Abstract
Background:
Behçet’s disease (BD) exhibits significant phenotypic diversity. The genetic basis of phenotypic variations in BD has not yet been elucidated. Based on the high frequency of familial BD, we aimed to analyze the familial aggregation of various manifestations of BD in this study.
Methods:
Patients with BD from 3 Turkish tertiary rheumatology outpatient clinics were evaluated. Demographic and clinical characteristics of the familial group with either a first- or second-degree relative with BD and the non-familial group were compared. Afterward, patients in the familial disease group for 5 years or longer were divided into 2: an “index patient” and a “first-degree relative patient” and the presence of BD manifestations were compared between these 2 groups.
Results:
We identified 864 BD patients (mean age (SD): 47.9 (12) years, disease duration (SD): 83.7 (65.3) months) with 251 (29.1%) having a BD family history. Genital ulcers (P = .002) and papulopustular lesions (P < .001) were detected more frequently in the familial group. Also in the familial group, statistically significant correlations were detected between the index patient and the first-degree relative-patient in terms of erythema nodosum-like lesions (r: 0.398, P: .016), pathergy test positivity (r: 0.561, P: .002), peripheral joint involvement (r: 0.563, P < .001) and vascular involvement (r: 0.408, P: .014).
Conclusion:
Familial BD may differ from sporadic BD. Additionally, erythema nodosum-like lesions, pathergy test positivity, and vascular and joint involvement may tend to show familial aggregation.
Keywords: Behçet’s disease, familial similarities, MHC-I-opathies
Main Points
Behçet’s disease has a wide phenotypic spectrum. The genetic and environmental reasons underlying individual phenotypic selection are not fully known.
Behçet’s disease has a familial tendency to cluster.
In this study, intrafamilial phenotypic similarities were detected in familial Behçet’s disease.
Introduction
Behçet’s disease (BD) has a wide clinical spectrum ranging from bipolar aphthosis to ocular, neurologic, gastrointestinal, or vascular involvements.1 The unique geographic and demographic selection manner of BD manifestations suggests a strong influence of common genetic and environmental factors, and the contribution of these factors to the clinical heterogeneity cannot be underestimated. Phenotypic diversity is the fundamental challenge hitherto in both comprehending and managing the disease processes. In large BD populations, relatively consistent BD clusters were established to clarify this daunting complexity.2,3 In addition, defining the acne-arthritis-enthesitis triad supported the concept that organ involvements may be related to each other rather than random co-occurrences.4 Moreover, the detection of the papulopustular lesion/arthritis cluster in patients with a family history of BD suggested that some symptoms may be hereditary, independent of the disease itself.5 Concordantly, mucocutaneous findings, pathergy test positivity, and ocular involvement were more frequent in patients with HLA-B*51 positivity, which is the most robust genetic susceptibility marker.6 Additionally, new single nucleotide polymorphisms have recently been identified in BD that may be specifically associated with ocular and neurological involvements.7
The highest frequency of familial aggregation among BD populations was reported at 18%, but there are widely varying results in the literature.8 It emerges that the evolving imperative of conducting comparative analyses to elucidate shared and divergent phenotypic patterns between familial and non-familial BD cases could significantly enrich our comprehension of disease mechanisms and inform more nuanced clinical paradigms. Within the framework of familial BD, genetic and environmental factors underlying the clinical heterogeneity can be shared between family members, thus the phenotypic features may also be similar. Therefore, this study aimed to compare the clinical features of familial and non-familial BD and to determine the phenotypic similarity of family members with the disease.
Material and Methods
Patients with BD diagnosed according to either Behçet’s Syndrome International Study Group Criteria (ISGC) or International Criteria for Behçet’s Disease (ICBD) and followed up in 3 tertiary rheumatology outpatient clinics in Türkiye were included in this study.1 The whole data of all the patients were collected retrospectively by reviewing the charts from the 3 centers. Approval for this study was obtained from the Marmara University Faculty of Medicine Ethics Committee (09.2022.59, 30.09.2022). Informed consent was obtained from the patients who agreed to take part in the study.
