Abstract
Background
Neovascularization (NV) within plaques is widely recognized as an important indicator of plaque vulnerability; however, the relationship between NV and plaque rupture in patients with acute myocardial infarction (AMI) has not been extensively evaluated. The purpose of this study was to investigate the association between NV of culprit lesions and plaque rupture in patients with AMI.
Methods
This study included 384 patients diagnosed with AMI. All patients were divided into Non-NV and NV groups according to whether NV was present in the optical coherence tomography (OCT) images of the preoperative culprit lesions.
Results
Patients with NV lesions had thinner minimal fibrous cap thickness, smaller minimal lumen area and symmetry, longer lesion length, and larger plaque volume and plaque burden than patients with AMI without NV lesions (P < 0.05). Patients with both AMI and NV had a higher incidence of thin-cap fibroatheroma, cholesterol crystals, macrophages, thrombus, calcific plaque, lipid-rich plaque, and plaque rupture (P < 0.01). After adjusting for covariates, NV is independently associated with plaque rupture (OR 3.21, 95% CI 2.00-5.14; P < 0.001). For the evaluation of the model after adjusting the covariates, the receiver operating characteristic (ROC) curve, clinical calibration curve, and decision curve analysis (DCA) of the model show good discriminative ability, prediction accuracy, and clinical utility.
Conclusions
In patients with AMI, NV of culprit lesions is significantly correlated with plaque growth, formation of unstable plaque phenotypes, and plaque rupture. NV may be an effective predictor of plaque rupture in AMI patients.
Keywords: Neovascularization, Acute myocardial infarction, Plaque rupture, Optical coherence tomography
Introduction
As atherosclerotic cardiovascular diseases continue to rise on a global scale, the urgency to accurately identify high-risk factors triggering atherosclerotic plaque rupture and explore novel therapeutic targets has become increasingly pressing. Atherosclerosis is a vascular inflammatory condition that stems from progressive accumulation of plaques. It is characterized by local thickening of the vascular wall and plaque formation. Atherosclerosis is often asymptomatic in the early stages. However, as the disease advances, the plaques may become unstable, thereby precipitating severe acute cardiovascular events. Once a plaque ruptures and obstructs the coronary or carotid arteries, it can potentially trigger acute myocardial infarction, transient ischemic attack, or stroke [1]. These unstable plaques are principally characterized by a thin fibrous cap, large lipid core, and conspicuous neovascularization [2].
Existing literature has linked neovascularization to vulnerable plaques in the carotid artery, indicated that it may serve as an indicator of high-risk vulnerable plaques [3–5]. Some research teams have regarded neovascularization in plaques as a potential target for slowing down or reversing the progression of atherosclerotic diseases [6, 7]. Staub et al. [8, 9] found that there was no obvious correlation between the NV in plaques and traditional cardiovascular risk factors, but there was a certain relationship with patients who had experienced myocardial infarction, indicating that NV may be an independent predictor of plaque vulnerability. Multivariate analysis showed that the presence of NV in plaques was the most important marker associated with previous cardiovascular events, and its correlation even exceeded traditional risk factors such as age, hypertension, diabetes, and smoking.
These findings emphasize the importance of an in-depth study of NV in cardiovascular health management. However, research on the role of NV in plaque rupture in patients with AMI is lacking. The purpose of this study was to use OCT to elaborate on the association between NV and plaque rupture in patients with AMI from aspects such as plaque morphology.
Methods
Study design
This is a single-center, retrospective, observational study. A total of 614 patients diagnosed with AMI and undergoing OCT examination at the Affiliated Hospital of Zunyi Medical University between May 2021 and September 2023 were enrolled in this study. The diagnostic criteria for AMI conform to the fourth universal definition of Myocardial Infarction [10]. AMI is defined as the presence of myocardial injury (defined as an elevated cardiac troponin level, with at least one value exceeding the 99th percentile upper reference limit), and the clinical symptoms are consistent with myocardial ischemia. Some treatment measures, such as reperfusion therapy, must be implemented immediately. If there is persistent chest discomfort or symptoms suggesting ischemia and ST-segment elevation in at least two adjacent leads on the electrocardiogram (ECG), ST-segment elevation myocardial infarction (STEMI) is considered. For cases with typical ischemic electrocardiogram changes (new-onset or transient ST-segment depression ≥ 0.1 mV, or T-wave inversion ≥ 0.2 mV) but without ST-segment elevation on the ECG, and corresponding dynamic evolutions such as the elevation of myocardial injury markers (cTnT, cTnI, or CK-MB), it is considered non-ST-segment elevation myocardial infarction (NSTEMI). For NSTEMI cases, we first use ST-T changes in the ECG to preliminarily identify the lead groups corresponding to the ischemic area. For example, leads V1-V3 correspond to the left anterior descending artery (LAD), while leads II, III, and aVF correspond to the right coronary artery (RCA) or left circumflex artery (LCX). Second, during coronary angiography, the culprit vessel typically shows a lesion with ≥ 50-70% stenosis. Finally, OCT is performed on the culprit lesion to detect unstable plaques. The culprit vessel is determined through comprehensive analysis of these findings. In accordance with the Declaration of Helsinki, ethical approval was obtained from the Ethics Committee of the Affiliated Hospital of Zunyi Medical University, and written informed consent was obtained from all the participants. Based on the presence or absence of NV in the OCT images of the culprit lesions before PCI, eligible patients were divided into two groups: Non-NV (n = 176) and NV (n = 208).
