Abstract
Little is known about how a cell's apoptotic threshold is controlled after exposure to chemotherapy, although the p53 tumor suppressor has been implicated. We identified executioner caspase-6 as a transcriptional target of p53. The mechanism involves DNA binding by p53 to the third intron of the caspase-6 gene and transactivation. A p53-dependent increase in procaspase-6 protein level allows for an increase in caspase-6 activity and caspase-6-specific Lamin A cleavage in response to Adriamycin exposure. Specific inhibition of caspase-6 blocks cell death in a manner that correlates with caspase-6 mRNA induction by p53 and enhances long-term survival in response to a p53-mediated apoptotic signal. Caspase-6 is an executioner caspase found directly regulated by p53, and the most downstream component of the death pathway controlled by p53. The induction of caspase-6 expression lowers the cell death threshold in response to apoptotic signals that activate caspase-6. Our results provide a potential mechanism of lowering the death threshold, which could be important for chemosensitization.
Keywords: chemosensitivity‖apoptosis‖transcription‖tumor suppressor‖lamin A
The susceptibility of cancer cells to killing by chemotherapy is determined in part by activation of a cell death program regulated by the p53 tumor suppressor protein (1, 2). p53 can transcriptionally activate a number of target genes involved in cell death signaling including proapoptotic members of the BclII family (bax, bak, noxa), Aip1, death receptors, and others such as puma, pidd, perp, and pigs (3–5). The susceptibility to killing by chemotherapeutic agents such as Adriamycin (doxorubicin) was recently reported to be reduced in certain tumors such as melanoma or neuroblastoma through silencing of apoptotic regulators APAF1 and caspase-8 (6, 7). Interestingly, recent studies have demonstrated that APAF1 is directly regulated by p53 (8, 9). Because proteins such as APAF1 do not cause death directly, but rather the increased APAF1 expression can lower the threshold of drug-induced cell death (7), the possibility exists that there are targets in cell death signaling that might affect apoptotic threshold and may modulate drug-induced cell death.
The caspase family began when a number of cysteine-directed proteases were concurrently being discovered by several groups investigating the final mediators of cell death (10). All related to the interleukin-1 converting enzyme, these proteases act in a cascade to rapidly degrade basic components of the cell such as lamins A&B, fodrin, Rb, and poly(ADP-ribose) polymerase (11–14). In the cascade, the enzymes are divided into two levels—initiator and effector caspases (15, 16). The initiator caspases are either at the level of death receptors, sensing activation (caspases 8 and 10), at the mitochondria, sensing changes in mitochondrial potential (caspases 2 and 9), or at the endoplasmic reticulum, possibly sensing abnormal protein folding (caspase 12). These initiators cleave and activate the effector caspases, caspases 3, 6, and 7, which then proceed to digest critical substrates essential for cell viability. However, each of these effectors are nonredundant, possessing a specific target sequence and substrate specificity.
We show here that at least one component of the sensitization of cells to stress by p53 is through induction of caspase-6 protein. Inhibition of the enzyme reduces the sensitivity conferred by overexpression of p53. These results identify a pathway by which p53 is able to accelerate the apoptosis cascade by loading the cell with cell death proteases so that when an apoptotic signal is received, programmed cell death occurs rapidly.
Materials and Methods
Western Blotting and Antibodies.
Immunoblotting was carried out by using mouse anti-human p53 monoclonal (PAb1801; Oncogene), rabbit anti-human caspase-3 (Cell Signaling, Beverly, MA), mouse anti-human caspase-6 (B93–4; PharMingen), mouse anti-human caspase-7 (B94–1; PharMingen), mouse anti-human caspase-8 (B9–2; PharMingen), rabbit anti-human caspase-9 (PharMingen), mouse anti-human actin (C-2; Santa Cruz Biotechnology), mouse anti-human p21WAF1 (EA10; Oncogene), rabbit anti-human poly(ADP-ribose) polymerase (Roche Molecular Diagnostics), and rabbit anti-Lamin A (Cell Signaling).
Northern Blotting.
Total RNA was harvested by using RNEasy columns from Qiagen (Valencia, CA). Caspase-6 Northerns were probed with a radioactive, full-length, sequence-verified PCR product (Primers: human, 5′-ATGAGCTCGGCCTCGGGG-3′ and 5′-TTAGATTTTGGAAAGAAA-3′; mouse, 5′-ATGACAGAAACCGATGGC-3′ and 5′-CTACTTGCTAGGTTTGGG-3′). Probes for p21WAF1 Northerns, electrophoresis, blotting, hybridization, and washing have been described (32).
