Abstract
Purpose
The executive summary of the guideline aims to provide the most relevant recommendations on the diagnosis and treatment of invasive pulmonary aspergillosis in critically ill patients in the intensive care unit.
Methods
The guideline’s work included a systematic literature search, selection and assessment of the data relevant to the issues identified. Key questions included the areas of epidemiology, risk factors, diagnostics, and therapy. They were discussed analogous to a PICO scheme within the guideline committee, with subsequent working groups proposing recommendations for specific key questions, which were then again discussed and finalized by the entire guideline committee.
Results
In addition to the classic risk factors (persistent neutropenia, allogeneic stem cell transplantation, congenital or acquired immunodeficiency, etc.), decompensated liver cirrhosis, COPD, solid tumours and viral pneumonia (influenza, COVID-19) have been established as risk factors for critically ill patients in need of intensive care. If there is no adequate improvement or even further clinical deterioration of the respiratory status in critically ill patients, the presence of IPA should be considered and appropriate diagnostic tests should be initiated. Diagnostics should include a CT scan of the chest and a broncho-alveolar lavage with culture for moulds, testing for galactomannan and PCR. Isavuconazole and voriconazole are recommended as first-line treatment, liposomal amphotericin B as an alternative, with posaconazole (PCZ) or the echinocandins (as an add-on to azole or polyene treatment) being additional options for salvage treatment.
Conclusion
Invasive aspergillosis in critically ill patients represents a diagnostic and therapeutic challenge. If indicated, invasive aspergillosis should be considered and appropriate diagnostic tests initiated. Isavuconazole and voriconazole are recommended as first-line treatment, liposomal amphotericin B as an alternative.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-025-02572-2.
Keyword: Invasive pulmonary aspergillosis, Intensive care medicine, Critically ill patients, Guideline, Azoles, Drug interactions
Background
With the criteria for invasive fungal infections in intensive care patients published recently, a broad consensus on the (research) definition of invasive aspergillosis in critically ill patients in the intensive care unit (ICU) was established [1]. In order to create robust evidence, the guideline, which is primarily intended to harmonise research questions followed a conservative approach and recommendations are therefore very strict. In a clinical setting, deviations from these recommendations may be warranted and should be made in exceptional cases, when justified.
Methods
The S1 guideline aimed to provide a comprehensive overview of evidence-based recommendations on the diagnosis and treatment of invasive (pulmonary) aspergillosis and addresses physicians involved in the care of critically ill adult patients treated in the ICU. This compendium highlights the key diagnostic and treatment recommendations from the guideline that are most important for clinical practice.
Critical evaluation of evidence and Preparation of recommendations
The guideline presented is based on a systematic search, selection, and assessment of the data relevant to the identified issues. Due to the significant lack of randomised controlled trials (RCT) or comparable studies, it was generally not possible to assign recommendation grades or determine the quality of evidence. Therefore, as defined by the Arbeitsgemeinschaft wissenschaftlicher Fachgesellschaften (AWMF), the recommendations presented here are classified as expert opinions (S1 level).
Determination of guideline questions and Preparation of the recommendations
Key questions were formulated for the areas of epidemiology and risk factors as well as diagnostics and therapy. Relevant core questions were identified and discussed within the guideline committee analogous to a PICO-based scheme. Subsequently, working groups for identified key questions were set up, who performed a literature search and formulated specific recommendations, which then underwent a review process within the entire guideline group. The final manuscript was submitted to the boards of the scientific societies and approved for publication.
Systematic literature research
We used MEDLINE, Livivo and ScienceDirect for the literature search. Articles published before August 31st 2024 were taken into account. Search strings included: (“ICU OR intensive care OR critical care”) AND “aspergill* AND (“galactomannan OR aspergillus antigen OR LFA OR lateral flow OR glucan OR BDG”), (“Aspergill*) AND (invasive OR infection OR case OR patient OR report) AND (guideline OR treatment OR therapy OR diagnosis OR therapeutic drug monitoring”), (Aspergill*) AND (invasive OR infection) AND (ICU OR critical care OR critical illness OR intensive care) AND (risk OR factor OR epidemiology OR incidence OR mortality).
Key recommendations
Epidemiology
Invasive mould infections are predominantly caused by Aspergillus spp. The majority of infections (90%) are due to species from the A. fumigatus species complex, followed by species from the complexes A. flavus, A. niger, A. terreus [2–4]. The incidence of invasive pulmonary aspergillosis (IPA) varies depending on the patient group, geographical location, and also because, until recently [1], there was no consensus on the underlying diagnostic criteria. In addition to differentiate between colonisation and infection might be difficult when indirect methods such as antigen- or nucleic acid amplification tests are used and histology is not available [3]. The incidence of invasive pulmonary aspergillosis in critically ill patients in intensive care units is very likely underestimated, as suggested by results from retrospective autopsy studies, in which 2.8% had invasive aspergillosis, but only 40% of those cases had been identified ante mortem [5]. Significant differences also exist in the data on patient mortality, as IPA usually occurs in patients with pronounced disease severity and already significantly increased mortality risk [6]. In a retrospective cohort study with 1850 included patients in a medical intensive care unit in Leuven, Belgium, 6.9% of patients had microbiological or histopathological evidence of infection with Aspergillus spp. In this study the proportion of confirmed or probable IPA in patients without haematological cancer was 3.7%, in these the mortality rate was 90% [4].
Risk factors for invasive aspergillosis
In recent years, additional risk factors for IPA in critically ill patients [6] have been identified. In addition to the classic risk factors [7], like (prolonged) neutropenia (< 500/mm³), post allogeneic stem cell transplantation or presence of haematological or solid cancer, post organ transplantation and patients with congenital or acquired immunodeficiency (CGD, AIDS, HIV infection with neutropenia) [6, 7], these include, COPD [8], ARDS [9], mechanical ventilation, viral infections from influenza and SARS-CoV-2 [10, 11], liver cirrhosis [12, 13], prolonged steroid therapy. Table 1 provides an overview of the risk factors for invasive aspergillosis described in the literature. In the past, a number of algorithms and diagnostic criteria for IPA have been proposed. To harmonise further studies, recently an international consensus definition for IPA has been published [1].
Table 1.
