Abstract
Osteoblasts mineralize bone matrix by promoting hydroxyapatite crystal formation and growth in the interior of membrane-limited matrix vesicles (MVs) and by propagating the crystals onto the collagenous extracellular matrix. Two osteoblast proteins, tissue-nonspecific alkaline phosphatase (TNAP) and plasma cell membrane glycoprotein-1 (PC-1) are involved in this process. Mutations in the TNAP gene result in the inborn error of metabolism known as hypophosphatasia, characterized by poorly mineralized bones, spontaneous fractures, and elevated extracellular concentrations of inorganic pyrophosphate (PPi). PPi suppresses the formation and growth of hydroxyapatite crystals. PPi is produced by the nucleoside triphosphate pyrophosphohydrolase activity of a family of isozymes, with PC-1 being the only member present in MVs. Mice with spontaneous mutations in the PC-1 gene have hypermineralization abnormalities that include osteoarthritis and ossification of the posterior longitudinal ligament of the spine. Here, we show the respective correction of bone mineralization abnormalities in knockout mice null for both the TNAP (Akp2) and PC-1 (Enpp1) genes. Each allele of Akp2 and Enpp1 has a measurable influence on mineralization status in vivo. Ex vivo experiments using cultured double-knockout osteoblasts and their MVs demonstrate normalization of PPi content and mineral deposition. Our data provide evidence that TNAP and PC-1 are key regulators of the extracellular PPi concentrations required for controlled bone mineralization. Our results suggest that inhibiting PC-1 function may be a viable therapeutic strategy for hypophosphatasia. Conversely, interfering with TNAP activity may correct pathological hyperossification because of PPi insufficiency.
In the process of bone formation, osteoblasts mineralize the matrix by promoting the seeding of basic calcium phosphate crystals of hydroxyapatite in the sheltered interior of shed membrane-limited matrix vesicles (MVs) and by propagating hydroxyapatite mineral onto the collagenous extracellular matrix (osteoid; refs. 1 and 2). Tissue-nonspecific alkaline phosphatase (TNAP), an isozyme of a family of four homologous human alkaline phosphatase genes (3), plays a role in bone matrix mineralization. Deactivating mutations in the TNAP gene causes the inborn error of metabolism known as hypophosphatasia (4), characterized by poorly mineralized cartilage (rickets) and bones (osteomalacia), spontaneous bone fractures, and elevated extracellular inorganic pyrophosphate (PPi) concentrations (5). The severity and expressivity of hypophosphatasia depends on the nature of the TNAP mutation (6). TNAP is present in MVs (7), and it has been proposed that the inorganic phosphate (Pi)-generating activity of TNAP is required to generate the Pi needed for hydroxyapatite crystallization (8–10). However, the ability of TNAP to hydrolyze PPi also has been hypothesized to be important to promote osteoblastic mineralization (11, 12), because PPi suppresses the formation and growth of hydroxyapatite crystals (13). In fact, heritable extracellular PPi deficiencies are models of ectopic calcification such as ankylosing spinal hyperostosis and pathologic soft-tissue ossification (14–16). PPi is produced by the nucleoside triphosphate pyrophosphohydrolase (NTPPPH) activity of a family of isozymes that include PC-1, B10/PDNP3, and autotaxin (17–19). However, PC-1 seems to be the only NTPPPH present in MVs (20). TNAP knockout (KO) mice (21–23) recapitulate the heritable metabolic disease hypophosphatasia (5), whereas PC-1-null mice display hypermineralization abnormalities similar to cartilage calcification in osteoarthritis (14) and ossification of the posterior longitudinal ligament of the spine (15).
We previously identified PC-1 as the likely NTPPPH isozyme to act on the same pathway with TNAP as antagonistic regulators of extracellular PPi concentrations (20). In this paper, we have tested the hypothesis that bone abnormalities caused by the lack of TNAP could be counterbalanced by the removal of PC-1 and vice versa. We show that bone mineralization in double-KO mice lacking both TNAP and PC-1 is essentially normal, providing evidence that TNAP and PC-1 are key regulators of bone mineralization by determining the normal steady-state levels of PPi. Our work suggests that TNAP and PC-1 may be useful therapeutic targets for the treatment of bone mineralization abnormalities.
Materials and Methods
Akp2 and Enpp1 KO Mice.
The generation and characterization of the Akp2 KO mice has been reported (22, 23). These Akp2 KO mice were hybrids of C57BL/6 × 129/J mouse strains. The generation of the Enpp1 KO mice has been reported briefly (24). These Enpp1 KO mice were hybrids of C57BL/6 × 129/SvTerJ mouse strains. Akp2/Enpp1 double-heterozygous mice were rederived into a C57BL/6 background, and the colony was maintained by brother–sister mating.
Tissue Preparation and Morphological Analysis.
