Abstract
Background
[177Lu]Lu-EDTMP has emerged as a promising radiopharmaceutical for the palliation of pain caused by osseous metastases. This phase I/II study comprehensively evaluate the pharmacokinetics, biodistribution, clinical efficacy, and safety profile of [177Lu]Lu-EDTMP, in patients with skeletal metastases from breast and prostate cancer.
Methods
A total of 27 patients with skeletal metastases were included in the study. Pharmacokinetics and biodistribution were analyzed in 17 patients through whole-body gamma camera imaging and quantification at multiple time points. Clinical efficacy and safety were evaluated in 18 patients receiving either low (1.3 GBq) or high (2.6 GBq) administered activity. Pain palliation was assessed using visual analog scale scores, analgesic usage (frequency and type), mobility scores, and Karnofsky performance status. The safety profile was determined through hematological and biochemical monitoring over 12 weeks.
Results
[177Lu]Lu-EDTMP demonstrated rapid blood clearance, with negligible residual activity at 24 h. Urinary excretion accounted for over 30% of administered activity at 24 h. Bone uptake increased progressively to over 70%.
Scintigraphy revealed selective uptake in metastases, improving lesion-to-bone and lesion-to-soft tissue ratios. Both low (1.3 GBq) and high (2.6 GBq) activity groups showed significant pain relief, with faster, longer-lasting effects in the high-dose group, reducing opioid/NSAID use. No Grade III/IV myelotoxicity or major renal/hepatic events occurred.
Conclusion
[177Lu]Lu-EDTMP is a safe and effective bone pain palliation agent, with favorable pharmacokinetics, targeted skeletal uptake, and minimal toxicity. These findings support its potential use as an alternative radiopharmaceutical for pain palliation in patients with skeletal metastases.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00259-025-07218-x.
Keywords: Theranostics, [177Lu]-EDTMP, Bone pain palliating agent, Pharmacokinetics, Osseous metastatic patients
Introduction
Bone is the third most common site for metastatic cancer after the lung and liver [1]. It is estimated that skeletal metastases develop in 14–70% of all cancer patients, with autopsy-based studies reporting a 70% occurrence in patients with breast and prostate cancers [2]. Additionally, tumours such as lung, thyroid, kidney, and melanoma have a marked predilection for skeletal metastases [3]. Randomized trials in advanced cancer indicate that major skeletal-related events, such as fractures, spinal cord compression, or the need for surgery and radiotherapy, occur on average every 3 to 6 months [4, 5].
Skeletal metastases lead to severe pain, reduced mobility, and an overall decline in quality of life. Effective symptom management is a cornerstone of palliative care in oncology, especially as longer survival times due to advances in cancer therapies have led to a rising incidence of bone metastases and their associated complications. This increase presents significant socioeconomic challenges by placing additional burdens on healthcare systems while causing adverse psychological impacts on both patients and their families.
The treatment options for bone metastases to alleviate pain and improve patient outcomes include the use of analgesics, bisphosphonates, external beam radiotherapy, and targeted radiopharmaceuticals emitting particulate radiation. Radiopharmaceuticals are particularly effective for the management of bone pain and may also impact the progression of bone lesions [6].
Bone-targeting radiopharmaceuticals are indicated for refractory pain caused by sclerotic or mixed-type skeletal metastases and for disseminated disease where repeated local treatments become impractical, as outlined in international guidelines [7]. It is essential to correlate foci of increased radiotracer uptake on [99mTc]Tc- Methylene Diphosphonate (MDP) bone scans with the patient’s symptoms. Pain arising from other causes such as vertebral collapse, nerve root entrapment, fractures, or visceral pain will not respond to radionuclide therapy.
Several radionuclides, such as Radium-223, Strontium-89, and Phosphorus-32, are selectively absorbed by metastatic bone sites, enabling their use in targeted therapy for bone pain palliation. Notably, [223Ra]Ra therapy has been associated with improved survival outcomes in patients with prostate cancer [8]. Bisphosphonates, which have a natural affinity for hydroxyapatite crystals in bones, can also be conjugated with radionuclides to deliver targeted therapy to skeletal metastases [7].
Lutetium-177 has gained substantial significance in targeted radionuclide therapy. Its beta emissions provide therapeutic efficacy while its relatively long half-life (6.7 days) allows for logistical advantages in production, transport, and administration. [177Lu]Lu is widely used to label peptides such as DOTATATE or DOTATOC, which target somatostatin receptors overexpressed in neuroendocrine tumors (NETs). Similarly, [177Lu]Lu-PSMA therapy is a promising treatment for men with advanced prostate cancer that has become resistant to conventional therapies [9–11].
Ethylenediaminetetramethylene phosphonic acid (EDTMP) is a phosphonate-based chelator that exhibits high affinity for bone, making it an ideal carrier for radionuclides in metastatic bone disease. When labeled with [177Lu]Lu, this radiopharmaceutical ([177Lu]Lu-EDTMP) combines the therapeutic advantages of beta radiation with the targeting specificity of bone-seeking agents. This combination provides effective palliation of metastatic bone pain with minimal side effects compared to traditional therapies [6].
Preliminary studies suggest that [177Lu]Lu-EDTMP is a highly effective agent for bone pain palliation in metastatic cancer patients. Its favorable biolocalization characteristics—such as rapid blood clearance, selective skeletal uptake, and high lesion affinity—along with its prolonged post-production shelf-life, make it particularly suitable for use in remote medical centers [12].
The present phase I/II study aims to:
Determine the pharmacokinetics and biodistribution of [177Lu]Lu-EDTMP in prostate and breast cancer patients with skeletal metastases.
Evaluate the clinical efficacy for bone pain palliation and assess biological toxicity at low (1.3 GBq, 35 mCi) and high (2.6 GBq, 70 mCi) administered activities.
Materials and methods
A total of 27 patients with histologically confirmed breast or prostate cancer and osteoblastic or mixed bone lesions took part in this phase I/II study. All patients had symptomatic bone metastases verified by [99mTc]Tc-MDP scans, a Karnofsky performance status ≥ 50%, and an expected survival of at least 3 months. The characteristics of the study population are summarized in Table 1.
Table 1.