Risk factors for invasive (pulmonary) aspergillosis
| Risk factors for IPA | Literature | |
|---|---|---|
| ICU specific criteria* |
Respiratory viral infection - Influenza - COVID-19 |
[1, 11, 70, 71] |
| AIDS, HIV infection with neutropenia | [1, 9, 71–73] | |
| Liver disease, especially advanced liver cirrhosis, acute on chronic liver failure | [1, 6, 13] | |
| Solid tumour | [1, 73] | |
| COPD | [1, 4, 6, 74] | |
| (Prolonged) neutropenia (< 500/mm³) | [7, 19] | |
| Haematological malignancy | [7, 12, 19] | |
| Allogeneic stem cell transplantation | [7, 12, 19] | |
| Prolonged treatment with corticosteroids ** | [7, 75] | |
|
Other immunosuppressive drugs - T-cell immunosuppressants, e.g. calcineurin inhibitors, TNF-alpha inhibitors, lymphocyte-specific monoclonal antibodies: Calcineurin inhibitors, TNF-alpha inhibitors, lymphocyte-specific monoclonal antibodies, immunosuppressive nucleoside analogues - B-cell immunosuppressants, e.g. ibrutinib |
[7, 19] | |
| Acute and chronic graft-versus-host reaction after allogeneic stem cell transplantation | [7, 19] | |
| Solid organ transplantation, especially lung transplantation | [7, 75–74] | |
| Congenital immunodeficiency | [7] | |
| ARDS | [6] | |
| Smoking | [6] | |
| Alcohol abuse | [6] | |
| Colonisation with Aspergillus spp. | [78, 79] | |
| Environmental exposure to moulds (construction activity, plant soil, food) | [19] | |
Diagnostics
Indication
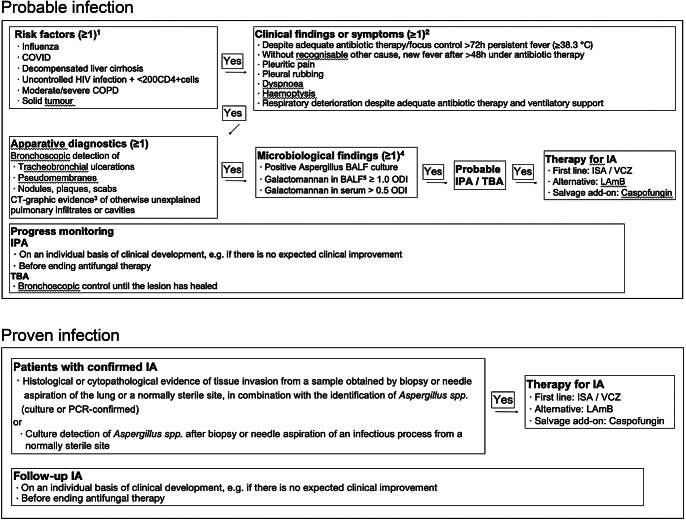
With the publication of the criteria for invasive fungal infections in ICU patients at the end of March 2024, a broad consensus on the (research) definition of IPA in critically ill patients in the ICU has been established [1]. In order to create robust evidence, the guideline, which is primarily intended to harmonise research questions, is very conservative and the recommendations are therefore very strict. In a clinical setting, deviations from these recommendations may be appropriate and should be made in exceptional cases, when justified. Taking these aspects into account, our proposed diagnostic (and therapeutic) algorithm is shown in Fig. 1.
Fig. 1.
Diagnostic and therapeutic algorithm for invasive (pulmonary) aspergillosis. 1 Classic risk factors (e.g. allogeneic stem cell transplantation) are covered by the established EORTC/MSG definition [7]. 2 oligosymptomatic course possible, especially in isolated TBA. 3 not in isolated TBA. 4 in isolated TBA, galactomannan and culture from bronchoalveolar lavage may be negative, in which case diagnosis from tracheal aspiration/bronchial lavage is recommended. 5 BALF highly preferred over serum. IPA invasive aspergillosis, BALF bronchoalveolar lavage fluid, COPD chronic obstructive pulmonary disease, COVID-19 coronavirus disease 2019, CT computed tomography, HIV human immunodeficiency virus, ICU intensive care unit, IPA invasive pulmonary aspergillosis, ODI optical density index, TBA tracheobronchial aspergillosis, ISA isavuconazole, VCZ voriconazole, LAmB liposomal amphotericin B. Due to the lack of standardization, the consensus recommendation [1] does not yet generally favor NAT for diagnostics. However, in experienced centers, it can be helpful in identifying species and resistance markers.
Radiological imaging
Recommendations for the radiological diagnosis of fungal diseases were recently published [14, 15]. Radiological imaging provides a major contribution to the differential diagnosis of the causes of respiratory deterioration in intensive care patients. Bedside techniques like ultrasound or supine chest radiography do not provide sufficient information on early detection, characterisation, or monitoring of fungal infections. The following techniques are recommended for suspected IPA with or without extrapulmonary manifestations. If haematogenous spread is suspected, further imaging of the extra-thoracic regions may be necessary, for which recommendations are given below.
Lungs:
Native thin-slice CT, if possible, using low-dose technology. An angio-CT to investigate possible vascular invasion is recommended in case of haemoptysis. The characterisation of the infiltrates allows for a certain classification of the underlying causes, yet cannot replace microbiological diagnostics. Monitoring with low-dose CT is recommended if new symptoms develop, a failure to achieve the expected improvement or a deterioration of the clinical status is observed.
Brain:
The recommended examination modality is MRI with contrast medium. CT should only be performed in an acute situation, in particular to search for haemorrhage. If the CT remains unremarkable, MRI should be added, if the clinical situation allows it and no contraindications exist.
Paranasal sinuses:
Invasive aspergillosis of the paranasal sinuses may be oligosymptomatic, and clinical examination in intensive care patients is difficult. Therefore, either CT or MRI should be performed when in doubt. The MRI however, is more sensitive for visualisation of invasion of fungal mass into the orbits or brain. Furthermore, repeated CT scans of the eye can trigger cataract particularly in young people [16, 17].
Abdomen:
In ICU patients, the potentially greater insight gained from MRI of the abdomen is limited by the impaired adherence to repeated breath-holding. Therefore, multiphasic contrast-enhanced CT is recommended for ICU patients.
