Abstract
Introduction
The rising prevalence of diabetes has driven extensive research into effective management strategies, emphasizing the importance of integrating self-management routines into daily life. This study presents real-world observations on the impact of the mySugr® app, used in conjunction with the Accu-Chek® Instant blood glucose monitoring device, on glycemic control and patient satisfaction in India.
Methods
This retrospective, observational, non-interventional study was conducted at 29 sites in India, involving people with diabetes (PwD) who used the mySugr® app in conjunction with the Accu-Chek® Instant glucose meter for at least 3 months. Data from electronic health records and paper-based records were analyzed. The primary objective was to evaluate changes in glycated hemoglobin (HbA1c) levels over 3 months. Additionally, the study assessed the frequency of hypoglycemic and hyperglycemic events, changes in HbA1c based on monitoring frequency, and the use of insulin and non-insulin therapies. Patient satisfaction with the mySugr® app was also assessed.
Results
A total of 111 PwD were included and had an average age of 53.2 years. The mean HbA1c level significantly decreased from 8.8% to 7.5% (p < 0.0001) in PwD using the mySugr® app in conjunction with the Accu-Chek® Instant glucose meter for at least 3 months. Frequent monitoring (≥ 6 times per week) resulted in a greater HbA1c reduction (1.5%-points) compared to less frequent monitoring (1.0%-point). Insulin-treated PwD showed a larger HbA1c reduction (1.6%-points) compared to those not on insulin (0.8%-points). PwD reported an average of 1.9 hypoglycemic and 20.0 hyperglycemic events.
Conclusion
The mySugr® app, used in conjunction with the Accu-Chek® Instant glucose meter, demonstrated improved glycemic control. Future research should focus on larger, diverse samples and long-term evaluations to confirm these findings and explore the cost-effectiveness of integrating such applications into routine diabetes care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-025-01768-x.
Keywords: Diabetes mellitus, mySugr® app, HbA1c, Glycemic control, PwD, End-user satisfaction, Digital health tools, Asian Indians, SMBG, HbA1c
Plain Language Summary
This study aimed to evaluate the impact of the mySugr® mobile health application, used in conjunction with the Accu-Chek® Instant glucose meter, on blood sugar control and patient satisfaction among people with diabetes in India. Conducted at 29 healthcare centers, the study included 111 participants who used the app for at least 3 months. The results showed a significant reduction in average glycated hemoglobin (HbA1c) levels from 8.8% to 7.5%, with more frequent blood glucose monitoring leading to greater improvements. Insulin-treated participants experienced a larger decrease in HbA1c compared to those not on insulin. Participants reported an average of 1.9 low blood sugar events and 20 high blood sugar events during the study. The app received positive feedback for its usability, scoring 70 out of 100 on the System Usability Scale (SUS). The study concluded that the mySugr® app, when used with the Accu-Chek® Instant glucose meter, effectively improves blood sugar control and is well received by users. Future research should focus on larger, diverse samples and long-term evaluations to confirm these findings and explore the cost-effectiveness of integrating such applications into routine diabetes care.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-025-01768-x.
Key Summary Points
| Why carry out this study? |
| India has a high and growing burden of type 2 diabetes, with many individuals lacking effective self-management tools. |
| There is a need to evaluate real-world digital health solutions that support glycemic control and patient engagement. |
| This study assessed the impact of the mySugr® app, used with the Accu-Chek® Instant glucose meter, on glycemic outcomes and patient satisfaction in people with diabetes in India. |
| What was learned from the study? |
| The study found a significant reduction in mean glycated hemoglobin (HbA1c) from 8.8% to 7.5% over 3 months, with greater reductions in those using insulin or monitoring more frequently. |
| Digital tools like the mySugr® app can enhance self-monitoring, support treatment adjustments, and improve patient satisfaction, suggesting their potential for broader integration into diabetes care. |
Introduction
Type 2 diabetes mellitus (T2DM) has emerged as a major public health challenge in India, with over 100 million individuals currently affected and projections indicating a rise to 124 million by 2045 [1]. Despite the availability of effective pharmacological treatments, glycemic control remains suboptimal in a large proportion of people with diabetes (PwD), primarily because of inadequate self-management, limited access to care, and poor treatment adherence [2, 3]. Self-monitoring of blood glucose (SMBG) is the cornerstone of diabetes self-management (DSM), enabling timely therapeutic adjustments and improved glycemic outcomes [3, 4]. However, in India, barriers such as low awareness, limited digital literacy, and fragmented care models hinder the widespread adoption of structured SMBG [5–9]. In recent years, mobile health (mHealth) applications have shown promise in supporting DSM by offering real-time tracking, personalized feedback, and improved communication between patients and healthcare providers [10–13]. The mySugr® app is one such digital tool designed to enhance SMBG and patient engagement. While global studies have demonstrated the effectiveness of mHealth apps in improving glycated hemoglobin (HbA1c) and treatment adherence [10–14], real-world evidence from India remains limited [14–19]. This study aims to address this gap by evaluating the impact of the mySugr® app, used in conjunction with the Accu-Chek® Instant glucose meter, on glycemic control and patient satisfaction among PwD in India. Through a retrospective, real-world analysis, we assess changes in HbA1c, frequency of glycemic events, and user-reported outcomes over 3 months.