Characteristics of study population
| Characteristics of study population | Values |
|---|---|
| Total patients | 27 |
| Total male patients | 18 |
| Total female patients | 09 |
| Male Ca breast patients | 01 |
| Female Ca breast patients | 09 |
| Ca prostate patients | 17 |
| Median age (yrs) | 65.16 ± 13.95 |
| Median height (cm) | 163.22 ± 8.77 |
| Median weight (kg) | 64.72 ± 7.16 |
| Duration of follow up period | 12 weeks |
Study design and groups
Participants were split into two major groups randomly:
Pharmacokinetics and Biodistribution Group
This group included 17 patients and was further subdivided into two subgroups:
Subgroup 1 (n = 9):
In this sub group, patients were divided into groups of three and injected with escalating activities of [177Lu]Lu-EDTMP. Specifically, the administered activity was designed to deliver estimated bone marrow doses equivalent to 75%, 100%, and 125% of the 200 cGy reference value for bone marrow, as predicted by the "test dose" study calculations described below.
Blood/urine sampling, plus gamma camera imaging at multiple intervals (30 min, 4 h, 8 h, 24 h, 48 h, 7 days, 14 days).
Bone uptake was calculated using Excel-based software for quantitative assessment.
Subgroup 2 (n = 17; 8 new patients and the 9 patients from Subgroup 1):
Patients were randomly assigned to receive either:
Low activity of [177Lu]Lu-EDTMP (1.3 GBq, 35 mCi), or
High activity of [177Lu]Lu-EDTMP (2.6 GBq, 70 mCi).
Urinary excretion, soft tissue uptake, and bone uptake were determined using scintigraphic methods.
-
2.
Clinical Efficacy and Toxicity Group
This group comprised 18 patients, including 8 patients from Subgroup 2 of the pharmacokinetics and biodistribution study, who had already received either low or high activity, and 10 additional new patients. The patients were further divided into two arms:
Arm 1 (n = 9): Received low activity of [177Lu]Lu-EDTMP (1.3 GBq, 35 mCi).
Arm 2 (n = 9): Received high activity of [177Lu]Lu-EDTMP (2.6 GBq, 70 mCi).
Inclusion and exclusion criteria
Inclusion Criteria
Patients meeting the following criteria were eligible for the study:
Histologically confirmed diagnosis of breast or prostate cancer.
Hormone refractory prostate cancer, defined as:
Two consecutive rises in serum Prostate-Specific Antigen (PSA) done measured 1 week apart, or
Disease progression on [99mTc]Tc-MDP bone scintigraphy, with the appearance of ≥ 2 new lesions.
Breast cancer with bone metastases.
Predominantly osteoblastic skeletal metastases as demonstrated by increased uptake (“hot foci”) on a [99mTc]Tc-MDP bone scan performed within 4 weeks prior to [177Lu]Lu-EDTMP administration. Painful sites described by the patient must correlate with the hot foci on the bone scan.
Life expectancy ≥ 3 months.
Karnofsky’s index ≥50%
Written informed consent, provided by the patient.
Exclusion criteria
Pregnancy and breastfeeding.
Risk of pathological bone fracture requiring immediate intervention.
Previous External Beam Radiation Therapy on >30% of the bone marrow.
Hematological values outside the following limits:
Hemoglobin (Hb) < 10 g/dL.
White blood cell count (WBC) < 3 × 10⁹/L.
Neutrophils < 1.5 × 10⁹/L.
Platelets < 100 × 10⁹/L.
Serum bilirubin > 1.5 times the upper normal limit (UNL).
Serum creatinine > 2 times the UNL.
Previous radiotherapy or chemotherapy within the last 4 weeks.
Prior radiopharmaceutical therapy within the previous 2 years.
Extensive metastatic disease demonstrated as a superscan on bone scintigraphy.
Presence of Central Nervous System or epidural metastases.
Any life-threatening comorbid illness.
History of bleeding disorders that could be exacerbated by the study medication.
Concurrent illnesses that could interfere with study results or completion.
Patients undergoing neural block treatments for bone pain.
Patient preparation and injection
For the escalating activity schedule, a tracer activity of 75 MBq (2 mCi) of [177Lu]Lu-EDTMP was administered, and whole-body scans were performed within 1 h (pre-void) and at 24 h post-injection. The desired red marrow absorbed dose was determined based on the bone marrow estimated dose of 100 cGy, 150 cGy, 200 cGy, 250 cGy, and 300 cGy.
Patient-specific parameters, including body height, weight, and desired red marrow absorbed activity, were incorporated into an Excel-based software program to calculate the therapeutic activity for each patient. The calculated activity, measured using an activity calibrator, was administered via a three-way cannula after ensuring adequate hydration and recording baseline vital signs. The injection procedure involved slow administration of the radiopharmaceutical over 30 s, followed by a 10 mL saline flush. Pre- and post-injection syringe activities were measured to determine the actual injected activity. Vital signs were monitored every 30 min for 2 h post-injection to ensure patient safety.
Imaging
Whole-body images were acquired using a dual-head gamma camera (GE Infinia with XelerisTM workstation, GE Healthcare, Milwaukee, WI, USA) in both anterior and posterior projections at 30 min (pre-void), 4 h, 8 h, 24 h, 48 h, 7 days, and 14 days after [177Lu]Lu-EDTMP injection. The scans at 7 and 14 days were conducted primarily for visual analysis to observe the retention of activity. For analytical purposes, only data from the 4 h, 24 h, and 48 h time points were used.
The imaging parameters included the use of a medium energy general-purpose (MEGP) collimator, a scan speed of 10 cm/min, a total scan length of 200 cm, and a total scan time of approximately 20 min. The energy window was centered at 208 keV with a ± 7.5% margin. The matrix size used was 256 × 1024 with a zoom factor of 1, and body contouring was disabled. To maintain consistency, the position of head 1 was noted for each patient to standardize imaging conditions across time points. All images were digitally stored for post-processing and further analysis.
Blood and urinary clearance
Estimation of blood clearance
Blood clearance was assessed using serial blood samples of 2 mL each, collected at 2 min, 4 min, 8 min, 15 min, 30 min, 2 h, 4 h, 8 h, and 24 h post-injection. Blood samples were prepared in 10 tubes, each filled with 100 µL of blood using a heparinized syringe. The tubes were weighed before and after filling using an electronic balance to determine the exact volume. Radioactivity in the blood samples was measured using a gamma counter (Packard Cobra II Auto Gamma Counter, Packard Instrument Company—now part of PerkinElmer, US), and the total blood activity was normalized as a percentage of the injected activity to generate the blood clearance curve.
