than one-third of patients admitted to hospitals with acute coronary syndrome receive echocardiography.317 In Australia, fewer than four of five survivors of acute coronary syndrome receive 75% or more of recommended secondary prevention medications, with disparities between male and female patients.318 Even when these medications are prescribed, discontinuation rates are unacceptably high.319,320 Poor adherence to recommended therapies and process measures mean that disparities are even greater within countries where fewer clinical trials are performed.
Panel 7: Key targets for research.
Health services research in atherosclerotic coronary artery disease (ACAD) is required to improve health outcomes by increasing the delivery of existing clinically effective therapies
The cost-effectiveness of clinical interventions needs to be identified to inform real-world prices and encourage appropriate allocation of scarce resources
Collection methods of population health data should be applied to health-care systems to allow modelling for resilience testing
Research into strategies and policies should be funded to increase the numbers of women and under-represented ethnic and racial groups in the ACAD workforce
Although differences in outcomes are often linked to socioeconomic factors, in some instances, wealthy populations have worse ACAD outcomes.321 For example, 30-day mortality after acute myocardial infarction is roughly 50% higher in the USA than in Canada.322 These discrepancies have many causes but are also partly related to systems of health-care delivery. Recognition of these variations, coupled with focused efforts by health-care services to increase implementation of evidence-based, clinically effective interventions for ACAD in health-care systems and care delivery has the potential to drastically improve outcomes and reduce disparities among populations.
Key problems and targets for health-care delivery
There is tremendous potential to improve ACAD health outcomes by simply improving the delivery of existing clinically effective therapies. The use of quality indicators helps to improve health-care delivery.323 Traditional physician office-based care is inferior to both community-based care and remote methods of managing hypertension and hyperlipidaemia in patients for primary and secondary prevention.324 Despite the proven efficacy of hypertension, hyperlipidaemia, and antiplatelet therapy for people with ACAD, substantial evidence shows that current health-care systems are inadequate to deliver ACAD treatments effectively. Equitable access, advances to address variability of image quality, and diagnostic accuracy of ACAD imaging will be of crucial importance, with increased emphasis on earlier detection of coronary atheroma. Financial investment to improve access and technological advances in imaging to enhance accuracy and efficiency will be needed to ensure that imaging-guided care and early intervention is a realistic global prospect.
In most cases, implementation of a novel approach to improve ACAD outcomes at a population level meets the definition of complex interventions according to multiple criteria.325 Investment into complex intervention research in the field of ACAD is urgently required to broaden the perspective beyond the unbiased estimates derived from randomised controlled trials of clinical efficacy. Methods to assess whether an intervention is implementable, cost-effective, and adaptable to be upscaled and applied in different contexts will need to be incorporated (panel 8). Emerging methods of implementation science that have appropriate theoretical frameworks can importantly identify barriers and enablers to efficacy, scalability, and sustainability.326–328 Although randomised controlled trials might be required, important information regarding implementation can be derived from a broader range of study designs including observational, quasi-experimental, and hybrid designs simultaneously incorporating measures of both implementation and clinical efficacy.329
Panel 8: Evaluation of health-care delivery for atherosclerotic coronary artery disease.
Gaps in delivery of health care for atherosclerotic coronary artery disease (ACAD)
Worse ACAD outcomes of unclear cause
Low adherence to performance metrics
Scarce resources misallocated to low value therapies
Treatments misallocated to patients at low risk
Primary prevention risk scores not correctly indicating risk in different populations
Health-care workforce insufficiently prepared to address care gaps, such as control of ACAD risk factors
Overdependence on traditional care models
Minimal ACAD workforce at the primary care level
Poor allocation of health-care workers in diverse settings
Suggested research methods to address gaps
Inductive qualitative methods
Pragmatic trials of health-care delivery mechanisms (eg, nudges) to increase adherence; and pragmatic trials of alternative health-care delivery mechanisms as alternatives to traditional care (eg, home hospital, blood pressure checks in barbershops, and electronic consults)
Decision analysis and cost-effectiveness simulations that use both effect estimates and costs that apply to local settings, including low-income and middle-income countries
Observational comparative effectiveness research using large observational datasets adequately powered for heterogeneous treatment effects
Trials of educational interventions to serve vulnerable populations
Inclusive transnational clinical registries that reflect a full range of diverse populations in different countries, including low-income and middle-income countries
Care delivery in resource-limited settings
All health-care service delivery operates in a setting of constrained resources and no single society has the capacity to cater to all the health-care needs of every individual. This difficulty is increasing due to a growing and ageing population with increased comorbidity and high expectations of health care, coupled with rapid resource-intensive technological advances in diagnostics and therapeutics. Variable governmental investments in healthcare (often a reduction in funding) are also frequently observed and result in inadequacies across populations at risk of ACAD.
In that context, health-care services aligning to maximise value and deliver the best outcome in return for each unit of investment is imperative. More than 20% of health-care service delivery is estimated to be wasteful, returning no improvement in outcome, or even causing harm.330 Within a constrained system, every individual service provided has an opportunity cost. Prices and real-world effectiveness of drugs and devices likely differ in different countries; therefore, cost-effectiveness analyses developed in some countries might not apply in other settings.
Measurement of the value of interventions through carefully conducted health economic evaluations is an important step in prioritisation of health-care services. Rather than an effort to reduce costs, appreciation of value stimulates innovation and facilitates research investment to promote the use of novel effective interventions, which is of particular importance for ACAD (for which the clinical benefits of some interventions are uncertain, clinical equipoise persists, and the benefits and harms of any treatment or diagnostic test are multiplied at scale due to the large burden of disease). Applying an entirely utilitarian approach to the provision of health-care interventions is unlikely to be possible because that would require knowing the cost-effectiveness of each individual intervention. Nevertheless, health economic evaluation is of particular importance when the clinical benefits of an intervention are uncertain and clinical equipoise persists.
Outcomes that facilitate health economic evaluation (eg, validated measures of health-related quality of life) should be routinely incorporated into clinical trials. Recognising that cost, health priorities, and conventional measures of value (such as willingness-to-pay thresholds) vary between communities is crucial. Economic models being published in a way that facilitates translation to other health-care settings through transparent presentation of results and provision of access to shared repositories of patient-level data is imperative. Failure to include these outcomes or present results in this manner is a considerable waste of research efforts.
Patient-reported quality-of-life measures are susceptible to bias in open-label trials, which is a problem in studies of novel procedures and devices or established therapies with perceived efficacy. Although placebo-controlled studies with blinding are challenging, they have the potential to provide important data, especially where clinical equipoise persists.
The role of clinical practice guidelines in reframing ACAD
Clinical practice guidelines from authoritative organisations (eg, the American College of Cardiology, the American Heart Association, the European Society of Cardiology and various national associations and governments) summarise available evidence to support clinical decision making and wider policy. Such guidelines are powerful instruments to initiate change, and the groups devising them will be essential to reach the target of a shift in focus from ischaemia to atherosclerosis.
Although clinical guidelines often limit their scope to individual clinician–patient decisions without consideration of the broader health-care service, the guidelines have the capacity to systematically lead clinicians away from low-value treatments. The move towards including health economic evaluation into clinical guidelines should be encouraged more broadly.331 Guidelines summarise which diagnostic and therapeutic approaches are known to be effective; however, given the importance of implementation science, guidelines should also explain how best to increase uptake of existing effective strategies for atherosclerosis. We recommend that all guidelines emphasise that prevention, reversal, or cure should be the aim of the treatment of atherosclerosis.
A shift from ischaemia to prevention of atherosclerosis requires not only changes in individual management but also changes in health-care systems. The provision of early invasive treatment of both non-ST elevation myocardial infarction and ST elevation myocardial infarction is an example of how both individual clinicians and health-care systems are able to change in response to overwhelming necessity.332 In North America and western Europe, ST-elevation myocardial infarction mortality is as low as 2% of patients.333 Primary PCI utilisation for ST-elevation myocardial infarction reperfusion in Sweden is among the highest worldwide (>90% of patients).33 Such successful implementation of treatment to different models of care could be used as a template for underperforming regions or health-care providers.334 Despite the undeniable success of the network of specialist centres delivering high-quality invasive care for late-stage atherosclerosis, willingness to embrace further transition towards better prevention and ultimately reduced need for such invasive approaches will be essential for success. To reduce the burden of and events from atherosclerosis, many of the essential interventions will be delivered in outpatient settings. As such, understanding how to improve uptake and adherence to outpatient medications will be crucial to risk factor control.
Workforce
Effective workforce delivery
ACAD care requires intensive involvement of health-care personnel. Bias towards management of late-stage ACAD has promoted investment in these services, such as coronary care units and acute coronary syndrome and cardiogenic shock networks. These services remain essential and require greater investment to address regional and global inequalities. However, a greater focus on prevention, early detection, reducing progression of ACAD, and occurrence of ACAD events will require more balanced investment within community and ambulatory care.
Although evaluation of new therapies follows well tested and regulated processes, predictable workforce changes are often implemented too late and without evidence or subsequent evaluation. Furthermore, global economic pressures and a health-care sector weakened by the COVID-19 pandemic are causing unrest and fractious relationships between health-care professionals and their employers. Thus, the future cardiovascular medicine workforce faces huge challenges. With a shift to focus on ACAD, delivering effective prediction and prevention early in the life course, while also expanding capacity to provide complex care to an ageing population with comorbidities, is necessary. This change necessitates an increased workforce in primary prevention, able to deliver interventions and prevention at large scale, and to accommodate new therapeutic options and pathways—all within budgetary constraints across varying economic environments. The growth in the global burden of modifiable risk factors for ACAD mandate that existing office-based medical care models of risk factor management need to be disrupted for benefits to be appreciated across large populations.
We define the coronary artery disease workforce as all health-care providers whose role includes predicting, preventing, diagnosing, or treating ACAD. Comprehensive data on the numbers and types of health-care providers in the coronary artery disease workforce are challenging to find. Some professional bodies publish data on the number of cardiologists, but these data only include a proportion of the coronary artery disease workforce. Separating data on primary care physicians, nurses, allied health clinicians, radiographers, and other health-care providers into those who contribute to coronary artery disease care and those who do not, is impossible. What is clear from the little data available is that there are wide geographical variations in health-care providers delivering current coronary artery disease care.335 To research effectiveness of different workforce models, data on care delivery and patient outcomes must be collected, as well as on job satisfaction, job retainment, and the cost of different models of care. Such data are the necessary first step to future research that can support understanding of how to optimise the ACAD workforce across different settings.
Most health-care systems (especially in high-income countries) need patients to attend health-care facilities in person. This attendance requires large numbers of people to travel to a vast health-care structure, which creates substantial environmental impacts and disadvantages for some groups of people, including those who are older, from low-income backgrounds, and from rural areas. Health care must ideally be provided closer to patients, which requires upskilling and resourcing of community-based teams and evaluation of new ways of delivering care. Involvement of other health-care and non-health-care professionals, non-telemedicine digital health delivery, chatbots, and other approaches is essential.324 The WHO HEARTS global programme outlined directives for team-based care, with identification of tasks that could be shared or shifted to non-physician health-care providers, and is important to the provision of care in all levels of resource settings.336 Research that specifically tests new ways of delivering care to improve clinical and patient-reported outcomes and their effectiveness in diverse settings is required. For example, the HOPE-4 randomised controlled trial tested a model of care using non-physician health-care workers alongside primary care physicians and family within 30 communities in Colombia and Malaysia.337 The intervention resulted in reduced cardiovascular risk, absolute reductions in systolic blood pressure and LDL cholesterol, and exemplified the type of workforce-delivered care that can be tested through different research methods in diverse economic settings.
Although better community care is urgently needed, expansion of specialty care is increasing due to innovation in genetics, pharmacotherapy, medical devices, and imaging technologies. Traditional physician-delivered care limits the number of patients a single clinician can manage. Given that no single health professional can support all elements of coronary artery disease care, ACAD teams that provide integrated community and specialist care are required. New ways of training the coronary artery disease workforce on assistive technologies offer promise of improved efficiencies in delivery and cost. Virtual simulators, remote proctoring, and robotics might allow for more efficient skill development while reducing the proportion of routine care delivered by non-specialists. The effect of assistive technologies on patient outcomes and workforce efficiency requires study. The COVID-19 pandemic forced a rapid transition to hybrid delivery of education and training, which future research might focus on for the long-term training of the coronary artery disease workforce.
