Abstract
(p)ppGpp is the master regulator of bacterial stress responses, orchestrating cellular physiology via the stringent response to promote survival and adaptation. In response to nutritional challenges and stress, (p)ppGpp extensively rewires the transcriptome. Here, we demonstrate that (p)ppGpp production in Pseudomonas aeruginosa is gradual and relative to stress severity, rather than binary (on/off). Transcriptomic analysis reveals that (p)ppGpp ensures proportionate cellular responses to stress by imposing a layer-by-layer regulation of gene expression. These effects intensify as (p)ppGpp levels rise, with up to a quarter of the genome differentially regulated at maximal levels. Initial increases in (p)ppGpp reduce growth and metabolism while suppressing motility and pyocyanin production. At higher levels, biofilm-related genes are upregulated at the expense of virulence genes, promoting the formation of condensed biofilms. Finally, (p)ppGpp-driven reprogramming induces antimicrobial tolerance, particularly under biofilm conditions, independently of its effects on growth.
Subject terms: Bacteriology, Biofilms, Pathogens
Introduction
Pseudomonas aeruginosa exhibits remarkable versatility in adapting to a wide range of terrestrial and aquatic habitats, contributing to its ecological success. Beyond its environmental niche, P. aeruginosa has emerged as a significant opportunistic pathogen, responsible for severe acute and chronic infections in various clinical settings. Its versatility as an opportunistic pathogen makes it a significant threat in healthcare settings, where it can cause severe infections in immunocompromised individuals, burn patients, and those with cystic fibrosis (CF)1,2. In CF patients, P. aeruginosa demonstrates resilience by adopting a biofilm mode of growth in the respiratory tract, where it persists despite aggressive antimicrobial therapy3. The ongoing inflammation and structural changes in the lungs of CF patients exert strong selective pressures, yet specific P. aeruginosa clones establish lifelong infections in these individuals2,4,5.
P. aeruginosa has developed sophisticated molecular mechanisms to rapidly sense and adapt to nutrient limitations or other environmental stresses. This adaptability is facilitated by the activation of the stringent response, which relies on nucleotide-based signalling pathways to efficiently coordinate essential cellular processes and mount a swift response—not only in P. aeruginosa but also in other major bacterial pathogens6,7. Central to the stringent response are two hyperphosphorylated derivatives of GTP and GDP, known as guanosine pentaphosphate and guanosine tetraphosphate (collectively referred to as (p)ppGpp). In Beta- and Gammaproteobacteria, the synthesis and hydrolysis of (p)ppGpp are mediated by the enzymes RelA and SpoT, namesakes of the widely distributed RelA-SpoT Homologue (RSH) family of enzymes8–10. The most studied member of the RSH family is RelA. Its (p)ppGpp synthetic activity depends on the accumulation of deacylated tRNAs, triggered by direct amino acid starvation11–14.
With the onset of the stringent response, (p)ppGpp coordinates diverse adaptations to rapidly synchronise vital biological processes necessary for growth and stress survival15–19. This is accomplished through direct binding to a variety of enzymes and modifying their activity18,20,21. One of the best studied targets of (p)ppGpp is the RNA polymerase (RNAP). In Gammaproteobacteria, together with its cofactor DksA, (p)ppGpp binds to RNAP to rewire the transcriptome22,23. In addition to the transcriptional machinery, (p)ppGpp targets key components that are essential for replication, translation, ribosome assembly and metabolism18,20,21. The (p)ppGpp response plays an essential role in regulating bacterial virulence, facilitates survival during host invasion, and has been implicated in antibiotic tolerance and resistance16,24–29.
To better understand the downstream regulatory networks of (p)ppGpp in P. aeruginosa, we performed transcriptomic analysis before and after varying levels of amino acid starvation. Our findings reveal a novel aspect of the (p)ppGpp response, showing it is not a simple on/off switch. Instead, (p)ppGpp levels rise in proportion to the severity of stress, accompanied by a gradual slowdown in growth. This increase in (p)ppGpp leads to differential gene regulation, with more genes becoming involved as stress escalates. Under mild stress, elevated (p)ppGpp levels alter metabolism and negatively affect motility, promoting a sessile state. In more severe stress conditions, higher (p)ppGpp levels enhance quorum sensing and alginate-related genes, driving the formation of compact biofilms, while simultaneously downregulating ribosome biogenesis and expression of virulence factors. Additionally, we demonstrate that (p)ppGpp induces antibiotic tolerance, particularly under biofilm-forming conditions, independent of its effect on growth. These findings highlight the intricate regulatory role of (p)ppGpp in bacterial survival, adaptability, and antibiotic tolerance.
Results
SHX treatment results in growth arrest and (p)ppGpp accumulation in P. aeruginosa PA14
Serine hydroxamate (SHX) is a serine analogue that inhibits the acylation of seryl-tRNA30. Exposure to SHX causes accumulation of deacylated seryl-tRNA, which activates the RelA-dependent stringent response, increasing cellular (p)ppGpp levels and ultimately leading to growth arrest30–32. Initially, we tested the effect of adding a broad concentration range of SHX (10 – 1000 µM) to exponentially growing P. aeruginosa PA14. As shown in Fig. 1A, addition of SHX is inhibitory to growth in a dose-dependent manner. SHX treatment below 50 µM had no or only modest effects on growth (Supplementary Fig. 1). The growth inhibitory effect became more apparent with higher SHX treatments. With 100 and 500 µM SHX, the growth rates were 0.4 and 0.26 doublings per hour, respectively. SHX at concentrations above 1000 µM resulted in a severe growth perturbation followed by recovery and resumption of growth (discussed below) (Supplementary Fig. 1). The concentration of SHX required to achieve half-maximal inhibition of growth rate (IC50) was determined by fitting the data to the Hill equation, yielding a value of 128 ± 24 µM (Fig. 1A).
Fig. 1. Dose-dependent inhibition of P. aeruginosa PA14 growth and gradual (p)ppGpp accumulation with increasing serine hydroxamate (SHX) concentrations.
A Exponentially growing cultures of P. aeruginosa PA14 were treated with increasing levels of serine hydroxamate (SHX), and the growth rates were determined in triplicates. The 50% inhibitory concentration IC50 of SHX was calculated by using the Hill equation. B The cellular levels of (p)ppGpp were determined 30 min post treatment with increasing SHX levels (see ‘Materials and Methods’ section) and shown as a fraction over the total guanine nucleotide pool. Significance was tested by using the Wilcoxon test (***P ≤ 0.0005). C Linear relation between growth rate and the reciprocal of (p)ppGpp accumulation for different SHX treatments (0, 100 µM, 500 µM, and 1000 µM).
We measured the cellular levels of (p)ppGpp in P. aeruginosa PA14 during exponential growth and 30 min after the addition of SHX at three concentrations: 100 µM (just below the IC50, referred to as ‘mild’ stringent response) 500 µM (referred to as ‘intermediate’ stringent response), and 1000 µM (growth inhibiting, referred to as ‘acute’ stringent response). A mild stringent response resulted in a 1.33-fold increase in the (p)ppGpp fraction within the total guanine nucleotide pool (ppGpp + pppGpp + GTP) compared to levels observed during exponential growth. In contrast, induction of intermediate and acute stringent response led to significantly higher accumulation of the signalling nucleotides, with a 1.39-fold and 1.48-fold increase, respectively (Fig. 1B). Previous studies on Escherichia coli have shown that the cellular levels of (p)ppGpp are inversely correlated with growth rate33–35. In good agreement, we show that in P. aeruginosa PA14, the (p)ppGpp levels induced by increasing SHX treatments are also negatively correlated with the growth rates, with an R2-value of 0.95 ± 0.0014 (Fig. 1C). Overall, our data suggest that, in response to SHX treatment, the inhibitory effect on growth is dose dependent, and the accumulation of cellular levels of (p)ppGpp is graded.
(p)ppGpp imposes a layer-by-layer alteration in the transcriptome
Given the dose-dependent SHX effect on (p)ppGpp induction and growth, we set out to investigate the resulting transcriptional effects of increasing SHX concentrations. To this end, exponentially growing P. aeruginosa PA14 were subjected to mild, intermediate, and acute stringent response conditions (100, 500 or 1000 µM SHX treatment, respectively) for 30 min and their respective transcriptional profiles were compared to untreated cells (Supplementary Fig. 2).
Mild stringent response conditions revealed 227 differentially expressed genes (DEGs) (± 1.5-fold, adjusted p-value 0.05), representing just under 4% of the genome, with 85 up- and 142 downregulated genes (Fig. 2A, B). Exposure to intermediate stringent response conditions resulted in 1197 DEGs (20% of the genome, with 460 genes up- and 737 genes downregulated). Remarkably, the intermediate stringent response covered all but one of the genes affected by the lower SHX treatment. Almost all those differentially regulated genes and an additional 311 genes were affected when exposed to acute stringent response conditions (25% of the genome, 634 up- and 874 downregulated genes) (Fig. 2A, B). A substantial number of identified DEGs are of unknown function: 32 (14.1%) at mild, 203 (17%) at intermediate, and 257 (17%) at acute stringent response conditions (Supplementary Table 1). Moreover, as stress severity increased, we observed not only a layer-by-layer activation of differentially regulated genes, but also a graded effect in gene expression levels, with increasing stress leading to more pronounced up or downregulation (Supplementary Table 1). Thus, the transcriptional response is finely tuned to the level of stress, with both the magnitude of gene expression and the number of regulated genes being adjusted accordingly.