Determination of urinary clearance
Urine was collected over a 48-h period and fractionated into the following intervals: 0–4 h, 4–8 h, 8–24 h, and 24–48 h. For each interval, two vials of 10 mL urine were prepared and weighed before and after filling to determine the volume accurately. Activity in each sample was measured using a dose calibrator (Capintec CRC-15R Dose Calibrator, Capintec, Inc., US). Urinary excretion was calculated as a percentage of the total administered activity using the following formula:
Ap: percentage of injected activity in urine at time (t).
At: Activity in urine at time (t) × 100.
TT: Total administered activity.
Determination of bone uptake
Whole body count method (Excel-Based Software)
Patient-specific data, including age, gender, height, weight, and desired red marrow absorbed dose, along with background-corrected whole-body counts (anterior and posterior projections at 4 h, 24 h, and 48 h), were entered into an Excel-based program. Bone uptake at each time point was expressed as a percentage of the 30-min counts, defined as 100%. The mean values of bone uptake were calculated for further analysis.
Scintigraphic method
For each image, the activity levels of the whole body, urinary bladder, and adductor muscle of the thigh (representing soft tissue) were measured using a conventional region-of-interest (ROI) technique applied to both anterior and posterior projections. Additionally, a rectangular ROI was placed adjacent to the head and shoulders to account for bremsstrahlung radiation from the β-particles of the radionuclide. An example of ROI positions is provided in Fig. 1.
Fig. 1.
Regions of Interest (ROIs) for determination of bone uptake, soft tissue retention, and urinary excretion of [177Lu]Lu-EDTMP by Gamma Camera imaging
Using these measurements, the geometric mean for each ROI was calculated after applying corrections for radioactive decay, bremsstrahlung radiation, and pixel normalization. The total whole-body activity measured at 30 min post-injection was set as the baseline (100%) for each patient and served as the reference for subsequent activity calculations, expressed as percentages of this baseline value. At the 30-min time point, the whole-body activity was also considered the maximum soft tissue activity. In subsequent images, the activity of the adductor muscle was used as a marker of soft tissue retention over time. The ratio of soft tissue activity to the initial whole-body activity was therefore equivalent to the ratio of adductor muscle activity to its initial value at 30 min post-injection.
Urinary excretion was calculated by subtracting the whole-body activity at each subsequent time point from the baseline 30-min activity and adding the urinary bladder activity. Bone uptake at each time point was determined by subtracting both urinary excretion and soft tissue retention from the initial whole-body activity (100%).
Formulae for calculations are given below:
St: Soft tissue retention at time (t).
WT: Whole body counts at 30 min.
At: Adductor’s counts at time (t).
AT: Adductor’s counts at 30 min
Ut: Urinary excretion at time (t).
WT: Whole body counts at 30 min.
WB: Whole body counts at time (t).
Bt: Bladder’s counts at time (t)
Bt: Bone uptake at time (t).
WT: Whole body counts at 30 min.
St: Soft tissue retention at time (t).
Ut: Urinary excretion at time (t).
Uptake ratios between metastatic lesion, normal bone, and soft tissue
Equal-sized ROIs were drawn over metastatic lesions, normal bone, and the adductor muscle of the thigh to calculate uptake ratios, as shown in Fig. 2. The ratios included lesion-to-normal bone, lesion-to-soft tissue, and normal bone-to-soft tissue. Data were analyzed at 30 min, 4 h, 8 h, 24 h, and 48 h.
Fig. 2.

Regions of Interest (ROIs) for Determination of uptake ratios between metastatic lesions, normal bone, and soft tissue
Correlation studies
The correlation between injected activity (MBq) and percent bone uptake was analyzed by plotting scatter graphs with injected activity on the x-axis and bone uptake percentages (at 4, 24, and 48 h) on the y-axis. Spearman correlation coefficients and p-values were calculated to determine statistical significance. Similarly, the bone lesion score, derived from [99mTc]Tc-MDP scans, was correlated with the percent bone uptake at the same time points.
Safety and toxicity profile
Patients were monitored over a 12-week period to assess safety and toxicity. Hematological parameters, including Hb levels, white blood cell counts, and platelet counts, were closely monitored along with renal and liver function tests. Assessments were conducted at 1, 2, 4, 8, and 12 weeks. For prostate cancer patients, serum PSA levels were repeated at week 8.
Pain response assessment
Pain response was evaluated through multiple parameters, including the Visual Analog Scale (VAS), type and frequency of analgesics, mobility, and the Karnofsky Performance Status (KPS).
Statistical analysis
Mean values and standard deviations were calculated for all measured parameters, including blood clearance, urinary excretion, and bone uptake. Correlation coefficients (Spearman's r) and p-values were computed to assess the relationships between variables. Pain response was analyzed using analysis of variance (ANOVA) to determine significant reductions in VAS scores and analgesic usage over time.
Data availability
The datasets generated during and/or analyzed during the current study are available from Dr Shabana Saeed, responsible of data collection and analysis, on reasonable request.
Human ethics and consent to participate
The research was undertaken in compliance with the Helsinki Declaration. Formal written patient consent was not required for this type of study. The study was approved by the Pakistan Institute of Engineering and Applied Sciences (P.I.E.A.S.), the Ethical Review Committee of the Nuclear Medicine, Oncology, and Radiotherapy Institute (N.O.R.I.)
Results
Pharmacokinetics and biodistribution
Blood clearance of [177Lu]Lu-EDTMP
Blood clearance of [177Lu]Lu-EDTMP was rapid within the first 30 min, with only a negligible fraction of the injected activity (2.2%) remaining in the blood at 24 h. The time-activity curve for blood clearance is presented in Fig. 3.
Fig. 3.
Percentage of injected activity in blood over time
Urinary clearance of [177Lu]Lu-EDTMP
Urinary excretion occurred predominantly within the first 24 h, accounting for 99.6% of the injected activity. Over 48 h, a cumulative urinary excretion of 30.8 ± 2.5% of the administered activity was observed. Fractionated urinary clearance data are presented in Fig. 4, and cumulative urinary excretion values are shown in Fig. 5.
