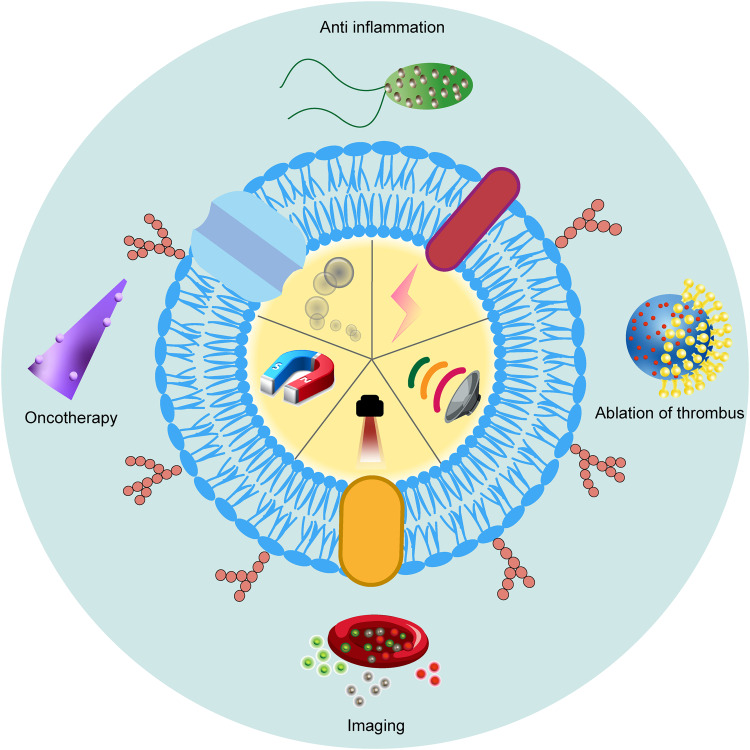
Abstract
The integration of synthetic micro/nanomotors (MNMs) with natural biomaterials derived from cell membranes has emerged as a highly promising strategy for advancing biological applications. The membrane-coated MNM offers distinct advantages over earlier MNMs dependent on chemical fuels such as hydrogen peroxide, which were prone to poor biocompatibility and harmful byproducts. These include enhanced biocompatibility, immune evasion via natural membrane surfaces, multifunctionality for drug delivery, self-propulsion in complex environments, self-degradation to reduce residual toxicity and inherent imaging capabilities enabling real-time tracking. This review provides a comprehensive overview of the integration of various types of micromotors with natural cell membranes commonly present in the circulatory system. Additionally, it summarizes the methodologies for the preparation, characterization and functional evaluation of biofilm-modified micromotors. The present article also critically examines current developments in biofilm-modified micromotors, addressing their challenges and limitations in enhancing clinical efficacy and facilitating transition to clinical trials in humans.
Keywords: micro/nanomotors, nanodrugs, membrane-coated, self-propulsion, drug delivery
Graphical Abstract
Introduction
MNMs are micro/nanoscale objects capable of using internal (chemical fuel,1 enzyme-powered2,3) or external energy (electric field,4 magnetic field,5 light,6 ultrasound,7 X-ray8) energies to create bubbles, local potential differences, diffusion gradients, temperature differences and surface tension gradients. These artificial MNMs have demonstrated exceptional capabilities to navigate through biofluids,9,10 while engaging in numerous critical tasks for biomedical applications11,12 including targeted drug delivery,13–16 immunomodulation,17,18 adjunctive therapies19 such as in vivo imaging,20 barrier penetration,21 diagnostics13,22,23 and precision surgery.16,24–27 However, exposed artificial motors deliver various disadvantages including simple detection and action by the reticuloendothelial system, which also leads to the rapid elimination from the body.28 Synthetic materials are sensitive to immunological responses and biofouling in complex biological systems, which hinder the function and efficiency of MNMs.29–32 Therefore, biocompatible and bionic setups are required for MNMs to function in the physiological milieu for an extended period.
Many researchers have explored bionic MNMs that utilize natural cell membranes to camouflage motors 33 to address this critical challenge. The preparation strategies for MNM primarily involve physical assembly (ultrasonication33 and extrusion)34, chemical modification (surface anchoring)35 and biological membrane reconstitution,29 focusing on modulating the affinity between the membrane and motor while preserving membrane functionality to meet biological application requirements. In 2011, Zhang et al introduced nanoparticles based on biomimetic materials.36 The research team synthesized RBC-NPs by extruding erythrocyte membranes. In addition, researchers have developed several alternative methods to coat cell membranes derived from stem cells,37,38 cancer cells,39 macrophages,40,41 neutrophils,42 natural killer cells,43 T cells44 and platelets.45 This approach utilizes the essential biological functions of innate cells including immune cloaking, precise antigen delivery, tissue-specific targeting, long-cycle characteristics and selective binding to bacterial toxins or pathogens. The combination of synthetic motors and the advantages of their dynamic motor has resulted in the development of cell-like micromotors, which exhibit the characteristics of progenitor cells and high efficacy in biocompatibility and autologous appearance.46 Due to their rapid advancement, bio-functional MNMs are becoming a key hotspot in biomedical research.15 The functionalization of cell membranes enables artificial motors to be biocompatible, which further allows micromotor research to move from lab tubes to biological systems. This review focuses on the unique capabilities, recent advancements and representative applications of cell membrane-functionalized micromotors.
Membrane-Functionalized MNMs
RBC Membrane-Functionalized MNMs
Erythrocytes play a vital role in the transportation of oxygen within the bloodstream and represent the majority of blood cells in vertebrates.47 Current evidence indicates that erythrocytes possess significant biocompatibility, biodegradability and non-immunogenicity, which can be highly suitable for bioengineering applications (Table 1).48–50 Erythrocytes eliminate toxins and circulate for prolonged periods.47 Membranes from mature erythrocytes are easier to separate and purify due to the absence of nuclei.51 Considering these characteristics, erythrocyte membranes are a highly suitable source for the development of MNMs.
Table 1.
Summary of RBC Membrane-Functionalized MNMs
| Substrate | Propulsion Source | Size | Speed | Environment | Application Field | Ref. |
|---|---|---|---|---|---|---|
| RBCs | Acoustically powered and magnetically navigated | 6±8 μm | 13-14 μms−1 | Whole blood | Absorption and neutralization of hemolytic toxins | [29] |
| RBCs | Acoustically powered and magnetically navigated | 400 μm with 6–8 nm thick fluid-like lipid bilayer | 14 μms-1, 27 μms−1 | Whole blood and distilled water | Absorption and neutralization of hemolytic toxins | [33] |
| RBCs | Bubble propulsion | 20 μm | 172 μms−1, 33 μm s−1 | Distilled water and albumin medium | Absorption and neutralization of hemolytic toxins | [52] |
| RBCs | Acoustically powered and magnetically navigated | 6-8μm | 14±(1–2) μms−1/155 μms−1 | 2.4MHz, a Triton lysis buffer solution: 2V/8V | Cargo transport, drug delivery and thermal imaging | [53] |
| RBCs | Near-infrared light drive: thermal gradient | ≈5μm | Max:19.8 μms−1 12.10 μms−1, 3.52 μms−1, 2.33 μms−1 |
PBS, medium, serum and blood | Ablation of thrombus | [54] |
| RBCs and Escherichia coli | Self-propulsion and magnetically navigated | ≈5μm | 10.2±3.5 μms−1 | Magnetic field (20 mT) | Cargo delivery | [55] |
| RBCs | Acoustically powered and magnetically navigated | 2.1±0.3μm | Max:56.5 μms−1 15.39 μms−1, 10.13 μms−1, 7.15 μms−1 | 2.15Hz, 0.5W: PBS, medium, serum and blood | Cancer therapy | [56] |
| RBCs | Acoustically powered | ≈400nm | 8.4 μms−1 | Distilled water (49 v) | Oxygen transport | [57] |
| RBCs | Bubble propulsion | ≈25μm | Not mentioned | Vivo: Intestine of mice | Oral antitoxicity vaccine | [58] |
| RBCs | Magnetically navigated | 9.0 ± 0.3 μm | 8.80±0.41μms−1 | 20 mT, 10 Hz | Drug Delivery and Image-Guided Therapy | [59] |
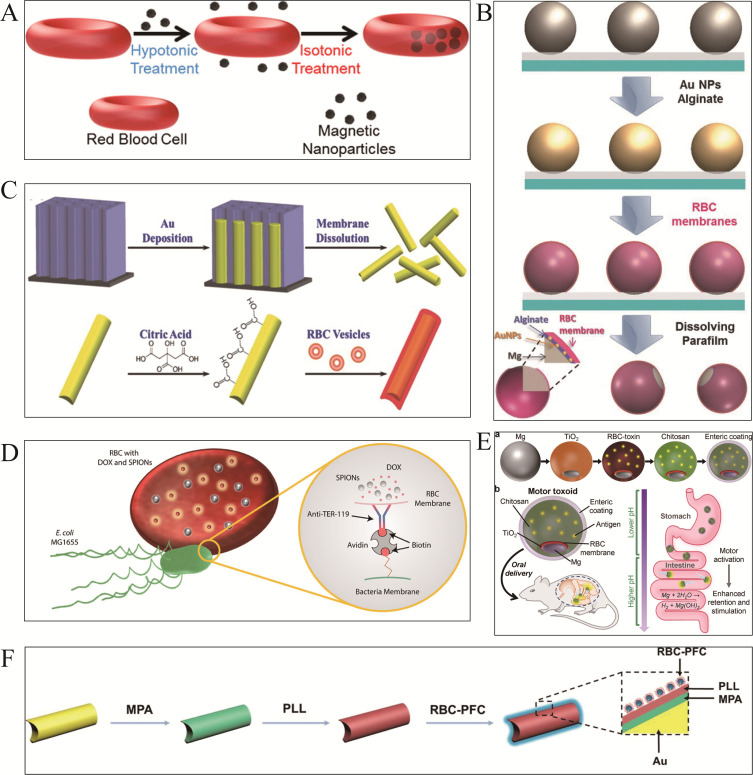
In 2014, Wu et al29 proposed and developed a bionic micromotor covered by the erythrocyte membrane (Figure 1A). This motor with cell camouflage was designed by combining ferric oxide nanoparticles (20 nm) into the vesicles of the erythrocyte membrane through a hypotonic dilution/encapsulation technique. Red blood cell motors preserve the discoid form of RBCs with a diameter of 6±8 μm. Magnetic fields exhibit stable, high-speed (13–14 μms-1), efficient and adaptable ultrasonic transportation in multiple biological media including blood, which can be an effective toxin bait for absorbing membrane-damaging toxins. Wu et al52 reported an RBC membrane-functionalized magnesium-based Janus micromotor (Figure 1B) constructed by integrating RBC membranes, gold nanoparticles (AuNPs) and alginate (ALG) onto the exposed surface area of Mg microparticles partially embedded in a paraffin film. Natural motile cells are driven by spontaneous magnesium-water reaction, high-current corrosion of AuNPs and pitting corrosion of coexisting chloride ions. Researchers have revealed that the magnesium core of RBC-Mg Janus motors remains intact after the magnesium core is completely dissolved in the alpha-toxin/albumin solution. The RBC membrane neutralizes alpha toxin and protects the cells. It was found that the detoxifying level was significantly enhanced. An alternative to double concave discs was constructed with a smaller rod-like motor33 (Figure 1C). Biomimetic motor sponges were designed under ultrasound using gold nanowires modified with a negative charge and erythrocyte membrane vesicles. Motor sponges possessed a “right-side-out membrane orientation”, facilitating the selective uptake of pore-forming toxins and protecting them from non-specific protein adsorption within their biological medium.
