Abstract
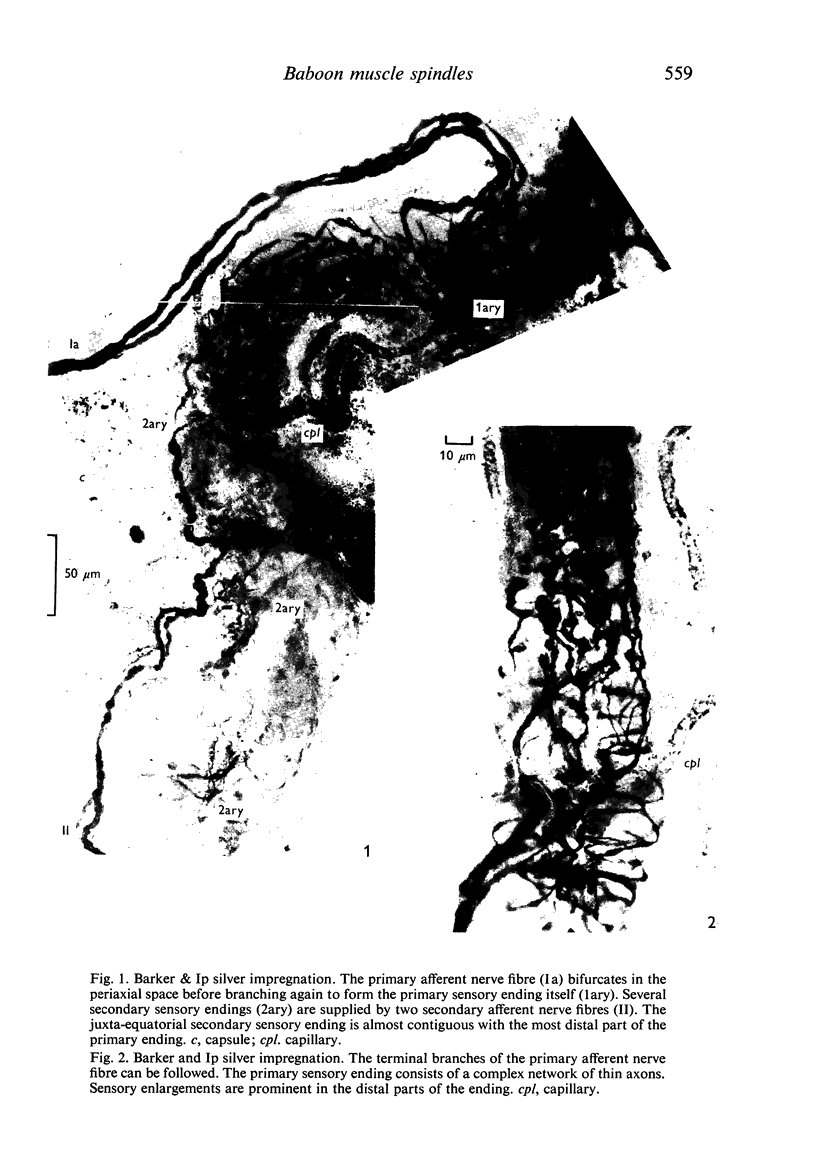
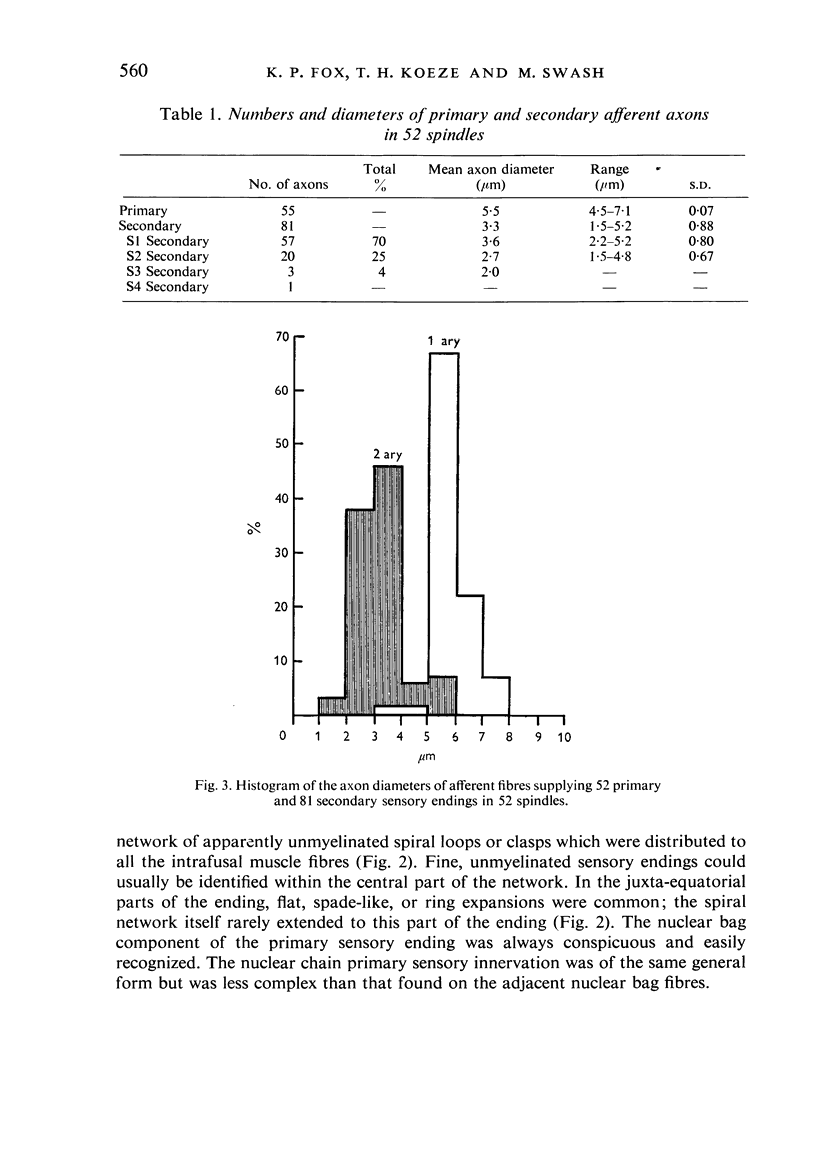
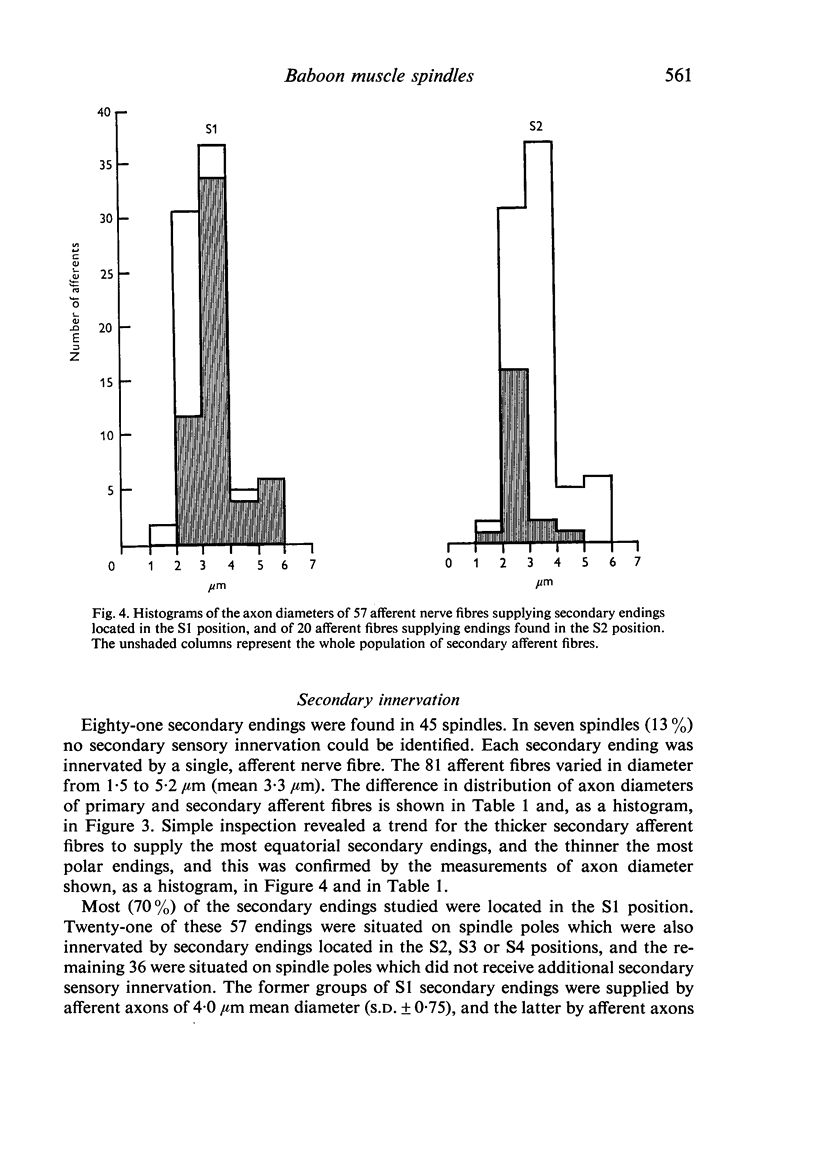
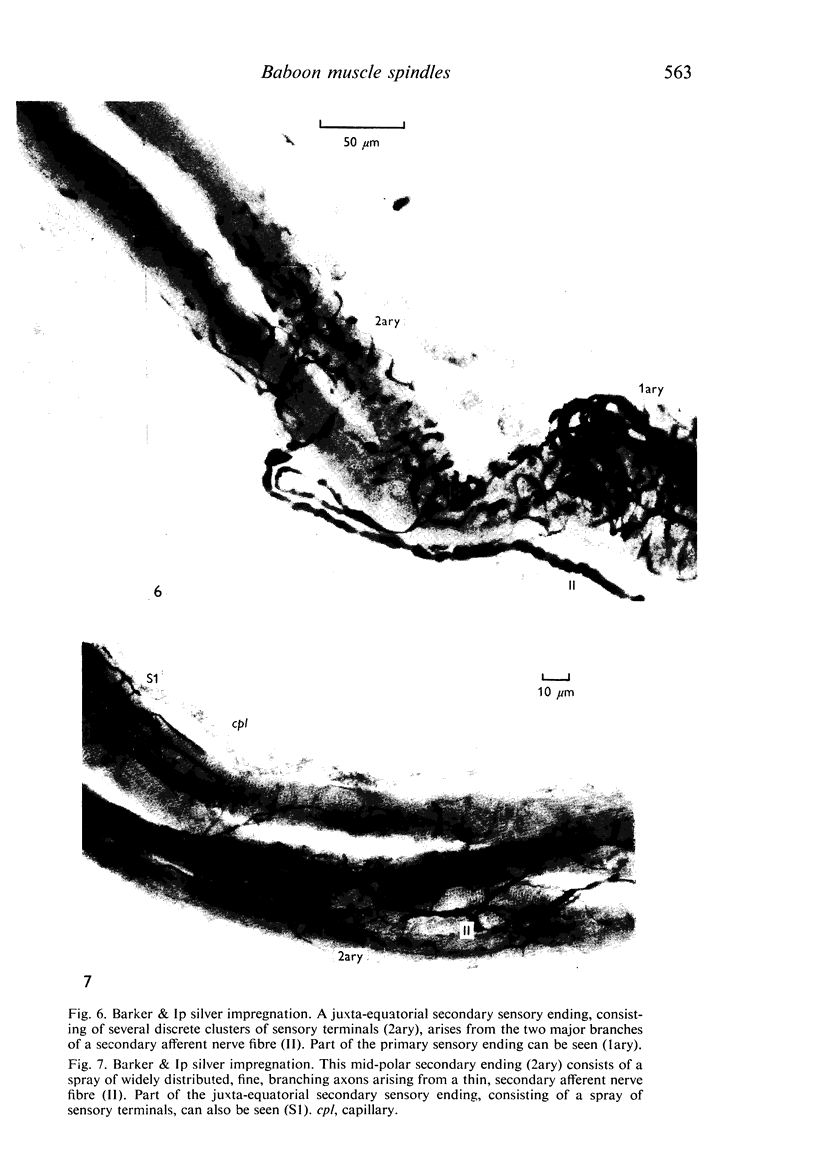
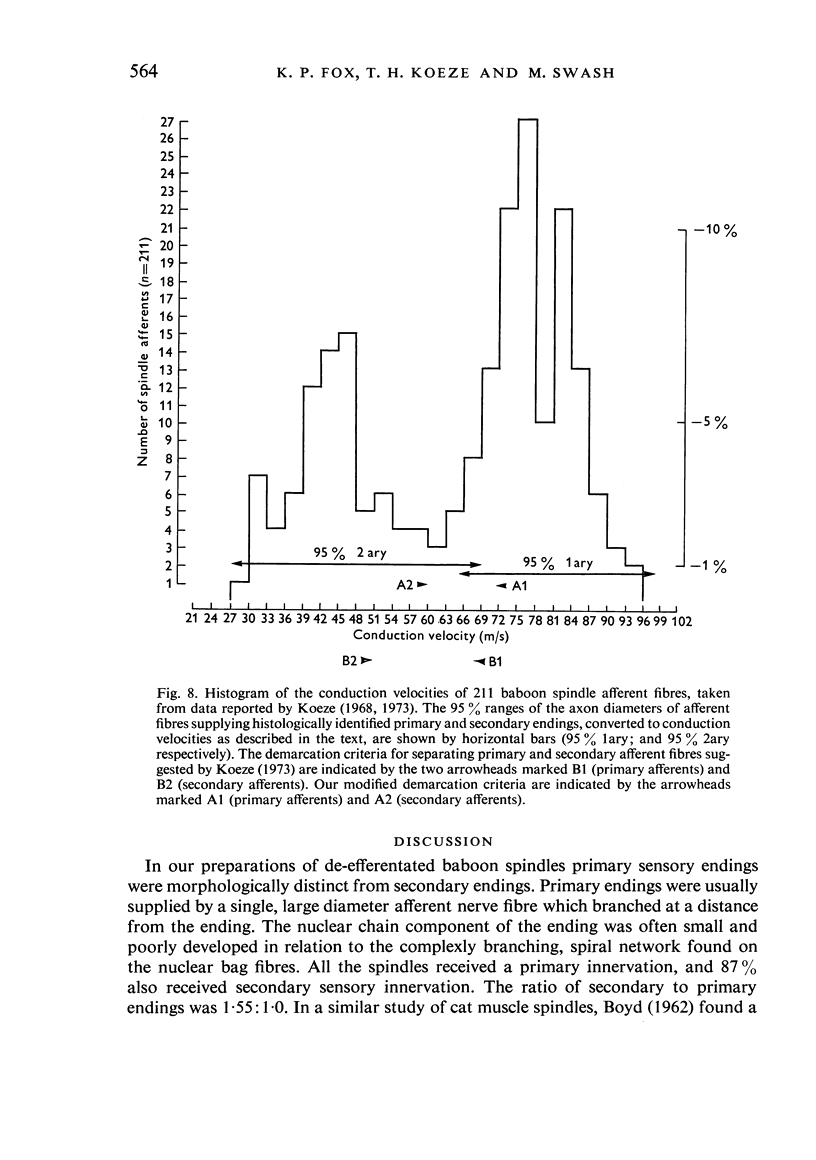
The number and distribution of primary and secondary sensory endings has been studied in 52 de-efferentated baboon muscle spindles and the axon diameters of the afferent fibres innervating these endings have been measured. Each spindle contained a single primary sensory ending; most of these endings were supplied by a single afferent nerve fibre. Each primary sensory ending consisted of a multi-branched network distributed on both nuclear bag and nuclear chain fibres. Beaded sensory terminals were prominent in the central part of the ending. Eighty one secondary endings were found in 45 spindles (87% of the number of spindles remained). Of