Workforce diversity
A diverse workforce with representative proportions of gender, socioeconomic class, and racial and ethnic groups leads to improved organisational performance, patient satisfaction, and patient outcomes.338,339 Despite these facts, cardiology has the lowest percentage of female staff of any medical specialty.340,341 Although under-representation of females in cardiology has been extensively documented, there are minimal global data reported on racial, ethnic, and cultural diversity. In the USA, self-reported data of ethnicity and race from numerous professional medical organisations shows low representation of diverse subgroups and Indigenous people.341 Barriers to workforce diversity include inflexible training or compensatory measures for parental or career responsibilities, conscious and unconscious bias, and gender-based or race-based discrimination.340,342,343 A non-diverse cardiology workforce perpetuates the under-representation of particular groups in clinical trials and health disparities seen in women and some racial and ethnic groups, thus narrowing trial generalisability. Addressing workforce diversity is essential in improving the overall quality of cardiovascular care. Strategies and policies designed to increase cardiology workforce diversity should be implemented and tested with transparent availability of data.
The ACAD workforce in high-income settings frequently attracts health-care professionals originally from, and often trained in, low-income settings. This migration of a skilled workforce calls for root-cause evaluation and context-specific approaches to job retention. Without further research, addressing the inequalities of care will be challenging.
Health policy and public education
Population-wide health policy and education have been modelled as high-value interventions to prevent and reduce ACAD burden on a large scale, with the potential to influence lifestyle behaviours such as diet, physical activity, and tobacco use.107,344 These longitudinal interventions are important in managing ACAD as a preventable chronic illness and reducing resultant morbidity and mortality. The combination of policy implementation alongside educational campaigns has been highly effective. Much can be learned, and potentially reproduced, from the substantial decline in tobacco use that followed the introduction of impactful policies (eg, smoke-free laws, health warnings on packaging, restricting youth access, and taxation policies) combined with mass media anti-smoking education campaigns.345 Similar policies and public education strategies should be expanded to other ACAD risk factors (panel 9). For example, better city planning policy to promote bike and walking paths could align with widespread public health messaging targeting sedentary behaviours.
Panel 9: Health policy and public education goals.
Public education needs to target the prevention of atherosclerotic coronary artery disease (ACAD) from during pregnancy, childhood, adolescence, and throughout the life course
Governments should prioritise funding of public health strategies for ACAD and a healthy built environment
Policy is needed to combat the global epidemic of obesity, diabetes, hypertension, and poor diet
In nearly all countries and regions, there is a documented epidemic of obesity, hypertension, and diabetes10 largely related to poor diet, inactivity, and an obesogenic environment. Metabolic risk begins early in life, with overweight and obesity affecting 390 million children and adolescents worldwide, and with numbers increasing in low-income and middle-income countries where malnutrition and underweight often coexist.26 For example, in east, south, and southeast Asia, the increase in obesity has accelerated in children, overtaking the adult trajectory.346 In India, where severe underweight remains prevalent, approximately 20% of children have overweight or obesity.347 Once overweight and obesity has been established in early life, consequent changes in behaviour and biology are difficult to overcome. Governments in low-income and middle-income countries where malnutrition has been the focus now need to shift priorities to also combat obesity in childhood. Government policies must have a key role in combating an obesogenic environment by limiting the availability, affordability, and promotion of unhealthy foods and drinks. For example, taxation can be placed on sugar-sweetened beverages to reduce their consumption348 and the income used to reduce the cost of fruits and vegetables. Governments should prioritise nutritional policy that reduces overweight, obesity, and subsequent health outcomes, despite lobbying from major food and beverage corporations. Conflicts of interest for all involved parties should be declared so that policy can be brought forward despite the influence of industry.
Sustained action is needed to further reduce smoking on a global scale. Concurrently, a rapidly evolving area in need of public education and more restrictive policy is e-cigarette use (or vaping), the use of which is expanding and overtaking rates of cigarette smoking, particularly in adolescents.349 Health policies are needed to restrict importation of e-cigarettes and inhaled nicotine, which often serves as a gateway to smoking tobacco, particularly for adolescents and young adults.
Public education has been integral to improving awareness and recognition of the early warning symptoms of acute coronary syndrome and the need to seek urgent care. However, as acute coronary syndrome is a sign of end-stage ACAD, public education in the contemporary era needs to focus on increasing population awareness of cardiovascular risk factors and the capacity for screening and diagnosis of early atherosclerosis. It is also necessary to improve awareness of stakeholders (eg, employers, community groups, or governments) on additional ways to prevent coronary artery disease through improved care pathways, air pollution levels, and city planning. Education campaigns can shift attitudes and build support for large-scale changes in the environment and policies on tobacco use and diet.350
Ascertaining which formats of public education are efficacious, cost-effective, scalable, and sustainable, as well as overcoming barriers to an individual’s understanding of ACAD, is important. Digital technologies and online platforms could be used to engage a wide range of people in diverse economies. Sex and gender, race, ethnicity, education, and health literacy all affect awareness, while cultural beliefs or mistrust of organisations affect response to public education.351–353 Other socioeconomic factors such as basic access to health care, economic and social stability, and geographical location, particularly in low-income and middle-income countries, can present barriers to adequate public health information. Public education campaigns should incorporate different cultures and languages to ensure diversity and cultural representation, necessitating involvement of consumers to create effective and relatable messaging about ACAD.
Public education campaigns around ACAD have been disrupted by misinformation, leading to confusion and misunderstanding. Media sensationalism about common cardiovascular medications (eg, statins) undermines public health advice. Public education campaigns must recognise and respond to misinformation, adapting to the platforms that spread it. For example, public education has been limited by use of older media such as print, television, and radio, which have fewer effects on informing and changing behaviours of the children and adolescents, individuals of low education and socioeconomic status, or inhabitants of rural locations.354,355 As mass media evolves and new technology emerges, future ACAD public education campaigns need to adapt, using evolving online platforms to reach a wider audience. Education that uses social media, videos, and gamification of information might appeal more to children and adolescents.356 Given that traditional ACAD risk factors begin in childhood and adolescence, public education campaigns need to start as early as possible and continue throughout the life course.
Health policy and public education strategies are often presumed to be efficacious and cost-effective due to their ability to reach large populations; however, these strategies have not been tested in the same way as other interventions. With the shift to an atheroma-centric view of ACAD, we believe that researchers should now begin to scientifically test the effect of the nature, frequency, content, and mode of delivery of health policy and public education programmes. Although current research in coronary artery disease education campaigns has relied on cross-sectional before and after self-reported awareness or behaviour change, future research methods could use big data and large-scale pragmatic cluster randomised controlled trials to look at changes in risk factor prevalence, major health outcomes, and economic benefits.356 Isolating the effects of health policy on risk factor prevalence and clinical outcomes is a challenge and an opportunity. One example is the improvement in hypertension control and antihypertensive prescribing noted in Canada from 1999 to 2006, which occurred after initiation of the Canadian Hypertension Education Program.357 Although not all improvements can be directly ascribed to the programme, improvements in hypertension diagnosis, management, control, and mortality rates were documented. Successful partnership of stakeholders such as governmental bodies, cardiovascular societies, and global organisations (eg, WHO) is necessary to enable improved reach and engagement on a global scale. Cardiovascular societies and advocacy organisations (eg, heart foundations) should use their public education campaigns to pressure governments to partner with communities, researchers, and organisations to enact and measure the effect of policy change.
Finding ways to encourage more people to participate in population-based research is a priority for inclusivity of those at risk of ACAD (panel 10). Future strategies could incorporate AI and machine learning algorithms to personalise public education based on available individual data, including imaging data showing preclinical ACAD. This approach aims to provide tailored recommendations for lifestyle modification, screening, and preventive measures. Coupled with measuring behaviour changes, these developing methods could purposefully encompass increased proportions of the population and aid in the applicability of ACAD health policy and public health interventions.
Panel 10: Key targets for research in health policy and public education.
Rigorously test new health policies to evaluate efficacy in reducing risk factors, burden of atherosclerotic coronary artery disease (ACAD), and improving ACAD outcomes, including a focus on cost-effectiveness
Establish the most effective methods using digital technologies and online platforms to engage a wide range of people in diverse economies
Find optimal ways to engage more people, particularly of diverse representation, to participate in population-based research
Conclusion
Refocusing and reframing the definition and discussion of coronary artery disease from late-stage ischaemia and acute coronary events to early detection of coronary artery atheroma and prevention of advanced ACAD has the potential to save 8·7 million lives globally every year. Acute coronary syndrome events must be recognised as a failure of upstream care, missed opportunities for early intervention, and should be seen as avoidable consequences of a preventable disease.
The current definition and ICD codes for ischaemic heart disease constrain the ability to devise clinical pathways for early diagnosis, effective prevention, and cure of the disease. We argue that it is necessary to measure and define coronary atherosclerosis at a much earlier stage, when the opportunity to make an impact is greatest. By the time ischaemia and obstruction develop, prevention is no longer possible, and the effectiveness of interventions on morbidity and mortality are greatly reduced.
Coronary artery disease must be recognised across all stages of atherosclerosis, from the onset of atheroma through to end-stage disease. Throughout this Commission, we have aimed to redefine the conceptual framework of coronary artery disease—from the traditional model centred on ischaemia to a continuous disease with multiple stages throughout the life course. The disease begins with precursor features even in utero, which can develop through to childhood and adolescence, progressing as individuals age. Only after redefining coronary artery disease as ACAD can data based on this new definition be collected and used to inform future advances in health care.
Strategies must be developed to prevent the onset of atherosclerosis, diagnose the disease early, and improve the implementation of known effective strategies for those who have already developed the disease. Achieving these goals, and ensuring the delivery of global and equitable care, will require investment, training, and development of a workforce focused on early risk factor modification, diagnosis, and prevention of atherosclerosis rather than diagnosis and treatment of cardiovascular events and end-stage disease.
Research funding must be increased to match the global burden of disease, and investment must be made in the development of novel therapies that can prevent, revert, and eradicate the disease, along with imaging methods that accurately capture low-risk to high-risk disease states.
We hope that this Commission will reach patients and the public, policy makers, international societies, research funders, researchers, and health-care providers to advocate for the stabilisation, reversal, and future elimination of ACAD as a realistic and attainable goal. Together, these stakeholders can implement the actions that will take the goals of this Commission to the next step of reducing the global burden of this disease. The allocation of funding, resources, and workforce to the research highlighted in this Commission is urgently needed to combat the consequences of this preventable disease.
Figure 10: Summary of evidence on risk factor exposure with ACAD according to primordial, primary, and secondary prevention interventions.

Green=modifiable risk factors with multiple studies showing that intervention on this risk factor leads to improved cardiovascular outcomes. Yellow=risk factors with some data, but the efficacy of an intervention on cardiovascular outcomes has not been established. Red=minimal or no supporting data. ACAD=atherosclerotic coronary artery disease. DASH=Dietary Approaches to Stop Hypertension. NA=not applicable.
Figure 11: Guidance on screening and investigation of atherosclerotic coronary artery disease.

Key messages.