Figure 1.
RBC membrane-functionalized micromotors. (A) RBC-based magnetic-driven MNMs for toxin neutralization. Reproduced from Wu Z, Li T, Li J, et al. Turning erythrocytes into functional micromotors. ACS Nano. 2014;8(12):12041–12048. Copyright © 2014 American Chemical Society.29 (B) RBC-Mg Janus MNMs for toxin absorption. Reproduced from Wu Z, Li J, de Avila BE-F, et al. Water-powered cell-mimicking janus micromotor. Adv Funct Mater. 2015;25(48):7497–7501. Copyright © 2015 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.52 (C) RBC was mimicking rod-like MNMs for toxins neutralization. Reproduced from Wu Z, Li T, Gao W, et al. Cell-membrane-coated synthetic nanomotors for effective biodetoxification. Adv Funct Mater. 2015;25(25):3881–3887. Copyright © 2015 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.33 (D) RBC-based bacterial microswimmer. Reproduced from Alapan Y, Yasa O, Schauer O, et al. Soft erythrocyte-based bacterial microswimmers for cargo delivery. Sci Rob. 2018;3(17):eaar4423. Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.55 (E) RBC-PFC MNMs for oxygen transport. a) The assembly process of RBC-PFC MNMs. b) The detailed structural diagram of RBC-PFC MNMs and their simulated effects in the intestine. Reproduced from Zhang F, Zhuang J, de Avila BEF, et al. A nanomotor-based active delivery system for intracellular oxygen transport. ACS Nano. 2019;13(10):11996–12005. Copyright © 2019 American Chemical Society.57 (F) RBC membrane-coated Mg-Tio2 MNMs as an oral antitoxicity vaccine. Reproduced from Wei X, Beltrán-Gastélum M, Karshalev E, et al. Biomimetic micromotor enables active delivery of antigens for oral vaccination. Nano Lett. 2019;19(3):1914–1921. Copyright © 2019 American Chemical Society.58
Erythrocyte membranes can eliminate and neutralize toxins, alter the surface of motors and deliver a variety of biological functions. Yunus et al recently designed a bacterial flagella-driven RBC hybrid microswimmer for drug delivery55 (Figure 1D). Bioengineered sports bacterium Escherichia coli MG1655 is used to attach RBCs loaded with doxorubicin (DOX) and superparamagnetic iron oxide nanoparticles (SPION). The results revealed that the release of DOX was significantly enhanced at lower pH values, especially at pH=3. The acidic tumor microenvironment promotes the pharmacological effects of anti-cancer drugs. It has been reported that erythrocyte membrane motors can transport gas molecules. The motor-based gas delivery system by Joseph et al combined sound power Au NWs rapid advance and the RBC membrane with fluorinated carbon nanoemulsion (RBC-PFC) carrying high-capacity oxygen to facilitate active oxygen transport within the cell (Figure 1E).57 RBC-PFC nanomotors (Motor-PFC) were designed with a 400 nm diameter and 8.4 μms-1 rotating speed. The motor-PFC may penetrate the cell membrane and continuously release intracellular oxygen. Ultrasound irradiation enhances oxygen transport in J774 macrophages and rapidly promotes cell survival by moving the Motor-PFC. Therefore, motor-PFC may be an efficient active transporter for intracellular oxygen and other therapeutic gas molecules. Recently, Wei et al synthesized an RBC membrane-coated Mg-TiO2 motor (Figure 1F) 58 with Mg as the core for power supply. Erythrocyte membrane coating neutralizes the toxin and loads onto MNMs for motor antigenicity. In the in vivo experiment, mice were orally administered the vaccine to study its distribution and retention in the gastrointestinal tract and the ability to induce an immune response to generate IgA antibodies.60 MNMs are activated and carried in the surrounding fluid upon entering the intestine, which further enhances the retention and penetration of the antigenic payload. The results demonstrated that motor toxoids elicit a greater production of IgA antibodies targeting alpha-toxin, which represents significant progress in the application of nanomotors in immunizations. Erythrocyte membrane acts as a substrate to alter the surface of other motors for photodynamic therapy,59 thrombus ablation,45 thermal imaging53 and other biomedical applications.
Leukocyte Membrane-Functionalized MNMs
Leukocytes are the primary immune cells in the body and are classified as granulocytes (mainly neutrophils), monocytes-macrophages, lymphocytes, natural killer (NK) cells and dendritic cells (DCs). Leukocytes exhibit active locomotion using pseudopods to move within and outside the blood vessels.54 Moreover, leukocytes exhibit chemotaxis and targeted motility in the presence of chemokines.56 Consequently, leukocytes have recently been studied as bionic MNMs that can significantly target inflammatory and cancer microenvironments (Table 2).61–63
Table 2.
Summary of Leukocyte Membrane-Functionalized MNMs
| Substrate | Fuel | Size | Speed | Environment | Application Field | Ref. |
|---|---|---|---|---|---|---|
| Leukocyte | Acoustically powered and magnetically navigated | 7.03±0.6 μm | 52.9 μms−1, 35.6 μms−1 | Serum, blood | Cancer therapy | [64] |
| Macrophage cells | Bubble propulsion:H2 | 23-44 μm | 127.3 μms−1 | Simulated gastric fluid (pH 1.3) | Endotoxin neutralization | [65] |
| Macrophage cells | Near-infrared light drive: thermal gradient | ≈80 nm | 6.44 μms−1, 4.71 μms−1, 1.46 μms−1 | 2.92Wcm−2:PBS, CM, FBS | Cancer therapy | [66] |
| Macrophage cells | Bubble propulsion: O2 | ≈50 nm | 43.2μ ms−1 | Vivo: a mouse model with acute pneumonia | Acute pneumonia | [67] |
| Macrophage cells | Photoacoustic (PA) imaging guided and magnetic guided | 182±3 nm | 10 μms−1 | A gradient Magnetic field (19.2 Tm-1) | Cancer therapy | [63] |
| Macrophage cells | Bubble propulsion: NO | 102.59 nm | Not mentioned | Blood; vivo: in a Mouse myocardial injury model | Heart repair and regeneration | [34] |
| Neutrophil | Self-propulsion | Not provided | 0.165 μms−1 | Agarose Hydrogel containing E. coli | Drug delivery | [68] |
| Neutrophil | Self-propulsion | ≈5-6 μm | 104.6±11.2 μms-1, >110 μms−1 | 22°C Tris-acetate-phosphate medium, simulated lung fluid | Acute bacterial pneumonia | [69] |
| Neutrophil | Magnetically navigated | ≈105 nm | 400 μms−1 | Rotating Magnetic field (RMF) | Cancer therapy | [70] |
Macrophage Cell Membrane-Functionalized Micromotors
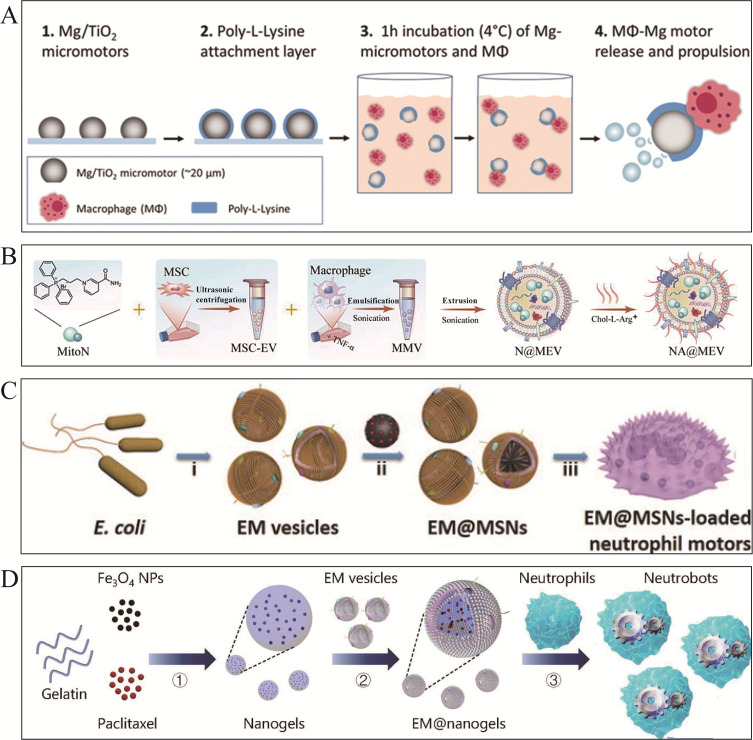
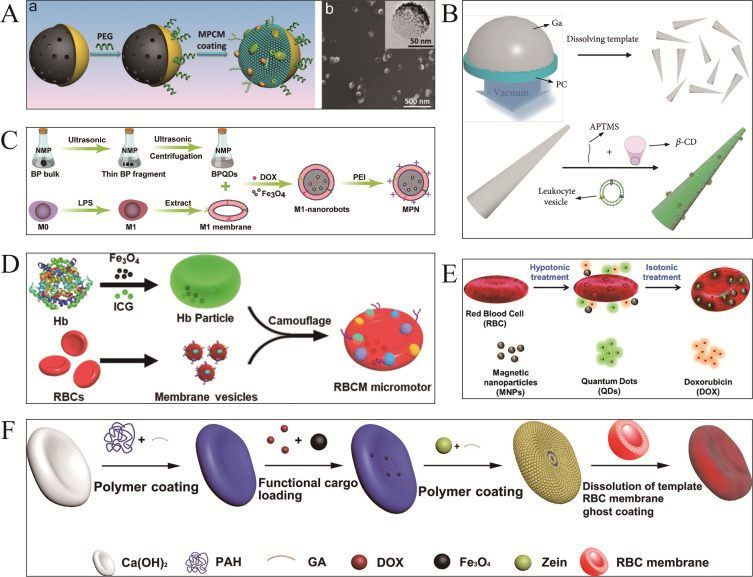
Macrophages regulate the immune system, which engulfs and eliminates a variety of pathological organisms. In addition, macrophages have distinct receptors for biotoxins including endotoxins. Zhang et al62 proposed the idea of hybridizing macrophages with magnesium micromotors (Figure 2A). A layer of titanium dioxide (TiO2) and polylysine (PLL) was coated on Mg particles, resulting in a diameter of approximately 23–44 μm. Mg core and protons may form a hydrogen bubble tail, which increases motor functions in acidic environments. The Mφ-Mg hybrid motor effectively neutralizes endotoxins, neutralizing about 50% in the first two minutes, which reveals high detoxification capability.41 Recently, Zhang et al34 constructed a bionic nitric oxide synthase-responsive nanomotor (NA@MEV) for enhancing cardiac repair and regeneration (Figure 2B), which was coated with a hybrid cell-derived extracellular vesicle (N@MEV) composed of mesenchymal stem cell-derived extracellular vesicles, macrophage cell membrane vesicles and ROS scavenger MitoN. Inflammatory signals from cardiac damage trigger hybrid extracellular vesicles to target and enhance the injury site.66 Therefore, researchers have investigated the application of macrophage membrane-encapsulated MNMs for targeted therapy.
Figure 2.