Borgharkar and Das [8] report that a significant proportion of the Indian population with type 2 diabetes has poor glycemic control. Specifically, the study found that 76.6% of people with type 2 diabetes in India have uncontrolled glucose levels. This highlights the urgent need for effective diabetes management strategies to reduce the risk of complications [3].
Effective self-management, especially through SMBG, is crucial for achieving optimal glycemic control and preventing complications associated with diabetes. Comprehensive strategies and interventions are urgently needed to support DSM. Studies have shown that integrated lifestyle interventions, including diet, exercise, psychological support, and medical management, can significantly improve glycemic control and quality of life [2, 3, 5, 6, 9]. The Integrated Personalised Diabetes Management (iPDM) approach is a comprehensive method that combines personalized treatment plans with advanced digital tools to manage diabetes effectively. In recent years, digital applications, particularly mHealth (mobile health) apps, have emerged as essential tools for DSM. These applications offer a wide range of features, including blood glucose tracking, medication management, diet and nutrition logging, physical activity monitoring, data sharing with healthcare providers and family members or caregivers, and interactive learning modules. These functionalities assist individuals with diabetes in monitoring their glucose levels, managing their medications, and making healthier lifestyle choices [10–12]. Globally, multiple studies have demonstrated the effectiveness of these apps in managing T2DM [10–14]. Participants who used these interventions showed significantly greater reductions in HbA1c levels and weight compared to control groups [10]. In India and other South Asian countries, although limited, studies have indicated a notable decrease in HbA1c levels following the use of these apps [14–19]. Systematic reviews and trials have highlighted the benefits of mHealth apps, though the effectiveness of these interventions varied across different studies. Some reviews concluded that mHealth apps are a viable option with the potential to enhance health, particularly in terms of glycemic control, when compared to standard care [20–22].
As the prevalence of diabetes continues to rise, leveraging digital health technologies becomes increasingly important in enhancing self-management and improving outcomes for people living with diabetes in India [23, 24]. Therefore, the mySugr® app was designed to assist individuals in managing diabetes by offering innovative solutions and providing tools for tracking blood glucose levels, logging meals, and activities. mySugr® is an mHealth application introduced by Roche Diabetes Care, certified with the CE mark, and registered with the US Food and Drug Administration.
Since DSM is significantly influenced by the attitudes and behaviors of PwD, alternative research methodologies are necessary to more effectively evaluate behavior-based interventions, such as the use of mHealth applications, in a real-world setting. To gather this evidence, a retrospective observational study was conducted to evaluate the impact of the mySugr® app on glycemic control and user satisfaction.
Methods
Study Design
This phase IV retrospective, observational, non-interventional study was conducted at 29 healthcare centers across various regions of India between April 2023 and July 2024. The study focused on evaluating people with diabetes (PwD) who utilized the mySugr® app in conjunction with the Accu-Chek® Instant blood glucose meter for a minimum duration of 3 months. Data were sourced and analyzed from both electronic health records and paper-based records of the participants. The primary objective was to assess changes in HbA1c levels over 3 months. Secondary objectives included evaluating the frequency of hypoglycemic and hyperglycemic events, and the satisfaction of PwD/end-users with the mySugr® app. The primary endpoint was the mean change in HbA1c levels among diabetes patients using the mySugr® application over 3 months. Secondary endpoints encompassed the frequency of hypoglycemic and hyperglycemic events, and responses to the mySugr® application questionnaire. Exploratory endpoints, including additional secondary endpoints, were examined post hoc as necessary.
Feedback on patient satisfaction was gathered from healthcare professionals (HCPs) based on their clinical opinions regarding the use of the mySugr® app by PwD.
Study Population
Participants were PwD eligible for the study if they were adults (≥ 18 years) of any gender with a diagnosis of T2DM, had been using the mySugr® app for a minimum of 3 months, had completed the satisfaction survey for the app, and were willing to provide informed consent. HCPs were included if they held an MD degree and were specialized in treating PwD. Exclusion criteria encompassed incomplete key data points in electronic medical records (EMR) or patient-based records (PBR).