Fig. 2. Differential regulation of genes upon activation of the stringent response.
A Number of genes that were negatively (red, left side) or positively (green, right) regulated 30 min post treatment of exponentially growing cultures of P. aeruginosa PA14 exposed to increasing concentrations of serine hydroxamate (SHX). B Venn diagram showing the overlapping numbers of genes that were differentially regulated upon exposure to different SHX concentrations.
(p)ppGpp impacts diverse cellular processes
We used the KEGG pathway36 and Gene Ontology (GO)37–39 databases to perform functional enrichment analysis on the DEGs identified across the treatment conditions. As (p)ppGpp levels increased, more pathways were engaged (Supplementary Fig. 3A, B). The general trend observed with stringent response activation is a downregulation of pathways essential for active metabolism and proliferation, and an upregulation of those involved in survival and stress management. Elevated (p)ppGpp levels negatively impact pathways like oxidative phosphorylation, the TCA cycle, glycolysis, fatty acid, peptidoglycan, and lipopolysaccharide biosynthesis, nucleic acid metabolism, flagellar assembly, secretion systems (type I, II, III, and VI), and ribosome biogenesis (Supplementary Fig. 4). Notably, ribosome biogenesis was unaffected by lower SHX treatments and was only downregulated under acute conditions (Supplementary Table 1, Supplementary Fig. 4).
In contrast, while several amino acid metabolic pathways were downregulated, serine metabolism was upregulated, allowing cells to overcome SHX-induced serine starvation and resume growth (Supplementary Fig. 1). Other upregulated pathways included fatty acid degradation, amino acid and geraniol degradation, aminoacyl-tRNA biosynthesis, and nitrogen metabolism. Pathways related to alginate and polysaccharide biosynthesis, cell adhesion, and biofilm formation were also positively regulated. These broad changes in pathway activity prompted further investigation into their key components and the regulatory factors differentially expressed upon stringent response activation.
Our transcriptomic analysis revealed that with the activation of the stringent response, genes essential for flagellar assembly and export (fliI, fliJ, fliQ, and fliR) were downregulated (Supplementary Table 1, Supplementary Fig. 4), as was flgK, crucial for flagellar hook assembly40. Deletion of flgK has been shown to disrupt flagellum function, abolishing swimming and swarming motilities41,42. Similarly, motC and motD, encoding the MotCD complex that controls flagellar motility43, were downregulated, while flgM, the FliA-specific anti-sigma factor (σ28), was upregulated. Overexpression of flgM is known to impair FliA-dependent flagellar gene expression, reducing motility44.
Further analysis showed that lasI and rhlR, involved in acyl-homoserine lactone (AHL)-mediated QS (las and rhl)45, were significantly upregulated (Supplementary Table 1, Supplementary Fig. 4). Sigma factor algU which modulates alginate biosynthesis genes46,47, was also upregulated during the stringent response. Ectopic expression of algU was previously linked to increased alginate production48. Furthermore, expression of algB, which is required for alginate biosynthesis49 and modulated by AlgU47, was upregulated. Post-transcriptional activation of AlgU is dependent on AlgW50, which was positively regulated by (p)ppGpp, and the anti-housekeeping sigma factor (RpoD, σ51), algQ, another alginate regulator52, was also upregulated (Supplementary Fig. 4).
RhlR acts as a regulator of rhlAB transcription53. The expression of rhlAB is the key requirement for rhamnolipid synthesis in P. aeruginosa54,55. Rhamnolipid biosynthesis is essential for swarming motility56–58, as well as various aspects of biofilm development and maintenance59,60. Moreover, rhlG encodes an NADPH-dependent β-ketoacyl reductase, which is essential for the biosynthesis of fatty acids that serve as substrates for rhamnolipid production61,62. In addition to above-mentioned rhlR, expression of rhlA and rhlG was also upregulated upon activation of the stringent response.
Although AHL QS is upregulated, our transcriptomic data showed that the 2-heptyl-3-hydroxy-4-(1H)-quinolone (PQS) quorum-sensing system was suppressed by (p)ppGpp. Both the entire pqsABCDE operon, which encodes the PQS biosynthesis genes, and the phenazine biosynthesis operon were downregulated (Supplementary Table 1, Supplementary Fig. 4). The PQS system is essential for phenazine production, and inactivation of pqsE alone is sufficient to eliminate phenazine synthesis, including the redox-active pyocyanin63,64.
Further key regulatory genes, which impact diverse cellular functions, were activated by (p)ppGpp in P. aeruginosa. One such gene was cbrB, a global regulator involved in regulating metabolism, motility, biofilm formation, virulence, and antibiotic resistance, highlighting its central role in adaptive responses to stress65. Another gene upregulated by (p)ppGpp was dsbA, which plays a crucial role in type III secretion, intracellular survival, and defence against host immune clearance66. Additionally, the expression of rsmA, a key post-transcriptional regulator, was significantly downregulated even under mild activation of the stringent response67. RsmA, along with its homolog RsmN, modulates translation initiation at target mRNAs, influencing virulence and the switch between acute and chronic infection states. The downregulation of rsmA during (p)ppGpp activation shifts the balance towards chronic persistence, which is essential for the long-term survival of the pathogen within the host68,69. RsmA controls motility, biofilm formation, and virulence, and an rsmA null mutant exhibits a complete loss of swarming motility70.
The wspD and wspE genes, which are part of the Wsp signalling system responsible for surface sensing and biofilm formation, were also regulated by (p)ppGpp. These genes activate cyclic-di-GMP production through WspR under certain conditions to promote biofilm formation51. However, expression of wspD and wspE was negatively affected by (p)ppGpp induction, suggesting that the stringent response influences biofilm formation through multiple regulatory layers.
Finally, the expression of ribosome modulation factor (rmf) was upregulated in response to SHX treatment, particularly under acute stringent conditions, while hibernation promoting factor (hpf) did not show similar upregulation (Supplementary Table 1). Previous studies have shown rmf expression is (p)ppGpp-dependent in E. coli71, and high levels of the transcript were observed in P. aeruginosa biofilms72. RMF deactivates ribosomes until nutrient conditions improve73. Finally, expression of bosR, a recently described regulator of biofilm formation74, was upregulated with the onset of acute stringent response (Supplementary Table 1). Both RMF and BosR are implicated in the maintenance of biofilms72,74,75.
This complex network of gene regulation underscores the significant impact of (p)ppGpp in balancing virulence, motility, and biofilm formation, ultimately supporting the survival and persistence of P. aeruginosa in various environments. Our findings reveal that elevated (p)ppGpp levels lead to extensive transcriptional changes that significantly impact bacterial phenotypes. Therefore, we further investigated the downstream phenotypes associated with (p)ppGpp elevation, focusing on motility, rhamnolipid biosynthesis, biofilm formation, exopolysaccharide and pyocyanin production, and antibiotic tolerance.
(p)ppGpp impairs swimming and swarming motilities
In P. aeruginosa, lipopolysaccharides, rhamnolipid biosurfactants, and a functional flagellum are essential for efficient swimming and swarming motilities41,42,56–58,76. Lipopolysaccharides maintain membrane integrity and facilitate surface interactions, while rhamnolipids reduce surface tension to promote swarming. The flagellum is crucial for both bacterial movement and surface colonization. The extensive effects of (p)ppGpp on the expression of flagellar machinery components prompted us to investigate these motilities further.