Leukocyte membrane-functionalized MNMs. (A) Mφ-Mg hybrid MNMs for toxin absorption. Reproduced from Zhang F, Mundaca-Uribe R, Gong H, et al. A macrophage-magnesium hybrid biomotor: fabrication and characterization. Adv Mater. 2019;31(27):e1901828. Copyright © 2019 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.62 (B) NA@MEV MNMs for cardiac repair and regeneration. Reproduced from Zhang N, Fan M, Zhao Y, et al. Biomimetic and NOS-responsive nanomotor deeply delivery a combination of MSC-EV and mitochondrial ROS scavenger and promote heart repair and regeneration. Adv Sci. 2023;10(21):e2301440. Copyright © 2023 The Authors. Advanced Science published by Wiley‐VCH GmbH. Creative Commons CC BY license.34 (C) EM@MNMs-loaded neutrophil MNMS for drug delivery. i) Preparation and synthesis of EM vesicles. ii) Preparation of EM@MSNs via vesicle fusion strategy. iii) Co-incubate EM@MSNs with neutrophils to prepare hybrid neutrophil micromotors. Reproduced from Shao J, Xuan M, Zhang H, Lin X, Wu Z, He Q. Chemotaxis-guided hybrid neutrophil micromotors for targeted drug transport. Angew Chem Int Ed Engl. 2017;56(42):12935–12939. Copyright © 2017 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim.64 (D) Neutrophils-membraned EM@nanogels MNMs for blood-brain-barrier crossing. Reproduced from Zhang H, Li Z, Gao C, et al. Dual-responsive biohybrid neutrobots for active target delivery. Sci Rob. 2021;6(52). Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.71
Neutrophil Cell Membrane-Functionalized Micromotors
However, internalized nanodrugs face crucial challenges in penetrating into inflamed tissues and premature outflow, which limits their translational outcomes. To address this critical issue, Yue et al64 synthesized hybrid neutrophil micromotors that transported drug-loaded Mesoporous silica nanoparticles (MSNs) by wrapping them in E. coli membranes (EMs) and incubating with neutrophils (NEs) (Figure 2C). It was found that encapsulating DOX in EM@MSNs significantly reduced the leakage of DOX. Furthermore, neutrophils migrate to the inflamed areas through chemotaxis, which further produces a superior level of MNMs in drug delivery. A new study has established a micromotor identified as a “neutrobot” that is responsive to dual stimuli and utilizes neutrophil71 (Figure 2D). E. coli membrane is wrapped with paclitaxel (PTX)-loaded magnetic nanogels and then engulfed with NEs. E. coli membranes also serve as encapsulating mechanisms. Chemotaxis and magnetic propulsion activate the microrobot to cross the blood-brain barrier and release drug-loaded nanogels. Therefore, neutrobots can be promising drug transporters for alleviating inflammatory tumors, particularly intracranial tumors.
Platelet Membrane-Functionalized MNMs
Platelets produced from developed erythrocyte cytoplasmic components in the bone marrow regulate hemostasis, thrombosis and inflammatory response.68,70 However, early studies have revealed that platelet membrane-coated nanoparticles can significantly enhance the ability to adhere to pathogens,65,67,69 reduce cellular uptake by several immune cells,68,72 improve targeting and affinity in tumors.73 Platelet membranes have recently been employed to develop autonomous micromotors (Table 3).74
Table 3.
Summary of Platelet Membrane-Functionalized MNMs
| Substrate | Driving Force | Size | Speed | Concentration | Application Field | Ref. |
|---|---|---|---|---|---|---|
| PLTs | Magnetically navigated | ≈3-5 μm | ≈18.5,17,14.5,12.5 μms-1 | 55 Hz, 15 mT: water, plasma, serum and blood | Bacteria isolation | [45] |
| PLTs | Acoustically powered | 400 nm (1.5–2.0 μm) | 46 μms-1; 35 μms-1 | Water, the whole blood | Targeting and neutralization of pathogenic bacteria and toxins | [75] |
| PLTs | Near-infrared light drive: thermal gradient | 410 nm | Not mentioned | Vivo: a rat model with abdominal thrombosis | Thrombus ablation | [76] |
| PLTs | Self-propulsion | 1.4–2.6 μm | ≈7 μms-1 | Urea concentrations (200 mM) | Drug delivery | [35] |
| PLTs | Near-infrared light drive: thermal gradient | ≈450 nm | Not mentioned | Vivo: a rabbit model with atherosclerosis | Drug delivery and Atherosclerosis therapy | [77] |
| PLTs | Near-infrared light drive: thermal gradient | ≈2 μm | 12.2 μms-1 | NIR light irradiated (808 nm, 2.5 W cm-2) | Cancer Therapy | [78] |
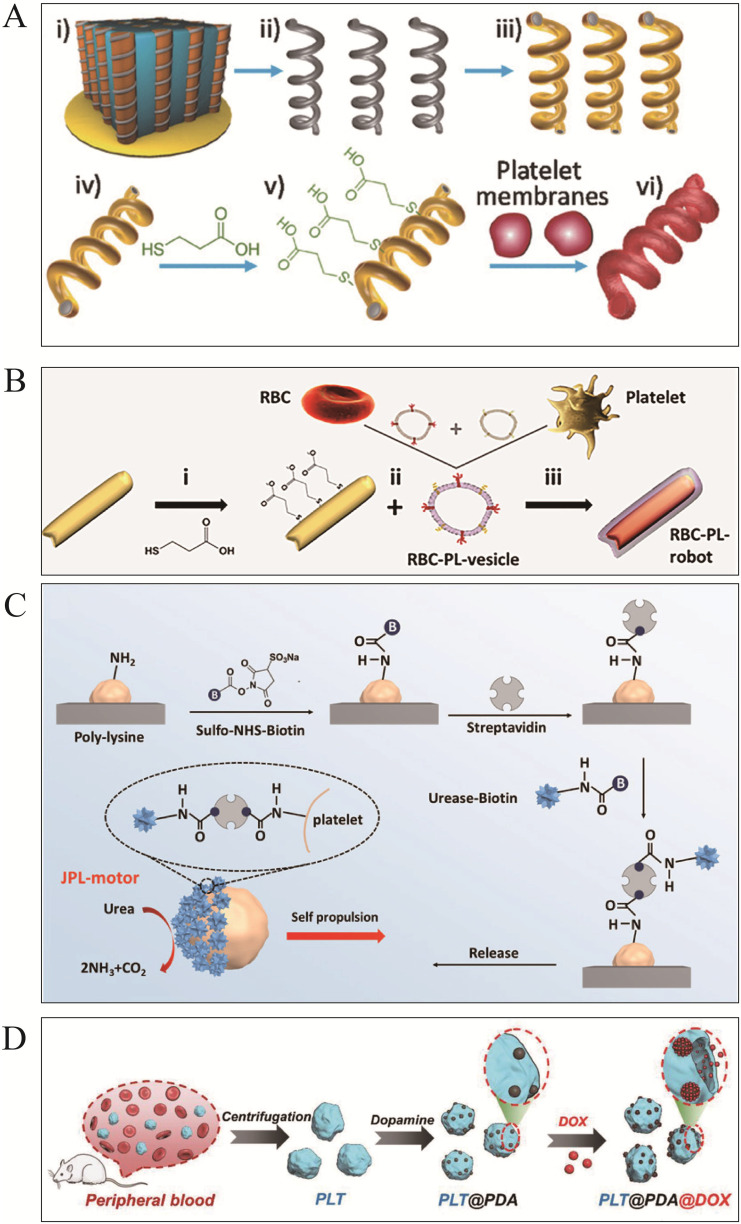
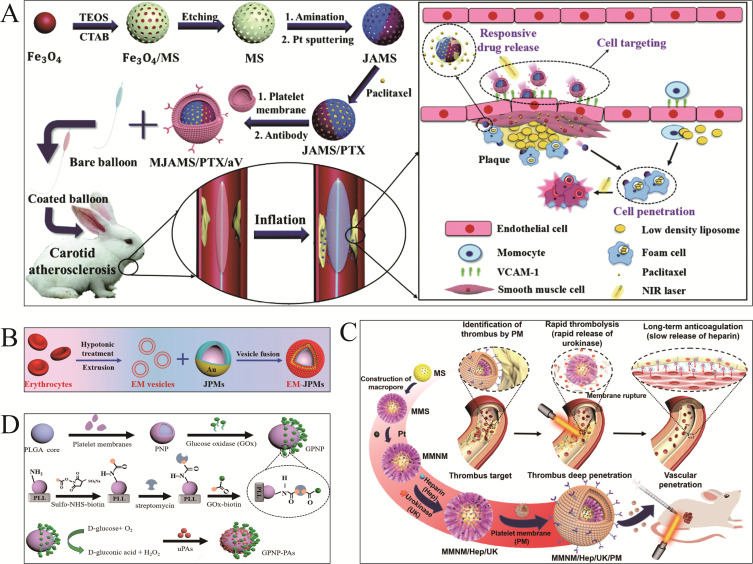
In 2018, Chang et al45 employed ultrasonic incubation to fuse platelet vesicles with magnetic helical MNMs surfaces, called “Pt-nanomotors” (Figure 3A). Pt-nanomotors displayed strong anti-fouling properties and motility (12.5 μms−1). Experimental data revealed that platelet membrane-functionalized MNMs effectively adsorbed and isolated Shiga toxin (STX) and MRSA252 bacteria. This affinity and efficient mobility provide nanorobots with significant detoxifying capability, which offers a novel therapeutic approach for the treatment of infectious disorders. Analogously, Fuel-free RBC-PL hybrid membrane functionalized nanomotors (RBC-PL-motors)79 (Figure 3B) were synthesized by mixing AuNW motors with erythrocyte-PL hybrid membrane source vesicles using a template-supported AuNW electrochemical deposition scheme under an ultrasonic field. Ultrasound-guided RBC-PL-motors have been shown to be more effective due to their dual detoxifying properties including binding and eliminating PL-attached pathogens such as MRSA and neutralizing bacterial toxins.
Figure 3.
PLT membrane-functionalized micromotors. (A) PLT-based magnetic-driven MNMs for toxin neutralization and bacteria separation. i) Pd/Cu co-electrodeposition in polycarbonate membrane pores (pore size: 400 nm). ii) Removal of Cu phase by nitric acid etching and release of helical Pd nanomotors. iii) Ni/Au bilayer deposition on the surface of pd helical nanostructures. iv) Collection and separation of helical nanomotors. v) Modification of the surface of bare helical nanomotors with 3 - mercaptopropionic acid (MPA). vi) Anchoring of PL-vesicles to the surface of MPA-modified helical nanomotors. Reproduced from Li J, Angsantikul P, Liu W, et al. Biomimetic platelet-camouflaged nanorobots for binding and isolation of biological threats. Adv Mater. 2018;30(2):1704800. Copyright © 2017 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.45 (B) RBC-PL hybrid MNMs for detoxification. i) Modification of the gold surface of gold nanowire (AuNW) Robots with MPA. ii) Preparation of hybrid membrane by fusing erythrocyte membrane and platelet membrane at a 1:1 protein mass ratio and its coating of MPA - modified nanorobots. iii) Preparation of red blood cell-platelet membrane robots (RBC-PL-robots) by 5 minutes ultrasonication. Reproduced from Esteban-Fernández de Ávila B, Angsantikul P, Ramírez-Herrera DE, et al. Hybrid biomembrane-functionalized nanorobots for concurrent removal of pathogenic bacteria and toxins. Sci Rob. 2018;3(18). Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.79 (C) Urease-catalyzed JPL-Motor for chemotherapeutic agent carrier. Reproduced from Tang S, Zhang F, Gong H, et al. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery. Sci Rob. 2020;5(43). Copyright © 2020, Science Robotics.35 (D) PLT@PDA-DOX MNMs for targeted cancer therapy. Reproduced from Li T, Chen T, Chen H, et al. Engineered platelet-based micro/nanomotors for cancer therapy. Small. 2021;17(52):e2104912. Copyright © 2021 Wiley‐VCH GmbH.80
Furthermore, JPL-Motor35 (Figure 3C) is a unique self-driving platelet MSN manufactured as a Janus structure with a 1.4–2.6 μm diameter and is catalyzed by urease. Platelets are suitable carriers for delivering chemotherapeutic drugs including DOX and antibacterial agents, due to their high binding effectiveness to cancerous cells and pathogens. Experimental studies revealed that the JPL-Motor can autonomously function at 50–200 mM urea concentrations driven by urease fuel decomposition. A pioneering research conducted by Li et al80 constructed engineered PLT micromotors (PLT@PDA-DOX) (Figure 3D) by universal self-polymerizing modification of polydopamine (PDA). P-selectin on PLTs allows the PLT@PDA-DOX motor to target the tumor location by binding to the increased cancer cell CD44 receptor. PLTA and PLT-derived microparticles (PMP@PLTA-DOX) can produce asymmetric thermal effects under NIR light irradiation and drive the motor to penetrate deep into cancer cells (MCF cells). The PLT@PDA-DOX motor has significantly enhanced the efficacy of targeted cancer therapies.