Clinical data
Clinical data, including patient demographics, medical history, and laboratory test results, were extracted from the electronic medical record system of the hospital. Relevant sociodemographic characteristics, such as age, sex, diabetes mellitus, hypertension, smoking history, AMI type, and heart function, were documented. Laboratory analyses included measurements of total bilirubin, triglycerides, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, uric acid, estimated glomerular filtration rate, creatinine, white blood cells, red blood cells, hemoglobin, and platelets. Furthermore, the utilization of pharmaceutical agents such as aspirin and P2Y12 inhibitors, statins, beta blockers, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker was recorded.
OCT image acquisition and analysis
The OCT images were acquired using an OCT catheter (Dragonfly Duo, St. Jude Medical) and frequency domain OCT (Silicon OPTIS; St. Jude, St. Paul, US). Upon insertion of the OCT catheter into the distal portion of the culprit lesion along the guidewire, a contrast agent was administered to displace the blood within the target vessel. The pullback rate was set to 18 mm/s, accompanied by a rotation rate of 100 frames/s. The OCT images were independently analyzed by two interventional cardiologists to ensure confidentiality of other clinical information. In instances where discrepancies in the interpretation of OCT images arose between the two interventional cardiologists, a third expert in OCT imaging was consulted to adjudicate the analysis results. Interobserver agreement for culprit lesion classification was assessed using Cohen’s kappa(κ = 0.73, 95% CI 0.67–0.81). For continuous variables (Minimal FCT, MLA, Lesion length, Plaque volume, Plaque burden, Symmetricity), intraclass correlation coefficient was 0.78 (95% CI 0.69–0.86).
Quantitative and qualitative analysis of the OCT
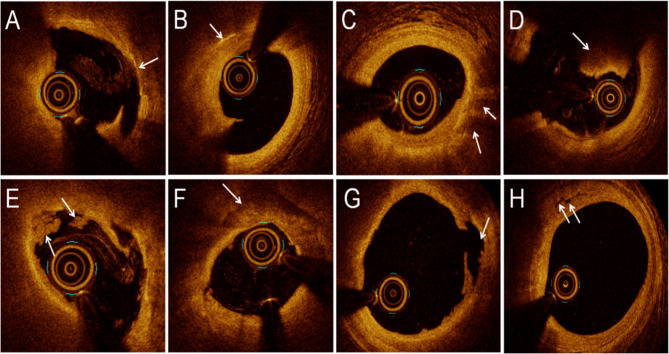
The Quantitative and qualitative analyses of the cross-sectional images obtained through OCT were conducted using OCT analysis software developed by LightLab Imaging Inc. (Westford, MA). Quantitative analysis included measurement of minimal fibrous cap thickness (Minimal FCT), minimal lumen area (MLA), lesion length, plaque volume, plaque burden, and symmetricity. MLA was defined as the smallest area within the length of one plaque. The lumen area was delineated by the lumen boundary, which was determined semi-automatically by the system and could be adjusted manually if necessary. In the qualitative analysis, thin-cap fibroatheroma (TCFA) was described as a lipid-rich plaque with a fibrous cap thickness at the thinnest part of < 65 μm [11]. Cholesterol crystals were identified as thin, linear regions of high signal intensity with high backscattering within a plaque. Lipid was defined as a signal-poor region with a poorly defined or diffuse border, and lipid-rich plaque was defined as a plaque with a maximal lipid arc of > 90°. Macrophages were defined by the presence of highly scattered focal regions within the fibrous cap. Thrombus was defined as an irregular mass with a minimum diameter of at least 250 μm adherent to the vessel wall or floating within the lumen; the red thrombus had high backscatter and no signal shadow; the white thrombus had abundant signal and low backscatter [12]. Calcific plaques appear as areas of strong reflection, high signal with posterior signal attenuation, or shadowing with relatively well-defined borders [13]. Plaque rupture was defined as discontinuity of the fibrous cap of the lipid plaque, accompanied by the formation of a cavity. Plaque erosion was defined as an intact fibrous cap without plaque rupture accompanied by thrombosis and an identifiable plaque structure under the thrombosis or an intact fibrous cap, absence of thrombosis in the culprit lesion, irregular luminal surface or presence of thrombosis at the lesion, indistinct plaque structure at the thrombus, and absence of superficial lipids or calcification proximal or distal to the thrombus [14]. NV refers to small vesicles or tubular structures that are found on at least three consecutive cross-sectional OCT images and have no connection to the lumen [15, 16]. Representative OCT images are shown in Fig. 1.
Fig. 1.
