Abstract
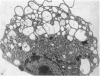
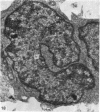
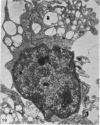
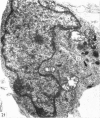
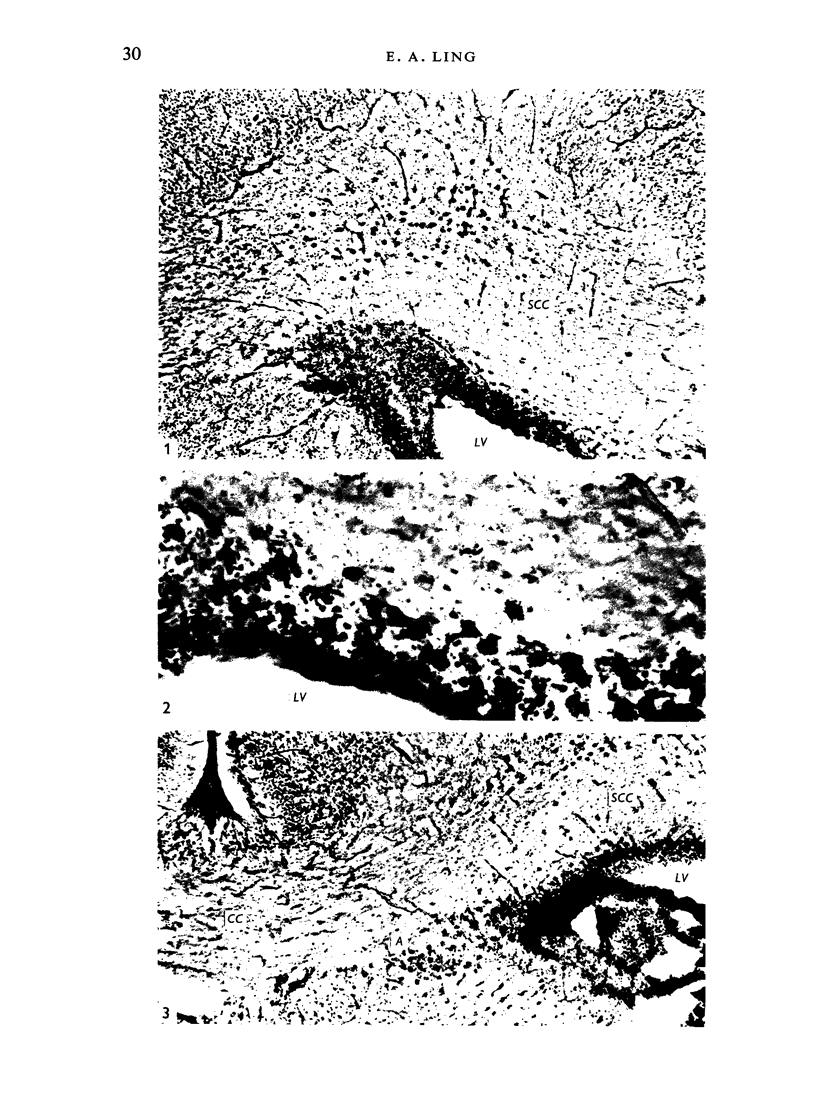
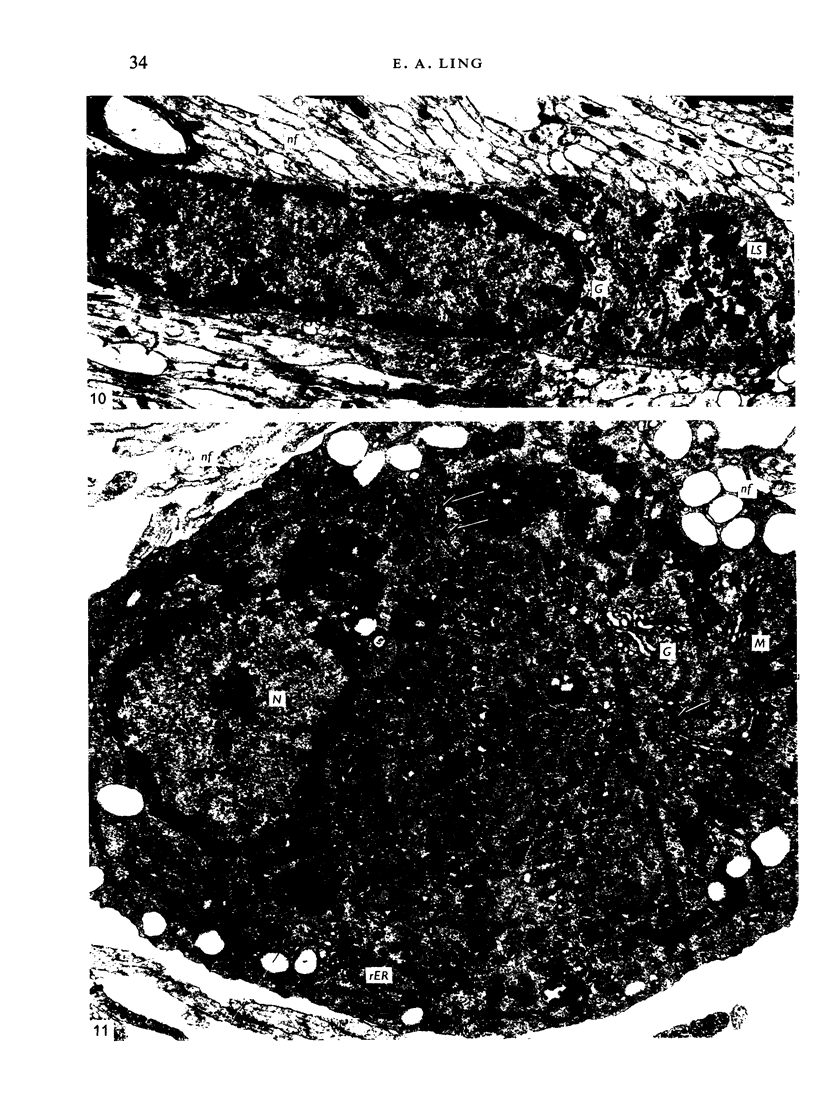
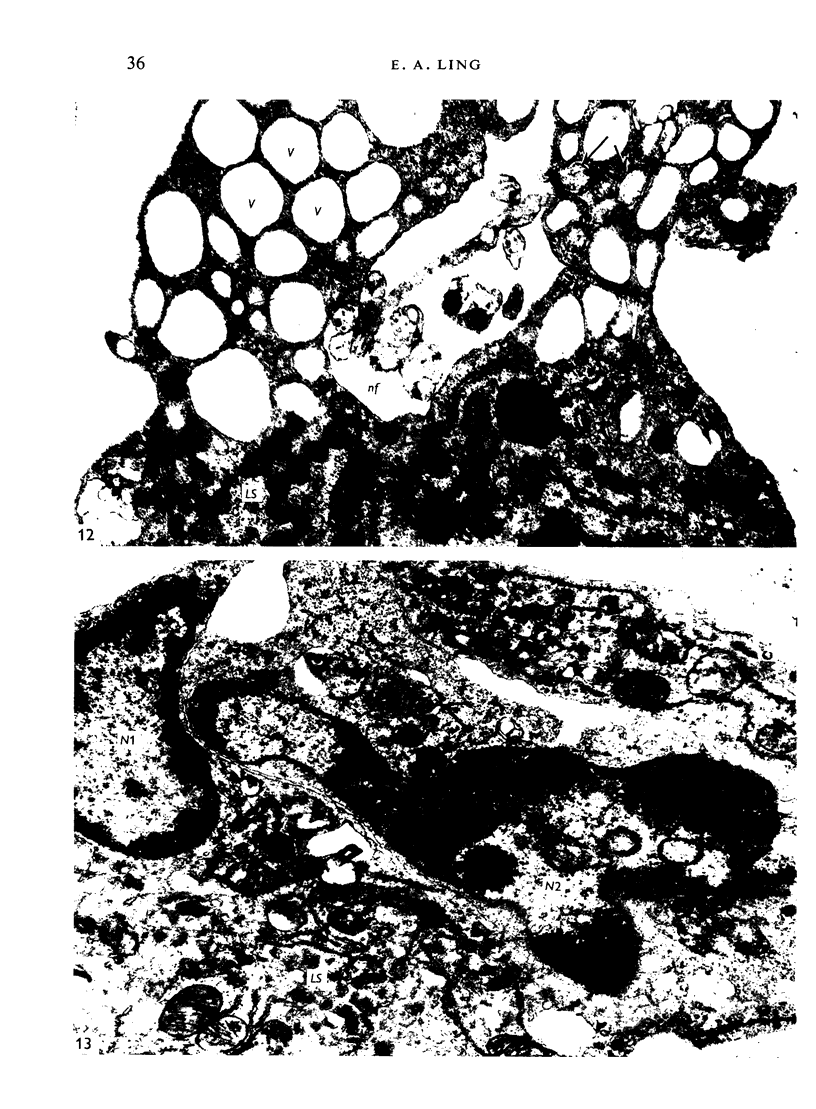
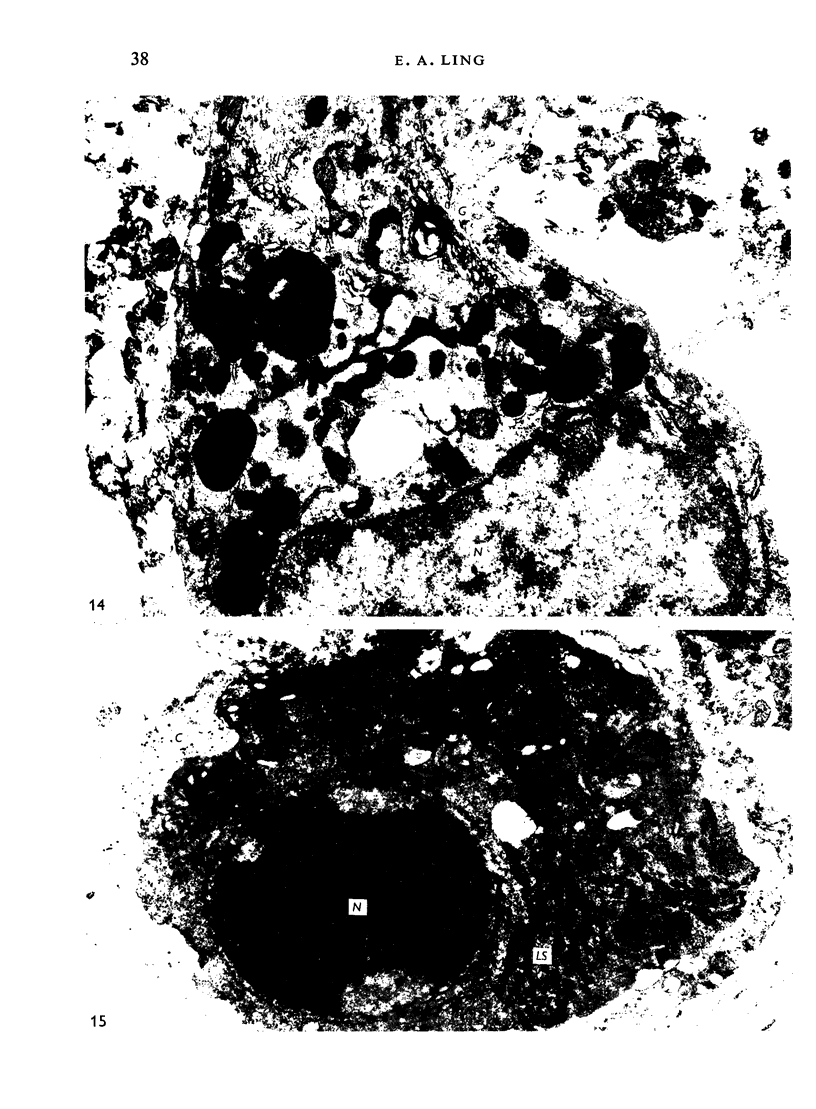
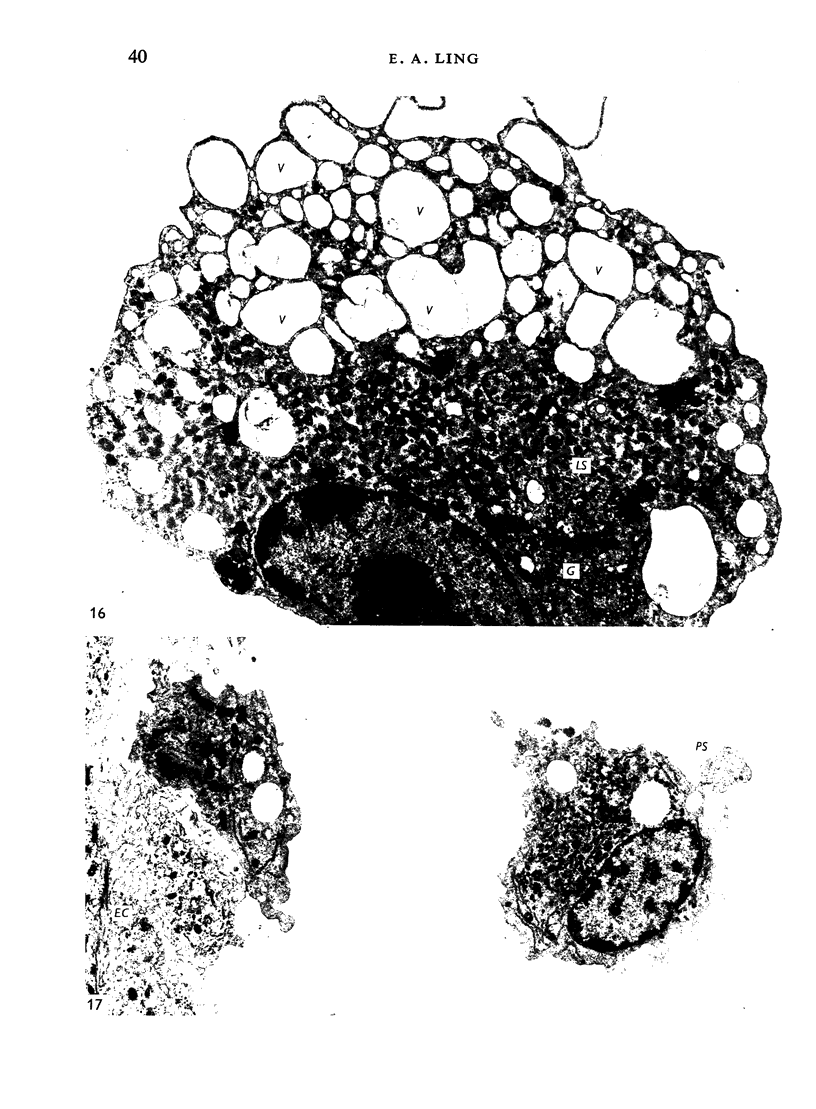
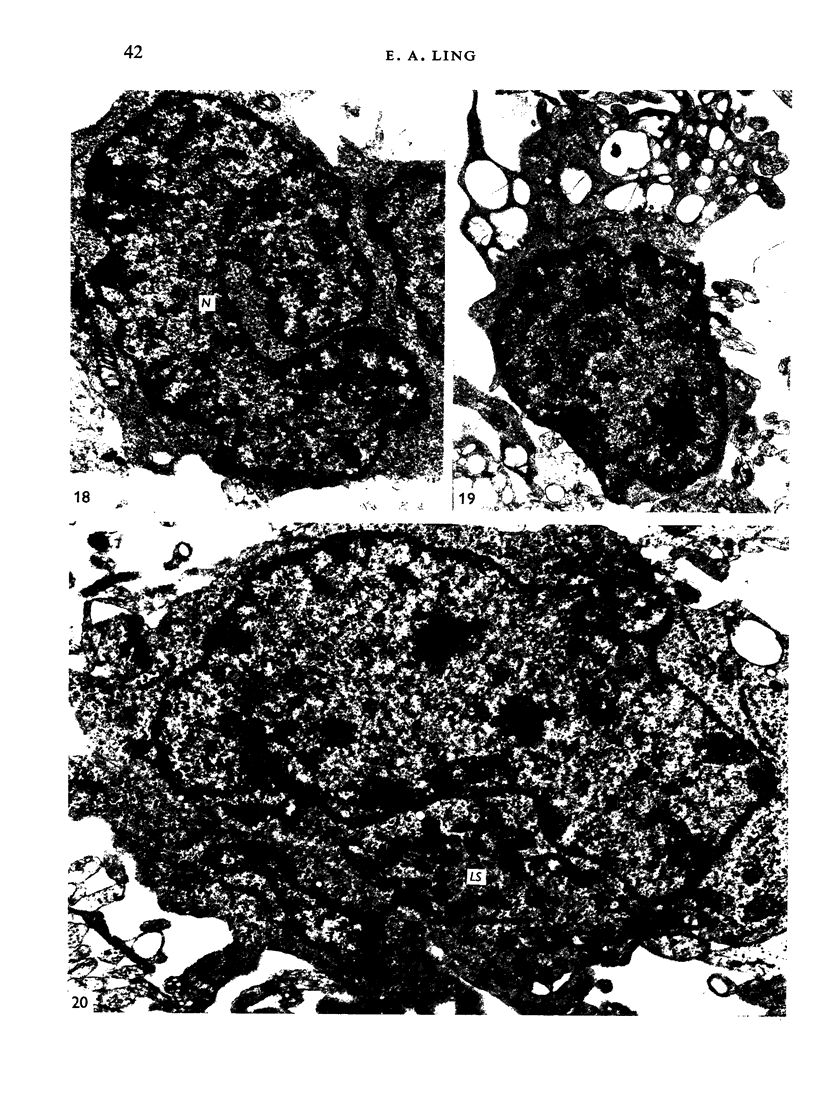
The distribution and form of amoeboid microglia in the brain of neonatal rats have been studied with the light and electron microscope. With the silver carbonate method of Rio-Hortega, two major 'colonies' of amoeboid microglia are identified: (1) in the supraventricular corpus callosum in which the nerve fibres are widely spaced, and (2) at the medial angle of the lateral ventricle inferior to the corpus callosum. Scattered amoeboid cells are also seen in the cavum septum pellucidum and in the lumen of the lateral ventricle. Associated with the subependyma forming the roof of the lateral ventricle there are also numerous amoeboid cells. Ultrastructural studies show that the subependyma includes cellular elements with features intermediate between those of immature subependymal cells and full-blown amoeboid microglia. It is suggested that the latter are derived from the subependymal cells and that, once they are formed, they leave the subependyma and migrate into the corpus callosum and