Significance
DNA glycosylase OGG1 is increasingly recognized for the roles beyond genome maintenance. Here, we identify a function for OGG1 in regulating immune tolerance by promoting the differentiation of inducible regulatory T cells (iTregs). OGG1 activates Foxp3 transcription through both recruitment of Smad3 and epigenetic remodeling, which reveals a previously unappreciated mechanism linking oxidative DNA damage sensing to immune regulation, and reflects an evolutionary adaptation of aerobic organisms. A naturally occurring OGG1 variant (S326C) enhances this function and is associated with reduced incidence of inflammatory bowel disease (IBD) in humans. Pharmacologic inhibition of OGG1’s enzymatic activity alleviates IBD in mice. These findings uncover an epigenetic function of OGG1 and highlight its potential as a therapeutic target in chronic inflammatory diseases.
Keywords: OGG1, iTreg differentiation, Foxp3, Smad3, IBD
Abstract
8-oxo-7,8-dihydroguanine (8-oxoG), the most frequent form of oxidative-DNA-base lesion caused by ROS, is recognized and repaired by 8-oxoguanine DNA glycosylase 1 (OGG1) through base excision repair (BER) pathway. Beyond its role in DNA repair, OGG1 has been shown to promote transcriptional activation of proinflammatory mediators and contribute to both acute and chronic lung inflammation. However, pioneering studies have shown an anti-inflammation role for OGG1 in inflammatory bowel disease (IBD), but its underlying molecular mechanism remains unclear. In the present study, we unveiled that OGG1 plays an important role in the differentiation of inducible regulatory T cells (iTregs). Binding of OGG1 to 8-oxoG facilitated the recruitment of Smad3 to the Foxp3 promoter, leading to the transcriptional activation. Moreover, OGG1 binding promoted demethylation of CpG sites in the conserved noncoding sequence 2 (CNS2) region of Foxp3 by decreasing Dnmt1 occupancy and enhancing recruitment of Tet1/2. Notably, the S326C variant—a naturally occurring polymorphism in humans—was more effective than the wild-type protein in promoting iTreg differentiation and showed a negative correlation with IBD incidence. Furthermore, treatment with O8, a selective OGG1 inhibitor that blocks base excision activity without affecting substrate binding, significantly alleviated IBD in a mouse model, suggesting a promising therapeutic strategy. Together, these findings extend the understanding of OGG1’s epigenetic role in transcriptional regulation and highlight its protective function in inflammatory diseases, potentially shaped by aerobic evolution.
Organisms with aerobic metabolism inevitably encounter reactive oxygen species (ROS), which arise either from the endogenous metabolic processes or in response to the challenges from environmental stressors. Among biomacromolecules, DNA is particularly vulnerable to ROS due to the low redox potential of its nucleobases. Guanine possessing the lowest redox potential is the most susceptible to oxidation (1, 2). The resultant base lesion 8-oxo-7, 8-dihydroguanine (8-oxoG) is commonly regarded as a biomarker of oxidative stress and is typically repaired through the 8-oxoguanine DNA glycosylase 1 (OGG1)-initiated base excision repair (BER) pathway (3, 4). However, recent studies have discovered and characterized a noncanonical function of OGG1 in the transcriptional regulation of inflammatory cytokines and chemokines, independent of its repair activity (5–7).
Numerous studies have demonstrated that upon stimulation of inflammatory agents, OGG1 binds to its substrates including those in the promoter regions of proinflammatory cytokine and chemokine genes without immediately excising the lesion (8–10). In turn, OGG1 facilitates the recruitment of site-specific transcription factors such as NF-κB and SP1 leading to rapid transcriptional activation of proinflammatory mediators (11). While these findings highlight a proinflammatory role of OGG1, a recent study demonstrated that Ogg1−/− mice were more susceptible to dextran sulfate sodium (DSS)-induced acute ulcerative colitis, a widely used experimental model of human inflammatory bowel disease (IBD) (12). This pioneering study proposed a protective role of OGG1 in a different inflammatory context, which seemingly conflicts with its previously described proinflammatory function. Importantly, the molecular mechanism underlying OGG1’s protective role in IBD remains unclear.
The balance between iTregs and helper 17 cells (Th17) is essential for immune homeostasis. Deficiency or dysfunction in Treg cells—a critical immunosuppressive subset—is closely related to IBD pathogenesis (13, 14). Treg cells include thymus-derived natural Tregs (nTregs) and peripherally induced Tregs (iTregs) (15). iTregs are differentiated from activated T (Th0) cells in response to transforming growth factor β1 (TGFβ1) signaling. Foxp3 is a core transcription factor that governs the differentiation and maturation of iTregs (16). During this process, TGFβ1 activates the transcription factor Smad2/3 through TGFβ receptor (TGFR), which then forms a heterotrimeric complex with Smad4. This complex binds to the promoter and conserved noncoding sequence 1 (CNS1) of the Foxp3 gene, promoting transcription activation necessary for iTreg establishment and maintenance (17–19). Additionally, Foxp3 transcription is regulated by epigenetic modifications, such as DNA methylation. The absence of DNA methyltransferase 1 (Dnmt1) facilitates Foxp3 expression (20), while, active DNA demethylation of Foxp3 promoter and CNS2 by Tet1/2 is required for the stabilization and maintenance of Foxp3 expression (21, 22).
Interestingly, unlike other CD4+ T cell subsets, iTregs exhibit elevated levels of intracellular ROS (23), likely due to their reliance on oxidative phosphorylation metabolism (24). Furthermore, increased ROS levels are required for Foxp3 expression and iTreg differentiation. For instance, deficiency of peroxiredoxin 2 (Prx2), an intracellular antioxidant enhances Foxp3 expression, and increases the number of Tregs in Prx2-deficient mice (25). Similarly, exogenous H2O2 has been shown to promote iTreg differentiation in vitro (24). Given OGG1’s role in transcriptional regulation under oxidative stress, we hypothesize that during iTreg differentiation, increase of intracellular ROS results in the accumulation of 8-oxoG within the regulatory regions of Foxp3, followed by OGG1 binding. The engagement of transiently inactive OGG1 with its substrate may serve as a hub for the recruitment/exclusion of chromatin modifiers and transcription factors, thereby enhancing transcriptional activation of Foxp3, facilitating iTreg differentiation and ameliorating bowel inflammation. This study not only aims to uncover a function of OGG1 in adaptive immunity-related inflammatory diseases, and to provide a new insight into the evolutionary significance of guanine oxidation, but to prove a potential utility of OGG1 inhibitor in prevention and treatment of IBD.
1. Results
1.1. OGG1 Is Essential for iTreg Differentiation in Mice with IBD.
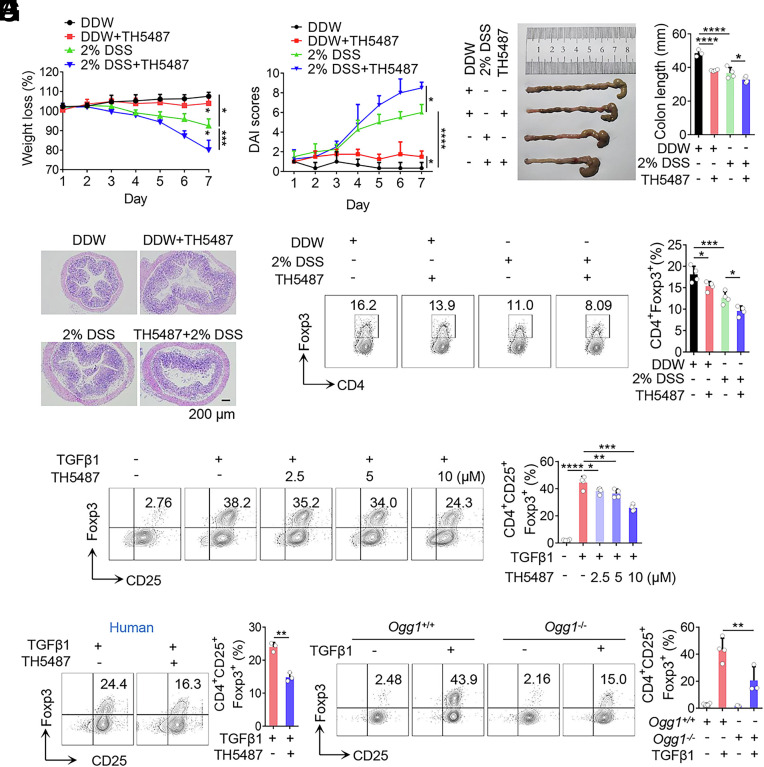
To determine whether the protective role of OGG1 in IBD as observed by previous studies is associated with iTreg differentiation, a classical mouse IBD model was established. Mice were intraperitoneally administered with or without TH5487, a specific inhibitor that blocks the binding of OGG1 to its DNA substrate (10) or vehicle control (DMSO). Compared to the vehicle control, TH5487-treated mice exhibited significantly aggravated colitis, characterized by body weight loss, increased disease activity index (DAI) scores, shortened colon length, inflammatory cell infiltration, edema and injury to mucosa, as well as enhanced crypt damage (Fig. 1 A–D). Similarly, Ogg1 knockout mice displayed comparable histopathological features (SI Appendix, Fig. S1 A–D), consistent with the previous findings (12). Notably, the frequency of colonic Tregs in the TH5487 treated mice was significantly lower than that in the DMSO-treated group with or without DSS administration (Fig. 1E). Ogg1 knockout mice also had fewer colonic Tregs than wild-type controls even under basal conditions, with this difference further significantly amplified upon DSS challenge (SI Appendix, Fig. S1E). Collectively, these results suggest that OGG1 promotes iTreg induction, which may contribute to its protective role in IBD pathogenesis.
Fig. 1.
Inhibition or depletion of OGG1 decreases iTreg differentiation and exacerbates IBD in the mouse model. (A–E) Inhibition of OGG1 reduces Treg frequency in colon and aggravates mouse IBD. Mice were administered DSS (2%, w/v) in double-distilled water (DDW). Concurrently, the OGG1 inhibitor TH5487 was administered (30 mg/kg/d) intraperitoneally every other day. The solvent DMSO was used as the negative control. Colitis was systematically evaluated daily. Body weight (A) and disease activity index (DAI) scores (B)—based on body weight loss, stool consistency, and the presence of blood in the stool—were recorded daily. Seven days later, entire colons were harvested from differently treated mice for length assessment (C) and hematoxylin and eosin (H and E) staining to assess histopathological changes (D). (Scale bar, 200 μm.) iTreg frequency in colonic lamina propria were analyzed by flow cytometry (FCM) (E). (F) Inhibition of OGG1 reduces iTreg differentiation. Naïve CD4+ T cells were cultured under Th0 or iTreg polarization conditions with varying concentrations of TH5487 for 3 d and analyzed by FCM for CD4+CD25+Foxp3+ iTregs (Left) followed by quantification (Right). (G) OGG1 inhibition suppresses human iTreg differentiation. Naive CD4+ T cells isolated from human peripheral blood were cultured under iTreg polarization conditions, prior to analysis for CD4+CD25+Foxp3+ iTregs by FAM (Left) and quantification (Right). (H) OGG1 depletion significantly suppresses iTreg differentiation. Naïve CD4+ T cells derived from Ogg1+/+ or Ogg1−/− mice were cultured as described in the legend to panel (F) and stained for CD4+CD25+Foxp3+ iTreg were analyzed by FAM (Left) and quantified (Right). (A–E) One representative experiment out of four biological replicas is shown. Data represent mean ± SD (n = 4), with significance determined by two-way ANOVA test (A and B) or one-way ANOVA test (C and E). (F–H) Quantification shows mean ± SD from four (F and H) or three (G) independent experiments, with significance determined by one-way ANOVA test (F and H) or t test (G). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To further explore the implication of OGG1 in iTreg differentiation, an in vitro iTreg induction model was employed. Treatment with TH5487 or SU0268—another specific inhibitor that prevents OGG1 binding to genomic 8-oxoG—resulted in a dose-dependent reduction in iTreg differentiation (Fig. 1F and SI Appendix, Fig. S2A). Importantly, a similar decrease in iTreg generation was observed during human iTreg differentiation (Fig. 1G). Consistently, iTreg differentiation was impaired in Ogg1 knockout cells (Fig. 1H), whereas, OGG1 overexpression increased iTreg differentiation (SI Appendix, Fig. S2B). Together, these data suggest that the beneficial effect of OGG1 in alleviating IBD is likely resulted from its role in promoting iTreg differentiation.
