Abstract
Background
Alcohol abuse and dependence represents a most serious health problem worldwide with major social, interpersonal and legal interpolations. Besides benzodiazepines, anticonvulsants are often used for the treatment of alcohol withdrawal symptoms. Anticonvulsants drugs are indicated for the treatment of alcohol withdrawal syndrome, alone or in combination with benzodiazepine treatments. In spite of the wide use, the exact role of the anticonvulsants for the treatment of alcohol withdrawal has not yet bee adequately assessed.
Objectives
To evaluate the effectiveness and safety of anticonvulsants in the treatment of alcohol withdrawal.
Search methods
We searched Cochrane Drugs and Alcohol Group' Register of Trials (December 2009), PubMed, EMBASE, CINAHL (1966 to December 2009), EconLIT (1969 to December 2009). Parallel searches on web sites of health technology assessment and related agencies, and their databases.
Selection criteria
Randomized controlled trials (RCTs) examining the effectiveness, safety and overall risk‐benefit of anticonvulsants in comparison with a placebo or other pharmacological treatment. All patients were included regardless of age, gender, nationality, and outpatient or inpatient therapy.
Data collection and analysis
Two authors independently screened and extracted data from studies.
Main results
Fifty‐six studies, with a total of 4076 participants, met the inclusion criteria. Comparing anticonvulsants with placebo, no statistically significant differences for the six outcomes considered.
Comparing anticonvulsant versus other drug, 19 outcomes considered, results favour anticonvulsants only in the comparison carbamazepine versus benzodiazepine (oxazepam and lorazepam) for alcohol withdrawal symptoms (CIWA‐Ar score): 3 studies, 262 participants, MD ‐1.04 (‐1.89 to ‐0.20), none of the other comparisons reached statistical significance.
Comparing different anticonvulsants no statistically significant differences in the two outcomes considered.
Comparing anticonvulsants plus other drugs versus other drugs (3 outcomes considered), results from one study, 72 participants, favour paraldehyde plus chloral hydrate versus chlordiazepoxide, for the severe‐life threatening side effects, RR 0.12 (0.03 to 0.44).
Authors' conclusions
Results of this review do not provide sufficient evidence in favour of anticonvulsants for the treatment of AWS. There are some suggestions that carbamazepine may actually be more effective in treating some aspects of alcohol withdrawal when compared to benzodiazepines, the current first‐line regimen for alcohol withdrawal syndrome. Anticonvulsants seem to have limited side effects, although adverse effects are not rigorously reported in the analysed trials.
Keywords: Humans, Alcohol Withdrawal Delirium, Alcohol Withdrawal Delirium/drug therapy, Alcohol Withdrawal Seizures, Alcohol Withdrawal Seizures/drug therapy, Anticonvulsants, Anticonvulsants/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Anticonvulsants for alcohol withdrawal syndrome
There are limited data on anticonvulsants versus placebo for alcohol withdrawal syndrome, while comparisons with other drugs show no clear differences.
This Cochrane review summarizes evidence from forty‐eight randomised controlled trials evaluating the effectiveness and safety of anticonvulsants in the treatment of alcohol withdrawal symptoms. There are limited data comparing anticonvulsants versus placebo and no clear differences between anticonvulsants and other drugs in the rates of therapeutic success. Data on safety outcomes are sparse and fragmented. There is a need for larger, well‐designed studies in this field.
Summary of findings
Summary of findings for the main comparison. Anticonvulsant versus Placebo for alcohol withdrawal.
| Anticonvulsant versus Placebo for alcohol withdrawal | ||||||
| Patient or population: patients with alcohol withdrawal Settings: Intervention: Anticonvulsant versus Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Anticonvulsant versus Placebo | |||||
| Alcohol Withdrawal Seizures post treatment ‐ Any Anticonvulsant vs. Placebo | Study population | RR 0.61 (0.31 to 1.2) | 883 (9 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 110 per 1000 | 67 per 1000 (34 to 132) | |||||
| Medium risk population | ||||||
| 167 per 1000 | 102 per 1000 (52 to 200) | |||||
| Alcohol Withdrawal Seizures post treatment ‐ Phenytoin vs. Placebo | Study population | RR 0.78 (0.35 to 1.77) | 381 (4 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 180 per 1000 | 140 per 1000 (63 to 319) | |||||
| Medium risk population | ||||||
| 176 per 1000 | 137 per 1000 (62 to 312) | |||||
| Adverse events | Study population | RR 1.56 (0.74 to 3.31) | 663 (7 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 50 per 1000 | 78 per 1000 (37 to 165) | |||||
| Medium risk population | ||||||
| 34 per 1000 | 53 per 1000 (25 to 113) | |||||
| Dropout ‐ Any Anticonvulsant vs. Placebo | Study population | RR 0.82 (0.5 to 1.34) | 344 (7 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 208 per 1000 | 171 per 1000 (104 to 279) | |||||
| Medium risk population | ||||||
| 182 per 1000 | 149 per 1000 (91 to 244) | |||||
| Dropout ‐ Chlormethiazole vs. Placebo | Study population | RR 1.05 (0.22 to 5.11) | 140 (3 studies) | ⊕⊕⊕⊝ moderate5 | ||
| 44 per 1000 | 46 per 1000 (10 to 225) | |||||
| Medium risk population | ||||||
| 21 per 1000 | 22 per 1000 (5 to 107) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealmnt adequate only in 1 on 9 included studies 2 Allcation concealment adequate in 1/4 studies 3 In 3 studies results were not estimable due no adverse events occurred 4 Allocation concealment adequate in 2 on 7 included studies 5 only 3 studies considered
Summary of findings 2. Anticonvulsant versus Other Drugs for alcohol withdrawal.
| Anticonvulsant versus Other Drugs for alcohol withdrawal | ||||||
| Patient or population: patients with alcohol withdrawal Settings: Intervention: Anticonvulsant versus Other Drugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Anticonvulsant versus Other Drugs | |||||
| Alcohol withdrawal seizures ‐ Any Anticonvulsant vs any Other | Study population | RR 0.58 (0.22 to 1.58) | 880 (12 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 27 per 1000 | 16 per 1000 (6 to 43) | |||||
| Medium risk population | ||||||
| 19 per 1000 | 11 per 1000 (4 to 30) | |||||
| Adverse events ‐ Any Anticonvulsant vs any Other | Study population | RR 0.71 (0.45 to 1.12) | 726 (14 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 287 per 1000 | 204 per 1000 (129 to 321) | |||||
| Medium risk population | ||||||
| 188 per 1000 | 133 per 1000 (85 to 211) | |||||
| Dropout ‐ Any Anticonvulsant vs any Other | Study population | RR 0.92 (0.67 to 1.26) | 1359 (20 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 114 per 1000 | 105 per 1000 (76 to 144) | |||||
| Medium risk population | ||||||
| 99 per 1000 | 91 per 1000 (66 to 125) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment adequate in 2 on 12 included studies 2 In 5 studies no events and results not estimable 3 Allocation concealment adeqate in 4/14 included studies 4 Allocation concealment adequate in 5 on 20 included studies
Background
Description of the condition
Alcohol abuse and dependence represents a most serious health problem worldwide with major social, interpersonal and legal interpolations. Dependence on alcohol is associated with both physiological symptoms such as tolerance and withdrawal, and behavioural symptoms such as impaired control over drinking (Hasin 1990). Alcohol withdrawal syndrome is a cluster of symptoms that occurs in alcohol‐dependent people after cessation or reduction in alcohol use that has been heavy or prolonged. The clinical presentation varies from mild to serious and the onset of symptoms typically occurs a few hours after the last alcohol intake. The most common manifestations are tremor, restlessness, insomnia, nightmares, paroxysmal sweats, tachycardia, fever, nausea, vomiting, seizures, hallucinations (auditory, visual, tactile), increased agitation, tremulousness and delirium. These symptoms involve a wide range of neurotransmitter circuits that are implicated in alcohol tolerance and reflect a homeostatic readjustment of the central nervous system (De Witte 2003; Koob 1997; Nutt 1999; Slawecki 1999). Long‐term alcohol consumption affects brain receptors that undergo adaptive changes in an attempt to maintain normal function. Some of the key changes involve reduced brain gamma‐amino butyric acid (GABA) levels and GABA‐ receptor sensitivity (Dodd 2000; Gilman 1996; Kohl 1998; Petty 1993) and activation of glutamate systems (Tsai 1995), which lead to nervous system hyperactivity in the absence of alcohol. The advances in knowledge of neurobiology and neurochemistry have prompted the use of drugs in the treatment of alcohol dependence and withdrawal that act through these GABA pathways.
Description of the intervention
Besides benzodiazepines, anticonvulsants are often used for the treatment of alcohol withdrawal symptoms. A meta‐analysis of studies concerning pharmacological therapies of alcohol withdrawal (Mayo‐Smith 1997) has suggested that carbamazepine, an anticonvulsant agent that is widely used in particular in Europe, may have modest beneficial effects on selected signs and symptoms of withdrawal. Carbamazepine may also be considered as adjunctive therapy to benzodiazepines, the classic treatment for alcohol withdrawal.
How the intervention might work
Anticonvulsants drugs are indicated for the treatment of alcohol withdrawal syndrome, alone or in combination with benzodiazepine treatments. In spite of the wide use, the exact role of the anticonvulsants for the treatment of alcohol withdrawal has not yet been adequately assessed. Moreover not all patients may need pharmacological treatment and it is unknown whether different anticonvulsants and different regimens of administration (e.g. symptom‐triggered versus fixed schedule) may have the same merits.