Cell Culture.
Culture conditions for H460, H460-neo, H460–E6, HCT116, HCT16-neo, HCT16-E6, HCT16-P53−/−, DLD1, SKBR3, MCF7, SW480, U2OS, M3, and HCC1937 have been described (32–34). SW13 adrenal carcinoma cells were grown in Leibowitz's medium supplemented with 10% FBS and 100 mg/ml pennicilin/streptomycin and grown in low CO2 environment. Adriamycin was obtained through the University of Pennsylvania pharmacy and added to the medium of cells at a concentration of 100, 200, or 300 ng/ml for the indicated time points. UV irradiation was performed in a Stratalinker at 20 Jm−2. The benzyloxylcarbonyl-valine-glutamate-isoleucine-aspartate-fluoromethyl ketone (VEID–FMK) caspase-6 inhibitor (R & D Systems) was added to cultures where appropriate at a concentration of 20 μM.
Adenovirus Infection and Propagation.
The construction and propagation of LacZ- and p53-expressing adenoviruses have been described (32). Infection of cell lines were performed at the following multiplicities of infection (mois): 20 moi for H460 and HCT116; 40 moi for HCT116-neo, HCT116-E6, HCT116-p53−/− H460-neo, H460-E6, MCF7, and U2OS; and 50 moi for SW480, DLD1, HCC1937, and SKBR3. These mois allowed for >90% infectivity in each cell line.
Electrophoretic Mobility Shift Assay.
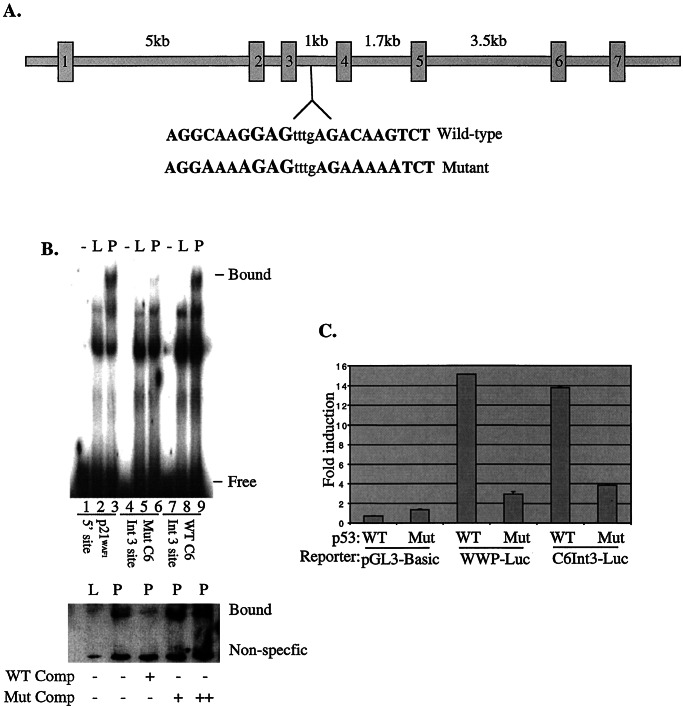
The genomic organization of caspase-6 was determined by searching the National Center for Biotechnology Information (NCBI) database. Consensus p53 DNA binding sites were found by searching the genomic sequence of caspase-6 using macvector. The intron 3 binding site was prepared by constructing complimentary oligonucleotides with the candidate binding site—AGGCAAGGAGtttgAGACAAGTAT—in the middle. A mutant version of this site was prepared by exchanging the conserved C and G in each consensus half site (a/g-a/g-a/g-C-a/t-a/t-G-t/c-t/c-t/c) to A. These were either radioactively labeled or labeled with biotin at the 5′ end. Biotinylated oligos were detected with streptavidin-linked horseradish peroxidase and the Pierce LightShift kit.
Luciferase Assay and Transfection.
A sequence verified 300-bp fragment of caspase-6 intron 3 containing the candidate p53 binding site was amplified from H460 genomic DNA and cloned into pGL3-Basic and pGL3-Promoter (Promega). These and vector alone or the p21WAF1 luciferase reporter were transfected into SW480 cells by using Superfect (Qiagen) at a concentration of 0.1 μg. This was cotransfected with 0.1–0.2 μg pCEP4, pCEP4-p53, or pCEP4-p53273mut and 0.1 μg cytomegalovirus–β-galactosidase for transfection efficiency. Luciferase activity was measured on a Fisher Luminometer.