Atherosclerotic coronary artery disease (ACAD) clinical pathways must be refocused away from ischaemia and towards atherosclerosis
ACAD death rates are forecasted to increase by 19·2% in lower-middle-income countries and by 4·2% in upper-middle-income countries between 2022 and 2050. ACAD is projected to remain the leading cause of death, responsible for the death of 10·5 million people annually by 2050
The focus of management of coronary artery disease needs to shift from the late stages of the disease, coronary artery obstruction and resultant ischaemia and infarction, towards strategies aimed at early prevention, regression, and cure of atherosclerosis
ACAD should be seen as a lifetime continuum from early life through to older age
This new perspective would move the focus from diagnosis after the development of ischaemia or a cardiovascular event towards defining lifetime risk for an individual or a population at the earliest opportunity
The onset of ACAD can be prevented with early risk factor modification
ACAD and associated cardiovascular events must be recognised as preventable. Complete elimination of known behavioural and metabolic risk factors by 2050 would reduce the rate of ACAD deaths by 82·1% and save 8·7 million lives per year globally
Effective strategies for early screening and detection of ACAD are needed
These strategies should aim to maximise the effect of therapies to delay, halt, and revert the process of atherosclerosis by using targeted screening and detection earlier in the life course
Implementation of current approaches to ACAD needs to be improved
Implementation of evidence-based knowledge in ACAD is poor and variable, contributing to avoidable death, disability, and waste of resources
Stakeholders should support implementation efforts to maximise the uptake of known effective strategies for ACAD prevention and treatment globally and equitably
Local and global disparities in prevention, diagnosis, treatment, and outcomes of all people at risk of and with ACAD must be addressed
Research in ACAD is not representative of diverse populations and might not be transferable to all settings
Practical and pragmatic research must be promoted and conducted by stakeholders that is inclusive of routine health care using decentralised and adaptive platforms of interventions that allow a greater proportion of diverse patients to be randomised within clinical trials with lifelong follow-up
New therapies to eradicate atherosclerosis must be developed
The eradication of atherosclerosis is possible with transformative research
A global standard of data collection and dissemination to inform population-based decision making in ACAD should be established
A systematic international approach can generate and make data accessible for research and policy
The necessary resources and legislation should be provided by stakeholders to embed sustainable, integrated, standardised, accessible, and accurate databases of key health indicators in ACAD within all countries, with fair and easily accessible mechanisms
The ACAD health-care workforce and research infrastructure should be aligned towards early detection and prevention
The current workforce is focused on diagnosis and treatment of late-stage disease. This focus is variable, inequitable, and not resilient to economic, social, and environmental challenges and catastrophes
The global workforce must be trained to redirect delivery of care to prevention, detection, and management of earlier stages of ACAD, considering the relevant national context
Research funding must reflect the global burden of ACAD
Current research resources and investment are not commensurate to the global burden of morbidity and mortality attributable to ACAD. Without additional investment, the global incidence of acute coronary syndromes will double by 2050
Research funding must increase to match the burden of disease and support transformational research to improve outcomes worldwide
Acknowledgments
We thank Ruqayya Nasir Sani for advice on addressing the cardiovascular health needs of sub-Saharan Africa. We are pleased to acknowledge the partners of the Commission: St Mary’s Coronary Flow Trust (London, UK); the Division of Clinical Medicine and School of Medicine and Population Health, University of Sheffield (Sheffield, UK); The British Heart Foundation Data Science Centre (London, UK); the estate of Reena Dyer; Queen Mary University of London and the associated institutions of NIHR Barts Biomedical Research Centre, the Cardiovascular Clinical Trials Unit, the William Harvey Research Institute, the William Harvey Clinical Research Centre, and the Cardiovascular Devices Hub (London, UK); Richard Stack Professorship Endowment, Duke University School of Medicine (Durham, NC, USA); GISE–ETS Italian Foundation for Research and Innovation in Cardiology (Italy); Fundacion Interhospitalaria Para La Investigacion Cardiovascular (Madrid, Spain); Società Italiana di Cardiologia (Rome, Italy); Fondazione ETNA (Excellence Through Newest Advances; Catania, Italy); the Faculty of Medicine and Health, University of Sydney (Sydney, NSW, Australia); Queensland University of Technology (Brisbane, QLD, Australia); the Cardiovascular Research Institute (Dublin, Ireland); the University of Medicine and Health Sciences, Royal College of Surgeons in Ireland (Dublin, Ireland); the Duke-NUS Medical School (Singapore); and NIHR Imperial Biomedical Research Centre (London, UK).
Footnotes
Declaration of interests
SZ reports grants from Abbott Vascular and personal fees from Novartis and Boston Scientific. JHW reports grants from the American Heart Association and National Institutes of Health; consulting fees from the Institute for Clinical and Economic Review, Pfizer, and Patient Centered Outcomes Research Institute; personal fees from Huff Powell Bailey; support for attending meetings and travel from the American College of Cardiology; and has a leadership and fiduciary role with the New England Comparative Effectiveness Public Affairs Council. VK reports support from Queen Mary University. BZ reports support from the David Geffen School of Medicine and VA Greater Los Angeles Healthcare System; grants from the National Institutes of Health, Veterans Administration, and American Heart Association; and personal fees from the Heart Failure Society of America, Circulation: Cardiovascular Quality and Outcomes, and Scholars in Medicine. WAP reports a leadership and fiduciary role within the Cardiac Society of Australia and New Zealand. TJAC reports support for attending meetings and travel from the University of Sheffield, British Heart Foundation Data Science Centre, and the estate of Mrs Reena Dyer. DCa reports consulting fees from Abbott Vascular; personal fees from Terumo, Sanofi Aventis, NovoNordisk, and Medtronic; and board participation for MedAlliance. NRS reports support for attending meetings and travel from Vanderbilt University Medical Center (for the submitted work); support from Society for Cardiovascular Angiography and Interventions and Zoll (outside the submitted work); grants from the National Institutes of Health, Society for Cardiovascular Angiography and Interventions, and Shockwave; personal fees from Philips, Abbott, Zoll, and Youman & Caputo; has a leadership role with the Women in Innovations Committee and the Society for Cardiovascular Angiography and Interventions; has stock options in Stallion Catheter; and stipend for serving as an associate editor for the Journal of the American Heart Association. RS reports grants from the National Institute for Health and Care Research and participation on a board for the UK Longitudinal Linkage Consortium. DCh reports personal fees from SpectraWave Medical Imaging. NG reports grant support from Abbott; personal fees from Abbott, ShockWave, Abiomed, Philips, and Boston Scientific; board participation for Boston Scientific and Abbott; and a leadership role with the European Association of Percutaneous Cardiovascular Interventions Scientific Documents Committee. SRM reports grant support from Abbott; personal fees from Amgen, Bristol Myers Squibb, Novartis, NovoNordisk, and Janssen; and board participation for Merck. JS reports stock in Anagram Kommunikation and Symptoms Europe. SJN reports grants from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Cyclarity, Eli Lilly, Esperion, Resverlogix, New Amsterdam Pharma, Novartis, InfraReDx, and Sanofi-Regeneron; personal fees from Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi-Regeneron, Vaxxinity, Sequiris, and NovoNordisk; is listed as inventor on the patent for effects of PCSK9 inhibition on coronary atherosclerosis; has a leadership and fiduciary role with Cardiac Society of Australia and New Zealand; and is director of Evidence to Practice (non-profit meeting company). LJS reports a leadership and fiduciary role with the Society of Cardiovascular Computed Tomography and that HeartFlow granted access to their software. MRP reports grants from HeartFlow, Johnson & Johnson, National Heart, Lung and Blood Institute, and Patient Centered Outcomes Research Institute and personal fees from Bayer Healthcare, Janssen, Novartis, and Medscape. RKA-L reports grant support from the British Heart Foundation; personal fees from Abbott Vascular, Fondazione Internazionale Menarini, Shockwave, Cathworks, Medtronic, Philips, and Servier Pharmaceuticals; and board participation for Janssen Pharmaceuticals and Cathworks. All other authors declare no competing interests.
Contributor Information
Sarah Zaman, Westmead Applied Research Centre, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia; Department of Cardiology, Westmead Hospital, Sydney, NSW, Australia.
Jason H Wasfy, Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Vikas Kapil, William Harvey Research Institute, Centre for Cardiovascular Medicine and Devices, NIHR Barts Biomedical Research Centre, Queen Mary University of London, St Bartholomew’s Hospital, London, UK.
Boback Ziaeian, Division of Cardiology, David Geffen School of Medicine at UCLA, University of California, Los Angeles, CA, USA.
William A Parsonage, Australian Centre for Health Services Innovation, Queensland University of Technology, Brisbane, QLD, Australia; Department of Cardiology, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia.
Sira Sriswasdi, Center of Excellence in Computational Molecular Biology, Chulalongkorn University, Pathum Wan, Bangkok, Thailand; Faculty of Medicine, Chulalongkorn University, Pathum Wan, Bangkok, Thailand.
Timothy J A Chico, School of Medicine and Population Health, University of Sheffield, Sheffield, UK; British Heart Foundation Data Science Centre, Health Data Research UK, London, UK.
Davide Capodanno, Division of Cardiology, Azienda Ospedaliero Universitaria Policlinico, University of Catania, Catania, Italy.
Róisín Colleran, Department of Cardiology and Cardiovascular Research Institute, Mater Private Network, Dublin, Ireland; School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, University of Medicine and Health Sciences, Dublin, Ireland.
Nadia R Sutton, Department of Internal Medicine, and Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, USA.
Lei Song, Department of Cardiology, National Clinical Research Centre for Cardiovascular Diseases, Fuwai Hospital, Beijing, China; Peking Union Medical College (Chinese Academy of Medical Sciences), Beijing, China.
Nicole Karam, Cardiology Department, European Hospital Georges Pompidou, Paris City University, Paris, France.
Reecha Sofat, Department of Pharmacology and Therapeutics, University of Liverpool, Liverpool, UK.
Chiara Fraccaro, Division of Cardiology, Department of Cardiac, Thoracic, Vascular Sciences and Public Health, University of Padua, Padua, Italy.
Daniel Chamié, Section of Cardiovascular Medicine, Yale School of Medicine, Yale University, New Haven, CT, USA.
Mirvat Alasnag, Cardiac Center, King Fahd Armed Forces Hospital, Jeddah, Saudi Arabia.
Takayuki Warisawa, Department of Cardiology, NTT Medical Center Tokyo, Tokyo, Japan.
Nieves Gonzalo, Cardiology Department, Hospital Clínico San Carlos, Universidad Complutense de Madrid, Madrid, Spain.
Walid Jomaa, Cardiology B Department, Fattouma Bourguiba University Hospital, University of Monastir, Monastir, Tunisia.
Shamir R Mehta, Population Health Research Institute, Hamilton Health Sciences, McMaster University Medical Centre, Hamilton, ON, Canada.
Elizabeth E S Cook, Duke Clinical Research Institute, Duke University School of Medicine, Durham, NC, USA.
Johan Sundström, Uppsala University, Uppsala, Sweden; The George Institute for Global Health, University of New South Wales, Sydney, NSW, Australia.
Stephen J Nicholls, Victorian Heart Institute, Monash University, Melbourne, VIC, Australia.
Leslee J Shaw, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Manesh R Patel, Duke Clinical Research Institute, Duke University School of Medicine, Durham, NC, USA; Division of Cardiology, Department of Medicine, Duke University School of Medicine, Durham, NC, USA.
Rasha K Al-Lamee, National Heart and Lung Institute, Imperial College London, London, UK.