Familial/Non-Familial Behçet’s Disease Classifications
Patients were classified into 2 groups: familial and non-familial BD, depending on whether their first- or second-degree relatives had BD according to either ISGC or ICBD. Demographic data, age at diagnosis, and organ involvement of the familial and non-familial groups were compared. Organ involvements were reported as cumulative burden throughout the disease course.
Comparison of Intrafamilial Phenotypic Similarity in Familial Groups
Patients with familial-BD group and their first-degree BD relatives who were followed up in the same center were included in this analysis. Since new organ involvement can often be encountered during follow-up in BD, patients with a disease duration of less than 5 years were excluded. Although no difference in the results of the statistical analyses is expected, to make the analyses more understandable, the patient with a longer follow-up period in the family was named the “index patient” and the one with less follow-up time the “first-degree relative patient.” Thus, the patient group that included the index patients was classified as the “index group,” and the other one as the “first-degree relative group.” The demographic characteristics and the clinical manifestations of BD were compared between the 2 family members. Nominal correlation analysis was performed to assess the phenotypic similarity of each manifestation between the “index group” and the “first-degree relative group.” In addition, the odds ratio (95% CI) giving the phenotype of the “index patient” being the same with the “first-degree relative patient” was calculated.
Data were analyzed using Statistical Package for the Social Sciences 22.0 (SPSS, Chicago, IL, USA). The continuous variables were expressed as mean (SD) and median (interquartile range) for standard and non-normal distribution, respectively. Frequency (%) was used for the description of categorical variables. The comparison of variables was evaluated by the Mann–Whitney U test, independent-samples t-test, Wilcoxon test, or chi-square test.
Results
Comparison Between Familial and Non-Familial Behçet’s Disease Group
Of a total of 864 BD (mean age (SD): 47.9 (12) years, disease duration (SD): 83.7 (65.3) months and male/female ratio: 368/496) patients, 251 (29.1%) had a family history of BD and 135 (16%) of them had first-degree relatives affected. When we compared the familial and non-familial BD groups, the disease duration was statistically significantly longer in the familial group (P < .001), which was also significantly younger (P = .013) at the time of diagnosis. In addition, genital ulcers (P = .002) and papulopustular lesions (P < .001) were detected significantly more frequently in the familial group. Immunosuppressive therapy was received by 125 patients (49.8%) in the familial group and 342 patients (55.8%) in the non-familial group. There was no statistically significant difference between the 2 groups (P = .301) (Table 1). According to Holm–Bonferroni adjusted analysis, disease duration was significantly longer in the familial BD group (P = .015), and the frequency of genital ulcers and papulopustular lesions was significantly higher in the familial group (P = .03 and P = .015, respectively). However, the difference in age at diagnosis between the 2 groups did not reach statistical significance (P = .182).
Table 1.
Comparison of Characteristics and Disease Features Between Familial and Non-Familial Behçet’s Disease Group
| Familial BD Group (n = 251) |
Non-Familial BD Group (n = 613) |
P | |
|---|---|---|---|
| Disease duration (month), mean (SD) | 101.9 (79.9) | 76.8 (57) | <.001 |
| Gender (F/M rate) | 107/144 | 261/352 | .989 |
| Age at diagnosis, mean (SD) | 28.5 (9.1) | 30.3 (9.3) | .013 |
| Time between symptom onset and diagnosis (year), mean (SD)* | 3.16 (5.1) | 3 (5.1) | .78 |
| HLA-B51 positivity, n (%)** | 15 (62.5) | 25 (58.8) | .68 |
| Oral aphthous stomatitis, n (%) | 250 (99.6) | 603 (99.6) | .142 |
| Genital ulcer, n (%) | 208 (84.2) | 452 (74.3) | .002 |
| Papulopustular lesion, n (%) | 157 (63.6) | 299 (51) | <.001 |
| Erythema nodosum, n (%) | 129 (51.4) | 310 (50.8) | .878 |
| Pathergy positivity, n (%) | 87 (41.6) | 213 (40.1) | .706 |
| Joint involvement, n (%) | 31 (12.4) | 61 (10) | .299 |
| Major organ involvement, n (%) | 131 (52.2) | 352 (57.4) | .819 |
| Vascular BD, n (%) | 63 (25.2) | 190 (31) | .09 |
| Uveitis, n (%) | 75 (29.9) | 202 (33) | .38 |
| Neuro-Behçet, n (%) | 17 (6.8) | 64 (10.4) | .096 |
| Entero-Behçet, n (%) | 3 (1.2) | 10 (1.6) | .767 |
| Immunosuppressive treatment, n (%) | 125 (49.8) | 342 (55.8) | .301 |
*Data were able to be retrieved for 147 familial patients and 460 non-familial patients.