Whole-mount skeletal preparations where prepared by removing the skin and viscera of the mice before they were immersed in 100% ethanol for 1 week and in acetone for 1 week. Specimens were transferred into a 100% ethanol solution containing 0.01% Alizarin Red S (Sigma), 0.015% Alcian Blue 8GX (Sigma), and 0.5% acetic acid and were incubated for 3 weeks. They were destained with 1% (vol/vol) KOH/50% (vol/vol) glycerol solution. Cleared specimens were stored in 100% glycerol.
The lumbar spines of 10-day-old mice were fixed in PBS containing 10% (vol/vol) formalin for 5 days. After washes in PBS-based sucrose solutions, samples were embedded in optimal cutting temperature (OCT) compound. Sections (16 μm) were stained with the von Kossa procedure, as described (25). The femurs of 10-day-old mice were fixed in 2.5% (vol/vol) glutaraldehyde in neutral 0.1 M cacodylate buffer for 24 h. Sections (1 μm) were stained with hematoxylin/eosin.
Isolation of Primary Osteoblasts and Matrix Vesicles.
Calvarial bones from 3-day-old pups were incubated three times for 10 min in a solution containing 4 mM EDTA, 137 mM NaCl, 2.7 mM KCl, 3 mM NaH2PO4 at pH 7.2. Specimens underwent seven 10-min digestions with EDTA-free buffer containing 180 μg/ml collagenase type 2 (Worthington). The last five fractions were pooled and seeded at 3–4 × 104 cells per cm2 in α-MEM (GIBCO/BRL) containing 10% (vol/vol) heat-inactivated FCS. When the primary calvarial cultures were confluent, the complete α-MEM media was supplemented with L-ascorbic acid (50 μg/ml) every third day and with either β-glycerophosphate (2.5 mM; ref. 26) or sodium phosphate (2.5 mM; ref. 18). The pericellular MV fraction was collected by collagenase digestion for 2 h at 37°C and sequential centrifugation, as described (20).
PPi and Mineralization Assays.
PPi concentrations were determined by differential adsorption on activated charcoal of UDP-D-[6-3H]glucose (Amersham Pharmacia) from the reaction product 6-phospho [6-3H]gluconate, as described (19).