Why it is important to do this review
The need to research non‐benzodiazepines effective in Alcohol Withdrawal is related to the risks of side‐effects and the potential of abuse of benzodiazepine (Leggio 2008).
Since there are several anticonvulsant agents and a large amount of evidence of their use in the management of alcohol withdrawal has been published during the last years, an updated systematic is needed in order to clarify the exact role of anticonvulsants in the management of alcohol withdrawal.
The purpose of this systematic review is to examine the evidence in the effectiveness and safety of anticonvulsants in the treatment of alcohol withdrawal symptoms. Results of a previous version of a Cochrane Systematic review (Polycarpou 2005) on anticonvulsants efficacy and safety are not conclusive. New trials have been published and the review needs update.
This review has a parallel one on benzodiazepines for alcohol withdrawal (Amato 2010) and together they are part of a series of reviews and protocols on the efficacy of pharmacological treatment (Acamprosate GHB, nitrous oxide, magnesium) for alcohol withdrawal (Gillman 2007; Leone 2010; Fox 2003; Tejani 2010).
Objectives
The objectives of this systematic review are:
To evaluate the effectiveness of anticonvulsants in the treatment of alcohol withdrawal.
To evaluate the safety of anticonvulsants in the treatment of the alcohol withdrawal symptoms (AWS).
Methods
Criteria for considering studies for this review
Types of studies
Randomized Controlled Trials (RCT) and Controlled Clinical Trials (CCT) evaluating the efficacy, safety and overall risk‐benefit of anticonvulsants for the treatment of alcohol withdrawal.
Types of participants
Alcohol dependent patients diagnosed in accordance with appropriate standardized criteria (e.g., criteria of Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐R) or ICD) who experienced alcohol withdrawal symptoms regardless of the severity of the withdrawal manifestations. All patients were included regardless of age, gender, nationality, and outpatient or inpatient therapy. The history of previous treatments was considered, but it was not an eligibility criterion.
Types of interventions
‐ Experimental intervention
Anticonvulsants drugs alone or in combination with other drugs
‐ Control Intervention
Placebo; Other pharmacological interventions
Types of comparisons
anticonvulsant versus placebo;
anticonvulsant versus other drugs;
different anticonvulsants between themselves;
anticonvulsant combined with other drugs versus other drugs.
anticonvulsant 1 combined with other drugs versus anticonvulsant 2
Types of outcome measures
Primary outcomes
Efficacy outcomes
Alcohol withdrawal seizures as number of subjects experiencing seizures
Alcohol withdrawal delirium as number of subjects experiencing delirium
Alcohol withdrawal symptoms as measured by prespecified scales(as the CIWA‐Ar score)
Global improvement of overall alcohol withdrawal syndrome as measured in pre‐specified scales ( as number of patients with global improvement, global doctors assessment of efficacy, Patients assessment of efficacy)
Craving as measured by prespecified scales
Safety outcomes
Adverse events as number of subjects experiencing at least one adverse event
Severe, life‐threatening adverse events as measured by number of subjects experiencing severe, life threatening adverse events
Acceptability outcomes
Dropout
Dropout due to adverse events
Secondary outcomes
Additional medication needed
Length of stay in intensive therapy
Mortality
Quality of life
Search methods for identification of studies
Electronic searches
Relevant trials were obtained from the following sources:
Cochrane Drugs and Alcohol Group' Register of Trials (December 2009)
PubMed (January 1966– December 2009)
EMBASE (January 1988– December 2009)
CINAHL (January 1982– December 2009)
EconLIT (1969 to December 2009)
We compiled detailed search strategies for each database searched, for detail see Appendix 1; Appendix 2; Appendix 3; Appendix 4
Searching other resources
We also searched:
the reference lists of all relevant papers to identify further studies.
conference proceedings likely to contain trials relevant to the review.
We contacted investigators seeking information about unpublished or incomplete trials.All searches included non‐English language literature and studies with English abstracts were assessed for inclusion. When considered likely to meet inclusion criteria, studies were translated.
Data collection and analysis
Selection of studies
Two authors independently screened the titles and abstracts of all publications, obtained through the search strategy. All potentially eligible studies were obtained as full articles and two authors independently assessed these for inclusion. In doubtful or controversial cases, all identified discrepancies were discussed and reached consensus on all items.
Data extraction and management
Two authors independently extracted data from published sources, where differences in data extracted occurred this was resolved through discussion. Where required additional information was obtained through collaboration with the original authors.
Assessment of risk of bias in included studies
The risk of bias assessment for RCTs and CCTs in this review was performed using four out of the six criteria recommended by the Cochrane Handbbok (Higgins 2008). The recommended approach for assessing risk of bias in studies included in Cochrane Review is a two‐part tool, addressing four specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data). The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry. This is achieved by answering a pre‐specified question about the adequacy of the study in relation to the entry, such that a judgement of "Yes" indicates low risk of bias, "No" indicates high risk of bias, and "Unclear" indicates unclear or unknown risk of bias. To make these judgments we will use the criteria indicated by the handbook adapted to the addiction field. See Appendix 5 for details.
The domains of sequence generation and allocation concealment (avoidance of selection bias) will be addressed in the tool by a single entry for each study.
Blinding of participants, personnel and outcome assessor (avoidance of performance bias and detection bias) was considered separately for objective outcomes (e.g. drop out, drop out due to adverse events, seizures, delirium, adverse events) and subjective outcomes (e.g. duration and severity of signs and symptoms of withdrawal, craving, psychiatric symptoms; improvements assessed by doctors and patients).
Incomplete outcome data (avoidance of attrition bias) was considered for all outcomes except for the drop out from the treatment, which is very often the primary outcome measure in trials on addiction.
Measures of treatment effect
Dichotomous outcomes were analysed calculating the Relative risk (RR) for each trial with the uncertainty in each result being expressed by their confidence intervals. Continuous outcomes were analysed calculating the MD or the SMD with 95%CI. In case of missing standard deviation of the differences from baseline to the end of treatment, the standard deviation were imputed using the standard deviation of the mean at the end of treatment for each group.
Assessment of heterogeneity
Statistically significant heterogeneity among primary outcome studies will be assessed with Chi‐squared (Q) test and I‐squared (Higgins 2008). A significant Q ( P<.05) and I‐squared of at least 50% will be considered as statistical heterogeneity
Assessment of reporting biases
Funnel plot (plot of the effect estimate from each study against the sample size or effect standard error) was not used to assess the potential for bias related to the size of the trials, because all the included studies had small sample size and not statistically significant results.
Data synthesis
The outcomes from the individual trials have been combined through meta‐analysis where possible (comparability of intervention and outcomes between trials) using a fixed effect model unless there was significant heterogeneity, in which case a random effect model have been used.
If all arms in a multi‐arm trial are to be included in the meta‐analysis and one treatment arm is to be included in more than one of the treatment comparisons, then we divided the number of events and the number of participants in that arm by the number of treatment comparisons made. This method avoid the multiple use of participants in the pooled estimate of treatment effect while retaining information from each arm of the trial. It compromise the precision of the pooled estimate slightly.
Sensitivity analysis
To assess the effect of methodological quality on the results, we first performed a graphical inspection of any effect sorting the results on the forest plots according to risk of bias for sequence generation, allocation concealment, blinding (subjective outcomes) ; if we found a difference in the results between studies at low, unclear, high risk of bias, we performed a sensitivity analysis excluding studies at high risk of bias
Results
Description of studies
Results of the search
We identified 993 reports from all electronic databases searched excluding duplicate, 899 were excluded on basis of title and abstract; 91 articles were retrieved in full text for more detailed evaluation, 35 of which were excluded, 56 satisfied all the criteria to be included in the review. Three studies are awaiting assessment because we are trying to find the full text. SeeFigure 1 to see the Flow chart showing the identification of included trials.
1.