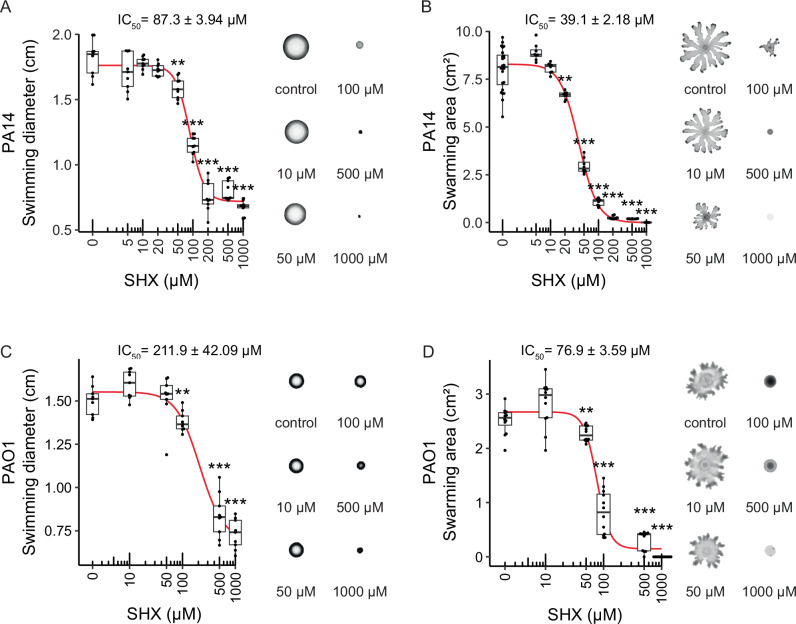
We first explored the phenotypic consequences of the incremental activation of the stringent response on swimming and swarming motilities. Upon exposure to increased SHX concentrations, we observed a negative effect on motility. At concentrations above 200 µM both swimming and swarming phenotypes were completely abolished (Fig. 3A, B). Swarming of P. aeruginosa PA14 was more sensitive to SHX treatment compared to swimming with an IC50 of 39.1 ± 2.18 µM and 87.3 ± 3.94 µM, respectively (Fig. 3A, B). We then tested the effect of (p)ppGpp on motility of the P. aeruginosa type strain PA01. We observed that both swimming (Fig. 3C) and swarming (Fig. 3D) of PA01 strain was also negatively affected with increased (p)ppGpp levels. Similar to PA14, swarming was more sensitive to SHX treatments than swimming, with IC50 values of 76.9 ± 3.59 µM and 211.9 ± 42.09 µM, respectively. To further explore the generality of this phenomenon, we subjected the two common lab strains of E. coli—MG1655 and BW25113—to increasing concentrations of SHX. In good agreement with our findings in P. aeruginosa, (p)ppGpp also impairs the swimming and swarming motility of the E. coli strains (Supplementary Fig. 5).
Fig. 3. (p)ppGpp induction negatively affects motility.
The ability of P. aeruginosa PA14 and PA01 to swim (A and C, respectively) or swarm (B and D, respectively) was determined in the presence of increasing serine hydroxamate (SHX) concentrations. Increase in the SHX concentration, associated with more severe induction of the stringent response, abolished both motility phenotypes. The experiments were repeated at least eight times and representative images of swimming and swarming motility agar plates are shown on the right side of each panel. The 50% inhibitory concentration (IC50) of SHX was calculated by using the Hill equation. Significance was tested by using the Wilcoxon test (*P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.0005).
Next, we assessed the effect of activating the stringent response on biosurfactant production, including rhamnolipids. Exponentially grown P. aeruginosa PA14 cultures were briefly treated with modest concentrations of SHX (below 200 µM) and then exposed to an oil droplet to detect surfactant production. As shown in Supplementary Fig. 6, slight elevations in cellular (p)ppGpp levels resulted in reduced surface tension and the formation of a clear zone due to oil displacement. Overall, our data align with the effects of (p)ppGpp on the transcriptome and suggest that elevated levels of (p)ppGpp impair motility, promoting a sessile lifestyle, while positively regulating biosurfactant biosynthesis.
(p)ppGpp promotes biofilm formation
Previous studies on (p)ppGpp-deficient P. aeruginosa strains have shown that these signalling nucleotides, in conjunction with quorum sensing, are essential for optimal biofilm formation and cell viability within biofilms45,77–79. Consistently, our enrichment analysis revealed that the stringent response positively regulates biofilm-associated pathways.
To determine whether the observed transcriptional changes translated into altered biofilm formation, we assessed the effect of increasing (p)ppGpp concentrations on biofilm formation in P. aeruginosa. Cultures were grown statically in 96-well plates for 12 h, followed by exposure to increasing concentrations of SHX and an additional 36 h of incubation. As shown in Fig. 4A, B, under mild stringent response conditions, biofilm formation in P. aeruginosa PA14 and PA01 strains remained unaffected. However, with higher SHX doses, resulting in intermediate to acute stringent responses, both strains formed more condensed biofilms. In contrast, the clinical isolate CH4780 showed a stronger response to increasing (p)ppGpp levels, with enhanced biofilm formation already observed under mild stringent response conditions (Fig. 4C). Notably, although each strain has different biofilm-forming capabilities, (p)ppGpp promoted further biofilm formation across all strains, with the extent of promotion varying between them. This differential response likely reflects strain-specific variations in (p)ppGpp signalling and/or biofilm pathways. Despite these differences, our findings underscore the fundamental role of (p)ppGpp signalling in biofilm regulation, highlighting its capacity to enhance biofilm formation across diverse strains.
Fig. 4. (p)ppGpp induction promotes formation of more condensed biofilms.
P. aeruginosa PA14 (A), PA01 (B), and CH4780 (C) cultures were statically incubated for 12 h and subsequently treated with increasing doses of SHX followed by an additional 36-hour incubation. Live/dead stained biofilms were imaged using confocal laser scanning microscopy followed by quantification of their respective biovolumes. The experiments were repeated minimum 8 times and the significance were tested by using the Wilcoxon test (*P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.0005). Representative images of the biofilms (top and cross-sectional views) for the SHX treated and non-treated cultures are shown on the right. Living cells are displayed in green (Syto9), dead cells in red (propidium iodide).
(p)ppGpp supresses pyocyanin production and promotes rugosity
We next tested the effect of (p)ppGpp on the production of phenazines. Consistent with our transcriptomic analysis, treatment of early and late planktonically grown P. aeruginosa PA14 (6 and 24 h, respectively) with SHX resulted in a significant reduction of pyocyanin production (Fig. 5A). Mild 100 µM SHX treatment reduced pyocyanin production by approximately half, while more acute activation of the stringent response resulted in complete absence of the green pigmentation in cultures (Fig. 5A).
Fig. 5. (p)ppGpp induction negatively affects phenazine pyocyanin production and promotes rugosity of colony biofilms.
A Treatment with increasing concentrations of SHX impaired the pyocyanin production of early and late planktonic P. aeruginosa PA14. The 50% inhibitory concentration IC50 of SHX was calculated by using the Hill equation. B Colony morphology of P. aeruginosa PA14 and PA01 strains with (p)ppGpp induction (500 µM SHX) or without (control) after seven days of growth.
Strains unable to produce phenazines exhibit increased exopolysaccharide production and altered colony morphologies, characterized by pronounced wrinkle formation (rugosity) on nutrient-rich solid medium80–82. Given the negative effect of (p)ppGpp on phenazine production, we tested SHX treatment’s impact on colony biofilms. As shown in Fig. 5B, supplementing the solid growth medium with SHX triggered colony wrinkle formation in P. aeruginosa PA14. SHX treatment also induced wrinkle formation in PA01 (Fig. 5B), indicating a (p)ppGpp-dependent effect regardless of the strains’ polysaccharide composition (PA01 primarily uses Psl, while PA14, which has a deletion in the psl operon, uses Pel83,84). The effect of (p)ppGpp on colony morphology mirrors that observed in phenazine-null mutant strains80–82. Overall, our findings suggest that (p)ppGpp signalling enhances rugosity, with suppression of phenazine production likely contributing to increased biofilm matrix production and changes in colony morphology.
(p)ppGpp induces antibiotic tolerance
The (p)ppGpp response has been associated with antibiotic tolerance (reviewed in refs. 26,29. To investigate the effect of increasing cellular levels of (p)ppGpp on antibiotic tolerance, we assessed the impact of SHX treatment on the killing efficiency of tobramycin and ciprofloxacin. These antibiotics belong to distinct functional classes, targeting the translation machinery and DNA gyrase/topoisomerase, respectively. Exponentially growing cultures were treated with 1000 µM SHX for 30 min to induce an acute stringent response or left untreated before exposure to 8-fold MIC of tobramycin or ciprofloxacin. In P. aeruginosa PA14, SHX treatment increased ciprofloxacin tolerance by approximately 1-log compared to the untreated control (Fig. 6A). However, SHX pre-treatment did not significantly affect tobramycin killing efficiency.
Fig. 6. (p)ppGpp induction promotes antimicrobial tolerance.
Kill curves of P. aeruginosa PA14 (A) and E. coli MG1655 (B) cultures treated with different antibiotics with or without 1000 µM SHX pre-treatment. Graphs indicate the mean of three independent replicates, and the error bars indicate the standard error. C, D P. aeruginosa PA14 biofilms, with or without 1000 µM SHX treatment, were exposed to increasing concentrations of tobramycin and ciprofloxacin and the survivors were scored 48 after hours.
We then assessed the killing efficiency of tobramycin and ciprofloxacin in E. coli MG1655. Consistent with previous findings in E. coli85,86, induction of an acute stringent response conferred strong tolerance to both antibiotics (Fig. 6B). SHX treatment provided over 3-log protection against tobramycin during the first 2–3 h, which diminished 5 h after stringent response induction. For ciprofloxacin, (p)ppGpp induction consistently conferred around 2-log protection (Fig. 6B). These effects were not due to changes in antibiotic susceptibility, as SHX treatment did not alter the MICs of tobramycin or ciprofloxacin in either E. coli or P. aeruginosa (Supplementary Table 2).