Transcutaneous CT-guided biopsy.
In view of the critical condition of ICU patients, a biopsy should be performed after careful risk-benefit assessment. Nonetheless, transcutaneous CT-guided biopsy is a reliable method for confirming the diagnosis, which might be preferable to empirical therapy if long-term therapy and possible secondary prophylaxis are required.
Follow-up.
It is recommended to repeat the imaging to monitor the response to therapy in the further course on an individual basis of the clinical development [16–19]. Especially before stopping therapy, a follow-up imaging is recommended.
Bronchoscopy
The list of differential diagnosis to pathogen-induced deterioration of lung function in ICU patients is extensive (secretions, pulmonary oedema, bleeding, etc.). In addition to its diagnostic role, bronchoscopy is often also of therapeutic importance in the ICU setting. The risk of respiratory deterioration during a bronchoscopy is generally low [20], which is why invasive diagnostics should be performed at a low threshold, especially in intubated patients. In the case of tracheobronchial aspergillosis, bronchoscopy offers the advantage of direct visualisation and the possibility of targeted biopsies. Microbiological cultures, PCR and serological tests should be carried out on the broncho-alveolar lavage fluid (BALF) obtained in regions suspected of being IPA in CT.
Culture
A positive BALF culture for Aspergillus spp. is associated with IPA in up to 50% of patients in the ICU [21], in addition to this, culture allows for species identification and phenotypic resistance testing to identify azole resistant isolates. However, culture alone has a low sensitivity of 20–50% [22] and can not differentiate between colonisation and infection.
Serology
ELISA based testing for galactomannan from serum demonstrated a reasonable test performance in neutropenic patients [23]. In contrast to this in non-neutropenic patients the infection is usually not angio-invasive, and the test has a much lower sensitivity [24, 25]. This explains why galactomannan testing in non-neutropenic ICU patients should only be performed from respiratory materials [26]. Using a 1.0 ODI cut-off a multicentre study in ICU patients could demonstrate a sensitivity of 80% and a specificity of 97% [27]. Lateral flow assays for bedside testing have also been evaluated in ICU with similar performance measures [28].
The testing of beta-D-glucan (BDG) in the BALF as a panfungal biomarker for the diagnosis of IPA in the intensive care unit is not recommended, due to low specificity and false positive test results [29, 30].
Nucleic acid amplification technique (NAT)
Molecular detection methods for the detection of Aspergillus spp. have been described from blood (also plasma, serum) and from deep airway materials, but the evidence for the benefit in critically ill patients in intensive care units is currently insufficient [1]. In addition, NAT does not differentiate between colonisation and infection. However, in combination with sequencing, it can be used for the targeted detection of azole resistance-associated mutations (RAMs) in the cyp51A gene. Studies on NAT from BALF have shown limited sensitivity and specificity, even in high-risk populations. However, this increased significantly when the PCR was combined with detection of the vitality of the fungal spores (galactomannan test) [31, 32]. In a systematic review regarding immunocompromised patients, molecular detection methods from blood showed a sensitivity and specificity of 79.2% and of 79.6%, respectively, for a single positive test result and 59.6% and 95.1% for two consecutive positive test results [33].
The Supplement Table 1 provides an overview of the various microbiological tests, their strengths and weaknesses.
Treatment
Systemic therapy
The recommended first-line treatment for possible, probable and proven invasive pulmonary aspergillosis is either voriconazole (VCZ) or isavuconazole (ISA) intravenously [19, 34]. The reason for this is the landmark study for VCZ from 2002, which established VCZ as the standard medication [35]. However, VCZ can have disadvantages in patients treated in ICU, so that the use of ISA, as the alternative first-line treatment option, may be justified. With inhibition of various cytochrome P450 (CYP) enzymes, such as CYP2C19, CYP2C9 and CYP3A4, VCZ is a drug most frequently associated with major drug-drug interactions (DDI) in the ICU [36]. The main side effect of both, VCZ and ISA, is an increase in transaminases. Phototoxicity and neurological side effects as well as QTc prolongation have also been described with VCZ, whereas QTc shortening is reported for ISA. Limited data are available for ISA for intensive care use outside the treatment of haematology patients, however ISA has a more favourable pharmacokinetic profile compared to VCZ and is associated with fewer toxicities [33]. Therefore, ISA can be considered an attractive alternative first-line treatment; however, it is important to remember that ISA itself is metabolised via CYP3A4 and is therefore not entirely free from DDIs, although those are generally less pronounced compared to VCZ.
Liposomal amphotericin B (LAmB) is an alternative option for the treatment of IPA in the intensive care unit [17, 19]. However, LAmB is nephrotoxic and can therefore lead to a deterioration in renal function (which is usually reversible), particularly in patients who already have acute kidney injury. LAmB should also be used empirically as initial therapy in cases of suspected azole resistance due to local epidemiology and considered when relevant DDIs with azoles are expected [37]. Possible alternative second-line options could be posaconazole (PCZ) or the echinocandins. Echinocandins should not be used as first-line monotherapy. However, if no other options are available, they can also be used as add-on salvage therapy in combination with azole antifungals [38]. Our proposed diagnostic (and therapeutic) algorithm is shown in Fig. 1.
Inhalation therapy
Inhalation of antifungal drugs is generally not recommended. It may be used off-label in individual cases but is technically very complex, especially in intubated patients. Best evidence exists for lung transplant patients with infection of the anastomosis [39]. For details, we refer to the information on antimicrobial inhalation therapy from the German Respiratory Society [40].
Surgical therapy
With the exception of rare individual cases, the use of surgical therapy is limited in the intensive care setting.
Drug-Drug interactions
Due to the inevitable polypharmacy in the intensive care setting, DDIs are frequent and affect approximately 30% of patients treated with mould-active antifungals [41].
These interactions involve alterations in metabolism and clearance, most frequently due to CYP-inhibition by azoles; resulting in risks for undesirable side effects, mitigation and amplification of other co-prescribed agents. However, in intensive care medicine, an individualised and interdisciplinary assessment of DDI is essential. This evaluation should consider clinical context, available monitoring options, potential risks and the efficacy of alternative therapies [42]. The use of DDI databases [42] as screening tools, in particular for azole therapies, is beneficial as they offer general recommendations on dosage adjustments and DDI management [43]. Potential DDI and their effects on the metabolism of agents commonly prescribed in the ICU setting are summarized in Table 2. However, these should not be regarded as comprehensive and can only serve as a guiding reference.