To quantify matrix calcification by the cultured calvarial osteoblasts, we used Alizarin Red S binding assay as described (27). In brief, calvarial osteoblasts (5,000 cells per well) were plated in a volume of 0.1 ml of medium in a flat bottom 96-well plate (Falcon). At each time point, the medium was removed, and 0.05 ml of diluted Alizarin Red S [0.5% (vol/vol) Alizarin Red S, pH 5.0] was added and incubated at 23°C for 10 min. The plates were washed four times with PBS before the addition of 0.1 ml of 10% (wt/vol) cetylpyridinium chloride for 10 min to release the remaining calcium-bound Alizarin Red S. The solution was collected, diluted at a ratio of 1:10 and read at OD570 on a SpectraMAX microplate reader (Molecular Devices).
Results
Rescue from Bone Abnormalities in Vivo.
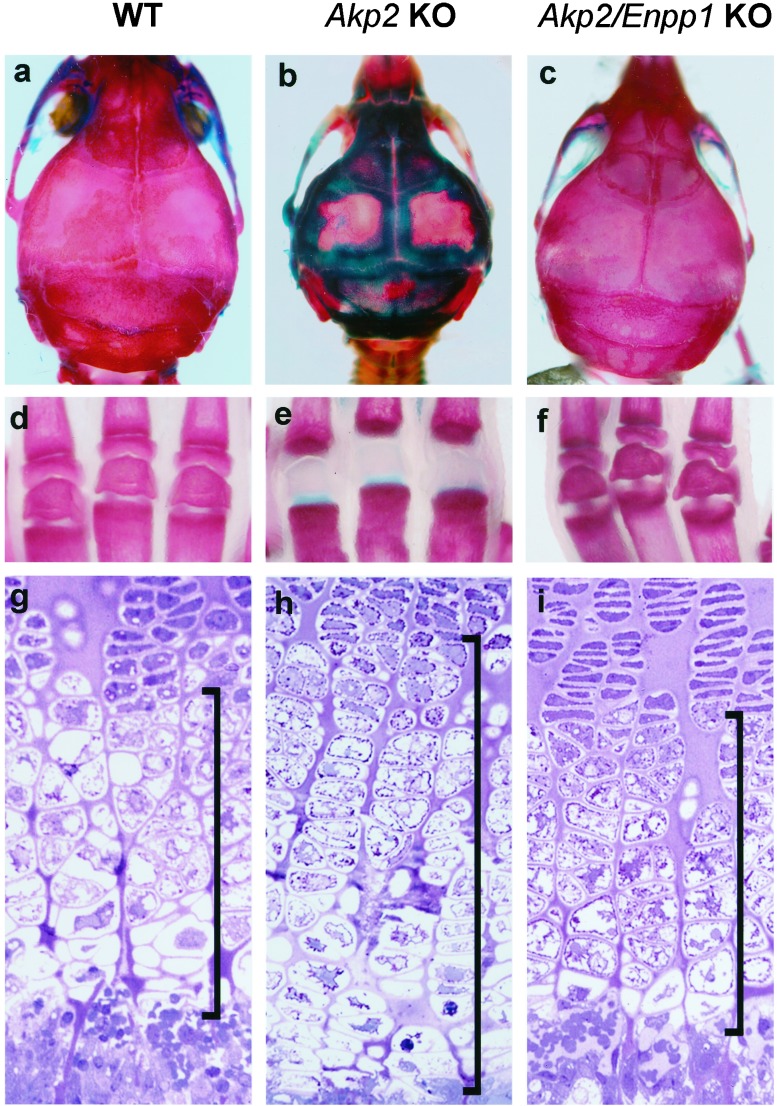
We observed clear evidence of rescue from rickets and osteomalacia in Akp2/Enpp1 double-KO mice. The unmineralized areas in the calvaria (shown by the lack of Alizarin Red staining) and the absence of secondary ossification centers in the hind limb phalanges characteristic of the Akp2 KO mice have been corrected in the Akp2/Enpp1 double-KO mice (Fig. 1 a–f). We also found correction in the length and histological appearance of the growth plates of the double-KO mice (Fig. 1 g–i). Akp2 KO mice show a characteristic thickening of the hypertrophic zone of the growth plates in the tibia (23). We observed a restoration of normal growth-plate thickness and cellularity in the Akp2/Enpp1 double-KO mice. Interestingly, double-homozygous mice live up to 25 day without vitamin B6 administration (21, 23, 25), whereas we have never observed an Akp2 KO mouse surviving longer than 14 days in our colony (n = 325) in the absence of vitamin B6 supplementation.
Figure 1.
Correction of skeletal mineralization and growth-plate structure in Akp2/Enpp1 double-KO mice. Whole-mount skeletal preparations of the calvaria (a–c) and hind limb phalanges (d–f) show skeletal correction in Akp2/Enpp1 double-KO mice. The tissues were stained with Alizarin Red that detects bone mineral and Alcian Blue that stains unmineralized osteoid at pH 5.5. WT, calvaria, and phalanges of a 20-day-old WT mouse (a and d). At 3 weeks of age, the Enpp1 KO mice (not shown), do not show any evidence of hypermineralization in the calvaria or phalanges, and their whole-mount preparations are indistinguishable from those of WT mice. Akp2 KO, calvaria and phalanges of a 20-day-old Akp2 KO mouse (b and e). Note the absence of secondary ossification centers in the phalanges; Akp2/Enpp1 KO, calvaria and phalanges of a 20-day-old Akp2/Enpp1 double-KO mouse displaying correction of the mineralization defects (c and