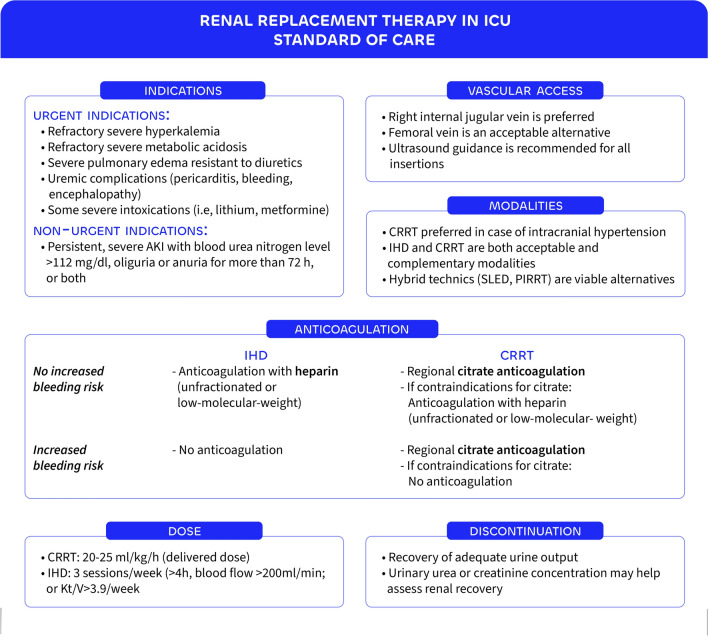
Over the past two decades, the approach to renal replacement therapy (RRT) in critically ill patients has shifted markedly—from early, largely empirical practices toward more individualized, evidence-based strategies. Large randomized-controlled trials (RCTs) have demonstrated the safety of applying a watch-and-wait strategy in the absence of urgent complications, while technical advances and refined dosing recommendations have improved safety and efficiency. This evolution supports a patient-centered model of care, integrating clinical judgment, therapeutic goals, and available resources to guide initiation, modality choice, and discontinuation of RRT. This article presents the key elements of the current standard of care for RRT in the intensive care setting. Figure 1 summarizes these elements (anticoagulation strategies and vascular access considerations are addressed in the figure but not discussed in the main text).
Fig. 1.
Renal replacement therapy in ICU. RRT = renal replacement therapy, AKI = acute kidney injury, CRRT = continuous renal replacement therapy, IHD = intermittent hemodialysis, SLED = sustained low-efficiency dialysis, PIRRT = prolonged intermittent renal replacement therapy, Kt/V = ([K] = clearance, [t] = time, and [V] = volume).
Initiation and timing
The optimal initiation of RRT during acute kidney injury (AKI) is guided primarily by clinical indications rather than a predefined timing. Four urgent indications necessitate immediate RRT to treat life-threatening complications: severe hyperkalemia unresponsive to standard medical treatment, severe metabolic acidosis not correctable by bicarbonate infusion (or by ventilatory compensation), refractory fluid overload unresponsive to diuretics leading to pulmonary edema, and specific uremic complications, such as encephalopathy or pericarditis. In the absence of these emergencies, a delayed RRT initiation strategy based on close clinical and biological monitoring should be preferred. Indeed, large RCTs (AKIKI [1], IDEAL-ICU [2], and STARRT-AKI [3]) have demonstrated that an early RRT initiation strategy (i.e., starting RRT before the occurrence of life-threatening complication) does not improve survival and may impair renal recovery. Notably, around 40% of patients managed with a delayed strategy never required RRT. However, excessive delays (more than 3 days of oligo-anuria or a serum urea concentration above 40 mmol/L) are associated with increased mortality, as highlighted in the AKIKI-2 study [4]. Therefore, the current standard of care supports a prudent, indication-driven initiation of RRT, balancing the need to avoid both unnecessary procedures and harmful delays. Personalized approaches are under investigation.