Flow chart showing identification of studies
Included studies
56 studies met the inclusion criteria, with a total of 4076 participants. For a description of the characteristics of the included studies, see Characteristics of included studies table
Country of origin of the included studies
33 studies were conducted in Europe, 18 in North America, 4 in Australia/New Zealand, and one in Asia.
Number of studies per type of comparison
Anticonvulsant versus placebo (No. = 17 studies, 17 comparisons) (Alldredge 1989; Bjorkqvist 1976; Blanchard 1985; Bonnet 2003; Burroughs 1985a; Chance 1991; Gann 2004; Glatt 1966; Golbert 1967; Koethe 2007; Krupitsky 2007; Lambie 1980; Murphy 1983; Rathlev 1994; Reoux 2001; Sampliner 1974; Stanhope 1989)
Anticonvulsant versus other drug (No. = 32 studies, 36 comparisons) (Agricola 1982; Borg 1986; Borg 1986; Burroughs 1985a; Burroughs 1985a; Burroughs 1985b; Burroughs 1985b; Choi 2005; Dencker 1978; Elsing 1996; Elsing 2009; Golbert 1967; Kaim 1972; Kaim 1972; Kalyoncu 1996; Koppi 1987; Kramp 1978; Krupitsky 2007; Lapierre 1983; Longo 2002; Lucht 2003; Madden 1969; Malcolm 1989; Malcolm 2002; Malcolm 2007; Manhem 1985; McGrath 1975; Murphy 1983; Nimmerichter 2002; Radouco‐Thomas 1989; Robinson 1989; Santo 1985; Stuppaeck 1992; Stuppaeck 1998; Thompson 1975; Tubridy 1988)
Different anticonvulsants between themselves (No.= 10 studies, 11 comparisons) (Flygering 1984; Golbert 1967; Kaim 1972; Krupitsky 2007; Krupitsky 2007; Mariani 2006; Ritola 1981; Rosenthal 1998; Schik 2005; Seifert 2004; Teijeiro 1975)
Anticonvulsant combined with other drug versus other drug (No. = 6 studies, 7 comparisons) (Balldin 1986; Golbert 1967; Lucht 2003; Myrick 2000; Spies 1996; Spies 1996; Rothstein 1973)
Anticonvulanst combined with other drug vs different anticonvulsant (No=1 study) (Croissant 2009)
For a more detailed information about the comparisons considered in the studies, see Additional Table 3; Table 4; Table 5; Table 6; Table 7
1. Comparisons Anticonvulsants versus placebo.
| Author | Treatment (Anticonvulsant) | Control |
| Alldredge 1989 | Phenytoin | Placebo |
| Bjorkvist 1976 | Carbamazepine (34) | Placebo |
| Blanchard 1985 | Phenobarbital | Placebo |
| Bonnet 2003 | Gabapentin | Placebo |
| Burroughs 1985 a | Chlormethiazole | Placebo |
| Chance 1991 | Phenytoin | Placebo |
| Gann 2004 | Chlormethiazole | Placebo |
| Glatt 1966 | Chlormethiazole | Placebo |
| Golbert 1967 | Promazine | Placebo |
| Koethe 2007 | Oxcarbazepine | Placebo |
| Krupitsky 2007 | Topiramate | Placebo |
| Lambie 1980 | Valproate | Placebo |
| Murphy 1983 | Chlormethiazole | Placebo |
| Rathlev 1994 | Phenytoin | Placebo |
| Reoux 2001 | Divalproex | Placebo |
| Sampliner 1974 | Phenytoin | Placebo |
| Stanhope 1989 | Carbamazepine | Placebo |
2. Comparisons Anticonvulsants versus Other.
| Author | Treatment (Anticonvulsant) | Control (Other) |
| Borg 1986 | Amobarbital | Oxazepam (benzodiazepine) |
| Borg 1986 | Amobarbital | Melperone (antipsychotic) |
| Kramp 1978 | Barbital | Diazepam (benzodiazepine) |
| Agricola 1982 | Carbamazepine | Tiapride (antipsychotic) |
| Kalyoncu 1996 | Carbamazepine | Diazepam (benzodiazepine) |
| Malcom 1989 | Carbamazepine | Oxazepam (benzodiazepine) |
| Malcom 2002 | Carbamazepine | Lorazepam (benzodiazepine) |
| Stuppaeck 1992 | Carbamazepine | Oxazepam (benzodiazepine) |
| Burroughs 1985 a | Chlormethiazole | Bromocriptine (dopamine agonist) |
| Burroughs 1985 a | Chlormethiazole | Chlordiazepoxide (benzodiazepine) |
| Burroughs 1985 b | Chlormethiazole | Bromocriptine (dopamine agonist) |
| Burroughs 1985 b | Chlormethiazole | Chlordiazepoxide (benzodiazepine) |
| Dencker 1978 | Chlormethiazole | Piracetam (CNS stimulant) |
| Elsing 1996 | Chlormethiazole | GHB (gamma‐Hydroxybutyric acid) |
| Elsing 2009 | Chlormethiazole | GHB (gamma‐Hydroxybutyric acid) |
| Lapierre 1983 | Chlormethiazole | Chlordiazepoxide (benzodiazepine) |
| Lucht 2003 | Chlormethiazole | Diazepam (benzodiazepine) |
| Madden 1969 | Chlormethiazole | Trifluoperazine (antipsychotic) |
| Manhem 1985 | Chlormethiazole | Clonidine (adrenergic agonist) |
| McGrath 1975 | Chlormethiazole | Chlordiazepoxide (benzodiazepine) |
| Murphy 1983 | Chlormethiazole | Tiapride (antipsychotic) |
| Nimmerichter 2002 | Chlormethiazole | GHB (gamma‐Hydroxybutyric acid) |
| Robinson 1989 | Chlormethiazole | Clonidine (adrenergic agonist) |
| Tubridy 1988 | Chlormethiazole | Alprazolam (benzodiazepine) |
| Longo 2002 | Depakote | Chlordiazepoxide or Loranzepam (benzodiazepine) |
| Malcom 2007 | Gabapentin | Lorazepam (benzodiazepine) |
| Koppi 1987 | Meprobamate | Caroverine (spasmolytic) |
| Kaim 1972 | Paraldehyde | Perhenazine (antipsychotic) |
| Kaim 1972 | Paraldehyde | Chlordiazepoxide (benzodiazepine) |
| Thompson 1975 | Paraldehyde | Diazepam (benzodiazepine) |
| Golbert 1967 a | Promazine | Chlordiazepoxide (benzodiazepine) |
| Raduco‐Thomas 1989 | Tetrabamate | Chlordiazepoxide (benzodiazepine) |
| Santo 1985 | Tetrabamate | Tiapride (Antipsychotic) |
| Choi 2005 | Topiramate | Lorazepam (benzodiazepine) |
| Krupitsky 2007 | Topiramate | Diazepam (benzodiazepine) |
| Author | Treatment (Anticonvulsant) | Control |
| Stuppaeck 1998 | Vigabatrin | Oxazepam (benzodiazepine) |
3. Comparisons Anticonvulsant 1 versus Anticonvulsant 2.
| Author | Treatment (Anticonvulsant 1) | Control (Anticonvulsant 2) |
| Flygering 1984 | Carbamazepine | Barbital |
| Scik 2005 | Carbamazepine | Oxcarbazepine |
| Mariani 2006 | Chlormethiazole | Phenobarbital |
| Ritola 1981 | Chlormethiazole | Carbamazepine |
| Seifert 2004 | Chlormethiazole | Carbamazepine |
| Teijeiro 1975 | Heminiurine | Phenobarbital+ Ferbamate (tranquillizes) |
| Kaim 1972 | Paraldehyde | Pentobarbital |
| Golbert 1967 b | Promazine | Paraldehyde and Chloral hydrate (sedative) |
| Krupitsky 2007 | Topiramate | Memantine |
| Krupitsky 2007 | Topiramate | Lamotrigine |
| Rosenthal 1998 | Valproate | Phenobarbital |
4. Anticonvulsant + Other versus Other.
| Author | Treatment (Anticonvulsant+Other) | Control |
| Balldin 1986 | Carbamazepine +Chlorprothixen (antipsychotic) | Clonidine (adrenergic agonist) |
| Lucht 2003 | Carbamazepine+Tiapride (antipsychotic) | Diazepam (benzodiazepine) |
| Spies 1996 | Chlormethiazole+Haloperidol (antipsychotic) | Clonidine (adrenergic agonist)+fFunitrazepam (benzodiazepine) |
| Spies 1996 | Chlormethiazole+Haloperidol (antipsychotic) | Funitrazepam (benzodiazepine)+ Haloperidol (antipsychotic) |
| Rothstein 1973 | Diphenylhydantoin | Tiamine (antipsychotic)+ Chlordiazepoxide (benzodiazepine) |
| Myrick 2000 | Divalproex+Lorazepam (benzodiazepine) | Lorazepam (benzodiazepine) |
| Golbert 1967 | Paraldehyde and Chloral hydrate (sedative) | Chlordiazepoxide (benzodiazepine) |
5. Comparison Anticonvulsant 1 + Other versus Anticonvulsant 2.
| Author | Treatment (Anticonvulsant 1+other) | Control (Anticonvulsant 2) |
| Croissant 2009 | Oxcarbazepine + Tiapride (antipsychotic) | Chlormethiazole |
Excluded studies
35 studies did not meet the criteria for inclusion in this review. The grounds for exclusion were: type of intervention not in the inclusion criteria: 13 studies; study design not in the inclusion criteria: 12 studies; type of outcomes measures not in the inclusion criteria: 6 studies; duplicate publication: 2 studies, outcome measures presented in a way not suitable for meta‐analysis: 2 studies. SeeExcluded studies Table
Risk of bias in included studies
All the studies were randomised controlled trials.
Allocation
The sequence generation was adequate in 17 studies, unclear in 34 and inadequate in 5 studies; the allocation concealment was adequate in 9 studies, unclear in 42 and inadequate in 5 studies
Blinding
Blinding for subjective outcomes was adequate in 33 studies, it was unclear in 10 and inadequate in 13
Blinding for objective outcomes was adequate in 52 studies and unclear in 4 studies
Incomplete outcome data
Incomplete outcome data were addressed in 48 studies, it was unclear in 6 studies and were not addressed in 2 studies.
See Included studies Table and Figure 2 and Figure 3 for a more detailed description of risk of bias across the studies.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
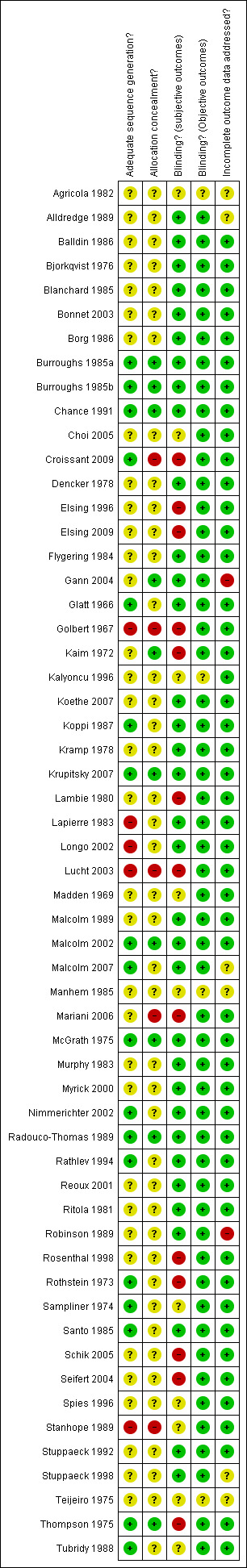
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
With a graphical inspection of the forest plots sorting studies according to the risk of bias, we didn't find any systematic difference in the results between studies at high risk of bias and studies at low or unclear risk of bias. So sensitivity analysis excluding studies at high risk of bias was not performed
Effects of interventions
We only performed meta‐analysis for the studies that had directly comparable interventions and used exactly the same rating scales for continuous outcome measures or had the same binary outcomes. The rest of the data retrieved from the studies (single comparison data) were not synthesized quantitatively. The following results refer to the cases where quantitative synthesis was performed.
The Results are split into four sections referring to the four main comparisons:
Anticonvulsant versus Placebo,
Anticonvulsant versus Other Drug,
Anticonvulsant 1 versus Anticonvulsant 2,
(Anticonvulsant + Other drug) versus Other Drug.
(Anticonvlusant 1 + other drug) versus Anticonvulsant 2
The outcomes are categorized as primary efficacy outcomes and secondary efficacy outcomes, according to the protocol. We dived them according to efficacy, safety and acceptability.
We decided to not consider separately the comparison between anticonvulsants and benzodiazepines because this is due in the parallel review (Amato 2010) on benzodiazepines for alcohol withdrawal
For a summary of results of some important outcomes see Summary of findings table 1 and Summary of findings table 2
Comparison 1 Anticonvulsant versus placebo:
Efficacy
1.1 Alcohol withdrawal seizures
1.1.1 Any Anticonvulsants versus placebo, 9 studies (Alldredge 1989; Bonnet 2003; Chance 1991; Koethe 2007; Lambie 1980; Murphy 1983; Rathlev 1994; Sampliner 1974; Stanhope 1989), 883 participants, RR 0.61 (0.31 to 1.20), the result is not statistically significant, see Analysis 1.1 or Figure 4
1.1. Analysis.