f). (g–i) Nondecalcified sections of tibial growth plates of 10-day-old mice stained with hematoxylin/eosin. The WT tibial growth plate (g) shows normal proliferative zone cells at the top. Lightly stained hypertrophic zone cells are seen in the middle of the field, and the metaphysis is present at the bottom. In contrast, the tibial growth plate from Akp2 KO mice (h) shows a characteristic thickening, especially of the hypertrophic zone, as seen (23). Note the restoration of normal growth-plate thickness and cellularity in the tibial growth plate of Akp2/Enpp1 double-KO mice (i). Bars in the figure indicate the thickness of the hypertrophic zone of the growth plate. (Magnification ×310.)
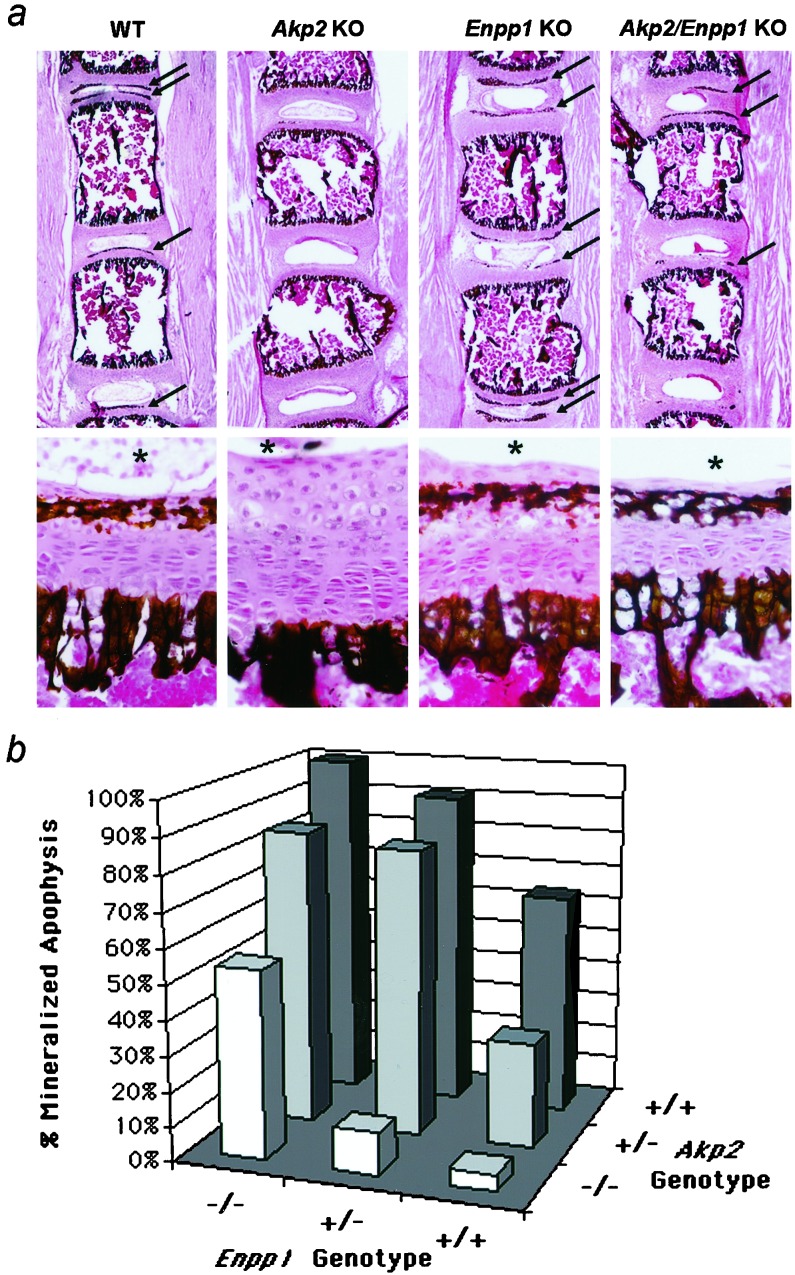
We examined the lumbar spine of all genotypes and found differences in the degree of mineralization in the vertebral apophyses. Fig. 2a shows a low and a high magnification view of the apophyses of each genotype. We then counted and determined the ratio of the number of ossification centers containing visible mineral deposits to those containing no visible deposits (Fig. 2b). Ten-day-old Enpp1 KO mice have precipitated mineral in every lumbar vertebral apophyses (100%), whereas in the corresponding areas of age-matched Akp2-null mice, mineral deposits are virtually absent (6%). More than half the lumbar vertebrae of the Akp2/Enpp1 double-KO mice had mineral deposits, a ratio comparable to that found in the wild-type (WT) mice. Each functional allele of Akp2 and Enpp1 contributes to the mineralization status of the spine (Fig. 2b). Note that even carriers (heterozygotes) of Akp2- and Enpp1-null mutations are affected.
Figure 2.
Nondecalcified lumbar spine sections stained by the von Kossa procedure to visualize phosphate deposits. (a) Low (×16) and high (×240) magnification view of the 10-day-old vertebral apophyses of WT, Akp2 KO, Enpp1 KO, and Akp2/Enpp1 double-KO mice. Arrows point to mineralized secondary ossification centers in sample sections at low magnification. Note the absence of mineral deposits in sections from the Akp2 KO mice. *, relative position of the nucleus pulposus as a point of reference in the high magnification panels. (b) Graph plotting the contribution of each Akp2 and Enpp1 allele to the mineralization status of the lumbar vertebrae. The percentage of mineralized apophyses is plotted as a function of the Akp2 and Enpp1 genotypes.
