Abstract
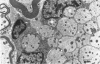
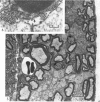
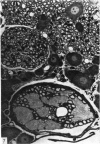
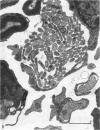
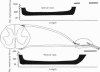
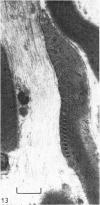
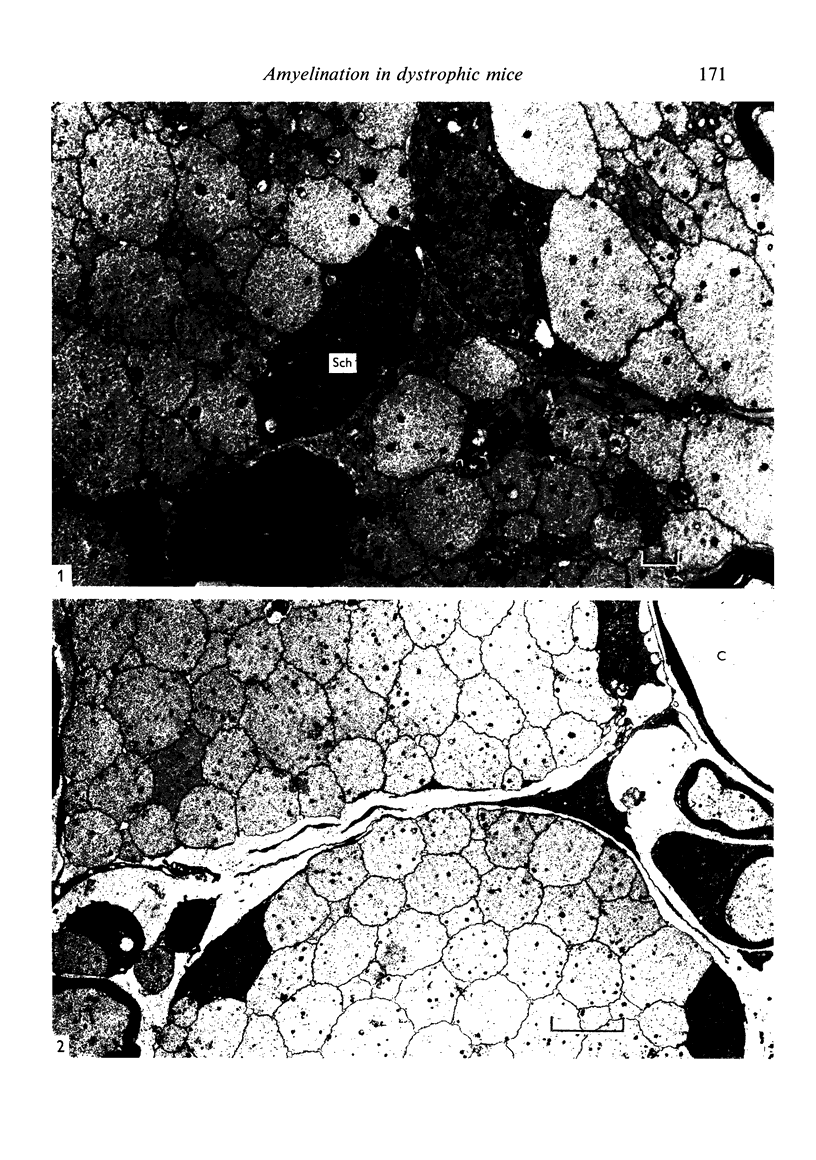
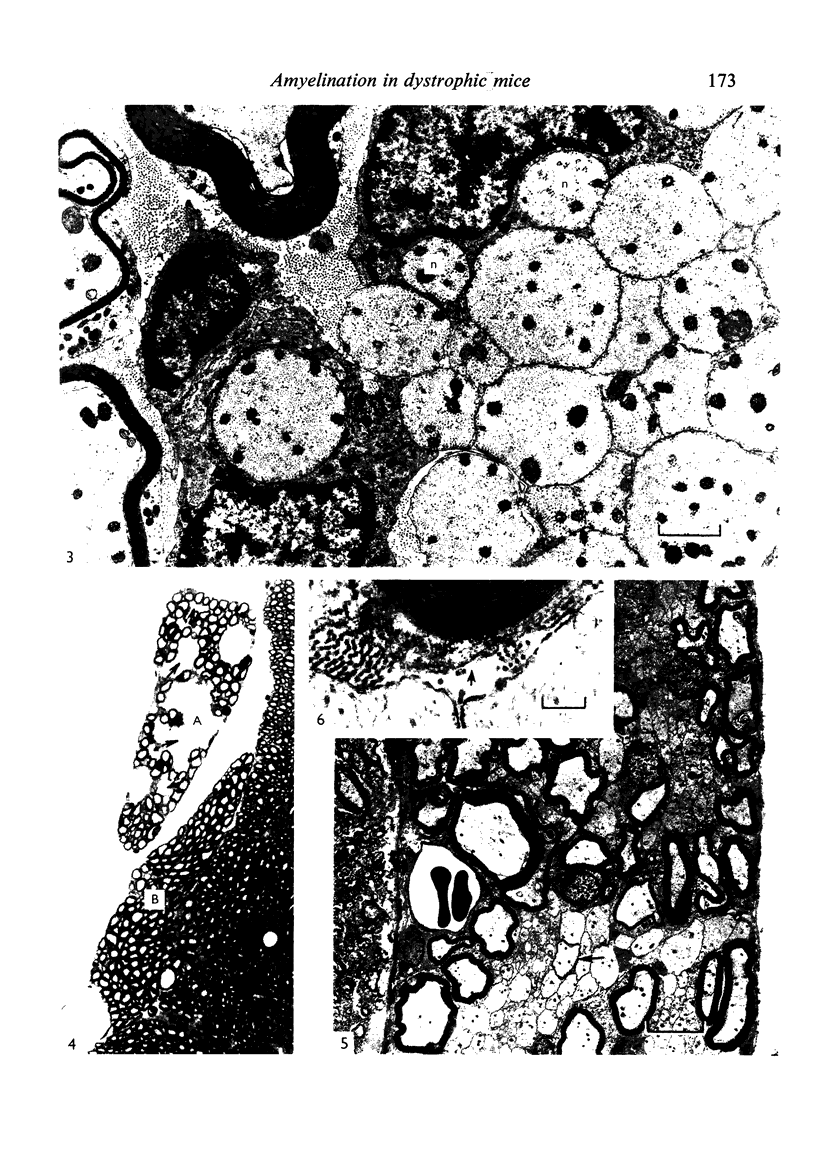
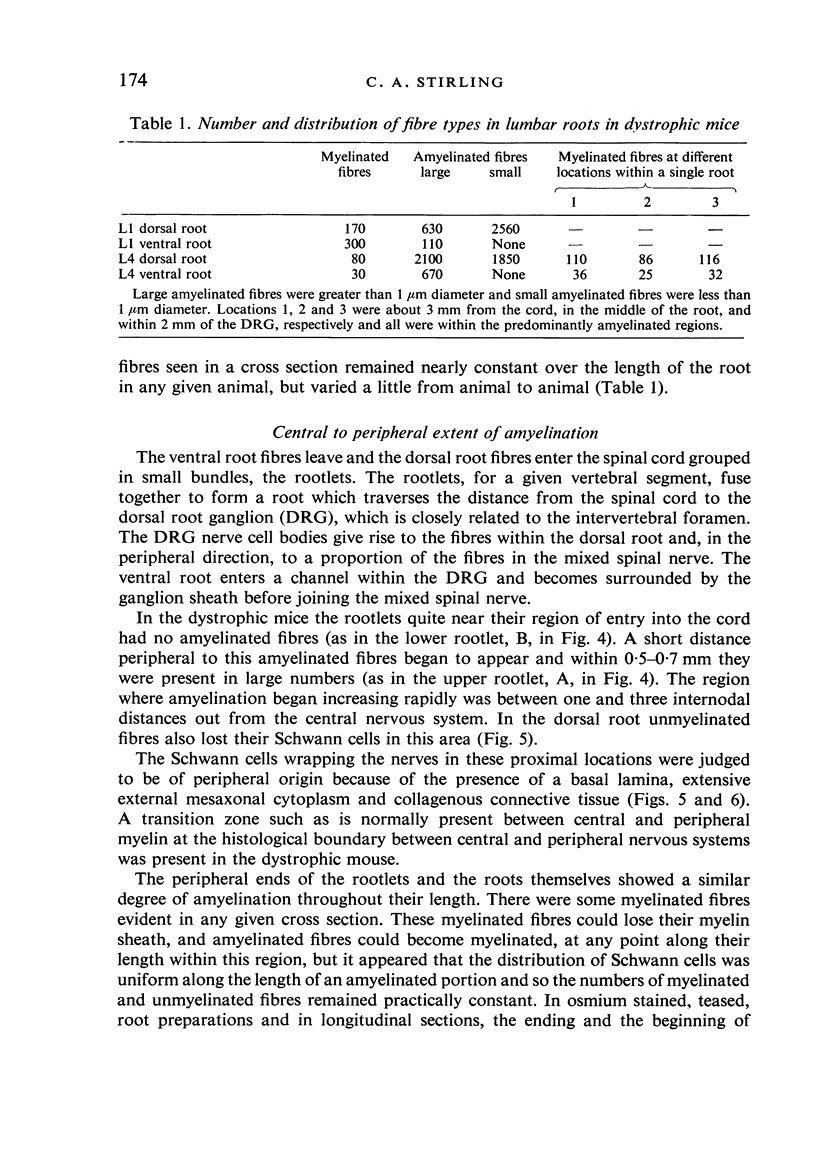
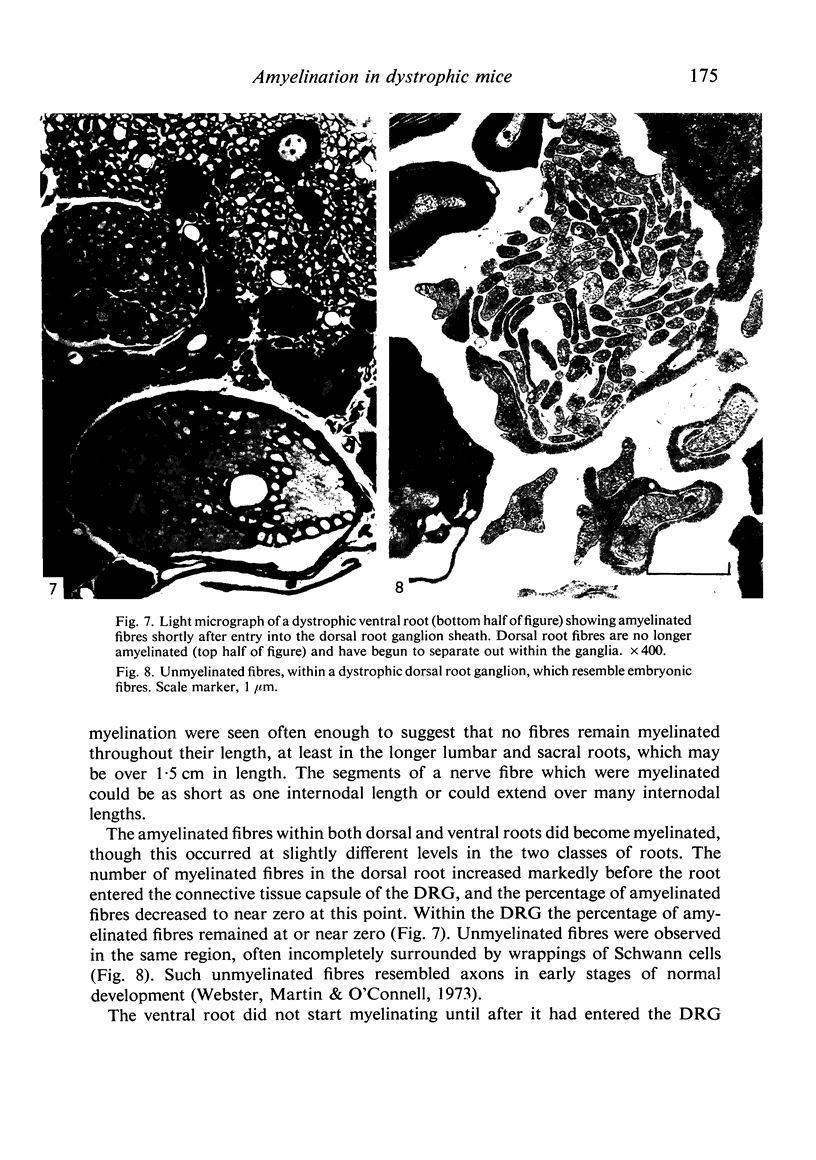
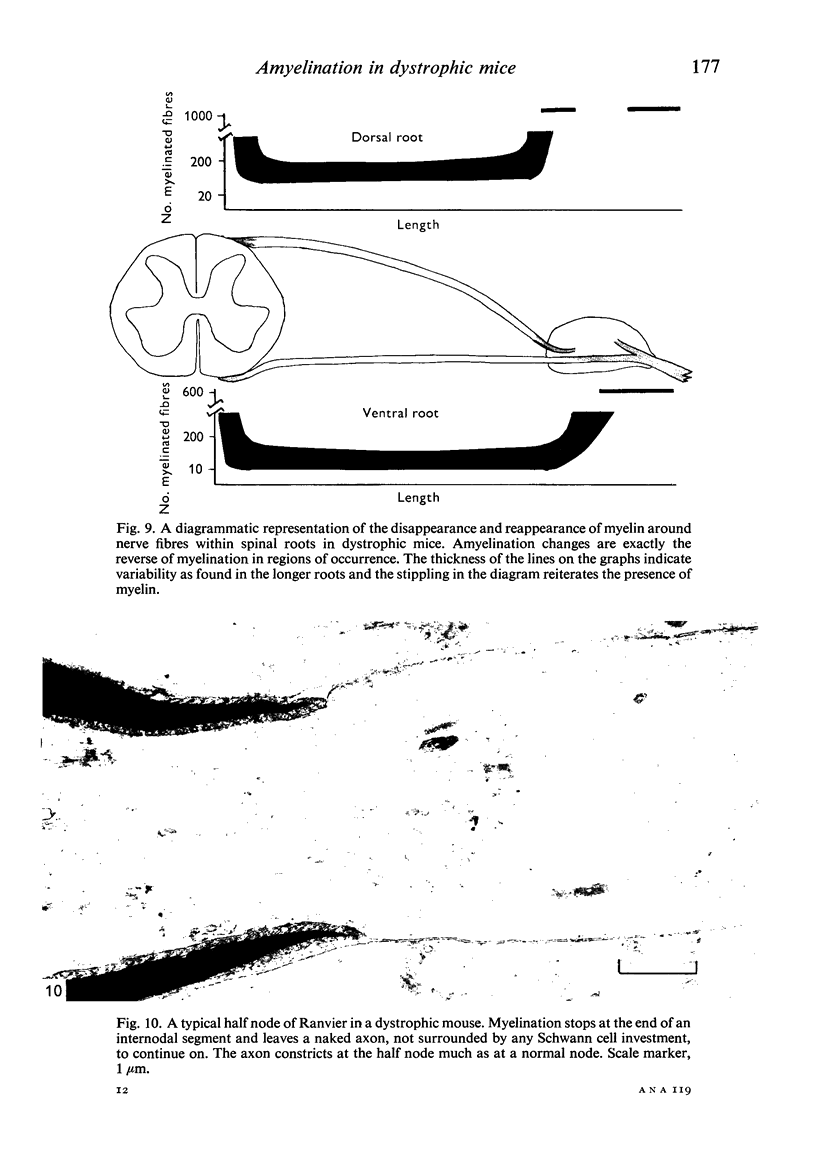
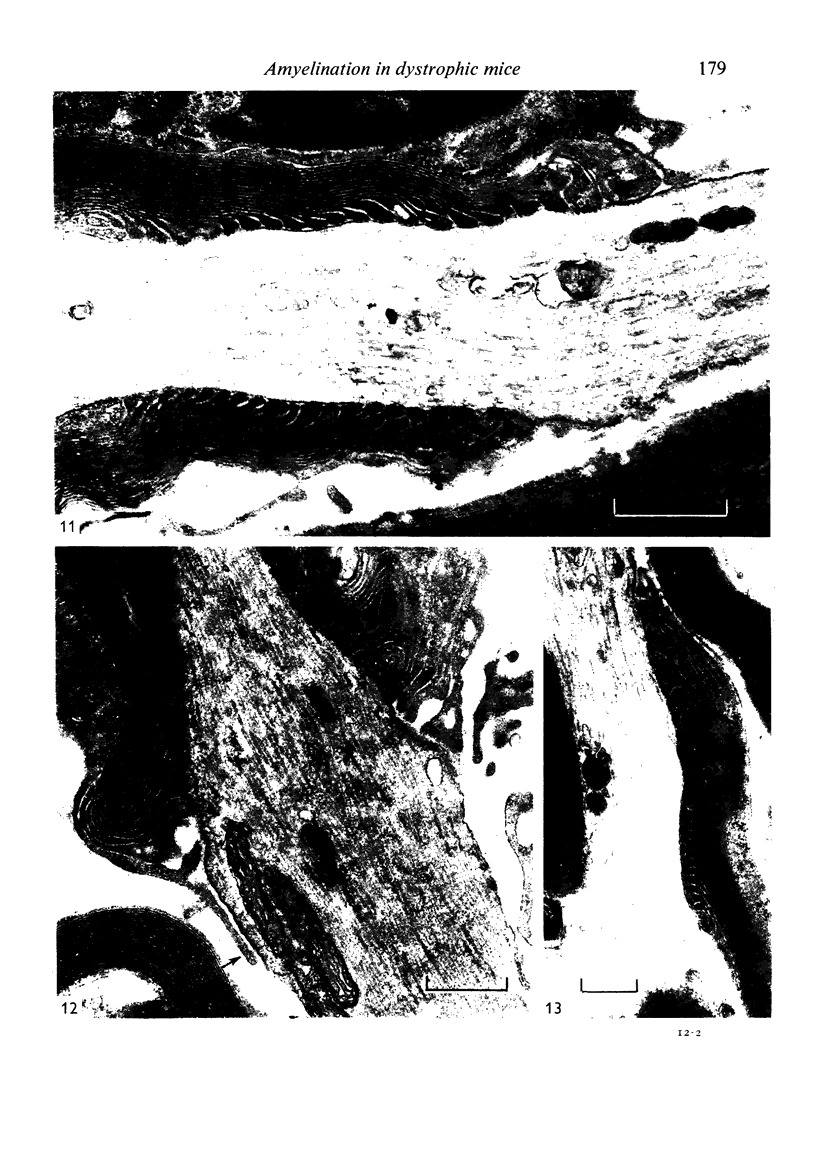
Dorsal and ventral spinal roots at cervical, thoracic, lumbar and sacral levels in dystrophic, dy/dy, mice of both 129/ReJ and C57Bl/6J phenotypes showed a complete lack of Schwann cell sheaths of any sort around the majority of their nerve fibres. This condition, termed amyelination, is more extensive (up to 1-5 cm in length) in the longer lumbar and sacral roots than