1.2. OGG1 Contributes to Transcriptional Activation of Genes for iTreg Differentiation.
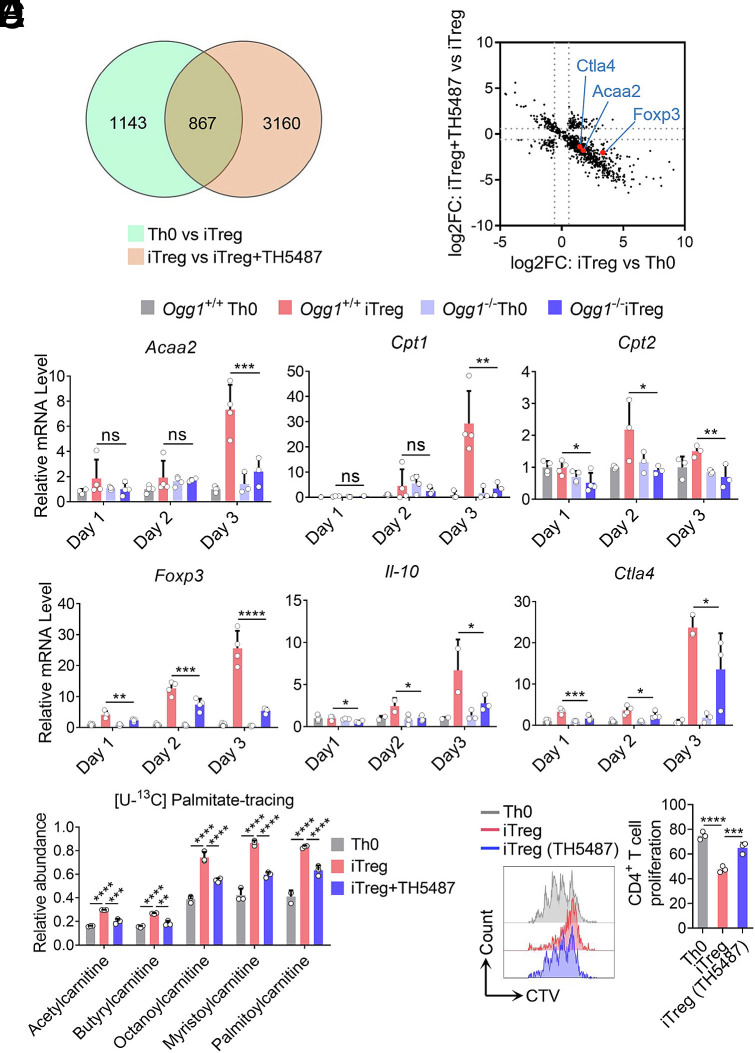
iTreg differentiation depends on the expression of the key transcription factor Foxp3, and the establishment of fatty acid oxidation (FAO) metabolism (26–30). To elucidate the molecular mechanism by which OGG1 promotes iTreg differentiation, RNA-sequencing analysis was performed. The data showed that 867 genes were upregulated in iTreg compared to Th0 cells, and their expression decreased upon TH5487 treatment (Fig. 2A). Among these genes, Foxp3, Ctla4 (an immunosuppressive function-related gene), and Acaa2 (a key enzyme controlling FAO) were highly representative (Fig. 2B). KEGG enrichment analysis further revealed that fatty acid metabolism was the most significant affected pathway (SI Appendix, Fig. S3).
Fig. 2.
OGG1 is implicated in transcriptional activation of genes for lineage differentiation, fatty acid oxidation, and suppressive function of iTregs. (A and B) RNA sequencing analysis identifies the gene expression profile regulated by OGG1. Naïve CD4+ T cells were cultured under Th0 or iTreg polarization conditions with or without TH5487 (10 μM) for three days. Total RNA was extracted followed by RNA-Sequencing. A Venn diagram shows the number of iTreg differentiation-related genes affected by OGG1 (A). Scatter plots show the distribution of differentially expressed genes in Th0 cells, iTregs, and TH5487-treated iTregs [adjusted P value < 0.05 (B)]. (C) OGG1 depletion inhibits the transcription of Foxp3 and other genes related to iTreg metabolism or function. Naïve CD4+ T cells derived from Ogg1+/+ or Ogg1−/− mice were cultured as described in the legend to (A and B). Total RNA was extracted, converted to cDNA, and analyzed by qPCR for gene expression. (D) Inhibition of OGG1 reduces FAO. Naïve CD4+ T cells were treated with TH5487 (10 μM) and cultured as in legend to (A and B) in the presence of [U-13C] palmitate (100 μM) for 48 h. Cells were collected and analyzed for FAO using ultra-high-performance liquid chromatography–high-resolution mass spectrometry (UHPLC-HRMS) analysis. n = 4, two-way ANOVA test, mean ± SD. (E) Inhibition of OGG1 impairs the suppressive function of iTregs. Naïve CD4+ T cells from Foxp3YFP mice were treated with TH5487 (10 μM) and cultured under Th0 or iTreg polarization conditions for three days. Pure iTregs were sorted by FCM and cocultured with CellTrace Violet (CTV)-stained naïve CD4+ T cells in the presence of Dynabeads® Mouse T-Activator CD3/CD28 for 72 h. CD4+ T cell proliferation was analyzed by FCM (Left) and quantified (Right). (C and E) Data represent mean ± SD from four (C) or three (E) independent experiments. Statistical significance was determined by two-way ANOVA (C) or one-way ANOVA test (E). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns, not significant.
Accordingly, the expression levels of the Foxp3, FAO-related genes, and genes associated with iTreg immunosuppressive function were validated by qPCR. The results showed that Foxp3 transcription was strongly dependent on OGG1 (Fig. 2C). In contrast, when OGG1 was overexpressed, the transcription of Foxp3 was significantly increased (SI Appendix, Fig. S4A). Importantly, the effect of OGG1 in promoting Foxp3 transcription was also observed during human iTreg differentiation (SI Appendix, Fig. S4B). Additionally, the expression of genes in the FAO pathway and those linked to iTreg immunosuppressive function such as Cpt1/2, Acaa2, Ctla4, Il10 was significantly reduced following OGG1 depletion. (Fig. 2C and SI Appendix, Fig. S4C). To further evaluate the role of OGG1 on FAO, we performed a [U-13C] palmitate-tracing experiment, which showed that OGG1 inhibition significantly reduced FAO efficiency (Fig. 2D). Moreover, to assess suppressive function of iTregs, we used Foxp3YFP reporter mice to isolate pure iTregs. The results showed that OGG1 inhibition decreased the suppressive function of iTregs in a T cell proliferation assay (Fig. 2E and SI Appendix, Fig. S4D). Taken together, these data suggest that OGG1 enhances transcriptional activation of key genes involved in iTreg differentiation and function.
1.3. Nonproductive Binding Rather Than Catalytic Activity of OGG1 Is Required for Its Function in Upregulation of Foxp3 Transcription.
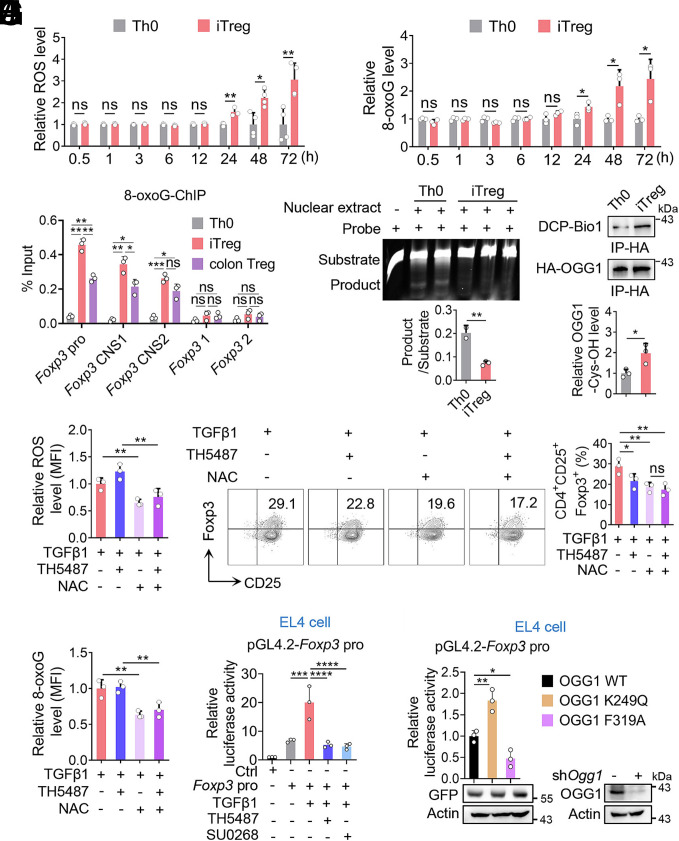
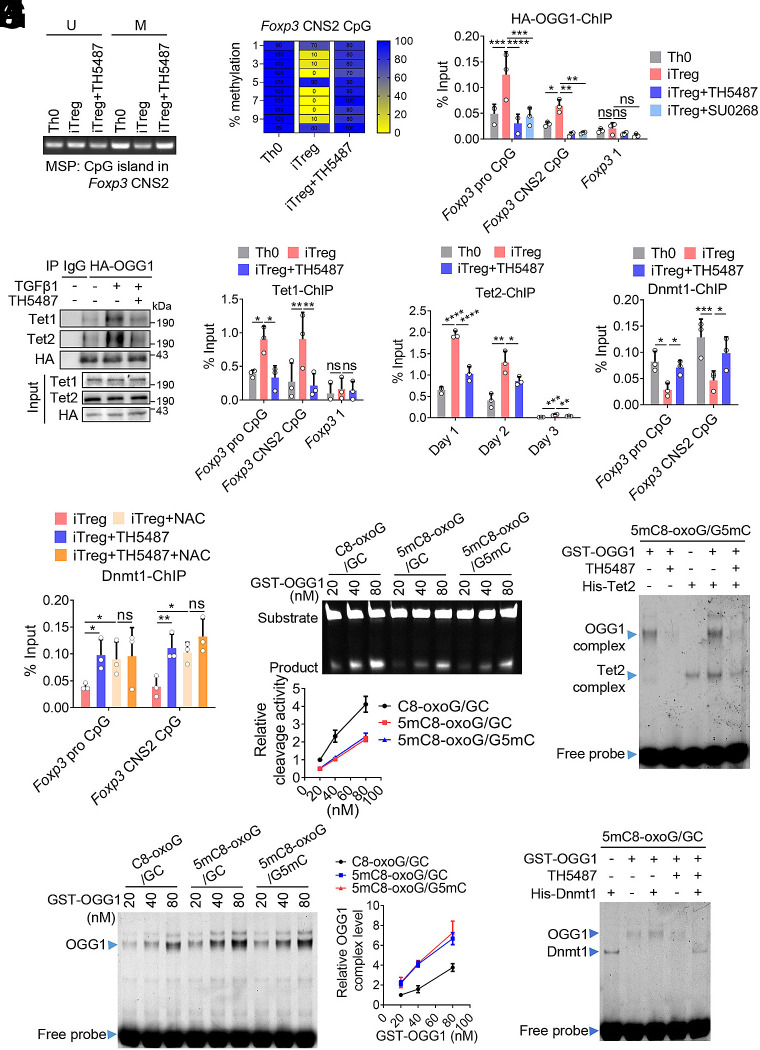
Given that binding of OGG1 to 8-oxoG lesion in the regulatory regions is required for its role in transcription modulation, we first examined levels of cellular ROS and genomic 8-oxoG accumulation during iTreg differentiation. During iTreg differentiation, both cellular ROS and genomic 8-oxoG levels were increased, which were significantly higher than those in Th0 cells with elevated levels detectable at 24 h and persisting through day 3 (Fig. 3 A and B). Notably, 8-oxoG enrichment was 6- to 10-fold higher in iTregs and colonic Tregs than in Th0 cells at key regulatory regions of Foxp3, including the promoter, CNS1, and CNS2 regions. In contrast, no significant accumulation of 8-oxoG was observed within the Foxp3 coding regions (Fig. 3C). These findings suggest that 8-oxoG accumulation may play an important role in the transcriptional regulation of Foxp3. Additionally, we assessed the enzymatic activity and the oxidation status of OGG1, which are essential for the switch of OGG1 from initiating BER to recruiting transcription factors (5, 31). Nuclear extracts were prepared for a cleavage assay, which showed a reduction in OGG1 catalytic activity in iTregs (Fig. 3D). To assess the oxidation state of OGG1, we generated a transgenic mouse expressing the 3 × HA-tagged OGG1 as the commercially available OGG1 antibody (Ab) does not effectively recognize nondenatured murine OGG1, and is unsuitable for immunoprecipitation (IP) (SI Appendix, Fig. S5A). EMSA and cleavage assays using purified recombinant proteins confirmed that the insertion of 3 × HA does not interfere with OGG1’s ability to bind 8-oxoG or its base excision (SI Appendix, Fig. S5 B and C). Using anti-HA antibody (Ab) for IP, we found that OGG1 in iTregs exhibited significant cysteine oxidation (Fig. 3E). The combined data suggest that the function of OGG1 in promoting iTreg differentiation may not depend on its base excision activity.
