Abstract
Hydrocephalus (HC) is a failure of brain and cerebrospinal fluid (CSF) homeostasis often associated with dilation of the CSF-filled ventricles (ventriculomegaly). Hallmarks of HC include aberrant CSF dynamics, neural stem cell dysfunction resulting in impaired neurogenesis and corticogenesis, biomechanical instability at the brain-CSF interface, and disrupted synaptogenesis and neural circuitry. Pleiotropic mechanisms, including genetic and environmental insults to the brain, contribute to neurodevelopmental comorbidities. Hypothesis generation from genome-wide, single cell multi-omic analyses coupled to experimental validation using induced pluripotent stem cell-derived cerebral organoids will refine molecular classification of HC subtypes and may lead to precision-based surgical and pharmacologic treatments.
One-Sentence Summary
The molecular hallmarks of hydrocephalus provide a framework for mechanistic studies to re-classify human disease subtypes and develop precision therapies.
INTRODUCTION
Hydrocephalus (HC) is the most common indication for childhood brain surgery 1. HC is a disorder of aberrant brain development and cerebrospinal fluid (CSF) homeostasis that is often associated with dilation of the cerebroventricular system (ventriculomegaly), increases in intracranial pressure or both 1, 2. HC includes primary (genetic or developmental) 3, 4 and acquired forms 5 across the age spectrum 6–8. Systemic and intracranial infections are the leading causes of HC across the world. However, numerous molecular mechanisms and genetic factors underlie congenital HC 6, 7. Current HC treatments are limited to surgical procedures such as ventriculoperitoneal (VP) shunting, endoscopic third ventriculostomy (ETV), or ETV with choroid plexus cauterization (CPC) 2, 9. Although age, etiology, treatment history and other factors may help to predict rates of surgical success 10, failure rates remain unacceptably high with approximately 25% requiring re-operation within the first post-operative year 11. Some children undergo more than 100 neurosurgical procedures over the course of their abbreviated life spans 11,12. Rational design of pharmacologic treatments could substantially alleviate morbidity and mortality associated with HC but these efforts have been impeded by an incomplete mechanistic understanding of the disease.
The path of CSF production and flow is classically represented as secretion by the choroid plexus (ChP) (Fig. 1A), with unidirectional flow from the lateral ventricles through the foramina of Monro to the third ventricle, through the cerebral aqueduct of Sylvius into the fourth ventricle, followed by CSF reabsorption into the subarachnoid space through the foramina of Luschka and Magendie (Fig. 1B). While useful as a general framework for ventricular system anatomy, this model is widely challenged and neglects emerging contributors to CSF flow, including SVZ neural stem cells (NSCs), ependymal cilia, and the glia-lymphatic (glymphatic) system (Fig. 1C), including the CSF connection to the peri-neural space 12. For example, defects in ependymal cilia can cause HC in animal models through disruption of cilia organization, beat frequency, and alterations in CSF flow dynamics with pleiotropic effects on brain development and function 13. However the role of cilia as a primary pathophysiologic driver of human HC remains contested. In addition, the contribution of the glymphatic system 14 to HC is attributed to attenuated CSF waste clearance from germinal matrix hemorrhage and/or inflammation 14, 15, but additional mechanistic studies are needed. Finally, the nasopharyngeal lymphatic plexus and meningeal lymphatic vessels may represent alternative routes of CSF drainage in mice 16, 17, but the role of these systems in human pathophysiology remain unexplored. These emerging mechanisms challenge dogmatic models of CSF flow and highlight our incomplete understanding of the relationships connecting CSF flow, brain development, and HC pathophysiology.
Figure 1. Overview of the human cerebroventricular system.

(A) Illustration of the choroid plexus (ChP) epithelium with component cell types. (B) Systemic movement of cerebrospinal fluid (CSF) produced by the ChP epithelium. CSF is thought to traverse the subarachnoid space and to be reabsorbed into the systemic circulation via the arachnoid granulations (B) or the cranial meninges and the glymphatic system (C). Abbreviations: aqueduct of Sylvius (AS), cerebrospinal fluid (CSF), ChP (CP), foramen of Monro (FM), foramen of Luschka (FM), fourth ventricle (4V), lateral ventricle (LV), interstitial fluid (ISF), third ventricle (3V), and subventricular zone (SVZ).
Molecular studies reveal HC as a phenotypically heterogenous disorder with impact on both “drain and brain.” Here we outline molecular hallmarks of human HC, to serve as a framework for understanding human brain development, HC pathobiology, and to identify remaining knowledge gaps. The hallmarks of HC include genetic predisposition, NSC dysfunction, alterations in corticogenesis, perturbation of brain volume and growth, biomechanical disruption of brain architecture, defects in synaptogenesis and neural circuitry, aberrant CSF dynamics and secretion, and augmentation of ChP developmental trajectories (Figure 2). Much of our molecular understanding of HC is derived from in vivo and in vitro model systems. Although valuable, these approaches do not fully recapitulate the diverse genetic, ancestral, physiologic, or phenotypic characteristics of human HC. Recent advances in generation and study of human induced pluripotent stem cell (iPSC)-derived cerebral organoids suggest that many mechanisms of HC will soon be directly testable in human systems. We will discuss in detail relevant considerations in leveraging this model system to advance our understanding of HC.
Figure 2. The hallmarks of human hydrocephalus,

defined as neural stem cell (NSC) dysfunction, alterations in corticogenesis, brain volume and growth perturbation, biomechanical disruption, defects in synaptogenesis and brain circuitry, aberrant cerebrospinal fluid dynamics and choroid plexus development, genetic predisposition, and ventriculomegaly and comorbid traits.
GENETIC PREDISPOSITION
The numerous challenges to understanding genetic contributions to HC include locus heterogeneity, the sporadic nature of HC and phenotypic complexity among HC patients. Moreover, HC is commonly accompanied by neurodevelopmental traits (for example autism spectrum disorders (ASD), epilepsy, cognitive dysfunction, etc.) with pleiotropic mechanisms. Strategies to overcome these challenges include Mendelian genetic analysis using whole-exome sequencing (WES) of rigorously phenotyped human cohorts to identify inherited and de novo mutations, as well as copy number and other structural variations (SV) across the genome. This approach enables precise identification of disease-causing mutations (at single base pair resolution in select cases), and has been successful in identifying causative genes for HC 3, 18, ASD 19, and epilepsy 20. WES is unbiased, systematic and reproducible, enabling robust characterization of implicated loci. HC risk genes TRIM71, SMARCC1, PTEN, FOXJ1, and PIK3CA have been identified using this approach 21. As larger cohorts are sequenced and additional genetic mechanisms are explored, diagnostic refinement of congenital HC by genetic subtype and endophenotype might enable precision medicine-based treatment, as WES was diagnostically informative in approximately 38% of cases 22.
Genome-wide and transcriptome-wide association studies (GWAS, TWAS) identify single nucleotide polymorphisms (SNPs) and gene-based associations, respectively. GWAS typically requires a large sample size and best captures associations with common cording or non-coding variants (minor allele frequency > 0.1%), typically low effect size. In addition, GWAS typically relies on array-based SNP detection and imputation methods based on reference population genetic architecture 23. In contrast, TWAS (which aggregates the effect of single variants across a gene locus), benefits from a higher p-value threshold, reflecting the lower number of statistical tests (for thousands of genes vs. millions of variants). TWAS also accounts for tissue-specific gene regulatory information (benefitting from reference tissue platforms such as the Genotype-Tissue Expression (GTEx) project 24), and can infer molecular mechanism owing to directionality of the gene-tissue pair association with the phenotype 25. While GWAS identifies single SNPs, often in non-coding regions of often obscure functional impact, TWAS can suggest tissue- and gene-level directionality to guide hypotheses to elucidate underlying molecular mechanisms. Integration of these human genetic approaches with ‘omic’ technologies such as neuroimaging linked to genetics and scRNA, epigenetic, and proteomic atlases of the developing brain can provide complementary and convergent means to define HC mechanisms. For example, a TWAS of HC identified maelstrom (MAEL) in the cortex as the gene-tissue pair reaching experiment-wide significant association with HC. This finding was supported by rare variant analysis, integrative genomic analysis of neuroimaging phenotypes, ChP transcriptome analysis in a mouse model of HC, and CSF proteomics from patients with HC 4. In summary, use of multiple parallel approaches is and will continue to be paramount for productive study of human cohorts.
NEURAL STEM CELL DYSFUNCTION
The global consequences to the developing brain of molecular errors within NSCs include HC 26, 27. Human genetics studies of HC have identified de novo and inherited mutations, copy number variations and other alterations in genes with roles in NSC maintenance, renewal and development, such asTRIM71, PTEN, PIK3CA, SMARCC1, FOXJ1, and MAEL 4, 18, 21. Mutations in these genes can disrupt NSC function through divergent mechanisms, as illustrated by the examples below. HC-associated mutations in TRIM71, an RNA binding protein and E3 ubiquitin-ligase, alters NSC differentiation and commitment to neural lineage through repression of β-catenin and lysine demethylase 1a (LSD1) translation 28, 29. Long non-coding RNA (lnRNA) Trinc1 (an inhibitor of Trim71 gene expression) blocks TRIM71-dependent fibroblast growth factor/extracellular signal-regulated kinase (FGF/ERK) signaling and NSC self-renewal 30. PTEN phosphatase and PIK3CA kinase regulate the production and subcellular localization of phosphatidyl-inositol 3,4,5-triphosphate (PIP3), thereby controlling NSC fate, proliferation, and identity. These enzymes also play pleiotropic roles in brain overgrowth phenotypes, among other neurodevelopmental pathologies 31, 32. SMARCC1 is a key regulator of transcription through chromatin remodeling. Smarcc1 deficiency in mice results in embryonic lethality due to exencephaly 33, and SMARCC1 dysfunction in mouse telencephalon results in aberrant epigenetic regulation in NSCs, causing global alterations in transcription and chromatin state 34, 35. Loss of function Smarcc1 mutations in Xenopus also result in midbrain overgrowth and CSF obstruction, inducing ventriculomegaly without forebrain abnormalities 35. Transcription factor FOXJ1 is required for cilia formation and differentiation of ChP epithelial and ependymal cells in the postnatal brain, but is not expressed in RGCs 36. These data together suggest that convergent, disruptive pathways of NSC differentiation broadly underly congenital HC.