Sample Size
The study included 111 PwD who used the mySugr® app for at least 3 months between April 2023 and July 2024. Data collection involved gathering demographic information (age, biological sex), medical history (diagnosis, differential diagnosis, medication history, concomitant medications), clinical data (presenting complaints, signs and symptoms, laboratory reports), and assessment details (clinical assessment and pharmacological management plan). Additionally, data from the mySugr® app, including HbA1c, the frequency of hypoglycemic and hyperglycemic events, were collected along with PwD/end-users’ satisfaction survey results. All data were collected using a pre-defined case report format and entered into a pre-defined Microsoft Excel format for statistical analysis.
Data Analysis
Continuous data were summarized using descriptive statistics, including the number of PwD, mean, standard deviation, median, minimum, and maximum. Categorical data were summarized using frequency counts and percentages. All statistical tests were conducted at the 5% significance level, unless indicated otherwise.
The following secondary efficacy analyses were performed to assess the improvement in glucose levels over 3 months among PwD using the mySugr® app. Each statistical test was conducted separately at a significance level of 5%. The mean glucose levels at 3 months were statistically compared to the mean glucose levels at baseline using a paired-difference t test.
The study utilized the System Usability Scale (SUS) [25], a standardized questionnaire used to evaluate perceived usability. It consists of 10 items, each offering five response options ranging from “strongly Disagree” to “strongly agree.” The questionnaire has a mixed tone: odd-numbered items are positively worded, while even-numbered items are negatively worded. To score the SUS, the score for each item is determined, which falls between 0 and 4. For positively worded items (odd-numbered), 1 point is subtracted from the scale position to find the score. For negatively worded items (even numbered), subtract the scale position from 5 to get the score (e.g., if the allocated score is 1, then it is calculated as 5 − 1). To calculate the overall SUS score, the sum of these item scores is multiplied by 2.5. This produces a final score between 0 (very poor usability) and 100 (excellent usability), in increments of 2.5 points. These SUS scores are then translated into percentile ranks and letter grades to provide a more detailed interpretation of system usability. The letter grades are categorized as A, 85–100 (excellent); B, 70–84 (good); C, 50–69 (okay); D, 25–49 (poor); F, 0–24 (awful). This process provides a standardized measure of system usability [25].
The detailed SUS scoring method is described in the supplementary material (Table S1).
Participants with missing values for key parameters such as baseline or follow-up HbA1c or comorbidity data were excluded from the final analysis to maintain data integrity.
The study protocol was reviewed and approved by the ethics committee of institutions (Royal Pune Independent Ethics Committee, IEC No. RPIEC150824) involved in planning the study and in data recruitment. Written informed consent was obtained from all participants in the study before study initiation.
Results
Participant Demographics and Baseline Characteristics
A total of 111 people with diabetes (PwD) were enrolled in this retrospective observational study conducted across 29 healthcare centers in India. The mean age of participants was 53.2 years (SD 11.4), with an age range spanning from 26 to 82 years. Among the 81 PwD with documented diabetes history, the average duration of diabetes was 10.9 years (SD 6.3), with a range from 0.01 to 31 years. The remaining 30 participants did not have a recorded duration of diabetes (date of initial diagnosis).
Comorbid conditions were prevalent among the study population, with hypertension (n = 16) and diabetic neuropathy (n = 12) being the most frequently reported. These baseline characteristics reflect a diverse and clinically relevant population, representative of real-world diabetes management scenarios in India. Table 1 provides a detailed summary of demographic and clinical baseline characteristics.
Table 1.
PwD demographics and baseline characteristics
| Characteristic | Value |
|---|---|
| Total PwD enrolled | 111 |
| Age (years; mean ± SD) | 53.2 ± 11.4 |
| Age range (years) | 26–82 |
| Duration of diabetes (years; mean ± SD) | 10.9 ± 6.3 |
| Duration range (years) | 0.01–31 |
| Baseline HbA1c (%) (years; mean ± SD) | 8.8 ± 1.9 |
| HbA1c range (%) | 5.6–13.6 |
HbA1c glycated hemoglobin, SD standard deviation, PwD people with diabetes
In the analysis of blood glucose monitoring frequency, a total of 111 PwD were initially considered. However, 19 PwD did not meet the inclusion criteria and were subsequently excluded from the analysis. Therefore, the final analysis for blood glucose monitoring frequency was conducted on 92 PwD.