Caspase-6 Activity Assay.
H460 cells were plated at 1 × 107 cells/T75 flask and treated as described. Cell lysates were prepared with lysis buffer provided by the manufacturer (R & D Systems). Cell lysate was incubated with a p-Nitroaniline (a colorimetric compound) conjugated VEID substrate specific for caspase-6 for 1 h. Reactions were then measured for substrate cleavage by absorbance at 405 nm.
Apoptosis Detection.
VEID–FMK or DMSO was added to cell media 3 h before treatment of cells with Ad-p53 and Adriamycin at a concentration of 20 μM. Cells were harvested 24 h after infection and treatment with adenovirus, stained with propidium iodide, and analyzed for DNA content by flow cytometry. Cells containing less than 2 N DNA content were labeled as apoptotic. For colony assays, cells were infected with Ad-p53 for 12 h, treated DMSO or 20 μM VEID–FMK for 1 h and then incubated with Adriamycin for 24 h. After 24 h, media was removed and replaced with fresh media without Adriamycin. Additional doses of VEID–FMK were added every 24 h for 14 days. Remaining cells were stained with Coomassie blue.
Results
Induction of Caspase-6 Expression by p53.
We investigated the possibility that p53 may transcriptionally regulate the expression of one or more caspases in a manner that may determine apoptotic threshold. The hypothesis was that increased production of any particular procaspase would not by itself lead to high levels of death unless there was a second signal to activate the caspase. In a screen evaluating the protein expression levels of various initiator and executioner death-inducing caspases (Fig. 1A), we found that caspase-6 is uniquely up-regulated in response to p53 overexpression. The induction of caspase-6 protein occurred in a time-dependent manner after p53 overexpression, in a manner similar to induction of the cyclin-dependent kinase inhibitor p21WAF1 (Fig. 1B). This induction appeared to take place at the transcriptional level as we observed a time-dependent up-regulation of caspase-6 mRNA after p53 overexpression (Fig. 1C). Induction of caspase-6 protein appeared to be more readily detectable than RNA in some cases, for example in SW13 cells, where cell death may be occurring and eliminating the transcript. p53 appeared to up-regulate caspase-6 mRNA expression in a variety of human cancer cell lines (Fig. 1D). Of all of the cell lines tested, MCF7 breast cancer cells failed to show an induction of caspase-6 mRNA, but showed robust p21WAF1 up-regulation, consistent with the idea that the exogenous p53 is competent in transcriptional activation of other known targets. The failure of p53 to up-regulate caspase-6 expression in MCF7 cells is of interest but the mechanism remains unknown.
Figure 1.
Induction of caspase-6 by p53. (A) H460 cells were infected with 20 moi of Ad-p53 and harvested for total protein at 6, 12, 18, and 24 h after infection. Lysates were electrophoresed on SDS/PAGE and immunoblotted with the appropriate antibodies. (B) SW13, H460, and SW480 cells were infected with 20, 10, and 50 moi Ad-p53, respectively, and harvested for total protein at 4, 6, 12, 18, and 24 h after infection. Total protein was immunoblotted for caspase-6 and p21WAF1. (C) SW13, H460, and SW480 cells were infected with 20, 10, and 50 moi Ad-p53, respectively, and harvested for total RNA at 4, 6, 12, 18 and 24 h after infection. Northern blotting was performed for caspase-6 and p21WAF1. (D) Nine cell lines were infected with the appropriate moi of Ad-p53 (as indicated with a “P”) (20 moi, H460, HCT116, SW13; 50 moi, DLD1, SKBR3, MCF7, SW480 U2OS, HCC1937) or a control Ad-LacZ adenovirus (indicated with a “L”) for 24 h and then harvested for total RNA. Northern blotting was performed for caspase-6 and p21WAF1.
Dependence on Wild-Type p53 on Caspase-6 Induction.