A TWAS of human HC identified MAEL, an evolutionarily divergent gene that regulates piRNA-mediated transposon silencing, expression in the brain cortex as an experiment-wide predictor of HC across all gene-tissue pairs tested. Gene set enrichment analysis of statistically significant HC-associated genes identified NSC regulation as a shared mechanism 4. MAEL may contribute to HC risk by regulating transcription through rearrangement of transposons 37, 38, potentially inducing mutations to shape cell identity and augment genome size by altering the genomic loci of DNA sequences. Loss of MAEL can recruit RNA polymerase II to increase transcription and steady-state levels of transposon RNAs. The accompanying increase in heterochromatin spreading despite modest changes in Histone-3 lysine-9 trimethylation (H3K9me3) patterns 39 suggests that MAEL may act independently or downstream of H3K9me3. Indeed, H3K9me3-dependent changes were among the most significantly enriched gene sets (gene ontology) and H3K9me3 methyltransferases among the genes most highly differentially expressed comparing HC cases and controls 4. However, the precise mechanism(s) by which H3K9me3-dependent alterations cause HC remain unelucidated. These data collectively implicate NSC function, proliferation, differentiation, and renewal as key mechanistic drivers of HC.
NORMAL DEVELOPMENT AT THE CSF-CORTICAL INTERFACE
Normal development of the cerebral cortex and ventricular system reflects a highly coordinated series of molecular, genetic, and cell-signaling events at the brain-CSF interface. The brain parenchyma and the CSF-producing ChP share a common tissue origin in the NSC at the dorsal midline and rhombic lip 40, 41. Dorsal pallial NSCs give rise to neurons and glia of the cerebral cortex and the lateral ventricle ChP.42–44. Distinct NSC subsets can self-renew through symmetric cell division and then differentiate to generate de novo all neural and neuroglial cell lineages 45. Defects within the NSC compartment or its precursor lineages, including RGCs, oRGCs and IPCs can cause alterations in cortical patterning, folding, and cell-type identity 46, with global functional consequences in cognition and neurodevelopment 47. Epigenetic alterations within the NSC compartment may also impact corticogenesis and drive HC pathogenesis 48.
Human neuroepithelial cells arise from neuroectoderm after neural tube closure (between days 28–32 post-conception) and give rise to much of the brain parenchyma (Figure 3A). Neuroepithelial cells generate multiple progenitor cell types, including radial glia cells (RGCs) beginning around gestational week (GW) 7 49, ChP epithelial cells around GW8 50, 51, and ependymal cells around GW20 52. RGCs rapidly proliferate to expand around GW10 to generate neurons and intermediate progenitor cells (IPCs) that in turn generate immature neurons 53. RGCs also produce more specialized RGC subtypes such as outer radial glia cells (oRGCs) by GW18 44, 54. Together, RGCs, oRGCs, and IPCs are the progenitors responsible for producing cortical neurons through the stereotyped inside-out process in which deep-layer neurons are born before the neurons that reside in more superficial layers 55. After generating neurons and neuronal progenitors, RGCs switch to a gliogenic role, producing astrocytes and oligodendrocyte precursor cells by the second trimester 56.
Figure 3.
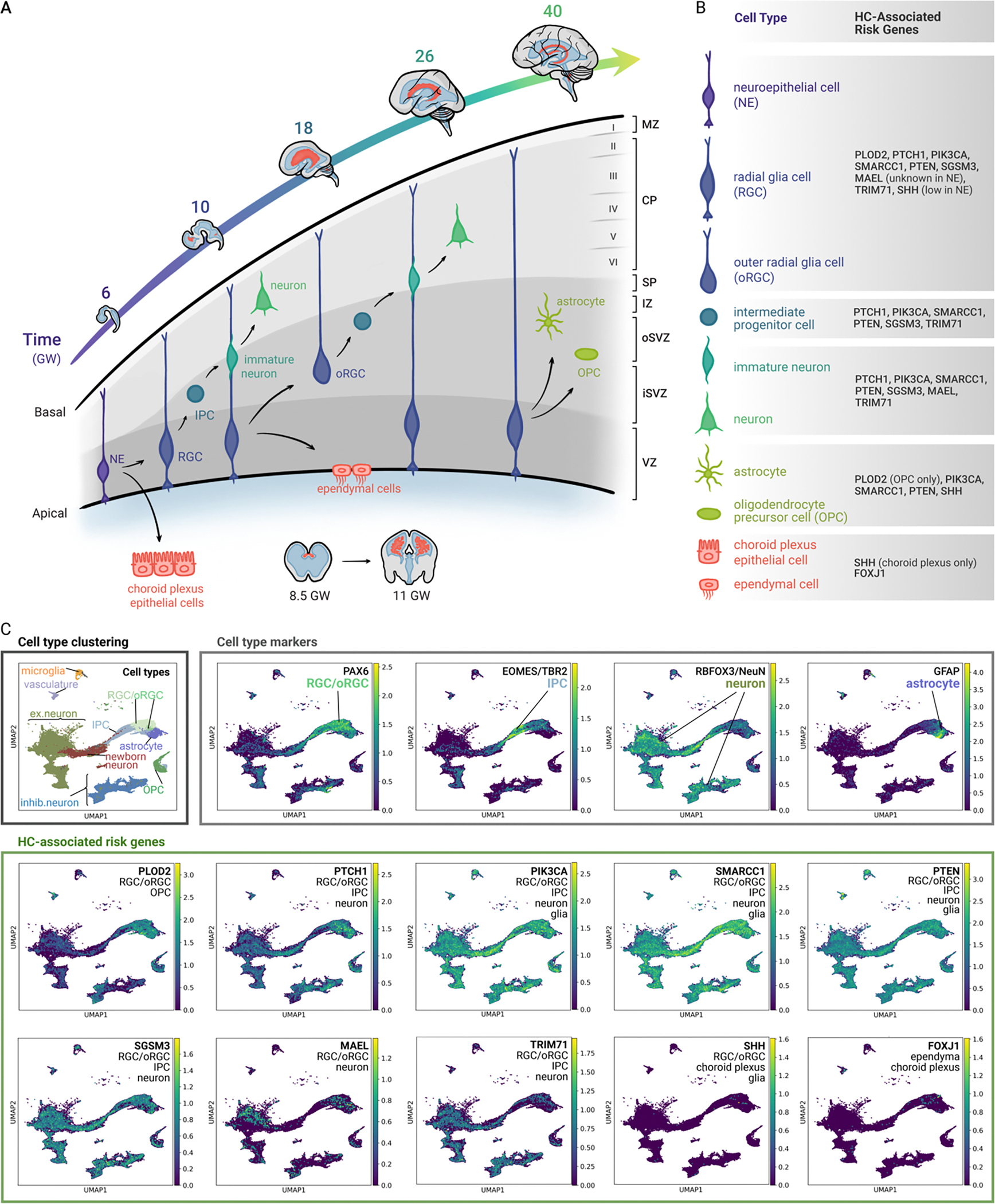
Overview of human neocortical development and HC-associated genes. (A) Cellular differentiation during cortical development between gestational weeks (GW) 4–40. NSCs successively expand, generate progenitors, and differentiate to form the principal components of the human cortex and ventricular system 43, 48. (B) Summary of cell types and their HC-associated gene expression. (C) UMAP clustering of HC-associated gene expression in neural progenitor cells (RGCs, oRGCs, IPCs, ORGs) and early differentiated neural cell types during human brain development. Processed scRNA-seq data of human neocortical samples was obtained from Wang, et al. 2024 58. The data subset was of cells originating from pre-natal samples (including first, second, and third trimester). Cell types were re-annotated to reflect stages of neural differentiation by combining annotated cell-types from the original publication using SCANPY 147. Expression values reflect log normalized UMI counts with a size factor of 10,000. Known markers of the annotated cell types (PAX6, TBR2, NeuN, GFAP) are plotted to demonstrate annotation specificity, and are compared with expression plots of 10 HC-linked genes.
Gliogenesis is a protracted process of differentiation that extends following neurogenesis through early childhood. Cerebrovascular and immune cell development in the human brain, though of possible relevance to HC, are beyond the scope of this review.
NSCs are the common precursor of neurons, neuroglia, ChP epithelial cells, and ependymal cells. Disruption of this lineage may impede CSF homeostasis by several mechanisms. For example, mutations that affect the earliest-stage NSCs could induce overgrowth and ventricular obstruction, alter CSF production and regulation, or perturb ependymal cells in the CSF-brain barrier. Forebrain expansion, neuroepithelial differentiation into oRGCs, and disproportionately long gliogenic periods are highly species-specific, dynamic processes essential for cortical formation, maturation, and growth. For example, mutations in L1CAM have been hypothesized to cause X-linked HC through aberrant radial neuronal migration 57. In contrast, mutations that affect later-born, more highly specified progenitors (including RGCs, neuroblasts, or glioblasts) may regulate particular cell types implicated in disease.
The timeline of human cortical development at the cellular level is central to HC. Identification of cell types expressing HC-associated genes during development may therefore help to clarify disease mechanisms. The expression of individual, congenital HC-associated genes, identified through unbiased human genetic studies 3, 4, 18, is enriched in divergent cell subpopulations in the brain neocortex (Figure 3B–C), suggesting cell-type specific roles for HC-associated genes 58. We surmise that mapping expression patterns of human HC-associated genes 3, 4, 18 to the developing human brain may yield mechanistic insights. For example, MAEL expression is highest in cortex (GTEx, Human Protein Atlas, and 4), while other HC-associated genes (PLOD2, PTCH1, PIK3CA, SMARCC1, PTEN, SGSM3) map onto specific subpopulations of cells in the human prenatal brain neocortex 59 (Figure 3C). Although these human studies did not validate that NSCs express HC-associated genes, mouse studies suggest that many of the genes expressed by human RGC/oRGCs could also be expressed in NSCs 60. Other HC-associated genes (SHH, FOXJ1), although not strongly captured in the prenatal brain dataset 59, display cell-type specific expression. SHH ligand is detectable in CSF, and source cells include epithelial cells, blood vessels postmitotic neurons, Cajal-Retzius cells, and ChP 61. FOXJ1 is expressed by multiciliated cells, including ChP epithelial cells and ependymal cells that line the brain ventricles (GTEx, Human Protein Atlas). Mapping HC risk genes to epigenetic atlases of developing human brain can reveal additional mechanistic insights into how genetic errors cause HC and comorbidities 4, 62–66. Moreover, to our knowledge no whole genome sequencing (WGS) studies of HC have been performed to elucidate the disease contributions of non-coding, regulatory regions or SVs. The highly human-specific and elaborately orchestrated genetic and molecular events underlying NSC development, CSF regulation/dynamics and corticogenesis will require human models to delineate HC mechanisms.