References
- 1.Silverman ME. William Herberden and some account of a disorder of the breast. Clin Cariol 1987; 10: 211–13. [DOI] [PubMed] [Google Scholar]
- 2.Hektoen L. Embolism of the left coronary artery; sudden death. Med Newsl 1892; 61: 210. [Google Scholar]
- 3.Obrastzov WP, Straschesko ND. Zur Kenntnis der Thrombose der Koronararterien des Herzens. Z Klin Med 1910; 71: 116–32. [Google Scholar]
- 4.Herrick JB. Certain clinical features of sudden obstruction of the coronary arteries. J Am Med Assoc 1912; 59: 2015–20. [Google Scholar]
- 5.Goetz RH, Rohman M, Haller JD, Dee R, Rosenak SS. Internal mammary-coronary artery anastomosis—a nonsuture method employing tantalum rings. J Thorac Cardiovasc Surg 1961; 41: 378–86. [PubMed] [Google Scholar]
- 6.Gruntzig A Transluminal dilatation of coronary-artery stenosis. Lancet 1978; 1: 263. [DOI] [PubMed] [Google Scholar]
- 7.Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023; 44: 3720–826. [DOI] [PubMed] [Google Scholar]
- 8.Vrints C, Andreotti F, Koskinas KC, et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur Heart J 2024; 45: 3415–537. [DOI] [PubMed] [Google Scholar]
- 9.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021; 42: 3227–337. [DOI] [PubMed] [Google Scholar]
- 10.GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024; 403: 2162–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: a forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024; 403: 2204–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayor M, Brown KJ, Vasan RS. The molecular basis of predicting atherosclerotic cardiovascular disease risk. Circ Res 2021; 128: 287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol 2000; 20: 1177–78. [DOI] [PubMed] [Google Scholar]
- 14.Xu S, Ilyas I, Little PJ, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev 2021; 73: 924–67. [DOI] [PubMed] [Google Scholar]
- 15.Borén J, Chapman MJ, Krauss RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2020; 41: 2313–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridker PM, MacFadyen JG, Thuren T, Libby P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur Heart J 2020; 41: 2153–63. [DOI] [PubMed] [Google Scholar]
- 17.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis 2011; 214: 3–10. [DOI] [PubMed] [Google Scholar]
- 18.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 2016; 118: 620–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takei H, Strong JP, Yutani C, Malcom GT. Comparison of coronary and aortic atherosclerosis in youth from Japan and the USA. Atherosclerosis 2005; 180: 171–79. [DOI] [PubMed] [Google Scholar]
- 20.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol 2022; 80: 2361–71. [DOI] [PubMed] [Google Scholar]
- 21.The Institute for Health Metrics and Evaluation. GBD results. 2021. https://vizhub.healthdata.org/gbd-results/ (accessed Jan 13, 2025).
- 22.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J Am Coll Cardiol 2020; 76: 2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I—general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001; 104: 2746–53. [DOI] [PubMed] [Google Scholar]
- 24.World Bank Group. Toward a healthy and harmonious life in China: stemming the rising tide of non-communicable diseases. 2011. https://www.worldbank.org/en/news/feature/2011/07/26/toward-health-harmonious-life-china-stemming-rising-tide-of-non-communicable-diseases (accessed Jan 13, 2025).
- 25.Ikeda N, Saito E, Kondo N, et al. What has made the population of Japan healthy? Lancet 2011; 378: 1094–105. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Obesity and overweight. 2024. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed Jan 13, 2025).
- 27.WHO. WHO global report on trends in prevalence of tobacco use 2000–2025, fourth edition. 2021. https://www.who.int/publications/i/item/9789240039322 (accessed Jan 13, 2025). [Google Scholar]
- 28.Chew NWS, Chong B, Kuo SM, et al. Trends and predictions of metabolic risk factors for acute myocardial infarction: findings from a multiethnic nationwide cohort. Lancet Reg Health West Pac 2023; 37: 100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023; 402: 203–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao H, Ekou A, Niamkey T, et al. Acute coronary syndromes in sub-Saharan Africa: a 10-year systematic review. J Am Heart Assoc 2022; 11: e021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. Health inequality monitor. 2025. https://www.who.int/data/inequality-monitor/manual (accessed Jan 13, 2025).
- 32.McGivern L, Shulman L, Carney JK, Shapiro S, Bundock E. Death certification errors and the effect on mortality statistics. Public Health Rep 2017; 132: 669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figtree GA, Vernon ST, Hadziosmanovic N, et al. Mortality in STEMI patients without standard modifiable risk factors: a sex-disaggregated analysis of SWEDEHEART registry data. Lancet 2021; 397: 1085–94. [DOI] [PubMed] [Google Scholar]
- 34.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153: 1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Cabo F, Fuster V, Silla-Castro JC, et al. Subclinical atherosclerosis and accelerated epigenetic age mediated by inflammation: a multi-omics study. Eur Heart J 2023; 44: 2698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.UN. World population prospects 2024. 2024. https://population.un.org/wpp/ (accessed Jan 13, 2025).
- 37.Hinton TC, Adams ZH, Baker RP, et al. Investigation and treatment of high blood pressure in young people: too much medicine or appropriate risk reduction? Hypertension 2020; 75: 16–22. [DOI] [PubMed] [Google Scholar]
- 38.Sabapathy K, Mwita FC, Dauya E, et al. Prevalence of hypertension and high-normal blood pressure among young adults in Zimbabwe: findings from a large, cross-sectional population-based survey. Lancet Child Adolesc Health 2024; 8: 101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mas-Llado C, Gonzalez-Del-Hoyo M, Siquier-Padilla J, et al. Representativeness in randomised clinical trials supporting acute coronary syndrome guidelines. Eur Heart J Qual Care Clin Outcomes 2023; 9: 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossello X, Mas-Lladó C, Pocock S, et al. Sex differences in mortality after an acute coronary syndrome increase with lower country wealth and higher income inequality. Rev Esp Cardiol 2022; 75: 392–400. [DOI] [PubMed] [Google Scholar]
- 41.Vogel B, Acevedo M, Appelman Y, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet 2021; 397: 2385–438. [DOI] [PubMed] [Google Scholar]
- 42.Lim GB. Role of sex hormones in cardiovascular diseases. Nat Rev Cardiol 2021; 18: 385. [DOI] [PubMed] [Google Scholar]
- 43.Ke W, Rand KA, Conti DV, et al. Evaluation of 71 coronary artery disease risk variants in a multiethnic cohort. Front Cardiovasc Med 2018; 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammond EC, Horn D. Smoking and death rates: report on forty-four months of follow-up of 187 783 men. 2. Death rates by cause. J Am Med Assoc 1958; 166: 1294–308. [DOI] [PubMed] [Google Scholar]
- 45.Willett WC, Green A, Stampfer MJ, et al. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med 1987; 317: 1303–09. [DOI] [PubMed] [Google Scholar]
- 46.Duncan MS, Freiberg MS, Greevy RA Jr, Kundu S, Vasan RS, Tindle HA. Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA 2019; 322: 642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.WHO. WHO global report on trends in prevalence of tobacco use 2000–2030. 2024. https://www.who.int/publications/i/item/9789240088283 (accessed Jan 13, 2025).
- 48.Jomaa W, Chamtouri I, Amdouni N, Turki A, Ben Hamda K. Temporal trends and prognostic impact of reperfusion modalities in Tunisian patients presenting with ST-elevation myocardial infarction: a 20-year analysis. Tunis Med 2024; 102: 387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bray GA, Kim KK, Wilding JPH, World Obesity Federation. Obesity: a chronic relapsing progressive disease process—a position statement of the World Obesity Federation. Obes Rev 2017; 18: 715–23. [DOI] [PubMed] [Google Scholar]
- 50.Di Angelantonio E, Bhupathiraju SN, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016; 388: 776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubino F, Cummings DE, Eckel RH, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol 2025; published online Jan 14. 10.1016/S2213-8587(24)00316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Z, Macpherson J, Gray SR, et al. Are people with metabolically healthy obesity really healthy? A prospective cohort study of 381 363 UK Biobank participants. Diabetologia 2021; 64: 1963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J 2020; 41: 221–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powell-Wiley TM, Poirier P, Burke LE, et al. A scientific statement from the American Heart Association. Circulation 2021; 143: e984–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manoharan MP, Raja R, Jamil A, et al. Obesity and coronary artery disease: an updated systematic review 2022. Cureus 2022; 14: e29480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thubrikar MJ, Robicsek F. Pressure-induced arterial wall stress and atherosclerosis. Ann Thorac Surg 1986; 59: 1594–603. [DOI] [PubMed] [Google Scholar]
- 57.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387: 957–67. [DOI] [PubMed] [Google Scholar]
- 58.Beaney T, Schutte AE, Stergiou GS, et al. May measurement month 2019: the global blood pressure screening campaign of the International Society of Hypertension. Hypertension 2020; 76: 333–41. [DOI] [PubMed] [Google Scholar]
- 59.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016; 134: 441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boateng EB, Ampofo AG. A glimpse into the future: modelling global prevalence of hypertension. BMC Public Health 2023; 23: 1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab 2011; 14: 575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossello X, Raposeiras-Roubin S, Oliva B, et al. Glycated hemoglobin and subclinical atherosclerosis in people without diabetes. J Am Coll Cardiol 2021; 77: 2777–91. [DOI] [PubMed] [Google Scholar]
- 63.Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007; 370: 1829–39. [DOI] [PubMed] [Google Scholar]
- 64.Libby P The changing landscape of atherosclerosis. Nature 2021; 592: 524–33. [DOI] [PubMed] [Google Scholar]
- 65.Bentham J, Singh GM, Danaei G, et al. Multi-dimensional characterisation of global food supply from 1961–2013. Nat Food 2020; 1: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.NCD Risk Factor Collaboration (NCD-RisC). Repositioning of the global epicentre of non-optimal cholesterol. Nature 2020; 582: 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simony SB, Mortensen MB, Langsted A, Afzal S, Kamstrup PR, Nordestgaard BG. Sex differences of lipoprotein(a) levels and associated risk of morbidity and mortality by age: the Copenhagen general population study. Atherosclerosis 2022; 355: 76–82. [DOI] [PubMed] [Google Scholar]
- 68.Martignoni FV, Rl Júnior JE, Marques IR, et al. The association of lipoprotein(a) and coronary artery calcium in asymptomatic patients: a systematic review and meta-analysis. Eur J Prev Cardiol 2024; 31: 732–41. [DOI] [PubMed] [Google Scholar]
- 69.Sarnak MJ, Amann K, Bangalore S, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol 2019; 74: 1823–38. [DOI] [PubMed] [Google Scholar]
- 70.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol 2003; 41: 47–55. [DOI] [PubMed] [Google Scholar]
- 72.Ridker PM, Everett BM, Thuren T, et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–31. [DOI] [PubMed] [Google Scholar]
- 73.Chan K, Wahome E, Tsiachristas A, et al. Inflammatory risk and cardiovascular events in patients without obstructive coronary artery disease: the ORFAN multicentre, longitudinal cohort study. Lancet 2024; 403: 2606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018; 392: 929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 2002; 288: 2015–22. [DOI] [PubMed] [Google Scholar]
- 76.Bønaa KH, Njølstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med 2006; 354: 1578–88. [DOI] [PubMed] [Google Scholar]
- 77.Luo Z, Tang K, Huang G, et al. Homocysteine concentration in coronary artery disease and severity of coronary lesions. J Cell Mol Med 2024; 28: e18474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guéant JL, Guéant-Rodriguez RM, Oussalah A, Zuily S, Rosenberg I. Hyperhomocysteinemia in cardiovascular diseases: revisiting observational studies and clinical trials. Thromb Haemost 2023; 123: 270–82. [DOI] [PubMed] [Google Scholar]
- 79.Liu H, Chen X, Hu X, et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome 2019; 7: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xue H, Chen X, Yu C, et al. Gut Microbially produced indole-3-propionic acid inhibits atherosclerosis by promoting reverse cholesterol transport and its deficiency is causally related to atherosclerotic cardiovascular disease. Circ Res 2022; 131: 404–20. [DOI] [PubMed] [Google Scholar]
- 81.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012; 380: 219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winzer EB, Woitek F, Linke A. Physical activity in the prevention and treatment of coronary artery disease. J Am Heart Assoc 2018; 7: e007725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med 2021; 385: 1067–77. [DOI] [PubMed] [Google Scholar]