Fig. 3.
Nonproductive binding rather than catalytic activity of OGG1 is required for its promoting effect on Foxp3 transcription. (A and B) ROS and 8-oxoG levels increase during iTreg differentiation. Naïve CD4+ T cells were cultured under Th0 or iTreg polarization conditions for three days. Cells were harvested at the indicated time points, incubated with the cell-permeable ROS probe DCFH-DA and analyzed by FCM to quantify ROS level (A). Subsequently, cells were stained with an anti-8-oxoG antibody and analyzed by FCM (B). (C) 8-oxoG is enriched at the regulatory regions of Foxp3 in both iTregs and colonic Tregs. Th0 cells and iTregs were obtained as in legend to (A) and colonic Tregs were isolated from the colon of Foxp3YFP mice. The enrichment of 8-oxoG at the Foxp3 promoter, CNS1 and CNS2 regions was assessed by ChIP using anti-8-oxoG antibody. Primers targeting the Foxp3 coding region were used as a negative control. (D) The cleavage activity of OGG1 decreases in iTregs compared to Th0 cells. Nuclear extracts from cells cultured for 48 h as described to (A) were incubated with 100 fmol 8-oxoG-containing, Cy5-labeled DNA oligos derived from Foxp3 promoter and subjected to an oligonucleotide incision assay. (E) OGG1 is oxidized in iTregs. Naïve CD4+ T cells from 3 × HA-OGG1 mice were cultured as in the legend to (A) for 48 h, then lysed in the presence of DCP-Bio1. Levels of DCP-Bio1-tagged OGG1 were determined by immunoprecipitation followed by western blot analysis. (F and G) NAC treatment decreases ROS and 8-oxoG levels. Naïve CD4+ T cells were cultured as in legend to (A) with TH5487 (10 μM) and/or NAC (1 mM) and assessed by FCM for ROS (F) and 8-oxoG (G) levels. (H) Removal of ROS decreases iTreg differentiation. Cells cultured as in legend to (F) were analyzed for iTreg differentiation by FCM (Left) and quantified (Right). (I) Inhibition of OGG1-8-oxoG interaction suppresses Foxp3 promoter activity. EL4 cells electroporated with pGL-4.2 or pGL-4.2-Foxp3 pro plasmids were treated with TH5487 (10 μM) or SU0268 (10 μM) in the presence of TGFβ1 for 12 h. Foxp3 transcriptional activity was assessed using a dual luciferase reporter assay. (J) Enhanced OGG1 binding to 8-oxoG promotes Foxp3 transcriptional activity. EL4 cells were electrotransfected with pLKO.1-shOgg1 along with pEGFP-N1-OGG1, pEGFP-N1-OGG1K249Q, or pEGFP-N1-OGG1F319A plasmids to generate OGG1 mutant cells, followed by transfected with pGL-4.2-Foxp3 pro plasmids. Foxp3 transcriptional activity was evaluated by dual luciferase assay. (A–I) Quantification shows mean ± SD from three (A–G and I) or four (H) independent experiments, with significance determined by two-way ANOVA test (A and B) or one-way ANOVA test (C–I). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns, not significant. (C and D) One representative experiment out of three is shown.
To test this, we treated cells with OGG1 inhibitor TH5487, which reduced iTreg differentiation. Similarly, scavenging ROS by N-Acetyl-L-cysteine (NAC) significantly decreased OGG1 binding to DNA, and iTreg differentiation. However, the combination of NAC and TH5487 did not result in further suppression of iTreg differentiation (Fig. 3 F–H), suggesting that OGG1 binding to 8-oxoG is ROS-dependent and contributes to iTreg differentiation. To further investigate the mechanism, we performed dual luciferase reporter assay in both EL4 and HEK293T cells. TGF-β1 significantly enhanced Foxp3 promoter activity, which was significantly suppressed by treatment with TH5487 or SU0268 (Fig. 3I and SI Appendix, Fig. S6A). To determine whether OGG1’s enzymatic activity is necessary for Foxp3 transcriptional activation, we used two OGG1 mutants: OGG1K249Q, which lacks enzymatic activity but remains strong binding with 8-oxoG (32), and OGG1F319A, which lacks substrate binding capacity (33). These constructs along with WT OGG1 were overexpressed in OGG1-knockdown EL4 and HEK293T cells. Luciferase dual reporter assay showed that the K249Q mutant enhanced Foxp3 promoter activity, significantly more than WT OGG1, whereas the F319A mutant lowered it (Fig. 3J and SI Appendix, Fig. S6B). Together, these data indicate that OGG1 promotes Foxp3 transcription through noncatalytic 8-oxoG-dependent DNA binding.
1.4. Binding of OGG1 to Foxp3 Promoter Augments the Smad3 Recruitment During iTreg Differentiation.
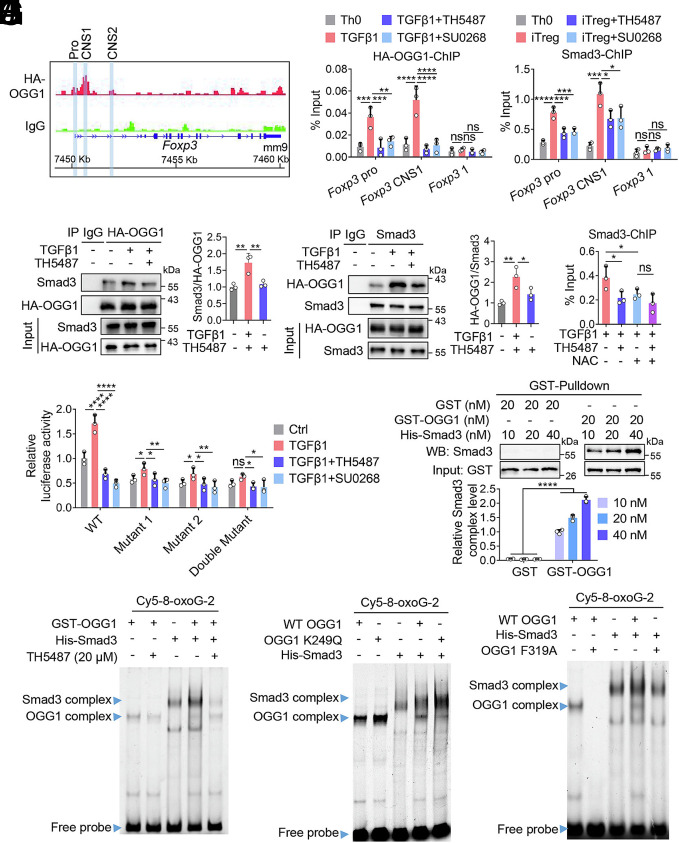
To elucidate how OGG1 regulates transcriptional activation of Foxp3, we performed CUT&RUN assays targeting OGG1. Naïve CD4+ T cells isolated from 3 × HA-OGG1 transgenic mice were cultured under iTreg-polarizing conditions, and chromatin was precipitated using an anti-HA Ab. The CUT&RUN analysis revealed that OGG1 binds to the Foxp3 promoter, CNS1 and CNS2 regions (Fig. 4A). In addition, OGG1 binding was detected at the promoter of Cxcl1, a known OGG1 target gene (34), but not at the Il17f promoter (SI Appendix, Fig. S6 C and D), supporting the specificity of these interactions. Our ChIP experiments further verified OGG1 binding to the Foxp3 promoter and CNS1; however, this binding was abolished upon TH5487 or SU0268 treatment (Fig. 4B). Moreover, when ROS were cleared using NAC, binding of OGG1 to Foxp3 promoter was significantly decreased. No synergistic effect was observed between TH5487 and NAC, indicating that OGG1 binds to 8-oxoG lesions within the Foxp3 promoter in response to ROS (SI Appendix, Fig. S6E).
Fig. 4.
OGG1 is essential for the recruitment of Smad3 to Foxp3. (A) CUT&RUN analysis showing OGG1 binding at the Foxp3 locus. Naïve CD4+ T cells derived from 3 × HA-OGG1 mice were cultured under iTreg polarization condition for 48 h. CUT&RUN was performed using anti-HA antibody, with IgG as a negative control. OGG1 binding peaks are shown at the Foxp3 promoter, CNS1 and CNS2 regions. Screenshots are from the Integrative Genomics Viewer. (B) OGG1 binds specifically to the promoter and CNS1 of Foxp3. Naïve CD4+ T cells derived from 3 × HA-OGG1 mice were treated with TH5487 (10 μM) or SU0268 (10 μM) and cultured under Th0 or iTreg polarization condition for 48 h. The binding of OGG1 to the promoter and CNS1 of Foxp3 was assessed by ChIP using anti-HA antibody. The primers targeting the Foxp3 coding region were used as a negative control. (C) Inhibition of OGG1 binding to 8-oxoG reduces its interaction with Smad3 in iTregs. Naïve CD4+ T cells from 3 × HA-OGG1 mice were cultured as in legend to (B) with or without TH5487 (10 μM). Co-IP was performed by immunoprecipitating either OGG1 (Left) or Smad3 (Right), followed by western blotting and quantified. IgG served as a negative control. (D) Inhibition of OGG1 binding to 8-oxoG suppresses the recruitment of Smad3 to the Foxp3 promoter and CNS1. Naïve CD4+ T cells were cultured as in legend to (B) and Smad3 binding was assessed by ChIP using an anti-Smad3 antibody. The primers targeting the Foxp3 coding region were used as a negative control. (E) Decreasing ROS levels impairs binding of Smad3 to Foxp3. Naïve CD4+ T cells were treated with TH5487 (10 μM) and/or NAC (1 mM) and cultured under iTreg polarization condition for 48 h. Smad3 binding to the CNS1 of Foxp3 was assessed by ChIP. (F) OGG1 enhances Foxp3 transcriptional activity by recruitment of Smad3. HEK293T cells transfected with pGL-4.2-Foxp3pro, pGL-4.2-Foxp3pro-Mutant 1, pGL-4.2-Foxp3pro-Mutant 2, or pGL-4.2-Foxp3pro-Double Mutant plasmids were treated with TH5487 (10 μM) or SU0268 (10 μM) in the presence of TGFβ1 for 12 h. Foxp3 transcriptional activity was evaluated by dual luciferase report assay. (G) OGG1 directly binds to Smad3. GST-OGG1 protein (20 nM) were incubated with His-Smad3 protein (10, 20, 40 nM) and the interaction was examined by GST-Pulldown and western blotting. (H) OGG1 promotes recruitment of Smad3 to the Foxp3 promoter. GST-OGG1 and/or His-Smad3 were incubated with 100 fmol 8-oxoG-containing, Cy5-labeled DNA oligos derived from the Foxp3 promoter with or without TH5487 (10 μM). Protein/DNA complex formation was analyzed by EMSA. (I and J) Modulation of OGG1 binding to 8-oxoG impacts the recruitment of Smad3 to Foxp3 promoter. GST-OGG1 K249Q (I) or GST-OGG1 F319A (J) and/or His-Smad3 were incubated with 100 fmol 8-oxoG-containing, Cy5-labeled DNA oligos derived from Foxp3 promoter, protein/DNA complexes were analyzed by EMSA. (B, and D–G) Quantification shows mean ± SD from three independent experiments. Statistical significance was determined by two-way ANOVA (B, D, F, and G) or one-way ANOVA test (E). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns, not significant. (C, and G–J) One representative experiment of three is shown.