elsewhere. With the metallic stain, the amoeboid microglia present a wide diversity of appearances, some of which bear a close resemblance to typical microglia. It is therefore suggested that amoeboid microglia change into typical microglia. The present study clearly demonstrates that amoeboid microglia are active phagocytes. Their cytoplasm is heavily loaded with secretory granules (lysosomes) and give a positive reaction with PAS and acid phosphatase.
Full text
PDF








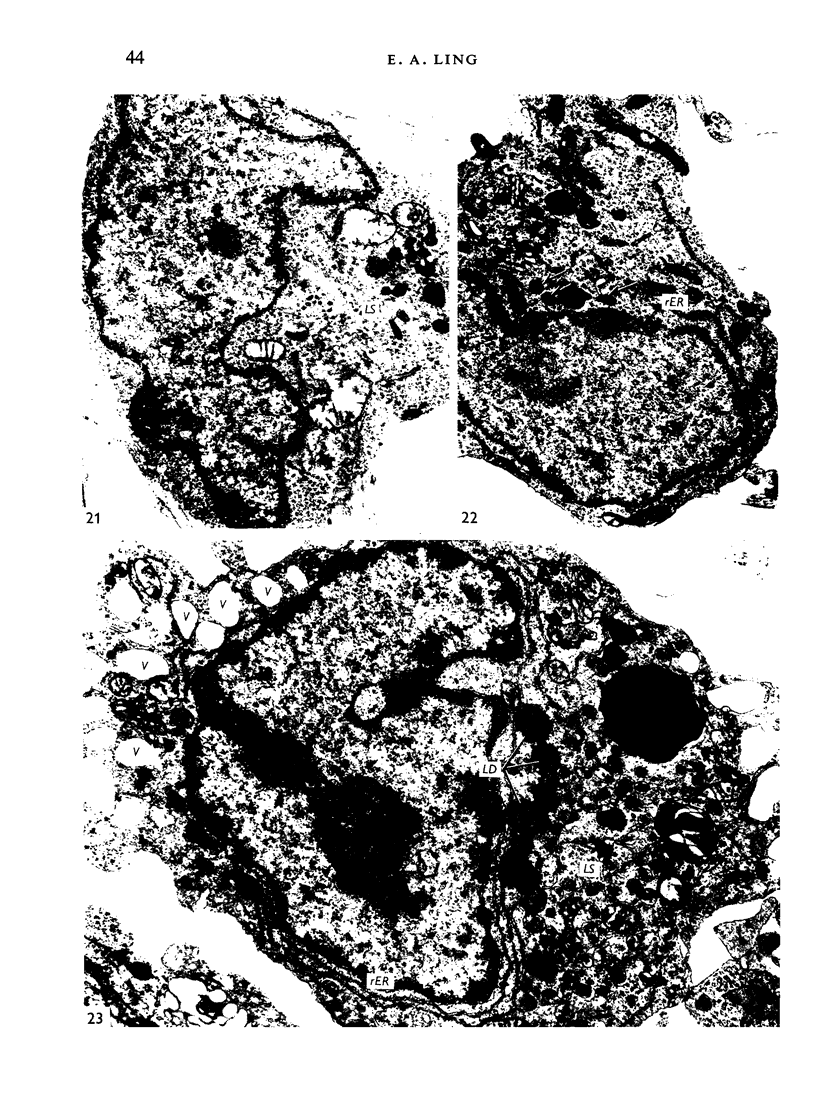

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN E. K., Jr, WALKER B. E. Incorporation of thymidine-H3 by cells in normal and injured mouse spinal cord. J Neuropathol Exp Neurol. 1962 Oct;21:597–609. doi: 10.1097/00005072-196210000-00007. [DOI] [PubMed] [Google Scholar]
- Barón M., Gallego A. The relation of the microglia with the pericytes in the cat cerebral cortex. Z Zellforsch Mikrosk Anat. 1972;128(1):42–57. doi: 10.1007/BF00306887. [DOI] [PubMed] [Google Scholar]
- Booz K. H., Felsing T. Uber ein transitorisches, perinatales subependymales Zellsystem der weissen Ratte. Z Anat Entwicklungsgesch. 1973;141(3):275–288. [PubMed] [Google Scholar]
- CEDERGREN B., HARARY I. IN VITRO STUDIES ON SINGLE BEATING RAT HEART CELLS. VI. ELECTRON MICROSCOPIC STUDIES OF SINGLE CELLS. J Ultrastruct Res. 1964 Dec;11:428–442. doi: 10.1016/s0022-5320(64)80074-5. [DOI] [PubMed] [Google Scholar]