BRAIN VOLUME AND GROWTH PERTUBATION
The goal of treatment for pediatric patients with HC is to optimize brain growth and cognitive development, both primary outcomes in randomized controlled trials of surgical treatments for HC 9, 67. Brain growth trajectories in HC correlate with neurodevelopmental outcomes in HC 67. However, determining ‘normal’ brain growth patterns is challenging. Understanding how alterations in HC risk genes contribute to growth of brain, white matter, grey matter 68 and cortical patterning 69 is essential to inform precision treatments and to define successful outcomes. For example, patients with a genetic syndrome characterized by attenuated cortical development and mild ventriculomegaly in the setting of normal intracranial pressure may not require CSF diversion but, rather, referral to early intervention services for neurocognitive development. On the other hand, permanent CSF shunting may be indicated for patients with loss-of-function mutations in a key regulatory protein involved in CSF clearance. Precision medicine approaches for HC 70 have the potential to reduce morbidity and mortality associated with the disease and treatment complications. Indeed, integrative neuroimaging and genomics analyses have identified alterations in brain architecture as a key feature of neurodevelopmental diseases comorbid with HC 68, 69, 71, 72. The top gene-level associations with HC include statistically significant enrichment for genes involved in total brain and white matter volumes, with MAEL among the topmost statistically significant genes 4. However, these population genetic models were developed from human genetic studies linked to neuroimaging phenotypes 73 lacking both birth-age data and adjustments for development. Brain charts coupled with multi-omics analysis across age, anthropometric, and population scales will further refine classification of HC subtypes.
HC rarely occurs in isolation, and the extent to which pleiotropic genetic mechanisms contribute to comorbid neurodevelopmental phenotypes is unknown. Detailed neuroimaging studies linked to genetic information may resolve these questions. Ventriculomegaly, but not necessarily HC, has been observed in patients with ASD, cognitive dysfunction, and neuropsychiatric disease 74–77. ‘Normal’ ventricular size is highly subjective, and while brain charts across the age spectrum have been developed (with significant effort) 78, these models have not been widely incorporated into clinical practice. For example, disruptive mutations in the PI3K-Akt-mTOR signaling pathway have been observed in patients with HC, macrocephaly, developmental delay, and ASD. As dysregulation of this pathway can alter corticogenesis, ventriculomegaly in these cases is likely a harbinger of a primary structural brain disorder, rather than a primary driver of HC.
BIOMECHANICAL DISRUPTION OF BRAIN PARENCHYMA
Emerging data on perturbations in the development of brain architecture support direct roles for biomechanical disruption underlying subtypes of congenital HC. For example, HC-associated de novo mutations in Trim71 and disruption of TRIM71 activity in the murine NSC compartment cause cortical hypoplasia and hypercompliant cortex with compensatory ventricular enlargement 79, 80. Mice harboring Trim71 mutations displayed impaired CSF outflow secondary to acquired aqueductal stenosis that could be ameliorated by CSF diversion. In contrast, the aqueduct in patients with L1CAM mutations remained stenotic even after CSF shunting 79. These data suggest a distinct mechanism by which altered cortical compliance and biomechanics may cause HC, such that the genetic etiology of HC determines, in large part, surgical treatment response. Independent mechanistic studies of Trim71 further supporting the importance of defects in the NSC compartment have implicated impaired epigenetic mechanisms 28, 29, 81 as responsible for phenotypes of Trim71-deficient mice (and humans harboring Trim71 loss-of-function mutations 79, 80). These findings disrupt the classical paradigm of HC, in which defects of CSF production and absorption were primary pathologic drivers of disease, and suggest primary disruption of brain parenchymal structural integrity as a causative mechanism for development of HC
SYNAPTOGENESIS AND CIRCUIT DYSRUPTION
It remains unclear whether increased intracranial pressure and subsequent changes in CSF dynamics after CSF diversion are primary causes of circuit disruptions associated with HC; or, alternatively, if HC-associated genetic errors result in pleiotropic effects leading to ‘passenger’ phenotypes. For example, mutations in genes within the PI3K signaling pathway disrupt corticogenesis and are associated with the HC comorbidities of brain overgrowth and epilepsy 82, 83. Genomic alterations within the PI3K pathway have also been identified by molecular phenotyping of congenital HC patients 3, 18, suggesting a pleiotropic basis for the wide phenotypic spectrum observed in humans with HC. Many independent mechanistic studies of the PI3K-PTEN signaling axis, a critical driver of synapse development 84, have identified defects in synaptogenesis and brain circuitry alterations caused by disruptions in this pathway. Disruptions in NSCs may delay gliogenesis or cause downstream defects in glial differentiation, impair processes required for synaptogenesis (astrocytes), and produce defects in circuit transduction through disrupted myelin production caused by oligodendrocyte dysfunction. Finally, aberrant cortical neurogenesis leads to imbalance between excitatory neurons and subpallial-generated inhibitory neurons, or to defects in neuronal migration, both known mechanisms contributing to epilepsy 85.
Until recently, the mechanism(s) by which PI3K-PTEN alterations cause both HC and comorbid disorders such as ASD, epilepsy, and impaired cognition have remained elusive. Mice harboring de novo Pten mutations observed in patients with ASD and HC recapitulate the cerebral ventriculomegaly seen in humans 86, 87. Pten-mutant mice developed aqueductal stenosis from proliferation of medial ganglionic eminence Nkx2.1 neural precursors and CSF hypersecretion 86, 87. Unbiased brain network analysis identified similar neural networks in Pten-mutant mice with HC, reflecting diminished activity of Nkx2.1 NSC-derived inhibitory interneurons. These phenotypes were rescued by postnatal pharmacologic inhibition of mTORC1 with Everolimus, which restored cortical architecture and decreased ventricular size 86, 87. These data allow proposal of a mechanism by which primary alterations in brain circuitry and HC are caused by genetic disruption of PI3K-PTEN signaling. They demonstrate a possible pharmacological strategy to ameliorate both circuit-level and ventriculomegaly phenotypes through shared mechanisms. Refined molecular characterization of HC endophenotypes will enable clinical application of such precision-based approaches.
ABERRANT CSF DYNAMICS, SECRETION, AND CHOROID PLEXUS DEVELOPMENT
Precise regulation of CSF dynamics plays an important role in cerebral cortical development, and impairment of CSF homeostasis has been implicated in the pathophysiology of HC. The ChP epithelium is the primary source of CSF 88, 89, based on observations from as far back as 1918, when neurosurgeon Walter Dandy first described surgical removal of ChP to decrease CSF production for HC treatment 90. Scarff later described endoscopic approaches to treat HC through ChP cauterization 91. However, the complex mechanisms of ChP-mediated ion and water transport remain debated 92, 93. The tightly regulated, concerted action of multiple integral membrane ion transport proteins such as Na+/K+-ATPase, Na+ K+ Cl− transporter NKCC1 and aquaporin water channels mediates transepithelial movement of solutes and water across the ChP epithelium 88, 92. Other enzymes such as carbonic anhydrase secondarily contribute to mature ChP secretion without direct mediation of trans-membrane transport. CSF secretion is achieved by the polarized localization of transport proteins in the ChP epithelium. For example, CSF [Na+] is regulated by the Na+/K+-ATPase and NKCC1 localized atypically on the ChP apical membrane, in contrast to their classically basolateral localization in other epithelia 94. Inhibitors of key ChP proteins such as ouabain (Na+/K+-ATPase), bumetanide and furosemide (NKCC1), and acetazolamide (carbonic anhydrase) decrease CSF secretion rates in vivo across model systems.
Increased cell mass or cell number due to ChP hyperplasia 95, ChP papilloma 96, and ChP carcinoma 97 can augment CSF production and obstruct CSF flow 98, leading to HC. Adult models of acquired HC secondary to hemorrhage 5, infection 99, and tumor 100 have demonstrated that increased CSF secretion in response to ChP inflammation is associated with up-regulated phosphorylation and activation of the SPAK kinase-regulated NKCC1 cotransporter, which exists in a protein complex with the Na+/K+-ATPase on the apical ChP membrane. Genetic or pharmacologic inhibition of Toll-like receptor 4 -associated ChP inflammation, as well as of the SPAK-NKCC1 complex, prevents development of ventriculomegaly in adult models of hemorrhagic and infectious HC 99, 101–103. NKCC1 activity is required for CSF secretion by the adult ChP 99, 101, 102, consistent with the decreased epithelial secretion and “slit-ventricle” brain morphology indicative of decreased intraventricular CSF volumes in patients with loss-of-function NKCC1 mutations 104. However, the vector of net NaCl transport by NKCC1 (into or out of the ChP epithelial cell) reflects prevailing transepithelial ion gradients and its bidirectional and electroneutral transport mechanism 105, but remains debated. In developing mouse CSF in which ion concentrations have not yet achieved adult levels, NKCC1 may also play a role in CSF K+ clearance 106, 107. Interestingly, genetic overexpression of NKCC1 early in development through adeno-associated viral (AAV) transduction attenuates post-hemorrhagic HC and obstructive HC in neonatal mice, which may be secondary to clearance of K+ 107, 108. Germline NKCC1 knockout animals display normal ventricular size 109, underscoring the complex, species-specific nature of CSF volume regulation. The lack of identified mutations in the NKCC1-SPAK driven pathway in human genetic studies of HC is not surprising, as the pathophysiologic and mechanistic basis of acquired and congenital HC are distinct 6. Importantly, the redundant pathways, molecular machinery, and mechanisms regulating ChP ion transport remain incompletely characterized. These data suggest NKCC1 acts as a “rheostat” to mediate CSF homeostasis, albeit through incompletely understood mechanisms.
The molecular toolkit required to address fundamental questions in ChP biology has, until recently, been lacking. Large-scale developing brain atlases from multiple species, offer increased resolution of NSCs and the ChP across neurodevelopment and enable unbiased insights into the function of these cell types 110–112. For example, FOXJ1 mutations may cause HC through disruption of ependymal cell differentiation and ciliary biosynthesis in the absence of obstruction of CSF flow 113. Although primary disruption in ciliary function due to impaired ependymal cell development can disrupt CSF bulk flow and the transepithelial ion transport required for CSF production 114, primary ciliopathies are not classically associated with HC in humans. Moreover, FOXJ1 in ChP is expressed predominantly at early developmental stages in non-motile, multi-ciliated cells 115, suggesting that defects in FOXJ1-dependent pathways may also alter CSF production and homeostasis. Radial glial over-production defects in SHH signaling 116 have also been shown to contribute to development of the hindbrain ChP 117, 118, as well as to HC 119 and to HC-related macrocephaly 120.
Single cell and spatial sequencing atlases 110 have identified a shared progenitor cell for both ChP epithelial and neuronal cells in early ChP development, confirmed by Wnt1 lineage tracing 110 and consistent with previous Nestin1 lineage tracing 121. Cell type composition, cell-cell interactions, and transition states shift considerably across the age spectrum. However, these analyses are currently restricted to the mouse brain, and the extent to which these paradigms apply to human ChP remains to be investigated. Further studies are needed to elucidate mechanisms of bi-directional CSF effects on neural development 88. Creation of a human model system could help resolve these mechanistic questions.