Representative optical coherence tomography images. A Thin-cap fibroatheroma. B Cholesterol crystals. C Macrophages. D Red thrombus. E White thrombus. F Calcific plaque. G Plaque rupture. H Neovascularization
Statistics analysis
To evaluate the data characteristics of this study, we utilized descriptive and inferential statistical analyses. Continuous variables conforming to a normal distribution are presented as the mean (standard deviation), whereas those that did not follow a normal distribution are denoted as the median (interquartile range). Categorical variables are expressed as proportions (percentages). Continuous variables with non-normal distributions were compared between the two groups using the rank-sum test. For categorical variables, the chi-squared test or Fisher’s exact test was used to determine statistical differences. Logistic regression analysis was performed to obtain the odds ratio (OR) and 95% confidence interval (CI) to explore the association between NV and incidence of plaque rupture. In this study, Model 1 was adjusted for traditional risk factors for coronary heart disease, such as age, sex, diabetes mellitus, hypertension, current smoking, total cholesterol, and low-density lipoprotein cholesterol. Model 2 adjusts for all variables in Model 1 plus thin-capped fibroatheroma, cholesterol crystals, macrophages, calcific plaque, and lipid-rich plaque. These OCT variables have been previously reported to be associated with plaque rupture [17]. Finally, the ROC curve, calibration curve, and DCA were used to evaluate the discriminative ability, prediction accuracy, and clinical utility of the model 2. A p-value of < 0.05 was considered statistically significant, and all analyses were performed using a two-sided approach. All statistical analyses were performed using R version 4.4.1.
Results
Clinical characteristics
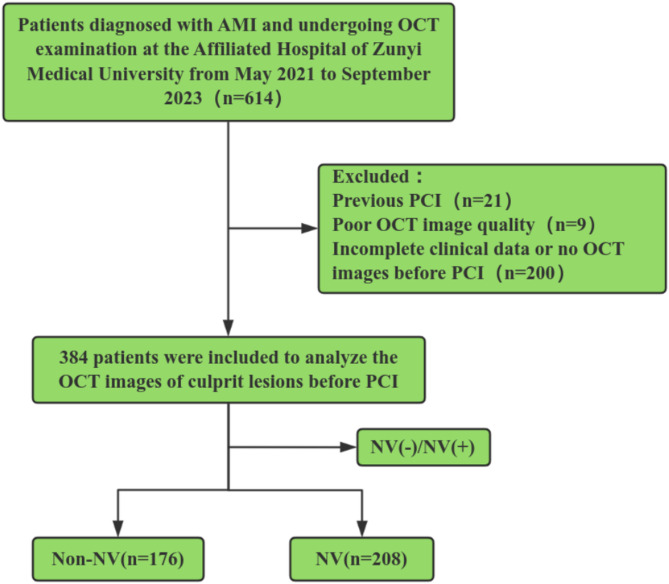
A total of 614 patients diagnosed with AMI and undergoing OCT examination were initially included in this study. Among the 614 patients with AMI, 21 patients were excluded due to a history of prior PCI, 9 because of poor quality OCT images, and 200 because of incomplete clinical data (It refers to the situation that some data such as medical history, laboratory test results, and medications at discharge cannot be found or are lost) or on OCT images before PCI. Finally, 384 patients with AMI were enrolled in the study (Fig. 2). According to the presence or absence of NV in the culprit lesion identified by OCT, patients were divided into NV group and Non-NV group. There were 208 patients in the NV group and 176 patients in the Non-NV group.
Fig. 2.
Study flow chart. Abbreviations: AMI: acute myocardial infarction. NV: Neovascularization. OCT: optical coherence tomography. PCI: percutaneous coronary intervention. NV(-)/NV(+): group the subjects according to the presence or absence of NV
No significant differences were observed in age, sex, diabetes mellitus, hypertension, smoking history, AMI type, heart function, laboratory test results (including measurements of total bilirubin, triglycerides, high-density lipoprotein cholesterol, uric acid, estimated glomerular filtration rate, creatinine, white blood cells, red blood cells, hemoglobin, and platelets), or discharge medication (all P > 0.05). However, statistically significant differences were observed in total cholesterol and low-density lipoprotein cholesterol (P = 0.006, P < 0.001), as presented in Table 1.
Table 1.
Patient baseline characteristics
| Total (n = 384) | Non-NV (n = 176) | NV (n = 208) | P-value | |
|---|---|---|---|---|
| Age, years | 58(50–68) | 59(52–69) | 57(50–65) | 0.091 |
| Male | 313 (81.5) | 141 (80.1) | 172 (82.7) | 0.517 |
| Diabetes mellitus | 66 (17.2) | 27 (15.3) | 39 (18.8) | 0.378 |
| Hypertension | 178 (46.3) | 80 (45.4) | 98 (47.1) | 0.745 |
| Current smoker | 229 (59.6) | 100 (56.8) | 129 (62.0) | 0.301 |
| STEMI | 271 (70.6) | 116 (65.9) | 155 (74.5) | 0.065 |
| LVEF(%) | 54(44–60) | 55 (46–60) | 54 (43–60) | 0.107 |
| Laboratory fingdings | ||||
| Total bilirubin,µmol/L | 10.90(8.18–14.43) | 11.30(8.10−14.45) | 10.55 (8.28–14.43) | 0.478 |