- CEDERGREN B., HARARY I. IN VITRO STUDIES ON SINGLE BEATING RAT HEART CELLS. VII. ULTRASTRUCTURE OF THE BEATING CELL LAYER. J Ultrastruct Res. 1964 Dec;11:443–454. doi: 10.1016/s0022-5320(64)80075-7. [DOI] [PubMed] [Google Scholar]
- EDWARDS G. A., CHALLICE C. E. The fine structure of cardiac muscle cells of ntwborn and suckling mice. Exp Cell Res. 1958 Aug;15(1):247–250. doi: 10.1016/0014-4827(58)90085-5. [DOI] [PubMed] [Google Scholar]
- FIELD E. J. Observations on the development of microglia together with a note on the influence of cortisone. J Anat. 1955 Apr;89(2):201–208. [PMC free article] [PubMed] [Google Scholar]
- HIBBS R. G. Electron microscopy of developing cardiac muscle in chick embryos. Am J Anat. 1956 Jul;99(1):17–51. doi: 10.1002/aja.1000990103. [DOI] [PubMed] [Google Scholar]
- Huntington H. W., Terry R. D. The origin of the reactive cells in cerebral stab wounds. J Neuropathol Exp Neurol. 1966 Oct;25(4):646–653. doi: 10.1097/00005072-196610000-00010. [DOI] [PubMed] [Google Scholar]
- JAMIESON J. D., PALADE G. E. SPECIFIC GRANULES IN ATRIAL MUSCLE CELLS. J Cell Biol. 1964 Oct;23:151–172. doi: 10.1083/jcb.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONIGSMARK B. W., SIDMAN R. L. ORIGIN OF BRAIN MACROPHAGES IN THE MOUSE. J Neuropathol Exp Neurol. 1963 Oct;22:643–676. doi: 10.1097/00005072-196310000-00006. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Hattori H., Fujita S. Autoradiographic studies on histogenesis of brain macrophages in the mouse. J Neuropathol Exp Neurol. 1972 Jul;31(3):502–518. doi: 10.1097/00005072-197207000-00008. [DOI] [PubMed] [Google Scholar]