in the shorter roots or in the proximal regions of the sciatic nerve which are also affected to some extent. Amyelination does not appear to be a consequence of myelin or Schwann cell degeneration, as debris is uncommon. Heterozygous carriers are not affected in any obvious way. Myelinated fibres, with Schwann cells of peripheral origin, occur immediately adjacent to the spinal cord in both dorsal and ventral roots, while in dorsal roots unmyelinated fibres also occur, as in normal animals. Amyelinated fibres begin to appear a few internodal lengths away from the cord and are present until near, or within, the dorsal root ganglion, where they become myelinated again. The portion of an axon which has no myelin begins at a normal appearing paranodal region (termed a half node of Ranvier) at the end of a myelin internode. Resumption of myelination likewise begins at a half node. A few myelinated axons may be seen in any given cross section of a root, but as a rule a given myelinated fibre does not remain myelinated throughout the whole length of the root. It is suggested that the nerve lesions develop where the nerves are lengthening rapidly as the animal grows and changes its shape. How these nerve changes release to those in muscle is conjectural.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker B. Q. A phase and electron microscopic study of dystrophic muscle. I. The pathological changes in the two-week-old Bar Harbor 129 dystrophic mouse. J Neuropathol Exp Neurol. 1967 Apr;26(2):259–275. doi: 10.1097/00005072-196704000-00006. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Nicholls J. G. Changes in extracellular potassium concentration produced by neuronal activity in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):555–569. doi: 