Correction of Mineralization and PPi Levels ex Vivo.
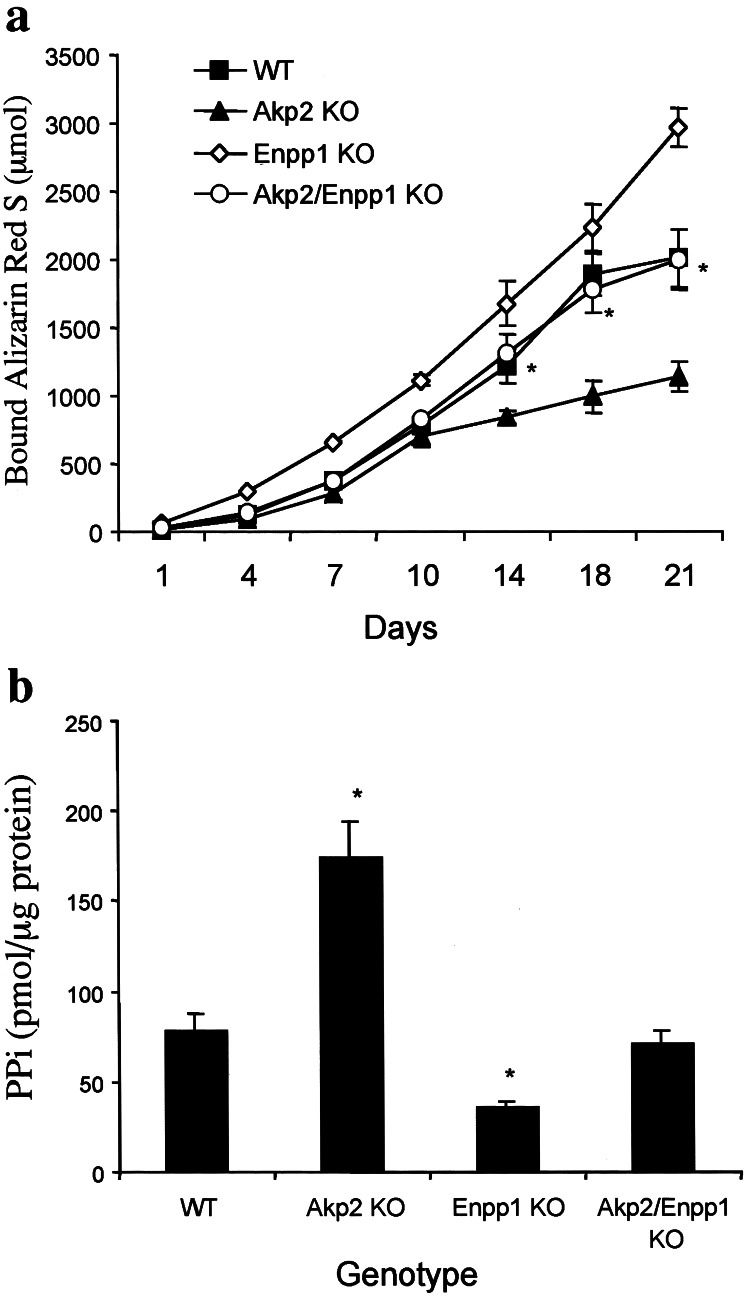
We then examined the ability of Akp2/Enpp1 double-KO primary osteoblasts to mineralize bone nodules in vitro. Similar to the Akp2 KO primary osteoblasts, the Akp2/Enpp1 double-KO osteoblasts cultured with β-glycerophosphate as the principal phosphate source showed an absence of mineralization (not shown). However, incubating primary osteoblasts with sodium phosphate as the free phosphate source, a condition that better mimics the in vivo environment, allows for the detection of differences in the mineralization ability of osteoblasts of differing genotypes (Fig. 3a). Akp2 KO osteoblasts cultured for 21 days precipitated significantly less mineral than WT osteoblasts, whereas osteoblasts of Enpp1 KO mice precipitated significantly more mineral than control cells. A normal mineralization capacity was restored in the Akp2/Enpp1 double-KO osteoblasts. Because inorganic pyrophosphate is a potent inhibitor of mineralization, we also quantified the amount of PPi in the MVs isolated from the cultured osteoblasts (Fig. 3b). MVs from Akp2 KO osteoblasts had increased amounts of PPi, whereas those from the Enpp1 KO osteoblasts had decreased levels of PPi. The MVs derived from the Akp2/Enpp1 double-KO osteoblasts exhibited PPi concentrations that were comparable to those of WT MVs.
Figure 3.
Primary Akp2/Enpp1 double-KO osteoblasts show a correction in their mineralization capacity and normalized PPi concentrations in MVs. (a) Akp2 KO osteoblasts show poor mineralization after 21 days in culture, whereas the cultures of Enpp1 KO osteoblasts have a higher mineralization capacity compared with WT osteoblasts. Remarkably, the Akp2/Enpp1 double-KO and the WT cultures display indistinguishable mineralization properties (n = 7 mice each genotype, in replicates of 4). *, P < 0.05 compared with single gene KO osteoblasts. (b) The histogram displays the concentration of PPi in the MVs of each genotype. Akp2/Enpp1 double-KO MVs show PPi levels that are equivalent to those in WT vesicles. In contrast, MVs from single KO osteoblasts show either an elevation (Akp2 KO) or a reduction (Enpp1 KO) in their PPi concentration (n = 5 mice, in replicates of 3) relative to TTPP mice. *, P < 0.05 compared with WT MVs.