We tested the relationship between p53 and caspase-6 by using several systems that are commonly used to study p53 target gene activation. The results show that a murine lymphoma cell line with temperature sensitive p53 up-regulates caspase-6 mRNA at the wild-type p53 permissive temperature (Fig. 2A). A second system relied on the use of the human lung cancer cell line H460 and the colon cancer cell line HCT116, for which there are available isogenic clones that express human papillomavirus E6 to degrade endogenous p53. We used this system and found that caspase-6 mRNA or protein levels were increased after Adriamycin exposure in the neo cells but not in the E6 cells (Fig. 2 B and C). In addition to these systems, we also saw an increase in caspase-6 expression in HCT116 p53+/+ cells after Adriamycin treatment and a mild induction after etoposide. In the HCT116-p53−/− cells, this effect was not seen, indicating a dependence on p53. We also tested the ability of caspase-6 to be induced by DNA damage in a mutant p53 expressing line, SW480. Although H460 cells induced caspase-6 in response to Adriamycin, SW480 cells were deficient in this respect (Fig. 2D).
Figure 2.
Induction of caspase-6 depends on the presence of wild-type p53. (A) Mouse M3 cells were cultured at 37°C (mutant p53) or 32°C (wild-type p53) for 24 h. Total RNA was harvested, and Northern blotting was performed for caspase-6 mRNA expression. (B–D) H460-neo, H460-E6, HCT116-Neo, HCT116-E6, HCT116-p53+/+, HCT116-p53−/−, H460, or SW480 cells were cultured in the presence of the indicated amount of Adriamycin for 24 h, harvested for total RNA and protein, and analyzed for the presence of caspase-6 transcript or protein and p53 and p21WAF1 protein.
Binding of Caspase-6 Promoter Sequences by p53.
We searched the public database for potential p53 DNA-binding response elements located within the human caspase-6 genomic locus. We identified a candidate p53 binding site within the third intron of caspase-6, which contains an 85% match to the required consensus (a/g-a/g-a/g-C-a/t-a/t-G-t/c-t/c-t/c), including all conserved residues (Fig. 3A). We used a wild-type and a mutant version of the element, and observed a strong supershift with the wild-type element and p53 antibody (Fig. 3B, lane 9). The shift is comparable to a supershift observed by using the well-known upstream 5′-site from the p21WAF1 promoter (Fig. 3B, lane 3). We used a cold intron-3-derived oligo at a 100-fold excess concentration, we were able to compete away this binding, whereas the same effect was not seen at either 100- or 200-fold excess of a mutant oligo.
Figure 3.
p53 binds and activates transcription of caspase-6 through a intronic element. (A) The genomic structure of human caspase-6 consists of 7 exons spaced by 6 introns varying in size from 5 kb to 200 bp. Within intron 3 lies an 85% match to the consensus p53 binding site. Bases in bold do not align with the consensus p53 binding site. (B) Thirty-bp oligomers representing the p53 binding site located at the 5′ end of the p21WAF1 promoter (lanes 1–3), the caspase-6 intron 3 site (lanes 7–9), or a mutant intron 3 site (lanes 4–6) were labeled with 32P and incubated with nuclear extracts of Saos2 that had been infected with Ad-LacZ (L) or Ad-p53 (P) or incubated with no extract (−). Complexes were electrophoresed on a 4% TGE gel, dried and autoradiographed. The same oligomers were labeled with biotin and incubated with Ad-LacZ (L) or Ad-p53 (P) lysates and incubated in a 100 fold molar excess of unlabeled wild-type oligo (WT Oligo) or a 100-fold (+) or 200-fold (++) molar excess of unlabeled mutant oligo (Mut Oligo). Samples were electrophoresed on a 6% TBE gel, transferred to a nitrocellulose membrane and shifted oligo was detected with streptavidin-labeled horseradish peroxidase. (C) A 300-bp fragment of caspase-6 intron 3 was placed upstream of the luciferase gene in pGL-Basic and transfected (0.1 μg) into SW480 cells with either pCEP4-p53 (WT) or pCEP4-p53273mutant (Mut). Luciferase activity was determined 24 h later. Empty vector (PGL-Basic) or p21WAF1 promoter (pWWP-Luc) was used as controls.
Expanding on these observations, we generated a caspase-6 promoter–luciferase reporter that contained a 300-bp fragment of the intronic region that binds p53. This construct was responsive to wild-type p53 (Fig. 3C), but not to a tumor-derived mutant of p53 that is incapable of binding DNA. The induction was similar to that of the p21WAF1 promoter–reporter construct. These data indicate that the p53 DNA binding element within intron 3 of caspase-6 is capable of recruiting p53 to activate transcription.