| Triglycerides, mmol/L | 1.75(1.15–2.76) | 1.60 (1.07–2.38) | 1.86 (1.20–2.84) | 0.082 |
| Total cholesterol, mmol/L | 5.05(4.21–5.82) | 4.84 (4.06–5.55) | 5.18 (4.33–6.05) | 0.006 |
| HDL-C, mmol/L | 1.08(0.94–1.23) | 1.08 (0.94–1.25) | 1.07 (0.95–1.20) | 0.382 |
| LDL-C, mmol/L | 3.08(2.55–3.59) | 2.85(2.41–3.41) | 3.20(2.63–3.77) | < 0.001 |
| Uric acid,µmol/L | 358(287–431) | 343 (280,420) | 373 (291–438) | 0.317 |
| eGFR, ml/min/1.73m2 | 90.89(68.47−109.01) | 91.3 (68.61−110.24) | 90.67 (68.47−108.77) | 0.899 |
| Cr,µmol/L | 78(68–90) | 78 (68–89) | 77 (68–91) | 0.734 |
| WBC, 10^9/L | 9.20(7.06–11.12) | 9.05 (7.08–11.18) | 9.27 (7.05–11.08) | 0.923 |
| RBC,10^12/L | 4.58(4.18–4.93) | 4.57 (4.18–4.96) | 4.60 (4.18–4.92) | 0.777 |
| HB, g/L | 141(128–152) | 141 (128–151) | 142 (129–152) | 0.623 |
| PLT,10^9/L | 205(165–245) | 206 (164–238) | 201 (165–256) | 0.481 |
| Medications at discharge | ||||
| Aspirin | 378 (98.4) | 174 (98.8) | 204 (98.1) | 0.692 |
| P2Y12 inhibitor | 356 (92.7) | 160 (90.9) | 196 (94.2) | 0.212 |
| Statin | 371 (96.6) | 170 (96.6) | 201 (96.6) | 0.981 |
| Beta blocker | 310 (80.7) | 141 (80.1) | 169 (81.3) | 0.779 |
| ACEI or ARB | 302 (78.7) | 133 (75.6) | 169 (81.3) | 0.176 |
Data are presented as median (interquartile range) or n (%). NV Neovascularization, STEM ST-segment elevation myocardial infarction, LVEF Left ventricular ejection fraction, HDL–C High-density lipoprotein cholesterol, LDL-C Low-density lipoprotein cholesterol, eGFR Estimated glomerular filtration rate, Cr Creatinine, WBC White blood cell, RBC Red blood cell, HB Hemoglobin, PLT Platelet, ACEI Angiotensin-converting enzyme inhibitor, ARB Angiotensin receptor blocker
OCT analysis
In the quantitative analysis, significant differences were observed in the minimal fibrous cap thickness, minimal lumen area, lesion length, plaque volume, plaque burden, and symmetricity (P < 0.05), as presented in Table 2. Compared with the non-NV group, the NV group had a thinner minimum fibrous cap (P = 0.003), smaller lumen area (P = 0.006), longer lesion length (P < 0.001), larger plaque volume (P < 0.001), heavier plaque burden (P < 0.001), and less symmetricity (P = 0.015). In the qualitative analysis, no significant differences were observed in plaque erosion (P = 0.447). However, statistically significant differences were identified in TCFA, cholesterol crystals, macrophages, thrombus, calcific plaque, lipid-rich plaque, and plaque rupture (all P < 0.05). Importantly, the proportion of TCFA in the NV group was higher than that in the Non-NV group (53.9% vs. 42.6%, P = 0.028). The incidence of plaque rupture was significantly higher in the NV group (69.2% vs. 43.2%, P < 0.001) (Fig. 3).
Table 2.
OCT characteristics between Non-NV and NV groups
| Total (n = 384) | Non-NV (n = 176) | NV (n = 208) | P-value | |
|---|---|---|---|---|
| Quantitative assessment | ||||
| Minimal FCT,µm | 69.12(44.20−90.03) | 74.01 (51.05–93.14) | 57.00 (40.01,88.25) | 0.003 |
| MLA, mm2 | 1.46(0.88–2.56) | 1.71 (0.98–2.88) | 1.31 (0.86–2.13) | 0.006 |
| Lesion length, mm | 21.70 (12.35–33.65) | 18.2 (9.75−31.00) | 24.55 (13.80−37.05) | < 0.001 |
| Plaque volume, mm3 | 126.50 (81.97–193.90) | 105.00 (62.53−156.65) | 149.00 (94.58–208.70) | < 0.001 |
| Plaque burden, % | 56.50 (51.50−62.73) | 54.15 (47.98–59.92) | 57.9 (52.98–64.20) | < 0.001 |
| Symmetricity | 0.71(0.63–0.79) | 0.74 (0.65–0.81) | 0.7 (0.62–0.78) | 0.015 |
| Qualitative assessment | ||||
| TCFA | 187 (48.7) | 75 (42.6) | 112 (53.9) | 0.028 |
| Cholesterol crystals | 127 (33.1) | 46 (26.1) | 81 (38.9) | 0.008 |
| Macrophages | 317 (82.6) | 137 (77.8) | 180 (86.5) | 0.025 |
| Thrombus | 322 (83.9) | 133 (75.6) | 189 (90.9) | < 0.001 |
| Calcific plaque | 223 (58.1) | 86 (48.9) | 137 (65.9) | < 0.001 |
| Lipid-rich plaque | 298 (77.6) | 127 (72.2) | 171 (82.2) | 0.019 |
| Plaque rupture | 220 (57.3) | 76 (43.2) | 144 (69.2) | < 0.001 |
| Plaque erosion | 92 (24.0) | 39 (22.2) | 53 (25.5) | 0.447 |
Data are presented as median (interquartile range) or n (%) or mean (standarddeviation). NV Neovascularization, Minimal FCT Minimal fibrous cap thickness, MLA Minimal lumen area, TCFA Thin-cap fibroatheroma
Fig. 3.