Primary Outcome: Glycemic Control (Absolute Reduction in HbA1c)
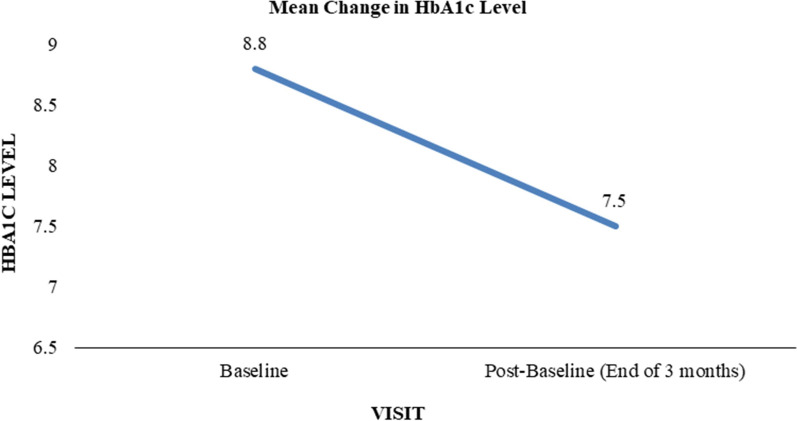
The primary endpoint of the study was the change in HbA1c levels over 3 months of using the mySugr® mobile health application in conjunction with the Accu-Chek® Instant blood glucose meter. At baseline, the mean HbA1c level was 8.8% (SD 1.9). After 3 months of app usage, the mean HbA1c significantly decreased to 7.5% (SD 1.0), representing a mean reduction of 1.3%-points (SD 1.6). This change was statistically significant (p < 0.0001), indicating a clinically meaningful improvement in glycemic control.
This reduction in HbA1c underscores the potential of digital health tools to support better diabetes self-management and improve clinical outcomes in a real-world setting. Figure 1 illustrates the mean change in HbA1c levels from baseline to the end of the study period.
Fig. 1.
Mean change in HbA1c levels. HbA1c glycated hemoglobin
Secondary Outcomes
Frequency of Hypoglycemic and Hyperglycemic Events
During the 3-month observation period, participants recorded an average of 1.9 hypoglycemic events (defined as blood glucose < 70 mg/dL) and 20.0 hyperglycemic events (defined as blood glucose > 180 mg/dL). The number of hypoglycemic events ranged from 0 to 30, while hyperglycemic events ranged from 0 to 124. The most frequently reported number of hypoglycemic events was 0, indicating that many participants did not experience low blood glucose episodes during the study. In contrast, the most common number of hyperglycemic events was 10.
Age-specific analysis revealed that PwD aged 20–30 years experienced the highest mean number of hypoglycemic events (mean 7), while those aged 30–40 years had the highest mean number of hyperglycemic events (mean 24.9). These findings suggest that while hypoglycemia was relatively infrequent, hyperglycemia remained a common challenge, particularly among younger adults.
Subgroup Analysis
Age-Based HbA1c Reduction
Subgroup analysis based on age revealed that PwD aged 40–50 years experienced the most substantial improvement in glycemic control, with a mean HbA1c reduction of 1.6%-points. This suggests that middle-aged adults may derive particular benefit from structured self-monitoring and digital engagement in diabetes management. This age group may benefit from a combination of factors such as higher digital literacy, greater motivation to manage health due to mid-life responsibilities, and better adherence to self-monitoring and treatment regimens, which could contribute to the observed improvement [14, 16, 32].
Blood Glucose Monitoring Frequency
To assess the impact of monitoring frequency on glycemic outcomes, participants were stratified into two groups: those who monitored their blood glucose levels ≥ 6 times per week (frequent monitors, n = 44) and those who monitored < 6 times per week (less frequent monitors, n = 48). Nineteen participants were excluded from this analysis because of incomplete data.
Frequent monitors demonstrated a greater reduction in HbA1c, from a baseline of 8.9% to 7.4%, representing a mean decrease of 1.5%-points. In contrast, less frequent monitors showed a reduction from 8.4% to 7.4%, a mean decrease of 1.0%-point. Both reductions were statistically significant (p < 0.0001, paired t test). However, the between-group comparison did not reach statistical significance, likely because of the relatively small sample sizes, which may have limited the statistical power to detect a difference. Figure 2 presents the HbA1c changes stratified by monitoring frequency.
Fig. 2.
Mean change in HbA1c levels based on blood glucose monitoring (BGM) frequency. HbA1c glycated hemoglobin
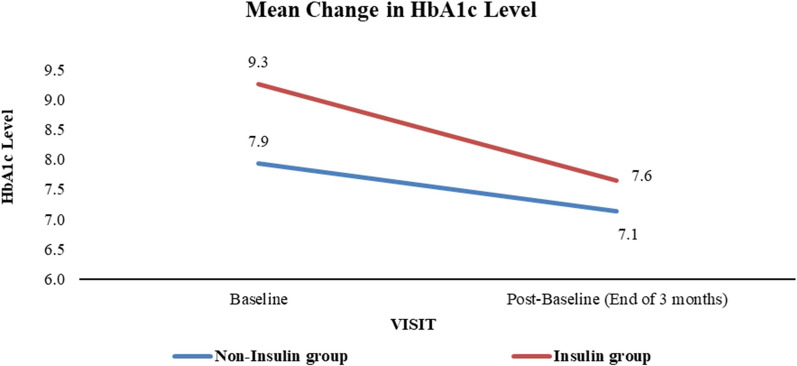
Insulin Use and HbA1c Reduction
Participants were also stratified according to insulin use. The insulin-treated group (n = 41), who were already on insulin therapy before the study, had a higher baseline HbA1c of 9.3%, which decreased to 7.6% after 3 months, reflecting a mean reduction of 1.6%-points. The non-insulin group (n = 70) showed a reduction from 7.9% to 7.1%, a mean decrease of 0.8%-points. Both reductions were statistically significant (p < 0.0001, paired t test). Although the insulin group demonstrated a more pronounced improvement, the between-group comparison did not achieve statistical significance, again likely because of sample size limitations. Figure 3 illustrates the HbA1c changes by insulin use.