- LEAK L. V., BURKE J. F. THE ULTRASTRUCTURE OF HUMAN EMBRYONIC MYOCARDIUM. Anat Rec. 1964 Aug;149:623–649. doi: 10.1002/ar.1091490408. [DOI] [PubMed] [Google Scholar]
- Lewis P. D. The fate of the subependymal cell in the adult rat brain, with a note on the origin of microglia. Brain. 1968;91(4):721–736. doi: 10.1093/brain/91.4.721. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Paterson J. A., Privat A., Mori S., Leblond C. P. Investigation of glial cells in semithin sections. I. Identification of glial cells in the brain of young rats. J Comp Neurol. 1973 May 1;149(1):43–71. doi: 10.1002/cne.901490104. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Tan C. K. Amoeboid microglial cells in the corpus callosum of neonatal rats. Arch Histol Jpn. 1974 Mar;36(4):265–280. doi: 10.1679/aohc1950.36.265. [DOI] [PubMed] [Google Scholar]
- Lockett M. F. Hormonal actions of the heart and of lungs on the isolated kidney. J Physiol. 1967 Dec;193(3):661–669. doi: 10.1113/jphysiol.1967.sp008386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUIR A. R. An electron microscope study of the embryology of the intercalated disc in the heart of the rabbit. J Biophys Biochem Cytol. 1957 Mar 25;3(2):193–202. doi: 10.1083/jcb.3.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUIR A. R. FURTHER OBSERVATIONS ON THE CELLULAR STRUCTURE OF CARDIAC MUSCLE. J Anat. 1965 Jan;99:27–46. [PMC free article] [PubMed] [Google Scholar]