Fig. 4.
Percentage of injected activity excreted in urine over time
Fig. 5.
Cumulative urinary excretion of [177Lu]Lu-EDTMP over 48 h
Bone uptake
Bone uptake was significant and increased over time. The mean bone uptake was 69.33 ± 12.95% at 4 h, rising to 71.33 ± 11.02% at 24 h, and further to 72.77 ± 12.24% at 48 h. Individual patient values and mean bone uptake are summarized in Table 2.
Table 2.
Bone uptakes of individual patients and their mean values
| Patients | Bone uptake 4 h. % |
Bone uptake 24 h. % |
Bone uptake 48 h. % |
|---|---|---|---|
| 1 | 80 | 85 | 85 |
| 2 | 51 | 52 | 50 |
| 3 | 78 | 75 | 88 |
| 4 | 51 | 64 | 63 |
| 5 | 70 | 77 | 70 |
| 6 | 88 | 87 | 85 |
| 7 | 60 | 64 | 71 |
| 8 | 76 | 70 | 76 |
| 9 | 70 | 68 | 67 |
| Mean | 69.33 ± 12.95% | 71.33 ± 11.02% | 72.77 ± 12.24% |
Soft tissue retention and urinary excretion
As urinary excretion and soft tissue retention decreased over time, bone uptake increased correspondingly. At 4 h post-injection, soft tissue retention was 36.77 ± 12.08% and urinary excretion was 27.07 ± 13.12%. By 24 h, these values decreased to 33.80 ± 9.08% and 25.56 ± 11.18%, respectively. Meanwhile, bone uptake increased from 37.22 ± 14.77% to 43.01 ± 12.74%. Trends for soft tissue retention, urinary excretion, and bone uptake are shown in Figs. 6 and 7.
Fig. 6.
Bone uptake, soft tissue retention, and urinary excretion of [177Lu]Lu-EDTMP by Gamma Camera imaging
Fig. 7.
Percentage of soft tissue retention at 4 and 24 h
Uptake ratios between metastatic lesion, normal bone and soft tissues
The ratios of lesion-to-normal bone, lesion-to-soft tissue, and normal bone-to-soft tissue increased progressively, reaching a near-plateau at 24 h post-injection, with minimal change observed between 24 and 48 h. The time-dependent uptake ratios are presented in Fig. 8.
Fig. 8.
Uptake ratios: lesion-to-bone, lesion-to-soft tissue, and normal bone-to-soft tissue
Correlation studies
Injected activity vs percentage of bone uptake
No significant correlation was observed between the injected activity and the percentage of bone uptake. The correlation, evaluated using both the whole-body count method and the scintigraphic method at 4, 24, and 48 h, was statistically insignificant at the 0.05 level (two-tailed).
Bone lesion score vs percentage of bone uptake
A significant positive correlation was noted between the bone lesion score (derived from [99mTc]Tc-MDP scans) and the percentage of bone uptake. This correlation was significant at the 0.05 level (two-tailed) using both the whole-body count and scintigraphic methods.
[99mTc]Tc-MDP vs [177Lu]Lu-EDTMP Images.
Excellent concordance was observed between the images acquired using [99mTc]Tc-MDP and [177Lu]Lu-EDTMP at all evaluated time points (30 min, 4 h, 8 h, 24 h, 48 h, 7 days, and 14 days). Representative images are shown in Fig. 9.
Fig. 9.
Concordance between [99mTc]Tc-MDP (a) and [177Lu]Lu-EDTMP (b) images
Clinical efficacy
Visual analog scale
Pain relief was greater and more sustained in the high-activity group compared to the low-activity group. The summary of VAS scores is provided in Table 3.
Table 3.
Pain palliation on basis of visual analog score
| Week 2 | Week 4 | Week 6 | Week 8 | Week 12 | |
|---|---|---|---|---|---|
| High Activity | 37.28 ± 2.31% | 42.79 ± 2.54% | 44.70 ± 2.50% | 37.07 ± 2.35% | 44.70 ± 2.38% |
| Low Activity | 46.61 ± 1.51% | 40.89 ± 2.64% | 37.08 ± 3.18% | 37.08 ± 4.42% | 39.08 ± 4.50% |
| Onset of Response | Within 1 week both with low and high activity | ||||
| Duration of response |
10.22 ± 3.07 weeks with high activity (Range: 4–12 weeks) 7.44 ± 5.41 weeks with low activity (Range: 1–12 weeks) |
||||
| Max. Response |
4 to 6 weeks and at 12 weeks with high activity 2 to 4 weeks with low activity |
||||
Frequency of analgesic use
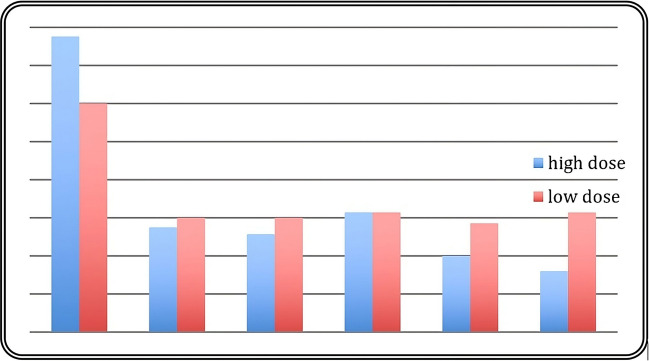
A significant reduction in analgesic use was observed in both groups, with a more pronounced decline in the high-activity group. The normalized analgesia scores demonstrated superior improvement in the high-activity group during the follow-up period. Detailed results are illustrated in Fig. 10.
Fig. 10.
Frequency of analgesic use in low- and high-activity groups
Type of analgesics
After therapy, the use of analgesics decreased significantly in the high-activity group. By week 12, in the low-activity group, 55.55% required no analgesics, 33.33% used aspirin/paracetamol, and 11.11% required morphine derivatives, while in the high-activity group, 66.66% needed no analgesics, and 33.33% required only aspirin/paracetamol. Summarized data are presented in Table 4.
Table 4.