Table 2.
Extract of important drug-drug interactions for mould-active Azole agents
| Drug substance Class | Drug substance examples | Interaction |
|---|---|---|
| Anti-infectives | Clarithromycin | ↓ Metabolism, ↑ Exposure and ↑ Effect of the macrolide (e.g. QTc prolongation) |
| Rifampicin, rifabutin | ↑ Azole metabolism, ↓ Azole exposure and ↓ Efficacy of azole | |
| Chemotherapeutics | Cyclophosphamide, ifosfamide, protein kinase inhibitors | ↓ Metabolism/efflux, ↑ Effects of chemotherapeutic agent (e.g. toxicity) |
| Immunosuppressants | Calcineurin/mTOR inhibitors | ↓ Metabolism/efflux, ↑ Exposure and ↑ Effects of immunosuppressants |
| Sedatives | Benzodiazepines | ↓ Metabolism, ↑ Exposure and ↑ Effects of benzodiazepine |
| Analgesics | Opioids | ↓ Metabolism, ↑ Exposure and ↑ Effects of opioid |
| Anticonvulsants | Phenytoin, carbamazepine | ↑ Azole metabolism, ↓ Azole exposure and ↓ Efficacy of azole |
| Neuroleptics | Quetiapine, haloperidol | ↓ Metabolism, ↑ Exposure and ↑ Effects of neuroleptics (e.g. QTc prolongation) |
| Cardiac | Amiodarone, Ca2+ channel blockers, digoxin, ivabradine | ↓ Metabolism, ↑ Exposure and ↑ Effects of cardiac drugs (e.g. QTc prolongation) |
| HIV therapeutics | Ritonavir, efavirenz | Complex interaction (inhibitor/substrate), possibly ↓ Efficacy of the azole |
| Oral anticoagulants | Oral anti-Xa inhibitors, phenprocoumon | ↓ Metabolism, ↑ Exposure and ↑ Effects of anticoagulants |
Therapeutic drug monitoring (TDM)
Voriconazole
VCZ shows pronounced intra- and inter-individual pharmacokinetic variability due to its non-linear elimination. In addition to DDI, polymorphisms of the CYP2C19 enzyme can also contribute to fluctuations in VCZ concentrations [44]. Several studies have demonstrated a correlation between VCZ serum concentration and efficacy. Thus, based on systematic reviews and a recent meta-analysis, a therapeutic target range of 1-5.5 mg/l is currently being recommended [45]. For patients with severe infections (e.g. multifocal or disseminated), central nervous system (CNS) involvement, and infections with pathogens with an elevated MIC, an increased target range of 2–6 mg/l is recommended [34, 46–48]. Supra-therapeutic VCZ concentrations have been associated with neuro- and hepatotoxicity [49], and a meta-analysis identified VCZ levels > 6 mg/l as strong predictors for toxicity [45]. VCZ concentrations should ideally be monitored within the first 5 days of therapy, with repeats four days after dose adjustments.
Isavuconazole
ISA TDM is currently not recommended in routine practice due to its dose-proportional pharmacokinetics, moderate inter-patient variability, and lack of defined efficacy or toxicity thresholds [50]. In the SECURE trial, fewer than 3% of patients had ISA levels outside the 1–7 mg/L range [51] and Desai et al. found no significant link between ISA levels and mortality or treatment response. However, TDM may be useful in specific ICU populations at risk for altered potentially sub-therapeutic drug exposure, such as those on renal replacement therapy, extracorporeal membrane oxygenation, or with high BMI [52, 53]. Due to its long half-life, TDM should be performed after 5–7 days. While > 1 mg/L is generally recommended (as > 90% of patients in SECURE achieved this), some experts suggest aiming for > 2 mg/L based on pharmacokinetic/dynamic models [54]. Toxicity has been shown to occur at levels > 4.8 mg/L [55, 56]. However, data from the SECURE trial and a study including intensive care patients, did not establish a clear correlation between ISA exposure and adverse reaction rates, highlighting the conflicting evidence [57].
Posaconazole
In retrospective analyses of patients who received PCZ for prophylaxis or IPA therapy, breakthrough infections and a reduced response to therapy occurred more frequently in patients with low PCZ concentrations [58]. Furthermore, in a prospective study, PCZ TDM had a positive effect on the reduction of breakthrough infections [59].
For the treatment of IPA, achieving a mean PCZ concentration of 1.25 mg/l resulted in an improved response rate in patients receiving PCZ for salvage therapy [60]. In ICU patients, up to 35% of the measured PCZ concentrations were below the targeted range for IPA therapy [61]. Of note, PCZ concentrations in bronchoalveolar fluid can reach levels up to 40 times higher than in plasma [62]. This observation might explain why some studies failed to demonstrate a correlation between low PCZ plasma concentrations and treatment failure, as pulmonary drug penetration may still be sufficient despite low systemic levels. Yet, available guidelines currently recommend maintaining trough levels > 1 mg/l for the treatment of IPA [63]. To avoid toxicity, including hepatotoxicity and development of pseudo hypoaldosteronism, an upper limit of 2 or 4 mg/l is being discussed [51, 63, 64]. If TDM is performed, it is recommended on days 5–7 of therapy, although further monitoring may be required depending on the results and clinical context [63].
Liposomal amphotericin B
Besides preclinical PK/PD-studies [65–67], clear data on target levels for LAmB in ICU patients with IPA do not exist and are further complicated by different LAmB kinetics in tissue und serum as well as accumulation in lung tissue [68, 69]. Therefore, TDM is currently not recommended.
Echinocandins
Since most of the available data is based on animal models of candidiasis rather than IPA, TDM for echinocandins is not currently recommended.
Conclusion
Invasive (pulmonary) aspergillosis in critically ill ICU patients presents a diagnostic and therapeutic challenge. If there is no improvement or if the respiratory status deteriorates further, the presence of IPA should be considered and appropriate diagnostic tests should be initiated. This should include a chest CT scan and a bronchoscopy with subsequent galactomannan testing and culture for moulds. In addition, TDM should be performed when appropriate for dose optimization.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
D.W. wrote the main manuscript text, all authors reviewed the manuscript and were involved in the preparation of the guideline whose summary is given here.