Induction of Caspase-6 Activity by p53.
We further tested the hypothesis that p53-dependent caspase-6 regulation may lower apoptotic threshold after exposure to a chemotherapeutic drug. We found that either p53 overexpression or exposure to Adriamycin can induce some caspase-6 activity assessed by using a colorimetric assay for caspase-6 specific activity (Fig. 4A). However, the combination of Adriamycin and p53 induced significantly more caspase-6 activity (Fig. 4A). Because one mechanism for the increased caspase-6 activity when both p53 and Adriamycin are used could be higher levels of caspase-6 mRNA and protein, we attempted to exclude this possibility. Our results revealed that neither caspase-6 mRNA nor protein expression is higher with the combination of p53 plus Adriamycin as compared with p53 alone (Fig. 4B lane 4 compared with lane 2). Interestingly, however, most of the procaspase-6 protein has been processed, and therefore activated, in the cells that had been treated with both Ad-p53 and Adriamycin. These observations are consistent with the notion that p53 induces caspase-6 expression, but it is likely that other apoptotic pathways induced by Adriamycin stimulate the processing and activation of the latent caspase-6 leading to the observed sensitization by lowering the death threshold by allowing an amplified caspase-6 response. We used an endogenous caspase-6-specific substrate (Lamin A), and found that whereas Adriamycin alone produces low levels of cleaved Lamin A, and p53 alone produced no detectable cleaved Lamin A, and the combination of Adriamycin and p53 produced high levels of cleaved Lamin A (Fig. 4C). The high levels of cleaved Lamin A in cells overexpressing wild-type p53 and treated with Adriamycin cannot be explained simply by having more caspase-6, but rather by having more active caspase-6, i.e., higher caspase-6 activity.
Figure 4.
p53 induction of caspase-6 activity. (A) H460 cells were treated with 200 ng/ml Adriamycin, infected with 20 moi Ad-p53, left untreated or treated with both for 24 h and harvested for total protein. Lysates were incubated with a caspase-6 substrate peptide linked to p-Nitroaniline (VEID-pNA) and then analyzed for absorbance at 405 nm. (B and C) H460 cells were treated as described and harvested for total RNA or protein and immunoblotted for caspases-3 and -6, poly(ADP-ribose) polymerase, and Lamin A, or Northern blotting was performed for caspase-6. (C Lower) Cells were harvested at 0, 6, 12, and 24 h after treatment. Lanes 1–4 in B and C indicate the following treatments: 1, untreated; 2, 20 moi Ad-p53 infection; 3, 200 ng/ml Adriamycin treatment; 4, 20 moi Ad-p53/200 ng/ml Adriamycin.
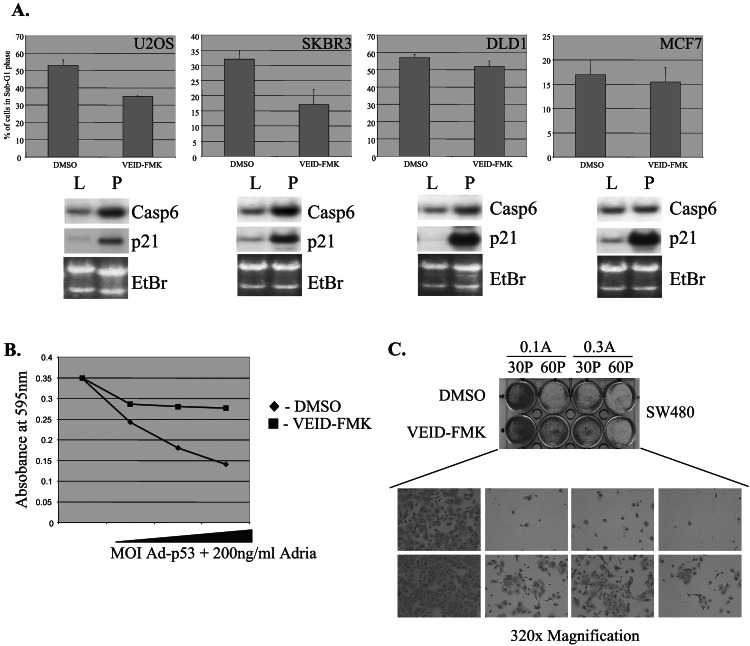
To investigate the potential importance of caspase-6 regulation by p53 to p53-mediated sensitization to Adriamycin-induced cell death, we used the specific peptide inhibitor of caspase-6, VEID–FMK. Caspase-6 is unique among the caspases in its target sequence—VEID. Although there exist caspase inhibitors that are cross-inhibitory with caspase-6 and other caspases, such as the tetra-peptide-aldehydes YVAD-CHO and DEVD-CHO (18–20), the VEID–FMK peptide has been found to be relatively specific to caspase-6. In a cell free system, a concentration of 100 nM of VEID–FMK is sufficient to inhibit caspase-6, whereas 100- to 1,000-fold higher concentrations are required to cross inhibit other caspases such as 7 and 10 (21). In tissue culture, higher concentrations of the inhibitor are