Finally, we investigated whether stringent response induction affects antibiotic tolerance under biofilm-forming conditions. Biofilms of P. aeruginosa PA14 were treated with 1000 µM SHX to induce an acute stringent response, followed by exposure to increasing concentrations of tobramycin or ciprofloxacin. (p)ppGpp induction conferred at least a 1-log increase in protection against both antibiotics across all tested concentrations (Fig. 6C, D). Notably, for tobramycin, SHX treatment provided over 2-log protection at 128 µg/ml and approximately 1-log protection at 256 µg/ml, but did not support survival at higher concentrations. For ciprofloxacin, (p)ppGpp induction conferred over 1-log protection at 128 and 256 µg/ml, and at 512 µg/ml, all untreated cells died, while only SHX-treated cells survived. Overall, our findings demonstrate that (p)ppGpp induction enhances antibiotic tolerance. This effect is significantly more pronounced in biofilms, whereas planktonic cells exhibit no tolerance to tobramycin and only low tolerance to ciprofloxacin, suggesting that (p)ppGpp-induced tolerance is not solely due to growth arrest.
Discussion
Elevated levels of (p)ppGpp orchestrate pleiotropic adaptations in bacteria in response to nutrient limitations and external stresses15,17,18,22. These adaptations involve extensive transcriptome reprogramming and post-transcriptional modulation of numerous cellular effectors11,18,20–22,87,88. Our findings reveal that in P. aeruginosa, (p)ppGpp levels as well as the subsequent (p)ppGpp-dependent transcriptional responses are graded and proportional to the severity of starvation stress. Mild activation of the stringent response regulates a core set of genes, while acute stress and higher (p)ppGpp levels expand this regulatory network, influencing up to a quarter of the genome. Furthermore, fold changes in the expression of differentially expressed genes (DEGs) intensify with increasing alarmone levels, allowing for a finely tuned, proportionate response to stress.
This graded regulatory mechanism may extend beyond P. aeruginosa. Previous studies in E. coli have provided preliminary evidence for such responses, with metabolic genes responding to subtle increases in (p)ppGpp before activation of the general stress regulon (RpoS) at maximal levels89. Similarly, in vitro transcriptional assays have shown that (p)ppGpp exhibits a dose-dependent effect on both negatively and positively regulated promoters, such as the ribosomal rrnB P1 and the threonine operon promoter, respectively90. Our findings expand on these observations, offering a more comprehensive view of graded gene regulation by (p)ppGpp and suggesting that this strategy may be widely conserved across bacterial species to ensure appropriately scaled responses to varying stress levels.
In addition to its role in transcriptional regulation, (p)ppGpp exerts significant post-transcriptional control by binding directly to numerous enzyme targets, either orthosterically (competing with substrates in the active site) or allosterically (binding to regulatory sites)11,18,20,21,87,88. These interactions influence key bacterial processes such as translation, replication, and metabolism. The affinity of (p)ppGpp for its target enzymes is central to its regulatory action, with its effects scaling in response to intracellular concentrations. Low levels of (p)ppGpp enable fine-tuned modulation of metabolic processes, while higher concentrations result in more pronounced repression of growth-related functions, ensuring bacterial survival under stress. We speculate that the affinity of (p)ppGpp for RNA polymerase may vary depending on the promoter architecture. This differential affinity could provide a mechanism for the graded transcriptional response, where the effect of (p)ppGpp on transcription initiation is proportional to the local context of the promoters it interacts with. Overall, these nuanced, graded regulatory mechanisms are key features of (p)ppGpp, enabling bacteria to adapt to varying stress intensities while maintaining cellular homeostasis and preserving growth potential once the stressor is resolved.
Motility provides a clear example of how (p)ppGpp influences bacterial behaviour through graded regulatory responses. Previous studies have shown that swarming motility is perturbed in (p)ppGpp-deficient strains, while swimming remains unaffected41,77,79,91. However, as (p)ppGpp levels increase, both motilities are negatively impacted, with swarming being more sensitive (Fig. 3). A similar graded response is observed with rhamnolipid production, a key factor in swarming. While rhamnolipids are essential for the swarming phenotype56–58, excessive production—whether through exogenous addition92, gene dysregulation56, or induction by (p)ppGpp (Supplementary Fig. 6)—can disrupt motility. These observations underscore the importance of finely tuned regulation of cellular responses, highlighting how (p)ppGpp coordinates the balance of motility and rhamnolipid levels to ensure appropriate phenotypic outcomes.
(p)ppGpp negatively affects growth, and reduced growth rates and lower metabolic activity have been linked to antimicrobial tolerance24,26,85,86,92–94. In line with previous findings85,86, we observed that starvation-induced cessation of E. coli planktonic growth enhances tolerance (Fig. 6B). However, we did not observe the same phenotype for P. aeruginosa (Fig. 6A). In the case of P. aeruginosa, despite efficient inhibition of growth, we observed only a modest 1-log protection against ciprofloxacin and no protection against tobramycin (Fig. 6A). Our findings indicate that growth inhibition alone does not necessarily lead to increased tolerance in P. aeruginosa, suggesting that the accepted paradigm that links low growth rates to tolerance is rather simplistic and warrants further studies. Nevertheless, (p)ppGpp-induction under biofilm-forming conditions protected P. aeruginosa populations against both tobramycin and ciprofloxacin (Fig. 6C, D). This suggests that beyond merely reducing metabolism and growth, (p)ppGpp might induce an active reprogramming, which includes the induction of biofilm-associated genes, that contribute to enhanced survival and antibiotic recalcitrance.
To survive and successfully cause an infection, all bacterial pathogens, including P. aeruginosa, rely on (p)ppGpp signalling to sense, respond, and adapt to the nutritional limitations as well as the host’s immune responses15–17,25,27. Our data suggests a model where the (p)ppGpp-mediated stringent response promotes P. aeruginosa survival, biofilm formation and antimicrobial tolerance (Fig. 7). After the establishment of an infection, initial mild stress reduces growth and metabolism (Figs. 1 and 2, Supplementary Table 1) as well as flagellar assembly to impair motility phenotypes, favouring a sessile lifestyle (Fig. 3). As the severity of stress intensifies, the expression of AHL-mediated QS systems and alginate-related genes are enhanced, promoting biofilm formation (Fig. 4). Bacterial cells, particularly those deeper embedded in the biofilm structures, experience greater stress and more intense nutrient limitations, which further intensifies the stringent response95. The heightened activation of the stringent response drives the hallmarks of a biofilm-associated chronic infection, including enhanced expression of biofilm-related genes and a cessation virulence factors expression, such as type I, II, III, and VI secretion systems, PQS-mediated QS, and phenazine biosynthesis (Fig. 5, Supplementary Fig. 4). Furthermore, the maximal (p)ppGpp response supports survival under such harsh conditions and activates the expression of genes involved in biofilm maintenance. The overall effects lead to metabolic alterations rendering the pathogens more resilient toward antimicrobial treatments, host defences, and phagocytosis, while ensuring viability during prolonged stress under biofilm growth conditions (Fig. 6C, D)28,96–100. Identifying genes involved in the stringent response not only advances our understanding of (p)ppGpp-mediated regulatory networks but also highlights potential targets for the development of novel antimicrobials and anti-biofilm therapies.
Fig. 7. (p)ppGpp induction leads to graded regulation of gene expression.
External stresses, including nutrient starvation, activate the stringent response, triggering the production of the signalling nucleotides (p)ppGpp from ATP and GTP/GDP. The levels of (p)ppGpp in the cell increase with stress severity inducing a two-pronged effect on gene expression. First, a layer-by-layer activation of differentially regulated genes occurs, with more genes affected as stress intensity increases. Second, there is a graded effect on gene expression, with higher (p)ppGpp levels resulting in more pronounced upregulation (green) or downregulation (red) of the affected genes. This results in the upregulation (green) or downregulation (red) of numerous essential cellular processes, ensuring a progressively scaled response to stress and enabling a proportionate, adaptive response to varying conditions.
Methods
Materials and methods overview
All bacterial strains, chemicals, reagents, kits, devices, and informatics tools used in this study are detailed in Supplementary Table 3. The following sections provide a detailed description of the specific methods employed in each experiment.
Bacterial strains and growth conditions
Bacterial strains were cultivated as follows unless otherwise stated. P. aeruginosa UCBPP-PA14, PA01, CH7480 or E. coli MG1655 and BW25113 were grown overnight in either LB (1 L; 5 g yeast extract, 7.5 g NaCl, 10 g tryptone) or M9 minimal medium (1 mM MgSO4, 0.1 mM CaCl2, 8.6 mM NaCl, 18.7 mM NH4Cl, 22 mM KH2PO4, 42.2 mM Na2HPO4, 0.01 mM FeSO4, 20 mM Glucose). After overnight incubation (16 h) at 37 °C, cells were diluted to the stated OD600 into fresh media and grown while shaking at 180 rpm. The cell density of the suspension was determined by measuring the optical density using a spectrophotometer (Libra S22; ID 80-2115-20, Biochrom). Where indicated, cultures were treated with DL-serine hydroxamate (SHX; ID S4503, Merck).
RNAseq sample preparation
To obtain SHX-treated bacterial samples for RNAseq analysis, P. aeruginosa UCBPP-PA14 was grown overnight in 10 ml of LB. The resulting cultures were washed twice in PBS after centrifugation at 10,000 × g for 5 min, before dilution to 0.1 OD600 into 200 ml M9 medium in a 1 l flask. When OD600 reached 0.5, the culture was split into 10 ml aliquots in 50 ml flasks. Aliquots were then treated with the indicated concentrations of SHX and incubated at 37 °C for a further 30 min.