Change in type of analgesics over 12 weeks
| Visit | Grade 0 % |
Grade I % |
Grade II % |
Grade III % |
Grade IV % |
|---|---|---|---|---|---|
| Low Activity | |||||
| Baseline | 11.11 | 44.44 | 44.44 | ||
| Week 2 | 55.55 | 33.33 | 11.11 | ||
| Week 4 | 22.22 | 5.555 | 22.22 | ||
| Week 6 | 11.11 | 88.88 | |||
| Week 8 | 55.55 | 44.44 | |||
| Week 12 | 55.55 | 33.33 | 11.11 | ||
| High Activity | |||||
| Baseline | 11.11 | 44.44 | 33.33 | 11.11 | |
| Week 2 | 11.11 | 55.55 | 33.33 | 11.11 | |
| Week 4 | 22.22 | 66.66 | 11.11 | 11.11 | |
| Week 6 | 22.22 | 66.66 | 11.11 | 11.11 | |
| Week 8 | 55.55 | 33.33 | 11.11 | 11.11 | |
| Week 12 | 66.66 | 33.33 | |||
Mobility
Following therapy, a significant improvement in mobility was noted, particularly in the high activity group. By week 12, in the high-activity group, 44.44% achieved Grade 0 (freely mobile), while in the low-activity group, 11.11% reached Grade 0. Results are summarized in Table 5.
Table 5.
Mobility in low and high activity group
| Visit | Grade 0 | Grade I | Grade II | Grade III | Grade IV |
|---|---|---|---|---|---|
| High activity | |||||
| Baseline | 66.66% | 33.33% | |||
| Week 2 | 66.66% | 11.11% | 22.22% | ||
| Week 4 | 11.11% | 66.66% | 11.11% | 11.11% | |
| Week 6 | 22.22% | 55.55% | 11.11% | ||
| Week 8 | 33.33% | 44.44% | 11.11% | 11.11% | |
| Week 12 | 44.44% | 33.33% | 11.11% | 11.11% | |
| Low activity | |||||
| Baseline | 22.22% | 22.22% | 55.55% | ||
| Week 2 | 11.11% | 33.33% | 22.22% | 33.33% | |
| Week 4 | 777.77% | 22.22% | |||
| Week 6 | 44.44% | 55.55% | |||
| Week 8 | 11.11% | 22.22% | 44.44% | 11.11% | |
| Week 12 | 11.11% | 33.33% | 33.33% | 22.22% | |
Safety profile
In the low activity group, 2 out of 9 patients experienced a Hb level drop below 10 g/dL, but no cases were observed with Hb < 8 g/dL. One patient developed thrombocytopenia with a platelet count of 56,000 (below the threshold of 60,000), and another patient developed leukopenia with a leukocyte count of 2,800 (below the 4,000 threshold). Statistical analysis revealed no significant decrease in Hb levels (P = 0.87, F = 0.36). However, the decreases in platelet (P = 0.04, F = 2.64) and leukocyte counts (P = 0.01, F = 3.32) were significant at the 0.05 level. Both platelet and leukocyte counts recovered spontaneously within 4 weeks, requiring no medical intervention (Fig. 11).
Fig. 11.
Mean hemoglobin, white blood cell count, and platelet values over 8 weeks in the low-activity group
In the high activity group, two out of nine patients reported a Hb level below 10 g/dL, though none had levels < 8 g/dL. No cases of thrombocytopenia with platelet counts < 60,000 were noted. However, five patients developed leukopenia with leukocyte counts < 3,000, although no instances of leukocyte counts < 2,000 were observed. The decrease in Hb levels (P = 0.77, F = 0.50) and platelet counts (P = 0.06, F = 2.25) was not statistically significant. In contrast, the reduction in leukocyte counts was highly significant (P = 0.0002, F = 6.11) at the 0.05 level. Similar to the low activity group, platelet and leukocyte counts returned to baseline levels within four weeks without any need for medical intervention (Fig. 12).
Fig. 12.
Mean hemoglobin, white blood cell count, and platelet values over 8 weeks in the high-activity group
Effect on physiological parameters
There were no significant changes in heart rate, blood pressure, respiratory rate, or rhythm abnormalities were observed during the 48-h post-injection monitoring compared to baseline values.
Discussion and conclusion
Bone metastasis is a severe complication of various solid malignancies, representing the most common form of metastatic bone disease. The true incidence remains debated, influenced by the prevalence of specific cancers in different populations. Prostate cancer in men and breast cancer in women are the leading sources of bone metastases. Bone pain, affecting 66% of affected patients, is the most debilitating complication [13].
Managing metastatic bone disease aims to alleviate pain and improve quality of life, requiring a multidisciplinary approach. Treatments are classified into systemic (e.g., NSAIDs, opioids, bisphosphonates, chemotherapy, and radiopharmaceuticals) and local therapies (e.g., external beam radiation and surgery) [14, 15]. The ideal therapy must be fast, safe, effective, and well-tolerated [16]. Moderate, localized skeletal symptoms are usually managed with conventional analgesics prescribed according to the World Health Organization (WHO) pain ladder, followed by single-fraction external beam radiotherapy for persistent, limiting discomfort. However, this approach becomes less effective in the context of progressive skeletal metastases, which often result in poorly localized or migratory multisite pain. Wide-field hemi-body radiotherapy is an effective treatment option, but its potential benefits are often outweighed by significant bone marrow and gastrointestinal toxicity. Bisphosphonates target osteoclast-mediated bone resorption and reduce the rate of skeletal complications arising from osteolytic metastases. In cases of metastases affecting multiple sites, radiopharmaceutical therapy with bone-seeking radiopharmaceuticals is a valuable approach. A major challenge, however, lies in ensuring adequate radiation delivery to bone lesions while minimizing marrow suppression, which is influenced by the tissue penetration of β− particles emitted by radionuclides. [17, 18].
Sodium phosphate ([32P]Na32PO4), the first radiopharmaceutical used for bone pain palliation, presents the drawback of significant bone marrow suppression due to its high-energy β− particles [19, 20]. Strontium-89, in the form of [89Sr]SrCl₂, is an effective bone pain palliative agent due to its selective accumulation at skeletal lesion sites by replacing Ca2⁺ [21, 22]. However, its use is limited by high production costs and the lack of imageable gamma photons [23]. [153Sm]Sm-EDTMP and [186Re]Re-HEDP radiopharmaceuticals have favorable decay characteristics, with [153Sm]Sm-EDTMP being easier to produce. However, production losses can be a concern due to the short half-life of these radionuclides [24, 25].