Cerebral organoids as a model to elucidate mechanisms underlying HC
Many in vitro, ex vivo, and in vivo (Xenopus 35, 122, 123, fish 124–126, rat 101, 127, mouse 128, 129, and pig 130) models have been developed to elucidate fundamental mechanisms underlying hallmark features of HC including, among others, alterations in ependymal barrier function 131, cortical development 132 and CSF dynamics 133. In view of the varied mechanisms and pathways implicated in HC and its comorbid phenotypes, a human model system at least partially recapitulating these complexities may enable a more detailed and translationally relevant mechanistic understanding of HC. Moreover, a human model system could serve as a screening platform for pharmacologic development. Human iPSC-derived cerebral organoids, in vitro 3-dimensional structures resembling developing human brain, have recently emerged as a promising experimental tool to answer fundamental questions about normal and abnormal brain development and disease 134. Cerebral organoids are self-organizing, recapitulate the spatial organization of the brain, and display transcriptional and epigenetic states that mirror brain development 135. These organoids have been critical tools to aid our mechanistic understanding of HC-related phenotypes including microcephaly 136, macrocephaly 137, ASD 138, host-pathogen interactions 139, altered CSF production and selectively modified transport of small molecules 140. Moreover, cerebral organoids form ventricular zones that resemble cerebral ventricles 141. Disruption of ZEB2, a gene crucial for neuroepithelial cell development, results in enlarged neuroepithelial buds resembling ventriculomegaly in cortical organoids 141. Leveraging this model system to study HC pathogenic features would be a logical next step to bridge the gap between mechanisms and patients.
Genetic forms of HC are driven primarily by disruption of the NSC compartment and subsequent defects in cortical growth, patterning, and development. Extended culture protocols will aid our understanding of the long-term consequences of genetically encoded early developmental dysfunction on downstream molecular consequences 142. Genetically tractable iPSCs can be used to understand the functional impact of patient-specific mutations using gene editing technologies. Such cell lines can also elucidate the consequences to cortical development of genetic errors at the NSC stage, and the impact on neuronal synaptic function through organoid-wide electrophysiological recording. Modulation of tissue morphology has also been shown to influence brain organoid development, a feature which may mirror the effect of permanent CSF shunting on brain structural architecture 143. Important limitations of cerebral organoids include the absence of circulating immune cells, limited nutrient and oxygen diffusion through vascular networks, absent myelination, low reproducibility from batch effects, and variation in iPSC lines necessitating stringent controls 135.
The mechanistically distinct acquired HC is driven by alterations in bulk CSF flow (production/absorption mismatch) and dysregulation of CSF dynamics. ChP-like cerebral organoids recapitulate the epithelial and stromal cell types observed in vivo. These organoids form a blood-CSF-barrier-like tight junction interface, secrete CSF-like fluid, and produce a protein signature like human CSF, recommending organoids for study of the effects of hemorrhage or infection on HC. Drawbacks to ChP organoids as an experimental model for acquired HC include the lack of a vascular network and circulating immune cells, lack of the physiological contribution of adjacent brain structure to CSF composition, and inability to analyze human ChP from surgical samples owing to safety concerns (for example, bleeding, stroke, etc.). Nonetheless, composition of CSF-like fluid from ChP organoids appears to resemble that of human CSF, and transcriptomic studies of iPSC-derived ChP mirror human ChP datasets 140. Finally, ChP organoids have been used to study infectious diseases like SARS-CoV-2 144, highlighting their utility in understanding brain infections. Reconstitution experiments introducing genetically modified and/or additional cell types (such as microglia) into organoid cultures will provide a platform for well-controlled, and hypothesis-driven experiments not feasible in other systems. Rigor and reproducibility of such experiments will require the broader scientific community to adhere to strict, clearly defined nomenclature and methods of organoid generation, culture, and assembly 145, 146.
Concluding remarks
We define the molecular hallmarks of human HC as a framework for future mechanistic studies, but many open questions remain. The most urgent unmet need is identification of therapeutic targets that reduce the requirement for and/or guide choice of neurosurgical interventions. We hypothesize these targets will emerge from: (1) careful study of cell-type and stage-specific roles of HC-associated genetic aberrations in human brain tissue; (2) molecular characterization of implicated genetic mechanisms in improved human and animal models; and (3) identification and validation of HC-associated genes in carefully phenotyped human cohorts. Such approaches will enable personalized characterization of genetic contributions to HC, will enhance validation of emerging pathogenic causes of HC in defined ancestral genomic backgrounds, and will provide resources for the broader HC research community.
ACKNOWLEDGEMENTS
We apologize to colleagues whose studies we could not include due to space limitations. This work was supported by the National Institutes of Health (NIH) R21NS135321, Neurosurgery Research and Education Foundation (NREF) & American Academy of Neurological Surgery Grant, UAB Civitan Scholar Award, Thrasher Early Career Award, Mission Brain Foundation Scholarship, NIH Fogarty Global Health Fellowship (Harvard University & University of Cape Town), and Oppenheimer Memorial Trust (A.T.H.); National Science Foundation (NSF) Graduate Research Fellowship Program (GRFP) and ARC Institute PhD Fellowship (B.Z.); NIH 5-T32 GM141828–02 (A.R.);The Gabriel Foundation, South African Medical Research Council, and the University of Cape Town Neuroscience Institute (M.G.); Hydrocephalus Association, Pediatric Hydrocephalus Foundation, NIH R01NS129823 (M.K.L.); Medical Research Council Core Funding and European Research Council Starting Grant (M.A.L.); Hydrocephalus Association; Brain Research Foundation Seed Grant; McCormick and Gabilan Faculty Award, Shurl and Kay Curci Foundation; NIH R01MH136258 (R.M.F.); and NIH R01NS111029, R01NS109358, and R01NS117609 (K.T.K.).
Footnotes
COMPETING INTERESTS
M.A.L. is an inventor on a patent application (WO2020152272A1) submitted by United Kingdom Research and Innovation that covers choroid plexus organoids.
References
- 1.Dewan MC, Rattani A, Mekary R, Glancz LJ, Yunusa I, Baticulon RE, Fieggen G, Wellons JC, Park KB, Warf BC. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg. 2018:1–15. Epub 2018/04/28. doi: 10.3171/2017.10.Jns17439. [DOI] [PubMed] [Google Scholar]
- 2.Kahle KT, Kulkarni AV, Limbrick DD Jr., Warf BC. Hydrocephalus in children. Lancet. 2016;387(10020):788–99. Epub 2015/08/11. doi: 10.1016/s0140-6736(15)60694-8. [DOI] [PubMed] [Google Scholar]
- 3.Jin SC, Dong W, Kundishora AJ, Panchagnula S, Moreno-De-Luca A, Furey CG, Allocco AA, Walker RL, Nelson-Williams C, Smith H, Dunbar A, Conine S, Lu Q, Zeng X, Sierant MC, Knight JR, Sullivan W, Duy PQ, DeSpenza T, Reeves BC, Karimy JK, Marlier A, Castaldi C, Tikhonova IR, Li B, Peña HP, Broach JR, Kabachelor EM, Ssenyonga P, Hehnly C, Ge L, Keren B, Timberlake AT, Goto J, Mangano FT, Johnston JM, Butler WE, Warf BC, Smith ER, Schiff SJ, Limbrick DD Jr., Heuer G, Jackson EM, Iskandar BJ, Mane S, Haider S, Guclu B, Bayri Y, Sahin Y, Duncan CC, Apuzzo MLJ, DiLuna ML, Hoffman EJ, Sestan N, Ment LR, Alper SL, Bilguvar K, Geschwind DH, Günel M, Lifton RP, Kahle KT. Exome sequencing implicates genetic disruption of prenatal neuro-gliogenesis in sporadic congenital hydrocephalus. Nat Med. 2020;26(11):1754–65. Epub 2020/10/21. doi: 10.1038/s41591-020-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hale AT, Bastarache L, Morales DM, Wellons JC 3rd, Limbrick DD Jr., Gamazon ER. Multi-omic analysis elucidates the genetic basis of hydrocephalus. Cell Rep. 2021;35(5):109085. Epub 2021/05/06. doi: 10.1016/j.celrep.2021.109085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimy JK, Reeves BC, Damisah E, Duy PQ, Antwi P, David W, Wang K, Schiff SJ, Limbrick DD Jr., Alper SL, Warf BC, Nedergaard M, Simard JM, Kahle KT. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nat Rev Neurol. 2020;16(5):285–96. Epub 2020/03/11. doi: 10.1038/s41582-020-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hale AT, Boudreau H, Devulapalli R, Duy PQ, Atchley TJ, Dewan MC, Goolam M, Fieggen G, Spader HL, Smith AA, Blount JP, Johnston JM, Rocque BG, Rozzelle CJ, Chong Z, Strahle JM, Schiff SJ, Kahle KT. The genetic basis of hydrocephalus: genes, pathways, mechanisms, and global impact. Fluids and barriers of the CNS. 2024;21(1):24. . doi: 10.1186/s12987-024-00513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahle KT, Klinge PM, Koschnitzky JE, Kulkarni AV, MacAulay N, Robinson S, Schiff SJ, Strahle JM. Paediatric hydrocephalus. Nature Reviews Disease Primers. 2024;10(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Räsänen J, Heikkinen S, Mäklin K, Lipponen A, Kuulasmaa T, Mehtonen J, Korhonen VE, Junkkari A, Grenier-Boley B, Bellenguez C. Risk Variants Associated With Normal Pressure Hydrocephalus: Genome-Wide Association Study in the FinnGen Cohort. Neurology. 2024;103(5):e209694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkarni AV, Schiff SJ, Mbabazi-Kabachelor E, Mugamba J, Ssenyonga P, Donnelly R, Levenbach J, Monga V, Peterson M, MacDonald M, Cherukuri V, Warf BC. Endoscopic Treatment versus Shunting for Infant Hydrocephalus in Uganda. N Engl J Med. 2017;377(25):2456–64. . doi: 10.1056/NEJMoa1707568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. Journal of Neurosurgery: Pediatrics. 2010;6(4):310–5. [DOI] [PubMed] [Google Scholar]