Post treatment, 1 ml of each treated culture along with the untreated control was mixed at a 1:1 ratio with RNAprotect Bacteria Reagent (ID 76506, Qiagen) and incubated for 5 min at room temperature (RT). The treated cell pellets were collected by centrifugation for 10 min at 17000xg, the supernatant was discarded, and the samples were stored at -80 °C until use.
To lyse the cells, 100 µl Tris-EDTA (1x TE: 10 mM Tris-HCl pH 8.0, 0.1 mM EDTA; TE Buffer, ThermoFisher Scientific; ID 12090015) containing 800 µg/ml lysozyme was added to the samples followed by vortexing for 30 s. After an incubation for 10 min at RT with occasional brief vortexing, 350 µl RLT buffer (from the RNeasy Mini Kit; ID 74106, Qiagen) supplemented with 1% β-mercaptoethanol. The samples were inverted several times and incubated at -80 °C an hour. Lysates were subsequently thawed on ice and loaded onto QIAshredder columns (ID 79656, Qiagen) and centrifuged for 2 min at 14,000 rpm. Total RNA was then isolated from the flow-through using the RNeasy Mini Kit (ID 74106, Qiagen) according to manufacturer’s instructions. This was performed in triplicate.
Preparation of RNAseq libraries
The preparation of the sequencing libraries from total RNA was performed according to refs. 101,102. In brief, 1 µg of total RNA was mixed with 10x FastAP buffer (ID EF0651, ThermoFisher Scientific) and incubated for 90 s at 94 °C for fragmentation (fragment size 100–300 bp). DNAse treatment was then performed with TurboDNAse (ID AM1907, Invitrogen) and samples underwent a subsequent purification using magnetic beads in a bead-buffer ratio of 2:1 according to manufacturer’s instructions (RNAClean XP; ID A63987, Beckman Coulter).
T4 RNA Ligase 1 (ID M0437M, NEB) was used to ligate the 5’ phosphorylated barcode oligonucleotides to the fragmented RNA (sequences of barcode oligonucleotides given in Supplementary Table 4), ligation mixes were incubated 2 min at 70 °C. Barcoded samples were then pooled together and purified using the RNA Clean & Concentrator Kit (ID R1017, Zymo Research). rRNA was depleted from the pooled samples using RiboZero (ID RZMB12324, Illumina) and followed by a bead purification as described above. RNA fragments were transcribed into cDNA using the SMARTScribe® Reverse Transcriptase (ID 639536, Takara) with incubation at 42 °C for 60 min, then 70 °C for 10 min to inactive the enzyme. After subsequent exonuclease treatment (Exonuclease I; ID M0293L, NEB) and bead purification, the Illumina index barcode P5 and the library specific primer X01 were introduced into the sequence of the library fragments during the PCR enrichment using the AccuPrime Taq DNA Polymerase (ID 12346-086, Invitrogen). After a final bead purification step, the sequencing library preparation was completed. A Fragment Analyzer (Agilent) was used to determine fragment sizes present in the resulting library and the nucleic acid concentration in the sample was ascertained by Qubit 2.0 (Thermo Fisher Scientific). The final sequencing library was submitted to paired-end 50 bp Illumina sequencing to the Genome Analytics Facility (HZI) using a NovaSeq 6000 system.
Processing of the sequencing raw data and determination of the differentially expressed genes (DEG)
FastQC was used to check data quality (v0.11.9). For all samples approx. 6 million reads or more were obtained with a read length with more than 40 bp and the FastQC-internal average quality score of 36. Reads were trimmed and mapped to the PA14 reference genome (NC 008463.1) with bowtie2 (v1.3.1)103, only uniquely mapped reads were allowed for further consideration. Annotation of genomic features was performed using featureCounts (v2.0.1)104. For analysis of differentially expressed genes, the R package edgeR was used with the function glmQLFit105. The package implemented Benjamini-Hochberg procedure was used to correct the p-value for multiple testing. Genes were considered as significantly regulated with adjusted p-value ≥ 0.05. Raw and processed data are accessible at Gene Expression Omnibus (GEO) with the accession number GSE283319.
Functional enrichment analysis
For the pathway annotation of P. aeruginosa genes, website-based version of KOBAS-i (v3.0, https://bioinfo.org/kobas/genelist/) was used106. Significantly altered (SHX treated versus untreated samples; FDR adjusted p value ≤ 0.05) were submitted in the UniProtKB format to the Gene-list Enrichment variant of the web tool (Species: Pseudomonas aeruginosa UCBPP-PA14, Input type: UniProtKB AC). Results were then imported into R for further processing and calculation of the enrichment factor using the hypergeometric test (testing provided by KOBAS-i). For functional enrichment analysis of transcriptomes, the Gene Ontology System38,39 was used using all significantly differentially regulated genes. Genes were subjected to the GO biological functions using a custom R script the enrichment factor per biological process was calculated using a hypergeometric model (R function phyper).
RNAseq data visualization
To depict the overlaps in significantly regulated genes the R package eulerr was used (10.32614/CRAN.package.eulerr). To visualise differentially expressed genes for certain cellular pathways, relative changes in expression levels (log2-fold change, adjusted p-value ≥ 0.05) were used as input for a custom R script applying the R package pathview (https://pathview.r-forge.r-project.org/)107.
Growth rate determination
The growth of cultures grown for the purpose of RNAseq sample collection (described above) was monitored to determine the growth rates of SHX-treated samples. Cell density (OD600), as a proxy for growth, was measured regularly both before and after SHX treatment. Doubling time was calculated using the exponential portion of the growth curve. The experiment was performed in three biological replicates.
Microscopy of treated biofilms
Biofilm experiments were performed as previously described with slight modifications108. A P. aeruginosa PA14, PA01, or CH7480 overnight cultures were adjusted to an OD600 of 0.002 in fresh LB medium and 100 µL bacterial suspensions were used as inoculum for the biofilm growth within each well of a 96-well microtiter plate (µClear, Greiner Bio-One), sealed with an air-permeable foil (BREATseal cover foil, Greiner Bio-One). Plates were incubated at 37 °C for 12 h statically before addition of SHX (100 µM, 500 µM, 1000 µM and 2000 µM). After a further 12 h incubation, cultures were dyed with using the LIVE/DEAD® BacLight® Bacterial Viability Kit (Molecular Probes, Life Technologies), differentiating living cells (2.1 µM Syto9, green fluorescence) and dead cells (12.5 µM propidium iodide (PI), red fluorescence). After another 24 h of biofilm growth (48 h incubation in total), automated microscopy was performed with a confocal laser scanning microscope SP8 (Leica Microsystems) equipped with a HC PL APO 40×/1.10 W water immersion objective and the according application suite LAS X including the Matrixscreener module. Z-stacks covering 36 µm in height with a z-step size of 2 µm were acquired in the center of each well and with the following laser/HyD-detector settings: Syto9, 5% of a 488 nm laser and emission in the range of 500–550 nm and PI, 15% of a 561 nm laser and emission in the range of 675–725 nm. For automated image acquisition, a number of pre-defined detector gain settings were assigned to avoiding under- and over-exposed images for the different treatments. Image analysis was performed with a customized programmed solution for the Developer XD 64 software (Definiens) and visualization of the sectioning projections with the software Imaris (version 7.6, Bitplane).
Swarming and swimming motility assays
Motility assays for P. aeruginosa were conducted following the procedures outlined previously41,109. P. aeruginosa PA14 and PA01 overnight cultures were washed with PBS and diluted to OD600 0.1 in fresh M9 minimal medium supplemented 20 mM Glucose and incubated at until OD600 0.5. An aliquot of the growing culture was concentrated by centrifugation in PBS to 2 OD600. 2 µL of the adjusted culture was spotted onto swarming plates (BM2, 0.4% glucose, 0.5% agar) and incubated at 30 °C in a humidity chamber. Swimming plates (BM2, 0.4% glucose, 0.1% CAS amino acids 0.1% agar) were inoculated by insertion of an impregnated toothpick into the agar and were subsequently incubated at 37 °C before imaging. Incubation time for both swimming and swarming for 16 to 18 h.
Motility assays for E. coli MG1655 and BW25113 strains were conducted similar to the motility experiments for P. aeruginosa but with some changes in the liquid and solid motility medium as previously described110. In brief, 1 ml liquid motility medium (1% tryptone, 0.25% NaCl, and 0.5% Glucose) were inoculated with a single colony and incubated for 6 h, bacterial cultures were adjusted to OD600 2 in PBS. Swarming and swimming plates were also prepared as previously described110 and were then inoculated analogous to the procedure described above for P. aeruginosa. Swarming plates were incubated at 30 °C under humid atmosphere for approx. 24 h and swimming plates were incubated at 30 °C for 8 h.
Plates were imaged using an Epson Perfection V850 Pro document scanner. Swarming area and diameter of the swimming area on the plates were measured using the Fiji image processing software.