Comparison 1 Anticonvulsant versus Placebo, Outcome 1 Alcohol Withdrawal Seizures post treatment.
4.

Forest plot of comparison: 1 Anticonvulsant versus Placebo, outcome: 1.1 Alcohol Withdrawal Seizures post treatment.
1.1.2 Phenytoin versus placebo, 4 studies, (Alldredge 1989; Chance 1991; Rathlev 1994; Sampliner 1974), 381 participants, RR 0.78 (0.35 to 1.77), the result is not statistically significant, see Analysis 1.1 or Figure 4
Safety
1.2 Adverse events as number of participants with at least one adverse event
7 studies (Bjorkqvist 1976; Bonnet 2003; Burroughs 1985b; Chance 1991; Krupitsky 2007; Lambie 1980; Stanhope 1989), 516 participants, RR 1.56 (0.74, 3.31), the result is not statistically significant, see Analysis 1.2 or Figure 5
1.2. Analysis.

Comparison 1 Anticonvulsant versus Placebo, Outcome 2 Adverse events.
5.
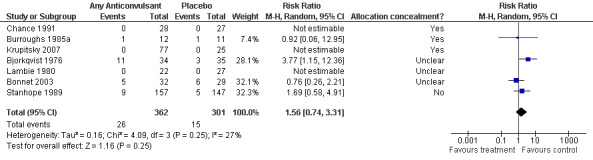
Forest plot of comparison: 1 Anticonvulsant versus Placebo, outcome: 1.4 Adverse events (N. of patients with at least one adverse event).
Acceptability
1.3 Dropout
1.5.1 Any Anticonvulsants versus placebo , 7 studies (Bjorkqvist 1976; Blanchard 1985; Bonnet 2003; Burroughs 1985a; Gann 2004; Glatt 1966; Reoux 2001), 344 participants, RR 0.82 (0.50 to 1.34), the result is not statistically significant, see Analysis 1.3 or Figure 6
1.3. Analysis.

Comparison 1 Anticonvulsant versus Placebo, Outcome 3 Dropout.
6.

Forest plot of comparison: 1 Anticonvulsant versus Placebo, outcome: 1.5 Dropout.
1.1.2 Chlormethiazole versus placebo , 3 studies (Burroughs 1985a; Gann 2004; Glatt 1966), 140 participants, RR 1.05 (0.22 to 5.11), the result is not statistically significant, see Analysis 1.3 or Figure 6
1.4 Dropout due to adverse events
8 studies (Bjorkqvist 1976; Blanchard 1985; Bonnet 2003; Burroughs 1985a; Chance 1991; Koethe 2007; Lambie 1980; Stanhope 1989), 623 participants, 0.67 (0.13, 3.36), the result is not statistically significant, see Analysis 1.4 or Figure 7
1.4. Analysis.

Comparison 1 Anticonvulsant versus Placebo, Outcome 4 Dropout due to adverse events.
7.

Forest plot of comparison: 1 Anticonvulsant versus Placebo, outcome: 1.6 Dropout due to adverse events
Comparison 2 Anticonvulsant versus Other:
Efficacy
2.1 Alcohol withdrawal seizures
2.1.1 Any Anticonvulsants versus any Other , 12 studies (Agricola 1982; Borg 1986; Kaim 1972; Koppi 1987; Kramp 1978; Lucht 2003; Murphy 1983; Nimmerichter 2002;Radouco‐Thomas 1989 ;Robinson 1989; Stuppaeck 1992; Tubridy 1988), 880 participants, RR 0.58 (0.22 to 1.58), the result is not statistically significant, see Analysis 2.1 or Figure 8
2.1. Analysis.

Comparison 2 Anticonvulsant versus Other Drugs, Outcome 1 Alcohol withdrawal seizures.
8.
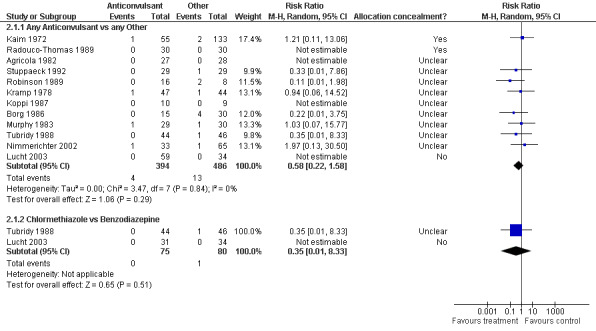
Forest plot of comparison: 2 Anticonvulsant versus Other, outcome: 2.1 Alcohol Withdrawal Seizures
2.1.2 Chlormethiazole versus Benzodiazepine, 2 studies (Lucht 2003; Tubridy 1988), 155 participants, RR 0.35 (0.01 to 8.33), the result is not statistically significant, see or Figure 8
2.2 Alcohol withdrawal delirium
2.2.1 Any Anticonvulsants versus any Other , 6 studies (Kalyoncu 1996; Lucht 2003; Manhem 1985;McGrath 1975; Murphy 1983; Stuppaeck 1992; ), 394 participants, RR 0.88 (0.23, 3.42), the result is not statistically significant, see Analysis 2.2 or Figure 9
2.2. Analysis.