**A total of 176 BD patients have available HLA-B51 data.
Analysis of Phenotypic Similarity of Relatives to Each Other in the Familial Group
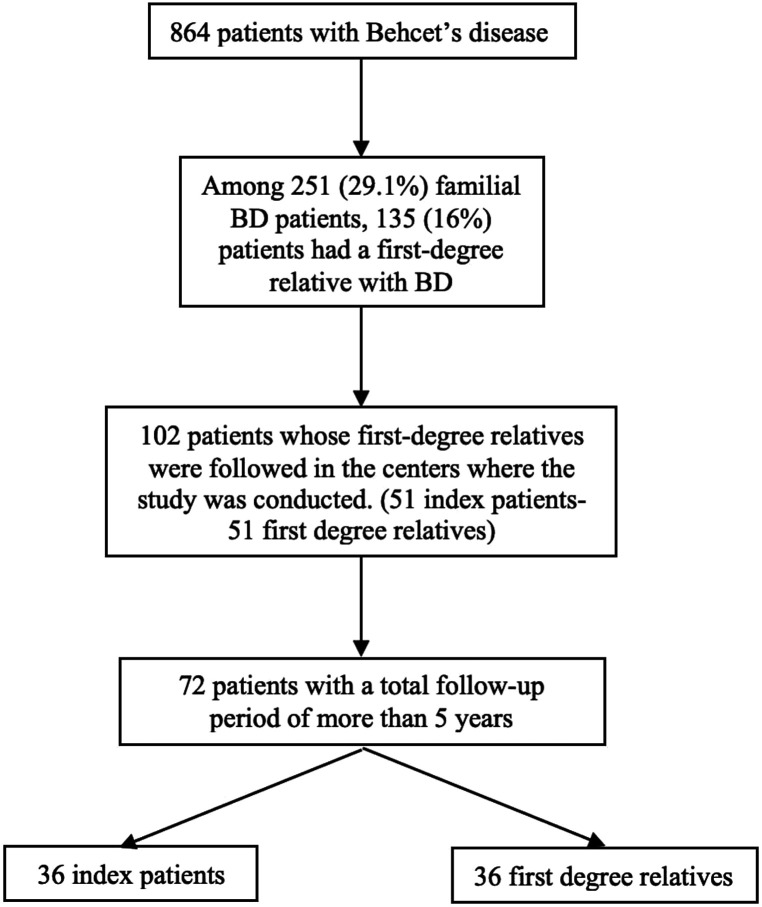
In the familial group, there were 51 index patients (and 51 relatives) who were followed up with their relatives in the same center. Data from 36 (72 patients in total) of these 51 index and first-degree relative pairs with a follow-up period of 5 years or more were included in the final analysis (Figure 1). Of the 36 patient pairs in the familial group, 29 (80.5%) were siblings, and 27 (75%) were of the same gender (Table 2).
Figure 1.
Flow chart of familial Behçet’s disease frequency determination.
Table 2.