- 11.Reddy GK, Bollam P, Caldito G. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World neurosurgery. 2014;81(2):404–10. [DOI] [PubMed] [Google Scholar]
- 12.Ligocki AP, Vinson AV, Yachnis AT, Dunn WA Jr., Smith DE, Scott EA, Alvarez-Castanon JV, Baez Montalvo DE, Frisone OG, Brown GAJ, Pessa JE, Scott EW. Cerebrospinal fluid flow extends to peripheral nerves further unifying the nervous system. Sci Adv. 2024;10(36):eadn3259. . doi: 10.1126/sciadv.adn3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olstad EW, Ringers C, Hansen JN, Wens A, Brandt C, Wachten D, Yaksi E, Jurisch-Yaksi N. Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Current Biology. 2019;29(2):229–41. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. The Lancet Neurology. 2018;17(11):1016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karimy JK, Reeves BC, Damisah E, Duy PQ, Antwi P, David W, Wang K, Schiff SJ, Limbrick DD Jr, Alper SL. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nature Reviews Neurology. 2020;16(5):285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi D, Park E, Choi J, Lu R, Yu JS, Kim C, Zhao L, Yu J, Nakashima B, Lee S, Singhal D, Scallan JP, Zhou B, Koh CJ, Lee E, Hong YK. Piezo1 regulates meningeal lymphatic vessel drainage and alleviates excessive CSF accumulation. Nat Neurosci. 2024. Epub 20240325. doi: 10.1038/s41593-024-01604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon JH, Jin H, Kim HJ, Hong SP, Yang MJ, Ahn JH, Kim YC, Seo J, Lee Y, McDonald DM, Davis MJ, Koh GY. Nasopharyngeal lymphatic plexus is a hub for cerebrospinal fluid drainage. Nature. 2024;625(7996):768–77. Epub 20240110. doi: 10.1038/s41586-023-06899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furey CG, Choi J, Jin SC, Zeng X, Timberlake AT, Nelson-Williams C, Mansuri MS, Lu Q, Duran D, Panchagnula S, Allocco A, Karimy JK, Khanna A, Gaillard JR, DeSpenza T, Antwi P, Loring E, Butler WE, Smith ER, Warf BC, Strahle JM, Limbrick DD, Storm PB, Heuer G, Jackson EM, Iskandar BJ, Johnston JM, Tikhonova I, Castaldi C, López-Giráldez F, Bjornson RD, Knight JR, Bilguvar K, Mane S, Alper SL, Haider S, Guclu B, Bayri Y, Sahin Y, Apuzzo MLJ, Duncan CC, DiLuna ML, Günel M, Lifton RP, Kahle KT. De Novo Mutation in Genes Regulating Neural Stem Cell Fate in Human Congenital Hydrocephalus. Neuron. 2018;99(2):302–14.e4. Epub 2018/07/10. doi: 10.1016/j.neuron.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, DiLullo NM, Parikshak NN, Stein JL. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485(7397):237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helbig KL, Farwell Hagman KD, Shinde DN, Mroske C, Powis Z, Li S, Tang S, Helbig I. Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genetics in Medicine. 2016;18(9):898–905. [DOI] [PubMed] [Google Scholar]
- 21.Jin SC, Dong W, Kundishora AJ, Panchagnula S, Moreno-De-Luca A, Furey CG, Allocco AA, Walker RL, Nelson-Williams C, Smith H. Exome sequencing implicates genetic disruption of prenatal neurogliogenesis in sporadic congenital hydrocephalus. Nature medicine. 2020;26(11):1754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg ABW, Mehta NH, Allington G, Jin SC, Moreno-De-Luca A, Kahle KT. Molecular Diagnostic Yield of Exome Sequencing in Patients With Congenital Hydrocephalus: A Systematic Review and Meta-Analysis. JAMA Netw Open. 2023;6(11):e2343384. Epub 20231101. doi: 10.1001/jamanetworkopen.2023.43384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantor RM, Lange K, Sinsheimer JS. Prioritizing GWAS results: a review of statistical methods and recommendations for their application. The American Journal of Human Genetics. 2010;86(1):6–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consortium G, Ardlie KG, Deluca DS, Segrè AV, Sullivan TJ, Young TR, Gelfand ET, Trowbridge CA, Maller JB, Tukiainen T. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science (New York, NY). 2015;348(6235):648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wainberg M, Sinnott-Armstrong N, Mancuso N, Barbeira AN, Knowles DA, Golan D, Ermel R, Ruusalepp A, Quertermous T, Hao K. Opportunities and challenges for transcriptome-wide association studies. Nature genetics. 2019;51(4):592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerra MM, Henzi R, Ortloff A, Lichtin N, Vio K, Jimenez AJ, Dominguez-Pinos MD, Gonzalez C, Jara MC, Hinostroza F. Cell junction pathology of neural stem cells is associated with ventricular zone disruption, hydrocephalus, and abnormal neurogenesis. Journal of Neuropathology & Experimental Neurology. 2015;74(7):653–71. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez EM, Guerra MM, Vío K, González C, Ortloff A, Bátiz LF, Rodríguez S, Jara MC, Muñoz RI, Ortega E. A cell junction pathology of neural stem cells leads to abnormal neurogenesis and hydrocephalus. Biological research. 2012;45(3):231–41. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Novak MK, Pepin RM, Maschhoff KR, Hu W. Different congenital hydrocephalus-associated mutations in Trim71 impair stem cell differentiation via distinct gain-of-function mechanisms. PLoS biology. 2023;21(2):e3001947. Epub 20230209. doi: 10.1371/journal.pbio.3001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q, Novak MK, Pepin RM, Maschhoff KR, Worner K, Chen X, Zhang S, Hu W. A congenital hydrocephalus-causing mutation in Trim71 induces stem cell defects via inhibiting Lsd1 mRNA translation. EMBO reports. 2023;24(2):e55843. Epub 20221227. doi: 10.15252/embr.202255843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li YP, Duan FF, Zhao YT, Gu KL, Liao LQ, Su HB, Hao J, Zhang K, Yang N, Wang Y. A TRIM71 binding long noncoding RNA Trincr1 represses FGF/ERK signaling in embryonic stem cells. Nat Commun. 2019;10(1):1368. Epub 20190325. doi: 10.1038/s41467-019-08911-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science (New York, NY). 2001;294(5549):2186–9. [DOI] [PubMed] [Google Scholar]
- 32.Rivière J-B, Mirzaa GM, O’Roak BJ, Beddaoui M, Alcantara D, Conway RL, St-Onge J, Schwartzentruber JA, Gripp KW, Nikkel SM. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nature genetics. 2012;44(8):934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harmacek L, Watkins-Chow DE, Chen J, Jones KL, Pavan WJ, Salbaum JM, Niswander L. A unique missense allele of BAF155, a core BAF chromatin remodeling complex protein, causes neural tube closure defects in mice. Developmental neurobiology. 2014;74(5):483–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayanan R, Pirouz M, Kerimoglu C, Pham L, Wagener RJ, Kiszka KA, Rosenbusch J, Seong RH, Kessel M, Fischer A, Stoykova A, Staiger JF, Tuoc T. Loss of BAF (mSWI/SNF) Complexes Causes Global Transcriptional and Chromatin State Changes in Forebrain Development. Cell Rep. 2015;13(9):1842–54. Epub 20151119. doi: 10.1016/j.celrep.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 35.Singh AK, Allington G, Viviano S, McGee S, Kiziltug E, Ma S, Zhao S, Mekbib KY, Shohfi JP, Duy PQ, DeSpenza T Jr., Furey CG, Reeves BC, Smith H, Sousa AMM, Cherskov A, Allocco A, Nelson-Williams C, Haider S, Rizvi SRA, Alper SL, Sestan N, Shimelis H, Walsh LK, Lifton RP, Moreno-De-Luca A, Jin SC, Kruszka P, Deniz E, Kahle KT. A novel SMARCC1 BAFopathy implicates neural progenitor epigenetic dysregulation in human hydrocephalus. Brain. 2023. Epub 20231221. doi: 10.1093/brain/awad405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacquet BV, Salinas-Mondragon R, Liang H, Therit B, Buie JD, Dykstra M, Campbell K, Ostrowski LE, Brody SL, Ghashghaei HT. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development. 2009;136(23):4021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sienski G, Dönertas D, Brennecke J. Transcriptional silencing of transposons by Piwi and maelstrom and its impact on chromatin state and gene expression. Cell. 2012;151(5):964–80. Epub 20121115. doi: 10.1016/j.cell.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soper SF, van der Heijden GW, Hardiman TC, Goodheart M, Martin SL, de Boer P, Bortvin A. Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev Cell. 2008;15(2):285–97. doi: 10.1016/j.devcel.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sienski G, Donertas D, Brennecke J. Transcriptional silencing of transposons by Piwi and maelstrom and its impact on chromatin state and gene expression. Cell. 2012;151(5):964–80. Epub 2012/11/20. doi: 10.1016/j.cell.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Currle DS, Cheng X, Hsu CM, Monuki ES. Direct and indirect roles of CNS dorsal midline cells in choroid plexus epithelia formation. Development. 2005;132(15):3549–59. Epub 20050623. doi: 10.1242/dev.01915. [DOI] [PubMed] [Google Scholar]
- 41.Hunter NL, Dymecki SM. Molecularly and temporally separable lineages form the hindbrain roof plate and contribute differentially to the choroid plexus. Development. 2007;134(19):3449–60. Epub 20070829. doi: 10.1242/dev.003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2(4):287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 43.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annual review of neuroscience. 2009;32:149–84. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464(7288):554–61. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 45.Uhlen M, Zhang C, Lee S, Sjostedt E, Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, Sanli K, von Feilitzen K, Oksvold P, Lundberg E, Hober S, Nilsson P, Mattsson J, Schwenk JM, Brunnstrom H, Glimelius B, Sjoblom T, Edqvist PH, Djureinovic D, Micke P, Lindskog C, Mardinoglu A, Ponten F. A pathology atlas of the human cancer transcriptome. Science (New York, NY). 2017;357(6352). Epub 2017/08/19. doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 46.Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455(7211):351–7. [DOI] [PubMed] [Google Scholar]
- 47.Vanderhaeghen P, Polleux F. Developmental mechanisms underlying the evolution of human cortical circuits. Nature Reviews Neuroscience. 2023;24(4):213–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MuhChyi C, Juliandi B, Matsuda T, Nakashima K. Epigenetic regulation of neural stem cell fate during corticogenesis. International Journal of Developmental Neuroscience. 2013;31(6):424–33. [DOI] [PubMed] [Google Scholar]