Comparison 2 Anticonvulsant versus Other Drugs, Outcome 2 Alcohol withdrawal delirium.
9.
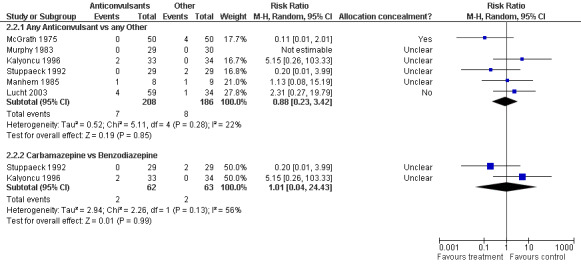
Forest plot of comparison: 2 Anticonvulsant versus Other, outcome: 2.2 Alcohol Withdrawal Delirium
2.2.2 Carbamazepine versus Benzodiazepine, 2 studies (Kalyoncu 1996; Stuppaeck 1992), 125 participants, RR 1.01 (0.04 to 24.43), the result is not statistically significant, see Analysis 2.2 or Figure 9
2.3 CIWA‐Ar score at 48 hours
2.3.1 Any Anticonvulsants versus any Other , 4 studies (Malcolm 1989; Malcolm 2002; Nimmerichter 2002; Stuppaeck 1992), 358 participants, MD ‐0.21 (‐0.95 to 0.53), the result is not statistically significant, see Analysis 2.3
2.3. Analysis.
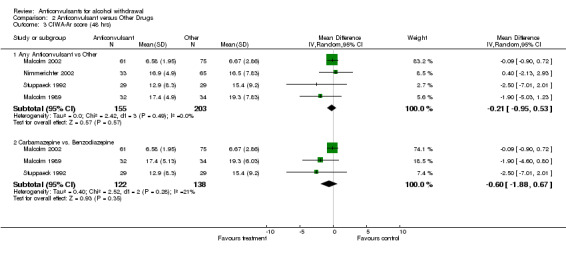
Comparison 2 Anticonvulsant versus Other Drugs, Outcome 3 CIWA‐Ar score (48 hrs).
2.3.2 Carbamazepine versus Benzodiazepine, 3 studies (Malcolm 1989; Malcolm 2002; Stuppaeck 1992), 260 participants MD ‐0.60 (‐1.88 to 0.67), the result is not statistically significant, see Analysis 2.3
2.4 CIWA‐Ar score at the end of treatment
2.4.1 Any Anticonvulsants versus any Other , 4 studies (Malcolm 1989; Malcolm 2002; Nimmerichter 2002; Stuppaeck 1992), 358 participants, MD ‐0.73 (‐1.76 to 0.31), the result is not statistically significant, see Analysis 2.4
2.4. Analysis.

Comparison 2 Anticonvulsant versus Other Drugs, Outcome 4 CIWA‐Ar score (end of treatment).
2.4.2 Carbamazepine versus Benzodiazepine , 3 studies (Malcolm 1989; Malcolm 2002; Stuppaeck 1992), 260 participants MD ‐1.04 (‐1.89 to ‐0.20), the result is statistically significant in favour of carbamazepine, see Analysis 2.4
2.5 Global Doctor’s Assessment of Efficacy
2 studies (Kramp 1978; Tubridy 1988), 181 participants, RR 0.97 (0.88 to 1.08), the result is not statistically significant, see Analysis 2.5
2.5. Analysis.

Comparison 2 Anticonvulsant versus Other Drugs, Outcome 5 Global Doctor's Assessment of Efficacy.
Safety
2.6 Adverse events as number of participants with at least one adverse event
2.6.1 Any Anticonvulsants versus any Other (Agricola 1982;Burroughs 1985a; Burroughs 1985b;Elsing 2009; Krupitsky 2007; Lapierre 1983; Longo 2002; Lucht 2003; Nimmerichter 2002; Radouco‐Thomas 1989 ;Robinson 1989; Santo 1985; Stuppaeck 1992; Tubridy 1988), 14 studies, 726 participants, RR 0.71(0.45 to 1.12), the result is not statistically significant, see Analysis 2.6 or Figure 10
2.6. Analysis.

Comparison 2 Anticonvulsant versus Other Drugs, Outcome 6 Adverse events.
10.

Forest plot of comparison: 2 Anticonvulsant versus Other, outcome: 2.14 Adverse events (number of patient with at least one adverse event)
2.6.2 Chlormethiazole versus Benzodiazepine , (Burroughs 1985a; Burroughs 1985b; Lapierre 1983; Lucht 2003; Tubridy 1988), 5 studies, 235 participants, RR 0.75 (0.35 to 1.59), the result is not statistically significant, see Analysis 2.6 or Figure 10
2.7 Severe, life‐treating adverse events
2.17.1 Any Anticonvulsants versus any Other , 12 studies (Agricola 1982; Burroughs 1985a; Burroughs 1985b; Dencker 1978;Elsing 2009; Koppi 1987; Krupitsky 2007; Lapierre 1983; Radouco‐Thomas 1989; Santo 1985; Thompson 1975; Tubridy 1988), 578 participants, RR 2.38 (0.33 to 17.24), the result is not statistically significant, see Analysis 2.7 or Figure 11
2.7. Analysis.

Comparison 2 Anticonvulsant versus Other Drugs, Outcome 7 Severe, life‐treatening adverse events.
11.

Forest plot of comparison: 2 Anticonvulsant versus Other, outcome: 2.15 Severe, life‐treating adverse events
2.7.2 Chlormethiazole versus Benzodiazepine , (Burroughs 1985a; Burroughs 1985b; Lapierre 1983; Tubridy 1988), 4 studies, 170 participants, RR 0.69 (95% CI, 0.09 to 5.33), the result is not statistically significant, see Analysis 2.7 or Figure 11
Acceptability
2.8 Dropout
2.8.1 Any Anticonvulsants versus any Other , 20 studies (Agricola 1982; Borg 1986; Burroughs 1985a; Burroughs 1985b; Dencker 1978; Elsing 2009; Kaim 1972; Kalyoncu 1996; Koppi 1987; Kramp 1978; Lucht 2003; Manhem 1985;McGrath 1975; Murphy 1983; Nimmerichter 2002; Radouco‐Thomas 1989 ;Robinson 1989; Santo 1985; Stuppaeck 1992; Tubridy 1988), 1359 participants, RR 0.92 (0.67, 1.26), the result is not statistically significant, see Analysis 2.8 or Figure 12
2.8. Analysis.

Comparison 2 Anticonvulsant versus Other Drugs, Outcome 8 Dropout.
12.

Forest plot of comparison: 2 Anticonvulsant versus Other, outcome: 2.18 Dropout.
2.8.2 Chlormethiazole versus Benzodiazepine, 5 studies (Burroughs 1985a; Burroughs 1985b; Lucht 2003;McGrath 1975; Tubridy 1988), 311 participants, RR 0.68 (0.37, 1.24), the result is not statistically significant, see Analysis 2.8 or Figure 12
2.9 Dropout due to adverse events
2.9.1 Any Anticonvulsants versus any Other , 10 studies (Agricola 1982; Burroughs 1985a; Burroughs 1985b; Dencker 1978; Elsing 2009; Kaim 1972; Koppi 1987; Lapierre 1983; Stuppaeck 1992; Tubridy 1988), 596 participants, RR 2.28 (0.67 to 7.70), the result is not statistically significant, see Analysis 2.9 or Figure 13
2.9. Analysis.

Comparison 2 Anticonvulsant versus Other Drugs, Outcome 9 Dropout due to adverse events.
13.
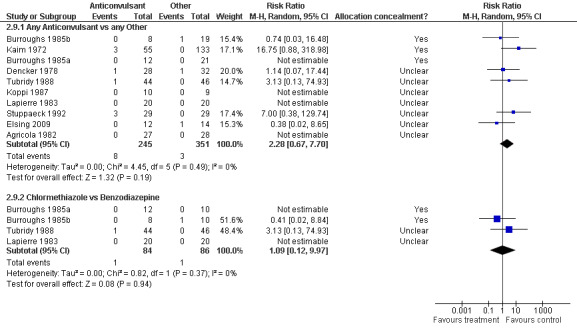
Forest plot of comparison: 2 Anticonvulsant versus Other, outcome: 2.19 Dropout due to adverse events.
2.9.2 Chlormethiazole versus Benzodiazepine, 4 studies (Burroughs 1985a; Burroughs 1985b; Lapierre 1983; Tubridy 1988), 170 participants, RR 1.09 (0.12 to 9.97), the result is not statistically significant, see Analysis 2.9 or Figure 13
Comparison 3 Anticonvulsant 1 versus Anticonvulsant 2:
Safety
3.1 Adverse events as number of participants with at least one adverse event
3.1.1 Carbamazepine versus Chlormethiazole , 2 studies (Lucht 2003; Ritola 1981), 121 participants, RR 3.12 (0.50 to 19.27), the result is not statistically significant
3.1.2 Carbamazepine versus Barbital , 1 study (Flygering 1984), 61 participants, RR 1.81 (0.70 to 4.68), the result is not statistically significant
3.1.3 Carbamazepine versus Oxcarbazepine , 1 study (Schik 2005), 29 participants, the result is not estimable because there were no side effects in both groups
3.1.4 Chlormethiazole versus Pentobarbital , 1 study (Mariani 2006), 27 participants, RR 2.80 (0.12 to 63.20), the result is not statistically significant
See Analysis 3.1 or Figure 14
3.1. Analysis.

Comparison 3 Anticonvulsant 1 versus Anticonvulsant 2, Outcome 1 Adverse events.
14.