Frequencies of Familial Behçet’s Disease Aggregation According to First-Degree Relation Types
| Same gender siblings, n (%) | 21 (58.3) |
| Different gender siblings, n (%) | 8 (22.2) |
| Father–son, n (%) | 4 (11.1) |
| Mother–daughter, n (%) | 2 (5.6) |
| Mother–son, n (%) | 1 (2.8) |
When we analyzed the phenotypic similarity of each patient with their relative individually in the familial group, statistically significant correlations were detected between the index patient and the first-degree relative-patient in terms of gender (OR (95% CI): 6.3 (1.3-31.1), r: 0.358, P: .032), presence of erythema nodosum-like lesion (OR (95% CI): 5.8 (1.3-25.6), r: 0.398, P: .016), pathergy test positivity (OR (95% CI): 13.3 (2.2-82), r: 0.561, P: .002), peripheral joint involvement (OR (95% CI): 15.6 (2.7-91.6), r: 0.563, P < .001), and vascular involvement (OR (95% CI): 11.7 (1.2-114.6), r: 0.408, P: .014). No statistically significant family co-occurrence was found in genital ulcers, papulopustular lesions, uveitis, neuro-Behçet, or entero-Behçet (Table 3).
Table 3.
Analysis of Similarity of Behçet’s Disease Manifestations Within the Families
| Presence in Index Group | Presence in First Degree Relatives-Group | Co-Occurrence in Family Members | Concordance in Both Family Members | Odds Ratio* (95% CI) | r | P | |
|---|---|---|---|---|---|---|---|
| Male gender, n (%) | 26 (72.2) | 27 (75) | 22 (61.1) | 27 (75) | 6.3 (1.3-31.1) | 0.358 | .032 |
| Oral aphthous ulcers, n (%) | 36 (100) | 36 (100) | 36 (100) | 36 (100) | – | – | |
| Genital ulcers, n (%) | 28 (77.8) | 33 (91.7) | 26 (72.2) | 27 (75) | 1.9 (0.2-23.6) | 0.081 | .64 |
| Papulopustular lesions, n (%) | 21 (58.3) | 25 (69.4) | 14 (38.9) | 18 (50) | 0.7 (0.2-3.1) | −0.071 | .679 |
| Erythema nodosum like lesions, n (%) | 13 (36.1) | 13 (36.1) | 8 (22.2) | 26 (72.2) | 5.8 (1.3-25.6) | 0.398 | .016 |
| Pathergy positivity, n (%)** | 18 (62.1) | 18 (62.1) | 15 (51.8) | 23 (79.3) | 13.3 (2.2-82) | 0.561 | .002 |
| Major organ involvement, n (%) | 22 (61.1) | 16 (44.4) | 10 (27.8) | 18 (50) | 1.1 (0.3-4.3) | 0.025 | .883 |
| Uveitis, n (%) | 8 (22.2) | 7 (19.4) | 3 (8.3) | 27 (75) | 3.6 (0.6-21.4) | 0.244 | .152 |
| Peripheral joint involvement, n (%) | 17 (47.2) | 13 (36.1) | 11 (30.6) | 28 (77.8) | 15.6 (2.7-91.6) | 0.563 | <.001 |
| Vascular BD, n (%) | 14 (38.9) | 6 (16.7) | 5 (13.9) | 26 (72.2) | 11.7 (1.2-114.6) | 0.408 | .014 |
| Neuro-Behçet, n (%) | 3 (8.3) | 0 (0) | 0 (0) | 33 (91.7) | |||
| Entero-Behçet, n (%) | 3 (8.3) | 5 (13.9) | 0 (0) | 28 (77.8) | 0.121 | .482 |
*Odds ratio of a first-degree relative having the same phenotype as the index case is given.