Fig. 3.
Mean change in HbA1c levels based on insulin use. HbA1c glycated hemoglobin
These findings suggest that insulin-treated PwD may benefit more from structured monitoring and digital engagement, possibly because of the greater need for precise glucose control and dose adjustments.
System Usability and Satisfaction: PwD' Satisfaction with the mySugr® App
User satisfaction was assessed using the SUS, a validated tool for evaluating perceived usability. The overall SUS score for the mySugr® app was 70, which is above the average benchmark of 68 and reflects “acceptable usability with room for improvement,” according to standard SUS interpretation [25]. This indicates that the app was generally well received by users.
Approximately 50% of PwD expressed a desire to use the app frequently, and 51% found it easy to use. Additionally, 44% reported feeling confident in using the app. These findings suggest that the app’s interface and functionality were accessible and user-friendly, which is critical for sustained engagement and long-term adherence to self-monitoring practices. Figure 4 summarizes the responses to the satisfaction survey.
Fig. 4.
PwD/end-user satisfaction survey result. PwD people with diabetes
Healthcare Provider (HCP) Feedback
In a survey conducted with HCPs, the effectiveness of the mySugr® app in managing diabetes was evaluated. The survey aimed to gather insights from HCPs on the perceived benefits of the app in reducing HbA1c levels among PwD. A total of 29 HCPs participated in the survey, providing valuable feedback on their experiences with the mySugr app. All HCPs reported observing significant benefits associated with the use of the mySugr® app, including:
Improved adherence: PwD demonstrated better adherence to treatment regimens, facilitated by the app’s reminders and logging features.
Enhanced glucose control: HCPs observed notable improvements in glucose levels of PwD, attributing this to the app’s ability to provide real-time data sharing, which enabled timely interventions and adjustments to therapy.
Optimized treatment management: The app supported more informed clinical decision-making, particularly in insulin dose titration and medication adjustments.
HCPs emphasized that the app’s ability to visualize glucose trends and patterns helped both clinicians and patients identify areas for improvement and make data-driven decisions. The integration of self-monitoring data into routine care workflows was seen as a key advantage, enhancing the personalization of diabetes management.
Discussion
This real-world, retrospective study evaluated the impact of the mySugr® mobile health application, used in conjunction with the Accu-Chek® Instant glucose meter, on glycemic control and user satisfaction among people with type 2 diabetes in India. The findings demonstrated a statistically significant reduction in HbA1c levels over 3 months, with a mean decrease from 8.8% to 7.5% (p < 0.0001), supporting the effectiveness of structured SMBG when integrated with a digital health platform [14–16].
A key strength of this study lies in its subgroup analyses. Participants who monitored their blood glucose more frequently (≥ 6 times per week) experienced a greater HbA1c reduction (1.5%-points) compared to those with less frequent monitoring (1.0%-point). Similarly, insulin-treated individuals showed a more pronounced HbA1c reduction (1.6%-points) than those not on insulin (0.8%-points). These findings reinforce the role of personalized monitoring strategies and suggest that the app may be particularly beneficial for individuals requiring intensive glycemic management [27–32].
The integration of SMBG data through the app facilitated essential adjustments to medication regimens, contributing to improved glucose control and better outcomes for PwD. The app’s ability to document glycemic trends enabled HCPs to make informed adjustments to anti-diabetes treatments, including insulin and oral antidiabetic drugs (OADs). This was especially valuable in cases of inconsistent monitoring, where traditional lab tests were insufficient [30, 31].
The study also captured the frequency of hypoglycemic and hyperglycemic events, with an average of 1.9 and 20.0 events, respectively, over 3 months. While hyperglycemia remained more prevalent, the relatively low incidence of hypoglycemia suggests that the app-supported SMBG approach may help users avoid overtreatment and maintain safer glucose levels [30, 31].