Discussion
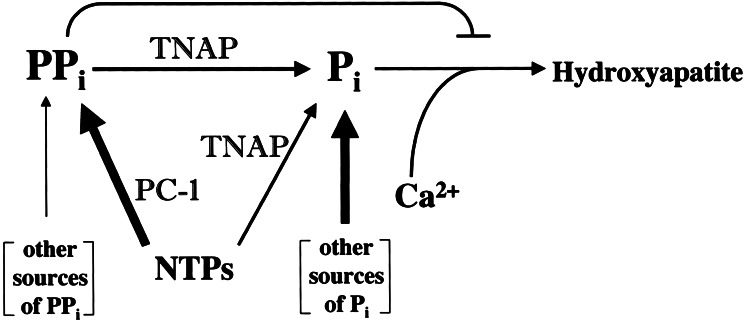
It has been proposed that the activity of TNAP is required to generate the Pi needed for hydroxyapatite crystallization (8–10), and ATP and PPi have been explored as potential substrates for this effect (11, 28). However, the ability of TNAP to hydrolyze PPi also has been hypothesized to be important to promote calcification by hydrolyzing this potent inhibitor of mineralization (11–13, 29, 30). Our data provide evidence that in osteoblasts, TNAP and PC-1 are central, directly antagonistic regulators of the concentration of the matrix calcification inhibitor PPi. Restoration of extracellular PPi to normal levels in Akp2/Enpp1 double-KO mice, in conjunction with correction of the hypomineralization resulting from the TNAP deficiency, points to the key role of PPi in this phenotypic rescue (Fig. 4). Our data support the hypothesis that the extracellular PPi pool largely determines the rate of hydroxyapatite crystal formation in bone tissue. PC-1 is a major contributor to the extracellular PPi pool, whereas the action of TNAP restricts PPi concentration and also contributes to the extracellular Pi pool available for the formation of hydroxyapatite crystals. The concerted action of TNAP and PC-1 ensures that the optimal concentration of PPi is achieved under normal conditions. In hypophosphatasia, the PPi pool increases because of lack of TNAP's inorganic pyrophosphatase activity, and rickets/osteomalacia ensue as PPi's inhibition of hydroxyapatite deposition is augmented. In PC-1 deficiency, the extracellular PPi pool decreases because of reduced production of PPi, and hypermineralization takes place as PPi inhibition of hydroxyapatite deposition is relaxed. The simultaneous deletion of TNAP and PC-1 correct the levels of extracellular PPi, and normal mineralization is re-established.
Figure 4.
Schematic representation of the counterregulatory functions of TNAP and PC-1 in modulating extracellular PPi concentrations. (NTPs, nucleoside triphosphates). In the bone matrix, PC-1 is the major producer of PPi (thick arrows), which in turn has an inhibitory effect on hydroxyapatite deposition. TNAP has a positive influence on mineralization primarily by controlling the size of the inhibitory pool of PPi through its inorganic pyrophosphatase activity. TNAP also generates Pi by using NTPs and PPi as substrates, but other more major sources of Pi (thick arrow), e.g., intestinal absorption, are likely to contribute the bulk of the Pi needed for hydroxyapatite deposition.
Our data allow us to conclude that TNAP's primary function in mineralizing tissues is to act in concert with PC-1 to fine tune PPi concentrations to maintain steady-state levels of PPi adequate for controlled mineralization. Additionally, through hydrolysis of PPi and nucleoside triphosphates, TNAP also may contribute to the pool of Pi available for deposition as hydroxyapatite. However, the fact that the TNAP-null mice, albeit inefficiently, still deposit bone mineral argues that the Pi needed for deposition is supplied by other, more major, sources, e.g., intestinal absorption (31). The bone nodule mineralization assay using β-glycerophosphate as the phosphate source reveals a lack of in vitro mineralization by Akp2 KO primary osteoblasts, as we have reported (26), as well as by the Akp2/Enpp1 double-KO osteoblasts (data not shown). The explanation is trivial, however, because free Pi cannot be produced from this organic compound in the absence of TNAP. However, the fact that MVs from patients with hypophosphatasia contain hydroxyapatite mineral in their interior (32) indicates that TNAP is not required for the initial deposition of bone mineral, and that phosphated organic compounds are not likely crucial substrates for TNAP's role in bone mineralization. A variation of the in vitro mineralization assay that uses free inorganic phosphate as the principal phosphate source (18) better mimics the physiological environment where there is an excess of free phosphate resulting from intestinal absorption (31). This assay has allowed us to detect differences in the ability of primary osteoblasts of different genotypes to mineralize. Importantly, we found that the restoration of in vitro mineralization correlates perfectly with the in vivo correction of mineralization abnormalities in the Akp2/Enpp1 double-KO mice and with the normalized levels of inorganic pyrophosphate resulting in the Akp2/Enpp1 double- KO MVs. Thus, a Pi-generating role for TNAP seems to be secondary to its inorganic pyrophosphatase function in bone tissue, in agreement with previous indications (29, 30).
To date, there is no effective medical treatment for hypophosphatasia (5). Attempts to treat the disease by using oral administration of Mg2+, Zn2+, or Pi supplementation have been unsuccessful, as have been the administration of parathyroid hormone to attempt to stimulate TNAP activity (33–35). Infusing hypophosphatasia patients with normal serum (36), Paget's serum (37), and purified alkaline phosphatase (38) also have had disappointing results. Our data identify PC-1 as a useful target for interference in the treatment of hypophosphatasia. By deleting the Enpp1 gene, we have restored PPi concentrations to a normal level and corrected osteomalacia. We fully expect that in vivo administration of inhibitors of PC-1 activity, e.g., reactive blue-2 or pyridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid 4-sodium (39), could be equally successful in treating rickets and osteomalacia in hypophosphatasia patients.