[177Lu]Lu-EDTMP, with its favorable decay characteristics, is emerging as a promising alternative radiopharmaceutical for bone pain palliation. The emission of gamma photons with appropriate energy and relatively low abundance enables simultaneous scintigraphic imaging and dosimetry studies, improving treatment planning. Additionally, [177Lu] low β⁻ particle energy minimizes bone marrow suppression, providing significant advantages over other radioisotopes [17, 18]. With its longer half-life and potential for large-scale production, [177Lu]Lu-EDTMP could serve as a cost-effective alternative to [153Sm]Sm-EDTMP for bone pain palliation.
The present phase I/II study focused on the pharmacokinetics, biodistribution, toxicity profile, and clinical efficacy of [177Lu]Lu-EDTMP in breast and prostate cancer patients.
Safety aspects and pharmacokinetics
The pharmacokinetic profile mirrored that of [153Sm]Sm-EDTMP, highlighting optimized biodistribution and minimized systemic exposure [26]. The blood clearance of [177Lu]Lu-EDTMP was rapid, with only 2.2% remaining in the bloodstream at 24 h, consistent with previous pre-clinical and clinical studies [26–30]. This value is lower compared with the blood clearance measured by Singh et al. [31]. Kidneys and the bladder are the most sensitive non-target organs as uptake was prominent, with 99.74% excretion occurring within the first 24 h, compared to 96.6% in animal studies [29]. Urinary clearance both by sampling and scintigraphy at 4 and 24 h is lower than that of [153Sm]Sm-EDTMP and [186Re]Re-HEDP, as calculated by Brenner et al. [32]. The excretion rate varied between patients, but there was a general trend toward lower urinary clearance in patients with more extensive bone metastasis.
The safety profile of [177Lu]Lu-EDTMP was generally favorable, with mild and transient Grade II myelotoxicity observed, particularly in patients with extensive metastatic bone disease. Recovery occurred without intervention, and no significant hepatotoxicity or nephrotoxicity was noted. Our analysis of the study data indicates that underlying conditions such as pre-treatment low counts, marrow replacement by extensive disease, tumor type (prostate cancer), and the percentage of skeletal uptake exert a greater impact than the amount of radionuclide administered. Our data confirm that [177Lu]Lu-EDTMP has lower toxicity than [153Sm]Sm-EDTMP, as transient Grade 3 or Grade 4 hematological toxicity was observed in 10% of patients with [153Sm]Sm-EDTMP [33]. These findings support the use of [177Lu]Lu-EDTMP as a safe and effective option for managing bone pain in prostate and breast cancer patients, offering similar efficacy to [153Sm]Sm-EDTMP but with a more favorable toxicity profile [34–40].
Clinical aspects
A sensitive and precise method to quantify bone uptake is essential. Fogelman et al. introduced a method for quantifying bone uptake to measure whole-body retention of bone-seeking tracers at 24 h post-injection, which has been extensively used [41]. Unfortunately, this method is of limited value because whole-body retention measurements are not routinely available in nuclear medicine departments [42]. Brenner et al. proposed a method to calculate skeletal uptake, soft tissue retention, and urinary excretion of bone-seeking radiopharmaceuticals based on whole-body scanning [32, 34]. The original method developed for [99mTc]Tc-diphosphonates was modified by introducing an additional background region of interest (ROI) to correct for Bremsstrahlung caused by β particles. We followed this method in the present study to quantify the bone uptake, soft tissue retention, and urinary excretion of [177Lu]Lu-EDTMP.
Bone uptake ratios showed rapid and progressive localization in bone lesions over time, with maximum ratios at 48 h and effective retention up to 14 days. No activity retention was observed in organs other than bone, reinforcing the targeted nature of the treatment. This selectivity is critical in minimizing radiation exposure to surrounding healthy tissues [35]. Poor correlation between bone uptake and the amount of injected activity again implies that bone uptake depends upon the level of osteoblastic activity (remodeling) in metastatic lesions. The highest uptake of [177Lu]Lu-EDTMP was seen in the patient with the most extensive metastatic lesions. Qualitatively, complete concordance was found between the images of [99mTc]Tc-MDP and [177Lu]Lu-EDTMP at 30 min, 4 h, 8 h, 24 h, 48 h, 7 days, and 14 days.
Preclinical studies have established that no toxicity occurs up to activities of 2.6 GBq of [177Lu]Lu-EDTMP. A reasonable best compromise between expected efficacy in terms of bone pain palliation and bone marrow toxicity in patients with painful bone metastasis from either prostate or breast cancer would be administering activities of [177Lu]Lu-EDTMP in the range of 1.3 to 2.6 GBq [34].
In the present study, patients were divided into two arms to study clinical efficacy. The onset of pain relief was observed within one week in both the low (1.3 GBq) and high (2.6 GBq) activity groups, with longer and more significant pain relief in the high-activity group. Wide variation was observed among patients in both arms in terms of response rate, duration of pain relief, and relapse. Notably, patients with a smaller metastatic burden experienced substantial relief even with lower doses. Reduced analgesic use and improved mobility were more prominent in the high-activity group, reflecting the enhanced clinical efficacy of the higher dosage.
These findings are consistent with other research comparing [177Lu]Lu-EDTMP and [153Sm]Sm-EDTMP, demonstrating that both treatments deliver similar radiation doses to bone metastases and achieve comparable palliative pain response rates (75%–80%) [39–44]. Additionally, combined therapy with both radiopharmaceuticals has proven safe, offering pain relief or reduction in most patients [35]. Furthermore, [177Lu]Lu-EDTMP has shown improved toxicity profiles and significant efficacy, with treatment leading to notable improvements in visual analog score (VAS), overall pain response, and Karnofsky performance status (KPS) [34–36].
Moreover, PSA levels decreased significantly in both activity groups, supporting the combination of radiopharmaceuticals with other anti-tumor therapies for enhanced efficacy [45]
Conclusion
Our study suggest that [177Lu]Lu-EDTMP demonstrates significant potential as an effective and safe option for bone pain palliation in cancer patients with skeletal metastases. Its favorable biokinetic properties, including substantial bone uptake, rapid blood clearance, efficient urinary excretion, swift soft tissue clearance, and prolonged bone retention of radioactivity, make it an ideal alternative to existing therapies such as [153Sm]Sm-EDTMP. Furthermore, the clinical efficacy of [177Lu]Lu-EDTMP is notably enhanced with higher doses (2.6 GBq), showing improved pain relief compared to lower doses (1.3 GBq), without the occurrence of Grade III toxicity.