needed to inhibit caspase-6 because of membrane permeability issues, while still remaining specific to caspase-6 (21). Here we used a concentration of 20 μM, an amount that has been described in several reports to specifically inhibit caspase-6 (21–23). We used four different human cancer cell lines and found that the degree to which the caspase-6-specific inhibitor blocks cell death correlated well with the degree to which caspase-6 mRNA was induced (Fig. 5A). Interestingly, MCF7 cells did not display increased caspase-6 expression in response to p53 (but did show high induction of p21). MCF7 cells were not sensitized to Adriamycin-induced killing by p53, and the observed death in these cells was not blocked by the caspase-6-specific inhibitor. These findings may reflect another genetic pathway that is altered in these cells; however, future studies are needed to determine whether this effect is caused by the lack of caspase-6 induction. We also observed that increasing doses of p53 in the presence of Adriamycin leads to progressive degrees of death which are blocked by the caspase-6 inhibitor (Fig. 5B). These results suggest that a potential mechanism by which p53 induces cell death is through induction of caspase-6 and the level to which caspase-6 contributes to the overall apoptosis is correlative with the amount to which p53 activates the caspase-6 gene.
Figure 5.
Caspase-6-dependent chemosensitization of cells by p53. (A) U2OS, SKBR3, DLD1, and MCF7 cells were infected with Ad-p53, treated with Adriamycin, and cultured in the presence of either VEID–FMK or DMSO as a negative control. After 24 h, cells were harvested and collected for FACS analysis. Cell death was scored by the percentage of cells containing less than 2 N DNA content. Parallel cultures infected with Ad-LacZ (L) or Ad-p53 (P) were harvested for total RNA and caspase-6 or p21WAF1 mRNA expression was analyzed by Northern blotting. (B) SW480 cells were plated in a 96-well dish at 5 × 104 cells per well and infected with increasing amounts of Ad-p53 (10, 20, 50, or 100 moi) in the presence of either VEID–FMK or DMSO as a negative control. After 24 h, cells were stained with the MTT dye (Sigma) and analyzed for absorbance at 595 nm. (C) SW480 cells were plated at 5 × 104 cells per well in a 12-well dish, infected with either 30 (30 P) or 60 (60 P) moi of Ad-p53 for 12 h, treated with 20 μM VEID–FMK or DMSO as control for 1 h, and then treated with either 100 ng/ml (0.1 A) or 300 ng/ml (0.3 A) Adriamycin. Twenty-four hours later, media was removed and then replaced with media without Adriamycin and fresh VEID–FMK/DMSO. Fresh caspase inhibitor was added every day for 14 days, after which the cells were stained with Coomassie blue.
Finally, to establish that caspase-6 is an important factor in arriving at the cell death endpoint induced by Ad-p53/Adriamycin treatment, we performed long-term survival assays of cells treated with the Ad-p53/Adriamycin combination with or without a caspase-6 inhibitor. After infection and treatment of SW480 cells with Ad-p53 and Adriamycin, respectively, cells were fed VEID–FMK or DMSO as a carrier control for 14 days. Cells were then stained for colony formation with Coomassie blue (Fig. 5C). Abrogation of caspase-6 activity with the specific inhibitor resulted in significantly more colony formation, and therefore more long-term survival, than with the DMSO control. Therefore, ample caspase-6 activity is required for p53 to fully chemosensitize cells to Adriamycin.