- Manasek F. J. Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. J Morphol. 1968 Jul;125(3):329–365. doi: 10.1002/jmor.1051250306. [DOI] [PubMed] [Google Scholar]
- Manasek F. J. Embryonic development of the heart. II. Formation of the epicardium. J Embryol Exp Morphol. 1969 Nov;22(3):333–348. [PubMed] [Google Scholar]
- Mark G. E., Strasser F. F. Pacemaker activity and mitosis in cultures of newborn rat heart ventricle cells. Exp Cell Res. 1966 Nov-Dec;44(2):217–233. doi: 10.1016/0014-4827(66)90427-7. [DOI] [PubMed] [Google Scholar]
- Matsuyama H., Komatsu N., Senda R. Electron microscopic studies on the developing telencephalic wall of the rat fetus. Okajimas Folia Anat Jpn. 1973 Dec;50(5):273–293. doi: 10.2535/ofaj1936.50.5_273. [DOI] [PubMed] [Google Scholar]
- Matthews M. A., Kruger L. Electron microscopy of non-neuronal cellular changes accompanying neural degeneration in thalamic nuclei of the rabbit. I. Reactive hematogenous and perivascular elements within the basal lamina. J Comp Neurol. 1973 Apr 1;148(3):285–312. doi: 10.1002/cne.901480302. [DOI] [PubMed] [Google Scholar]