Forest plot of comparison: 3 Anticonvulsant 1 versus Anticonvulsant 2, outcome: 3.1 Adverse events (number of patients with at least one adverse event).
Acceptability
3.2 Dropout
3.2.1 Carbamazepine versus Chlormethiazole , 2 studies (Lucht 2003; Ritola 1981), 121 participants, RR 0.51 (0.08 to 3.11), the result is not statistically significant
3.2.2 Carbamazepine versus Barbital , 1 study (Flygering 1984), 60 participants, RR 0.94 (0.34 to 2.57), the result is not statistically significant
3.2.3 Carbamazepine versus Oxcarbazepine , 1 study (Schik 2005), 29 participants, RR 3.20 (0.14 to 72.62), the result is not statistically significant
3.2.4 Pentobarbital versus Paraldehyde , 1 study (Kaim 1972), 96 participants, RR 0.37 (0.03 to 3.97), the result is not statistically significant
3.1.4 Chlormethiazole versus Pentobarbital , 1 study (Mariani 2006), 27 participants, RR 1.39 (0.28 to 7.05), the result is not statistically significant
See Analysis 3.2 or Figure 15
3.2. Analysis.

Comparison 3 Anticonvulsant 1 versus Anticonvulsant 2, Outcome 2 Dropout.
15.

Forest plot of comparison: 3 Anticonvulsant 1 versus Anticonvulsant 2, outcome: 3.2 Dropout.
Comparison 4 (Anticonvulsant + Other drug) versus Other Drug:
Efficacy
4.1Alcohol withdrawal delirium
3 studies (Golbert 1967; Lucht 2003; Rothstein 1973), 311 participants, RR 0.79 (0.18 to 3.52), the result is not statistically significant, see Analysis 4.1 or Figure 16
4.1. Analysis.

Comparison 4 (Anticonvulsant + Other) versus Other, Outcome 1 Alcohol withdrawal delirium.
16.

Forest plot of comparison: 4 (Anticonvulsant + Other) versus Other, outcome: 4.1 Alcohol Withdrawal Delirium post treatment.
Safety
4.2 Severe, life‐threatening adverse events
1 study, (Golbert 1967; ), 49 participants, RR 0.13 (0.02 to 0.89), the result is statistically significant in favour of the association Paraldehyde+ chloral hydrate in respect of chlordiazepoxide, see Analysis 4.2
4.2. Analysis.

Comparison 4 (Anticonvulsant + Other) versus Other, Outcome 2 Severe, life‐threatening adverse events.
Acceptability
4.3 Dropout
Three studies (Golbert 1967; Lucht 2003; Spies 1996), 267 participants, RR 0.56 (0.18 to 1.72), the result is not statistically significant, see Analysis 4.3 or Figure 17
4.3. Analysis.

Comparison 4 (Anticonvulsant + Other) versus Other, Outcome 3 Dropout.
17.

Forest plot of comparison: 4 (Anticonvulsant + Other) versus Other, outcome: 4.3 Dropout.
Comparison 5 (Anticonvulsant 1 + Other drug) versus Anticonvulsant 2
Efficacy
1 study( Croissant 2009), 56 participants, compared Oxcarbaxepine plus Tiapride versus Chlormethiazole. No alcohol withdrawal seizures or delirium happened in both groups. See Analysis 5.1 and Analysis 5.2
5.1. Analysis.

Comparison 5 Anticonvulsant1 + other vs anticonvulsant 2, Outcome 1 alcohol withdrawal seizures.
5.2. Analysis.

Comparison 5 Anticonvulsant1 + other vs anticonvulsant 2, Outcome 2 alcohol withdrawal delirium.
Safety
5.3. Adverse events
1 study (Croissant 2009) 56 participants, compared Oxcarbaxepine plus Tiapride versus Chlormethiazole, RR 0.68 (0.45 to 1.04) , the result is not statistically significant, see Analysis 5.3
5.3. Analysis.
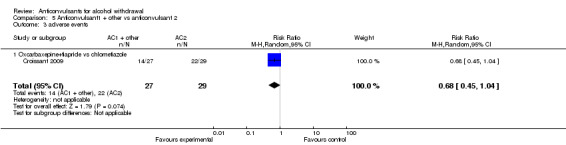
Comparison 5 Anticonvulsant1 + other vs anticonvulsant 2, Outcome 3 adverse events.
5.4Severe, life‐threatening adverse events
1 study ( Croissant 2009), 56 participants, compared Oxcarbaxepine plus Tiapride versus Chlormethiazole. No severe adverse events happened in both groups.Analysis 5.4
5.4. Analysis.