Discussion
Our results reveal that an executioner caspase can be directly regulated by p53. Recently, APAF1 has been found to be directly regulated by p53. It appears that p53 regulates not only proapoptotic members of the bcl2 family (bax, bak, noxa), Aip1, death receptors, and others such as puma, pidd, perp, and pigs, but also regulates very downstream components of the death pathway such as APAF1 and caspase-6. Although all of these contributors certainly affect the speed at which a cell will undergo apoptosis, none is of a general nature that would allow for hastened cell death whether a cell underwent apoptosis via death receptors, mitochondrial breakdown, oxidative DNA damage, or other forms of cells death. We present here evidence that p53 can affect the threshold at which cell death occurs via any caspase-dependent cell death pathway through the induction of caspase-6, an effector caspase.
Although the other effector caspases, caspases 3 and 7, were not modulated by p53 expression, caspase-6 was induced severalfold reproducibly in a variety of cell lines at the RNA level. Recently, another caspase, caspase-1, has been described as a transcriptional target of p53 (24). Although this enzyme is the founding member of this protein family, it appears to be structurally related to other caspases but is not believed to play a role in apoptosis induction under physiological conditions. Caspase-1 generally is involved with the processing of inflammatory cytokines such as interleukin-1B into active cytokines, and causes cell death on exogenous overexpression (25).
The substrates that caspase-6 are known to cleave are growing in numbers, including the nuclear envelope protein Lamin A and several transcription factors (26). It has been shown that an uncleavable Lamin A inhibits the dissolution of the nucleus and its contents during apoptosis (12). The breakdown of genomic DNA is one of the key events that occurs during programmed cell death, and that event is preceded by nuclear envelope breakdown to allow entrance of proteases and DNases. The speed at which this dissolution occurs may help determine the rapidity of apoptosis induced by p53. Interestingly, before the discovery of Lamin A as a capsase-6 substrate, cleavage of Lamin A was described to be resultant of activation of an interleukin-1 converting enzyme protease downstream of p53 (12).
The chromosomal localization of caspase-6 suggests that alterations could occur that may affect the ability of p53 to chemosensitize cells. Caspase-6 is localized to chromosome 4q25, a region of common loss of heterozygosity and deletion in colorectal, oral, cervical and, most commonly, in breast cancer (27, 28), leading to much speculation about the possible existence of a tumor suppressor. Interestingly, p53 was unable to induce caspase-6 expression in MCF7 cells, a breast cancer cell line. Although at this time it is unknown why p53 is unable to induce caspase-6, future study will determine whether the caspase-6 genomic locus is altered in MCF7 cells and whether caspase-6 is encompassed in the deletions found in this region in breast and oral cancers. A key role for caspase-6 has also been uncovered in the apoptosis of neuronal cells. Caspase-6 is a primary effector of neuronal cell death and the predominant protein involved in the cleavage of amyloid precursor protein (29). Future studies will need to specifically investigate the role of caspase-6 in p53-induced neuronal cell death, where Bax has previously been implicated (30). Previous studies have placed a constitutively active form of caspase-6 (Rev-Caspase-6) upstream of the hTERT promoter and used it to infect glioma cells. Although normal brain tissue is unaffected because of the lack of telomerase expression, glioma cells are killed at a rapid pace because of the ample expression of active caspase-6 (31).
In conclusion, we have found caspase-6 regulation by p53 as the most downstream component of the death pathway to date identified as a p53 transcriptional target. In many instances described here, Ad-p53 infection, and thereby induction of caspase-6 mRNA, is not enough to kill cells. Elevated levels of latent caspase-6 protein provide a mechanism by which the cell death threshold can be lowered, thereby enhancing p53-dependent cell death during cancer chemotherapy.
Acknowledgments
We thank Tim Burns, Rob McDonald, and Joanna Sax for technical assistance and helpful discussions, and Dave Dicker for excellent assistance with flow cytometry. W.S.E.-D. is an Assistant Investigator of the Howard Hughes Medical Institute.
Abbreviations
- VEID–FMK
benzyloxylcarbonyl-valine-glutamate-isoleucine-aspartate-fluoromethyl ketone
- moi
multiplicity of infection
Footnotes
Present address: Curagen Corporation, Branford, CT 06406.