The app’s visual representation of SMBG readings—through graphs and charts—helped users identify patterns and trends, making complex data more accessible and actionable. This not only improved self-management but also enhanced communication with HCPs, leading to more effective treatment adjustments and glucose control [32].
Real-time monitoring capabilities, enabled by Bluetooth connectivity, allowed HCPs to access glucose data directly from the app, eliminating the need for manual logs. This streamlined workflow supported timely clinical decisions and personalized treatment planning, contributing to improved outcomes [32].
User satisfaction was another critical component of this study. The usability of the mySugr® app was rated positively by users, with a SUS score of 70, indicating good usability. Approximately half of the participants expressed confidence in using the app and a desire to continue its use, encouraging consistent monitoring and management of their condition. These findings are critical, as sustained engagement with digital tools is essential for long-term glycemic control [25, 32].
HCP feedback further validated the clinical utility of the app. Providers reported improved adherence, better glucose control, and more effective treatment adjustments based on app-generated data. The ability to access real-time SMBG data enabled more informed clinical decisions and facilitated personalized treatment planning. This dual perspective—from both patients and providers—underscores the app’s potential to enhance communication, engagement, and decision-making in diabetes care [32].
While CGM technologies are gaining traction globally, their adoption in India remains limited because of cost, infrastructure, and awareness barriers [8, 23]. In this context, the mySugr® app offers a scalable, affordable alternative that can deliver meaningful clinical benefits. The study’s findings are particularly relevant for low- and middle-income countries (LMICs), where digital health tools can bridge critical gaps in diabetes management and support iPDM strategies [10, 11, 33].
Despite these promising results, the study has several limitations. Its retrospective design may introduce selection and recall biases, and the relatively small sample size limits generalizability. The 3-month duration, while sufficient to observe short-term changes, does not capture the long-term sustainability of glycemic improvements. Additionally, as this was a real-world observational study, potential confounding factors such as changes in treatment regimens, dietary habits, physical activity levels, and physician counseling were not controlled. These variables may have independently influenced glycemic outcomes, making it difficult to attribute improvements solely to the use of the mySugr® app. Incomplete EMRs or PBRs may have affected data accuracy, and the exclusion of incomplete records could introduce selection bias. Self-reported outcomes, such as satisfaction and app usage frequency, may also be subject to reporting bias.
To address these limitations, future research should focus on prospective studies with larger, more diverse populations and longer follow-up periods. Such studies should also incorporate objective measures of app engagement, control for confounding variables, and assess the cost-effectiveness of integrating digital tools like the mySugr® app into national diabetes care frameworks. Collecting detailed data on lifestyle and treatment changes will be essential to isolate the specific impact of digital health interventions on glycemic control.
Conclusion
While SMBG supported by digital tools like the mySugr® app is associated with improved glycemic outcomes, these benefits are most effectively realized when paired with timely and informed treatment adjustments. The app acts as a vital enabler of this process, bridging the gap between PwD and healthcare professionals, fostering shared decision-making, and empowering individuals to take an active role in their diabetes management journey.
Despite these advantages, challenges such as non-compliance with intensive monitoring, data reliability concerns, technological barriers, and cost constraints need to be addressed. By implementing strategies to overcome these hurdles, digital tools like the mySugr app can play a vital role in transforming diabetes care, particularly in India.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Anirban Mujumdar, Dr. Arvind Gupta, Dr. Anuj Maheshwari, Dr. Anantharaman R, Dr. Arthur Joseph Asirvatham, Dr. Banshi Saboo, Dr. Debmalya Sanyal, Dr. Jothydev Kesavadev, Dr. K M Prassana Kumar, Dr. Manoj Chawla, Dr. Nagendar Reddy J, Dr. Rishi Shukla, Dr. S.K. Mathur, Dr. S.S. Srikanta, Dr. Sujay Ghosh, Dr. Tom Babu, Dr. Vijay Vishwanathan, Dr. Yash Patel, Dr. Yogesh Kadam, Dr. Kavitha Muniraj, Dr. Rajiv Kovil, Prerna Goyal, Dr. B.M. Makkar, Dr. Supratik Bhattacharya, Dr. Bhahu Pravin Naidu, Dr. Sanjay Agarwal, Beatrice Anne, Dr. Vishal Gala and Dr. Caterina Presenti for their expertise, support, and guidance in the completion of this study. We are deeply grateful for their contributions. The authors would like to acknowledge the use of the System Usability Scale (SUS) in this study. The SUS was originally developed by John Brooke at Digital Equipment Corporation in 1986 and is copyrighted by Digital Equipment Corporation. The scale is freely available for use in usability assessments, provided proper citation is given. We have adhered to these requirements in our study [25].
Medical Writing/Editorial Assistance
Medical writing and editorial support were provided by Dr. Neha Deshpande and Ms. Priyanka Barathe from EVERSANA APAC, and this assistance was funded by Roche Diabetes Care, India.