- Matthews M. A., Kruger L. Electron microscopy of non-neuronal cellular changes accompanying neural degeneration in thalamic nuclei of the rabbit. II. Reactive elements within the neuropil. J Comp Neurol. 1973 Apr 1;148(3):313–346. doi: 10.1002/cne.901480303. [DOI] [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Identification of microglia in light and electron microscopy. J Comp Neurol. 1969 Jan;135(1):57–80. doi: 10.1002/cne.901350104. [DOI] [PubMed] [Google Scholar]
- Obinata T., Yamamoto M., Maruyama K. The identification of randomly formed thin filaments in differentiating muscle cells of the chick embryo. Dev Biol. 1966 Oct;14(2):192–213. doi: 10.1016/0012-1606(66)90013-3. [DOI] [PubMed] [Google Scholar]
- Paterson J. A., Privat A., Ling E. A., Leblond C. P. Investigation of glial cells in semithin sections. 3. Transformation of subependymal cells into glial cells, as shown by radioautography after 3 H-thymidine injection into the lateral ventricle of the brain of young rats. J Comp Neurol. 1973 May 1;149(1):83–102. doi: 10.1002/cne.901490106. [DOI] [PubMed] [Google Scholar]
- Penfield W. Microglia and the Process of Phagocytosis in Gliomas. Am J Pathol. 1925 Jan;1(1):77–90.15. [PMC free article] [PubMed] [Google Scholar]
- Privat A., Leblond C. P. The subependymal layer and neighboring region in the brain of the young rat. J Comp Neurol. 1972 Nov;146(3):277–302. doi: 10.1002/cne.901460302. [DOI] [PubMed] [Google Scholar]