The radiopharmaceutical's selective accumulation in osteoblastic lesions, minimal toxicity, and improved clinical outcomes, such as reduced analgesic requirements and increased mobility, make it a promising candidate for multimodal therapy in metastatic bone disease. The biodistribution studies confirm that [177Lu]Lu-EDTMP is predominantly taken up by bone tissue, minimizing systemic exposure and reinforcing its therapeutic effectiveness.
Overall, [177Lu]Lu-EDTMP positions itself as a viable alternative for bone pain palliation, particularly in regions with limited access to other isotopes like [153Sm]Sm-EDTMP. Despite these promising results, further clinical trials are necessary to establish its long-term safety and therapeutic efficacy fully. The combination of its optimal physical, chemical, and biokinetic properties, along with minimal toxicity, underscores its potential as an ideal treatment option for cancer patients with bone metastases.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Shabana Saeed, Ghazal Jameel, and Farida Qureshi. The first draft of the manuscript was written by Francesco Giammarile and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The research was partially sponsored by the International Atomic Energy Agency (IAEA) through a specific Coordinated Research Project (E13033).
Declarations
Conflict of interest
The author declared no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.URL:http:/www.insightec.com/135-en-r10/BoneMetastasis.aspx (Pain Palliation of Bone Metastasis). Last accessed August 23, 2024.
- 2.Chiacchio S, Borso E, Alsharif AA, Boni G, Mariani G. Radiopharmaceuticals for pain palliation therapy in patients with skeletal metastasis, and their possible integration with chemotherapy. AJ. 2010;13(50):1–5. [Google Scholar]
- 3.Criteria for Palliation of Bone Metastases. TECDOC-1549. IAEA, Vienna, 2007. http://www.pub.iaea.org/MTCD/publications/PDF/te_1549_web.pdf. Last accessed August 23, 2024.
- 4.Robert EC. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20):6243–9. [DOI] [PubMed] [Google Scholar]
- 5.Luc AM, Vakaet L, Tom B. Pain control by ionizing radiation of bone metastasis. Int J Dev Biol. 2004;48:599–606. [DOI] [PubMed] [Google Scholar]
- 6.Choi JY. Treatment of bone metastasis with bone-targeting radiopharmaceuticals. Nucl Med Mol Imaging. 2018;52:200–7. 10.1007/s13139-017-0509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handkiewicz-Junak D, Poeppel TD, Bodei L, Aktolun C, Ezziddin S, Giammarile F, et al. EANM guidelines for radionuclide therapy of bone metastases with beta-emitting radionuclides. Eur J Nucl Med Mol Imaging. 2018;45:846–59. 10.1007/s00259-018-3947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado Bolton RC, Giammarile F. Bone radionuclide therapy and increased survival with radium-223 is the way to go for nuclear medicine: the offer that oncologists cannot refuse. Eur J Nucl Med Mol Imaging. 2018;45:822–3. 10.1007/s00259-017-3913-z. [DOI] [PubMed] [Google Scholar]
- 9.Almeida LS, Etchebehere ECSC, García Megías I, Calapaquí Terán AK, Hadaschik B, Colletti PM, et al. PSMA radioligand therapy in prostate cancer: where are we and where are we heading? Clin Nucl Med. 2024;49:45–55. 10.1097/RLU.0000000000004919. [DOI] [PubMed] [Google Scholar]
- 10.Kratochwil C, Fendler WP, Eiber M, Baum R, Bozkurt MF, Czernin J, et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2019;46:2536–44. 10.1007/s00259-019-04485-3. [DOI] [PubMed] [Google Scholar]
- 11.Kratochwil C, Fendler WP, Eiber M, Hofman MS, Emmett L, Calais J, et al. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2023;50:2830–45. 10.1007/s00259-023-06255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elboga U, Kilbas B, Sahin E, Cayırlı YB, Eryilmaz K, Begec T, Bakar HE, Mercanoglu G, Celen YZ. An automated synthesis of 177Lu-EDTMP as an efficient bone-seeking therapeutic radiopharmaceutical. Eur Rev Med Pharmacol Sci. 2021;25(14):4829–34. [DOI] [PubMed] [Google Scholar]
- 13.Askari E, Harsini S, Vahidfar N, Divband G, Sadeghi R. 177Lu-EDTMP for metastatic bone pain palliation: a systematic review and meta-analysis. Cancer Biother Radiopharm. 2021;36:383–90. [DOI] [PubMed] [Google Scholar]
- 14.American Cancer Society. Cancer facts and figures. 2007. URL: http://www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf. Last accessed August 23, 2024.
- 15.Joseph G. Rajendran. Therapeutic Raidioisotopes. In: Silberstein EB, eds. Nuclear Medicine Therapy, 3rd ed. Informa health care New York, NY, 2007.
- 16.URL: http://www.mydr.com.au/printerfriendly.asp?article=4190. Last accessed August 23, 2024.
- 17.Thonos L, Mylona S, Galani P, et al. Radiofrequency ablation of osseous metastases for the palliation of pain. Skeletal Radiol. 2008;37(3):189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkert WA, Hoffman TJ. Therapeutic radiopharmaceuticals. Chem Rev. 1999;99:22–69. [DOI] [PubMed] [Google Scholar]
- 19.Hosain F, Spencer RP. Radiopharmaceuticalsfor palliation of metastatic osseous lesions: Biologic and physical background. Semin Nucl Med. 1992;22:11. [DOI] [PubMed] [Google Scholar]
- 20.Cheung A, Driedger AA. Evaluation of radioactive phosphorus in the palliation of metastatic bone lesions from carcinoma of breast and prostate. Radiology. 1980;134:209. [DOI] [PubMed] [Google Scholar]
- 21.O’Mara RE. New P-32 compounds in therapy of bone lesions. In: Therapy in nuclear medicine (Spencer RP, ed.). Grune and Stratton, New York, 1978, p. 257.