Modalities
Once the decision to initiate RRT is made, several modalities are available, including continuous RRT (CRRT), intermittent hemodialysis (IHD), hybrid techniques such as sustained low-efficiency dialysis (SLED) or prolonged intermittent RRT (PIRRT), or peritoneal dialysis in resource-limited settings. The optimal choice remains subject to ongoing debate. Current KDIGO guidelines recommend the use of CRRT in hemodynamically unstable patients due to its presumed better hemodynamic tolerance. Nonetheless, other expert groups emphasize that local expertise, staff training, and resource availability should primarily guide modality selection. While CRRT provides gradual solute and fluid removal, RCTs [5] and meta-analyses [6] have not shown a consistent survival benefit or improved renal recovery compared to IHD. A secondary analysis of the STARRT-AKI trial [7] suggested that CRRT may be associated with a reduced risk of death or dialysis dependence at 90 days (adjusted OR 0.81; 95% CI 0.66–0.99) which was more pronounced in patients with accelerated initiation of RRT. Similarly, a large claims-based cohort study involving over 34,000 patients found lower RRT dependency at hospital discharge among those treated with CRRT versus IHD (26.5% vs. 29.9%, p < 0.0001) [8]. In contrast, a secondary analysis of AKIKI and IDEAL-ICU trials [9] suggested that CRRT might be associated with increased mortality. The contradictory nature of these results highlights the limitations of observational data and post hoc analyses, especially in the context of confounding by indication and treatment heterogeneity. This uncertainty has led to the design of the ongoing ICRAKI trial (NCT05586503), an RCT specifically intended to clarify the impact of RRT modality on clinically meaningful outcomes, including survival and renal recovery.
Dose
Regardless of the modality, the dose of RRT is recognized as a key determinant of its effectiveness. It primarily reflects small solute clearance and the correction of complications related to electrolyte and acid–base imbalances. Large multicenter RCTs and several systematic reviews have not demonstrated a beneficial effect of higher versus lower RRT doses [10, 11]. Higher doses may even delay renal recovery at day 28 [relative risk 1.15 (95% confidence interval 1.00–1.33); P = 0.05] [12]. Regarding very high-dose CRRT (high-volume hemofiltration)—mainly studies in sepsis/septic shock patients and typically defined as a total effluent flow rate ≥ 45 mL/kg/h—systematic reviews of RCTs have shown no clinical benefit over standard CRRT dosing [13]. Additionally, small retrospective studies from Japan have evaluated the effects of lower-than-standard CRRT dosing (total effluent flow rate between 10 and 20 mL/kg/h) [14]. These studies suggest that lower doses may be tolerated and can achieve comparable control of electrolyte, acid–base, and metabolic parameters. However, their findings regarding major clinical outcomes remain unknown, and RCTs on low-dose RRT are ongoing. Current recommendations, such as those from the KDIGO guidelines, suggest targeting a CRRT dose of 20–25 mL/kg/h. For intermittent hemodialysis (IHD) or prolonged intermittent RRT, a weekly Kt/V of approximately 3.9 is recommended.
Discontinuation
Discontinuation of RRT is indicated if renal recovery is occurring. Since creatinine and other uremic toxins are removed by RRT, the recovery of endogenous glomerular filtration rate is difficult to obtain. Based on large cohort studies, a spontaneous urinary output of > 500 ml/h or 2.4 L when using diuretics is widely accepted criterion to try discontinuation [15]. Since prolonged or unnecessary RRT may even harm renal recovery, biomarkers are investigated on their ability to help in decision-making (upon those are NGAL, proenkephalin, and CCL14) for the optimal time-point for discontinuation. Beside urinary output at time of discontinuation, urinary output at beginning of RRT, duration RRT, and preexisting CKD are prognostic factors which can be taken into consideration. The use of diuretics however, does not appear to hasten deliberation from RRT [16].
In the coming years, RRT practices in the ICU are expected to evolve toward greater personalization, guided by patient-specific clinical and biological markers. Individualized timing of initiation, as proposed in the recent modeling studies, may optimize outcomes by targeting therapy more precisely. Ongoing trials aim to clarify the optimal modality choice, while other studies are exploring the benefits of lower RRT dosing strategies. Additionally, efforts to standardize and optimize RRT discontinuation will help refine decision-making in this critical phase. Together, these advances promise a more tailored and effective approach to RRT in critical care.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. None.
Declarations
Not applicable.
Conflicts of interest
SG declares no competing interest regarding the submitted manuscript.