10.1113/jphysiol.1969.sp008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Caddy K. W., Pallot D. J., Pehrson U. M., Stirling C. A. The neurological lesion in the dystrophic mouse. Brain Res. 1974 Aug 23;76(3):534–536. doi: 10.1016/0006-8993(74)90830-0. [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Jenkison M. Abnormalities of peripheral nerves in murine muscular dystrophy. J Neurol Sci. 1973 Feb;18(2):227–247. doi: 10.1016/0022-510x(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Gallup B., Dubowitz V. Letter: Failure of "dystrophic" neurones to support functional regeneration of normal or dystrophic muscle in culture. Nature. 1973 Jun 1;243(5405):287–289. doi: 10.1038/243287a0. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Wilson P. Mechanical properties of dystrophic mouse muscle. J Neurol Neurosurg Psychiatry. 1971 Oct;34(5):512–520. doi: 10.1136/jnnp.34.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Michelson A. M., Russell E. S., Harman P. J. Dystrophia Muscularis: A HEREDITARY PRIMARY MYOPATHY IN THE HOUSE MOUSE. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1079–1084. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE R. S., MURPHY E. D. Survival of strain 129 dystrophic mice in parabiosis. Am J Physiol. 1960 Dec;199:1097–1100. doi: 10.1152/ajplegacy.1960.199.6.1097. [DOI] [PubMed] [Google Scholar]
- Pachter B. R., Davidowitz J., Breinin G. M. A phase-electron microscopic study of extraocular muscle dystrophy in the mouse. Invest Ophthalmol. 1972 Sep;11(9):715–722. [PubMed] [Google Scholar]
- Peterson A. C. Chimaera mouse study shows absence of disease in genetically dystrophic muscle. Nature. 1974 Apr 12;248(449):561–564. doi: 10.1038/248561a0. [DOI] [PubMed] [Google Scholar]
- STEVENS L. C., RUSSELL E. S., SOUTHARD J. L. Evidence on inheritance of muscular dystrophy in an inbred strain of mice using ovarian transplantation. Proc Soc Exp Biol Med. 1957 May;95(1):161–164. doi: 10.3181/00379727-95-23153. [DOI] [PubMed] [Google Scholar]
- Salafsky B. Functional studies of reeenerated muscles from normal and dystrophic mice. Nature. 1971 Jan 22;229(5282):270–272. doi: 10.1038/229270a0. [DOI] [PubMed] [Google Scholar]
- Salafsky B., Stirling C. A. Altered neural protein in murine muscular dystrophy. Nat New Biol. 1973 Nov 28;246(152):126–128. doi: 10.1038/newbio246126a0. [DOI] [PubMed] [Google Scholar]
- Webster H. D., Martin R., O'Connell M. F. The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Dev Biol. 1973 Jun;32(2):401–416. doi: 10.1016/0012-1606(73)90250-9. [DOI] [PubMed] [Google Scholar]