Cancer Cell Membrane-Functionalized Micromotor
Cancer cells exhibit the main characteristic of homotypic binding, leading to the formation of tumors and clumps.81 Accumulating research suggests that chemotherapeutic agents can be efficiently delivered through nanoparticle drug delivery systems.81,82 Several advantages of these systems include high solubility, targeted drug delivery and enzyme protection.83 Intriguingly, a pioneering study revealed that membrane-coated nanoparticles enhance the homotypic binding of cancerous cells, thereby facilitating targeted therapy.75 Furthermore, Kroll et al explored that antigen components on the cell membrane can regulate anti-cancer immunity.78 Thus, the application of cell membrane coatings exhibits promising potential in drug targeting and imaging techniques for the treatment and management of cancers.76
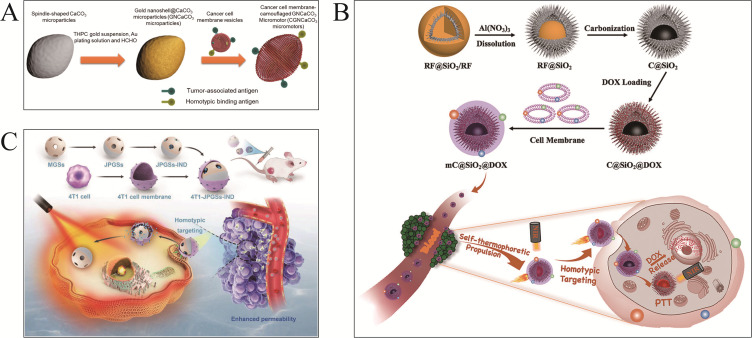
Zhang et al77 functionalized CaCO3 motors with gold nanoshells (Figure 4A). Nanoparticles were attached to membrane vesicles prepared from G422 mouse cancer cells. Micromotors can be detected by antigen-presenting cells using the cell membrane coating. Micromotors can target cancerous cells by binding homologously with their membranes. Subcutaneous injection of CaCO3 motors resulted in anti-tumor immunity in mice within 24 hours. Zhou et al84 designed a semi-yolk, spiky-shell carbon-based@silicon (C@SiO2) nanomotor (MC@SiO2@DOX) coated with MCF-7 breast cancer cell membranes (Figure 4B). Thermal gradients in MC@SiO2@DOX cause efficient self-heating propulsion in near-infrared light. Tumor cell membrane coating enhances cell adhesion and absorption efficiency, resulting in faster migration (2.42 µms−1) and homologous target binding. Moreover, tumor cell membrane coating significantly improved photodynamic therapy and synergistic chemotherapy (intracellular light/pH responsive DOX release) and decreased MCF-7 cell proliferation. The outer shell may be utilized entirely for loading DOX, resulting in a higher drug efficiency with a capacity of 596 μgmg−1. Intriguingly, Wang et al85 developed a Janus nanomotor (Figure 4C) performed by near-infrared light in 4T1 cell membranes loaded with indomethacin (IND) via 217.1±1.79 nm mesoporous gold nanospheres. The external tumor cell membrane promotes homologous tumor enrichment. Near-infrared light driving motors may enhance photothermal therapy in tumors and IND release may inhibit the inflammatory response. Therefore, this strategy can potentially eradicate tumors while reducing therapeutic adverse effects.
Figure 4.
(A) Gold nanoshells modified CaCO3 MNMs for anti-tumor immunity. Reproduced from Zhang H, Li Z, Wu Z, He Q. Cancer Cell Membrane Camouflaged Micromotor. Adv Ther. 2019;2(12):1900096. Copyright © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.77 (B) MC@SiO2 @DOXMNMs for drug loading. Reproduced from Zhou M, Xing Y, Li X, Du X, Xu T, Zhang X. Cancer cell membrane camouflaged semi-yolk@spiky-shell nanomotor for enhanced cell adhesion and synergistic therapy. Small. 2020;16(39):e2003834. Copyright © 2020 Wiley‐VCH GmbH.84 (C) 4T1 cell-coated mesoporous gold nanospheres for tumor photothermal therapy. Reproduced from Wang H, Gao J, Xu C, et al. Light-driven biomimetic nanomotors for enhanced photothermal therapy. Small. 2023;e2306208. Copyright © 2023 Wiley‐VCH GmbH.85
Biological Applications of Cell-Membrane-Functionalized Micromotors
This section classifies applications based on different disease types to provide a comprehensive and organized overview.
Thrombosis
Thrombosis can be classified as arterial or venous thrombosis depending on the site of formation.86 The mechanisms of vascular and arterial thrombosis differ due to their physiological environment.87,88 Most of the arterial thrombosis is formed based on the rupture of atherosclerotic plaques.89,90
Currently, drug-eluting stents (DES)91 and drug-coated balloons (DCB)89 are the primary therapeutic options for the treatment of atherosclerosis. DES is initially effective, but it is a foreign body that can cause vascular restenosis over time.92 DCB, placed via catheter for local plaque treatment, avoids long-term foreign-body-related restenosis, offering clinical advantages.93 Thus, Mao et al94 studied DCB coatings combined with nanomotor therapy for the treatment of atherosclerosis. The researchers developed an autonomously moving, porous nanomotor coated with an anti-vessel cell adhesion molecule-1 antibody (JAMS/PTX/AV MNMs) (Figure 5A). Past studies have indicated that JAMS enhances nanoparticles deep into plaque, increases drug retention and attenuates the activation of inflammatory macrophages in atherosclerosis under near-infrared light.95 The inhibitory effect of paclitaxel-carrying MNMs appropriately supplied by the balloon on atherosclerotic blood vessel proliferation may provide additional evidence for the application of drug balloon coatings.
Figure 5.
MNMs for Thrombosis treatment. (A) JAMS/PTX/AV combined with DBC for atherosclerosis treatment. Reproduced from Huang Y, Li T, Gao W, et al. Platelet-derived nanomotor coated balloon for atherosclerosis combination therapy. J Mater Chem B. 2020;8(26):5765–5775. Copyright © 2020, Journal of Materials Chemistry.94 (B) RBC-membraned MNMs with gold shells for atherosclerosis treatment. Reproduced from Shao J, Abdelghani M, Shen G, Cao S, Williams DS, van Hest JCM. Erythrocyte membrane modified janus polymeric motors for thrombus therapy. ACS Nano. 2018;12(5):4877–4885. Copyright © 2018 American Chemical Society.96 (C) INR-driven MMNM/Hep/UK/PM for venous thrombosis treatment. Reproduced from Wan M, Wang Q, Wang R, et al. Platelet-derived porous nanomotor for thrombus therapy. Sci Adv. 2020;6(22):eaaz9014. Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).97 Copyright 2020, Science Advances. (D) GOX-driven and uPA-loaded MNMs for venous thrombolysis. Reproduced from Fang X, Ye H, Shi K, et al. GOx-powered janus platelet nanomotors for targeted delivery of thrombolytic drugs in treating thrombotic diseases. ACS Biomater Sci Eng. 2023;9(7):4302–4310. Copyright © 2023 American Chemical Society.98
Short-term thrombolytic recanalization and long-term anti-coagulant prophylaxis are the current treatment options for ameliorating venous thrombosis.99,100 Short half-lives of thrombolytic drugs, low drug utilization, poor drug penetration at thrombus sites and recurrence risk are current therapeutic challenges.101,102 Thus, the development of self-actuated nanomotors can overcome these challenging issues.
Shao and his colleagues96 developed layer-by-layer Janus polymer motors (EM-JPMs) using self-assembly. The EM-JPMs were capable of auto-motility and photothermal ablation of thrombi under NIR illumination. The EM-JPMs experienced rupture upon NIR irradiation, resulting in the release of Hep. The release of heparin contributed to the enhancement of the photothermal-mediated thrombolytic effect (Figure 5B). However, only specific qualitative trials did not exhibit substantial thrombolytic effects and in vivo thrombolysis was not assessed.
Wan and his colleagues developed a platelet membrane-modified mesoporous or macroporous silica/Pt nanomotor97 containing urokinase and heparin in the macropores and mesopores (Figure 5C). The resonance of the macroporous structure generates asymmetric Pt NPs under near-infrared irradiation to form thermophoresis-driven nanoparticles, which can rupture the thrombus and release urokinase and heparin. The movement of MNMs under near-infrared irradiation can penetrate thrombi and increase the thrombolytic effects.
Pan et al98 developed a glucose oxidase (GOX)-driven Janus nanomotor (GPNP-PA) loaded with urokinase plasminogen activator (uPA) (Figure 5D). GOX catalyzed the production of H2O2 from glucose and enhanced the targeting of the outer platelet membrane. It was shown that the GPNP-PAs treatment group had excellent thrombolytic action. In addition, GPNP-PAs are glucose-powered nanomachines that exhibit characteristics such as safety, cleanliness and accessibility. This type of nanomotor has a high degree of adaptability for delivering several therapeutic agents.
Surface-engineered cell membrane-cloaked MNMs demonstrate enhanced thrombus-targeting precision through rational ligand modification on their biointerfaces. This active targeting strategy promotes site-specific drug enrichment at vascular occlusion sites while reducing off-target distribution, improving thrombolytic performance and mitigating hemorrhagic complications. The endogenous membrane architecture endows MNMs with prolonged circulatory persistence via inherent immune-evasion properties, enabling sustained therapeutic action. Motor-enabled thrombolysis systems have shown superior clinical potential in preventing secondary thromboembolic events through regulating dynamic biodistribution over passive drug diffusion methods.