Colony morphology assays
Colony morphology assays were performed as described previously80–82. In brief, P. aeruginosa strains were grown overnight in LB medium, then 5 µl were spotted onto 1% agar plates containing 1% tryptone, supplemented with 40 µg/ml Congo red and 20 µg/ml Coomassie blue. Plates were imaged after 7 days of incubation at room temperature (25 °C). Representative images of biological replicates are shown.
Antibiotic killing assay of planktonically grown cultures
P. aeruginosa PA14 or E. coli MG1655 cultures were grown overnight in M9 medium. Cultures were then washed and diluted to OD600 0.005 in 100 ml of fresh media and grown at 37 °C with shaking at 180 rpm. When cultures reached OD600 0.125, the culture was split into two pre-warmed flasks and grown for 30 min in the presence or absence of 1000 µM SHX. After pre-treatment, the culture was split again into 10 ml flasks with or without antibiotics at 8x MIC, (16 and 1 µg mL for Tobramycin and Ciprofloxacin, respectively for PA14 and 16 and 0.25 µg mL, respectively for MG1655). Aliquots of 1 ml were then removed at given time points, centrifuged at 4000 rpm in a microcentrifuge and washed three times with 1x PBS to remove antibiotics, before serially diluting and plating on LB agar. CFU/ml were counted after 16, 24, 48 and 72 h of incubation at 37 °C, to ensure that colonies with a slow wake up rate were accounted for. Surviving cells are calculated as percentage survival compared to time 0, (after pre-treatment with SHX and before addition of the antibiotic).
Antibiotic killing assay of statically grown stationary phase cultures
Killing assays were performed as previously described100. Briefly, 1 ml overnight cultures of either P. aeruginosa PA14 or E. coli MG1655 were grown overnight in LB medium and diluted to 0.002 OD600 in fresh LB media. 145 µl of culture was added to each well in a 96-well µClear microtiter plate (Greiner Bio-One), sealed with an air-permeable foil and grown statically in a humidity chamber at 37 °C for 12 h. 5 µl of SHX, resulting in a final concentration of 1 mM, or 5 µl of sterile H2O as control was added to each well and incubated for a further 36 h (48 h incubation total), before addition of 10 µl of ciprofloxacin or tobramycin at indicated concentrations. After a further 24 h incubation treated cultures were resuspended by vigorous pipetting (60x), serially diluted and spotted onto solid LB agar for viability counting after incubation at 37 °C. CFU/ml were ascertained by counting after 24, 48 and 72 h of incubation.
MIC determination by microtiter broth dilution
To determine the MIC of indicate strains in the presence or absence of SHX in our experimental conditions. M9 medium was supplemented with the indicated concentrations of SHX as well as increasing 2-fold concentrations of either ciprofloxacin or tobramycin. Media was then inoculated with 5 × 105 CFU/ml (OD600 of approximately 0.0005) from an overnight culture, as per European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (http://www.eucast.org/ast of bacteria/mic determination) and grown in flat-bottomed 96-well plates. After growth for 24 h at 37 °C with shaking at 180 rpm, the presence or absence of bacterial growth was scored by eye.
Pyocyanin production
Pyocyanin production assays were performed as previously described111,112. Briefly, 1 ml of overnight P. aeruginosa PA14 cultures was grown in M9 medium. The cultures were then diluted to 0.001 OD600 in fresh M9 medium and grown shaking at 37 °C until reaching an OD600 of 0.01. The cultures were treated with increasing concentrations of SHX (10, 20, 100, 200, and 1000 µM) or water as a control. After 6 and 24 h of incubation at 37 °C, 1 ml of bacterial suspension was collected. After centrifugation, pyocyanin was extracted by adding 1 ml of chloroform. Next, 600 µl of the organic phase was mixed with 200 µl of 0.1 M HCl, vortexed, and the absorbance of the aqueous phase was measured at 520 nm. Pyocyanin quantification (µg/mL) was determined by multiplying the absorbance by a factor of 17.072. Values were normalized to the OD600 of the culture.
Oil displacement assay
The oil displacement assay was performed at previously described113 with modifications. P. aeruginosa UCBPP-PA14 was to exponential phase (0.3 OD600) before being treated with SHX (50, 100, and 200 µM) or left untreated for 1 h. Subsequently, 20 µl of the cultures was added on top of 200 µl of mineral oil which was previously put onto the surface of 20 ml of distilled water in a petri dish (150 mm in diameter) to form a thin membrane. Media and 5% SDS were used as a negative and positive control, respectively.
(p)ppGpp measurements by thin-layer chromatography (TLC)
This experiment was performed as described earlier11,32,114. Overnight cultures of P. aeruginosa PA14 were diluted 100-fold in 5 mL of MOPS minimal medium supplemented with 0.2% glucose and 0.2 mM K2HPO4. At approx. OD600 0.5 they were back diluted 10-fold and left to grow for approx. 2 generations (OD600 approx. 0.2) in the presence of H332PO4 (100 mCi/ml). Subsequently amino acid starvation was induced by the addition of 100, 500, 1000 µM SHX. Thirty-microliter samples were withdrawn before and 30 min after addition of SHX and quenched by 6 µl of ice-cold 2 M formic acid. After a 10-min centrifugation at 14,000 rpm at 4 °C, 10 µl aliquots were loaded on polyethyleneimine (PEI) cellulose thin-layer chromatography (TLC) plates (Merck, Sigma), and separated by chromatography in 1.5 M potassium phosphate (pH 3.4). The plates were then visualized by phosphorimaging (Typhoon FLA 9500 GE Healthcare). Spot intensities were quantified using ImageJ and the (p)ppGpp quotient was expressed as a fraction of the total guanosine pool, i.e. pppGpp+ppGpp/pppGpp+ppGpp+GTP.
Supplementary information
Acknowledgements
S.H. is funded by the Novo Nordisk Foundation (NNF 18OC0033946), and received funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC 2155 “RESIST”—Project ID 390874280, within the SFB/TRR-298-SIIRI—Project-ID 426335750 and in the SPP 2389 (HA 3299/9-1, AOBJ: 687646), and from the Ministry of Science and Culture of Lower Saxony (Niedersächsisches Ministerium für Wissenschaft und Kultur) BacData, ZN3428. We are also grateful to Lars Bindslev for assistance with the graphics used in Fig. 7.
Author contributions
M.R. and S.H. coordinated the study and drafted the manuscript with contributions from all authors. M.R., F.E., K.T. and S.H. designed experiments and analysed the data. M.R., F.E., K.T., M.G., M.M., and M.P. performed experiments.
Data availability
Transcriptome data have been deposited in the NCBI Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE283319. All raw data are deposited in Mendeley and can be accessed at 10.17632/v4c9m3ys3h.1.
Code availability
Scripts for RNA-seq data processing and analysis are available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Susanne Häussler, Mohammad Roghanian.
Contributor Information
Susanne Häussler, Email: susanne.haeussler@helmholtz-hzi.de.
Mohammad Roghanian, Email: mohammad.roghanian@regionh.dk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41522-025-00795-7.