PC-1-deficient mice represent a model of certain human diseases in which there is uncontrolled ossification of ligamentous tissues leading to loss of spine and peripheral joint mobility. One of these diseases is ossification of the posterior longitudinal ligament (OPLL; refs. 14 and 15). OPLL is a common disorder in Asia, particularly in Japan, where the incidence is 1.5% among people over 50 years of age. Polymorphisms of the PC-1 gene, of currently unknown functional significance, have been reported in Japanese subjects with OPLL (40, 41). The PC-1 KO mice are also an accepted animal model for diffuse idiopathic skeletal hyperostosis and ankylosing spinal hyperostosis (refs. 42 and 43). Treatments for enhanced ossification, except for surgery, are not well established. There is a need for drugs that target the action of osteoblasts by affecting their ability to ossify their surrounding matrix. Our results suggest the possible utility of interfering with TNAP activity as a therapeutic strategy for the treatment of pathologic cartilage calcification and hyperostosis due to local and systemic PC-1 deficiency.
Acknowledgments
This work was supported by National Institutes of Health Grants CA 42595, DE 12889, AR 47908, AG O7996, AR 40770, and AR 47347, and by grants from the Department of Veterans Affairs, Monash University, and from the National Health and Medical Research Council of Australia.
Abbreviations
- MV
matrix vesicle
- TNAP
tissue-nonspecific alkaline phosphatase
- Pi
inorganic phosphate
- PPi
inorganic pyrophosphate
- KO
knockout
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Anderson H C. Clin Orthop Relat Res. 1995;314:266–280. [PubMed] [Google Scholar]
- 2.Boskey A L. Connect Tissue Res. 1996;35:357–363. doi: 10.3109/03008209609029212. [DOI] [PubMed] [Google Scholar]
- 3.Henthorn P, Millán J L, Leboy P. In: Principles of Bone Biology. Seibel M J, Robins S P, Bilezikian J P, editors. San Diego: Academic; 1999. pp. 127–136. [Google Scholar]
- 4.Henthorn P S, Whyte M P. Clin Chem. 1992;38:2501–2505. [PubMed] [Google Scholar]
- 5.Whyte M P. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, Childs B, Kinzler K W, Vogelstein B, editors. New York: McGraw–Hill; 2001. pp. 5313–5329. [Google Scholar]
- 6.Zurutuza L, Muller F, Gibrat J F, Taillandier A, Simon-Bouy B, Serre J L, Mornet A. Hum Mol Genet. 1999;8:1039–1046. doi: 10.1093/hmg/8.6.1039. [DOI] [PubMed] [Google Scholar]
- 7.Hsu H H, Anderson H C. Proc Natl Acad Sci USA. 1978;75:3805–3808. doi: 10.1073/pnas.75.8.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robison R. Biochem J. 1923;17:286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majeska R J, Wuthier R E. Biochim Biophys Acta. 1975;391:51–60. doi: 10.1016/0005-2744(75)90151-5. [DOI] [PubMed] [Google Scholar]
- 10.Fallon M D, Whyte M P, Teitelbaum S L. Lab Invest. 1980;43:489–494. [PubMed] [Google Scholar]
- 11.Moss D W, Eaton R H, Smith J K, Whitby L G. Biochem J. 1967;102:53–57. doi: 10.1042/bj1020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezende A, Pizauro J, Ciancaglini P, Leone F. Biochem J. 1994;301:517–522. doi: 10.1042/bj3010517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer J L. Arch Biochem Biophys. 1984;231:1–8. doi: 10.1016/0003-9861(84)90356-4. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto M, Hosoda Y, Kojimahara K, Yamazaki T, Yoshimura Y. Pathol Int. 1994;44:420–427. doi: 10.1111/j.1440-1827.1994.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 15.Okawa A, Nakamura I, Goto S, Moriya H, Nakamura Y, Ikegawa S. Nat Genet. 1998;19:271–273. doi: 10.1038/956. [DOI] [PubMed] [Google Scholar]
- 16.Ho A M, Johnson M D, Kingsley D M. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 17.Terkeltaub R, Rosenbach M, Fong F, Goding J. Arthritis Rheum. 1994;37:934–941. doi: 10.1002/art.1780370624. [DOI] [PubMed] [Google Scholar]
- 18.Johnson K, Moffa A, Chen Y, Pritzker K, Goding J, Terkeltaub R. J Bone Miner Res. 1999;14:883–892. doi: 10.1359/jbmr.1999.14.6.883. [DOI] [PubMed] [Google Scholar]