NS has received honoraria or research support from Vantive Healthcare Corp, and Novartis outside the submitted work. MJ has received honoraria or research support from Baxter Healthcare Corp, AM-Pharma, CLS Behring, Fresenius, Takeda, and Novartis outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gaudry S, Hajage D, Schortgen F et al (2016) Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 375:122–133. 10.1056/NEJMoa1603017 [DOI] [PubMed] [Google Scholar]
- 2.Barbar SD, Clere-Jehl R, Bourredjem A et al (2018) Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 379:1431–1442. 10.1056/NEJMoa1803213 [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw S, Wald R, Adhikari N et al (2020) Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med 383:240–251. 10.1056/NEJMoa2000741 [DOI] [PubMed] [Google Scholar]
- 4.Gaudry S, Hajage D, Martin-Lefevre L et al (2021) Comparison of two delayed strategies for renal replacement therapy initiation for severe acute kidney injury (AKIKI 2): a multicentre, open-label, randomised, controlled trial. Lancet Lond Engl 397:1293–1300. 10.1016/S0140-6736(21)00350-0 [DOI] [PubMed] [Google Scholar]
- 5.Vinsonneau C, Camus C, Combes A et al (2006) Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet Lond Engl 368:379–385. 10.1016/S0140-6736(06)69111-3 [DOI] [PubMed] [Google Scholar]
- 6.Ye Z, Wang Y, Ge L et al (2021) Comparing renal replacement therapy modalities in critically Ill patients with acute kidney injury: a systematic review and network meta-analysis. Crit Care Explor 3:e0399. 10.1097/CCE.0000000000000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald R, Gaudry S, da Costa B et al (2023) Initiation of continuous renal replacement therapy versus intermittent hemodialysis in critically ill patients with severe acute kidney injury: a secondary analysis of STARRT-AKI trial. Intensive Care Med 49:1305–1316. 10.1007/s00134-023-07211-8 [DOI] [PubMed] [Google Scholar]
- 8.Koyner JL, Mackey RH, Echeverri J et al (2024) Initial renal replacement therapy (RRT) modality associates with 90-day postdischarge RRT dependence in critically ill AKI survivors. J Crit Care 82:154764. 10.1016/j.jcrc.2024.154764 [DOI] [PubMed] [Google Scholar]
- 9.Gaudry S, Grolleau F, Barbar S et al (2022) Continuous renal replacement therapy versus intermittent hemodialysis as first modality for renal replacement therapy in severe acute kidney injury: a secondary analysis of AKIKI and IDEAL-ICU studies. Crit Care 26:93. 10.1186/s13054-022-03955-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, et al (2008) Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359:7–20. 10.1056/NEJMoa0802639 [DOI] [PMC free article] [PubMed]
- 11.RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A et al (2009) Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361:1627–1638. 10.1056/NEJMoa0902413 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Gallagher M, Li Q et al (2018) Renal replacement therapy intensity for acute kidney injury and recovery to dialysis independence: a systematic review and individual patient data meta-analysis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc 33:1017–1024. 10.1093/ndt/gfx308 [DOI] [PubMed] [Google Scholar]
- 13.Borthwick EM, Hill CJ, Rabindranath KS et al (2017) High-volume haemofiltration for sepsis in adults. Cochrane Database Syst Rev 1:CD008075. 10.1002/14651858.CD008075.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yagi K, Fujii T, Kageyama A et al (2024) The effects of early-phase, low- or standard-intensity continuous renal replacement therapy on acid-base control and clinical outcomes: an observational study. Blood Purif 53:716–724. 10.1159/000539810 [DOI] [PubMed] [Google Scholar]
- 15.Uchino S, Bellomo R, Morimatsu H et al (2009) Discontinuation of continuous renal replacement therapy: a post hoc analysis of a prospective multicenter observational study. Crit Care Med 37:2576–2582. 10.1097/CCM.0b013e3181a38241 [DOI] [PubMed] [Google Scholar]
- 16.van der Voort PHJ, Boerma EC, Koopmans M et al (2009) Furosemide does not improve renal recovery after hemofiltration for acute renal failure in critically ill patients: a double blind randomized controlled trial. Crit Care Med 37:533–538. 10.1097/CCM.0b013e318195424d [DOI] [PubMed] [Google Scholar]