Author’s Contributions
All authors made substantial contributions to study conception, design, writing, reviewing, and approving the final draft and had full access to datasets and contributed to methodology, data curation, data analysis, validation, and visualisation, project supervision, resource planning, and provision of funding. Dr. V. Mohan: conceptualization, supervision, critical review of the manuscript. Dr. Sanjay Kalra: study design, data interpretation, manuscript review. Dr. Abin Augustine: study coordination, data analysis, drafting and revising the manuscript, project administration. Ms. Johanna Kober: data acquisition, interpretation, manuscript editing, and review. All authors had full access to the data, contributed to the study design, reviewed and approved the final manuscript, and agreed to be accountable for all aspects of the work.
Funding
The study design, conduct, data analysis, medical writing assistance, and publication, including the journal’s Rapid Service Fee, were funded by Roche Diabetes Care, India.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
V. Mohan declares no conflicts for this work. Sanjay Kalra is an Editorial Board member of Diabetes Therapy. Sanjay Kalra was not involved in the selection of peer reviewers for the manuscript, nor any of the subsequent editorial decisions. Abin Augustine is an employee of Roche Diabetes Care, India. Johanna Kober is an employee of Roche mySugr Austria.
Ethical Approval
The study protocol was reviewed and approved by the ethics committee of institutions (Royal Pune Independent Ethics Committee, IEC No. RPIEC150824) involved in planning the study and in data recruitment. Written informed consent was obtained from all participants in the study before study initiation.
Footnotes
Prior Presentation: A preliminary version of this study was presented as a basic poster at the Research Society for the Study of Diabetes in India (RSSDI) 2024 Annual Conference, held in Delhi, India, from November 14 to 17, 2024. The poster included minimal details and served as an initial presentation of the study findings.
References
- 1.Anjana RM, Unnikrishnan R, Deepa M, et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023;11(7):474–89. [DOI] [PubMed] [Google Scholar]
- 2.Anjana RM, Deepa M, Venkatesan U, et al. Achievement of guideline recommended diabetes treatment targets and health habits in people with self-reported diabetes in India (ICMR-INDIAB-13): a national cross-sectional study. Lancet Diabetes Endocrinol. 2022;10(6):430–41. [DOI] [PubMed]
- 3.Venkataraman K, Kannan AT, Viswanathan M. Challenges in diabetes management with particular reference to India. Int J Diabetes Dev Countries. 2009;29(3):103–9. [DOI] [PMC free article] [PubMed]
- 4.Mohan V, Deepa M, Anjana RM, Lanthorn H, Deepa R. Incidence of diabetes and pre-diabetes in a selected urban south Indian population (CUPS-19). J Assoc Physicians India. 2008;56:152–7. [PubMed]
- 5.Mohan V, Ramachandran A, Snehalatha C, Mohan R, Bharani G, Viswanathan M. High prevalence of maturity-onset diabetes of the young (MODY) among Indians. Diabetes Care. 1985;8(4):371–4. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan M, Shanmugam S, Rajagopal G, Kamala K. Are unhealthy diets contributing to the rapid rise of type 2 diabetes in India? J Nutr. 2023;153(4):940–8. [DOI] [PubMed]
- 7.Mohan V, Gokulakrishnan K, Deepa R, Shanthirani CS, Datta M. Association of physical inactivity with components of metabolic syndrome and coronary artery disease—the Chennai Urban Population Study (CUPS no. 15). Diabet Med. 2005;22(9):1206–11. [DOI] [PubMed]
- 8.Borgharkar SS, Das SS. Real-world evidence of glycemic control among patients with type 2 diabetes mellitus in India: the TIGHT study. BMJ Open Diabetes Res Care. 2019;7(1):e000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham AM, Sudhir PM, Philip M, Bantwal G. Efficacy of a brief self-management intervention in type 2 diabetes mellitus: a randomized controlled trial from India. Indian J Psychol Med. 2020;42(6):540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong E, Baptista S, Russell A, et al. My diabetes coach, a mobile app-based interactive conversational agent to support type 2 diabetes self-management: randomized effectiveness-implementation trial. J Med Internet Res. 2020;22(11):e20322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim SL, Ong KW, Johal J, et al. Effect of a smartphone app on weight change and metabolic outcomes in Asian adults with type 2 diabetes. JAMA Netw Open. 2021;4(6):e2112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Lee EY, Kim HS, Lee SH, Yoon KH, Cho JH. Effect of a mobile phone-based glucose-monitoring and feedback system for type 2 diabetes management in multiple primary care clinic settings: cluster randomized controlled trial. JMIR Mhealth Uhealth. 2020;8(2):e16266. [DOI] [PMC free article] [PubMed]