To further decipher how OGG1 binding promotes Foxp3 transcription, mass spectrometry analysis of OGG1-associated protein complexes was conducted. This analysis identified Smad3 –a classical transcription factor involved in Foxp3 expression –as an OGG1-binding partner. This interaction was further validated by Co-IP, and was significantly reduced by TH5487 treatment (Fig. 4C). ChIP assays showed that inhibition of OGG1 by TH5487 or SU0268 significantly impaired recruitment of Smad3 to the Foxp3 promoter and CNS1 region (Fig. 4D). Similar effects were observed upon OGG1 deletion (SI Appendix, Fig. S6F) or ROS removal by NAC (Fig. 4E). Furthermore, inhibition of OGG1 binding to 8-oxoG disrupted colocalization of Smad3 and OGG1 at the promoter and CNS1 region of Foxp3 (SI Appendix, Fig. S6G). These data suggest that Smad3 recruitment is facilitated by OGG1 binding to the 8-oxoG harbored within the Foxp3 promoter. When Smad3-binding sites in the Foxp3 promoter were mutated, OGG1’s ability to enhance Foxp3 promoter activity was significantly abolished (Fig. 4F and SI Appendix, Fig. S7A). Similarly, the effects of OGG1 mutants K249Q and F319A on Foxp3 transcription were significantly reduced in the absence of functional Smad3-binding sites (SI Appendix, Fig. S7B). Taken together, these data indicate that OGG1 binding to 8-oxoG promotes Smad3 recruitment, thereby enhancing Foxp3 transcription.
To further validate the role of OGG1 in facilitating Smad3 docking on the Foxp3 promoter, EMSA was performed by utilizing recombinant proteins. A GST-pull-down assay first confirmed that OGG1 directly interacts with Smad3 (Fig. 4G). Then, a probe with a sequence derived from Foxp3 promoter was synthesized for EMSA. OGG1 bound to the probe only in the presence of 8-oxoG; while Smad3 bound to probe with or without 8-oxoG. Notably, Smad3/DNA interaction was largely enhanced when OGG1 bound to 8-oxoG-containing probe (SI Appendix, Fig. S8 A and B). As expected, the addition of TH5487, which inhibits OGG1–probe interaction, significantly decreased the binding of Smad3 (Fig. 4H and SI Appendix, Fig. S8C). Additionally, the OGG1-K249Q mutant showed a higher binding to 8-oxoG-containing probes and promoted Smad3/DNA interaction to a higher level, compared with WT OGG1 (Fig. 4I); in contrast, the F319A mutation reduced the capacity of OGG1 in promoting Smad3 binding to DNA because this mutant was defective in recognizing 8-oxoG in DNA (Fig. 4J). Taken together, these findings demonstrate that binding of OGG1 to 8-oxoG lesions in Foxp3 promoter enhances the recruitment of Smad3, thereby promoting the transcriptional activation of Foxp3.
1.5. Binding of OGG1 to 8-oxoG in the Promoter and CNS2 Region Accelerates Foxp3 Demethylation.
Guanine oxidation is known to be closely related with cytosine methylation. Within methylated CpG dinucleotides, guanine paired with methylated cytosines is more susceptible to oxidation (35, 36). Given that the activation of Foxp3 transcription depends on demethylation of its promoter and CNS2 region (37, 38), we investigated whether binding of OGG1 to 8-oxoG could decrease DNA methylation at an epigenetic level, and subsequently facilitate Smad3 recruitment to promote Foxp3 transcription. Interestingly, the result of dot blotting showed that inhibition of OGG1 binding to 8-oxoG by TH5487 treatment significantly sustained 5mC level in iTregs (SI Appendix, Fig. S9A), suggesting that OGG1 binding to DNA may promote 5mC demethylation. Moreover, methylation-specific PCR (MSP) revealed decreased methylation of the CpG island within the Foxp3 CNS2 region, which was reversed by TH5487 (Fig. 5A). To more precisely assess DNA methylation at Foxp3 CNS2, we performed 5mC-ChIP and bisulfite sequencing. Compared to Th0 cells, iTregs exhibited significantly reduced methylation within the Treg cell specific demethylation region (TSDR) of Foxp3 CNS2, and this demethylation was substantially attenuated by TH5487 (Fig. 5B and SI Appendix, Fig. S9B). It has been documented that the demethylation of Foxp3 is mediated by the Tet (ten eleven translocation) family; whereas methylation is primarily catalyzed by DNA methyltransferase 1 (Dnmt1) (21, 22). To explore how OGG1 regulates methylation of the Foxp3 promoter and CNS2, we examined the effects of OGG1 binding with the substrate on the site occupation of these DNA modifiers. The data showed that OGG1 bound to the CpG island in the Foxp3 promoter and CNS2 region during iTreg differentiation, which was abolished by TH5487 or SU0268 treatment (Fig. 5C). Notably, TH5487 treatment significantly inhibited the interaction of OGG1 with Tet1/Tet2 and reduced their binding to Foxp3, particularly during the early stages of iTreg differentiation (Fig. 5 D–F and SI Appendix, Fig. S10 A and B). In contrast, binding of Dnmt1 to the CpG island in the Foxp3 promoter and CNS2 region was decreased during the differentiation of iTregs from Th0 cells, but was sustained when OGG1 binding was blocked by TH5487(Fig. 5G). This suggests that OGG1 occupancy may hinder binding of Dnmt1, thus promoting passive demethylation. In addition, NAC decreased OGG1 binding to the CpG island while having little effect on Dnmt1 binding. There was no synergistic effect between TH5487 and NAC treatment (Fig. 5H and SI Appendix, Fig. S10C), indicating that ROS-elicited binding of OGG1 to 8-oxoG interferes with Dnmt1 recruitment. Taken together, these data suggest binding of OGG1 to the substrate shields Dnmt1 while facilitating the homing of Tet 1/2.
Fig. 5.
Binding of OGG1 to 8-oxoG in the promoter and CNS2 region accelerates Foxp3 demethylation. (A and B) Inhibition of OGG1 increases the methylation of CpG island in Foxp3 CNS2. Naïve CD4+ T cells were cultured under Th0 or iTreg differentiation conditions with or without TH5487 for 48 h. Equivalent amounts of bisulfite-converted-DNA were analyzed for methylation by methylation-specific PCR (MSP). U: PCR with primers detecting unmethylated DNA; M: PCR with primers detecting methylated DNA (A). Bisulfite sequencing was performed across 10 CpG sites in the Foxp3 CNS2 region. Ten individual clones per sample were analyzed and methylation levels were calculated as the percentage of methylated CpG per clone (B). (C) OGG1 binds to the CpG island in the promoter and CNS2 regions of Foxp3. Naïve CD4+ T cells from 3 × HA-OGG1 mice were treated with TH5487 (10 uM) or SU0268 (10 uM) and cultured as described in (A). OGG1 binding was evaluated by ChIP assays. The primers targeting the Foxp3 coding region were used as a negative control. (D) Inhibition of OGG1 binding to 8-oxoG impairs its interaction with Tet1/Tet2. Naïve CD4+ T cells from 3 × HA-OGG1 mice were cultured as in the legend to (A), and 3 × HA-OGG1 were immunoprecipitated followed by western blotting analysis. IgG served as a negative control. (E–G) Inhibition of OGG1 binding to 8-oxoG reduces the recruitment of Tet1/2 and enhances the occupation of Dnmt1 to Foxp3. Cells were prepared as described in (A). Tet1 binding to the Foxp3 promoter and CNS2 region was evaluated by ChIP (E). The primers targeting the Foxp3 coding region were used as a negative control. The binding of Tet2 to the promoter of Foxp3 was evaluated by ChIP (F). The binding of Dnmt1 to the promoter and CNS2 of Foxp3 was analyzed by ChIP (G). (H) Decreasing ROS levels improves the recruitment of Dnmt1 to Foxp3. Naive CD4+ T cells were treated with NAC (1 mM) and/or TH5487 (10 μM) and cultured as in legend to (A). Dnmt1 binding to the promoter and CNS2 of Foxp3 was analyzed by ChIP assays. (I and J) 5mC on the same strand of 8-oxoG increases OGG1 occupancy on Foxp3 and suppresses its cleavage activity. GST-OGG1 was incubated with 100 fmol 8-oxoG-containing, Cy5-labeled oligos derived from Foxp3 promoter, including C8-oxoG/GC, 5mC8-oxoG/GC, or 5mC8-oxoG/G5mC. Protein/DNA complexes were visualized by EMSA (I). Cleavage activity was analyzed by oligonucleotide incision assay (J). (K and L) The binding of OGG1 to 8-oxoG improves recruitment of Tet2 and inhibits Dnmt1 occupancy at Foxp3. GST-OGG1 and/or His-Tet2 (K) or His-Dnmt1(L) were incubated with 100 fmol 8-oxoG and 5mC containing DNA oligos derived from Foxp3 promoter (5mC8-oxoG/G5mC) (K) or (5mC8-oxoG/GC) (L) in the absence or presence of TH5487 (10 μM). Formation of protein/DNA complexes was assessed by EMSA. (A, C, and E–J) Quantification shows mean ± SD from three independent experiments. Statistical significance was determined by one-way ANOVA (A) or two-way ANOVA test (C, and E–J). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns, not significant. (A, B, D, and I–L) One representative experiment of three is shown.
To further elucidate the molecular mechanism by which OGG1 influences DNA methylation, two sets of oligonucleotide probes with sequence derived from the CpG island in Foxp3 CNS2 region were synthesized. Since Tet2 binds to symmetrically methylated DNA and Dnmt1 binds to hemimethylated DNA (39, 40), one probe contained symmetric 5mC in both strands with an adjacent 8-oxoG, mimicking ROS-induced guanine oxidation in an activated T cell. The second probe contained 8-oxoG and 5mC in cis on the same strand, representing a postreplication state in proliferative cells. Intriguingly, EMSA and cleavage assay revealed that the presence of 5mC enhanced the engagement of OGG1 with 8-oxoG, but impaired lesion excision (Fig. 5 I and J). When Tet2 or Dnmt1 was introduced into the EMSA mixture, OGG1 binding to 8-oxoG significantly increased the recruitment of Tet2 and inhibited Dnmt1 binding. These effects were diminished upon TH5487 addition (Fig. 5 K and L). Together, these data suggest that OGG1 binding to 8-oxoG in response to ROS is essential for the demethylation of Foxp3 by modulating the interaction of Dnmt1 and Tet1/Tet2, thereby enhancing Foxp3 transcription. Since CNS2 demethylation is primarily linked to the Treg’s lineage stability, we also analyzed whether OGG1 contributes to Treg lineage stability by treating purified iTregs from Foxp3YFP mice with TH5487. The data showed that OGG1 inhibition significantly impaired the stability of iTregs (SI Appendix, Fig. S11 A and B), suggesting that OGG1 binding to 8-oxoG is important for sustaining Foxp3 transcription and preserving Treg lineage stability.