Funding
Open Access funding enabled and organized by Projekt DEAL.
The preparation of the guideline was financed exclusively with direct funds from the responsible professional association DGIIN and was limited to the costs of software for preparing the guideline and for the video conferences.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
D.W. has received consulting honoraria from Gilead Science GmbH; lecture honoraria from Gilead Science GmbH, Shionogi, Eumedica Pharmaceuticals AG, Pfizer Pharma GmbH, InfectoPharm Arzneimittel und Consilium GmbH, 3 M, Mundipharma GmbH, AstraZeneca GmbH and MSD Sharp & Dohme GmbH and research support from Shionogi. M.H. has received lecture honoraria from StreamedUp! GmbH, Shionogi, Medentech Ltd., Volumen, Mundipharma GmbH, AstraZeneca GmbH and research support from F2G Ltd., Gilead Science GmbH, Pfizer Pharma GmbH, Pulmocide Ltd., Mundipharma GmbH, IMMY and Melinta Therapeutics. P.K. has received consulting honoraria from Ambu GmbH, Gilead Science GmbH, Infill Healthcare Communications gmbH, Mundipharma GmbH, NOXXON N.V. and Pfizer Pharma GmbH; lecture honoraria from Akademie für Infektionsmedizin, Ambu GmbH, Bio-Rad Laboratories Inc., Datamed SA, European Confederation of Medical Mycology, Gilead Science GmbH, GPR Academy Rüsselsheim, HELIOS Kliniken, Jazz Pharmaceuticals Germany GmbH, medupdate GmbH, MedMedia GmbH, Pfizer Pharma GmbH, Scilink Comunicación Científica S.C., StreamedUp! GmbH, Universitätsklinikum der LMU München and research support from Gilead Science GmbH. C.K. has received consulting honoraria from Gilead Science GmbH and lecture honoraria from Shionogi, Pfizer Pharma GmbH, Gilead Science GmbH.F.L. has received lecture honoraria from Gilead Science GmbH, Shionogi, MSD Sharp & Dohme GmbH and Pfizer Pharma GmbH.S.M. has nothing to declare. R.S. has received consulting honoraria from Gilead Science GmbH; lecture honoraria from Schöchl Medical Education e.U., Biotest AG, Pfizer Pharma GmbH, Amomed Pharma, InfectoPharm Arzneimittel und Consilium GmbH, Shionogi, Tillotts Pharma GmbH, Mundipharma GmbH, HELIOS Kliniken, Sana Kliniken, DGI– Akademie für Infektionsmedizin, BDI-Veranstaltungsservice, Forum für Medizinische Fortbildung and research support from Biotest AG.M.W. has received consulting honoraria from B. Braun SE, Gilead Science GmbH, Boehringer Ingelheim International GmbH, MSD Sharp & Dohme GmbH, Shionogi, Eumedica Pharmaceuticals AG, Beckman Coulter Inc., Biotest AG, Sedana Medical AB, Swedish Orphan Biovitrum GmbH and Mundipharma GmbH; lecture honoraria from Pfizer Pharma GmbH, MSD Sharp & Dohme GmbH, Mundipharma GmbH and Gilead Science GmbH and research support from Dr. Franz Köhler Chemie GmbH.Ch.H. has received lecture honoraria from Gilead Science GmbH.O.K. has received consulting honoraria from Berufsgenossenschaft Rohstoffe und chemische Industrie and Laboratoire National de Santé Luxembourg; lecture honoraria from Pfizer Pharma GmbH, Gilead Science GmbH and FUJIFILM Wako Chemicals Europe GmbH and research support from FUJIFILM Wako Chemicals Europe GmbH, Virotech Diagnostics GmbH, Pfizer Pharma GmbH, MSD Sharp & Dohme GmbH and Basilea Pharmaceutica Ltd. Cl.H. has received consulting honoraria from Boehringer Ingelheim International GmbH; lecture honoraria from AstraZeneca GmbH, Pfizer Pharma GmbH and Boehringer Ingelheim; research support from Exscientia and holds shares in GlaxoSmithKline GmbH & Co. KG.M.K. holds shares in BioNTech SE.
References
- 1.Bassetti M, Giacobbe DR, Agvald-Ohman C, Akova M, Alastruey-Izquierdo A, Arikan-Akdagli S, et al. Invasive fungal diseases in adult patients in intensive care unit (FUNDICU): 2024 consensus definitions from ESGCIP, EFISG, ESICM, ECMM, MSGERC, ISAC, and ISHAM. Intensive Care Med. 2024. 10.1007/s00134-024-07341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lass-Florl C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses. 2009;52(3):197–205. 10.1111/j.1439-0507.2009.01691.x. [DOI] [PubMed] [Google Scholar]
- 3.Lass-Florl C, Steixner S. The changing epidemiology of fungal infections. Mol Aspects Med. 2023;94:101215. 10.1016/j.mam.2023.101215. [DOI] [PubMed] [Google Scholar]
- 4.Meersseman W, Vandecasteele SJ, Wilmer A, Verbeken E, Peetermans WE, Van Wijngaerden E. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170(6):621–5. 10.1164/rccm.200401-093OC. [DOI] [PubMed] [Google Scholar]
- 5.Jenks JD, Nam HH, Hoenigl M. Invasive aspergillosis in critically ill patients: review of definitions and diagnostic approaches. Mycoses. 2021;64(9):1002–14. 10.1111/myc.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taccone FS, Van den Abeele AM, Bulpa P, Misset B, Meersseman W, Cardoso T, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7. 10.1186/s13054-014-0722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of Cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71(6):1367–76. 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186(1):56–64. 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 9.Baddley JW, Stephens JM, Ji X, Gao X, Schlamm HT, Tarallo M. Aspergillosis in intensive care unit (ICU) patients: epidemiology and economic outcomes. BMC Infect Dis. 2013;13:29. 10.1186/1471-2334-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schauwvlieghe A, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–92. 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 11.Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149–62. 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluge S, Strauss R, Kochanek M, Weigand MA, Rohde H, Lahmer T, Aspergillosis. Emerging risk groups in critically ill patients. Med Mycol. 2021;60(1). 10.1093/mmy/myab064. [DOI] [PubMed]
- 13.Lahmer T, Pecanha-Pietrobom PM, Schmid RM, Colombo AL. Invasive fungal infections in acute and chronic liver impairment: A systematic review. Mycoses. 2022;65(2):140–51. 10.1111/myc.13403. [DOI] [PubMed] [Google Scholar]
- 14.Alexander BD, Lamoth F, Heussel CP, Prokop CS, Desai SR, Morrissey CO, et al. Guidance on imaging for invasive pulmonary aspergillosis and mucormycosis: from the imaging working group for the revision and update of the consensus definitions of fungal disease from the EORTC/MSGERC. Clin Infect Dis. 2021;72(Suppl 2):S79–88. 10.1093/cid/ciaa1855. [DOI] [PubMed] [Google Scholar]
- 15.Rademacher J, Ewig S, Grabein B, Nachtigall I, Abele-Horn M, Deja M, et al. Key summary of German National guideline for adult patients with nosocomial pneumonia- update 2024 funding number at the federal joint committee (G-BA): 01VSF22007. Infection. 2024. 10.1007/s15010-024-02358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aghai L, Heller H, Müller-Neumann M. Jahresbericht 2009 der Strahlenschutzkommission. 2010.