these endings, 70% were found in the S1 position, 25% in the S2 position and 4% in the S3 location. The afferent axons supplying the most equatorial of these endings were of thicker mean diameter than those supplying the most polar endings. In addition, the juxta-equatorial secondary endings were similar in form, although less regularly organized than the primary endings. The more polar secondary endings rarely fromed terminal sensory enlargements and usually took the form of a fine spray of unmyelinated branches. A non-parametric statistical comparison of physiological and anatomical data in baboon spindles has suggested that the demarcation criteria for separation of primary and secondary spindle afferents, using conduction velocity, should be modified. It is suggested that afferent fibres of conduction velocity less than 60 m/sec should be classified as secondary afferents, and fibres of conduction velocity greater than 72 m/sec should be classified as primary afferents.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FERNAND V. S. V., YOUNG J. Z. The sizes of the nerve fibres of muscle nerves. Proc R Soc Lond B Biol Sci. 1951 Dec 31;139(894):38–58. doi: 10.1098/rspb.1951.0045. [DOI] [PubMed] [Google Scholar]
- Friede R. L. Control of myelin formation by axon caliber (with a model of the control mechanism). J Comp Neurol. 1972 Feb;144(2):233–252. doi: 10.1002/cne.901440207. [DOI] [PubMed] [Google Scholar]
- Koeze T. H. Muscle spindle afferent studies in the baboon. J Physiol. 1973 Mar;229(2):297–317. doi: 10.1113/jphysiol.1973.sp010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeze T. H. The response to stretch of muscle spindle afferents of baboon's tibialis anticus and the effect of fusimotor stimulation. J Physiol. 1968 Jul;197(1):107–121. doi: 10.1113/jphysiol.1968.sp008549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod J. G., Wray S. H. Conduction velocity and fibre diameter of the median and ulnar nerves of the baboon. J Neurol Neurosurg Psychiatry. 1967 Jun;30(3):240–247. doi: 10.1136/jnnp.30.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey M. J. Free nerve endings in skeletal muscle of the cat. J Anat. 1969 Sep;105(Pt 2):231–254. [PMC free article] [PubMed] [Google Scholar]
- Swash M., Fox K. P. Muscle spindle innervation in man. J Anat. 1972 May;112(Pt 1):61–80. [PMC free article] [PubMed] [Google Scholar]
- Swash M., Fox K. P. The pathology of the human muscle spindle: effect of denervation. J Neurol Sci. 1974 May;22(1):1–24. doi: 10.1016/0022-510x(74)90050-1. [DOI] [PubMed] [Google Scholar]