Prevalence of OCT-Defined Plaque Characteristics in Lesions With or Without NV. Compared to lesions without NV, NV lesions have a higher prevalence of TCFA, cholesterol crystals, macrophages, thrombus, calcific plaque, lipid-rich plaque, and plaque rupture
The association between NV and plaque rupture
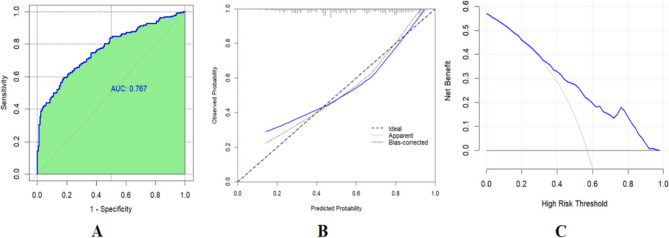
The association between NV and plaque rupture was tested through univariate analysis and two multivariate models, with clinical and OCT imaging characteristics adjusted. In the univariate regression analysis, NV (OR 3.82, 95% CI 2.50–5.86; P < 0.001) was significantly associated with plaque rupture. After adjusting for the following covariates: age, sex, diabetes mellitus, hypertension, current smoker, total cholesterol, and low-density lipoprotein cholesterol (Model 1), the presence of NV was independently associated with plaque rupture (P < 0.001). Additionally, based on Model 1, after further adjusting for the covariates, thin-capped fibroatheroma, cholesterol crystals, macrophages, calcific plaque, and lipid-rich plaque (Model 2), the presence of NV remained independently associated with plaque rupture (P < 0.001), as shown in Table 3. The ROC curve was used to evaluate the discriminative ability of Model 2. The area under the ROC curve (AUC) of the model was 0.767 (95% confidence interval [CI]: 0.721–0.814, Fig. 4 A). This indicates that Model 2 has good discriminative ability. The bootstrap self-sampling method with B = 1000 repetitions and the calibration curves were used to assess the predictive accuracy of Model 2. The clinical calibration curve (blue line) of Model 2 was close to the ideal calibration curve (dashed line) (Fig. 4B), and there was a good consistency between the predicted probabilities and the actually observed probabilities, indicating that Model 2 has good predictive accuracy. DCA was used to evaluate the clinical utility of Model 2. The results of the decision curve analysis showed that when the threshold probability was approximately between 28% and 95%, the curve was above the “treat all” (gray curve) and “treat none” (horizontal axis line) lines (Fig. 4 C). Model 2 had a good overall net benefit within this threshold range. These results indicate that Model 2 has good clinical validity.
Table 3.
Logistic regression analysis for plaque rupture
| Variable | Univariate regression | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| NV | 3.82 | 2.50–5.86 | < 0.001 | 3.71 | (2.39–5.76) | < 0.001 | 3.21 | 2.00-5.14 | < 0.001 |
Abbreviations: NV, neovascularization; CI, confidence interval; OR, odds ratio
Model 1: adjusted for age, sex, diabetes mellitus, hypertension, current smoker, total cholesterol, low density lipoprotein - cholesterol;
Model 2: adjusts for all variables in Model 1 plus thin-capped fibroatheroma, cholesterol crystals, macrophages, calcific plaque, and lipid-rich plaque
Fig. 4.
A The receiver operating characteristic (ROC) curve of Model 2; B The clinical calibration curve of Model 2; C The decision curve analysis (DCA) of Model 2
Discussion
In this study, we retrospectively analyzed the baseline characteristics and quantitative and qualitative features of OCT in patients with AMI. The following conclusions were drawn: (1) Compared to non-NV patients, NV patients had higher levels of total cholesterol and low-density lipoprotein cholesterol. (2) In NV patients, the fibrous caps of plaques were thinner, and the lumen loss and degree of lesions were more severe. Vulnerable plaques and ruptured plaques were more easily observed in patients with NV. (3) Multivariate logistic regression analysis revealed that NV is independently associated with plaque rupture. The model after adjusting for confounding factors still has good discriminative ability, prediction accuracy, and clinical utility.
A close correlation between lipid metabolism disorder and the occurrence of atherosclerosis has been fully confirmed [18]. In our study, the levels of total cholesterol and low-density lipoprotein cholesterol in patients with NV were higher than those in patients without NV. Some studies have also elucidated the relationship between blood lipid levels and NV. The research findings of Wang et al. [19] indicated that in the high-risk population for stroke, the elevation of total cholesterol, low-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol (non-HDL-C) is associated with an increased incidence of intraplaque neovascularization in carotid artery plaques. Ma et al. [20] pointed out that the formation of NV is a complex process that depends on the interaction among various vascular endothelial growth factors. Vascular endothelial growth factor (VEGF) is one of the most well-known proangiogenic factors. Salami et al. [21] demonstrated that the plasma levels of vascular endothelial growth factor in patients with hyperlipidemia increased. These studies have shown that there is a certain degree of association between the occurrence of NV and elevation of blood lipid levels, which is consistent with the results of our study. This indicates that for patients with NV, control of their blood lipid levels should be strengthened.