- 17.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1–38. 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Cornely OA, Koehler P, Arenz D, EQUAL Aspergillosis Score SCM. 2018: An ECMM score derived from current guidelines to measure QUALity of the clinical management of invasive pulmonary aspergillosis. Mycoses. 2018;61(11):833-6. 10.1111/myc.12820 [DOI] [PubMed]
- 19.Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63(4):e1–60. 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed]
- 20.Klein U, Karzai W, Zimmermann P, Hannemann U, Koschel U, Brunner JX, et al. Changes in pulmonary mechanics after fiberoptic Bronchoalveolar lavage in mechanically ventilated patients. Intensive Care Med. 1998;24(12):1289–93. 10.1007/s001340050764. [DOI] [PubMed] [Google Scholar]
- 21.van de Veerdonk FL, Kolwijck E, Lestrade PP, Hodiamont CJ, Rijnders BJ, van Paassen J, et al. Influenza-Associated aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2017;196(4):524–7. 10.1164/rccm.201612-2540LE. [DOI] [PubMed] [Google Scholar]
- 22.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387(10020):760–9. 10.1016/s0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 23.Leeflang MM, Debets-Ossenkopp YJ, Wang J, Visser CE, Scholten RJ, Hooft L, et al. Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev. 2015;2015(12):CD007394. 10.1002/14651858.CD007394.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Autier B, Prattes J, White PL, Valerio M, Machado M, Price J, et al. Aspergillus lateral flow assay with digital reader for the diagnosis of COVID-19-Associated pulmonary aspergillosis (CAPA): a multicenter study. J Clin Microbiol. 2022;60(1):e0168921. 10.1128/JCM.01689-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eigl S, Hoenigl M, Spiess B, Heldt S, Prattes J, Neumeister P, et al. Galactomannan testing and Aspergillus PCR in same-day Bronchoalveolar lavage and blood samples for diagnosis of invasive aspergillosis. Med Mycol. 2017;55(5):528–34. 10.1093/mmy/myw102. [DOI] [PubMed] [Google Scholar]
- 26.Heldt S, Prattes J, Eigl S, Spiess B, Flick H, Rabensteiner J, et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: performance of cytokines, asp LFD, and aspergillus PCR in same day blood and Bronchoalveolar lavage samples. J Infect. 2018;77(3):235–41. 10.1016/j.jinf.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Heer K, Gerritsen MG, Visser CE, Leeflang MM. Galactomannan detection in broncho-alveolar lavage fluid for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev. 2019;5(5):CD012399. 10.1002/14651858.CD012399.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercier T, Dunbar A, Veldhuizen V, Holtappels M, Schauwvlieghe A, Maertens J, et al. Point of care Aspergillus testing in intensive care patients. Crit Care. 2020;24(1):642. 10.1186/s13054-020-03367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M, Horvath A, Pruller F, Fickert P, Finkelman M, Kriegl L, et al. Fungal translocation measured by serum 1,3-ss-D-glucan correlates with severity and outcome of liver cirrhosis-A pilot study. Liver Int. 2023;43(9):1975–83. 10.1111/liv.15648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamoth F, Akan H, Andes D, Cruciani M, Marchetti O, Ostrosky-Zeichner L, et al. Assessment of the role of 1,3-beta-d-Glucan testing for the diagnosis of invasive fungal infections in adults. Clin Infect Dis. 2021;72(Suppl 2):S102–8. 10.1093/cid/ciaa1943. [DOI] [PubMed] [Google Scholar]
- 31.Mikulska M, Furfaro E, De Carolis E, Drago E, Pulzato I, Borghesi ML, et al. Use of Aspergillus fumigatus real-time PCR in Bronchoalveolar lavage samples (BAL) for diagnosis of invasive aspergillosis, including azole-resistant cases, in high risk haematology patients: the need for a combined use with Galactomannan. Med Mycol. 2019;57(8):987–96. 10.1093/mmy/myz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wehrle-Wieland E, Affolter K, Goldenberger D, Tschudin Sutter S, Halter J, Passweg J, et al. Diagnosis of invasive mold diseases in patients with hematological malignancies using Aspergillus, mucorales, and panfungal PCR in BAL. Transpl Infect Dis. 2018;20(5):e12953. 10.1111/tid.12953. [DOI] [PubMed] [Google Scholar]
- 33.Cruciani M, Mengoli C, Barnes R, Donnelly JP, Loeffler J, Jones BL, et al. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst Rev. 2019;9(9):CD009551. 10.1002/14651858.CD009551.pub4. [DOI] [PubMed] [Google Scholar]
- 34.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24:e1–38. 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–15. 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 36.Baniasadi S, Farzanegan B, Alehashem M. Important drug classes associated with potential drug-drug interactions in critically ill patients: highlights for cardiothoracic intensivists. Ann Intensive Care. 2015;5(1):44. 10.1186/s13613-015-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis. 2007;44(10):1289–97. 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 38.Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81–9. 10.7326/m13-2508. [DOI] [PubMed] [Google Scholar]
- 39.Monforte V, Roman A, Gavalda J, Bravo C, Tenorio L, Ferrer A, et al. Nebulized amphotericin B prophylaxis for Aspergillus infection in lung transplantation: study of risk factors. J Heart Lung Transpl. 2001;20(12):1274–81. 10.1016/s1053-2498(01)00364-3. [DOI] [PubMed] [Google Scholar]