- 49.Howard BM, Zhicheng M, Filipovic R, Moore AR, Antic SD, Zecevic N. Radial glia cells in the developing human brain. Neuroscientist. 2008;14(5):459–73. Epub 2008/05/10. doi: 10.1177/1073858407313512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bayer SA, Altman J. The human brain during the early first trimester. Boca Raton, Fla. London: CRC ; Taylor & Francis distributor; 2008. 522 p. p. [Google Scholar]
- 51.Liddelow SA. Development of the choroid plexus and blood-CSF barrier. Front Neurosci. 2015;9:32. Epub 2015/03/19. doi: 10.3389/fnins.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gould SJ, Howard S, Papadaki L. The development of ependyma in the human fetal brain: an immunohistological and electron microscopic study. Brain Res Dev Brain Res. 1990;55(2):255–67. Epub 1990/09/01. doi: 10.1016/0165-3806(90)90207-f. [DOI] [PubMed] [Google Scholar]
- 53.Pebworth MP, Ross J, Andrews M, Bhaduri A, Kriegstein AR. Human intermediate progenitor diversity during cortical development. Proc Natl Acad Sci U S A. 2021;118(26). Epub 2021/06/23. doi: 10.1073/pnas.2019415118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13(6):690–9. Epub 2010/05/04. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- 55.Rakic P Specification of cerebral cortical areas. Science. 1988;241(4862):170–6. Epub 1988/07/08. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 56.Yang L, Li Z, Liu G, Li X, Yang Z. Developmental Origins of Human Cortical Oligodendrocytes and Astrocytes. Neurosci Bull. 2022;38(1):47–68. Epub 2021/08/11. doi: 10.1007/s12264-021-00759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itoh K, Fushiki S. The role of L1 cam in murine corticogenesis, and the pathogenesis of hydrocephalus. Pathology international. 2015;65(2):58–66. [DOI] [PubMed] [Google Scholar]
- 58.Li W, Cheng W, Juan AM, Songcang C, Shaobo Z, Tanzila M, Shaohui W, Arantxa C-S, Qiuli B, Jonathan JA, Lilian Gomes de O, Mengyi S, Xinxin G, Guolong Z, Mercedes FP, Eric JH, Arturo A-B, Xin D, Jingjing L, Arnold RK. Molecular and cellular dynamics of the developing human neocortex at single-cell resolution. bioRxiv. 2024:2024.01.16.575956. doi: 10.1101/2024.01.16.575956. [DOI] [Google Scholar]
- 59.Wang L, Wang C, Moriano JA, Chen S, Zuo G, Cebrián-Silla A, Zhang S, Mukhtar T, Wang S, Song M, de Oliveira LG, Bi Q, Augustin JJ, Ge X, Paredes MF, Huang EJ, Alvarez-Buylla A, Duan X, Li J, Kriegstein AR. Molecular and cellular dynamics of the developing human neocortex at single-cell resolution. bioRxiv. 2024:2024.01.16.575956. doi: 10.1101/2024.01.16.575956. [DOI] [PubMed] [Google Scholar]
- 60.Chau KF, Shannon ML, Fame RM, Fonseca E, Mullan H, Johnson MB, Sendamarai AK, Springel MW, Laurent B, Lehtinen MK. Downregulation of ribosome biogenesis during early forebrain development. eLife. 2018;7. Epub 20180510. doi: 10.7554/eLife.36998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yabut OR, Pleasure SJ. Sonic Hedgehog Signaling Rises to the Surface: Emerging Roles in Neocortical Development. Brain Plast. 2018;3(2):119–28. Epub 20180810. doi: 10.3233/bpl-180064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bayraktar OA, Bartels T, Holmqvist S, Kleshchevnikov V, Martirosyan A, Polioudakis D, Ben Haim L, Young AM, Batiuk MY, Prakash K. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nature neuroscience. 2020;23(4):500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G. Spatio-temporal transcriptome of the human brain. Nature. 2011;478(7370):483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polioudakis D, de la Torre-Ubieta L, Langerman J, Elkins AG, Shi X, Stein JL, Vuong CK, Nichterwitz S, Gevorgian M, Opland CK. A single-cell transcriptomic atlas of human neocortical development during midgestation. Neuron. 2019;103(5):785–801. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seidlitz J, Váša F, Shinn M, Romero-Garcia R, Whitaker KJ, Vértes PE, Wagstyl K, Reardon PK, Clasen L, Liu S. Morphometric similarity networks detect microscale cortical organization and predict inter-individual cognitive variation. Neuron. 2018;97(1):231–47. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziffra RS, Kim CN, Ross JM, Wilfert A, Turner TN, Haeussler M, Casella AM, Przytycki PF, Keough KC, Shin D. Single-cell epigenomics reveals mechanisms of human cortical development. Nature. 2021;598(7879):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandell JG, Kulkarni AV, Warf BC, Schiff SJ. Volumetric brain analysis in neurosurgery: Part 2. Brain and CSF volumes discriminate neurocognitive outcomes in hydrocephalus. Journal of Neurosurgery: Pediatrics. 2015;15(2):125–32. [DOI] [PubMed] [Google Scholar]
- 68.Ball G, Seidlitz J, Beare R, Seal ML. Cortical remodelling in childhood is associated with genes enriched for neurodevelopmental disorders. NeuroImage. 2020;215:116803. [DOI] [PubMed] [Google Scholar]
- 69.Seidlitz J, Nadig A, Liu S, Bethlehem RA, Vértes PE, Morgan SE, Váša F, Romero-Garcia R, Lalonde FM, Clasen LS. Transcriptomic and cellular decoding of regional brain vulnerability to neurogenetic disorders. Nature communications. 2020;11(1):3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allington G, Duy PQ, Ryou J, Singh A, Kiziltug E, Robert SM, Kundishora AJ, King S, Haider S, Kahle KT. Genomic approaches to improve the clinical diagnosis and management of patients with congenital hydrocephalus. Journal of Neurosurgery: Pediatrics. 2021;29(2):168–77. [DOI] [PubMed] [Google Scholar]
- 71.Alexander-Bloch AF, Raznahan A, Vandekar SN, Seidlitz J, Lu Z, Mathias SR, Knowles E, Mollon J, Rodrigue A, Curran JE. Imaging local genetic influences on cortical folding. Proceedings of the National Academy of Sciences. 2020;117(13):7430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warrier V, Stauffer EM, Huang QQ, Wigdor EM, Slob EAW, Seidlitz J, Ronan L, Valk SL, Mallard TT, Grotzinger AD, Romero-Garcia R, Baron-Cohen S, Geschwind DH, Lancaster MA, Murray GK, Gandal MJ, Alexander-Bloch A, Won H, Martin HC, Bullmore ET, Bethlehem RAI. Genetic insights into human cortical organization and development through genome-wide analyses of 2,347 neuroimaging phenotypes. Nat Genet. 2023;55(9):1483–93. Epub 20230817. doi: 10.1038/s41588-023-01475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, Cortes A, Welsh S, Young A, Effingham M, McVean G, Leslie S, Allen N, Donnelly P, Marchini J. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–9. Epub 2018/10/12. doi: 10.1038/s41586-018-0579-z. Peptide Groove LLP. G.M. and P.D. are founders and directors of Genomics Plc. The remaining authors declare no competing financial interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ge YJ, Wu BS, Zhang Y, Chen SD, Zhang YR, Kang JJ, Deng YT, Ou YN, He XY, Zhao YL, Kuo K, Ma Q, Banaschewski T, Barker GJ, Bokde ALW, Desrivières S, Flor H, Grigis A, Garavan H, Gowland P, Heinz A, Brühl R, Martinot JL, Martinot MP, Artiges E, Nees F, Orfanos DP, Lemaitre H, Paus T, Poustka L, Hohmann S, Millenet S, Fröhner JH, Smolka MN, Vaidya N, Walter H, Whelan R, Feng JF, Tan L, Dong Q, Schumann G, Cheng W, Yu JT. Genetic architectures of cerebral ventricles and their overlap with neuropsychiatric traits. Nat Hum Behav. 2023. Epub 2023/10/20. doi: 10.1038/s41562-023-01722-6. [DOI] [PubMed] [Google Scholar]
- 75.Kyriakopoulou V, Davidson A, Chew A, Gupta N, Arichi T, Nosarti C, Rutherford MA. Characterisation of ASD traits among a cohort of children with isolated fetal ventriculomegaly. Nat Commun. 2023;14(1):1550. Epub 20230321. doi: 10.1038/s41467-023-37242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vojinovic D, Adams HH, Jian X, Yang Q, Smith AV, Bis JC, Teumer A, Scholz M, Armstrong NJ, Hofer E. Genome-wide association study of 23,500 individuals identifies 7 loci associated with brain ventricular volume. Nature communications. 2018;9(1):3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao B, Luo T, Li T, Li Y, Zhang J, Shan Y, Wang X, Yang L, Zhou F, Zhu Z. Genome-wide association analysis of 19,629 individuals identifies variants influencing regional brain volumes and refines their genetic co-architecture with cognitive and mental health traits. Nature genetics. 2019;51(11):1637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, Adler S, Alexopoulos GS, Anagnostou E, Areces-Gonzalez A, Astle DE, Auyeung B, Ayub M, Bae J, Ball G, Baron-Cohen S, Beare R, Bedford SA, Benegal V, Beyer F, Blangero J, Blesa Cábez M, Boardman JP, Borzage M, Bosch-Bayard JF, Bourke N, Calhoun VD, Chakravarty MM, Chen C, Chertavian C, Chetelat G, Chong YS, Cole JH, Corvin A, Costantino M, Courchesne E, Crivello F, Cropley VL, Crosbie J, Crossley N, Delarue M, Delorme R, Desrivieres S, Devenyi GA, Di Biase MA, Dolan R, Donald KA, Donohoe G, Dunlop K, Edwards AD, Elison JT, Ellis CT, Elman JA, Eyler L, Fair DA, Feczko E, Fletcher PC, Fonagy P, Franz CE, Galan-Garcia L, Gholipour A, Giedd J, Gilmore JH, Glahn DC, Goodyer IM, Grant PE, Groenewold NA, Gunning FM, Gur RE, Gur RC, Hammill CF, Hansson O, Hedden T, Heinz A, Henson RN, Heuer K, Hoare J, Holla B, Holmes AJ, Holt R, Huang H, Im K, Ipser J, Jack CR Jr., Jackowski AP, Jia T, Johnson KA, Jones PB, Jones DT, Kahn RS, Karlsson H, Karlsson L, Kawashima R, Kelley EA, Kern S, Kim KW, Kitzbichler MG, Kremen WS, Lalonde F, Landeau B, Lee S, Lerch J, Lewis JD, Li J, Liao W, Liston C, Lombardo MV, Lv J, Lynch C, Mallard TT, Marcelis M, Markello RD, Mathias SR, Mazoyer B, McGuire P, Meaney MJ, Mechelli A, Medic N, Misic B, Morgan SE, Mothersill D, Nigg J, Ong MQW, Ortinau C, Ossenkoppele R, Ouyang M, Palaniyappan L, Paly L, Pan PM, Pantelis C, Park MM, Paus T, Pausova Z, Paz-Linares D, Pichet Binette A, Pierce K, Qian X, Qiu J, Qiu A, Raznahan A, Rittman T, Rodrigue A, Rollins CK, Romero-Garcia R, Ronan L, Rosenberg MD, Rowitch DH, Salum GA, Satterthwaite TD, Schaare HL, Schachar RJ, Schultz AP, Schumann G, Schöll M, Sharp D, Shinohara RT, Skoog I, Smyser CD, Sperling RA, Stein DJ, Stolicyn A, Suckling J, Sullivan G, Taki Y, Thyreau B, Toro R, Traut N, Tsvetanov KA, Turk-Browne NB, Tuulari JJ, Tzourio C, Vachon-Presseau É, Valdes-Sosa MJ, Valdes-Sosa PA, Valk SL, van Amelsvoort T, Vandekar SN, Vasung L, Victoria LW, Villeneuve S, Villringer A, Vértes PE, Wagstyl K, Wang YS, Warfield SK, Warrier V, Westman E, Westwater ML, Whalley HC, Witte AV, Yang N, Yeo B, Yun H, Zalesky A, Zar HJ, Zettergren A, Zhou JH, Ziauddeen H, Zugman A, Zuo XN, Bullmore ET, Alexander-Bloch AF. Brain charts for the human lifespan. Nature. 