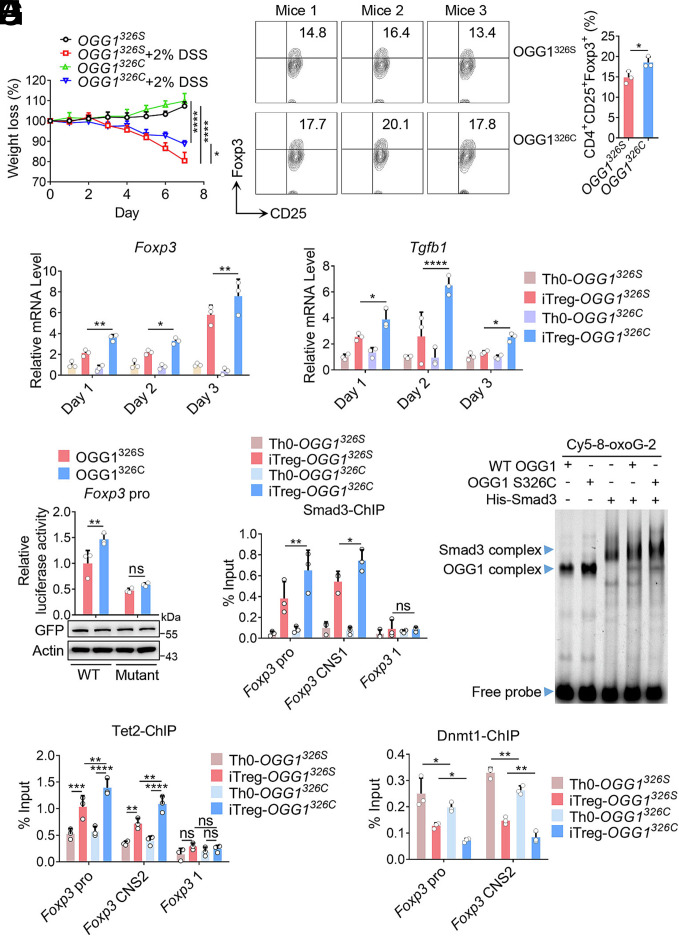
1.6. OGG1 S326C Variant Activates Foxp3 Transcription and Enhances iTreg Differentiation with Increased Efficiency.
The single nucleotide polymorphic (SNP) variants of OGG1 have attracted wide attention. To date, five SNP types have been reported to be associated with clinical diseases (41). Among these, the Cys-326 allele frequency ranges from 23 to 41% in Caucasians, and from 40% to 60% in Asians, and it has been linked to 147 diseases (42, 43). Due to OGG1 S326C variant reduced enzymatic activity (42), our previous study found that it forms a more stable complex with 8-oxoG-containing DNA compared to the wild-type protein (44). Given that binding of OGG1 to 8-oxoG is crucial for its role in transcription activation (7, 10), we hypothesized that the S326C variant likely enhances iTreg differentiation and contributes to IBD remission. To test this, we generated transgenic mice expressing either OGG1326S and OGG1326C. Compared with OGG1326S controls, OGG1326C mice showed increased Treg accumulation in colons, and demonstrated an increase in tolerance to DSS-induced colitis (Fig. 6A and SI Appendix, Fig. S12 A–D). In line with this, the OGG1 S326C variant enhanced iTreg differentiation, and increased transcription of Foxp3 and Tgfb1 (Fig. 6 B and C). A luciferase dual reporter assay further confirmed that OGG1 S326C variant significantly increased the transcriptional activity of Foxp3 promoter compared with the control, and this effect was significantly lower when Smad3 binding sites were mutated (Fig. 6D). ChIP and EMSA results showed that OGG1 S326C variant was more effectively in promoting the recruitment of Smad3 to both the promoter and CNS1 region of Foxp3 compared with the OGG1 326S in vivo and in vitro, respectively (Fig. 6 E and F). Additionally, OGG1 S326C variant also enhanced the binding of Tet2 and decreased that of Dnmt1 at the Foxp3 promoter and CNS2 region (Fig. 6 G and H). Together, these data demonstrate that OGG1 S326C enhances Foxp3 transcription and iTreg differentiation, thereby effectively serving as a protective factor in IBD.
Fig. 6.
OGG1 S326C variant is more potent to activate Foxp3 transcription and enhance iTreg differentiation. (A) The OGG1 S326C variant plays a protective role in mouse models of IBD. OGG1326S or OGG1326C mice were treated with or without 2% DSS in DW, and the body weight loss was recorded daily. (B and C) The OGG1 S326C variant promotes iTreg differentiation and enhances the transcription of Foxp3 and Tgfb1. Naïve CD4+ T cells from OGG1326S or OGG1326C mice were cultured under Th0 or iTreg polarization conditions for three days. CD4+CD25+Foxp3+ iTregs were analyzed by FCM (Left) and quantified (Right) (B). Total RNA was isolated at the indicated point for qPCR analysis of Foxp3 and Tgfb1 mRNA levels (C). (D) The OGG1 S326C variant enhances the transcriptional activity of the Foxp3 promoter. HEK293T cells transfected with pLKO.1-shOgg1 along with pEGFP-N1-OGG1 326S or pEGFP-N1-OGG1 326C plasmids to generate cells expressing the OGG1 variant. Cells were then transfected with pGL-4.2-Foxp3 pro or pGL-4.2-Foxp3 pro-Double Mutant plasmids. Foxp3 transcriptional activity was evaluated by dual luciferase report assay. (E and F) The S326C mutant enhances OGG1-mediated recruitment of Smad3. Cells cultured as described in (B) for 48 h and Smad3 binding to the Foxp3 promoter and CNS1 was analyzed by ChIP (E). The primers targeting the Foxp3 coding region were used as a negative control. GST-OGG1, GST-OGG1 S326C, and/or His-Smad3 were incubated with 100 fmol 8-oxoG-containing, Cy5-labeled DNA oligos derived from Foxp3 promoter. Protein–DNA complex formation was analyzed by EMSA (F). (G and H) The OGG1 S326C variant increases Tet2 recruitment and decreases Dnmt1 occupancy at Foxp3. Cells were cultured as described in (B) for 48 h and the binding of Tet2 (G) and Dmnt1 (H) to Foxp3 promoter and CNS2 was evaluated by ChIP. The primers targeting the Foxp3 coding region were used as a negative control. (A) Data represent mean ± SD (n = 4), with significance determined by two-way ANOVA test. Quantification shows mean ± SD from three independent experiments. Statistical significance was determined by the t test (B) or two-way ANOVA test (C–E, G, and H). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns, not significant. (F) One representative experiment out of three is shown.
To further explore the negative correlation between OGG1 and IBD in humans, we conducted a single variant association analysis utilizing whole-exome sequencing data from 327,684 individuals in the UK Biobank, focusing on the OGG1S326C (rs1052133) variant. This analysis yielded a P value of 0.102. A variant-set association analysis evaluating the cumulative effect of OGG1 missense variants on IBD, showed a P value of 0.067, approaching the conventional threshold for statistical significance (P < 0.05). These findings suggest that while the OGG1S326C variant is a primary contributor, other missense variants in OGG1 also influence disease susceptibility. We also compared the frequency of OGG1S326C between healthy individuals and IBD patients, from a local hospital cohort and all of whom were Asian. Peripheral blood DNAs from 55 IBD patients and 69 healthy subjects were sequenced. The frequency of OGG1S326C allele was 51.8% in IBD patients compared to 61.6% in healthy control subjects, indicating a trend toward a protective association, although this did not reach statistical significance, the p value was 0.155 using chi-squared test analysis (SI Appendix, Fig. S12E). Together, these results corroborate findings from the mouse model, and indicate that OGG1, particularly the OGG1S326C variant, plays a protective role in human IBD, possibly through the enhancement of iTreg differentiation.
1.7. OGG1 Inhibitor O8 Promotes iTreg Differentiation and Attenuates Mice IBD.
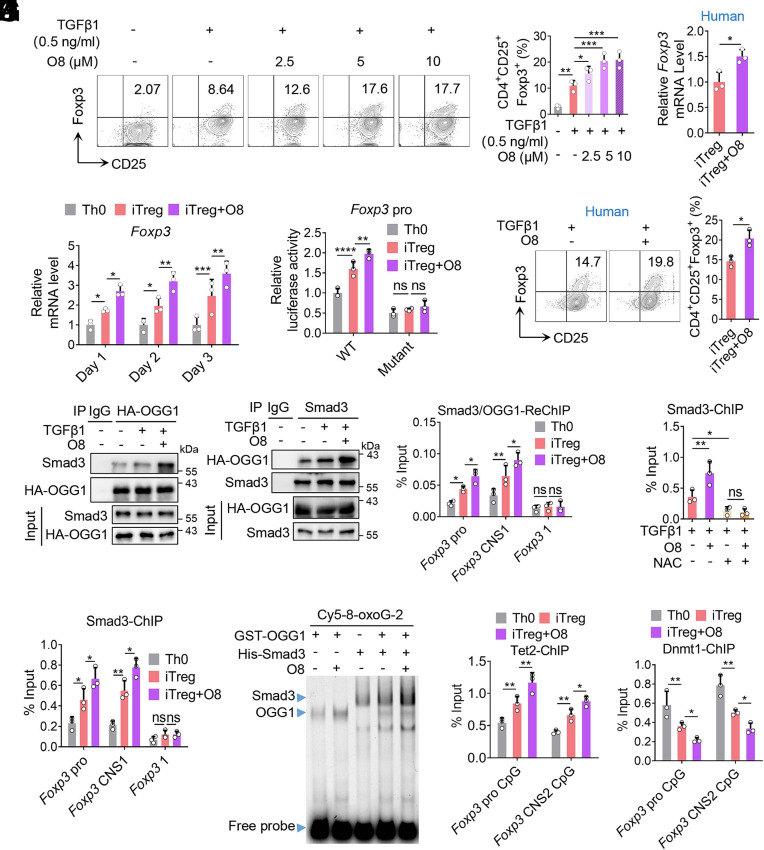
Since the engagement of OGG1 with 8-oxoG in DNA accounts for iTreg differentiation and alleviates colitis in the mouse model of IBD, we next evaluated the therapeutic potential of the OGG1 inhibitor O8. In contrast to TH5487 or SU0268, which act by blocking the binding of OGG1 to 8-oxoG lesions, O8 specifically inhibits Schiff base formation during the OGG1 catalytic cycle (a key step in BER), thereby suppressing enzymatic activity without affecting 8-oxoG recognition or DNA binding (5, 45). This results in prolonged engagement of OGG1 with DNA. The results showed that O8 treatment significantly promoted iTreg differentiation in vitro (Fig. 7A), and this effect was abolished when ROS and 8-oxoG formation was prevented by NAC treatment (SI Appendix, Fig. S13 A–C). In addition, O8 treatment enhanced the transcriptional activation of Foxp3 (Fig. 7 B and C). Importantly, the effects of O8 on enhancing Foxp3 transcription and iTreg differentiation were replicated in human T cells (Fig. 7 D and E). Mechanistically, the ChIP and Co-IP experiments showed that O8 enhanced the binding of OGG1 to the promoter and CNS1 region of Foxp3, as well as enhanced its interaction with Smad3. This resulted in a significant increase in binding of Smad3 and colocalization of OGG1 and Smad3 at these regions (Fig. 7 F–H and SI Appendix, Fig. S13D). Notably, when ROS were removed (thus disrupting OGG1–DNA binding), the O8-induced enhancement of Smad3 recruitment to Foxp3 was abolished (Fig. 7I and SI Appendix, Fig. S13E). EMSA further confirmed that O8 enhanced the formation of a complex between OGG1, Smad3 and a Foxp3 promoter probe containing 8-oxoG (Fig. 7J). In addition, ChIP data showed that O8 enhanced OGG1 binding to the CpG islands in both the Foxp3 promoter and CNS2 regions. This was associated with enhanced recruitment of Tet2 and decreased binding of Dnmt1 (Fig. 7 K and L and SI Appendix, Fig. S13F). The O8-mediated decreased recruitment of Dnmt1 was reversed when ROS was removed by NAC treatment (SI Appendix, Fig. S13 G and H). Collectively, these findings demonstrate that O8 promotes Foxp3 transcription and iTreg differentiation by enhancing the recruitment of Smad3 and Tet2 and reducing Dnmt1 binding at Foxp3 regulatory regions.