Comparison 5 Anticonvulsant1 + other vs anticonvulsant 2, Outcome 4 severe, life threatening adverse events.
Acceptability
5.5 Drop out
1 study (Croissant 2009) 56 participants, compared Oxcarbaxepine plus Tiapride versus Chlormethiazole, RR 5.36 (0.27 to 106.78) the result is not statistically significant. See Analysis 5.5
5.5. Analysis.
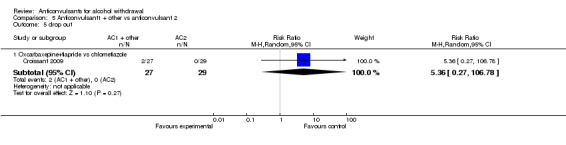
Comparison 5 Anticonvulsant1 + other vs anticonvulsant 2, Outcome 5 drop out.
Discussion
Summary of main results
Fifty‐six studies, with a total of 4076 participants, met the inclusion criteria for this review. However, despite the considerable number of randomised controlled trials, the large variety of outcomes and rating scales considerably limited a quantitative synthesis of data. A large chunk of information could not be synthesized.
‐ No statistically significant differences were found in the four outcomes considered in the comparisons of anticonvulsants versus placebo. Anticonvulsant showed a potentially protective benefit against seizures. The adverse events were non‐significantly more common among the anticonvulsant‐treated patients, but the discontinuations due to adverse events tended to be more common in the placebo‐group. None of these trends, however, reached statistical significance.
‐ For the anticonvulsant versus other drug, results favour anticonvulsants only in one of the nine outcomes considered: comparing carbamazepine with benzodiazepine (oxazepam and lorazepam), results favour carbamazepine for alcohol withdrawal symptoms rated with CIWA‐Ar score. None of the other comparisons reached statistical significance. This can suggests that anticonvulsants and specifically carbamazepine may actually be more effective in treating some aspects of alcohol withdrawal when compared to benzodiazepines, the current first‐line regimen for alcohol withdrawal syndrome. The incidence of seizures tended to be more common among the participants that were treated with other drugs (e.g. benzodiazepines) than anticonvulsants, but delirium tremens favoured the other‐treatment participants. Adverse events showed a potentially better profile for the anticonvulsants.
‐ Comparing different anticonvulsants (2 outcomes considered), when carbamazepine was compared to Chlormethiazole the incidence of adverse events tended to be more common among the carbamazepine‐treated participants, whereas withdrawals tended to be more common among the Chlormethiazole participants. When carbamazepine was compared to barbital, the side effects were not statistically significant but more common among the carbamazepine patients, and no difference was found regarding the withdrawals between the compared drugs. Out of eleven studies comparing different anticonvulsant agents, no participant died during the treatment period.
‐ Comparing anticonvulsants plus other drugs versus other drugs (3 outcomes considered), results favour paraldehyde plus chloral hydrate versus chlordiazepoxide, for the severe‐life threatening side effects, but only one study, with four arms, and 72 participants was included in this comparison.
‐ Comparing anticonvulsant plus other drug versus another anticonvulsant, no significant difference were found in all the outcome considered (seizures, delirium, adverse events and drop out). But only one study with 56 participants, which compared oxcarbazepine plus Tiapride versus Chlormethiazolewas included in this comparison.
Overall completeness and applicability of evidence
Our review has some important limitations. First, most studies involve a very small sample size and have been conducted in different years, and in diverse patient populations.
Another potential limitation is the unavoidable grouping of drugs. All anticonvulsants were analysed together compared to the other drugs. Differences between specific anticonvulsant agents could not be seen, because of the limited number of studies comparing different anticonvulsants between themselves. Other drugs included a large variety of medicines such as benzodiazepines, neuroleptics, hypnotics, etc. These drugs do not belong to the same class and do not share the same mechanism of action. The grouping of all these drugs together, could have led to the loss of possibly important between‐drugs differences concerning effectiveness or safety. While we made an effort to address drug‐specific comparisons as well, these data are definitely more limited and even more inconclusive. It was also not possible to examine dose‐response effects, since participants were not treated with even similar doses of various anticonvulsants across RCTs. Moreover, data on side effects should be interpreted cautiously, as they were derived from participants with different co‐morbidity. Many trials tended to exclude participants in severe, medical conditions such as hepatic, heart or lung disease. However, these participants may be more sensitive to various adverse events of anticonvulsants or comparator drugs.
Quality of the evidence
Although randomisation was an inclusion criterion, indicating a degree of methodological quality for these studies, many of them are quite old, the mode of randomisation is not described, allocation of concealment is often unclear and information on follow‐up is frequently missing. On the other hand, this makes the meta‐analysis potentially more useful, since small trends that could not reach statistical significance due to the small number of participants in these trials, could now be revealed after the data synthesis. Also highlighting reporting deficits is important for improving future research.
Authors' conclusions
Implications for practice.
Results of this review do not provide sufficient evidence in favour of anticonvulsants for the treatment of AWS. Anticonvulsants seem to have limited side effects, although adverse effects are not rigorously reported in the analysed trials.
Implications for research.
Although a significant number of trends has emerged, most of these were small and the data for most outcomes did not reach statistical significance; the need for further studies should be carefully evaluated on the basis of these findings. If these studies are going to be carried out, they should be limited to few important efficacy and safety measures such as the severity of the alcohol withdrawal syndrome, the incidence of seizures and delirium tremens, side effects, withdrawals and mortality. In the case of continuous outcomes the same rating scale (e.g. CIWA‐Ar) should preferably be used, in order to have more comparable information across studies and to be able to estimate the real effect of all these agents.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2010 | New citation required and conclusions have changed | new studies founded |
| 7 January 2010 | New search has been performed | substantially updated; authors changed |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 21 March 2008 | Amended | Converted to new review format. |
| 13 April 2005 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review is a substantial update of a previous version (Polycarpou 2005). The authors of the first version are not yet interested in updating this review that for that change the authorship.
Acknowledgements
We thank the author of the first version of the review that did an excellent work that was very helpful for this update and Zuzana Mitrova for providing helpful assistance during all the process
Appendices
Appendix 1. CENTRAL search strategy
Free text: (((alcohol*) AND (withdraw* or detox* or abstinen* or abstain*)) AND (anticonvulsant*)))
Appendix 2. PubMed search strategy
Alcohol‐related disorders [mesh]
((alcohol*) and (disorder* or withdr* or abstinen* or abstain* or detox* or neuropathy)) [tiab]
Alcohol‐Induced Disorders, Nervous System [mesh]
1 or 2 or 3
ANTICONVULSANTS [Mesh]
anticonvulsant* [tiab]
(ACTH or carbamazepine or clorazepate or clobazam or clonazepam or chlordiazepoxide or divalproex or ethosuximide or ethosuccimide or ethotoin or felbamate or fosphenytoin or gabapentin or lignocaine or lamotrigine or Levetiracetam or lidocaine or hydantoins or levetiracetam or mephobarbital or methsuximide or oxcarbazepine or paraldehyde or phenacemide or phenytoin or pregabalin or primidone or succinimide or tiagabine or topiramate or valproate or vigabatrin or zonisamide) [tw]
5 or 6 or 7
randomized controlled trial[pt]
controlled clinical trial[pt]
random*[tiab]
placebo[tiab]
drug therapy[mesh]
trial[tiab]
groups[tiab]
9 or 10 or 11 or 12 or 13 or 14 or 15
animals [mesh]
humans [mesh]
animals NOT (17 and 18)
16 NOT 19
4 AND 8 AND 20
Anticonvulsants/adverse effects [mesh] or Anticonvulsants/poisoning [mesh] or anticonvulsants/toxicity [mesh] or Anticonvulsants/contraindications [mesh]
adverse effects [subheadings]
Drug toxicity [mesh]
“adverse effect*”[tiab]
“side effect* [tiab]
serious or safety or surveillance or adverse or toxicity or complication or tolerability [tiab]
22 or 23 or 24 or 25 or 26 or 27
4 AND 8 AND 28
29 NOT 19
Appendix 3. EMBASE search strategy
exp alcohol withdrawal
exp withdrawal syndrome
((alcohol*) and (disorder* or withdr* or abstinen* or abstain* or detox* or neuropathy)).ti,ab
1 or 2 or 3
exp anticonvulsive agent/
(ACTH or carbamazepine or clorazepate or clobazam or clonazepam or chlordiazepoxide or divalproex or ethosuximide or ethosuccimide or ethotoin or felbamate or fosphenytoin or gabapentin or lignocaine or lamotrigine or Levetiracetam or lidocaine or hydantoins or levetiracetam or methsuximide or oxcarbazepine or paraldehyde or phenacemide or phenytoin or pregabalin or primidone or succinimide or tiagabine or topiramate or valproate or vigabatrin or zonisamide)
5 or 6
random*.ti,ab
placebo. Ti,ab
(singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab
crossover*.ti,ab
exp randomized controlled trial/
exp double blind procedure/
exp single blind procedure/
exp triple blind procedure/
exp crossover procedure/
exp Latin square design/
exp placebos/
exp multicenter study/
8/19
4 AND 7 AND 20
limit 21 to human
Appendix 4. CINAHL search strategy
MESH alcohol related disorders
MESH alcohol withdrawal delirium
TX ((alcohol*) and (disorder* or withdr* or abstinen* or abstain* or detox* or neuropathy))
1 or 2 or 3
MH ANTICONVULSANTS
MH Dipotassium
Valproic acid:ME
TX Acetazolamide or carbamazepine or Chlormethiazole or Clorazepate or Clorazepate or divalproex or or Ethosuximide or felbamate or fosphenytoin or gabapentin or lamotrigine or Levetiracetam or Metaclazepam or lidocaine or mephobarbital or lignocaine or methsuximide or oxcarbazepine or paraldehyde or pentobarbital or phenytoin or primidone or tiagabine or topiramate or valproate or vigabatrin or zonisamide
5 or 6 or 7 or 8
MH Random Assignment/
MH Clinical Trials/
TW random*
TW placebo*
TW group*
TW (singl* or doubl* or tripl* or trebl*) and (mask* or blind*)
MH crossover design
TW crossover*
TW allocate*
TW assign*
10/19 OR
4 and 9 and 20
Appendix 5. Criteria for risk of bias in RCTs and CCTs
| Item | Judgment | Description | |
| 1 | Was the method of randomization adequate? | Yes | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization |
| No | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | ||
| Unclear | Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’. | ||
| 2 | Was the treatment allocation concealed? | Yes | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomization); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| No | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | ||
| Unclear | Insufficient information to permit judgement of ‘Yes’ or ‘No’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement | ||
| 3/4 |
Was knowledge of the allocated interventions adequately prevented during the study? (blinding of patients, provider, outcome assessor) Objective outcomes Subjective outcomes |
Yes | Blinding of participants, providers and outcome assessor and unlikely that the blinding could have been broken; Either participants or providers were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias. No blinding, but the objective outcome measurement are not likely to be influenced by lack of blinding |
| No | No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; Either participants or outcome assessor were not blinded, and the non‐blinding of others likely to introduce bias |
||
| Unclear | Insufficient information to permit judgement of ‘Yes’ or ‘No’; | ||
| 5 |
Were incomplete outcome data adequately addressed? For all outcomes except retention in treatment or drop out |
Yes | No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; Missing data have been imputed using appropriate methods All randomized patients are reported/analyzed in the group they were allocated to by randomization irrespective of non‐compliance and co‐interventions (intention to treat) |
| No | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization; |
||
| Unclear | Insufficient reporting of attrition/exclusions to permit judgement of ‘Yes’ or ‘No’ (e.g. number randomized not stated, no reasons for missing data provided; number of drop out not reported for each group); |
Data and analyses
Comparison 1. Anticonvulsant versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alcohol Withdrawal Seizures post treatment | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Any Anticonvulsant vs. Placebo | 9 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.31, 1.20] |
| 1.2 Phenytoin vs. Placebo | 4 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.35, 1.77] |
| 2 Adverse events | 7 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.74, 3.31] |
| 3 Dropout | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Any Anticonvulsant vs. Placebo | 7 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.50, 1.34] |
| 3.2 Chlormethiazole vs. Placebo | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.22, 5.11] |