**Pathergy test results were found in only 58 patients (29 in each group).
Discussion
In our study, the frequency of familial BD in the BD population was 29.3%, the highest prevalence reported in the literature until now. The prevalence of familial BD has a wide range in the literature, between 1% and 18%, but the highest prevalence was also reported from Türkiye.8,9 In addition, the lambda value determined by Gül et al10 in a study on the familial aggregation of BD was 52.5, which is above that reported in spondyloarthritis (SpA), rheumatoid arthritis, and type 1 diabetes in the literature. However, the fact that Türkiye has the highest prevalence of BD in the world, with 370 per 100 000 inhabitants, may be one of the arguments for the high frequency of familial BD. The prevailing kinship bond in familial BD was that of first-degree siblinghood (brotherhood or sisterhood) in this study. Similarly, Gül et al10 and Ahn et al8 identified that the highest familial penetration of BD was among siblings. It might be argued that the multitude of common risk factors between siblings due to genetic inheritance from the same ancestor is one of the reasons leading to familial disease aggregation in BD.
We determined that the familial BD group was diagnosed at a younger age, and also genital ulcers and papulopustular lesions were more common than in non-familial group. In our study, the follow-up period of the familial BD group was significantly longer. Theoretically, a new manifestation may occur at later timepoints during the disease course, but the average follow-up period of both groups is longer than 5 years, suggesting that major phenotypic variability will not be expected in the subsequent follow-up period.
Interestingly, although not significant, major organ involvements (ocular, vascular, and neurological) were less frequent in the familial group with longer disease duration, suggesting a different phenotype in familial BD. Similar to our results, Karaca et al5 found that the frequency of uveitis and immunosuppressive use for the whole disease course was higher in non-familial BD. Moreover, Akpolat et al9 also detected the frequency of vascular involvement to be significantly higher in the non-familial BD group (7.4% vs. 28.8%). An increase in the number of familial BD patients with milder symptoms is somewhat unexpected in the context of genetic heritability. However, awareness of BD in their relatives may induce them to consult rheumatology centers with relatively fewer clinical symptoms and thus be diagnosed with BD with a less diverse phenotype, and that early treatments might prevent new manifestations, which is a speculative but possible explanation.
In a study comparing ocular involvement in 5 sibling pairs, the course of ocular involvement was similar in 2/5 patient pairs.11 Except this data, our study is the first to investigate the phenotypic similarity of BD within families. Other than BD, the presence of psoriatic arthritis in a first-degree relative also increases the risk of arthritis in psoriasis patients.12 Our study shows a concordance among family members for the expression of erythema nodosum, vascular involvement, joint involvement, and pathergy test positivity in BD. Furthermore, the affected family members were mostly of the same gender. This accentuates that the role of gender-specific factors should not be underestimated.
We did not see similarities in major organ involvement with respect to ocular, neurological, and intestinal involvement within the BD families. However, since aforementioned manifestations are uncommon involvements especially neuro and intestinal BD, it is not expected to detect intra-familial resemblance. Particularly in terms of ocular involvement, our data suggest that a correlation may be detected when examined in larger patient populations.
In ankylosing spondylitis (AS), Brophy et al13 showed that disease activity and functional index were found to be correlated between sibling pairs (correlation coefficients 0.27 and 0.36, respectively). In addition, children of parents with AS with iritis were more likely to develop iritis than children of patients without iritis. Similarly, parents with juvenile AS were more likely to have children with juvenile AS compared to parents without juvenile AS. Also, the heritability of radiological disease severity was 0.62 in this study. Finally, another speculative explanation is the presence of BD among MHC-I-opathies. The strongest genetic factors for BD align with the MHC-I-opathy spectrum disorders, involving genes such as HLA-B*51, endoplasmic reticulum aminopeptidase 1, IL23R, MICA, IL12, IL10, IFNGR1, and STAT4.14 These complex factors involve multiple cytokine pathways in the pathogenesis and encompass various elements of both the innate and adaptive immune systems.15 Genetic aspects of BD fit well with other SpAs as an MHC-I-associated disease and may be the dominant factor of heritability for the clinical phenotype among family members. Genetic factors associated with ocular or vascular phenotypes can be more diverse with sporadic penetrance in the family.