- 19.Johnson K, Vaingankar S, Chen Y, Moffa A, Goldring M, Sano K, Jin-Hua P, Sali A, Goding J, Terkeltaub R. Arthritis Rheum. 1999;42:1986–1997. doi: 10.1002/1529-0131(199909)42:9<1986::AID-ANR26>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 20.Johnson K A, Hessle L, Vaingankar S, Wennberg C, Mauro S, Narisawa S, Goding J W, Sano K, Millán J L, Terkeltaub R. Am J Physiol. 2000;279:R1365–R1377. doi: 10.1152/ajpregu.2000.279.4.R1365. [DOI] [PubMed] [Google Scholar]
- 21.Waymire K G, Mahuren J D, Jaje J M, Guilarte T R, Coburn S P, MacGregor G R. Nat Genet. 1995;11:45–51. doi: 10.1038/ng0995-45. [DOI] [PubMed] [Google Scholar]
- 22.Narisawa S, Fröhlander N, Millan J L. Dev Dyn. 1997;208:432–446. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Fedde K N, Blair L, Silverstein J, Coburn S P, Ryan L M, Weinstein R S, Waymire K, Narisawa S, Millan J L, MacGregor G R, Whyte M P. J Bone Miner Res. 1999;14:2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sali A, Favaloro J M, Terkeltaub R, Goding J W. In: Ecto-ATPases and Related Ectonucleotidases. Vanduffel L, Lemmens R, editors. Maastricht, The Netherlands: Shaker; 1999. pp. 267–282. [Google Scholar]
- 25.Narisawa S, Wennberg C, Millán J L. J Pathol. 2001;193:125–133. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH722>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 26.Wennberg C, Hessle L, Lundberg P, Mauro S, Narisawa S, Lerner U H, Millán J L. J Bone Miner Res. 2000;15:1879–1888. doi: 10.1359/jbmr.2000.15.10.1879. [DOI] [PubMed] [Google Scholar]
- 27.Johnson K, Hashimoto S, Lotz M, Pritzker K, Goding J, Terkeltaub R. Arthritis Rheum. 2001;44:1071–1081. doi: 10.1002/1529-0131(200105)44:5<1071::AID-ANR187>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Hsu H H, Anderson H C. Biochim Biophys Acta. 1977;500:162–172. doi: 10.1016/0304-4165(77)90056-3. [DOI] [PubMed] [Google Scholar]
- 29.Caswell A M, Whyte M P, Russell R G. Crit Rev Clin Lab Sci. 1991;28:175–232. doi: 10.3109/10408369109106862. [DOI] [PubMed] [Google Scholar]
- 30.Whyte M P. Endocr Rev. 1994;15:439–461. doi: 10.1210/edrv-15-4-439. [DOI] [PubMed] [Google Scholar]
- 31.Drezner M K. In: Principles of Bone Biology. Bilezikian J P, Raisz L G, Rodan G A, editors. San Diego: Academic; 1996. pp. 263–276. [Google Scholar]
- 32.Anderson H C, Hsu H H, Morris D C, Fedde K N, Whyte M P. Am J Pathol. 1997;151:1555–1561. [PMC free article] [PubMed] [Google Scholar]
- 33.Bongiovanni A M, Album M M, Root A W, Hope J W, Marino J, Spencer D M. Am J Med Sci. 1968;255:163–170. doi: 10.1097/00000441-196803000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Whyte M P, Teitelbaum S L, Murphy W A, Bergfeld M A, Avioli L V. Medicine (Baltimore) 1979;58:329–347. [PubMed] [Google Scholar]
- 35.Whyte M P, Magill H L, Fallon M D, Herrod H G. J Pediatr. 1986;108:82–88. doi: 10.1016/s0022-3476(86)80773-9. [DOI] [PubMed] [Google Scholar]
- 36.Whyte M P, Mahuren J D, Fedde K N, Cole F S, McCabe E R, Coburn S P. J Clin Invest. 1988;81:1234–1239. doi: 10.1172/JCI113440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whyte M P, McAlister W H, Patton L S, Magill H L, Fallon M D, Lorentz W B, Herrod H G. J Pediatr. 1984;105:926–933. doi: 10.1016/s0022-3476(84)80079-7. [DOI] [PubMed] [Google Scholar]
- 38.Whyte M P, Habib D, Coburn S P, Tecklenburg F, Ryan L, Fedde K N, Stinson R A. J Bone Miner Res. 1992;7,Suppl.:S155. [Google Scholar]
- 39.Grobben B, Claes P, Roymans D, Esmans E L, Van Onckelen H, Slegers H. Br J Pharmacol. 2000;130:139–145. doi: 10.1038/sj.bjp.0703289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura I, Ikegawa S, Okawa A, Okuda S, Koshizuka Y, Kawaguchi H, Nakamura K, Koyama T, Goto S, Toguchida J, et al. Hum Genet. 1999;104:492–497. doi: 10.1007/s004390050993. [DOI] [PubMed] [Google Scholar]
- 41.Koshizuka Y, Kawaguchi H, Ogata N, Ikeda T, Mabuchi A, Seichi A, Nakamura Y, Nakamura K, Ikegawa S. J Bone Miner Res. 2002;17:138–144. doi: 10.1359/jbmr.2002.17.1.138. [DOI] [PubMed] [Google Scholar]
- 42.Okawa A, Goto S, Moriya H. Calcif Tissue Int. 1999;64:239–247. doi: 10.1007/s002239900610. [DOI] [PubMed] [Google Scholar]
- 43.Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert W A, Superti-Furga A, Terkeltaub R. Am J Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]