Tumor-Related Diseases
Photothermal Therapy
This novel tumor therapy utilizes photothermal agents (PTA) to generate enough heat to eliminate tumor cells under near-infrared light irradiation.103 Low photothermal conversion efficiency, poor PTA stability, inadequate tumor accumulation and cell uptake, tumor thermostability, tumor recurrence and metastasis are still challenging to overcome utilizing PTT.103 Due to its photothermal conversion and near-infrared radiation absorption, nanostructure technology has contributed to cancer therapies in recent years.104
A Janus mesoporous silica nanoparticle motor (JMSNM) with 10 nm thick Au half-shells was developed to function in NIR light (Figure 6A).105 The macrophage cell membrane (MPCM) on the surface may disguise, inhibit biological adhesion and detect cancerous cells. NIR light induces localized heating that eliminates cancer cells. MPCM coating and self-propulsion provide a novel nanomotor-driven photothermal therapeutic strategy. Wang63 and his co-workers recently developed a needle-like white cell membrane-coated gallium nanoswimmer (LMGNS) (Figure 6B). LMGNS motors were found to sustain longer and move rapidly in biological conditions compared to bare-gallium nanomotors. In addition, LMGNS strongly target and penetrate cancerous cells under acoustic conditions. Pharmacokinetic studies indicated that LMGNS can be used as pH-sensitive cargo. LMGNS-Dox changes from needle-like to spherical, fuses and releases DOX in an acidic environment (pH 5.0). It was confirmed that leukocyte membrane camouflage induced cancer cells to absorb a significant amount of LMGNS-Dox. The short-term administration of LMGNS induces notable morphological alterations and necrosis in cancer cells. Membrane-functionalized MNMs exhibit tumor-homing specificity through biomolecular recognition, promoting spatially-precise accumulation at malignant lesions. This targeting mechanism optimizes PTA depth penetration while containing therapeutic activity within pathological regions to minimize systemic toxicity. Furthermore, these hybrid systems enable combinatorial chemo-photothermal regimens where laser-triggered hyperthermia facilitates drug liberation from the nanoplatforms, establishing synergistic therapeutic effects. The engineered membrane architecture additionally provides stimuli-responsive gatekeeping functions for on-demand cargo delivery through endogenous microenvironmental triggers.
Figure 6.
MNMs for Tumor Imaging and precise on-demand drug delivery. (A) Macrophage-cell-membrane-coated MNMs for the thermomechanical portion of cancer cell membranes. a) Schematic diagram of the preparation process of MPCM@JMSNMs. b) SEM image of JMSNMs. Inset: TEM image of a Single JMSNM. Reproduced from Xuan M, Shao J, Gao C, Wang W, Dai L, He Q. Self-propelled nanomotors for thermomechanically percolating cell membranes. Angew Chem Int Ed Engl. 2018;57(38):12463–12467. Copyright © 2018 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim.105 (B) Needle-like white cell membrane-coated gallium MNMs for Photothermal Therapy. Reproduced from Wang D, Gao C, Zhou C, Lin Z, He Q. Leukocyte membrane-coated liquid metal nanoswimmers for actively targeted delivery and synergistic chemophotothermal therapy. Research. 2020;2020:3676954. Copyright © 2020 Daolin Wang et al.63 C) Magnetic anticancer DOX@MPN for chemo-phototherapy. Reproduced from Song X, Qian R, Li T, et al. Imaging-Guided biomimetic M1 macrophage membrane-camouflaged magnetic nanorobots for photothermal immunotargeting cancer therapy. ACS Appl Mater Interfaces. 2022;14(51):56548–56559. Copyright © 2022 American Chemical Society.61 (D) RBC-based and Fe3O4-encapsulated hemoglobin MNMs for oxygen and PSs activity delivery. Reproduced from Gao C, Lin Z, Wang D, Wu Z, Xie H, He Q. Red blood cell-mimicking micromotor for active photodynamic cancer therapy. ACS Appl Mater Interfaces. 2019;11(26):23392–23400. Copyright © 2019 American Chemical Society.106 (E) Multi-cargo-loaded RBC micromotors for imaging. Reproduced from Wu Z, de Avila BE-F, Martin A, et al. RBC micromotors carrying multiple cargos towards potential theranostic applications. Nanoscale. 2015;7(32):13680–13686. Copyright © The Royal Society of Chemistry 2015.53 (F) Bio-RBC-based MNMs as a potential mobile thermal therapy and imaging tool. Reproduced from Hou K, Zhang Y, Bao M, et al. A multifunctional magnetic red blood cell-mimetic micromotor for drug delivery and image-guided therapy. ACS Appl Mater Interfaces. 2022;14(3):3825–3837. Copyright © 2022 American Chemical Society.59
Chemo-phototherapy is a synergistic technique that combines PT and chemotherapy, which delivers significant anti-cancer effects.107–109 The MPN (182±3nm) magnetic anti-cancer nanocomposite was synthesized by Song et al61 utilizing macrophage characteristics110,111 (Figure 6C). Exposure to oxygen degrades black phosphorus quantum dots (BPQDs) in MPNs with photothermal and photoacoustic imaging properties.112,113 To overcome this critical challenge, BPQDs were encapsulated in macrophage membranes. MPN may form macrophage proteins and evade the immune system. DOX@MPN possessed more significant chemotherapeutic effects on DOX release and ROS production in the tumor microenvironment under a magnetic field and NIR. The membrane-wrapped MNMs significantly improve the drug utilization rate by stabilizing the contents. Furthermore, photoacoustic (PA) and magnetic resonance imaging can enhance the visualization of the tumor microenvironment.
Photodynamic Therapy
Photodynamic therapy (PDT) has demonstrated promising efficacy in the treatment of malignancies by the conversion of biologically inert oxygen into reactive oxygen species (ROS), facilitating the eradication of cancerous cells.114,115 Hypoxia and poor targeting and aggregation of PSs still limit the efficacy of PDTs.116 Nanomedicine-based delivery systems improve the selectivity of PSs in tumors.117 PDT for cancer was developed using a red blood cell mimicking (RBCM) micromotor (Figure 6D). Hypotonic treatment causes red blood cells to leak hemoglobin, which leaves motors oxygen-dependent. The high levels of Fe3O4-encapsulated hemoglobin microparticles in RBCM compensate for this deficiency. Under acoustic fields, they can move at speeds up to 56.5 μms−1 in biological media using an external magnetic field to navigate the direction of motion. The RBCM micromotor significantly promotes the anticancer effect of PDT due to excellent loading, oxygen release, PS loading and directional movement. Therefore, this fuel-free RBCM micromotor provides an efficient, fast and novel approach to more precise and effective PT in the future.
Tumor Imaging and Precise On-Demand Drug Delivery
Synthetic MNMs have been employed for tumor imaging and therapy due to their active mobility.118–121 In 2015, Wu et al53 embedded quantum dots (QDs), iron oxide magnetic nanoparticles (MNPs) and DOX into red blood cell micromotors (Figure 6E) through hypotonic dilution encapsulation. Since MNPs are asymmetrically distributed, ultrasound pressure gradients may convert acoustic energy into kinetic energy and magnetic guiding under acoustic propulsion moves multi-loaded micromotors. Two wavelengths of QDS and DOX fluorescence allow the direct observation of their loading in the erythrocyte motor. Recently, Hou and his colleagues59 developed an LBL assembly method-based red blood cell-mimetic micromotor (RBCM) (Figure 6F). DOX and Fe3O4 nanoparticles on the RBCM surface promoted drug delivery and enhanced magnetic manipulation. MNMs can also be imaged with fluorescence contrast agents and MIR contrast agents, both of which can be stabilized through the stabilization of their signal expression. This method utilizes encapsulation of fluorescent dyes and MIR contrast agents within cell membranes and allows both to be verified for more complete and accurate localization.
Neurological Disorders
Precise targeted delivery of drugs to the brain lesions is critical for effective treatment of neurological disorders, yet remains challenging due to the blood-brain barrier (BBB) and the intricate anatomy of the brain.
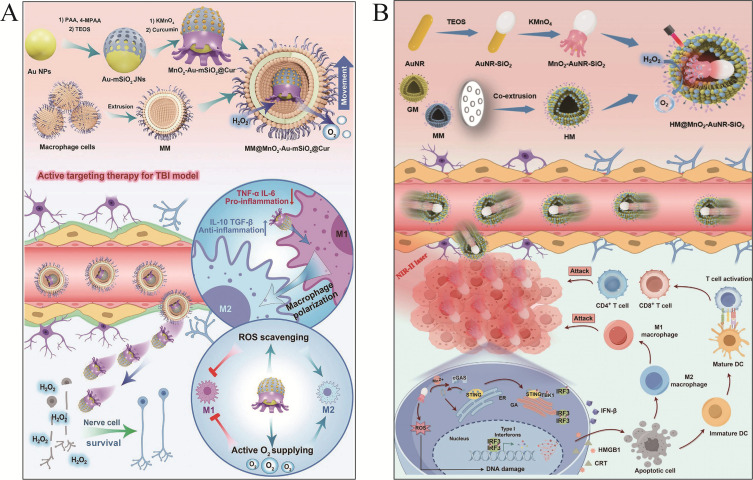
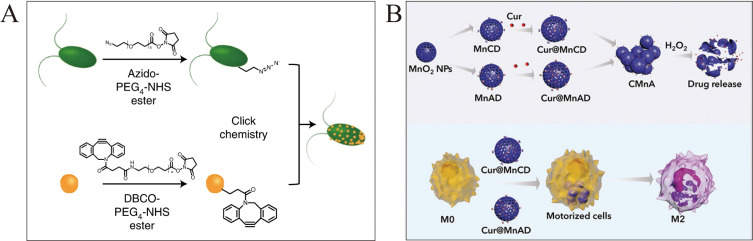
To overcome these critical challenges, Ye et al engineered a macrophage membrane-cloaked nanomotor (MM@MnO2-Au-mSiO2@Cur) for neuroinflammation therapy (Figure 7A).122 The asymmetric MM coating utilizes membrane transporters/adhesion molecules to bypass the BBB and target inflammation via chemotaxis. MnO2 catalyzes H2O2-to-O2 conversion for self-propulsion and oxidative stress reduction, while encapsulated curcumin shifts macrophages from M1 to M2 phenotypes. The preclinical validation confirmed precise BBB penetration, neuroinflammation mitigation, functional recovery promotion and biosafety. Expanding this design, the team developed a dual-driven nanomotor (HM@MnO2-AuNR-SiO2) for glioblastoma (Figure 7B). A hybrid MM/GM coating synergizes homologous tumor targeting (GM) with BBB penetration (MM), outperforming single-ligand approaches. Dual propulsion via H2O2-derived O2 bubbles and photothermal actuation enhances deep tumor penetration. The nanomotor induces immunogenic cell death (ICD) through ROS production and activates cGAS-STING signaling pathway via Mn2+ release, linking catalytic therapy to antitumor immunity. This multimodal approach enhances immune surveillance and suppresses recurrence, showing efficacy in GBM models. Membrane coatings enhance the targeting of MMNs, which improves their therapeutic effect.
Figure 7.
MNMs for neurological disorders. (A) macrophage membrane-cloaked nanomotor for neuroinflammation therapy. Reproduced from Ye J, Fan Y, She Y, et al. Biomimetic self-propelled asymmetric nanomotors for cascade-targeted treatment of neurological inflammation. Adv Sci. 2024;11(22):2310211. Copyright © 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH. Creative Commons CC BY license.122 (B) dual-driven nanomotor for glioblastoma. Reproduced from Ye J, Fan Y, Kang Y, et al. Biomimetic dual-driven heterojunction nanomotors for targeted catalytic immunotherapy of glioblastoma. Adv Funct Mater. 2025;35(9):2416265. Copyright © 2024 Wiley‐VCH GmbH.123
Acute Bacterial Pneumonia
Acute pneumonia is an inflammatory response to alveolar and interstitial lung tissue infection. A high mortality rate is associated with severe pulmonary dysfunction characterized by respiratory failure and hyperinflammation. Anti-inflammatory drugs are limited by their short systemic circulation time and poor pulmonary specificity. MNM drug delivery systems have been studied to efficiently deliver anti-inflammatory drugs to the lungs.