- ROSS R., BENDITT E. P. WOUND HEALING AND COLLAGEN FORMATION. IV. DISTORTION OF RIBOSOMAL PATTERNS OF FIBROBLASTS IN SCURVY. J Cell Biol. 1964 Aug;22:365–389. doi: 10.1083/jcb.22.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL G. V. The compound granular corpuscle or gitter cell: a review, together with notes on the origin of this phagocyte. Tex Rep Biol Med. 1962;20:338–351. [PubMed] [Google Scholar]
- Rash J. E., Biesele J. J., Gey G. O. Three classes of filaments in cardiac differentiation. J Ultrastruct Res. 1970 Dec;33(5):408–435. doi: 10.1016/s0022-5320(70)90171-1. [DOI] [PubMed] [Google Scholar]
- Rash J. E., Shay J. W., Biesele J. J. Cilia in cardiac differentiation. J Ultrastruct Res. 1969 Dec;29(5):470–484. doi: 10.1016/s0022-5320(69)90067-7. [DOI] [PubMed] [Google Scholar]
- Rash J. E., Shay J. W., Biesele J. J. Preliminary biochemical investigations of the intermediate filaments. J Ultrastruct Res. 1970 Dec;33(5):399–407. doi: 10.1016/s0022-5320(70)90170-x. [DOI] [PubMed] [Google Scholar]
- Roessmann U., Friede R. L. Entry of labeled monocytic cells into the central nervous system. Acta Neuropathol. 1968 Jun 7;10(4):359–362. doi: 10.1007/BF00690711. [DOI] [PubMed] [Google Scholar]
- SLAYTER H. S., WARNER J. R., RICH A., HALL C. E. THE VISUALIZATION OF POLYRIBOSOMAL STRUCTURE. J Mol Biol. 1963 Dec;7:652–657. doi: 10.1016/s0022-2836(63)80112-6. [DOI] [PubMed] [Google Scholar]