There were some limitations in our study. First, in the phenotypic similarity analysis, we were able to include only patients whose relatives were followed up in the same 3 centers, rather than all familial BD patients in our cohort. This limitation caused the number of patients we included in the phenotypic similarity analysis to be lower. For this reason, we did not have enough patients to analyze familial phenotypic aggregation differences according to the type of relationships and gender. Additionally, in our study, only individuals with first- and second-degree relatives diagnosed with BD were categorized under the familial BD group. The BD status of third-, fourth-, and fifth-degree relatives remains unknown, as the process of reliably assessing such distant familial connections presents inherent challenges and substantial limitations in obtaining accurate information. Furthermore, given that our primary hypothesis emphasizes the shared genetic and environmental factors underlying phenotypic similarity, the inclusion of more distant relatives could potentially dilute the discernment of these influences, as their relevance diminishes with increasing genetic and environmental divergence. The lack of genetic analysis of monogenic autoinflammatory diseases similar to the BD phenotype in our study is a limitation. However, monogenic diseases such as familial Mediterranean fever, A20 haploinsufficiency, and mevalonate kinase deficiency usually occur in childhood and are more common in families because they are single-gene diseases that are inherited as autosomal recessive or dominant. In addition, these patients are characterized by periodic attacks of fever, a feature of the autoinflammatory pattern.16 Crohn's disease (CD)-specific manifestations such as uveitis, pathergy positivity, and venous thrombotic involvement are detected less frequently in the aforementioned monogenic autoinflammatory diseases.16
In this study, the frequency of familial BD in the BD population was found to be 29.3%. The familial BD group was diagnosed at a younger age, and also genital ulcers and papulopustular lesions were more common than in non-familial group. Statistically significant correlations were also observed in gender, presence of erythema nodosum-like lesions, pathergy test positivity, joint involvement, and vascular BD phenotype between affected individuals within the BD families. Our results suggest that familial BD may have some unique differences from sporadic BD. These observations may pave the way for pathogenetic studies that can elucidate the natural course of the disease and may provide prognostic predictions for familial BD patients in clinical practice.
Funding Statement
The authors declared that this study has eceived no financial support.
Footnotes
Ethics Committee Approval: The datasets generated and/or analysed during the current study are not publicly available due to institutional data protection policies but are available from the corresponding author on reasonable request.
Informed Consent: Informed consent was obtained from the participants who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – K.A., F.A.O..; Design – K.A., F.A.O.; Supervision – K.A., F.A.O.; Resources – K.A., F.A.O.; Materials – K.A., A.E.B., R.D., B.C.U., D.K., E.A., G.K., T.B., N.Ş.Y.B., C.B., T.K., D.M., T.E., H.D., F.A.O.; Data Collection and/or Processing – K.A., A.E.B., R.D., B.C.U., D.K., E.A., G.K., T.B., N.Ş.Y.B., C.B., T.K., D.M., T.E., H.D., F.A.O.; Analysis and/or Interpretation – K.A., F.A.O.; Literature Search – K.A., F.A.O.; Writing – K.A., F.A.O.; Critical Review – K.A., D.M., H.D., F.A.O.
Declaration of Interests: DM has received grant funding and honoraria from AbbVie, Janssen, Eli Lilly, Novartis, and UCB. KA was financially supported by Amgen within the PARTNER fellowship program. All other authors declare no competing interests.
Data Availability Statement:
The data that support the findings of this case are available on request from the corresponding author.