2022;604(7906):525–33. Epub 2022/04/08. doi: 10.1038/s41586-022-04554-y. consultant for GlaxoSmithKline, Boehringer Ingelheim and Monument Therapeutics. G.S.A. has served on advisory boards of Eisai and Janssen and in speakers bureaus of Allergan, Takeda and Lundbeck. K.M.A. is an employee of Neumora Therapeutics. P.B.J. has consulted for MSD. L. Palaniyappan reports personal fees from Janssen Canada for participating in an Advisory Board (2019) and Continuous Professional Development events (2017–2020), Otsuka Canada for Continuous Professional Development events (2017–2020), SPMM Course Limited, UK for preparing educational materials for psychiatrists and trainees (2010 onwards), Canadian Psychiatric Association for Continuous Professional Development events (2018–2019); book royalties from Oxford University Press (2009 onwards); institution-paid investigator-initiated educational grants with no personal remunerations from Janssen Canada, Sunovion and Otsuka Canada (2016–2019); travel support to attend a study investigator’s meeting organized by Boehringer-Ingelheim (2017); travel support from Magstim Limited (UK) to speak at an academic meeting (2014); none of these activities are related to this work. T.R. has received honoraria from Oxford Biomedica. A.P.S. has consulted for Janssen, Biogen, Qynapse, and NervGen. R.T.S. has received consulting income from Octave Bioscience and compensation for scientific review duties from the American Medical Association, the US Department of Defense, the Emerson Collective, and the National Institutes of Health. R.A.S. has consulted for Janssen, AC Immune, NervGen and Genentech. D.J.S. has received research grants and/or consultancy honoraria from Discovery Vitality, Johnson & Johnson, Lundbeck, Sanofi, Servier, Takeda and Vistagen. J. Suckling has consulted for GW Pharmaceuticals, Claritas HealthTech, Fundacion La Caixa and Fondazione Cariplo. All other authors declare no competing interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duy PQ, Weise SC, Marini C, Li XJ, Liang D, Dahl PJ, Ma S, Spajic A, Dong W, Juusola J, Kiziltug E, Kundishora AJ, Koundal S, Pedram MZ, Torres-Fernández LA, Händler K, De Domenico E, Becker M, Ulas T, Juranek SA, Cuevas E, Hao LT, Jux B, Sousa AMM, Liu F, Kim SK, Li M, Yang Y, Takeo Y, Duque A, Nelson-Williams C, Ha Y, Selvaganesan K, Robert SM, Singh AK, Allington G, Furey CG, Timberlake AT, Reeves BC, Smith H, Dunbar A, DeSpenza T Jr., Goto J, Marlier A, Moreno-De-Luca A, Yu X, Butler WE, Carter BS, Lake EMR, Constable RT, Rakic P, Lin H, Deniz E, Benveniste H, Malvankar NS, Estrada-Veras JI, Walsh CA, Alper SL, Schultze JL, Paeschke K, Doetzlhofer A, Wulczyn FG, Jin SC, Lifton RP, Sestan N, Kolanus W, Kahle KT. Impaired neurogenesis alters brain biomechanics in a neuroprogenitor-based genetic subtype of congenital hydrocephalus. Nat Neurosci. 2022;25(4):458–73. Epub 2022/04/06. doi: 10.1038/s41593-022-01043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duy PQ, Jux B, Zhao S, Mekbib KY, Dennis E, Dong W, Nelson-Williams C, Mehta NH, Shohfi JP, Juusola J. TRIM71 mutations cause a neurodevelopmental syndrome featuring ventriculomegaly and hydrocephalus. Brain. 2024:awae175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Welte T, Tuck AC, Papasaikas P, Carl SH, Flemr M, Knuckles P, Rankova A, Bühler M, Großhans H. The RNA hairpin binder TRIM71 modulates alternative splicing by repressing MBNL1. Genes & development. 2019;33(17–18):1221–35. Epub 20190801. doi: 10.1101/gad.328492.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jansen LA, Mirzaa GM, Ishak GE, O’Roak BJ, Hiatt JB, Roden WH, Gunter SA, Christian SL, Collins S, Adams C. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain. 2015;138(6):1613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee JH, Huynh M, Silhavy JL, Kim S, Dixon-Salazar T, Heiberg A, Scott E, Bafna V, Hill KJ, Collazo A. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nature genetics. 2012;44(8):941–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Pang K, Zhou L, Cebrián-Silla A, González-Granero S, Wang S, Bi Q, White ML, Ho B, Li J. A cross-species proteomic map reveals neoteny of human synapse development. Nature. 2023:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Staley K Molecular mechanisms of epilepsy. Nature neuroscience. 2015;18(3):367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DeSpenza T Jr, Kiziltug E, Allington G, Barson D, O’Connor D, Robert SM, Mekbib KY, Nanda P, Greenberg A, Singh A. Dual impact of PTEN mutation on CSF dynamics and cortical networks via the dysregulation of neural precursors and their interneuron descendants. bioRxiv. 2023:2023.03.18.533275. [Google Scholar]
- 87.DeSpenza T Jr., Kiziltug E, Allington G, Barson DG, McGee S, O’Connor D, Robert SM, Mekbib KY, Nanda P, Greenberg ABW, Singh A, Duy PQ, Mandino F, Zhao S, Lynn A, Reeves BC, Marlier A, Getz SA, Nelson-Williams C, Shimelis H, Walsh LK, Zhang J, Wang W, Prina ML, OuYang A, Abdulkareem AF, Smith H, Shohfi J, Mehta NH, Dennis E, Reduron LR, Hong J, Butler W, Carter BS, Deniz E, Lake EMR, Constable RT, Sahin M, Srivastava S, Winden K, Hoffman EJ, Carlson M, Gunel M, Lifton RP, Alper SL, Jin SC, Crair MC, Moreno-De-Luca A, Luikart BW, Kahle KT. PTEN mutations impair CSF dynamics and cortical networks by dysregulating periventricular neural progenitors. Nat Neurosci. 2025;28(3):536–57. Epub 20250224. doi: 10.1038/s41593-024-01865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus–cerebrospinal fluid system. Nature Reviews Neuroscience. 2015;16(8):445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiological reviews. 2013;93(4):1847–92. [DOI] [PubMed] [Google Scholar]
- 90.Dandy WE. Extirpation of the choroid plexus of the lateral ventricles in communicating hydrocephalus. Annals of surgery. 1918;68(6):569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scarff JE. The treatment of nonobstructive (communicating) hydrocephalus by endoscopic cauterization of the choroid plexuses. J Neurosurg. 1970;33(1):1–18. doi: 10.3171/jns.1970.33.1.0001. [DOI] [PubMed] [Google Scholar]
- 92.Praetorius J, Damkier HH. Transport across the choroid plexus epithelium. American Journal of Physiology-Cell Physiology. 2017;312(6):C673–C86. [DOI] [PubMed] [Google Scholar]
- 93.Owler BK, Pitham T, Wang D. Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal fluid research. 2010;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Quinton P, Wright EM, Tormey JM. Localization of sodium pumps in the choroid plexus epithelium. The Journal of cell biology. 1973;58(3):724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robson EA, Dixon L, Causon L, Dawes W, Benenati M, Fassad M, Hirst RA, Kenia P, Moya EF, Patel M. Hydrocephalus and diffuse choroid plexus hyperplasia in primary ciliary dyskinesia-related MCIDAS mutation. Neurology: Genetics. 2020;6(4):e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujimura M, Onuma T, Kameyama M, Motohashi O, Kon H, Yamamoto K, Ishii K, Tominaga T. Hydrocephalus due to cerebrospinal fluid overproduction by bilateral choroid plexus papillomas. Child’s Nervous System. 2004;20:485–8. [DOI] [PubMed] [Google Scholar]
- 97.Pencalet P, Sainte-Rose C, Lellouch-Tubiana A, Kalifa C, Brunelle F, Sgouros S, Meyer P, Cinalli G, Zerah M, Pierre-Kahn A. Papillomas and carcinomas of the choroid plexus in children. Journal of neurosurgery. 1998;88(3):521–8. [DOI] [PubMed] [Google Scholar]
- 98.Karimy JK, Duran D, Hu JK, Gavankar C, Gaillard JR, Bayri Y, Rice H, DiLuna ML, Gerzanich V, Simard JM. Cerebrospinal fluid hypersecretion in pediatric hydrocephalus. Neurosurgical focus. 2016;41(5):E10. [DOI] [PubMed] [Google Scholar]
- 99.Robert SM, Reeves BC, Kiziltug E, Duy PQ, Karimy JK, Mansuri MS, Marlier A, Allington G, Greenberg AB, DeSpenza T. The choroid plexus links innate immunity to CSF dysregulation in hydrocephalus. Cell. 2023;186(4):764–85. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Y, Di C, Song S, Zhang Y, Lu Y, Liao J, Lei B, Zhong J, Guo K, Zhang N. Choroid plexus mast cells drive tumor-associated hydrocephalus. Cell. 2023;186(26):5719–38. e28. [DOI] [PubMed] [Google Scholar]
- 101.Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, Furey CG, Zhou X, Mansuri MS, Montejo J, Vera A, DiLuna ML, Delpire E, Alper SL, Gunel M, Gerzanich V, Medzhitov R, Simard JM, Kahle KT. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23(8):997–1003. Epub 20170710. doi: 10.1038/nm.4361. [DOI] [PubMed] [Google Scholar]
- 102.Zhang J, Bhuiyan MIH, Zhang T, Karimy JK, Wu Z, Fiesler VM, Zhang J, Huang H, Hasan MN, Skrzypiec AE. Modulation of brain cation-Cl− cotransport via the SPAK kinase inhibitor ZT-1a. Nature communications. 2020;11(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnsen L, Friis KA, Damkier HH. In vitro investigation of the effect of proinflammatory cytokines on mouse choroid plexus membrane transporters Ncbe and NKCC1. Fluids and barriers of the CNS. 2023;20(1):71. Epub 20231012. doi: 10.1186/s12987-023-00474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stödberg T, Magnusson M, Lesko N, Wredenberg A, Martin Munoz D, Stranneheim H, Wedell A. SLC12A2 mutations cause NKCC1 deficiency with encephalopathy and impaired secretory epithelia. Neurol Genet. 2020;6(4):e478. Epub 20200702. doi: 10.1212/nxg.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gregoriades JMC, Madaris A, Alvarez FJ, Alvarez-Leefmans FJ. Genetic and pharmacological inactivation of apical Na(+)-K(+)-2Cl(−) cotransporter 1 in choroid plexus epithelial cells reveals the physiological function of the cotransporter. Am J Physiol Cell Physiol. 2019;316(4):C525–c44. Epub 20181221. doi: 10.1152/ajpcell.00026.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fame RM, Xu H, Pragana A, Lehtinen M. Age-appropriate potassium clearance from perinatal cerebrospinal fluid depends on choroid plexus NKCC1. Fluids and barriers of the CNS. 2023;20(1):45. Epub 20230616. doi: 10.1186/s12987-023-00438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu H, Fame RM, Sadegh C, Sutin J, Naranjo C, Della S, Cui J, Shipley FB, Vernon A, Gao F, Zhang Y, Holtzman MJ, Heiman M, Warf BC, Lin PY, Lehtinen MK. Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development. Nat Commun. 2021;12(1):447. Epub 20210119. doi: 10.1038/s41467-020-20666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sadegh C, Xu H, Sutin J, Fatou B, Gupta S, Pragana A, Taylor M, Kalugin PN, Zawadzki ME, Alturkistani O, Shipley FB, Dani N, Fame RM, Wurie Z, Talati P, Schleicher RL, Klein EM, Zhang Y, Holtzman MJ, Moore CI, Lin PY, Patel AB, Warf BC, Kimberly WT, Steen H, Andermann ML, Lehtinen MK. Choroid plexus-targeted NKCC1 overexpression to treat post-hemorrhagic hydrocephalus. Neuron. 2023;111(10):1591–608.e4. Epub 20230308. doi: 10.1016/j.neuron.2023.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nature medicine. 2005;11(11):1205–13. [DOI] [PubMed] [Google Scholar]