- 84.Santosa A, Rosengren A, Ramasundarahettige C, et al. Psychosocial risk factors and cardiovascular disease and death in a population-based cohort from 21 low-, middle-, and high-income countries. JAMA Netw Open 2021; 4: e2138920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han H, Wang Y, Li T, et al. Sleep duration and risks of incident cardiovascular disease and mortality among people with type 2 diabetes. Diabetes Care 2023; 46: 101–10. [DOI] [PubMed] [Google Scholar]
- 86.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342: 1378–84. [DOI] [PubMed] [Google Scholar]
- 87.Dixon SB, Liu Q, Chow EJ, et al. Specific causes of excess late mortality and association with modifiable risk factors among survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet 2023; 401: 1447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tapia-Vieyra JV, Delgado-Coello B, Mas-Oliva J. Atherosclerosis and cancer—a resemblance with far-reaching implications. Arch Med Res 2017; 48: 12–26. [DOI] [PubMed] [Google Scholar]
- 89.Armstrong ADC, de Souza CDF, Santos JMD, et al. Urbanization and cardiovascular health among Indigenous groups in Brazil. Commun Med 2023; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pabon-Nau LP, Cohen A, Meigs JB, Grant RW. Hypertension and diabetes prevalence among US Hispanics by country of origin: the National Health Interview Survey 2000–2005. J Gen Intern Med 2010; 25: 847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huffman MD, Galloway JM. Cardiovascular health in indigenous communities: successful programs. Heart Lung Circ 2010; 19: 351–60. [DOI] [PubMed] [Google Scholar]
- 92.Stoner L, Stoner KR, Young JM, Fryer S. Preventing a cardiovascular disease epidemic among indigenous populations through lifestyle changes. Int J Prev Med 2012; 3: 230–40. [PMC free article] [PubMed] [Google Scholar]
- 93.Paul CL, Sanson-Fisher R, Stewart J, Anderson AE. Being sorry is not enough: the sorry state of the evidence base for improving the health of indigenous populations. Am J Prev Med 2010; 38: 566–68. [DOI] [PubMed] [Google Scholar]
- 94.Kianoush S, Rifai MA, Jain V, et al. Prevalence and predictors of premature coronary heart disease among Asians in the United States: a national health interview survey study. Curr Probl Cardiol 2023; 48: 101152. [DOI] [PubMed] [Google Scholar]
- 95.Patel AP, Wang M, Kartoun U, Ng K, Khera AV. Quantifying and understanding the higher risk of atherosclerotic cardiovascular disease among south Asian individuals: results from the UK Biobank prospective cohort study. Circulation 2021; 144: 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joseph P, Kutty VR, Mohan V, et al. Cardiovascular disease, mortality, and their associations with modifiable risk factors in a multi-national south Asia cohort: a PURE substudy. Eur Heart J 2022; 43: 2831–40. [DOI] [PubMed] [Google Scholar]
- 97.Khraishah H, Alahmad B, Ostergard RL Jr, et al. Climate change and cardiovascular disease: implications for global health. Nat Rev Cardiol 2022; 19: 798–812. [DOI] [PubMed] [Google Scholar]
- 98.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004; 109: 2655–71. [DOI] [PubMed] [Google Scholar]
- 99.Mirowsky JE, Carraway MS, Dhingra R, et al. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ Health 2017; 16: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marfella R, Prattichizzo F, Sardu C, et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N Engl J Med 2024; 390: 900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364: 937–52. [DOI] [PubMed] [Google Scholar]
- 102.Lloyd-Jones DM, Albert MA, Elkind M. The American Heart Association’s focus on primordial prevention. Circulation 2021; 144: e233–35. [DOI] [PubMed] [Google Scholar]
- 103.Crump C, Howell EA. Perinatal origins of cardiovascular health disparities across the life course. JAMA Pediatr 2020; 174: 113–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y, Gao E, Wu J, et al. Fetal macrosomia and adolescence obesity: results from a longitudinal cohort study. Int J Obes 2009; 33: 923–28. [DOI] [PubMed] [Google Scholar]
- 105.Napoli C, Glass CK, Witztum JL, Deutsch R, D’Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet 1999; 354: 1234–41. [DOI] [PubMed] [Google Scholar]
- 106.Marschner S, Pant A, Henry A, et al. Cardiovascular risk management following gestational diabetes and hypertensive disorders of pregnancy: a narrative review. Med J Aust 2023; 218: 484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation 2011; 124: 967–90. [DOI] [PubMed] [Google Scholar]
- 108.Rossello X, Dorresteijn JA, Janssen A, et al. Risk prediction tools in cardiovascular disease prevention: a report from the ESC Prevention of CVD Programme led by the European Association of Preventive Cardiology (EAPC) in collaboration with the Acute Cardiovascular Care Association (ACCA) and the Association of Cardiovascular Nursing and Allied Professions (ACNAP). Eur J Prev Cardiol 2019; 26: 1534–44. [DOI] [PubMed] [Google Scholar]
- 109.Damen JA, Pajouheshnia R, Heus P, et al. Performance of the Framingham risk models and pooled cohort equations for predicting 10-year risk of cardiovascular disease: a systematic review and meta-analysis. BMC Med 2019; 17: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alaa AM, Bolton T, Di Angelantonio E, Rudd JHF, van der Schaar M. Cardiovascular disease risk prediction using automated machine learning: a prospective study of 423 604 UK Biobank participants. PLoS One 2019; 14: e0213653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu W, Laranjo L, Klimis H, et al. Machine-learning versus traditional approaches for atherosclerotic cardiovascular risk prognostication in primary prevention cohorts: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes 2023; 9: 310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fahed AC, Wang M, Homburger JR, et al. Polygenic background modifies penetrance of monogenic variants for tier 1 genomic conditions. Nat Commun 2020; 11: 3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018; 50: 1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O’Sullivan JW, Raghavan S, Marquez-Luna C, et al. Polygenic risk scores for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2022; 146: e93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017; 377: 111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet 2014; 384: 591–98. [DOI] [PubMed] [Google Scholar]
- 117.Chou R, Cantor A, Dana T, et al. Statin use for the primary prevention of cardiovascular disease in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2022; 328: 754–71. [DOI] [PubMed] [Google Scholar]
- 118.Jarmul J, Pletcher MJ, Hassmiller Lich K, et al. Cardiovascular genetic risk testing for targeting statin therapy in the primary prevention of atherosclerotic cardiovascular disease: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes 2018; 11: e004171. [DOI] [PubMed] [Google Scholar]
- 119.Wang DD, Li Y, Afshin A, et al. Global improvement in dietary quality could lead to substantial reduction in premature death. J Nutr 2019; 149: 1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Filippou CD, Tsioufis CP, Thomopoulos CG, et al. Dietary Approaches to Stop Hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr 2020; 11: 1150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Agarwal A, Ioannidis JPA. PREDIMED trial of Mediterranean diet: retracted, republished, still trusted? BMJ 2019; 364: l341. [DOI] [PubMed] [Google Scholar]
- 122.WHO. WHO report on the global tobacco epidemic, 2023: protect people from tobacco smoke. 2023. https://iris.who.int/bitstream/handle/10665/372043/9789240077164-eng.pdf?sequence=1 (accessed Feb 11, 2025).
- 123.Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol 2018; 72: 3332–65. [DOI] [PubMed] [Google Scholar]
- 124.World Obesity, Global Obesity Observatory. World obesity atlas 2023. 2023. https://data.worldobesity.org/publications/?cat=19 (accessed Jan 14, 2025).
- 125.Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023; 389: 2221–32. [DOI] [PubMed] [Google Scholar]
- 126.Iannone A, Natale P, Palmer SC, et al. Clinical outcomes associated with drugs for obesity and overweight: a systematic review and network meta-analysis of randomized controlled trials. Diabetes Obes Metab 2023; 25: 2535–44. [DOI] [PubMed] [Google Scholar]
- 127.Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet 2024; 403: e21–31. [DOI] [PubMed] [Google Scholar]
- 128.LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018; 320: 1172–91. [DOI] [PubMed] [Google Scholar]
- 129.Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022; 387: 205–16. [DOI] [PubMed] [Google Scholar]
- 130.Jastreboff AM, Kaplan LM, Frías JP, et al. Triple-hormone-receptor agonist retatrutide for obesity—a phase 2 trial. N Engl J Med 2023; 389: 514–26. [DOI] [PubMed] [Google Scholar]
- 131.Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects study. N Engl J Med 2020; 383: 1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.He J, Ouyang N, Guo X, et al. Effectiveness of a non-physician community health-care provider-led intensive blood pressure intervention versus usual care on cardiovascular disease (CRHCP): an open-label, blinded-endpoint, cluster-randomised trial. Lancet 2023; 401: 928–38. [DOI] [PubMed] [Google Scholar]
- 133.Song J, Wang X, Wang B, et al. Learning implementation of a guideline based decision support system to improve hypertension treatment in primary care in China: pragmatic cluster randomised controlled trial. BMJ 2024; 386: e079143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.He G, Yang G, Huang X, Luo D, Tang C, Zhang Z. SGLT2 inhibitors for prevention of primary and secondary cardiovascular outcomes: a meta-analysis of randomized controlled trials. Heart Lung 2023; 59: 109–16. [DOI] [PubMed] [Google Scholar]
- 135.EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Global perspective of familial hypercholesterolaemia: a cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet 2021; 398: 1713–25. [DOI] [PubMed] [Google Scholar]
- 136.Pirillo A, Catapano AL, Norata GD. Monoclonal antibodies in the management of familial hypercholesterolemia: focus on PCSK9 and ANGPTL3 inhibitors. Curr Atheroscler Rep 2021; 23: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Momtazi-Borojeni AA, Jaafari MR, Badiee A, Sahebkar A. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis 2019; 283: 69–78. [DOI] [PubMed] [Google Scholar]
- 138.Fukami H, Morinaga J, Nakagami H, et al. Vaccine targeting ANGPTL3 ameliorates dyslipidemia and associated diseases in mouse models of obese dyslipidemia and familial hypercholesterolemia. Cell Rep Med 2021; 2: 100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017; 38: 2459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354: 1264–72. [DOI] [PubMed] [Google Scholar]
- 141.Lee RG, Mazzola AM, Braun MC, et al. Efficacy and safety of an investigational single-course CRISPR base-editing therapy targeting PCSK9 in nonhuman primate and mouse models. Circulation 2023; 147: 242–53. [DOI] [PubMed] [Google Scholar]
- 142.Virani SS, Newby LK, Arnold SV, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines. Circulation 2023; 148: e9–119. [DOI] [PubMed] [Google Scholar]
- 143.Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mendelson MM, Lyass A, O’Donnell CJ, D’Agostino RB Sr, Levy D. Association of maternal prepregnancy dyslipidemia with adult offspring dyslipidemia in excess of anthropometric, lifestyle, and genetic factors in the Framingham Heart Study. JAMA Cardiol 2016; 1: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Upadhyay A, Earley A, Lamont JL, Haynes S, Wanner C, Balk EM. Lipid-lowering therapy in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157: 251–62. [DOI] [PubMed] [Google Scholar]
- 146.Lee M, Hong YA, Myong JP, Lee K, Park MW, Kim DW. Trends and outcome of statin therapy in dialysis patients with atherosclerotic cardiovascular diseases: a population-based cohort study. PLoS One 2023; 18: e0286670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferguson KK, Meeker JD, McElrath TF, Mukherjee B, Cantonwine DE. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. Am J Obstet Gynecol 2017; 216: 527e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Catov JM, Snyder GG, Fraser A, et al. Blood pressure patterns and subsequent coronary artery calcification in women who delivered preterm births. Hypertension 2018; 72: 159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boivin A, Luo ZC, Audibert F, et al. Pregnancy complications among women born preterm. CMAJ 2012; 184: 1777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359: 2195–207. [DOI] [PubMed] [Google Scholar]
- 151.del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001; 44: 2737–45. [DOI] [PubMed] [Google Scholar]
- 152.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997; 145: 408–15. [DOI] [PubMed] [Google Scholar]
- 153.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–31. [DOI] [PubMed] [Google Scholar]
- 154.Augustemak de Lima LR, Petroski EL, Moreno YMF, et al. Dyslipidemia, chronic inflammation, and subclinical atherosclerosis in children and adolescents infected with HIV: the PositHIVe Health Study. PLoS One 2018; 13: e0190785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Triant VA, Perez J, Regan S, et al. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation 2018; 137: 2203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fröbert O, Götberg M, Erlinge D, et al. Influenza vaccination after myocardial infarction: a randomized, double-blind, placebo-controlled, multicenter trial. Circulation 2021; 144: 1476–84. [DOI] [PubMed] [Google Scholar]