References
- 1. Yazici H, Seyahi E, Hatemi G, Yazici Y. Behçet syndrome: a contemporary view. Nat Rev Rheumatol. 2018;14(2):107 119. ( 10.1038/nrrheum.2017.208) [DOI] [PubMed] [Google Scholar]
- 2. Soejima Y, Kirino Y, Takeno M, et al. Changes in the proportion of clinical clusters contribute to the phenotypic evolution of Behçet's disease in Japan. Arthritis Res Ther. 2021;23(1):49. ( 10.1186/s13075-020-02406-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zou J, Luo JF, Shen Y, Cai JF, Guan JL. Cluster analysis of phenotypes of patients with Behçet's syndrome: a large cohort study from a referral center in China. Arthritis Res Ther. 2021;23(1):45. ( 10.1186/s13075-021-02429-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hatemi G, Fresko I, Tascilar K, Yazici H. Increased enthesopathy among Behçet's syndrome patients with acne and arthritis: an ultrasonography study. Arthritis Rheum. 2008;58(5):1539 1545. ( 10.1002/art.23450) [DOI] [PubMed] [Google Scholar]
- 5. Karaca M, Hatemi G, Sut N, Yazici H. The papulopustular lesion/arthritis cluster of Behçet's syndrome also clusters in families. Rheumatol (Oxf Engl). 2012;51(6):1053 1060. ( 10.1093/rheumatology/ker423) [DOI] [PubMed] [Google Scholar]
- 6. Maldini C, Lavalley MP, Cheminant M, de Menthon M, Mahr A. Relationships of HLA-B51 or B5 genotype with Behçet's disease clinical characteristics: systematic review and meta-analyses of observational studies. Rheumatol (Oxf Engl). 2012;51(5):887 900. ( 10.1093/rheumatology/ker428) [DOI] [PubMed] [Google Scholar]
- 7. Casares-Marfil D, Esencan D, Alibaz-Oner F, et al. Clinical trait-specific genetic analysis in Behçet's disease identifies novel loci associated with ocular and neurological involvement. Clin Immunol. 2023;253:109657. ( 10.1016/j.clim.2023.109657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahn HS, Kim HJ, Kazmi SZ, et al. Familial risk of Behçet's disease among first-degree relatives: a population-based aggregation study in Korea. Rheumatol (Oxf Engl). 2021;60(6):2697 2705. ( 10.1093/rheumatology/keaa682) [DOI] [PubMed] [Google Scholar]
- 9. Akpolat T, Koç Y, Yeniay I, et al. Familial Behçet's disease. Eur J Med. 1992;1(7):391 395. [PubMed] [Google Scholar]
- 10. Gül A, Inanç M, Ocal L, Aral O, Koniçe M. Familial aggregation of Behçet's disease in Turkey. Ann Rheum Dis. 2000;59(8):622 625. ( 10.1136/ard.59.8.622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Onal S, Tugal-Tutkun I, Urgancioglu M, Gul A. Clinical course of ocular Behçet's disease in siblings. Ocul Immunol Inflamm. 2001;9(2):111 124. ( 10.1076/ocii.9.2.111.3971) [DOI] [PubMed] [Google Scholar]
- 12. Zabotti A, De Marco G, Gossec L, et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann Rheum Dis. 2023;82(9):1162 1170. ( 10.1136/ard-2023-224148) [DOI] [PubMed] [Google Scholar]
- 13. Brophy S, Hickey S, Menon A, et al. Concordance of disease severity among family members with ankylosing spondylitis? J Rheumatol. 2004;31(9):1775 1778. [PubMed] [Google Scholar]
- 14. Saadoun D, Bodaghi B, Cacoub P. Behçet's syndrome. N Engl J Med. 2024;390(7):640 651. ( 10.1056/NEJMra2305712) [DOI] [PubMed] [Google Scholar]
- 15. Abacar K, Macleod T, Direskeneli H, McGonagle D. How underappreciated autoinflammatory (innate immunity) mechanisms dominate disparate autoimmune disorders. Front Immunol. 2024;15:1439371. ( 10.3389/fimmu.2024.1439371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. An J, Marwaha A, Laxer RM. Autoinflammatory diseases: a review. J Rheumatol. 2024;51(9):848 861. ( 10.3899/jrheum.2023-1209) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this case are available on request from the corresponding author.



 Content of this journal is licensed under a
Content of this journal is licensed under a