References
- 1.Lowe S W, Bodis S, McClatchey A, Remington L, Ruley H E, Fisher D E, Housman D E, Jacks T. Science. 1994;266:807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- 2.Lowe S W, Ruley H E, Jacks T, Housman D E. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 3.Nakano K, Vousden K H. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, Lane D, Levine A J. Nature (London) 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Zhang L, Hwang P M, Kinzler K W, Vogelstein B. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 6.Teitz T, Wei T, Valentine M B, Vanin E F, Grenet J, Valentine V A, Behm F G, Look A T, Lahti J M, Kidd V J. Nat Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 7.Soengas M S, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman J G, Gerald W L, Lazebnik Y A, et al. Nature (London) 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 8.Robles A I, Bemmels N A, Foraker A B, Harris C C. Cancer Res. 2001;61:6660–6664. [PubMed] [Google Scholar]
- 9.Moroni M C, Hickman E S, Denchi E L, Caprara G, Colli E, Cecconi F, Muller H, Helin K. Nat Cell Biol. 2001;3:552–558. doi: 10.1038/35078527. [DOI] [PubMed] [Google Scholar]
- 10.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 11.Porter A G, Ng P, Janicke R U. BioEssays. 1997;19:501–507. doi: 10.1002/bies.950190609. [DOI] [PubMed] [Google Scholar]
- 12.Rao L, Perez D, White E. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson D W, Thornberry N A. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 14.Tan X, Martin S J, Green D R, Wang J Y. J Biol Chem. 1997;272:9613–9616. doi: 10.1074/jbc.272.15.9613. [DOI] [PubMed] [Google Scholar]
- 15.Thornberry N A. Br Med Bull. 1997;53:478–490. doi: 10.1093/oxfordjournals.bmb.a011625. [DOI] [PubMed] [Google Scholar]
- 16.Zheng T S, Hunot S, Kuida K, Flavell R A. Cell Death Differ. 1999;6:1043–1053. doi: 10.1038/sj.cdd.4400593. [DOI] [PubMed] [Google Scholar]
- 17.Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak T W. Mol Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 18.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, et al. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 19.Thornberry N A, Chapman K T, Nicholson D W. Methods Enzymol. 2000;322:100–110. doi: 10.1016/s0076-6879(00)22011-9. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Calvo M, Peterson E P, Leiting B, Ruel R, Nicholson D W, Thornberry N A. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 21.Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, Okazaki T, Yamamoto K, Sasada M. J Exp Med. 1998;187:587–600. doi: 10.1084/jem.187.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlesinger M, Jiang J D, Roboz J P, Denner L, Ling Y H, Holland J F, Bekesi J G. Biochem Pharmacol. 2000;60:1693–1702. doi: 10.1016/s0006-2952(00)00484-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang S, Simon G. Am J Physiol Cell Physiol. 2000;279:C341–C351. doi: 10.1152/ajpcell.2000.279.2.C341. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Radha V, Furukawa Y, Swarup G. J Biol Chem. 2001;276:10585–10588. doi: 10.1074/jbc.C100025200. [DOI] [PubMed] [Google Scholar]
- 25.Cryns V, Yuan J. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 26.Galande S, Dickinson L A, Mian I S, Sikorska M, Kohwi-Shigematsu T. Mol Cell Biol. 2001;21:5591–5604. doi: 10.1128/MCB.21.16.5591-5604.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X L, Uzawa K, Imai F L, Tanzawa H. Oncogene. 1999;18:823–825. doi: 10.1038/sj.onc.1202318. [DOI] [PubMed] [Google Scholar]
- 28.Shivapurkar N, Maitra A, Milchgrub S, Gazdar A F. Hum Pathol. 2001;32:169–177. doi: 10.1053/hupa.2001.21560. [DOI] [PubMed] [Google Scholar]
- 29.LeBlanc A, Liu H, Goodyer C, Bergeron C, Hammond J. J Biol Chem. 1999;274:23426–23436. doi: 10.1074/jbc.274.33.23426. [DOI] [PubMed] [Google Scholar]
- 30.Macleod K F, Hu Y, Jacks T. EMBO J. 1996;15:6178–6188. [PMC free article] [PubMed] [Google Scholar]
- 31.Komata T, Kondo Y, Kanzawa T, Hirohata S, Koga S, Sumiyoshi H, Srinivasula S M, Barna B P, Germano I M, Takakura M, et al. Cancer Res. 2001;61:5796–5802. [PubMed] [Google Scholar]
- 32.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 33.Tomlinson G E, Chen T T, Stastny V A, Virmani A K, Spillman M A, Tonk V, Blum J L, Schneider N R, Wistuba I I, Shay J W, et al. Cancer Res. 1998;58:3237–3242. [PubMed] [Google Scholar]
- 34.Wu G S, El-Deiry W S. Clin Cancer Res. 1996;2:623–633. [PubMed] [Google Scholar]