Fig. 7.
OGG1 inhibitor O8 enhances Foxp3 transcription and promotes iTreg differentiation. (A) O8 promotes iTreg differentiation. Naïve CD4+ T cells were cultured in Th0 or iTreg (with 0.5 ng/mL TGFβ1) polarization conditions with increasing concentrations of O8. CD4+CD25+Foxp3+ iTregs were analyzed by FCM (Left) and quantified (Right). (B) O8 upregulates the transcription of Foxp3. Cells treated with O8 (10 μM) were cultured as described in (A), and cDNA was analyzed for Foxp3 mRNA expression. (C) O8 increases the transcriptional activity of the Foxp3 promoter. HEK293T cells transfected with pGL-4.2-Foxp3 pro or pGL-4.2-Foxp3 pro-Double Mutant plasmids were treated with or without O8 (10 μM). Foxp3 transcriptional activity was evaluated by dual luciferase reporter assay. (D and E) O8 augments the transcription of Foxp3 and increases human iTreg differentiation. Naive CD4+ T cells isolated from human peripheral blood were cultured under iTreg polarization condition (with 0.5 ng/mL TGFβ1). CD4+CD25+Foxp3+ iTregs were analyzed by FCM (Left) and quantified (Right) (D). Foxp3 mRNA levels were analyzed by qPCR (E). (F) O8 increases the interaction between OGG1 and Smad3. Naïve CD4+ T cells derived from 3 × HA-OGG1 mice were treated with O8 and cultured as described in (B) for 48 h. Cells were lysed and OGG1 (Left) or Smad3 (Right) were immunoprecipitated and analyzed by western blotting. IgG served as a negative control. (G) O8 enhances Smad3 binding to the Foxp3 promoter and CNS1. Naïve CD4+ T cells treated with O8 were cultured as described in (B) for 48 h and Smad3 binding was evaluated by ChIP using anti-Smad3 antibody. The primers targeting the Foxp3 coding region were used as a negative control. (H) O8 promotes colocalization of OGG1 and Smad3 at the promoter and CNS1 of Foxp3. Cells prepared as described in (F) were analyzed by Re-ChIP using anti-HA and anti-Smad3 antibodies. The primers targeting the Foxp3 coding region were used as a negative control. (I) Decrease in ROS levels abolish the effect of O8 on recruitment of Smad3 to Foxp3. Cells treated with O8 (10 uM) and/or NAC (1 mM) were cultured as described in (B) for 48 h and ChIP was performed using an anti-Smad3 antibody. (J) O8 enhances the ability of OGG1 in recruiting Smad3 to Foxp3. GST-OGG1 and/or His-Smad3 were incubated with 100 fmol 8-oxoG-containing Cy5-labeled DNA oligos derived from Foxp3 promoter in the absence/presence of O8 (10 µM). Protein- DNA complex formation was analyzed by EMSA. (K and L) O8 increases Tet2 recruitment and reduces Dnmt1 binding to Foxp3. Cells were cultured as described in (B) for 48 h and the binding of Tet2 (K) or Dnmt1 (L) to the promoter and CNS2 of Foxp3 were analyzed by ChIP. (A–E, G–I, K, and L) Quantification shows mean ± SD from three independent experiments, Statistical significance was determined by one-way ANOVA (A and I), two-way ANOVA (B, C, G, H, K, and L), or unpaired t test (D and E). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns, not significant. (F and J) One representative experiment out of three is shown.
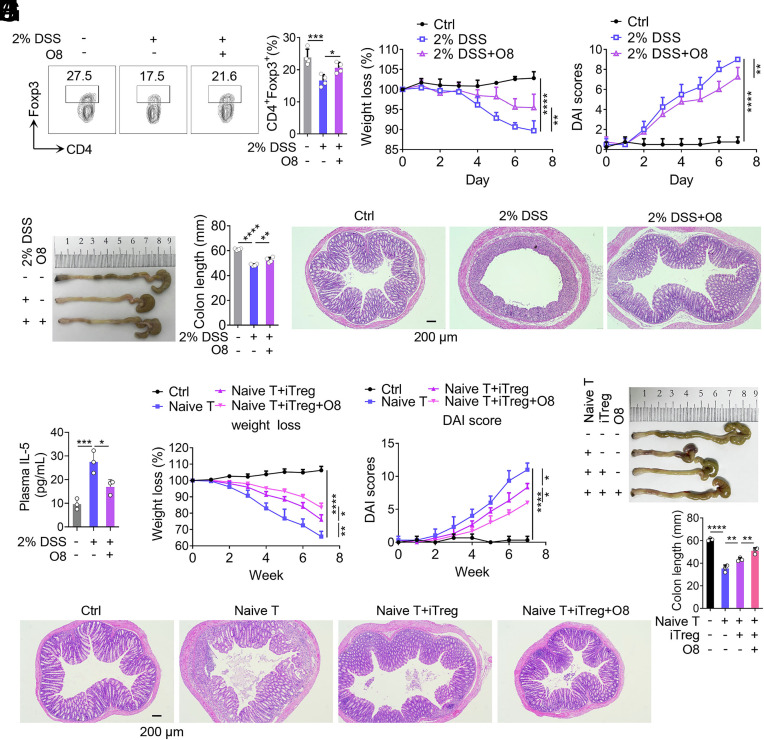
Next, we evaluated the therapeutical effect of O8 in a DSS-induced mouse model of IBD. O8 treatment significantly increased colonic Treg frequency and alleviated IBD symptoms (Fig. 8 A–E). Additionally, level of IL-5—a cytokine involved in eosinophil recruitment and previously reported to be significantly increased in Ogg1-deficient mice during DSS-induced colitis (12)—was significantly reduced by O8 treatment (Fig. 8F). To explore whether O8 offers an additional protective role in mice already expressing S326C variant of OGG1, DSS-induced IBD experiments were performed using both OGG1326S and OGG1326C mice with or without O8 injection. The data showed that OGG1326C mice exhibited significantly milder colitis compared to OGG1326S mice; However, O8 injection did not further alleviate IBD symptoms in OGG1326C mice (SI Appendix, Fig. S14 A–D). The results suggest that both O8 and S326C variant act via shared mechanism—enhancing the binding of OGG1 to 8-oxoG—and thus their effects are not additive. These findings point to a specific therapeutic potential of O8 in IBD patients carrying the OGG1 326S variant.
Fig. 8.
OGG1 inhibitor O8 alleviates colitis in the mouse model of IBD. (A–E) O8 alleviates DSS-induced mouse IBD. Mice were administered DSS (2%, w/v) in DDW. Simultaneously, OGG1 inhibitor (O8, 20 mg/kg) was administered via intraperitoneal injection every other day; solvent (DMSO) served as the negative control. After 7 d, Tregs in colonic lamina propria were analyzed by FCM (A). Body weight (B) and DAI scores (C)—based on body weight loss, stool consistency, and blood in the stool—were recorded daily. On day 7, colons were harvested for length assessment (D) and histopathological analysis after hematoxylin and eosin (H and E) staining (E). (Scale bar, 200 μm.) (F) Injection of O8 reduces plasma IL-5 levels. IL-5 levels in plasma were assayed using an ELISA kit. (G–J) Evaluation of colitis in mice, adoptively transferred with different cells. Body weight (G) and DAI scores (H) were recorded weekly. After 7 wk, colons were collected for length assessment (I) and histologically analyzed after H&E staining (J). (Scale bar, 200 mm.) (E and J) One representative experiment from four biological replicas is shown. (A–D, and F–I) Data are represented as mean ± SD (n = 4), with significance determined by one-way ANOVA test (A, D, F, and H) or two-way ANOVA test (B, C, G, and H). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Given that adoptive transfer of iTregs is a clinically validated therapy for several immune diseases including solid organ transplantation and type I diabetes (46–48), we further evaluated O8 in a T cell transfer-induced colitis model. In this model naive CD4+ T cells were transferred into Rag1−/− mice (49). As expected, mice receiving naive CD4+ T cells alone developed severe colitis, whereas cotransfer with iTregs significantly alleviated disease symptoms (Fig. 8 G–J). Notably, O8-treated iTregs along with naive CD4+ T cells ameliorated mouse colitis more effectively than untreated iTregs (Fig. 8 G–J). To assess whether O8 enhances the stability of Tregs in vivo, we performed adaptive transfer using CD45.2 Rag1−/− mice (SI Appendix, Fig. S15 A–E). Naïve CD4+ T cells isolated from the spleen of CD45.1 mice were cultured under iTreg differentiation condition in the presence or absence of OGG1 inhibitor O8 for 3 d to generate iTregs and iTreg+O8 group of cells. These iTregs along with naïve CD4+ T cells were adoptively transferred into Rag1−/− (CD45.2) mice. After 7 wk, CD45.1 positive cells from the colon of Rag1−/− (CD45.2) mice were isolated and analyzed for Foxp3 expression. In mice receiving untreated iTregs, the proportion of Foxp3+ cells was 5.3 ± 0.9%, representing 34.9% of the pretransfer level. In contrast, in the mice receiving iTreg+O8 group cells, the proportion of Foxp3+ cells was significantly higher at 9.7 ± 1.8%, accounting for 39.8% of the preinjection level. These results indicate that despite the inevitable decline in Foxp3+ Tregs over time, O8 treatment enhanced the functional persistence of adoptively transferred iTregs. Collectively, these findings demonstrate that the OGG1 inhibitor O8 promotes Foxp3 expression, iTreg differentiation, and Treg stability, and effectively attenuates disease in a murine model of colitis.
2. Discussion
ROS are deleterious only when excessive; however, at physiological levels, they act as signaling molecules by reversibly modifying redox-sensitive molecules including those with cysteine residues. This redox modulation is also central to the concept of ROS-mediated signaling in diverse physio pathological processes. Recent studies have expanded this paradigm, demonstrating that the oxidation of guanine within regulatory DNA regions can be regarded as an epigenetic-like mark. OGG1, conventionally recognized as a specific glycosylase that initiates BER pathway, is a specific “reader” of this DNA base modification. Upon binding to 8-oxoG, it can modulate the transcription of ROS-responsive genes without necessarily initiating repair (5–7). Here, we identified a role for OGG1 in transcriptional activation of Foxp3, a lineage-defining transcription factor essential in iTreg differentiation. We found that OGG1 bound to 8-oxoG in the Foxp3 promoter and CNS2 regions, promoting DNA demethylation and facilitating the recruitment of Smad3, thereby enhancing Foxp3 transcription. Our findings highlight a mechanism by which ROS-induced guanine oxidation is co-opted to regulate gene expression during iTreg differentiation, elucidating the requirement for ROS in this process and uncovering a protective role for OGG1 in IBD.