NV usually originates from the vasa vasorum in the blood vessel wall, which is located between the tunica media and tunica adventitia. When pathological factors stimulate angiogenesis, the vasa vasorum is activated and extends into the intima of the blood vessels or deep into the plaque to continue growing [22]. Hypoxia in the plaque environment is an effective inducer of NV. Under physiological conditions, NV allows oxygen and nutrients to enter the blood vessel walls. However, when it expands due to pathological stimuli, the NV within the plaque creates favorable conditions for plaque growth, serving as a pathway for cholesterol, inflammatory cells, red blood cells, the transient extracellular matrix, or other molecules that promote atherosclerosis to enter the plaque [23]. Uemura et al. [24] conducted a 7-month follow-up of patients with coronary heart disease who had undergone percutaneous coronary intervention. They found that TCFA and NV were potential predictors of subsequent plaque progression in patients with coronary heart disease. A study using optical OCT and IVUS conducted by Liu et al. [25] showed that intraplaque NV was still significantly associated with plaque progression, despite statin therapy. Our study showed that compared with patients without NV, patients with NV had a smaller minimal lumen area, longer lesion length, larger plaque volume, and higher plaque burden. This is consistent with previous studies, indicating that, for patients with AMI accompanied by NV, the degree of their lesions may be relatively severe.
Intraplaque NV usually consists of small blood vessels that are immature, delicate, and have an incomplete structure (lack of pericytes in the vessel wall). Therefore, they are extremely susceptible to the influence of the external environment and prone to leakage, rupture, and bleeding, which accelerates plaque progression [26]. The leakage effect of NV: Some inflammatory cells, such as macrophages and leukocytes, as well as some lipid components, can leak from the immature NV into the plaques, thus increasing the necrotic core of the plaques, accelerating plaque progression, and even leading to plaque rupture [27]. Intraplaque hemorrhage: Intraplaque hemorrhage caused by the rupture of NV, through the exudation of red blood cells, causes a large amount of red blood cell membranes rich in cholesterol to accumulate within the plaques, resulting in the deposition of free cholesterol in the plaques and the expansion of the necrotic core in atherosclerotic plaques [28]. Our study showed that the incidence rates of cholesterol crystals, macrophages, lipid-rich plaques, and calcific plaques were higher in patients with NV. This may be related to the unique effects of NV on the plaques. Here, we used data to illustrate that NV is closely associated with plaque vulnerability. In addition, during the formation of NV in plaques, there are inflammatory cells, such as macrophages, mast cells, and T cells. They may leak from the newly formed NV with impaired structural integrity and accumulate around them. These inflammatory cells are rich sources of cytokines, growth factors, cysteine proteases, and extracellular matrix metalloproteinases(MMPs) [29]. Activated macrophages produce extracellular MMPs and cysteine proteases via myeloperoxidase. The collagen-dissolving ability of these two enzymes can thin the fibrous cap of the plaque, which may ultimately lead to plaque rupture [30]. Correspondingly, in our study, patients with NV had thinner fibrous caps and more TCFA, and plaque ruptures were more common. After multivariate analysis, it was concluded that NV is an independent risk factor for plaque rupture and has good predictive value for plaque rupture. Currently, most studies on NV and atherosclerotic plaques are basic research, and few studies have been conducted on living patients. This study intuitively presented the relationship between NV and plaque vulnerability and plaque rupture using specific clinical data and effective statistical methods. A large number of previous [31–33] studies have shown that TCFA is representative of vulnerable plaques, and only a few articles [34, 35] have pointed out that NV, like TCFA, can also serve as a marker of vulnerable plaques. However, there are not many such articles, and in the future, larger-scale prospective studies are needed to prove the association between NV and vulnerable plaques as well as the relationship with plaque rupture. Recently, Ugusman et al. [6] proposed that targeted therapy of NV may be a promising treatment approach for enhancing plaque stability. Boswell-Patterson et al. [36] have also summarized the relevant methods of targeted therapy for NV within plaques. Finally, in the foreseeable future, it is likely that NV can be integrated into the prognostic management framework for AMI patients.
Advantages and limitations
This study has certain strengths and limitations. To the best of our knowledge, this may be the first time to use a predictive model approach to propose that NV in patients with AMI is independently associated with plaque rupture, and to analyze and evaluate the unique predictive value and clinical practicality of NV for plaque rupture. However, this study also has some limitations. First, this is a single-center, retrospective study; there are inherent potential biases. These results should be interpreted with caution, and further prospective multicenter studies are needed to verify these results. Second, thrombus aspiration was not performed during the preoperative OCT imaging, the penetration of OCT is limited, especially when lipid-rich plaques and red thrombi are present, the NV at the bottom of some plaques may be underestimated. In addition, in this study, no data analysis was conducted on some pathological factors that may lead to AMI, such as eruptive calcified nodules and SCAD. Therefore, we cannot rule out the possibility that unmeasured or unknown confounding factors may account for the associations observed in this study. In our study, it can be observed that not all culprit lesions have plaque rupture or erosion, especially in the non-NV group. Regarding this, we have mainly considered the following reasons: according to the existing literature [37, 38], eruptive calcified nodules represent the third major pathological mechanism leading to acute thrombosis (although the proportion is relatively low), and the prevalence of Spontaneous Coronary Artery Dissection (SCAD) in the population of young women with Acute Myocardial Infarction (AMI) can be as high as 25%. However, in this study, no systematic statistics were conducted on eruptive calcified nodules and SCAD. Therefore, it is speculated that some lesions without plaque rupture or erosion may be related to this. In the future, it is necessary to expand the sample size and the plaque characteristics to clarify the incidence of such lesions.