References
- 1.Gellatly, S. L. & Hancock, R. E. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog. Dis.67, 159–173 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Valentini, M., Gonzalez, D., Mavridou, D. A. & Filloux, A. Lifestyle transitions and adaptive pathogenesis of Pseudomonas aeruginosa. Curr. Opin. Microbiol.41, 15–20 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Costerton, J. W. Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol.9, 50–52 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Romling, U. et al. Epidemiology of chronic Pseudomonas aeruginosa infections in cystic fibrosis. J. Infect. Dis.170, 1616–1621 (1994). [DOI] [PubMed] [Google Scholar]
- 5.Marvig, R. L., Sommer, L. M., Molin, S. & Johansen, H. K. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet.47, 57–64 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Anderson, B. W., Fung, D. K. & Wang, J. D. Regulatory themes and variations by the stress-signaling nucleotide alarmones (p)ppGpp in bacteria. Annu. Rev. Genet.55, 115–133 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Pesavento, C. & Hengge, R. Bacterial nucleotide-based second messengers. Curr. Opin. Microbiol.12, 170–176 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Mittenhuber, G. Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA and SpoT proteins). J. Mol. Microbiol. Biotechnol.3, 585–600 (2001). [PubMed] [Google Scholar]
- 9.Atkinson, G. C., Tenson, T. & Hauryliuk, V. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One6, e23479 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jimmy, S. et al. A widespread toxin-antitoxin system exploiting growth control via alarmone signaling. Proc. Natl. Acad. Sci. USA117, 10500–10510 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roghanian, M. et al. p)ppGpp controls stringent factors by exploiting antagonistic allosteric coupling between catalytic domains. Mol. Cell81, 3310–3322.e3316 (2021). [DOI] [PubMed]
- 12.Winther, K. S., Roghanian, M. & Gerdes, K. Activation of the stringent response by loading of RelA-tRNA complexes at the ribosomal A-Site. Mol. Cell70, 95–105.e104 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Brown, A., Fernandez, I. S., Gordiyenko, Y. & Ramakrishnan, V. Ribosome-dependent activation of stringent control. Nature534, 277–280 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haseltine, W. A. & Block, R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc. Natl. Acad. Sci. USA70, 1564–1568 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauryliuk, V., Atkinson, G. C., Murakami, K. S., Tenson, T. & Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol.13, 298–309 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irving, S. E., Choudhury, N. R. & Corrigan, R. M. The stringent response and physiological roles of (pp)pGpp in bacteria. Nat. Rev. Microbiol.19, 256–271 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Potrykus, K. & Cashel, M. p)ppGpp: still magical?. Annu. Rev. Microbiol.62, 35–51 (2008). [DOI] [PubMed]
- 18.Gaca, A. O., Colomer-Winter, C. & Lemos, J. A. Many means to a common end: the intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis. J. Bacteriol.197, 1146–1156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnusson, L. U., Farewell, A. & Nystrom, T. ppGpp: a global regulator in Escherichia coli. Trends Microbiol.13, 236–242 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Steinchen, W., Zegarra, V. & Bange, G. p)ppGpp: magic modulators of bacterial physiology and metabolism. Front. Microbiol.11, 2072 (2020). [DOI] [PMC free article] [PubMed]
- 21.Steinchen, W. & Bange, G. The magic dance of the alarmones (p)ppGpp. Mol. Microbiol.101, 531–544 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Gourse, R. L. et al. Transcriptional Responses to ppGpp and DksA. Annu. Rev. Microbiol.72, 163–184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Vazquez, P., Dewey, C. N., Kitten, N., Ross, W. & Gourse, R. L. Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc. Natl. Acad. Sci. USA116, 8310–8319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole, K. Bacterial stress responses as determinants of antimicrobial resistance. J. Antimicrob. Chemother.67, 2069–2089 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Dalebroux, Z. D., Svensson, S. L., Gaynor, E. C. & Swanson, M. S. ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev.74, 171–199 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hobbs, J. K. & Boraston, A. B. p)ppGpp and the stringent response: an emerging threat to antibiotic therapy. ACS Infect. Dis.5, 1505–1517 (2019). [DOI] [PubMed]
- 27.Dalebroux, Z. D. & Swanson, M. S. ppGpp: magic beyond RNA polymerase. Nat. Rev. Microbiol.10, 203–212 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Nguyen, D. et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science334, 982–986 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deventer, A. T., Stevens, C. E., Stewart, A. & Hobbs, J. K. Antibiotic tolerance among clinical isolates: mechanisms, detection, prevalence, and significance. Clin. Microbiol. Rev.37, e0010624 (2024). [DOI] [PMC free article] [PubMed]
- 30.Tosa, T. & Pizer, L. I. Biochemical bases for the antimetabolite action of L-serine hydroxamate. J. Bacteriol.106, 972–982 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzger, S., Schreiber, G., Aizenman, E., Cashel, M. & Glaser, G. Characterization of the relA1 mutation and a comparison of relA1 with new relA null alleles in Escherichia coli. J. Biol. Chem.264, 21146–21152 (1989). [PubMed] [Google Scholar]
- 32.Turnbull, K. J., Dzhygyr, I., Lindemose, S., Hauryliuk, V. & Roghanian, M. Intramolecular interactions dominate the autoregulation of Escherichia coli stringent factor RelA. Front Microbiol.10, 1966 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryals, J., Little, R. & Bremer, H. Control of rRNA and tRNA syntheses in Escherichia coli by guanosine tetraphosphate. J. Bacteriol.151, 1261–1268 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarubbi, E., Rudd, K. E. & Cashel, M. Basal ppGpp level adjustment shown by new spoT mutants affect steady state growth rates and rrnA ribosomal promoter regulation in Escherichia coli. Mol. Gen. Genet.213, 214–222 (1988). [DOI] [PubMed] [Google Scholar]
- 35.Wu, C. et al. Cellular perception of growth rate and the mechanistic origin of bacterial growth law. Proc. Natl. Acad. Sci. USA119, e2201585119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanehisa, M., Sato, Y., Furumichi, M., Morishima, K. & Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res.47, D590–D595 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mi, H., Muruganujan, A., Ebert, D., Huang, X. & Thomas, P. D. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res.47, D419–D426 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet.25, 25–29 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Gene Ontology, C The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res.47, D330–D338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouteiller, M. et al. Pseudomonas Flagella: generalities and specificities. Int. J. Mol. Sci.22, 10.3390/ijms22073337 (2021). [DOI] [PMC free article] [PubMed]
- 41.Felgner, S. et al. Host-induced spermidine production in motile Pseudomonas aeruginosa triggers phagocytic uptake. Elife9, 10.7554/eLife.55744 (2020). [DOI] [PMC free article] [PubMed]
- 42.Ha, D. G., Kuchma, S. L. & O’Toole, G. A. Plate-based assay for swimming motility in Pseudomonas aeruginosa. Methods Mol. Biol.1149, 59–65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuchma, S. L. et al. Cyclic di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa PA14 requires the MotAB stator. J. Bacteriol.197, 420–430 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frisk, A., Jyot, J., Arora, S. K. & Ramphal, R. Identification and functional characterization of flgM, a gene encoding the anti-sigma 28 factor in Pseudomonas aeruginosa. J. Bacteriol.184, 1514–1521 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahator, S. D. & Zhang, L. Small is mighty–chemical communication systems in Pseudomonas aeruginosa. Annu. Rev. Microbiol.73, 559–578 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Flynn, J. L. & Ohman, D. E. Cloning of genes from mucoid Pseudomonas aeruginosa which control spontaneous conversion to the alginate production phenotype. J. Bacteriol.170, 1452–1460 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wozniak, D. J. & Ohman, D. E. Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. J. Bacteriol.176, 6007–6014 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim, S. K. et al. Inhibition of Pseudomonas aeruginosa alginate synthesis by ebselen oxide and its analogues. ACS Infect. Dis.7, 1713–1726 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Goldberg, J. B. & Dahnke, T. Pseudomonas aeruginosa AlgB, which modulates the expression of alginate, is a member of the NtrC subclass of prokaryotic regulators. Mol. Microbiol.6, 59–66 (1992). [DOI] [PubMed] [Google Scholar]
- 50.Cezairliyan, B. O. & Sauer, R. T. Control of Pseudomonas aeruginosa AlgW protease cleavage of MucA by peptide signals and MucB. Mol. Microbiol.72, 368–379 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Connor, J. R., Kuwada, N. J., Huangyutitham, V., Wiggins, P. A. & Harwood, C. S. Surface sensing and lateral subcellular localization of WspA, the receptor in a chemosensory-like system leading to c-di-GMP production. Mol. Microbiol.86, 720–729 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim, H. Y. et al. Alginate, inorganic polyphosphate, GTP and ppGpp synthesis co-regulated in Pseudomonas aeruginosa: implications for stationary phase survival and synthesis of RNA/DNA precursors. Mol. Microbiol.27, 717–725 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Medina, G., Juarez, K., Valderrama, B. & Soberon-Chavez, G. Mechanism of Pseudomonas aeruginosa RhlR transcriptional regulation of the rhlAB promoter. J. Bacteriol.185, 5976–5983 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soberon-Chavez, G., Aguirre-Ramirez, M. & Sanchez, R. The Pseudomonas aeruginosa RhlA enzyme is involved in rhamnolipid and polyhydroxyalkanoate production. J. Ind. Microbiol. Biotechnol.32, 675–677 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Zhu, K. & Rock, C. O. RhlA converts beta-hydroxyacyl-acyl carrier protein intermediates in fatty acid synthesis to the beta-hydroxydecanoyl-beta-hydroxydecanoate component of rhamnolipids in Pseudomonas aeruginosa. J. Bacteriol.190, 3147–3154 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caiazza, N. C., Shanks, R. M. & O’Toole, G. A. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J. Bacteriol.187, 7351–7361 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deziel, E., Lepine, F., Milot, S. & Villemur, R. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology149, 2005–2013 (2003). [DOI] [PubMed] [Google Scholar]