Previous studies have documented the involvement of guanine oxidation and binding of OGG1 in transcriptional regulation of proinflammatory cytokine or chemokine genes—primarily in the context of acute inflammation driven by neutrophil infiltration—our work identified a contrasting role in chronic inflammation. Mice deficient in Ogg1 (Ogg1−/−) display resistance to lipopolysaccharide (LPS)-induced inflammation and organ dysfunction, along with lower levels of chemokines and cytokines such as macrophage inflammatory protein-1 alpha (Mip-1α/CCL3), IL-12, and TNFα (50). Moreover, OGG1 inhibition by small molecule(s) or its siRNA knock down suppresses the expression of inflammatory mediators at mRNA and protein levels including TNFα, chemokine (C-X-C motif) ligand (CXCL)1, CXCL2, macrophage inflammatory protein-3 alpha (CCL20), IL-6, and IL-1β, after TNFα exposure or infection with respiratory syncytial virus in cultured human cells and mouse lungs (11, 51). Mechanistically, oxidative stress leads to transient inactivation of OGG1’s enzymatic function via cysteine oxidation (5, 31) allowing its transient accumulation at 8-oxoG in promoter regions, where facilitating binding of sequence-specific transcription factors such as NF-κB, Sp1 along with phosphorylated RNA polymerase II leading to expression from proinflammatory cytokine and chemokine genes (11). In contrast, our studies here revealed that during iTreg differentiation OGG1 binds to Foxp3 promoter and enhances the recruitment of Smad3 promoting Foxp3 expression and iTreg generation, thereby suppressing chronic inflammation in a murine IBD model. Specifically, the present study uncovers a role for OGG1 in transcriptional regulation of the lineage-specific gene during immune cell differentiation. Taken together, with prior studies our findings suggest that OGG1 binding with its substrate promotes the expression of distinct subsets of ROS-responding gene programs in different immune contexts. Thus, its proinflammatory role in acute inflammation and protective role in chronic inflammation may reflect an evolutionary conserved function aimed at maintaining organismal homeostasis under oxidative stress.
In addition to sequence-specific transcriptional regulation, our study broadens the understanding of OGG1’s epigenetic roles in transcriptional control. Previous research has shown that OGG1 binding to 8-oxoG can recruit chromatin remodelers such as chromodomain helicase DNA-binding protein 4 CHD4, which in turn recruit DNMT3A/3B, leading to cytosine methylation and gene silencing (52). In contrast, Foxp3 activation during iTreg differentiation depends on demethylation of CpG islands in its promoter and enhancer (CNS2) regions (37). While Dnmt1 promotes methylation, Tet1/2 are required for demethylation and the maintenance of Foxp3 expression (20–22). However, the mechanism by which Tet1/2 are directed to Foxp3 has remained unclear. Here, we demonstrate that the binding of OGG1 to 8-oxoG enhances the recruitment of Tet1/2 and prevents Dnmt1 occupancy, promoting demethylation and transcriptional activation of Foxp3. Notably, DNA methylation of vertebrates generally occurs in an evolutionarily conserved CpG dinucleotides context. Interestingly, the frequency of CpG dinucleotides is underrepresented in the vertebrate genome (0.99% in humans), much lower than other dinucleotide combinations (4.27 to 9.8%), possibly reflecting evolutionary pressure due to their chemical reactivity in aerobic cells. This chemical vulnerability may have been exploited by aerobic organisms to sense oxidative stress and regulate gene expression. Our data suggest that OGG1 reads this epigenetic signal, showing enhanced binding to 8-oxoG in a methylated CpG context while its catalytic activity is suppressed.
Genetic variation in OGG1 has clinical implications. The OGG1 S326C is present in ~20% in Caucasian, and 40 to 60% of Asian populations, and is associated with increased risk for various malignancies, including lung, gastric, prostate, and pharyngeal cancers, as well as the relapse of acute myeloid leukemia (41, 43). The disease susceptibility of OGG1 S326C has been attributed to its impaired base-excision function (42). However, our data suggest that OGG1 S326C variant displays even enhanced substrate engagement despite lacking catalytic activity, thereby augmenting its transcriptional role(s) (44). Indeed, we found that OGG1 S326C variant promotes the recruitment of Smad3 and the demethylation of Foxp3 regulatory sequences, thereby enhancing iTreg differentiation and conferring increased protection against IBD compared to the wild-type OGG1. This variant may partly explain the lower incidence of IBD in Asian populations, which merits further investigation in large-scale cohort studies. Conversely, enhanced iTreg generation may contribute to the immune suppressive tumor microenvironment, potentially explaining the increased tumor susceptibility observed in carriers of this variant.
Our study also highlights the translational potential of OGG1-targeted therapies as the present study along with those from databases suggested a negative correlation between OGG1 expression and IBD incidence. This strongly implies that OGG1 inhibitor O8 may have the potential in clinical utility to treat IBD. In the previous studies, TH5487 and SU0268 were used to inhibit the binding of OGG1 to intrahelical 8-oxoG as well as the expression of proinflammatory cytokines/chemokines, and to cease mouse lung inflammation, where O8 was applied as a negative control (10, 11). However, in the present study, O8 exhibited excellent inhibitory effects on IBD in mice by both intraperitoneal injection or adoptive transfer of O8-treated-iTreg. This greatly highlights the clinical translational significance of O8 as in vitro culture and adoptive transfer of iTregs has already achieved reliable and good efficacy in the clinical trials of other immune diseases, such as solid organ transplantation and type I diabetes (46–48). On the other hand, accumulating evidence has revealed that Tregs are also highly infiltrative in various tumor types, such as skin, pancreas, breast, and ovarian tumors in both humans and mice, and the infiltration of intratumoral Tregs was negatively correlated with the survival (53). Advanced cancers (stage III and IV) frequently display higher levels of Treg accumulation. Thus, reducing Tregs is a critical strategy for tumor treatment. Tregs in tumors can be derived from the external migration of Tregs and the differentiation of naive CD4+ T cells within the tumor (54). Since our data showed that TH5487 and SU0268 significantly inhibit iTreg differentiation. Implicitly, TH5487/SU0268 are likely to have important clinical translational significance in tumor treatment.
In summary, this study defines a mechanism by which OGG1 promotes transcriptional activation of Foxp3 via transcription factor recruiting and epigenetic remodeling during iTreg differentiation. Our findings emphasize the dual roles of OGG1 in inflammation and immunity, and underscore the therapeutic potential of selectively modulating OGG1 activity in distinct disease contexts. Together with prior research, the present study supports a concept that guanine oxidation can act as a genomic sensor of ROS, and OGG1 functions as a redox-responsive “reader” to shape the epigenetic landscape and gene expression.
3. Materials and Methods
3.1. Animals.
C57BL/6 (B6) mice, OGG1326S, OGG1326C,3 × HA-OGG1, Ogg1−/−, Rag1−/− (CD45.1 or CD45.2), and Foxp3YFPreporter mice were used. Animal use, treatment, and killing are in accordance with the Environmental and Facilities for Laboratory Animals (GB14925-2010) and the Guidelines for Ethical Review of Welfare of Laboratory Animals (GB/T 35892-2018), and approved by the local ethics committee (reference number: 202402017, the institutional animal care and use committee (IACUC) of the Northeast Normal University).
3.2. Induction of Mouse iTregs In Vitro.
Naïve CD4+ T cells isolated from the spleen of female mice were stimulated with Dynabeads® Mouse T-Activator CD3/CD28 and cultured in RPMI 1640 medium supplemented with 10% FBS, penicillin-streptomycin (500 U), β-mercaptoethanol (50 μM) to obtain Th0. For iTreg induction, rhTGFβ1 (2 ng/mL) was added. For details of cell induction, see SI Appendix, Materials and Methods.
3.3. Induction of Human iTregs In Vitro.
Human peripheral blood samples were obtained from the Jilin Blood Center (Changchun, China). All work with human blood samples was approved by the local ethics committee (The ethical committee of the Northeast Normal University) (Authorization number: 202402017), and informed consent was obtained from all subjects. After Ficoll (Sigma Aldrich) gradient, naïve CD4+ T cells were isolated with MojoSort™ human CD4+ Naïve T Cell Isolation Kit. For details of cell induction, see SI Appendix, Materials and Methods.
3.4. Flow Cytometry Analysis.
Cells were collected in PBS containing 1% FBS (v/v) and fixed/permeabilized using the Transcription Factor Buffer Set (562574, BD Biosciences) according to the manufacturer’s instruction. For details of cell staining and analysis, see SI Appendix, Materials and Methods.
3.5. Quantitative real-time PCR.
Total RNA was isolated using the TRIzol reagent (15596-018, Invitrogen). The details of reverse transcription and real-time quantitative PCR (RT-qPCR) were described in SI Appendix, Materials and Methods.
3.6. Plasmid Construction.
The details of the eukaryotic and prokaryotic expression plasmids’ construction were described in SI Appendix, Materials and Methods.
3.7. Retrovirus Transduction of CD4+ T Cells.
Platinum-E cells were transfected with pMIG-OGG1. The supernatant was harvested at 48, 72, and 96 h after transfection. Naïve CD4+ T cells were cultured under Th0-inducing condition as described above for 16-24 h and were spin infected for two rounds with retrovirus supernatants. For details, see SI Appendix, Materials and Methods.
3.8. Immunoprecipitation and Western Blotting.
Coimmunoprecipitation and western-blotting were performed as previously described (9). For details, see SI Appendix, Materials and Methods.
3.9. Expression and Purification of the Recombinant Proteins.
Proteins GST, GST-OGG1, GST-OGG1 F319A, GST-OGG1 K249Q, and GST-OGG1 S326C were induced in BL21 (DE3) Escherichia coli. For details, see SI Appendix, Materials and Methods.
3.10. Sequential ChIP Assays.
Chromatin immunoprecipitation (ChIP) was performed as described previously (55). For details, see SI Appendix, Materials and Methods.
3.11. Electrophoretic Mobility Shift Assay.
The oligonucleotides were synthesized by Sangon Biotech (Shanghai, China). The EMSA analysis was performed using the LightShift chemiluminescent EMSA kit (ThermoFisher Scientific, 20,148) as described previously (5). For details, see SI Appendix, Materials and Methods.
3.12. Oligonucleotide Incision Assay.
To examine the lesion incision abilities of OGG1, Cy5-labeled oligonucleotides containing 8-oxoG and (or) 5mC were used and oligonucleotide incision assay was performed as described previously (5). For details, see SI Appendix, Materials and Methods.
3.13. Luciferase Reporter Gene Assay.
To measure the activity of Foxp3 promoter, HEK293T or EL4 cells were used. Luciferase reporter gene assay was performed by Dual-Luciferase® Reporter Assay System (E1910, Promega). For details, see SI Appendix, Materials and Methods.
3.14. Extracellular Flux Analysis.
For the FAO-associated oxygen consumption rate (OCR) assay by Seahorse XFp Analyzer (Agilent), Palmitate-BSA reagent (Agilent) and Mito Stress Test Kits (Agilent) were used according to the manufacturers’ instructions with minor modifications (56). For details, see SI Appendix, Materials and Methods.
3.15. Intracellular ROS and 8-oxoG Analysis.
For ROS and 8-oxoG measurement, cell-permeable ROS probe DCFH-DA (HY-D0940, MCE) or anti-8-oxoG antibody (ab206461, Abcam) was used before flow cytometry analysis. For details, see SI Appendix, Materials and Methods.
3.16. RNA-Sequencing Analysis.
Total RNA was isolated with TRIzol (Invitrogen) and subjected to RNA-sequencing using Illumina Nextseq500 (75 bp paired end reads) by BGI Genomics Co., Ltd. For details, see SI Appendix, Materials and Methods.
3.17. Bisulfite Sequencing.
Bisulfite sequencing for analysis of DNA methylation was performed by Sangon Biotech company as described previously (57). For details, see SI Appendix, Materials and Methods.
3.18. CUT & RUN Analysis.
To assess OGG1 binding region on DNA, naive CD4+ T cells derived from 3 × HA-OGG1 mice were used to induce iTregs. CUT & RUN analysis was performed by Guangzhou Epibiotek Co., Ltd. For details, see SI Appendix, Materials and Methods.
3.19. In Vitro iTreg Suppression Assay.
Naïve CD4+ T cells from Foxp3YFP mice were used to obtain pure iTregs. iTreg suppression assay was performed as described previously (58). For details, see SI Appendix, Materials and Methods.
3.20. OGG1 Interactome Analysis by Mass Spectrometry.
Naïve CD4+ T cells derived from 3 × HA-OGG1 mice were cultured under iTreg differentiation conditions. Proteins complex (300 μg) immunoprecipitated with HA antibody or IgG were subjected to SDS-PAGE and then in-gel protein digestion and peptides recovery in Bioprofile Co., Ltd. For details, see SI Appendix, Materials and Methods.