- 157.Vallée A Association between cannabis use and ten-year estimated atherosclerotic cardiovascular disease risk in a middle-aged population survey. Eur J Intern Med 2023; 111: 69–76. [DOI] [PubMed] [Google Scholar]
- 158.Razaz N, Villamor E, Muraca GM, Bonamy AE, Cnattingius S. Maternal obesity and risk of cardiovascular diseases in offspring: a population-based cohort and sibling-controlled study. Lancet Diabetes Endocrinol 2020; 8: 572–81. [DOI] [PubMed] [Google Scholar]
- 159.Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr 2015; 113: 1–15. [DOI] [PubMed] [Google Scholar]
- 160.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018; 378: e34. [DOI] [PubMed] [Google Scholar]
- 161.Delgado-Lista J, Alcala-Diaz JF, Torres-Peña JD, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet 2022; 399: 1876–85. [DOI] [PubMed] [Google Scholar]
- 162.Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of preterm birth with risk of ischemic heart disease in adulthood. JAMA Pediatr 2019; 173: 736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1500–09. [DOI] [PubMed] [Google Scholar]
- 164.Raal FJ, Hovingh GK, Blom D, et al. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol 2017; 5: 280–90. [DOI] [PubMed] [Google Scholar]
- 165.Ridker PM, Rose LM, Kastelein JJP, et al. Cardiovascular event reduction with PCSK9 inhibition among 1578 patients with familial hypercholesterolemia: results from the SPIRE randomized trials of bococizumab. J Clin Lipidol 2018; 12: 958–65. [DOI] [PubMed] [Google Scholar]
- 166.Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 385: 331–40. [DOI] [PubMed] [Google Scholar]
- 167.Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 385: 341–50. [DOI] [PubMed] [Google Scholar]
- 168.Blom DJ, Harada-Shiba M, Rubba P, et al. Efficacy and safety of alirocumab in adults with homozygous familial hypercholesterolemia: the ODYSSEY HoFH Trial. J Am Coll Cardiol 2020; 76: 131–42. [DOI] [PubMed] [Google Scholar]
- 169.Alzahrani T, Pena I, Temesgen N, Glantz SA. Association between electronic cigarette use and myocardial infarction. Am J Prev Med 2018; 55: 455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gottlieb SS, Kop WJ, Thomas SA, et al. A double-blind placebo-controlled pilot study of controlled-release paroxetine on depression and quality of life in chronic heart failure. Am Heart J 2007; 153: 868–73. [DOI] [PubMed] [Google Scholar]
- 171.Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288: 701–09. [DOI] [PubMed] [Google Scholar]
- 172.O’Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol 2010; 56: 692–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004; 363: 1589–97. [DOI] [PubMed] [Google Scholar]
- 174.Taylor CB, Youngblood ME, Catellier D, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005; 62: 792–98. [DOI] [PubMed] [Google Scholar]
- 175.Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol 2015; 12: 627–42. [DOI] [PubMed] [Google Scholar]
- 176.Moran CA, Collins LF, Beydoun N, et al. cardiovascular implications of immune disorders in women. Circ Res 2022; 130: 593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342: 836–43. [DOI] [PubMed] [Google Scholar]
- 178.Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis 2011; 70: 482–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Conrad N, Verbeke G, Molenberghs G, et al. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 2022; 400: 733–43. [DOI] [PubMed] [Google Scholar]
- 180.Conrad N, Verbeke G, Molenberghs G, et al. AT-RISK: AuToimmune disorders and cardiovascular RISK. Eur Heart J 2022; 43 (suppl 2): 2862. [Google Scholar]
- 181.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021; 42: 3227–337. [DOI] [PubMed] [Google Scholar]
- 182.Lloyd-Jones DM, Ning H, Labarthe D, et al. Status of cardiovascular health in US adults and children using the American Heart Association’s new “Life’s Essential 8” metrics: prevalence estimates from the National Health and Nutrition Examination Survey (NHANES), 2013 through 2018. Circulation 2022; 146: 822–35. [DOI] [PubMed] [Google Scholar]
- 183.Krittanawong C, Kumar A, Wang Z, et al. Sleep duration and cardiovascular health in a representative community population (from NHANES, 2005 to 2016). Am J Cardiol 2020; 127: 149–55. [DOI] [PubMed] [Google Scholar]
- 184.Rizzo M, Berneis K, Spinas G, Rini GB, Carmina E. Long-term consequences of polycystic ovary syndrome on cardiovascular risk. Fertil Steril 2009; 91 (suppl): 1563–67. [DOI] [PubMed] [Google Scholar]
- 185.Cussons AJ, Stuckey BG, Watts GF. Cardiovascular disease in the polycystic ovary syndrome: new insights and perspectives. Atherosclerosis 2006; 185: 227–39. [DOI] [PubMed] [Google Scholar]
- 186.Galatou E, Mourelatou E, Hatziantoniou S, Vizirianakis IS. Nonalcoholic Steatohepatitis (NASH) and Atherosclerosis: explaining their pathophysiology, association and the role of incretin-based drugs. Antioxidants 2022; 11: 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2015; 132: 873–98. [DOI] [PubMed] [Google Scholar]
- 188.Streed CG Jr, Beach LB, Caceres BA, et al. Assessing and addressing cardiovascular health in people who are transgender and gender diverse: a scientific statement from the american heart association. Circulation 2021; 144: e136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aranda G, Halperin I, Gomez-Gil E, et al. Cardiovascular risk associated with gender affirming hormone therapy in transgender population. Front Endocrinol 2021; 12: 718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hay M, Stehli J, Martin C, et al. Sex differences in optimal medical therapy following myocardial infarction according to left ventricular ejection fraction. Eur J Prev Cardiol 2020; 27: 2348–50. [DOI] [PubMed] [Google Scholar]
- 191.Stehli J, Martin C, Brennan A, Dinh DT, Lefkovits J, Zaman S. Sex differences persist in time to presentation, revascularization, and mortality in myocardial infarction treated with percutaneous coronary intervention. J Am Heart Assoc 2019; 8: e012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Australian Institute of Health and Welfare. Cardiovascular disease in Australian women—a snapshot of national statistics. 2019. https://www.aihw.gov.au/reports/heart-stroke-vascular-diseases/cardiovascular-disease-in-women/contents/table-of-contents (accessed Jan 14, 2025).
- 193.Alzahrani T, Nguyen T, Ryan A, et al. Cardiovascular disease risk factors and myocardial infarction in the transgender population. Circ Cardiovasc Qual Outcomes 2019; 12: e005597. [DOI] [PubMed] [Google Scholar]
- 194.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376 162 patients. Am J Med 2012; 125: 882–87.e1. [DOI] [PubMed] [Google Scholar]
- 195.Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J 2013; 34: 2940–48. [DOI] [PubMed] [Google Scholar]
- 196.Castellano JM, Pocock SJ, Bhatt DL, et al. Polypill strategy in secondary cardiovascular prevention. N Engl J Med 2022; 387: 967–77. [DOI] [PubMed] [Google Scholar]
- 197.Chow CK, Klimis H, Thiagalingam A, et al. Text messages to improve medication adherence and secondary prevention after acute coronary syndrome: the TEXTMEDS randomized clinical trial. Circulation 2022; 145: 1443–55. [DOI] [PubMed] [Google Scholar]
- 198.Palmer MJ, Barnard S, Perel P, Free C. Mobile phone-based interventions for improving adherence to medication prescribed for the primary prevention of cardiovascular disease in adults. Cochrane Database Syst Rev 2018; 6: CD012675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Salzwedel A, Jensen K, Rauch B, et al. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: update of the Cardiac Rehabilitation Outcome Study (CROS-II). Eur J Prev Cardiol 2020; 27: 1756–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shields GE, Wells A, Doherty P, Heagerty A, Buck D, Davies LM. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart 2018; 104: 1403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kotseva K, De Backer G, De Bacquer D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol 2019; 26: 824–35. [DOI] [PubMed] [Google Scholar]
- 202.Resurrección DM, Moreno-Peral P, Gómez-Herranz M, et al. Factors associated with non-participation in and dropout from cardiac rehabilitation programmes: a systematic review of prospective cohort studies. Eur J Cardiovasc Nurs 2019; 18: 38–47. [DOI] [PubMed] [Google Scholar]
- 203.Chow CK, Redfern J, Hillis GS, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA 2015; 314: 1255–63. [DOI] [PubMed] [Google Scholar]
- 204.Hamilton SJ, Mills B, Birch EM, Thompson SC. Smartphones in the secondary prevention of cardiovascular disease: a systematic review. BMC Cardiovasc Disord 2018; 18: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ma Z, Mao C, Chen X, et al. Peptide vaccine against ADAMTS-7 ameliorates atherosclerosis and postinjury neointima hyperplasia. Circulation 2023; 147: 728–42. [DOI] [PubMed] [Google Scholar]
- 206.Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol 2016; 67: 2118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Patel J, Pallazola VA, Dudum R, et al. Assessment of coronary artery calcium scoring to guide statin therapy allocation according to risk-enhancing factors: the multi-ethnic study of atherosclerosis. JAMA Cardiol 2021; 6: 1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003; 228: 826–33. [DOI] [PubMed] [Google Scholar]
- 209.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol 2005; 46: 807–14. [DOI] [PubMed] [Google Scholar]
- 210.Okwuosa TM, Greenland P, Ning H, et al. Distribution of coronary artery calcium scores by Framingham 10-year risk strata in the MESA (Multi-Ethnic Study of Atherosclerosis) potential implications for coronary risk assessment. J Am Coll Cardiol 2011; 57: 1838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Al-Kindi SG, Costa M, Tashtish N, et al. No-charge coronary artery calcium screening for cardiovascular risk assessment. J Am Coll Cardiol 2020; 76: 1259–62. [DOI] [PubMed] [Google Scholar]
- 212.Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol 2011; 57: 1622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lindholt JS, Søgaard R, Rasmussen LM, et al. Five-year outcomes of the Danish Cardiovascular Screening (DANCAVAS) trial. N Engl J Med 2022; 387: 1385–94. [DOI] [PubMed] [Google Scholar]
- 214.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2021; 144: e368–454. [DOI] [PubMed] [Google Scholar]
- 215.Williams MC, Wereksi R, Tuck C, et al. Coronary CT angiography-guided management of patients with stable chest pain: 10-year outcomes from the SCOT-HEART randomised controlled trial in Scotland. Lancet 2025; 405: 329–37. [DOI] [PubMed] [Google Scholar]
- 216.Curzen N, Nicholas Z, Stuart B, et al. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: the FORECAST randomized trial. Eur Heart J 2021; 42: 3844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Peng AW, Dudum R, Jain SS, et al. Association of coronary artery calcium detected by routine ungated CT imaging with cardiovascular outcomes. J Am Coll Cardiol 2023; 82: 1192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Iribarren C, Chandra M, Lee C, et al. Breast arterial calcification: a novel cardiovascular risk enhancer among postmenopausal women. Circ Cardiovasc Imaging 2022; 15: e013526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chen KW, Wang YC, Liu MH, et al. Artificial intelligence-assisted remote detection of ST-elevation myocardial infarction using a mini-12-lead electrocardiogram device in prehospital ambulance care. Front Cardiovasc Med 2022; 9: 1001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dawson LP, Nehme E, Nehme Z, et al. Chest pain management using prehospital point-of-care troponin and paramedic risk assessment. JAMA Intern Med 2023; 183: 203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Dawson LP, Smith K, Cullen L, et al. Care models for acute chest pain that improve outcomes and efficiency: JACC state-of-the-art review. J Am Coll Cardiol 2022; 79: 2333–48. [DOI] [PubMed] [Google Scholar]
- 222.Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020; 382: 1395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356: 1503–16. [DOI] [PubMed] [Google Scholar]
- 224.Spertus JA, Jones PG, Maron DJ, et al. Health-status outcomes with invasive or conservative care in coronary disease. N Engl J Med 2020; 382: 1408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Reynolds HR, Shaw LJ, Min JK, et al. Outcomes in the ISCHEMIA trial based on coronary artery disease and ischemia severity. Circulation 2021; 144: 1024–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nurmohamed NS, Min JK, Anthopolos R, et al. Atherosclerosis quantification and cardiovascular risk: the ISCHEMIA trial. Eur Heart J 2024; 45: 3735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gaba P, Gersh BJ, Muller J, Narula J, Stone GW. Evolving concepts of the vulnerable atherosclerotic plaque and the vulnerable patient: implications for patient care and future research. Nat Rev Cardiol 2023; 20: 181–96. [DOI] [PubMed] [Google Scholar]
- 228.Kedhi E, Berta B, Roleder T, et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: the COMBINE OCT-FFR trial. Eur Heart J 2021; 42: 4671–79. [DOI] [PubMed] [Google Scholar]