Zhang et al124 developed an algae-nanoparticle-motor (Figure 8A) to administer antibiotics in vivo to treat lung infections. Past studies have revealed that encapsulated neutrophil membranes can escape alveolar macrophages.76 Encapsulation of ciprofloxacin (Cip) within motors produces prolonged retention and sustained release in the lung. Microorganisms and nanomotors have significant potential for improving antibiotic delivery to treat lung infections. The MNNs exhibit autonomous actions, increased lifespans in the particular environment, microbial autofluorescence and potential for targeted actions.
Figure 8.
MNMs for acute Bacterial Pneumonia treatment. (A) Algae-neutrophil hybrid MNMs are used for antibiotic transport. Reproduced from Zhang F, Zhuang J, Li Z, et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat Mater. 2022;21(11):1324–1332. Copyright © 2022 Nature Materials..124 (B) Macrophage-based MNMs for anti-inflammation. Reproduced from Yue L, Gao C, Li J, et al. Chemotaxis-guided self-propelled macrophage motor for targeted treatment of acute pneumonia. Adv Mater. 2023;35(20):e2211626. Copyright © 2023 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.125
A self-propelled macrophage motor with chemotaxis characteristics has recently been developed (Figure 8B). Activated M2 macrophages can be used as vectors for targeted drug delivery, deep tissue penetration in inflamed lungs and local drug release for synergistic anti-inflammatory effects by polarizing M2 cells, anti-inflammatory curcumin and reducing the production of H2O2. Studies revealed that the motor reduced the risk of acute pneumonia complicated with heart disease in mice.126,127 New drugs can ameliorate the progression of multiple diseases and mitigate several complications.
Current Challenges and Future Prospects
The unique advantages of membrane-coated MNMs stem from their biomimetic camouflage and biocompatibility and their inherent smart and stimuli-responsive capabilities. MNMs achieve precise control by dynamically adjusting their locomotion modes or functionalities in response to environmental signals such as light, temperature, ultrasound and magnetic fields. Despite these advancements, several barriers must be addressed before MNMs can achieve widespread clinical application. First, membrane coatings can degrade and biofoul in complex environments due to biological instability. This issue can be addressed by enhancing membrane stability through crosslinking techniques, multilayer membrane structures and advanced engineering modifications to improve resistance to degradation. Second, while MNMs show improved immune evasion, challenges persist in optimizing their targeting specificity and avoiding clearance by the reticuloendothelial system. These can be mitigated by selecting membranes with specialized properties such as immune or cancer cell membranes, preserving functional membrane proteins and incorporating stimuli-responsive materials to enhance precision and efficacy. Third, the scalable and standardized production of MNMs remains a critical challenge, which can be overcome by employing automated microfluidic technologies and establishing reproducible protocols to ensure consistency in large-scale manufacturing. Moreover, the lack of comprehensive data on long-term safety, biodistribution and pharmacokinetics hinders regulatory approval and clinical adoption. The therapeutic effects of MNMs are currently confined to the pre-clinical and clinical research stages. More studies are highly required to evaluate and confirm their effectiveness and biological applications. There are currently no registered or publicly reported clinical trials involving MNMs. Clinical translation developments should be monitored and forwarded to academic research collaborations in order to contribute to the advancement of this field. Finally, integrating artificial intelligence (AI) offers transformative opportunities to overcome these barriers by optimizing MNM design, improving targeting through patient-specific data, and enabling adaptive monitoring during treatment. The advancement of MNMs towards clinical application can lead to breakthroughs in precision medicine by addressing these limitations.
The future of MNMs is highly promising with potential applications extending beyond their current use in targeted drug delivery and biomedical imaging. The ability of MNMs to navigate complex biological environments opens up possibilities for advanced therapeutic strategies including personalized medicine. Second, their inherent biocompatibility and functional versatility make them suitable for tissue engineering and regenerative medicine, aiding in precisely delivering growth factors or stem cells to damaged tissues. Third, MNMs could revolutionize environmental monitoring within the human body, acting as active sensors to detect biomarkers, pathogens and toxins in real-time. Fourth, they hold potential in non-medical fields such as environmental remediation, where their biomimetic properties and efficient propulsion mechanisms could be adapted for removing contaminants or delivering agents in challenging settings. Lastly, integrating MNMs with artificial intelligence and advanced robotics could lead to the development of autonomous nano-systems capable of intelligent decision-making, adaptive targeting and coordinated actions.
The path from in vitro studies to in vivo application has made some encouraging progress, but there is still a long way to go. In conclusion, this review will promote the further development of membrane-encapsulated micro- and nanorobotics approaches in the field of nanomedicine and further benefit clinical research.
Funding Statement
This review was supported by the Public Welfare Technology Research Funding Project of Zhejiang (LTGY24H100002, LLSSY24H160003 and LGF21H310002), Public Welfare Technology Research Funding Project of Lishui (2021SJZC022, 2020GYX18 and 2020GYX23) and Key Research and Development Project of Lishui (2023zdyf15, 2021ZDYF15 and 2022ZDYF23).
Disclosure
The authors declare no conflict of interest for publication in this journal.
References
- 1.Zhou C, Gao C, Lin Z, et al. Autonomous motion of bubble-powered carbonaceous nanoflask motors. Langmuir. 2020;36(25):7039–7045. doi: 10.1021/acs.langmuir.9b03398 [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Hortelão AC, Huang X, Sánchez S. Lipase-powered mesoporous silica nanomotors for triglyceride degradation. Angew Chem Int Ed. 2019;58(24):7992–7996. doi: 10.1002/anie.201900697 [DOI] [PubMed] [Google Scholar]
- 3.Tang M, Ni J, Yue Z, et al. Polyoxometalate-nanozyme-integrated nanomotors (POMotors) for self-propulsion-promoted synergistic photothermal-catalytic tumor therapy. Angew Chem Int Ed. 2024;63(6):e202315031. doi: 10.1002/anie.202315031 [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Xu D, Chen J, Peng N, Ma T, Liang F. Nanozymatic magnetic nanomotors for enhancing photothermal therapy and targeting intracellular SERS sensing. Nanoscale. 2023;15(31):12944–12953. doi: 10.1039/d3nr02739b [DOI] [PubMed] [Google Scholar]
- 5.Jiang S, Kaltbeitzel A, Hu M, Suraeva O, Crespy D, Landfester K. One-step preparation of fuel-containing anisotropic nanocapsules with stimuli-regulated propulsion. ACS Nano. 2020;14(1):498–508. doi: 10.1021/acsnano.9b06408 [DOI] [PubMed] [Google Scholar]
- 6.Guo J, Gallegos JJ, Tom AR, Fan D. Electric-field-guided precision manipulation of catalytic nanomotors for cargo delivery and powering nanoelectromechanical devices. ACS Nano. 2018;12(2):1179–1187. doi: 10.1021/acsnano.7b06824 [DOI] [PubMed] [Google Scholar]
- 7.Zhou D, Zhuang R, Chang X, Li L. Enhanced light-harvesting efficiency and adaptation: a review on visible-light-driven micro/nanomotors. Research. 2020;20206821595. doi: 10.34133/2020/6821595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Q, Zhang H, Liu H. External physical field-driven nanocatalytic cancer therapy. BMEMat. 2023;1(1):e12010. doi: 10.1002/bmm2.12010 [DOI] [Google Scholar]
- 9.Ismagilov RF, Schwartz A, Bowden N, Whitesides GM. Autonomous movement and self‐assembly. Angew Chem. 2002;114(4):674–676. [Google Scholar]
- 10.Paxton WF, Kistler KC, Olmeda CC, et al. Catalytic nanomotors: autonomous movement of striped nanorods. J Am Chem Soc. 2004;126(41):13424–13431. doi: 10.1021/ja047697z [DOI] [PubMed] [Google Scholar]
- 11.Tu Y, Peng F, Andre AAM, Men Y, Srinivas M, Wilson DA. Biodegradable hybrid stomatocyte nanomotors for drug delivery. ACS Nano. 2017;11(2):1957–1963. doi: 10.1021/acsnano.6b08079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y, Zhao H, Cai Y, Jurado-Sánchez B, Dong R. Recent advances in one-dimensional micro/nanomotors: fabrication, propulsion and application. Nanomicro Lett. 2022;15(1):20. doi: 10.1007/s40820-022-00988-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K, Guo J, Liang Z, Fan D. Artificial micro/nanomachines for bioapplications: biochemical delivery and diagnostic sensing. Adv Funct Mater. 2018;28(25):1705867. doi: 10.1002/adfm.201705867 [DOI] [Google Scholar]
- 14.Esteban-Fernandez de Avila B, Angsantikul P, Li J, et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat Commun. 2017. doi: 10.1038/s41467-017-00309-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Peng F, Yan X, et al. Medical micro- and nanomotors in the body. Acta Pharm Sin B. 2023;13(2):517–541. doi: 10.1016/j.apsb.2022.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kagan D, Benchimol MJ, Claussen JC, Chuluun-Erdene E, Esener S, Wang J. Acoustic droplet vaporization and propulsion of perfluorocarbon-loaded microbullets for targeted tissue penetration and deformation. Angew Chem Int Ed Engl. 2012;51(30):7519–7522. doi: 10.1002/anie.201201902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You K, Wang Q, Osman MS, et al. Advanced strategies for combinational immunotherapy of cancer based on polymeric nanomedicines. BMEMat. 2024;2(2):e12067. doi: 10.1002/bmm2.12067 [DOI] [Google Scholar]
- 18.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Article. Science. 2000;288(5473):2051. doi: 10.1126/science.288.5473.2051 [DOI] [PubMed] [Google Scholar]
- 19.Wan MM, Chen H, Wang Z, et al. Nitric oxide-driven nanomotor for deep tissue penetration and multidrug resistance reversal in cancer therapy. Adv Sci. 2021;8(3):2002525. doi: 10.1002/advs.202002525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao W, Zhao F, Xue J, Huang L. NIR-II absorbing organic nanoagents for photoacoustic imaging and photothermal therapy. BMEMat. 2023;1(1):e12009. doi: 10.1002/bmm2.12009 [DOI] [Google Scholar]
- 21.Gao C, Wang Y, Ye Z, Lin Z, Ma X, He Q. Biomedical micro-/nanomotors: from overcoming biological barriers to in vivo imaging. Adv Mater. 2021;33(6):2000512. doi: 10.1002/adma.202000512 [DOI] [PubMed] [Google Scholar]