3.21. 13C-Tracing Assessment.
13C-tracing assessments were performed as described previously (59) with slight modifications. For details, see SI Appendix, Materials and Methods.
3.22. Whole Exome Sequence Analysis of Missense Variants in OGG1 Associated with IBD.
We used the plink format files for WES data of 469,602 UK Biobank participants (UK Biobank Field #23158). The UK Biobank analyses were conducted using the UK Biobank under application 91486 (release date: 8 Jan 2024). For details, see SI Appendix, Materials and Methods.
3.23. OGG1 S326C Mutation Analysis in Human.
DNA of peripheral blood samples from healthy human and IBD patients was sequenced in Comate Bioscience Co., Ltd. For details, see SI Appendix, Materials and Methods.
3.24. Analysis of Colitis in Mice.
T cell transfer colitis based on adoptive transfer of naïve CD4+ T cells into Rag1−/− mice, and dextran sodium sulfate (DSS)-induced colitis model was performed as described previously (49). For details, see SI Appendix, Materials and Methods.
3.25. Data Analysis and Statistics.
All experiments were done at least three times independently. Statistical analysis was performed using GraphPad Prism version 8 (GraphPad Software). For details, see SI Appendix, Materials and Methods.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
National Natural Science Foundation of China Grants 32200729 (M.T.) and 32170591 (X.B). Key Special Project for International Science and Technology Innovation Cooperation between Governments under the National Key R&D Program 2025YFE0107900 (M.W.). Department of Science and Technology of Jilin Province Grant QT202304 (M.T.). Education Department of Jilin Province Grants JJKH20231314KJ (M.T.) and JJKH20250323KJ (X.Z.). United States National Institute of Allergic and Infectious Diseases AI062885 (I.B). National Universitys Basic Research Foundation of China 2412024QD023 (M.T.).
Author contributions
M.T., M.W., and X.B. designed research; M.T., F.H., X.W., X.Z., H.W., J.L., M.X., C.L., J.H., and D.L. performed research; Y.N. contributed new reagents/analytic tools; M.T., F.H., X.W., Z.L., and Z.Z. analyzed data; Y.N., I.B., M.W., and X.B. supervised research; and M.T., F.H., Z.L., Y.N., I.B., M.W., and X.B. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. H.C. is a guest editor invited by the Editorial Board.
Contributor Information
Min Wei, Email: weim750@nenu.edu.cn.
Xueqing Ba, Email: baxq755@nenu.edu.cn.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix. CUT&RUN data have been submitted to Sequence Read Archive (SRA) under accession number PRJNA1260081 (60). RNA-Seq data have been submitted to SRA under accession number PRJNA1260152 (61).
Supporting Information
References
- 1.Kasai H., et al. , Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis 7, 1849–1851 (1986). [DOI] [PubMed] [Google Scholar]
- 2.Neeley W. L., Essigmann J. M., Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem. Res. Toxicol. 19, 491–505 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Svilar D., Goellner E. M., Almeida K. H., Sobol R. W., Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage. Antioxid. Redox Signal. 14, 2491–2507 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Audebert M., Radicella J. P., Dizdaroglu M., Effect of single mutations in the OGG1 gene found in human tumors on the substrate specificity of the Ogg1 protein. Nucleic Acids Res. 28, 2672–2678 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao W., et al. , Enzymatically inactive OGG1 binds to DNA and steers base excision repair toward gene transcription. FASEB J. 34, 7427–7441 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming A. M., Ding Y., Burrows C. J., Oxidative DNA damage is epigenetic by regulating gene transcription via base excision repair. Proc. Natl. Acad. Sci. U.S.A. 114, 2604–2609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R., Hao W., Pan L., Boldogh I., Ba X., The roles of base excision repair enzyme OGG1 in gene expression. Cell. Mol. Life Sci. 75, 3741–3750 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ba X., et al. , The role of 8-oxoguanine DNA glycosylase-1 in inflammation. Int. J. Mol. Sci. 15, 16975–16997 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan L., et al. , 8-Oxoguanine targeted by 8-oxoguanine DNA glycosylase 1 (OGG1) is central to fibrogenic gene activation upon lung injury. Nucleic Acids Res. 51, 1087–1102 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visnes T., et al. , Small-molecule inhibitor of OGG1 suppresses proinflammatory gene expression and inflammation. Science (80-) 362, 834–839 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ba X., et al. , 8-Oxoguanine DNA glycosylase-1 augments proinflammatory gene expression by facilitating the recruitment of site-specific transcription factors. J. Immunol. 192, 2384–2394 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon H., et al. , Ogg1 deficiency alters the intestinal microbiome and increases intestinal inflammation in a mouse model. PLoS One 15, 1–23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noack M., Miossec P., Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 13, 668–677 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Lee G. R., The balance of Th17 versus Treg cells in autoimmunity. Int. J. Mol. Sci. 19, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayne C. G., Williams C. B., Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm. Bowel Dis. 19, 1772–1778 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W., et al. , Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–86 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derynck R., Zhang Y. E., Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425, 577–584 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Fontenot J. D., Gavin M. A., Rudensky A. Y., Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Hori S., Nomura T., Sakaguchi S., Control of regulatory T cell development by the transcription factor Foxp3. Science (80-) 299, 1057–1061 (2003). [PubMed] [Google Scholar]
- 20.Josefowicz S. Z., Wilson C. B., Rudensky A. Y., Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J. Immunol. 182, 6648–6652 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Yang R., et al. , Hydrogen sulfide promotes Tet1- and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity 43, 251–263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue X., et al. , Control of Foxp3 stability through modulation of TET activity. J. Exp. Med. 213, 377–397 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saksida T., Jevtić B., Djedović N., Miljkovic D., Stojanović I., Redox regulation of tolerogenic dendritic cells and regulatory T cells in the pathogenesis and therapy of autoimmunity. Antioxid. Redox Signal. 34, 364–382 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Chávez M. D., Tse H. M., Targeting mitochondrial-derived reactive oxygen species in T cell-mediated autoimmune diseases. Front. Immunol. 12, 1–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Won H. Y., et al. , Ablation of peroxiredoxin II attenuates experimental colitis by increasing FoxO1-induced Foxp3+ regulatory T cells. J. Immunol. 191, 4029–4037 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Barbi J., Pardoll D., Pan F., Metabolic control of the Treg/Th17 axis. Immunol. Rev. 252, 52–77 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalek R. D., et al. , Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacIver N. J., Michalek R. D., Rathmell J. C., Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31, 259–283 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao F., et al. , Butyrate enhances CPT1A activity to promote fatty acid oxidation and iTreg differentiation. Proc. Natl. Acad. Sci. U.S.A. 118, e2014681118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian M., et al. , ACLY ubiquitination by CUL3-KLHL25 induces the reprogramming of fatty acid metabolism to facilitate iTreg differentiation. Elife 10, 1–27 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bravard A., et al. , Redox regulation of human OGG1 activity in response to cellular oxidative stress. Mol. Cell. Biol. 26, 7430–7436 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaich M. A., et al. , Single-molecule analysis of DNA-binding proteins from nuclear extracts (SMADNE). Nucleic Acids Res. 51, E39 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Kemp P. A., Charbonnier J. B., Audebert M., Boiteux S., Catalytic and DNA-binding properties of the human Ogg1 DNA N-glycosylase/AP lyase: Biochemical exploration of H270, Q315 and F319, three amino acids of the 8-oxoguanine-binding pocket. Nucleic Acids Res. 32, 570–578 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao W., et al. , Effects of the stimuli-dependent enrichment of 8-oxoguanine DNA glycosylase1 on chromatinized DNA. Redox Biol. 18, 43–53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawai K., Wata Y., Hara M., Tojo S., Majima T., Regulation of one-electron oxidation rate of guanine by base pairing with cytosine derivatives. J. Am. Chem. Soc. 124, 3586–3590 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Ming X., et al. , Mapping structurally defined guanine oxidation products along DNA duplexes: Influence of local sequence context and endogenous cytosine methylation. J. Am. Chem. Soc. 136, 4223–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polansky J. K., et al. , DNA methylation controls Foxp3 gene expression. Eur. J. Immunol. 38, 1654–1663 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Sun X., Cui Y., Feng H., Liu H., Liu X., TGF-β signaling controls Foxp3 methylation and T reg cell differentiation by modulating Uhrf1 activity. J. Exp. Med. 216, 2819–2837 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu L., et al. , Crystal structure of TET2-DNA complex: Insight into TET-mediated 5mC oxidation. Cell 155, 1545–1555 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Kikuchi A., et al. , Structural basis for activation of DNMT1. Nat. Commun. 13, 1–7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gotoh N., et al. , Association between OGG1 S326C CC genotype and elevated relapse risk in acute myeloid leukemia. Int. J. Hematol. 108, 246–253 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Bravard A., et al. , Oxidation status of human ogg1-s326c polymorphic variant determines cellular DNA repair capacity. Cancer Res. 69, 3642–3649 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Simonelli V., et al. , Genotype-phenotype analysis of S326C OGG1 polymorphism: A risk factor for oxidative pathologies. Free Radic. Biol. Med. 63, 401–409 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Han J., et al. , OGG1S326C variant frequent in human populations facilitates inflammatory responses due to its extended interaction with DNA substrate. Proc. Natl. Acad. Sci. U.S.A. 122, e2426102122 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donley N., et al. , Small molecule inhibitors of 8-oxoguanine DNA glycosylase-1 (OGG1). ACS Chem. Biol. 10, 2334–2343 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bluestone J. A., et al. , Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 7, 315ra189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong S., et al. , The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight 6, 1–18 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marek-Trzonkowska N., et al. , Administration of CD4 +CD25 highCD127 - regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 35, 1817–1820 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furusawa Y., et al. , Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Mabley J. G., et al. , Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB J. 18, 1–18 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Pan L., et al. , Oxidized guanine base lesions function in 8-oxoguanine DNA glycosylase-1-mediated epigenetic regulation of nuclear factor κB-driven gene expression. J. Biol. Chem. 291, 25553–25566 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia L., et al. , CHD4 has oncogenic functions in initiating and maintaining epigenetic suppression of multiple tumor suppressor genes. Cancer Cell 31, 653–668.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H., Franco F., Ho P. C., Metabolic regulation of Tregs in cancer: Opportunities for immunotherapy. Trends Cancer 3, 583–592 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Li J., et al. , Aiduqing formula inhibits breast cancer metastasis by suppressing TAM/CXCL1-induced Treg differentiation and infiltration. Cell Commun. Signal. 19, 1–20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou Y., et al. , H4S47 o-GlcNAcylation regulates the activation of mammalian replication origins. Nat. Struct. Mol. Biol. 30, 800–811 (2023). [DOI] [PubMed] [Google Scholar]
- 56.Miguel V., Ramos R., García-Bermejo L., Rodríguez-Puyol D., Lamas S., The program of renal fibrogenesis is controlled by microRNAs regulating oxidative metabolism. Redox Biol. 40, 101851 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Floess S., et al. , Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 5, 0169–0178 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson M. O., et al. , Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell, 1–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson M. O., et al. , Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell 175, 1780–1795.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian M., et al. , OGG1 augments the transcriptional activation of Foxp3 to promote iTreg differentiation for IBD alleviation. NCBI SRA. http://www.ncbi.nlm.nih.gov/bioproject/1260081. Deposited 8 May 2025. [DOI] [PMC free article] [PubMed]
- 61.Tian M., et al. , OGG1 augments the transcriptional activation of Foxp3 to promote iTreg differentiation for IBD alleviation. NCBI SRA. http://www.ncbi.nlm.nih.gov/bioproject/1260152. Deposited 8 May 2025. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix. CUT&RUN data have been submitted to Sequence Read Archive (SRA) under accession number PRJNA1260081 (60). RNA-Seq data have been submitted to SRA under accession number PRJNA1260152 (61).