- 229.Lee JM, Choi G, Hwang D, et al. Impact of longitudinal lesion geometry on location of plaque rupture and clinical presentations. JACC Cardiovasc Imaging 2017; 10: 677–88. [DOI] [PubMed] [Google Scholar]
- 230.Johnson TW, Räber L, di Mario C, et al. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2019; 40: 2566–84. [DOI] [PubMed] [Google Scholar]
- 231.Chang HJ, Lin FY, Lee SE, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol 2018; 71: 2511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hwang D, Kim HJ, Lee SP, et al. Topological data analysis of coronary plaques demonstrates the natural history of coronary atherosclerosis. JACC Cardiovasc Imaging 2021; 14: 1410–21. [DOI] [PubMed] [Google Scholar]
- 233.Koo BK, Yang S, Jung JW, et al. Artificial intelligence-enabled quantitative coronary plaque and hemodynamic analysis for predicting acute coronary syndrome. JACC Cardiovasc Imaging 2024; 17: 1062–76. [DOI] [PubMed] [Google Scholar]
- 234.Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet 2017; 389: 2473–81. [DOI] [PubMed] [Google Scholar]
- 235.Howard JP, Wood FA, Finegold JA, et al. Side effect patterns in a crossover trial of statin, placebo, and no treatment. J Am Coll Cardiol 2021; 78: 1210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Strandberg TE, Kovanen PT, Lloyd-Jones DM, et al. Drugs for dyslipidaemia: the legacy effect of the Scandinavian Simvastatin Survival Study (4S). Lancet 2024; 404: 2462–75. [DOI] [PubMed] [Google Scholar]
- 237.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713–22. [DOI] [PubMed] [Google Scholar]
- 238.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018; 379: 2097–107. [DOI] [PubMed] [Google Scholar]
- 239.Räber L, Ueki Y, Otsuka T, et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA 2022; 327: 1771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rahimi K, Bidel Z, Nazarzadeh M, et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet 2021; 397: 1625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021; 398: 957–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018; 392: 1036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Xia Y, Hu Y, Tang L. Factor XIa inhibitors as a novel anticoagulation target: recent clinical research advances. Pharmaceuticals 2023; 16: 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017; 377: 1319–30. [DOI] [PubMed] [Google Scholar]
- 245.Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–800. [DOI] [PubMed] [Google Scholar]
- 246.Gragnano F, Cao D, Pirondini L, et al. P2Y12 inhibitor or aspirin monotherapy for secondary prevention of coronary events. J Am Coll Cardiol 2023; 82: 89–105. [DOI] [PubMed] [Google Scholar]
- 247.McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med 2018; 379: 1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Butler J, Jones WS, Udell JA, et al. Empagliflozin after acute myocardial infarction. N Engl J Med 2024; 390: 1455–66. [DOI] [PubMed] [Google Scholar]
- 249.Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res 2019; 124: 315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135–43. [DOI] [PubMed] [Google Scholar]
- 251.Ridker PM. C-reactive protein and risks of future myocardial infarction and thrombotic stroke. Eur Heart J 1998; 19: 1–3. [DOI] [PubMed] [Google Scholar]
- 252.Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020; 383: 1838–47. [DOI] [PubMed] [Google Scholar]
- 253.Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019; 381: 2497–505. [DOI] [PubMed] [Google Scholar]
- 254.Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019; 380: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jolly SS, d’Entremont MA, Lee SF, et al. Colchicine in acute myocardial infarction. N Engl J Med 2024. [DOI] [PubMed] [Google Scholar]
- 256.Sorbets E, Steg PG, Young R, et al. β-blockers, calcium antagonists, and mortality in stable coronary artery disease: an international cohort study. Eur Heart J 2019; 40: 1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Poole-Wilson PA, Lubsen J, Kirwan BA, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004; 364: 849–57. [DOI] [PubMed] [Google Scholar]
- 258.European Coronary Surgery Study Group. Long-term results of prospective randomised study of coronary artery bypass surgery in stable angina pectoris. Lancet 1982; 2: 1173–80. [PubMed] [Google Scholar]
- 259.Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994; 344: 563–70. [DOI] [PubMed] [Google Scholar]
- 260.Bittl JA, He Y, Jacobs AK, Yancy CW, Normand SL. Bayesian methods affirm the use of percutaneous coronary intervention to improve survival in patients with unprotected left main coronary artery disease. Circulation 2013; 127: 2177–85. [DOI] [PubMed] [Google Scholar]
- 261.Marui A, Kimura T, Nishiwaki N, et al. Comparison of five-year outcomes of coronary artery bypass grafting versus percutaneous coronary intervention in patients with left ventricular ejection fractions≤50% versus >50% (from the CREDO-Kyoto PCI/CABG Registry Cohort-2). Am J Cardiol 2014; 114: 988–96. [DOI] [PubMed] [Google Scholar]
- 262.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360: 2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Xaplanteris P, Fournier S, Pijls NHJ, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 2018; 379: 250–59. [DOI] [PubMed] [Google Scholar]
- 264.Brown DL, Boden WE. Impact of revascularisation on outcomes in chronic coronary syndromes: a new meta-analysis with the same old biases? Eur Heart J 2021; 42: 4652–55. [DOI] [PubMed] [Google Scholar]
- 265.Daoued Z, Ghedira F, Boudiche S, Ziadi J, Mourali MS, Denguir R. Multiple arterial coronary artery bypass grafting: perioperative complications, clinical and angiographic evolution. Tunis Med 2019; 97: 1258–67. [PubMed] [Google Scholar]
- 266.Mehta SR, Cannon CP, Fox KA, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA 2005; 293: 2908–17. [DOI] [PubMed] [Google Scholar]
- 267.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361: 13–20. [DOI] [PubMed] [Google Scholar]
- 268.Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med 2019; 381: 1411–21. [DOI] [PubMed] [Google Scholar]
- 269.ISIS-2 Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2: 349–60. [PubMed] [Google Scholar]
- 270.Abdallah MS, Wang K, Magnuson EA, et al. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA 2013; 310: 1581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Baron SJ, Chinnakondepalli K, Magnuson EA, et al. Quality-of-life after everolimus-eluting stents or bypass surgery for left-main disease: results from the EXCEL trial. J Am Coll Cardiol 2017; 70: 3113–22. [DOI] [PubMed] [Google Scholar]
- 272.Ernst E, Resch KL. Concept of true and perceived placebo effects. BMJ 1995; 311: 551–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol 2000; 53: 786–92. [DOI] [PubMed] [Google Scholar]
- 274.Wartolowska K, Judge A, Hopewell S, et al. Use of placebo controls in the evaluation of surgery: systematic review. BMJ 2014; 348: g3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018; 391: 31–40. [DOI] [PubMed] [Google Scholar]
- 276.Parisi AF, Folland ED, Hartigan P. A comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. N Engl J Med 1992; 326: 10–16. [DOI] [PubMed] [Google Scholar]
- 277.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87–165. [DOI] [PubMed] [Google Scholar]
- 278.Rajkumar CA, Foley MJ, Ahmed-Jushuf F, et al. A placebo-controlled trial of percutaneous coronary intervention for stable angina. N Engl J Med 2023; 389: 2319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rajkumar CA, Foley MJ, Ahmed-Jushuf F, et al. N-of-1 trial of angina verification before percutaneous coronary intervention. J Am Coll Cardiol 2024; 84: 1–12. [DOI] [PubMed] [Google Scholar]
- 280.Simader FA, Rajkumar CA, Foley MJ, et al. Symptoms as a predictor of the placebo-controlled efficacy of PCI in stable coronary artery disease. J Am Coll Cardiol 2024; 84: 13–24. [DOI] [PubMed] [Google Scholar]
- 281.Weldring T, Smith SM. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv Insights 2013; 6: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005; 293: 1223–38. [DOI] [PubMed] [Google Scholar]
- 283.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995; 25: 333–41. [DOI] [PubMed] [Google Scholar]
- 284.Ganesananthan S, Rajkumar CA, Foley M, Francis D, Al-Lamee R. Remote digital smart device follow-up in prospective clinical trials: early insights from ORBITA-2, ORBITA-COSMIC, and ORBITA-STAR. Eur Heart J Suppl 2022; 24 (suppl H): H32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Korjian S, McCarthy KJ, Larnard EA, et al. Drug-coated balloons in the management of coronary artery disease. Circ Cardiovasc Interv 2024; 17: e013302. [DOI] [PubMed] [Google Scholar]
- 286.Jinnouchi H, Torii S, Sakamoto A, Kolodgie FD, Virmani R, Finn AV. Fully bioresorbable vascular scaffolds: lessons learned and future directions. Nat Rev Cardiol 2019; 16: 286–304. [DOI] [PubMed] [Google Scholar]
- 287.Verheye S, Jolicœur EM, Behan MW, et al. Efficacy of a device to narrow the coronary sinus in refractory angina. N Engl J Med 2015; 372: 519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Foley MJ, Rajkumar CA, Ahmed-Jushuf F, et al. Coronary sinus reducer for the treatment of refractory angina (ORBITA-COSMIC): a randomised, placebo-controlled trial. Lancet 2024; 403: 1543–53. [DOI] [PubMed] [Google Scholar]
- 289.Arora RR, Chou TM, Jain D, et al. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol 1999; 33: 1833–40. [DOI] [PubMed] [Google Scholar]
- 290.Schmid JP, Capoferri M, Wahl A, Eshtehardi P, Hess OM. Cardiac shock wave therapy for chronic refractory angina pectoris. A prospective placebo-controlled randomized trial. Cardiovasc Ther 2013; 31: e1–6. [DOI] [PubMed] [Google Scholar]
- 291.Horvath KA, Cohn LH, Cooley DA, et al. Transmyocardial laser revascularization: results of a multicenter trial with transmyocardial laser revascularization used as sole therapy for end-stage coronary artery disease. J Thorac Cardiovasc Surg 1997; 113: 645–53, discussion 653–54. [DOI] [PubMed] [Google Scholar]
- 292.Henry TD, Bairey Merz CN, Wei J, et al. Autologous CD34+ stem cell therapy increases coronary flow reserve and reduces angina in patients with coronary microvascular dysfunction. Circ Cardiovasc Interv 2022; 15: e010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Povsic TJ, Henry TD, Traverse JH, et al. The RENEW trial: efficacy and safety of intramyocardial autologous CD34(+) cell administration in patients with refractory angina. JACC Cardiovasc Interv 2016; 9: 1576–85. [DOI] [PubMed] [Google Scholar]
- 294.Xing L, Yamamoto E, Sugiyama T, et al. EROSION study (effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion): a 1-year follow-up report. Circ Cardiovasc Interv 2017; 10: e005860. [DOI] [PubMed] [Google Scholar]
- 295.Niccoli G, Montone RA, Di Vito L, et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J 2015; 36: 1377–84. [DOI] [PubMed] [Google Scholar]
- 296.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011; 364: 226–35. [DOI] [PubMed] [Google Scholar]
- 297.Prati F, Romagnoli E, Gatto L, et al. Relationship between coronary plaque morphology of the left anterior descending artery and 12 months clinical outcome: the CLIMA study. Eur Heart J 2020; 41: 383–91. [DOI] [PubMed] [Google Scholar]
- 298.Erlinge D, Maehara A, Ben-Yehuda O, et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet 2021; 397: 985–95. [DOI] [PubMed] [Google Scholar]
- 299.Nicholls SJ, Kataoka Y, Nissen SE, et al. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging 2022; 15: 1308–21. [DOI] [PubMed] [Google Scholar]
- 300.Ahn JM, Kang DY, Lee PH, et al. Preventive PCI or medical therapy alone for vulnerable atherosclerotic coronary plaque: rationale and design of the randomized, controlled PREVENT trial. Am Heart J 2023; 264: 83–96. [DOI] [PubMed] [Google Scholar]
- 301.Stone GW, Maehara A, Ali ZA, et al. Percutaneous coronary intervention for vulnerable coronary atherosclerotic plaque. J Am Coll Cardiol 2020; 76: 2289–301. [DOI] [PubMed] [Google Scholar]
- 302.Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 2015; 66: 337–46. [DOI] [PubMed] [Google Scholar]
- 303.Misra A, Ramchandran A, Jayawardena R, Shrivastava U, Snehalatha C. Diabetes in south Asians. Diabet Med 2014; 31: 1153–62. [DOI] [PubMed] [Google Scholar]
- 304.Van Spall HGC, Averbuch T, Damman K, Voors AA. Risk and risk reduction in trials of heart failure with reduced ejection fraction: absolute or relative? Eur J Heart Fail 2021; 23: 1437–44. [DOI] [PubMed] [Google Scholar]
- 305.Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 2015; 36: 1163–70. [DOI] [PubMed] [Google Scholar]
- 306.Senn S Statistical pitfalls of personalized medicine. Nature 2018; 563: 619–21. [DOI] [PubMed] [Google Scholar]
- 307.Sturm AC, Knowles JW, Gidding SS, et al. Clinical genetic testing for familial hypercholesterolemia: JACC scientific expert panel. J Am Coll Cardiol 2018; 72: 662–80. [DOI] [PubMed] [Google Scholar]
- 308.Sundström J, Lind L, Nowrouzi S, et al. Heterogeneity in blood pressure response to 4 antihypertensive drugs: a randomized clinical trial. JAMA 2023; 329: 1160–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.PharmGKB. Annotation of CPIC guideline for clopidogrel and CYP2C19. 2022. https://www.pharmgkb.org/guidelineAnnotation/PA166104948#:~:text=The%20CPIC%20Dosing%20Guideline%20for,clinically%20indicated%20and%20no%20contraindication (accessed Jan 14, 2025).