- 22.Chałupniak A, Morales-Narváez E, Merkoçi A. Micro and nanomotors in diagnostics. Adv Drug Deliv Rev. 2015;95:104–116. doi: 10.1016/j.addr.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 23.Zheng S, Wang Y, Pan S, et al. Biocompatible nanomotors as active diagnostic imaging agents for enhanced magnetic resonance imaging of tumor tissues in vivo. Adv Funct Mater. 2021;31(24):2100936. doi: 10.1002/adfm.202100936 [DOI] [Google Scholar]
- 24.Srivastava SK, Medina-Sanchez M, Koch B, Schmidt OG. Medibots: dual-action biogenic microdaggers for single-cell surgery and drug release. Adv Mater. 2016;28(5):832–837. doi: 10.1002/adma.201504327 [DOI] [PubMed] [Google Scholar]
- 25.Xi W, Solovev AA, Ananth AN, Gracias DH, Sanchez S, Schmidt OG. Rolled-up magnetic microdrillers: towards remotely controlled minimally invasive surgery. Nanoscale. 2013;5(4):1294–1297. doi: 10.1039/c2nr32798h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halder A, Sun Y. Biocompatible propulsion for biomedical micro/nano robotics. Biosens Bioelectron. 2019;139111334. doi: 10.1016/j.bios.2019.111334 [DOI] [PubMed] [Google Scholar]
- 27.Li J, de Avila BE-F, Gao W, Zhang L, Wang J. Micro/nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci Rob. 2017;2(4):eaam6431. doi: 10.1126/scirobotics.aam6431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Liu X, Wang Y, et al. Biocompatibility of artificial micro/nanomotors for use in biomedicine. Nanoscale. 2019;11(30):14099–14112. doi: 10.1039/c9nr03393a [DOI] [PubMed] [Google Scholar]
- 29.Wu Z, Li T, Li J, et al. Turning erythrocytes into functional micromotors. ACS Nano. 2014;8(12):12041–12048. doi: 10.1021/nn506200x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z, Li L, Yang Y, et al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci Rob. 2019;4(32):eaax0613. doi: 10.1126/scirobotics.aax0613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Xiong Z, Zheng J, Zhan X, Tang J. Light-driven micro/nanomotor for promising biomedical tools: principle, challenge, and prospect. Acc Chem Res. 2018;51(9):1957–1965. doi: 10.1021/acs.accounts.8b00254 [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Xu J, Zhou Q, et al. Biodegradability of micro/nanomotors: challenges and opportunities. Adv Healthcare Mater. 2021;10(13):2100335. doi: 10.1002/adhm.202100335 [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Li T, Gao W, et al. Cell-membrane-coated synthetic nanomotors for effective biodetoxification. Adv Funct Mater. 2015;25(25):3881–3887. doi: 10.1002/adfm.201501050 [DOI] [Google Scholar]
- 34.Zhang N, Fan M, Zhao Y, et al. Biomimetic and NOS-responsive nanomotor deeply delivery a combination of MSC-EV and mitochondrial ROS scavenger and promote heart repair and regeneration. Adv Sci. 2023;10(21):e2301440. doi: 10.1002/advs.202301440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang S, Zhang F, Gong H, et al. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery. Sci Rob. 2020;5(43). doi: 10.1126/scirobotics.aba6137 [DOI] [PubMed] [Google Scholar]
- 36.Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, Medina-Sanchez M, Magdanz V, Schwarz L, Hebenstreit F, Schmidt OG. Sperm-hybrid micromotor for targeted drug delivery. ACS Nano. 2018;12(1):327–337. doi: 10.1021/acsnano.7b06398 [DOI] [PubMed] [Google Scholar]
- 38.Xu H, Medina-Sanchez M, Maitz MF, Werner C, Schmidt OG. Sperm micromotors for cargo delivery through flowing blood. ACS Nano. 2020;14(3):2982–2993. doi: 10.1021/acsnano.9b07851 [DOI] [PubMed] [Google Scholar]
- 39.Kroll AV, Fang RH, Jiang Y, et al. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv Mater. 2017;29(47):1703969. doi: 10.1002/adma.201703969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li R, He Y, Zhu Y, et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19(1):124–134. doi: 10.1021/acs.nanolett.8b03439 [DOI] [PubMed] [Google Scholar]
- 41.Thamphiwatana S, Angsantikul P, Escajadillo T, et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci U S A. 2017;114(43):11488–11493. doi: 10.1073/pnas.1714267114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Dehaini D, Zhang Y, et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13(12):1182. doi: 10.1038/s41565-018-0254-4 [DOI] [PubMed] [Google Scholar]
- 43.Zhu L, Gangadaran P, Kalimuthu S, et al. Novel alternatives to extracellular vesicle-based immunotherapy - exosome mimetics derived from natural killer cells. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S166–s179. doi: 10.1080/21691401.2018.1489824 [DOI] [PubMed] [Google Scholar]
- 44.Zhai Y, Wang J, Lang T, et al. T lymphocyte membrane-decorated epigenetic nanoinducer of interferons for cancer immunotherapy. Nat Nanotechnol. 2021;16(11):1271. doi: 10.1038/s41565-021-00972-7 [DOI] [PubMed] [Google Scholar]
- 45.Li J, Angsantikul P, Liu W, et al. Biomimetic platelet-camouflaged nanorobots for binding and isolation of biological threats. Adv Mater. 2018;30(2):1704800. doi: 10.1002/adma.201704800 [DOI] [PubMed] [Google Scholar]
- 46.de Avila BE-F, Gao W, Karshalev E, Zhang L, Wang J. Cell-like micromotors. Acc Chem Res. 2018;51(9):1901–1910. doi: 10.1021/acs.accounts.8b00202 [DOI] [PubMed] [Google Scholar]
- 47.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112(10):3939–3948. doi: 10.1182/blood-2008-07-161166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierigè F, Bigini N, Rossi L, Magnani M. Reengineering red blood cells for cellular therapeutics and diagnostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(5). doi: 10.1002/wnan.1454 [DOI] [PubMed] [Google Scholar]
- 49.Song Y, Shan L, Gbyli R, et al. Combined liver-cytokine humanization comes to the rescue of circulating human red blood cells. Science. 2021;371(6533):1019–1025. doi: 10.1126/science.abe2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villa CH, Anselmo AC, Mitragotri S, Muzykantov V. Red blood cells: supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv Drug Deliv Rev. 2016;106(Pt A):88–103. doi: 10.1016/j.addr.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Z, Sun L, Bian F, et al. Erythrocyte-inspired functional materials for biomedical applications. Adv Sci. 2023;10(6). doi: 10.1002/advs.202206150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Z, Li J, de Avila BE-F, et al. Water-powered cell-mimicking janus micromotor. Adv Funct Mater. 2015;25(48):7497–7501. doi: 10.1002/adfm.201503441 [DOI] [Google Scholar]
- 53.Wu Z, de Avila BE-F, Martin A, et al. RBC micromotors carrying multiple cargos towards potential theranostic applications. Nanoscale. 2015;7(32):13680–13686. doi: 10.1039/c5nr03730a [DOI] [PubMed] [Google Scholar]
- 54.Roberts RE, Hallett MB. Neutrophil cell shape change: mechanism and signalling during cell spreading and phagocytosis. Int J Mol Sci. 2019;20(6). doi: 10.3390/ijms20061383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alapan Y, Yasa O, Schauer O, et al. Soft erythrocyte-based bacterial microswimmers for cargo delivery. Sci Rob. 2018;3(17):eaar4423. doi: 10.1126/scirobotics.aar4423 [DOI] [PubMed] [Google Scholar]
- 56.Van Haastert PJM, Devreotes PN. Chemotaxis: signalling the way forward. review. Nat Rev Mol Cell Biol. 2004;5(8):626–634. doi: 10.1038/nrm1435 [DOI] [PubMed] [Google Scholar]
- 57.Zhang F, Zhuang J, de Avila BEF, et al. A nanomotor-based active delivery system for intracellular oxygen transport. ACS Nano. 2019;13(10):11996–12005. doi: 10.1021/acsnano.9b06127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei X, Beltrán-Gastélum M, Karshalev E, et al. Biomimetic micromotor enables active delivery of antigens for oral vaccination. Nano Lett. 2019;19(3):1914–1921. doi: 10.1021/acs.nanolett.8b05051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hou K, Zhang Y, Bao M, et al. A multifunctional magnetic red blood cell-mimetic micromotor for drug delivery and image-guided therapy. ACS Appl Mater Interfaces. 2022;14(3):3825–3837. doi: 10.1021/acsami.1c21331 [DOI] [PubMed] [Google Scholar]
- 60.Gao P, Morita N, Shinkura R. Role of mucosal IgA antibodies as novel therapies to enhance mucosal barriers. Semin Immunopathol. 2024;47(1):1. doi: 10.1007/s00281-024-01027-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song X, Qian R, Li T, et al. Imaging-Guided biomimetic M1 macrophage membrane-camouflaged magnetic nanorobots for photothermal immunotargeting cancer therapy. ACS Appl Mater Interfaces. 2022;14(51):56548–56559. doi: 10.1021/acsami.2c16457 [DOI] [PubMed] [Google Scholar]
- 62.Zhang F, Mundaca-Uribe R, Gong H, et al. A macrophage-magnesium hybrid biomotor: fabrication and characterization. Adv Mater. 2019;31(27):e1901828. doi: 10.1002/adma.201901828 [DOI] [PubMed] [Google Scholar]
- 63.Wang D, Gao C, Zhou C, Lin Z, He Q. Leukocyte membrane-coated liquid metal nanoswimmers for actively targeted delivery and synergistic chemophotothermal therapy. Research. 2020;2020:3676954. doi: 10.34133/2020/3676954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shao J, Xuan M, Zhang H, Lin X, Wu Z, He Q. Chemotaxis-guided hybrid neutrophil micromotors for targeted drug transport. Angew Chem Int Ed Engl. 2017;56(42):12935–12939. doi: 10.1002/anie.201706570 [DOI] [PubMed] [Google Scholar]
- 65.Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg PA. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005;105(9):3577–3582. doi: 10.1182/blood-2004-08-2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molinaro R, Corbo C, Martinez JO, et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016;15(9):1037–1046. doi: 10.1038/nmat4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kunde SS, Wairkar S. Platelet membrane camouflaged nanoparticles: biomimetic architecture for targeted therapy. Int J Pharm. 2021;598:120395. doi: 10.1016/j.ijpharm.2021.120395 [DOI] [PubMed] [Google Scholar]
- 68.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang S, Duan Y, Zhang Q, et al. Drug targeting via platelet membrane-coated nanoparticles. Small Struct. 2020;1(1). doi: 10.1002/sstr.202000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desai C, Koupenova M, Machlus KR, Sen Gupta A. Beyond the thrombus: platelet-inspired nanomedicine approaches in inflammation, immune response, and cancer. J Thromb Haemost. 2022;20(7):1523–1534. doi: 10.1111/jth.15733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H, Li Z, Gao C, et al. Dual-responsive biohybrid neutrobots for active target delivery. Sci Rob. 2021;6(52). doi: 10.1126/scirobotics.aaz9519 [DOI] [PubMed] [Google Scholar]