- 22.Robinson RG, Spicer JA, Preston DF, et al. Treatment of metastatic bone pain with stroncium-89. Nucl Med Biol. 1987;14:219. [DOI] [PubMed] [Google Scholar]
- 23.Shine J, Amir D, Soskolne WA, et al. Correlation between uptake of technetium, calcium, phosphate and mineralization in rat tibial bone repair. J Nucl Med. 1990;31:2011. [PubMed] [Google Scholar]
- 24.Pillai MRA, Chakraborty S, Das T, et al. Production logistics of 177Lu for radionuclide therapy. Appl Radiat Isot. 2003;59:109. [DOI] [PubMed] [Google Scholar]
- 25.Goeckeler WF, Edwards B, Volkert WA, et al. Skeletal localization of samarium-153 chelates: Potential therapeutic bone agents. J Nucl Med. 1987;28:495. [PubMed] [Google Scholar]
- 26.Rutty SGA, Arguelles MG, Bottazzini DL, Furnari JC, Gomez Parada I, Rojo AM, Vera RH. Lutetium-177-EDTMP for bone pain pallation. Preparation, biodistribution and pre-clinical studies. Publicado Radiochim. 2000;88(3–4):157–61. [Google Scholar]
- 27.Ketring AR. 153Sm-EDTMP and 186Re-HEDP as bone therapeutic radiopharmaceuticals. Nucl Med Biol. 1987;14:223. [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty S, Das T, Unni PR, et al. 177Lu-labeled polyaminophosphonates as potential agents for bone pain palliation. Nucl Med Commun. 2002;23:67–75. [DOI] [PubMed] [Google Scholar]
- 29.Máthé D, Balogh L, Polyák A, Király R, Márián T, Pawlak D, et al. Multispecies animal investigation on biodistribution, pharmacokinetics and toxicity of 177Lu-EDTMP, a potential bone pain palliation agent. Nuclear Med Biol. 2010;37(2):215–26. [DOI] [PubMed] [Google Scholar]
- 30.Bal C, Arora G, Kumar P, Damle N, Das T, Chakraborty S, Banerjee S, Venkatesh M, Zaknun JJ, Pillai MR. Pharmacokinetic, dosimetry and toxicity study of 1⁷⁷Lu-EDTMP in patients: phase 0/I study. Curr Radiopharm. 2016;9(1):71–84. [DOI] [PubMed] [Google Scholar]
- 31.Singh A, Richard A, Farhangi M, et al. Human pharmacokientics of samarium-153-EDTMP in metastatic cancer. J Nucl Med. 1989;30:1814–8. [PubMed] [Google Scholar]
- 32.Brenner W, Kampen WU, Kampen AM, Henze E. Skeletal uptake and soft-tissue retention of 186Re-HEDP and 153Sm-EDTMP in patients with metastatic bone disease. J Nucl Med. 2001;42:230–6. [PubMed] [Google Scholar]
- 33.Serafini AN. Samarium Sm-153 lexidronam for the palliation of bone pain associated with metastases. Cancer. 2000;88:2934–9. [DOI] [PubMed] [Google Scholar]
- 34.Brenner W, Bohuslavizki KH, Sieweke N, Tinnemeyer S, Clausen M, Henze E. Quantification of diphosphonate uptake based on conventional bone scanning. Eur J Nucl Med. 1997;24:1284–90. [DOI] [PubMed] [Google Scholar]
- 35.Yuan J, Liu C, Liu X, et al. Efficacy and safety of 177Lu-EDTMP in bone metastatic pain palliation in breast cancer and hormone refractory prostate cancer: A phase II study. Clin Nucl Med. 2013;38:88. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal KK, Singla S, Arora G, et al. 177 Lu-EDTMP for palliation of pain from bone metastases in patients with prostate and breast cancer: A phase II study. Eur J Nucl Med Mol Imaging. 2015;42:79. [DOI] [PubMed] [Google Scholar]
- 37.Alavi M, Omidvari S, Mehdizadeh A, et al. Metastatic bone pain palliation using 177Lu-ethylenediaminetetramethylene phosphonic acid. World J Nucl Med. 2015;14:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma S, Singh B, Koul A, et al. Comparative therapeutic efficacy of 153Sm-EDTMP and 177Lu-EDTMP for bone pain palliation in patients with skeletal metastases: Patients’pain score analysis and personalized dosimetry. Front Med. 2017;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thapa P, Nikam D, Das T, et al. Clinical efficacy and safety comparison of 177Lu-EDTMP with 153Sm-EDTMP on an equiactivity basis in patients with painful skeletal metastases. J Nucl Med. 2015;56:1513. [DOI] [PubMed] [Google Scholar]
- 40.Singh B, Prashar S, Koul A, et al. Comparative therapeutic efficacy of 177Lu-EDTMP and 153Sm-EDTMP in bone pain palliation in cancer patients with multiple skeletal metastases. J Nucl Med. 2016;57(Suppl 2):1441. [Google Scholar]
- 41.Fogelman I, Bessent RG, Turner JG, Citrin DL, Boyle IT, Greig WR. The use of whole body retention of Tc-99m diphosphonate in the diagnosis of metabolic bone disease. J Nucl Med. 1978;19:270–5. [PubMed] [Google Scholar]
- 42.Holmes RA. Quantification of skeletal Tc-99m labeled phosphates to detect metabokic bone disease. J Nucl Med. 1987;19:330–1. [PubMed] [Google Scholar]
- 43.Eary JF, Collins C, Stabin M, et al. Samarium-153-EDTMP biodistribution and dosimetry estimation. J Nucl Med. 1993;34:1031–6. [PubMed] [Google Scholar]
- 44.Hyldstrup L, Mogensen N, Jensen GF, McNair P, Transbol I. Urinary Tc-99m-diphosphonate excretion as a simple method to quantify bone metabolism. Scand J Clin Lab Invest. 1984;44:105–9. [DOI] [PubMed] [Google Scholar]
- 45.Alavi M, Khajeh-Rahimi F, Yousefnia H, et al. 177Lu/153Sm-Ethylenediamine tetramethylene phosphonic acid cocktail: a novel palliative treatment for patients with bone metastases. Cancer Biother Radiopharm. 2019;34:280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from Dr Shabana Saeed, responsible of data collection and analysis, on reasonable request.