- 310.PharmGKB. Annotation of CPIC guideline for atorvastatin and SLCO1B1. 2022. https://www.pharmgkb.org/guidelineAnnotation/PA166262221#:~:text=Summary,based%20on%20disease%2Dspecific%20guidelines (accessed Jan 14, 2025).
- 311.Jernberg T, Attebring MF, Hambraeus K, et al. The Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010; 96: 1617–21. [DOI] [PubMed] [Google Scholar]
- 312.Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY, Van Calster B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol 2019; 110: 12–22. [DOI] [PubMed] [Google Scholar]
- 313.European Medicines Agency. European Medicines Agency network strategy to 2025: protecting public health at a time of rapid change. 2020. https://www.ema.europa.eu/en/documents/report/european-union-medicines-agencies-network-strategy-2025-protecting-public-health-time-rapid-change_en.pdf (accessed Jan 14, 2025).
- 314.Medicines & Healthcare Products Regulatory Agency. Medicines and Healthcare products Regulatory Agency delivery plan 2021–2023. 2022. https://www.gov.uk/government/publications/the-medicines-and-healthcare-products-regulatory-agency-delivery-plan-2021-2023/medicines-and-healthcare-products-regulatory-agency-delivery-plan-2021-2023 (accessed Jan 14, 2025).
- 315.Joynt Maddox KE, Elkind MSV, Aparicio HJ, et al. Forecasting the burden of cardiovascular disease and stroke in the United States through 2050-prevalence of risk factors and disease: a presidential advisory from the American Heart Association. Circulation 2024; 150: e65–88. [DOI] [PubMed] [Google Scholar]
- 316.Tanguturi VK, Kennedy KF, Virani SS, Maddox TM, Armstrong K, Wasfy JH. Association between poverty and appropriate statin prescription for the treatment of hyperlipidemia in the United States: an analysis from the ACC NCDR PINNACLE registry. Cardiovasc Revasc Med 2020; 21: 1016–21. [DOI] [PubMed] [Google Scholar]
- 317.Ellis C, Gamble G, Devlin G, et al. The management of acute coronary syndrome patients across New Zealand in 2012: results of a third comprehensive nationwide audit and observations of current interventional care. N Z Med J 2013; 126: 36–68. [PubMed] [Google Scholar]
- 318.Hyun K, Negrone A, Redfern J, et al. Gender difference in secondary prevention of cardiovascular disease and outcomes following the survival of acute coronary syndrome. Heart Lung Circ 2021; 30: 121–27. [DOI] [PubMed] [Google Scholar]
- 319.Hudson M, Richard H, Pilote L. Parabolas of medication use and discontinuation after myocardial infarction—are we closing the treatment gap? Pharmacoepidemiol Drug Saf 2007; 16: 773–85. [DOI] [PubMed] [Google Scholar]
- 320.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation 2006; 113: 203–12. [DOI] [PubMed] [Google Scholar]
- 321.Timmis A, Vardas P, Townsend N, et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J 2022; 43: 716–99. [DOI] [PubMed] [Google Scholar]
- 322.Organisation for Economic Co-operation and Development. Health statistics. 2021. https://web-archive.oecd.org/2023-02-01/70274-health-statistics.htm (accessed Jan 14, 2025).
- 323.Schiele F, Aktaa S, Rossello X, et al. 2020 update of the quality indicators for acute myocardial infarction: a position paper of the Association for Acute Cardiovascular Care: the study group for quality indicators from the ACVC and the NSTE-acute coronary syndrome guideline group. Eur Heart J Acute Cardiovasc Care 2021; 10: 224–33. [DOI] [PubMed] [Google Scholar]
- 324.Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med 2018; 378: 1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021; 374: n2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Nieuwlaat R, Schwalm JD, Khatib R, Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease? Eur Heart J 2013; 34: 1262–69. [DOI] [PubMed] [Google Scholar]
- 327.Lane-Fall MB, Curran GM, Beidas RS. Scoping implementation science for the beginner: locating yourself on the “subway line” of translational research. BMC Med Res Methodol 2019; 19: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Glasgow RE, Vinson C, Chambers D, Khoury MJ, Kaplan RM, Hunter C. National Institutes of Health approaches to dissemination and implementation science: current and future directions. Am J Public Health 2012; 102: 1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Curran GM, Landes SJ, McBain SA, et al. Reflections on 10 years of effectiveness-implementation hybrid studies. Front Health Serv 2022; 2: 1053496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA 2012; 307: 1513–16. [DOI] [PubMed] [Google Scholar]
- 331.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on performance measures and task force on practice guidelines. J Am Coll Cardiol 2014; 63: 2304–22. [DOI] [PubMed] [Google Scholar]
- 332.Terkelsen CJ, Sørensen JT, Maeng M, et al. System delay and mortality among patients with STEMI treated with primary percutaneous coronary intervention. JAMA 2010; 304: 763–71. [DOI] [PubMed] [Google Scholar]
- 333.García-García C, Ribas N, Recasens LL, et al. In-hospital prognosis and long-term mortality of STEMI in a reperfusion network. “Head to head” analysis: invasive reperfusion vs optimal medical therapy. BMC Cardiovasc Disord 2017; 17: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Jomaa W, Hamdi S, Ben Ali I, et al. Risk profile and in-hospital prognosis in elderly patients presenting for acute ST-elevation myocardial infarction in the Tunisian context. Indian Heart J 2016; 68: 760–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Boniol M, Kunjumen T, Nair TS, Siyam A, Campbell J, Diallo K. The global health workforce stock and distribution in 2020 and 2030: a threat to equity and ‘universal’ health coverage? BMJ Glob Health 2022; 7: e009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.WHO. HEARTS: technical package for cardiovascular disease management in primary health care. 2016. https://www.who.int/publications/i/item/9789240001367 (accessed Jan 14, 2025).
- 337.Schwalm JD, McCready T, Lopez-Jaramillo P, et al. A community-based comprehensive intervention to reduce cardiovascular risk in hypertension (HOPE 4): a cluster-randomised controlled trial. Lancet 2019; 394: 1231–42. [DOI] [PubMed] [Google Scholar]
- 338.Lau ES, Hayes SN, Volgman AS, et al. Does patient-physician gender concordance influence patient perceptions or outcomes? J Am Coll Cardiol 2021; 77: 1135–38. [DOI] [PubMed] [Google Scholar]
- 339.Gomez LE, Bernet P. Diversity improves performance and outcomes. J Natl Med Assoc 2019; 111: 383–92. [DOI] [PubMed] [Google Scholar]
- 340.Burgess S, Shaw E, Ellenberger K, Thomas L, Grines C, Zaman S. Women in medicine: addressing the gender gap in interventional cardiology. J Am Coll Cardiol 2018; 72: 2663–67. [DOI] [PubMed] [Google Scholar]
- 341.Mehta LS, Fisher K, Rzeszut AK, et al. Current demographic status of cardiologists in the United States. JAMA Cardiol 2019; 4: 1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Kurdi H, Morgan H, Williams C. Women not in cardiology: where are we going wrong? A survey of the perceptions and barriers to training. Br J Cardiol 2020; 27: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Perera S, Aslam A, Burgess S, et al. Gender differences in medical student perceptions of a career in cardiology. Heart Lung Circ 2023; 32: 1250–56. [DOI] [PubMed] [Google Scholar]
- 344.Chang M, Hahn RA, Teutsch SM, Hutwagner LC. Multiple risk factors and population attributable risk for ischemic heart disease mortality in the United States, 1971–1992. J Clin Epidemiol 2001; 54: 634–44. [DOI] [PubMed] [Google Scholar]
- 345.Chugh A, Arora M, Jain N, et al. The global impact of tobacco control policies on smokeless tobacco use: a systematic review. Lancet Glob Health 2023; 11: e953–68. [DOI] [PubMed] [Google Scholar]
- 346.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017; 390: 2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Singh S, Awasthi S, Kapoor V, Mishra P. Childhood obesity in India: a two-decade meta-analysis of prevalence and socioeconomic correlates. Clin Epidemiol Glob 2023; 23: 101390. [Google Scholar]
- 348.Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ 2016; 352: h6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Birdsey J, Cornelius M, Jamal A, et al. Tobacco product use among US middle and high school students—national youth tobacco survey, 2023. MMWR Morb Mortal Wkly Rep 2023; 72: 1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Murukutla N, Cotter T, Wang S, et al. Results of a mass media campaign in South Africa to promote a sugary drinks tax. Nutrients 2020; 12: 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Boateng D, Wekesah F, Browne JL, et al. Knowledge and awareness of and perception towards cardiovascular disease risk in sub-Saharan Africa: a systematic review. PLoS One 2017; 12: e0189264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Nieblas-Bedolla E, Bream KDW, Rollins A, Barg FK. Ongoing challenges in access to diabetes care among the indigenous population: perspectives of individuals living in rural Guatemala. Int J Equity Health 2019; 18: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Austin C. The impact of social determinants of health of Australian Indigenous women on access and engagement in maternal child health services. J Adv Nurs 2023; 79: 1815–29. [DOI] [PubMed] [Google Scholar]
- 354.Sun D, Pang Y, Lyu J, Li L. Current progress and challenges to tobacco control in China. China CDC Wkly 2022; 4: 101–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Arikan I, Karakaya K, Erata M, et al. Fighting obesity campaign in Turkey: evaluation of media campaign efficacy. Cent Eur J Public Health 2014; 22: 170–74. [DOI] [PubMed] [Google Scholar]
- 356.Guillory J, Henes A, Farrelly MC, et al. Awareness of and receptivity to the fresh empire tobacco public education campaign among hip hop youth. J Adolesc Health 2020; 66: 301–07. [DOI] [PubMed] [Google Scholar]
- 357.McAlister FA, Feldman RD, Wyard K, et al. The impact of the Canadian Hypertension Education Programme in its first decade. Eur Heart J 2009; 30: 1434–39. [DOI] [PubMed] [Google Scholar]