- 40.e.v. DGfPuB. S3_Leitlinie Epidemiologie, Diagnostik und Therapie erwachsener Patienten mit nosokomialer Pneumonie. (2024). https://register.awmf.org/assets/guidelines/020-013l_S3_Epidemiologie-Diagnostik-Therapie-erwachsener-Patienten-nosokomiale-Pneumonie__2024-03.pdf. Accessed. [DOI] [PubMed]
- 41.Koeck JA, Hilgarth H, von Ameln-Mayerhofer A, Meyn D, Warlich R, Münstedt A, et al. Clinically relevant interactions with Anti-Infectives on intensive care Units-A multicenter Delphi study. Antibiot (Basel). 2021;10(11). 10.3390/antibiotics10111330. [DOI] [PMC free article] [PubMed]
- 42.Bakker T, Klopotowska JE, Dongelmans DA, Eslami S, Vermeijden WJ, Hendriks S, et al. The effect of computerised decision support alerts tailored to intensive care on the administration of high-risk drug combinations, and their monitoring: a cluster randomised stepped-wedge trial. Lancet. 2024;403(10425):439–49. 10.1016/s0140-6736(23)02465-0. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Ma D, Chen M, Hu Y, Chen X, Chen J, et al. Prevalence and clinical significance of potential drug-drug interactions among lung transplant patients. Front Pharmacol. 2024;15:1308260. 10.3389/fphar.2024.1308260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geist MJ, Egerer G, Burhenne J, Riedel KD, Mikus G. Induction of voriconazole metabolism by Rifampin in a patient with acute myeloid leukemia: importance of interdisciplinary communication to prevent treatment errors with complex medications. Antimicrob Agents Chemother. 2007;51(9):3455–6. 10.1128/aac.00579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Husain S, Sole A, Alexander BD, Aslam S, Avery R, Benden C, et al. The 2015 international society for heart and lung transplantation guidelines for the management of fungal infections in mechanical circulatory support and cardiothoracic organ transplant recipients: executive summary. J Heart Lung Transpl. 2016;35(3):261–82. 10.1016/j.healun.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Ueda K, Nannya Y, Kumano K, Hangaishi A, Takahashi T, Imai Y, et al. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol. 2009;89(5):592–9. 10.1007/s12185-009-0296-3. [DOI] [PubMed] [Google Scholar]
- 47.Siopi M, Mavridou E, Mouton JW, Verweij PE, Zerva L, Meletiadis J. Susceptibility breakpoints and target values for therapeutic drug monitoring of voriconazole and Aspergillus fumigatus in an in vitro Pharmacokinetic/pharmacodynamic model. J Antimicrob Chemother. 2014;69(6):1611–9. 10.1093/jac/dku023. [DOI] [PubMed] [Google Scholar]
- 48.Troke PF, Hockey HP, Hope WW. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob Agents Chemother. 2011;55(10):4782–8. 10.1128/aac.01083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsumoto K, Abematsu K, Shigemi A, Kanazawa N, Watanabe E, Yokoyama Y, et al. Therapeutic drug monitoring of voriconazole in Japanese patients: analysis based on clinical practice data. J Chemother. 2016;28(3):198–202. 10.1179/1973947815Y.0000000057. [DOI] [PubMed] [Google Scholar]
- 50.Andes D, Kovanda L, Desai A, Kitt T, Zhao M, Walsh TJ. Isavuconazole concentration in Real-World practice: consistency with results from clinical trials. Antimicrob Agents Chemother. 2018;62(7). 10.1128/AAC.00585-18. [DOI] [PMC free article] [PubMed]
- 51.Kaindl T, Andes D, Engelhardt M, Saulay M, Larger P, Groll AH. Variability and exposure-response relationships of isavuconazole plasma concentrations in the phase 3 SECURE trial of patients with invasive mould diseases. J Antimicrob Chemother. 2019;74(3):761–7. 10.1093/jac/dky463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertram R, Naumann HT, Bartsch V, Hitzl W, Kinzig M, Haarmeyer GS, et al. Clinical and demographic factors affecting trough levels of isavuconazole in critically ill patients with or without COVID-19. Mycoses. 2023;66(12):1071–8. 10.1111/myc.13653. [DOI] [PubMed] [Google Scholar]
- 53.Höhl R, Bertram R, Kinzig M, Haarmeyer GS, Baumgärtel M, Geise A, et al. Isavuconazole therapeutic drug monitoring in critically ill ICU patients: A monocentric retrospective analysis. Mycoses. 2022;65(7):747–52. 10.1111/myc.13469. [DOI] [PubMed] [Google Scholar]
- 54.Willeman T, Tonini J, Garnaud C, Bailly S, Gandia P, Stanke-Labesque F, et al. Refining the therapeutic range of posaconazole and isavuconazole for efficient therapeutic drug monitoring using a bioassay approach. Fundam Clin Pharmacol. 2020;34(2):279–87. 10.1111/fcp.12507. [DOI] [PubMed] [Google Scholar]
- 55.Furfaro E, Signori A, Di Grazia C, Dominietto A, Raiola AM, Aquino S, et al. Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. J Antimicrob Chemother. 2019;74(8):2341–6. 10.1093/jac/dkz188. [DOI] [PubMed] [Google Scholar]
- 56.Gomez-Lopez A, Sanchez Galiano S, Ortega Madueño S, Carballo Gonzalez C. Observed isavuconazole exposure: 5-year experience of Azole TDM from a Spanish reference laboratory. Med Mycol. 2023;61(8). 10.1093/mmy/myad086. [DOI] [PubMed]