Conclusion
In patients with AMI, patients with NV have more severe lumen loss and lesion degree, and often have more vulnerable plaques. Multivariable analysis shows that NV is independently associated with plaque rupture. The mechanism of action of NV in plaque rupture still requires further investigation.
Acknowledgements
I would like to express my gratitude to all those who helped me during the writing of this manuscript.
Authors’ contributions
Long Zhang designed the research and wrote this manuscript. Chancui Deng made rigorous revisions to the manuscript. Wei Zhang, Xiushi Li, Jie Xia, Caifeng Yang, Lingjun Zhou, and Bei Shi contributed to data collection and the improvement of the manuscript’s quality. Guanxue Xu exercised strict control over the quality and logicality of the manuscript.All authors reviewed the manuscript.
Funding
This study was supported by the Science and Technology Project Fund of the Guizhou Provincial Health Commission (Grant Nos. gzwkj2024-113, gzwkj2024-083) and the National Natural Science Foundation of China (Grant No. 82260106).
Data availability
The dataset analyzed during the current study are available on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Affiliated Hospital of Zunyi Medical University. All patients signed a written informed consent form to participate in the study prior to any procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Long Zhang and Chancui Deng contributed equally to this work and deserve a conjoint first authorship.
References
- 1.Baradaran H, Kamel H, Gupta A. The role of cross-sectional imaging of the extracranial and intracranial vasculature in embolic stroke of undetermined source. Front Neurol. 2022;13:982896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He Z, Luo J, Lv M, Li Q, Ke W, Niu X, et al. Characteristics and evaluation of atherosclerotic plaques: an overview of state-of-the-art techniques. Front Neurol. 2023;14:1159288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyu Q, Tian X, Ding Y, Yan Y, Huang Y, Zhou P, et al. Evaluation of carotid plaque rupture and neovascularization by Contrast-Enhanced ultrasound imaging: an exploratory study based on histopathology. Transl Stroke Res. 2021;12(1):49–56. [DOI] [PubMed] [Google Scholar]
- 4.Migdalski A, Jawien A. Neovascularization as a leading mechanism of intraplaque hemorrhage and carotid plaque destabilization: A narrative review. Curr Vasc Pharmacol. 2024;22(6):377–85. [DOI] [PubMed]
- 5.Miceli G, Basso MG, Pintus C, Pennacchio AR, Cocciola E, Cuffaro M et al. Molecular pathways of vulnerable carotid plaques at risk of ischemic stroke: A narrative review. Int J Mol Sci. 2024;25(8):4351. [DOI] [PMC free article] [PubMed]
- 6.Ugusman A, Hisam NSN, Othman NS, Anuar NNM, Hamid AA, Kumar J, et al. Pharmacological interventions for intraplaque neovascularization in atherosclerosis. Pharmacol Ther. 2024;261:108685. [DOI] [PubMed] [Google Scholar]
- 7.Perrotta P, Van der Veken B, Van Der Veken P, Pintelon I, Roosens L, Adriaenssens E, et al. Partial Inhibition of Glycolysis reduces atherogenesis independent of intraplaque neovascularization in mice. Arterioscler Thromb Vasc Biol. 2020;40(5):1168–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staub D, Patel MB, Tibrewala A, Ludden D, Johnson M, Espinosa P, et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke. 2010;41(1):41–7. [DOI] [PubMed] [Google Scholar]
- 9.Yan H, Wu X, He Y, Staub D, Wen X, Luo Y. Carotid intraplaque neovascularization on Contrast-Enhanced ultrasound correlates with cardiovascular events and poor prognosis: A systematic review and Meta-analysis. Ultrasound Med Biol. 2021;47(2):167–76. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl B, Mills NL. A new clinical classification of acute myocardial infarction. Nat Med. 2023;29(9):2200–5. [DOI] [PubMed] [Google Scholar]
- 11.Cao M, Zhao L, Ren X, Wu T, Yang G, Du Z, et al. Pancoronary plaque characteristics in STEMI caused by culprit plaque Erosion versus rupture: 3-Vessel OCT study. JACC Cardiovasc Imaging. 2021;14(6):1235–45. [DOI] [PubMed] [Google Scholar]
- 12.Shi X, Cai H, Wang F, Liu R, Xu X, Li M, et al. Cholesterol Crystals are Associated with Carotid Plaque Vulnerability: An Optical Coherence Tomography Study. J Stroke Cerebrovasc Dis. 2020;29(2):104579 [DOI] [PubMed]
- 13.Qin Z,Yu L, Zhang Y, Xu Q, Li C, Zhao S, et al. Coronary artery calcification and plaque stability: an optical coherence tomography study. Heliyon. 2023;9(12):e23191. [DOI] [PMC free article] [PubMed]
- 14.Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62(19):1748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian J, Hou J, Xing L, Kim SJ, Yonetsu T, Kato K, et al. Significance of intraplaque neovascularisation for vulnerability: optical coherence tomography study. Heart. 2012;98(20):1504–9. [DOI] [PubMed] [Google Scholar]
- 16.Vorpahl M, Nakano M, Virmani R. Small black holes in optical frequency domain imaging matches intravascular neoangiogenesis formation in histology. Eur Heart J. 2010;31(15):1889. [DOI] [PubMed] [Google Scholar]