- Schiebler T. H., Wolff H. H. Elektronenmikroskopische Untersuchungen am Herzmuskel der Ratte während der Entwicklung. Z Zellforsch Mikrosk Anat. 1966;69:22–40. [PubMed] [Google Scholar]
- Schmitt D. Uber glykoproteidhaltige amöboide Zellen im embryonalen Hühnergehirn. Eine licht- und elektronenmikroskopische Untersuchung zur Frage der Volumenreserve bei Wachstumsprozessen im Gehirn. Z Anat Entwicklungsgesch. 1973 Dec 31;142(3):341–358. [PubMed] [Google Scholar]
- Stensaas L. J., Reichert W. H. Round and amoeboid microglial cells in the neonatal rabbit brain. Z Zellforsch Mikrosk Anat. 1971;119(2):147–163. doi: 10.1007/BF00324517. [DOI] [PubMed] [Google Scholar]
- Vaughn J. E., Hinds P. L., Skoff R. P. Electron microscopic studies of Wallerian degeneration in rat optic nerves. I. The multipotential glia. J Comp Neurol. 1970 Oct;140(2):175–206. doi: 10.1002/cne.901400204. [DOI] [PubMed] [Google Scholar]
- Vaughn J. E., Peters A. A third neuroglial cell type. An electron microscopic study. J Comp Neurol. 1968 Jun;133(2):269–288. doi: 10.1002/cne.901330207. [DOI] [PubMed] [Google Scholar]
- Virágh S., Challice C. E. Origin and differentiation of cardiac muscle cells in the mouse. J Ultrastruct Res. 1973 Jan;42(1):1–24. doi: 10.1016/s0022-5320(73)80002-4. [DOI] [PubMed] [Google Scholar]
- WAINRACH S., SOTELO J. R. Electron microscope study of the developing chick embryo heart. Z Zellforsch Mikrosk Anat. 1961;55:622–634. doi: 10.1007/BF00384502. [DOI] [PubMed] [Google Scholar]