- 72.Zhuang J, Gong H, Zhou J, et al. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci Adv. 2020;6(13):eaaz6108. doi: 10.1126/sciadv.aaz6108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27(44):7043–7050. doi: 10.1002/adma.201503323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi J. Transforming platelets into microrobots. Sci Rob. 2020;5(43). doi: 10.1126/scirobotics.abc6582 [DOI] [PubMed] [Google Scholar]
- 75.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27(8):1482–1492. doi: 10.1093/annonc/mdw168 [DOI] [PubMed] [Google Scholar]
- 76.Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30(23):1706759. doi: 10.1002/adma.201706759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang H, Li Z, Wu Z, He Q. Cancer Cell Membrane〤amouflaged Micromotor. Adv Ther. 2019;2(12):1900096. [Google Scholar]
- 78.Kroll AV, Fang RH, Zhang L. Biointerfacing and applications of cell membrane-coated nanoparticles. Bioconjug Chem. 2017;28(1):23–32. doi: 10.1021/acs.bioconjchem.6b00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Esteban-Fernández de Ávila B, Angsantikul P, Ramírez-Herrera DE, et al. Hybrid biomembrane-functionalized nanorobots for concurrent removal of pathogenic bacteria and toxins. Sci Rob. 2018;3(18). doi: 10.1126/scirobotics.aat0485 [DOI] [PubMed] [Google Scholar]
- 80.Li T, Chen T, Chen H, et al. Engineered platelet-based micro/nanomotors for cancer therapy. Small. 2021;17(52):e2104912. doi: 10.1002/smll.202104912 [DOI] [PubMed] [Google Scholar]
- 81.Chen Z, Zhao P, Luo Z, et al. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10(11):10049–10057. doi: 10.1021/acsnano.6b04695 [DOI] [PubMed] [Google Scholar]
- 82.Fang RH, Hu CM, Luk BT, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14(4):2181–2188. doi: 10.1021/nl500618u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fang RH, Gao W, Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat Rev Clin Oncol. 2023;20(1):33–48. doi: 10.1038/s41571-022-00699-x [DOI] [PubMed] [Google Scholar]
- 84.Zhou M, Xing Y, Li X, Du X, Xu T, Zhang X. Cancer cell membrane camouflaged semi-yolk@spiky-shell nanomotor for enhanced cell adhesion and synergistic therapy. Small. 2020;16(39):e2003834. doi: 10.1002/smll.202003834 [DOI] [PubMed] [Google Scholar]
- 85.Wang H, Gao J, Xu C, et al. Light-driven biomimetic nanomotors for enhanced photothermal therapy. Small. 2023;e2306208. doi: 10.1002/smll.202306208. [DOI] [PubMed] [Google Scholar]
- 86.Asakura H. Pathophysiology and classification of thrombosis. Nihon Rinsho. 2014;72(7):1184–1190. [PubMed] [Google Scholar]
- 87.Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18(9):666–682. doi: 10.1038/s41569-021-00552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. 2023;147(8):e93–e621. doi: 10.1161/cir.0000000000001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jeger RV, Eccleshall S, Wan Ahmad WA, et al. Drug-coated balloons for coronary artery disease: third report of the international DCB consensus group. JACC: Cardiovasc Interv. 2020;13(12):1391–1402. doi: 10.1016/j.jcin.2020.02.043 [DOI] [PubMed] [Google Scholar]
- 90.Xiong GM, Ang H, Lin J, et al. Materials technology in drug eluting balloons: current and future perspectives. J Control Release. 2016;239:92–106. doi: 10.1016/j.jconrel.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 91.Nicolas J, Pivato CA, Chiarito M, Beerkens F, Cao D, Mehran R. Evolution of drug-eluting coronary stents: a back-and-forth journey from the bench to bedside. Cardiovasc Res. 2023;119(3):631–646. doi: 10.1093/cvr/cvac105 [DOI] [PubMed] [Google Scholar]
- 92.Aoki J, Tanabe K. Mechanisms of drug-eluting stent restenosis. Cardiovasc Interv Ther. 2021;36(1):23–29. doi: 10.1007/s12928-020-00734-7 [DOI] [PubMed] [Google Scholar]
- 93.Rittger H, Waliszewski M, Brachmann J, et al. Long-term outcomes after treatment with a paclitaxel-coated balloon versus balloon angioplasty: insights from the PEPCAD-DES study (treatment of drug-eluting stent [DES] in-stent restenosis with sequent please paclitaxel-coated percutaneous transluminal coronary angioplasty [PTCA] catheter). JACC: Cardiovasc Interv. 2015;8(13):1695–1700. doi: 10.1016/j.jcin.2015.07.023 [DOI] [PubMed] [Google Scholar]
- 94.Huang Y, Li T, Gao W, et al. Platelet-derived nanomotor coated balloon for atherosclerosis combination therapy. J Mater Chem B. 2020;8(26):5765–5775. doi: 10.1039/d0tb00789g [DOI] [PubMed] [Google Scholar]
- 95.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323 [DOI] [PubMed] [Google Scholar]
- 96.Shao J, Abdelghani M, Shen G, Cao S, Williams DS, van Hest JCM. Erythrocyte membrane modified janus polymeric motors for thrombus therapy. ACS Nano. 2018;12(5):4877–4885. doi: 10.1021/acsnano.8b01772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wan M, Wang Q, Wang R, et al. Platelet-derived porous nanomotor for thrombus therapy. Sci Adv. 2020;6(22):eaaz9014. doi: 10.1126/sciadv.aaz9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fang X, Ye H, Shi K, et al. GOx-powered janus platelet nanomotors for targeted delivery of thrombolytic drugs in treating thrombotic diseases. ACS Biomater Sci Eng. 2023;9(7):4302–4310. doi: 10.1021/acsbiomaterials.3c00387 [DOI] [PubMed] [Google Scholar]
- 99.Landman GW, Gans ROB. Oral rivaroxaban for symptomatic venous thromboembolism. letter. N Engl J Med. 2011;364(12):1178. [DOI] [PubMed] [Google Scholar]
- 100.Thomas, Hyers M. Antithrombotic therapy for venous thromboembolism. Chest. 1993;103(5):1636. [DOI] [PubMed] [Google Scholar]
- 101.Yang X, Wang Q, Zhang A, et al. Strategies for sustained release of heparin: a review. Carbohydr Polym. 2022;294:119793. doi: 10.1016/j.carbpol.2022.119793 [DOI] [PubMed] [Google Scholar]
- 102.Wu XD, Min J. Aspirin for thromboprophylaxis after a fracture: to the editor. New Engl J Med. 2023;2023:1. [DOI] [PubMed] [Google Scholar]
- 103.Zhao L, Zhang W, Wang X, Guan X, Zhang X, Ma J. Recent advances in selective photothermal therapy of tumor. J Nanobiotechnol. 2021;19(1):335. doi: 10.1186/s12951-021-01080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hussein EA, Zagho MM, Nasrallah GK, Elzatahry AA. Recent advances in functional nanostructures as cancer photothermal therapy. Int J Nanomed. 2018;13:2897–2906. doi: 10.2147/ijn.S161031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xuan M, Shao J, Gao C, Wang W, Dai L, He Q. Self-propelled nanomotors for thermomechanically percolating cell membranes. Angew Chem Int Ed Engl. 2018;57(38):12463–12467. doi: 10.1002/anie.201806759 [DOI] [PubMed] [Google Scholar]
- 106.Gao C, Lin Z, Wang D, Wu Z, Xie H, He Q. Red blood cell-mimicking micromotor for active photodynamic cancer therapy. ACS Appl Mater Interfaces. 2019;11(26):23392–23400. doi: 10.1021/acsami.9b07979 [DOI] [PubMed] [Google Scholar]
- 107.Yang Y, Yun K, Li Y, et al. Self-assembled multifunctional polymeric micelles for tumor-specific bioimaging and synergistic chemo-phototherapy of cancer. Int J Pharm. 2021;602:120651. doi: 10.1016/j.ijpharm.2021.120651 [DOI] [PubMed] [Google Scholar]
- 108.Zhao R, Zheng G, Fan L, et al. Carrier-free nanodrug by co-assembly of chemotherapeutic agent and photosensitizer for cancer imaging and chemo-photo combination therapy. Acta Biomater. 2018;70:197–210. doi: 10.1016/j.actbio.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 109.Hao Y, Wang L, Zhao Y, et al. Targeted imaging and chemo-phototherapy of brain cancer by a multifunctional drug delivery system. Macromol Biosci. 2015;15(11):1571–1585. doi: 10.1002/mabi.201500091 [DOI] [PubMed] [Google Scholar]
- 110.Hu C, Lei T, Wang Y, et al. Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials. 2020;255:120159. doi: 10.1016/j.biomaterials.2020.120159 [DOI] [PubMed] [Google Scholar]
- 111.Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater. 2020;32(40):e2002054. doi: 10.1002/adma.202002054 [DOI] [PubMed] [Google Scholar]
- 112.Gui R, Jin H, Wang Z, Li J. Black phosphorus quantum dots: synthesis, properties, functionalized modification and applications. Chem Soc Rev. 2018;47(17):6795–6823. doi: 10.1039/c8cs00387d [DOI] [PubMed] [Google Scholar]
- 113.Zhang X, Xie H, Liu Z, et al. Black phosphorus quantum dots. Angew Chem Int Ed Engl. 2015;54(12):3653–3657. doi: 10.1002/anie.201409400 [DOI] [PubMed] [Google Scholar]
- 114.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071 [DOI] [PubMed] [Google Scholar]
- 115.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–281. doi: 10.3322/caac.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–674. doi: 10.1038/s41571-020-0410-2 [DOI] [PubMed] [Google Scholar]
- 117.Ji B, Wei M, Yang B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics. 2022;12(1):434–458. doi: 10.7150/thno.67300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Q, Liu L, Huo H, et al. Nanosized janus AuNR-Pt motor for enhancing NIR-II photoacoustic imaging of deep tumor and Pt(2+) Ion-based chemotherapy. ACS Nano. 2022;16(5):7947–7960. doi: 10.1021/acsnano.2c00732 [DOI] [PubMed] [Google Scholar]
- 119.Chen S, Sun X, Fu M, et al. Dual-source powered nanomotor with integrated functions for cancer photo-theranostics. Biomaterials. 2022;288:121744. doi: 10.1016/j.biomaterials.2022.121744 [DOI] [PubMed] [Google Scholar]
- 120.Xu D, Hu J, Pan X, Sánchez S, Yan X, Ma X. Enzyme-powered liquid metal nanobots endowed with multiple biomedical functions. ACS Nano. 2021;15(7):11543–11554. doi: 10.1021/acsnano.1c01573 [DOI] [PubMed] [Google Scholar]
- 121.Xie L, Pang X, Yan X, et al. Photoacoustic imaging-trackable magnetic microswimmers for pathogenic bacterial infection treatment. ACS Nano. 2020;14(3):2880–2893. doi: 10.1021/acsnano.9b06731 [DOI] [PubMed] [Google Scholar]
- 122.Ye J, Fan Y, She Y, et al. Biomimetic self-propelled asymmetric nanomotors for cascade-targeted treatment of neurological inflammation. Adv Sci. 2024;11(22):2310211. doi: 10.1002/advs.202310211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ye J, Fan Y, Kang Y, et al. Biomimetic dual-driven heterojunction nanomotors for targeted catalytic immunotherapy of glioblastoma. Adv Funct Mater. 2025;35(9):2416265. doi: 10.1002/adfm.202416265 [DOI] [Google Scholar]
- 124.Zhang F, Zhuang J, Li Z, et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat Mater. 2022;21(11):1324–1332. doi: 10.1038/s41563-022-01360-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yue L, Gao C, Li J, et al. Chemotaxis-guided self-propelled macrophage motor for targeted treatment of acute pneumonia. Adv Mater. 2023;35(20):e2211626. doi: 10.1002/adma.202211626 [DOI] [PubMed] [Google Scholar]
- 126.Reyes LF, Restrepo MI, Hinojosa CA, et al. severe pneumococcal pneumonia causes acute cardiac toxicity and subsequent cardiac remodeling. Am J Respir Crit Care Med. 2017;196(5):609–620. doi: 10.1164/rccm.201701-0104OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet. 2013;381(9865):496–505. doi: 10.1016/s0140-6736(12)61266-5 [DOI] [PubMed] [Google Scholar]