- 110.Dani N, Herbst RH, McCabe C, Green GS, Kaiser K, Head JP, Cui J, Shipley FB, Jang A, Dionne D. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184(11):3056–74. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eze UC, Bhaduri A, Haeussler M, Nowakowski TJ, Kriegstein AR. Single-cell atlas of early human brain development highlights heterogeneity of human neuroepithelial cells and early radial glia. Nature neuroscience. 2021;24(4):584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lun MP, Johnson MB, Broadbelt KG, Watanabe M, Kang Y-j, Chau KF, Springel MW, Malesz A, Sousa AM, Pletikos M. Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. Journal of Neuroscience. 2015;35(12):4903–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hou CC, Li D, Berry BC, Zheng S, Carroll RS, Johnson MD, Yang HW. Heterozygous FOXJ1 Mutations Cause Incomplete Ependymal Cell Differentiation and Communicating Hydrocephalus. Cell Mol Neurobiol. 2023. Epub 20230824. doi: 10.1007/s10571-023-01398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus2005. [DOI] [PubMed] [Google Scholar]
- 115.Stubbs JL, Oishi I, Izpisúa Belmonte JC, Kintner C. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat Genet. 2008;40(12):1454–60. Epub 20081116. doi: 10.1038/ng.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hevner RF. Brain overgrowth in disorders of RTK-PI3K-AKT signaling: a mosaic of malformations. Semin Perinatol. 2015;39(1):36–43. Epub 20141126. doi: 10.1053/j.semperi.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang X, Ketova T, Fleming JT, Wang H, Dey SK, Litingtung Y, Chiang C. Sonic hedgehog signaling regulates a novel epithelial progenitor domain of the hindbrain choroid plexus 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hunter NL, Dymecki SM. Molecularly and temporally separable lineages form the hindbrain roof plate and contribute differentially to the choroid plexus 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimada IS, Somatilaka BN, Hwang S-H, Anderson AG, Shelton JM, Rajaram V, Konopka G, Mukhopadhyay S. Derepression of sonic hedgehog signaling upon Gpr161 deletion unravels forebrain and ventricular abnormalities. Developmental biology. 2019;450(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klein SD, Nguyen DC, Bhakta V, Wong D, Chang VY, Davidson TB, Martinez-Agosto JA. Mutations in the sonic hedgehog pathway cause macrocephaly-associated conditions due to crosstalk to the PI3K/AKT/mTOR pathway. American Journal of Medical Genetics Part A. 2019;179(12):2517–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shannon ML, Fame RM, Chau KF, Dani N, Calicchio ML, Géléoc GS, Lidov HGW, Alexandrescu S, Lehtinen MK. Mice Expressing Myc in Neural Precursors Develop Choroid Plexus and Ciliary Body Tumors. The American journal of pathology. 2018;188(6):1334–44. Epub 20180313. doi: 10.1016/j.ajpath.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Date P, Ackermann P, Furey C, Fink IB, Jonas S, Khokha MK, Kahle KT, Deniz E. Visualizing flow in an intact CSF network using optical coherence tomography: implications for human congenital hydrocephalus. Scientific reports. 2019;9(1):6196. Epub 20190417. doi: 10.1038/s41598-019-42549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dur AH, Tang T, Viviano S, Sekuri A, Willsey HR, Tagare HD, Kahle KT, Deniz E. In Xenopus ependymal cilia drive embryonic CSF circulation and brain development independently of cardiac pulsatile forces. Fluids and barriers of the CNS. 2020;17(1):72. Epub 20201211. doi: 10.1186/s12987-020-00234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang S, Emelyanov A, You MS, Sin M, Korzh V. Camel regulates development of the brain ventricular system. Cell Tissue Res. 2021;383(2):835–52. Epub 20200909. doi: 10.1007/s00441-020-03270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Olstad EW, Ringers C, Hansen JN, Wens A, Brandt C, Wachten D, Yaksi E, Jurisch-Yaksi N. Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development. Curr Biol. 2019;29(2):229–41.e6. Epub 20190103. doi: 10.1016/j.cub.2018.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fame RM, Chang JT, Hong A, Aponte-Santiago NA, Sive H. Directional cerebrospinal fluid movement between brain ventricles in larval zebrafish. Fluids and barriers of the CNS. 2016;13(1):11. Epub 20160621. doi: 10.1186/s12987-016-0036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lodhia KR, Shakui P, Keep RF. Hydrocephalus in a rat model of intraventricular hemorrhage. Acta neurochirurgica Supplement. 2006;96:207–11. doi: 10.1007/3-211-30714-1_45. [DOI] [PubMed] [Google Scholar]
- 128.Vogel P, Read R, Hansen G, Payne B, Small D, Sands A, Zambrowicz B. Congenital hydrocephalus in genetically engineered mice. Veterinary pathology. 2012;49(1):166–81. [DOI] [PubMed] [Google Scholar]
- 129.Crews L, Wyss-Coray T, Masliah E. Insights into the pathogenesis of hydrocephalus from transgenic and experimental animal models. Brain pathology. 2004;14(3):312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McAllister JP, Talcott MR, Isaacs AM, Zwick SH, Garcia-Bonilla M, Castaneyra-Ruiz L, Hartman AL, Dilger RN, Fleming SA, Golden RK. A novel model of acquired hydrocephalus for evaluation of neurosurgical treatments. Fluids and barriers of the CNS. 2021;18(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Del Bigio MR. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia. 1995;14(1):1–13. [DOI] [PubMed] [Google Scholar]
- 132.Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, Horvath TL, Vaccarino FM. Modeling human cortical development in vitro using induced pluripotent stem cells. Proceedings of the National Academy of Sciences. 2012;109(31):12770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Benninghaus A, Balédent O, Lokossou A, Castelar C, Leonhardt S, Radermacher K. Enhanced in vitro model of the CSF dynamics. Fluids and barriers of the CNS. 2019;16(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eichmüller OL, Knoblich JA. Human cerebral organoids—a new tool for clinical neurology research. Nature Reviews Neurology. 2022:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chiaradia I, Lancaster MA. Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Nature neuroscience. 2020;23(12):1496–508. [DOI] [PubMed] [Google Scholar]
- 136.Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang W, Ma L, Yang M, Shao Q, Xu J, Lu Z, Zhao Z, Chen R, Chai Y, Chen J-F. Cerebral organoid and mouse models reveal a RAB39b–PI3K–mTOR pathway-dependent dysregulation of cortical development leading to macrocephaly/autism phenotypes. Genes & development. 2020;34(7–8):580–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang P, Mokhtari R, Pedrosa E, Kirschenbaum M, Bayrak C, Zheng D, Lachman HM. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in cerebral organoids derived from iPS cells. Molecular autism. 2017;8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dutta D, Clevers H. Organoid culture systems to study host–pathogen interactions. Current opinion in immunology. 2017;48:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pellegrini L, Bonfio C, Chadwick J, Begum F, Skehel M, Lancaster MA. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science (New York, NY). 2020;369(6500):eaaz5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Benito-Kwiecinski S, Giandomenico SL, Sutcliffe M, Riis ES, Freire-Pritchett P, Kelava I, Wunderlich S, Martin U, Wray GA, McDole K, Lancaster MA. An early cell shape transition drives evolutionary expansion of the human forebrain. Cell. 2021;184(8):2084–102.e19. Epub 20210324. doi: 10.1016/j.cell.2021.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Giandomenico SL, Sutcliffe M, Lancaster MA. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nature protocols. 2021;16(2):579–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chiaradia I, Imaz-Rosshandler I, Nilges BS, Boulanger J, Pellegrini L, Das R, Kashikar ND, Lancaster MA. Tissue morphology influences the temporal program of human brain organoid development. Cell stem cell. 2023;30(10):1351–67.e10. Epub 2023/10/07. doi: 10.1016/j.stem.2023.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, James LC, Lancaster MA. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell stem cell. 2020;27(6):951–61. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pașca SP, Arlotta P, Bateup HS, Camp JG, Cappello S, Gage FH, Knoblich JA, Kriegstein AR, Lancaster MA, Ming GL, Muotri AR, Park IH, Reiner O, Song H, Studer L, Temple S, Testa G, Treutlein B, Vaccarino FM. A nomenclature consensus for nervous system organoids and assembloids. Nature. 2022;609(7929):907–10. Epub 20220928. doi: 10.1038/s41586-022-05219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sandoval SO, Cappuccio G, Kruth K, Osenberg S, Khalil SM, Méndez-Albelo NM, Padmanabhan K, Wang D, Niciu MJ, Bhattacharyya A. Rigor and reproducibility in human brain organoid research: Where we are and where we need to go. Stem cell reports. 2024;19(6):796–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wolf FA, Angerer P, Theis FJ. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19(1):15. Epub 20180206. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