- 57.Zurl C, Waller M, Schwameis F, Muhr T, Bauer N, Zollner-Schwetz I, et al. Isavuconazole treatment in a mixed patient cohort with invasive fungal infections: outcome, tolerability and clinical implications of isavuconazole plasma concentrations. J Fungi (Basel). 2020;6(2):90. 10.3390/jof6020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dolton MJ, Brüggemann RJ, Burger DM, McLachlan AJ. Understanding variability in posaconazole exposure using an integrated population Pharmacokinetic analysis. Antimicrob Agents Chemother. 2014;58(11):6879–85. 10.1128/aac.03777-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boppana M, Sengar M, Jain H, Gurjar M, Ambotkar M, Gota V, et al. A prospective study to evaluate the effect of therapeutic drug Monitoring-Based posaconazole prophylaxis on invasive fungal infection rate during acute myeloid leukemia induction therapy. Indian J Hematol Blood Transfus. 2024;40(2):204–12. 10.1007/s12288-023-01709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44(1):2–12. 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 61.Konig C, Gopfert M, Kluge S, Wichmann D. Posaconazole exposure in critically ill ICU patients: a need for action. Infection. 2023. 10.1007/s15010-023-02078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conte JE, Golden JA, Krishna G, McIver M, Little E, Zurlinden E. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob Agents Chemother. 2009;53(2):703–7. 10.1128/aac.00663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chau MM, Daveson K, Alffenaar JC, Gwee A, Ho SA, Marriott DJE, et al. Consensus guidelines for optimising antifungal drug delivery and monitoring to avoid toxicity and improve outcomes in patients with haematological malignancy and Haemopoietic stem cell transplant recipients, 2021. Intern Med J. 2021;51(Suppl 7):37–66. 10.1111/imj.15587. [DOI] [PubMed] [Google Scholar]
- 64.Andes D, Safdar N, Marchillo K, Conklin R. Pharmacokinetic-pharmacodynamic comparison of amphotericin B (AMB) and two lipid-associated AMB preparations, liposomal AMB and AMB lipid complex, in murine candidiasis models. Antimicrob Agents Chemother. 2006;50(2):674–84. 10.1128/aac.50.2.674-684.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takemoto K, Kanazawa K. AmBisome: relationship between the Pharmacokinetic characteristics acquired by liposomal formulation and safety/efficacy. J Liposome Res. 2017;27(3):186–94. 10.1080/08982104.2016.1205087. [DOI] [PubMed] [Google Scholar]
- 66.Hope WW, Kruhlak MJ, Lyman CA, Petraitiene R, Petraitis V, Francesconi A, et al. Pathogenesis of Aspergillus fumigatus and the kinetics of Galactomannan in an in vitro model of early invasive pulmonary aspergillosis: implications for antifungal therapy. J Infect Dis. 2007;195(3):455–66. 10.1086/510535. [DOI] [PubMed] [Google Scholar]
- 67.Siopi M, Mouton JW, Pournaras S, Meletiadis J. In vitro and in vivo Exposure-Effect relationship of liposomal amphotericin B against Aspergillus fumigatus. Antimicrob Agents Chemother. 2019;63(6). 10.1128/aac.02673-18. [DOI] [PMC free article] [PubMed]
- 68.Watanabe A, Matsumoto K, Igari H, Uesato M, Yoshida S, Nakamura Y, et al. Comparison between concentrations of amphotericin B in infected lung lesion and in uninfected lung tissue in a patient treated with liposomal amphotericin B (AmBisome). Int J Infect Dis. 2010;14:e220–3. 10.1016/j.ijid.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 69.Demartini G, L C, BmP P, F. S, and, Fraschini F. Penetration of amphotericin B in human lung tissue after single liposomal amphotericin B (AmBisome) infusion. J Chemother. 2005;17(1):82–5. 10.1179/joc.2005.17.1.82. [DOI] [PubMed] [Google Scholar]
- 70.Janssens I, Lambrecht BN, Van Braeckel E. Aspergillus and the lung. Semin Respir Crit Care Med. 2024;45(1):3–20. 10.1055/s-0043-1777259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wauters J, Baar I, Meersseman P, Meersseman W, Dams K, De Paep R, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med. 2012;38(11):1761–8. 10.1007/s00134-012-2673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller WT Jr., Sais GJ, Frank I, Gefter WB, Aronchick JM, Miller WT. Pulmonary aspergillosis in patients with AIDS. Clinical and radiographic correlations. Chest. 1994;105(1):37–44. 10.1378/chest.105.1.37. [DOI] [PubMed] [Google Scholar]
- 73.Sipsas NV, Kontoyiannis DP. Invasive fungal infections in patients with cancer in the intensive care unit. Int J Antimicrob Agents. 2012;39(6):464–71. 10.1016/j.ijantimicag.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in Cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405–21. 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewis RE, Kontoyiannis DP. Invasive aspergillosis in glucocorticoid-treated patients. Med Mycol. 2009;47(Suppl 1):S271–81. 10.1080/13693780802227159. [DOI] [PubMed] [Google Scholar]
- 76.Gavalda J, Len O, San Juan R, Aguado JM, Fortun J, Lumbreras C, et al. Risk factors for invasive aspergillosis in solid-organ transplant recipients: a case-control study. Clin Infect Dis. 2005;41(1):52–9. 10.1086/430602. [DOI] [PubMed] [Google Scholar]
- 77.Silveira FP, Husain S. Fungal infections in solid organ transplantation. Med Mycol. 2007;45(4):305–20. 10.1080/13693780701200372. [DOI] [PubMed] [Google Scholar]
- 78.Azoulay E, Afessa B. Diagnostic criteria for invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186(1):8–10. 10.1164/rccm.201204-0761ED. [DOI] [PubMed] [Google Scholar]
- 79.Jenks JD, Prattes J, Frank J, Spiess B, Mehta SR, Boch T, et al. Performance of the Bronchoalveolar lavage fluid Aspergillus Galactomannan lateral flow assay with cube reader for diagnosis of invasive pulmonary aspergillosis: A multicenter cohort study. Clin Infect Dis. 2021;73(7):e1737–44. 10.1093/cid/ciaa1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chastain DB, Spradlin M, Ahmad H, Henao-Martinez AF. Unintended consequences: risk of opportunistic infections associated with Long-term glucocorticoid therapies in adults. Clin Infect Dis. 2024;78(4):e37–56. 10.1093/cid/ciad474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.