- 58.Tremblay, J., Richardson, A. P., Lepine, F. & Deziel, E. Self-produced extracellular stimuli modulate the Pseudomonas aeruginosa swarming motility behaviour. Environ. Microbiol.9, 2622–2630 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Davey, M. E., Caiazza, N. C. & O’Toole, G. A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol.185, 1027–1036 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pamp, S. J. & Tolker-Nielsen, T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol.189, 2531–2539 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bredenbruch, F. et al. Biosynthetic pathway of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines. J. Bacteriol.187, 3630–3635 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campos-Garcia, J. et al. The Pseudomonas aeruginosa rhlG gene encodes an NADPH-dependent beta-ketoacyl reductase which is specifically involved in rhamnolipid synthesis. J. Bacteriol.180, 4442–4451 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diggle, S. P. et al. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol.50, 29–43 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Gallagher, L. A., McKnight, S. L., Kuznetsova, M. S., Pesci, E. C. & Manoil, C. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol.184, 6472–6480 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeung, A. T., Bains, M. & Hancock, R. E. The sensor kinase CbrA is a global regulator that modulates metabolism, virulence, and antibiotic resistance in Pseudomonas aeruginosa. J. Bacteriol.193, 918–931 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ha, U. H., Wang, Y. & Jin, S. DsbA of Pseudomonas aeruginosa is essential for multiple virulence factors. Infect. Immun.71, 1590–1595 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chihara, K., Barquist, L., Takasugi, K., Noda, N. & Tsuneda, S. Global identification of RsmA/N binding sites in Pseudomonas aeruginosa by in vivo UV CLIP-seq. RNA Biol.18, 2401–2416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moscoso, J. A., Mikkelsen, H., Heeb, S., Williams, P. & Filloux, A. The Pseudomonas aeruginosa sensor RetS switches type III and type VI secretion via c-di-GMP signalling. Environ. Microbiol.13, 3128–3138 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Mulcahy, H. et al. Pseudomonas aeruginosa RsmA plays an important role during murine infection by influencing colonization, virulence, persistence, and pulmonary inflammation. Infect. Immun.76, 632–638 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heurlier, K. et al. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J. Bacteriol.186, 2936–2945 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izutsu, K., Wada, A. & Wada, C. Expression of ribosome modulation factor (RMF) in Escherichia coli requires ppGpp. Genes Cells6, 665–676 (2001). [DOI] [PubMed] [Google Scholar]
- 72.Williamson, K. S. et al. Heterogeneity in Pseudomonas aeruginosa biofilms includes expression of ribosome hibernation factors in the antibiotic-tolerant subpopulation and hypoxia-induced stress response in the metabolically active population. J. Bacteriol.194, 2062–2073 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prossliner, T., Skovbo Winther, K., Sorensen, M. A. & Gerdes, K. Ribosome Hibernation. Annu. Rev. Genet.52, 321–348 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Dostert, M. et al. BosR: A novel biofilm-specific regulator in Pseudomonas aeruginosa. Front. Microbiol.13, 1021021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katharios-Lanwermeyer, S. & O’Toole, G. A. Biofilm maintenance as an active process: evidence that biofilms work hard to stay put. J. Bacteriol.204, e0058721 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindhout, T., Lau, P. C. Y., Brewer, D. & Lam, J. S. Truncation in the core oligosaccharide of lipopolysaccharide affects flagella-mediated motility in Pseudomonas aeruginosa PAO1 via modulation of cell surface attachment. Microbiology155, 3449–3460 (2009). [DOI] [PubMed] [Google Scholar]
- 77.Pletzer, D. et al. Surfing motility is a complex adaptation dependent on the stringent stress response in Pseudomonas aeruginosa LESB58. PLoS Pathog.16, e1008444 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steinchen, W. et al. Dual role of a (p)ppGpp- and (p)ppApp-degrading enzyme in biofilm formation and interbacterial antagonism. Mol. Microbiol. 10.1111/mmi.14684 (2021). [DOI] [PubMed]
- 79.Xu, X. et al. Role of ppGpp in Pseudomonas aeruginosa acute pulmonary infection and virulence regulation. Microbiol Res.192, 84–95 (2016). [DOI] [PubMed] [Google Scholar]
- 80.Dietrich, L. E. et al. Bacterial community morphogenesis is intimately linked to the intracellular redox state. J. Bacteriol.195, 1371–1380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dietrich, L. E., Teal, T. K., Price-Whelan, A. & Newman, D. K. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science321, 1203–1206 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okegbe, C. et al. Electron-shuttling antibiotics structure bacterial communities by modulating cellular levels of c-di-GMP. Proc. Natl. Acad. Sci. USA114, E5236–E5245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colvin, K. M. et al. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog.7, e1001264 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee, D. G. et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol.7, R90 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amato, S. M. & Brynildsen, M. P. Persister Heterogeneity arising from a single metabolic stress. Curr. Biol.25, 2090–2098 (2015). [DOI] [PubMed] [Google Scholar]
- 86.Amato, S. M., Orman, M. A. & Brynildsen, M. P. Metabolic control of persister formation in Escherichia coli. Mol. Cell50, 475–487 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Wang, B. et al. Affinity-based capture and identification of protein effectors of the growth regulator ppGpp. Nat. Chem. Biol.15, 756 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bange, G., Brodersen, D. E., Liuzzi, A. & Steinchen, W. Two P or not two P: understanding regulation by the bacterial second messengers (p)ppGpp. Annu. Rev. Microbiol.75, 383–406 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Traxler, M. F. et al. Discretely calibrated regulatory loops controlled by ppGpp partition gene induction across the ‘feast to famine’ gradient in Escherichia coli. Mol. Microbiol.79, 830–845 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mechold, U., Potrykus, K., Murphy, H., Murakami, K. S. & Cashel, M. Differential regulation by ppGpp versus pppGpp in Escherichia coli. Nucleic Acids Res.41, 6175–6189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vogt, S. L. et al. The stringent response is essential for Pseudomonas aeruginosa virulence in the rat lung agar bead and Drosophila melanogaster feeding models of infection. Infect. Immun.79, 4094–4104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pacios, O. et al. (p)ppGpp and its role in bacterial persistence: new challenges. Antimicrob. Agents Chemother.64, 10.1128/AAC.01283-20 (2020). [DOI] [PMC free article] [PubMed]
- 93.Kaldalu, N. et al. In vitro studies of persister cells. Microbiol. Mol. Biol. Rev.84, 10.1128/MMBR.00070-20 (2020). [DOI] [PMC free article] [PubMed]
- 94.Balaban, N. Q., Merrin, J., Chait, R., Kowalik, L. & Leibler, S. Bacterial persistence as a phenotypic switch. Science305, 1622–1625 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Stewart, P. S. & Franklin, M. J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol.6, 199–210 (2008). [DOI] [PubMed] [Google Scholar]
- 96.Hentzer, M. et al. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol.183, 5395–5401 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krieg, D. P., Helmke, R. J., German, V. F. & Mangos, J. A. Resistance of mucoid Pseudomonas aeruginosa to nonopsonic phagocytosis by alveolar macrophages in vitro. Infect. Immun.56, 3173–3179 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leid, J. G. et al. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J. Immunol.175, 7512–7518 (2005). [DOI] [PubMed] [Google Scholar]
- 99.Pedersen, S. S., Hoiby, N., Espersen, F. & Koch, C. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax47, 6–13 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thoming, J. G. & Haussler, S. Pseudomonas aeruginosa is more tolerant under biofilm than under planktonic growth conditions: a multi-isolate survey. Front. Cell. Infect. Microbiol.12, 851784 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhattacharyya, R. P. et al. Simultaneous detection of genotype and phenotype enables rapid and accurate antibiotic susceptibility determination. Nat. Med.25, 1858–1864 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shishkin, A. A. et al. Simultaneous generation of many RNA-seq libraries in a single reaction. Nat. Methods12, 323–325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics30, 923–930 (2014). [DOI] [PubMed] [Google Scholar]
- 105.Chen, Y., Chen, L., Lun, A. T. L., Baldoni, P. L. & Smyth, G. K. edgeR v4: powerful differential analysis of sequencing data with expanded functionality and improved support for small counts and larger datasets. Nucleic Acids Res.53, 10.1093/nar/gkaf018 (2025). [DOI] [PMC free article] [PubMed]
- 106.Bu, D. et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res.49, W317–W325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luo, W. & Brouwer, C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics29, 1830–1831 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Musken, M., Di Fiore, S., Romling, U. & Haussler, S. A 96-well-plate-based optical method for the quantitative and qualitative evaluation of Pseudomonas aeruginosa biofilm formation and its application to susceptibility testing. Nat. Protoc.5, 1460–1469 (2010). [DOI] [PubMed] [Google Scholar]
- 109.Bense, S. et al. Pseudomonas aeruginosa post-translational responses to elevated c-di-GMP levels. Mol. Microbiol.117, 1213–1226 (2022). [DOI] [PubMed] [Google Scholar]
- 110.Sudarshan, S., Hogins, J., Ambagaspitiye, S., Zimmern, P. & Reitzer, L. The nutrient and energy pathway requirements for surface motility of nonpathogenic and uropathogenic Escherichia coli. J. Bacteriol.203, 10.1128/JB.00467-20 (2021). [DOI] [PMC free article] [PubMed]
- 111.Krueger, J. et al. tRNA epitranscriptome determines pathogenicity of the opportunistic pathogen Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA121, e2312874121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thoming, J. G. et al. Parallel evolutionary paths to produce more than one Pseudomonas aeruginosa biofilm phenotype. NPJ Biofilms Microbiomes6, 2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morikawa, M., Hirata, Y. & Imanaka, T. A study on the structure-function relationship of lipopeptide biosurfactants. Biochim. Biophys. Acta1488, 211–218 (2000). [DOI] [PubMed] [Google Scholar]
- 114.Tian, C. et al. Rapid curtailing of the stringent response by toxin-antitoxin module-encoded mRNases. J. Bacteriol.198, 1918–1926 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Transcriptome data have been deposited in the NCBI Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE283319. All raw data are deposited in Mendeley and can be accessed at 10.17632/v4c9m3ys3h.1.
Scripts for RNA-seq data processing and analysis are available upon request.