- 17.Shimamura K, Kubo T, Akasaka T. Evaluation of coronary plaques and atherosclerosis using optical coherence tomography. Expert Rev Cardiovasc Ther. 2021;19(5):379–86. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Hong F, Ma C, Yang S. Hepatic lipid metabolism disorder and atherosclerosis. Endocr Metab Immune Disord Drug Targets. 2022;22(6):590–600. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Yao M, Zou M, Ge Z, Cai S, Hong Y, et al. Relationship between serum lipid profiles and carotid intraplaque neovascularization in a High-Stroke-Risk population: A Cross-Sectional study in China. J Am Heart Assoc. 2021;10(22):e021545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, Ni M, Hao P, Lu H, Yang X, Xu X, et al. Tongxinluo mitigates atherogenesis by regulating angiogenic factors and inhibiting Vasa vasorum neovascularization in Apolipoprotein E-deficient mice. Oncotarget. 2016;7(13):16194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salami A, El Shamieh S. Association between SNPs of Circulating vascular endothelial growth factor levels, hypercholesterolemia and metabolic syndrome. Med (Kaunas). 2019;55(8):464. [DOI] [PMC free article] [PubMed]
- 22.Phillippi JA. On Vasa vasorum: A history of advances in Understanding the vessels of vessels. Sci Adv. 2022;8(16):eabl6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarbell J, Mahmoud M, Corti A, Cardoso L, Caro C. The role of oxygen transport in atherosclerosis and vascular disease. J R Soc Interface. 2020;17(165):20190732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uemura S, Ishigami K, Soeda T, Okayama S, Sung JH, Nakagawa H, et al. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur Heart J. 2012;33(1):78–85. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Sun C, Gu X, Liu X, Wang X, Wang X, et al. Intraplaque neovascularization attenuated Statin benefit on atherosclerotic plaque in CAD patients: A follow-up study with combined imaging modalities. Atherosclerosis. 2019;287:134–9. [DOI] [PubMed] [Google Scholar]
- 26.Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, Kutys B, et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;53(17):1517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rademakers T, Douma K, Hackeng TM, Post MJ, Sluimer JC, Daemen MJ, et al. Plaque-associated Vasa vasorum in aged Apolipoprotein E-deficient mice exhibit proatherogenic functional features in vivo. Arterioscler Thromb Vasc Biol. 2013;33(2):249–56. [DOI] [PubMed] [Google Scholar]
- 28.Chistiakov DA, Orekhov AN, Bobryshev YV. Contribution of neovascularization and intraplaque haemorrhage to atherosclerotic plaque progression and instability. Acta Physiol (Oxf). 2015;213(3):539–53. [DOI] [PubMed] [Google Scholar]
- 29.Senders ML, Mulder WJM. Targeting myeloperoxidase in inflammatory atherosclerosis. Eur Heart J. 2018;39(35):3311–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieddu G, Formato M, Lepedda AJ. Searching for atherosclerosis biomarkers by proteomics: A focus on lesion pathogenesis and vulnerability. Int J Mol Sci. 2023;24(20):15175. [DOI] [PMC free article] [PubMed]
- 31.van Veelen A, van der Sangen NMR, Delewi R, Beijk MAM, Henriques JPS, Claessen B. Detection of vulnerable coronary plaques using invasive and Non-Invasive imaging modalities. J Clin Med. 2022;11(5):1361. [DOI] [PMC free article] [PubMed]
- 32.Mushenkova NV, Summerhill VI, Zhang D, Romanenko EB, Grechko AV, Orekhov AN. Current advances in the diagnostic imaging of atherosclerosis: insights into the pathophysiology of vulnerable plaque. Int J Mol Sci. 2020;21(8):2992. [DOI] [PMC free article] [PubMed]
- 33.Lee KY, Chang K. Understanding vulnerable plaques: current status and future directions. Korean Circ J. 2019;49(12):1115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung H, Kim W. Letter by Chung and Kim regarding article, Head-to-Head comparison of inflammation and neovascularization in human carotid plaques: implications for the imaging of vulnerable plaques. Circ Cardiovasc Imaging. 2017;10(10):e006995. [DOI] [PubMed]
- 35.Motoyama R, Saito K, Tonomura S, Ishibashi-Ueda H, Yamagami H, Kataoka H, et al. Utility of complementary magnetic resonance plaque imaging and Contrast-Enhanced ultrasound to detect carotid vulnerable plaques. J Am Heart Assoc. 2019;8(8):e011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boswell-Patterson CA, Hétu MF, Pang SC, Herr JE, Zhou J, Jain S, et al. Novel theranostic approaches to neovascularized atherosclerotic plaques. Atherosclerosis. 2023;374:1–10. [DOI] [PubMed] [Google Scholar]
- 37.Van den Hoogen IJ, Gianni U, Al Hussein Alawamlh O, Wijeratne R, Jinnouchi H, Finn A, et al. What atherosclerosis findings can CT see in sudden coronary death: plaque rupture versus plaque erosion. J Cardiovasc Comput Tomogr. 2020;14(3):214–8. [DOI] [PubMed] [Google Scholar]
- 38.Meng PN, Xu C, You W, Wu ZM, Xie DJ, Zhang H, et al. Spontaneous coronary artery dissection as a cause of acute myocardial infarction in young female population: A Single-center study. Chin Med J (Engl). 2017;130(13):1534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analyzed during the current study are available on reasonable request.