- 13.Bennett GG, Steinberg D, Askew S, et al. Effectiveness of an app and provider counseling for obesity treatment in primary care. Am J Prev Med. 2018;55(6):777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinman NJ, Shah A, Shah S, Phatak S, Viswanathan V. Improved medication adherence and frequency of blood glucose self-testing using an m-health platform versus usual care in a multisite randomized clinical trial among people with type 2 diabetes in India. Telemed e-Health. 2017;23(9):733–740. [DOI] [PubMed]
- 15.Raghavana A, Nanditha A, Satheesha K, et al. Improvement in glycemic control in patients with type 2 diabetes with treatment using an interactive mobile application—a pilot study from India. Prim Care Diabetes. 2022;16(6);844–8. [DOI] [PubMed]
- 16.Krishnakumar A, Verma R, Chawla R, et al. Evaluating glycemic control in patients of south Asian origin with type 2 diabetes using a digital therapeutic platform: analysis of real-world data. J Med Internet Res. 2021;23(3):e17908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muralidharan S, Ranjani H, Mohan Anjana R, et al. Engagement and weight loss: results from the mobile health and diabetes trial. Diabetes Technol Ther. 2019;21(9):507–13. [DOI] [PubMed]
- 18.Muralidharan S, Ranjani H, Anjana RM, et al. Change in cardiometabolic risk factors among Asian Indian adults recruited in a mHealth-based diabetes prevention trial. Digit Health. 2021;7:205520762110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranjani H, Nitika S, Hariharan R, et al. Systematic review and scientific rating of commercial apps available in India for diabetes prevention. J Diabetol. 2021;12(3):285.
- 20.Ballotari P, Ferrari F, Ballini L, Chiarenza A, Manicardi V, Rossi PG. Lifestyle-tailored interventions for South Asians with type 2 diabetes living in high-income countries: a systematic review. Acta Diabetol. 2017;54:784–94. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal P, Mukerji G, Desveaux L, et al. Mobile app for improved self-management of type 2 diabetes: multicenter pragmatic randomized controlled trial. JMIR mHealth uHealth. 2019;7(1):e10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Min J, Khuri J, et al. Effectiveness of mobile health interventions on diabetes and obesity treatment and management: systematic review of systematic reviews. JMIR mHealth uHealth. 2020;8(4):e15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming GA, Petrie JR, Bergenstal RM, Holl RW, Peters AL, Heinemann L. Diabetes digital app technology: benefits, challenges, and recommendations. A consensus report by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) diabetes technology working group. Diabetes Care. 2020;43(1):250–60. [DOI] [PubMed] [Google Scholar]
- 24.Tripathi D, Vikram NK, Chaturvedi S, Bhatia N. Development of “DiabetesSutra” a mobile application for lifestyle management of type 2 diabetes in India. J Diabetes Metab Disord. 2023;23:709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James R, Lewis JS. Item benchmarks for the System Usability Scale. J User Exp. 2018;13(3):158–167.
- 26.Katz ME, Mszar R, Grimshaw AA, et al. Digital health interventions for hypertension management in US populations experiencing health disparities. JAMA Netw Open. 2024;7(2):e2356070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telehealth Interventions to Improve Chronic Disease: https://www.cdc.gov/cardiovascular-resources/php/dataresearch/telehealth.html
- 28.Krakauer M, Botero JF, Lavalle-González FJ, Proietti A, Barbieri DE. A review of flash glucose monitoring in type 2 diabetes. Diabetol Metab Syndr. 2021;13(1):42. [DOI] [PMC free article] [PubMed]
- 29.Montero AR, Toro-Tobon D, Gann K, Nassar CM, Youssef GA, Magee MF. Implications of remote monitoring technology in optimizing traditional self-monitoring of blood glucose in adults with T2DM in primary care. BMC Endocr Disord. 2021;21(1):222. [DOI] [PMC free article] [PubMed]
- 30.Martens T, Bailey R, Ruedy KJ, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin a randomized clinical trial. JAMA. 2021;325(22):2262–72. [DOI] [PMC free article] [PubMed]
- 31.American Diabetes Association. Breakthrough studies on automated insulin delivery and CGM for type 2 diabetes unveiled at ADA scientific sessions. Arlington: American Diabetes Association; 2024. [Google Scholar]
- 32.Cuixart G, Corcoy R, González C. Can a mobile application improve glucose-related and patient-reported outcome measures (PROMs) in people with type 1 diabetes mellitus? A randomized controlled trial using the mySugr® app. Hormones. 2025;24(1):137–47. [DOI] [PMC free article] [PubMed]
- 33.Guldemond N. What is meant by ‘integrated personalised diabetes management’: a view into the future and what success should look like. Diabetes Obes Metab. 2024;26(S1):14–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.