| 4 Dropout due to adverse events | 8 | 649 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.13, 3.36] |
Comparison 2. Anticonvulsant versus Other Drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alcohol withdrawal seizures | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Any Anticonvulsant vs any Other | 12 | 880 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.22, 1.58] |
| 1.2 Chlormethiazole vs Benzodiazepine | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.33] |
| 2 Alcohol withdrawal delirium | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Any Anticonvulsant vs any Other | 6 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.23, 3.42] |
| 2.2 Carbamazepine vs Benzodiazepine | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.04, 24.43] |
| 3 CIWA‐Ar score (48 hrs) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Any Anticonvulsant vs Other | 4 | 358 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.95, 0.53] |
| 3.2 Carbamazepine vs. Benzodiazepine | 3 | 260 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.88, 0.67] |
| 4 CIWA‐Ar score (end of treatment) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Any Anticonvulsant vs any Other | 4 | 358 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.76, 0.31] |
| 4.2 Carbamazepine vs Benzodiazepine | 3 | 260 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.89, ‐0.20] |
| 5 Global Doctor's Assessment of Efficacy | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.08] |
| 6 Adverse events | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Any Anticonvulsant vs any Other | 14 | 726 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.45, 1.12] |
| 6.2 Chlormethiazole vs Benzodiazepine | 5 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.35, 1.59] |
| 7 Severe, life‐treatening adverse events | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Any Anticonvulsant vs anyOther | 12 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [0.33, 17.24] |
| 7.2 Chlormethiazole vs. Benzodiazepine | 4 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.09, 5.33] |
| 8 Dropout | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Any Anticonvulsant vs any Other | 20 | 1359 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.67, 1.26] |
| 8.2 Chlormethiazole vs Benzodiazepine | 5 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.37, 1.24] |
| 9 Dropout due to adverse events | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Any Anticonvulsant vs any Other | 10 | 596 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [0.67, 7.70] |
| 9.2 Chlormethiazole vs Benzodiazepine | 4 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.12, 9.97] |
Comparison 3. Anticonvulsant 1 versus Anticonvulsant 2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Carbamazepine vs. Chlormethiazole | 2 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [0.50, 19.27] |
| 1.2 Carbamazepine vs. Barbital | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.70, 4.68] |
| 1.3 Carbamazepine vs Oxcarbazepine | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Chlormethiazole vs. Pentobarbital | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 2.8 [0.12, 63.20] |
| 2 Dropout | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Carbamazepine vs. Chlormethiazole | 2 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.08, 3.11] |
| 2.2 Carbamazepine vs. Barbital | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.34, 2.57] |
| 2.3 Carbamazepine vs Oxcarbazepine | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 3.2 [0.14, 72.62] |
| 2.4 Pentobarbital vs. Paraldehyde | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.03, 3.97] |
| 2.5 Chlormethiazole vs. Pentobarbital | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.28, 7.05] |
Comparison 4. (Anticonvulsant + Other) versus Other.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alcohol withdrawal delirium | 3 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.18, 3.52] |
| 2 Severe, life‐threatening adverse events | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.89] |
| 3 Dropout | 3 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.18, 1.72] |
Comparison 5. Anticonvulsant1 + other vs anticonvulsant 2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 alcohol withdrawal seizures | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Oxcarbaxepine+tiapride vs chlometiazole | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 alcohol withdrawal delirium | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Oxcarbaxepine+tiapride vs chlometiazole | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 adverse events | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.45, 1.04] |
| 3.1 Oxcarbaxepine+tiapride vs chlometiazole | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.45, 1.04] |
| 4 severe, life threatening adverse events | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Oxcarbaxepine+tiapride vs chlometiazole | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 drop out | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Oxcarbaxepine+tiapride vs chlometiazole | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 5.36 [0.27, 106.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agricola 1982.
| Methods | Randomized controlled trial | |
| Participants | No. = 60; age = 20‐64 years (mean: 44.28) Inclusion criteria: alcoholic hospitalised patients with a severe withdrawal syndrome proceeding delirium tremens. Exclusion criteria: severe heart, liver or kidney diseases; use of psychotropic drugs; addicted or chronic abusers of other gender = 53 males; 7 females |
|
| Interventions | Group A (30) carbamazepine 200mg tid (no. = 30); Group B (30) Tiapride 200mg tid | |
| Outcomes | Withdrawal symptoms; overall evaluation of the clinical condition; assessment of therapeutic effectiveness both by the doctor and the patient; blood pressure and heart rate; SGOT, SGPT; tolerability; seizures | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? subjective outcomes | Unclear risk | Unclear |
| Blinding? Objective outcomes | Unclear risk | Unclear |
| Incomplete outcome data addressed? All outcomes except withdrawal | Unclear risk | Unclear |
Alldredge 1989.
| Methods | Randomized controlled trial | |
| Participants | No. = 90; gender = 85 males, 5 females; age = 30.3‐50.9. Inclusion criteria: patients admitted to the Emergency Department for a recent seizure occurred in the setting of acute withdrawal from alcohol. Exclusion criteria: history of seizures unrelated to alcohol use, severe head trauma, sedative or anticonvulsant use within 14 days, stimulant drug use within 3 days, significant electrolyte abnormalities, second‐or third‐degree atrioventricular heart block, bradycardia, or known hypersensitivity to hydantoin derivatives. |
|
| Interventions | Group A (45) diphenylhydantoin sodium 1000mg intravenously over 20 minutes; Group B (45) equivalent volume of 0.9% sodium chloride | |
| Outcomes | Efficacy : Seizure Recurrence; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "patients were randomly assigned to receive either diphenylhydantoin sodium injection or infusion of an equivalent volume of 0.9% sodium chloride" |
| Allocation concealment? | Unclear risk | "patients were randomly assigned to receive either diphenylhydantoin sodium injection or infusion of an equivalent volume of 0.9% sodium chloride" |
| Blinding? subjective outcomes | Low risk | study described as "double blind placebo controlled trial". "Both diphenylhydantoin and sodium chloride had an identical appearance" For patients with recurrent seizures, the treating physician could request that the study code be broken and subsequent treatment administered. Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | study described as "double blind placebo controlled trial". "Both diphenylhydantoin and sodium chloride had an identical appearance" For patients with recurrent seizures, the treating physician could request that the study code be broken and subsequent treatment administered. Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Unclear risk | "Ninety eligible patients completed the study between October 1982 and June 1988" COMMENT: it is not reported if more than 90 patients were randomised during the study period but they did not complete the study |
Balldin 1986.
| Methods | Randomized controlled trial | |
| Participants | No. = 38; Age = 28‐57 Inclusion criteria: Patients alcohol dependent (DMS‐III) Exclusion Criteria: somatic diseases; psychotic symptoms; use of alcohol together with sedatives and anxiolytics. |
|
| Interventions | Group A chlorprothixene 50mg x 3 daily+ Carbamazepin 200mg x 2 daily; Group B clonidine 300ìg x 2 daily | |
| Outcomes | Efficacy: Psychiatric rating CPRS; mood: (pleasantness‐unpleasantness) and somatic symptoms (including symptoms in the abstinence situation but also side‐effects from the medication) (self‐rating scales developed by Sjoberg and Persson & Sjoberg were used) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "patients were treated either with alpha2 agonist clonidine or chlorprothixene" COMMENT: it was not stated if the study was randomised |
| Allocation concealment? | Unclear risk | "patients were treated either with alpha2 agonist clonidine or chlorprothixene" COMMENT: it was not state if the study was randomised |
| Blinding? subjective outcomes | Low risk | "the design was double blind and double dummy". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "the design was double blind and double dummy". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "two patients in the chlorprothixene group were excluded from day two and four in the clonidine group ". reason fro drop out given |
Bjorkqvist 1976.
| Methods | Randomized controlled trial | |
| Participants | No. = 105; Gender: all male.; age 20‐60 years Inclusion criteria: out‐patients volunteers who sought treatment for alcohol withdrawal symptoms at five clinics; drinking for at least three days; cooperative; not in need of hospitalisation. Exclusion criteria: heart, liver, renal insufficiency; antihypertensive treatment; chronic narcotics; use of drugs |
|
| Interventions | Group A (34) carbamazepine; Group B (35) placebo | |
| Outcomes | Efficacy: Symptoms related to abstinence; evaluation of the patient's global feelings; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients were randomly allocated to two treatments" |
| Allocation concealment? | Unclear risk | "Patients were randomly allocated to two treatments" |
| Blinding? subjective outcomes | Low risk | "a double blind technique was used". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "a double blind technique was used". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 18 patients dropped out from the carbamazepine group and 18 from the the placebo group. Reason fro drop out given |
Blanchard 1985.
| Methods | Randomized controlled trial | |
| Participants | No. = 38; Gender:29 males/9 females; Age: 38 years; Inclusion criteria: Hospitalized patients for alcohol withdrawal symptoms; severity score for AWS minimum of 2. |
|
| Interventions | Group A Atrium 300 mg; Group B (18) placebo | |
| Outcomes | Efficacy: Evaluation of global efficacy; Safety: side effects | |
| Notes | Study in French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "patients were randomised in two parallel group" |
| Allocation concealment? | Unclear risk | "patients were randomised in two parallel group" |
| Blinding? subjective outcomes | Low risk | study described as "double blind". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | study described as "double blind". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 1 subject interrupted the treatment in the experimental group and 5 in the placebo group. reason for interruption given |
Bonnet 2003.
| Methods | Randomized controlled trial | |
| Participants | No. = 61; Age = 36.8‐51.8; Gender: 43 males/18 females; All Caucasian Inclusion criteria: Inpatients suffering from alcohol dependence (ICD‐10) with moderate or severe AWS (MAWS = or > 4) Exclusion Criteria: psychiatric condition requiring medication; abuse or dependence of other substances; |
|
| Interventions | Group A (32) gabapentin 400mg qid for 7 days; Group B (29) placebo. | |
| Outcomes | Efficacy: Amount of CLO required (first 24 h); course of MAWS (first 48 h); Accecptability: premature discontinuations (first 48h);drop out: Safety: adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "patients were randomised to receive either P or GP" |
| Allocation concealment? | Unclear risk | "patients were randomised to receive either P or GP" |
| Blinding? subjective outcomes | Low risk | study described as "double blind". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | study described as "double blind". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "efficacy was analysed for the intention to treat sample, including all subject randomised. Missing data were substituted by an estimated value, derived from regressing the number of CLO capsule on baseline MAWS (for primary effectiveness measure) or by carrying last observation forward" |
Borg 1986.
| Methods | Randomised controlled trial | |
| Participants | No. = 45; Gender: 100% male; age range: 29‐73 years Inclusion criteria: WHO criteria for alcohol dependence. Exclusion criteria: history of liver cirrhosis or psychotic disorders |
|
| Interventions | Group A (15) amobarbital; initial dose=800 mg/day reduced in steps of 100 mg/day during the week.Group B (15) oxazepam; initial dose=120 mg/day reduced in steps of 10 mg/day during the week, Group C (15) Melperone; 200 mg/day throughout the week | |
| Outcomes | Efficacy: seizures, CPRS scores ; Safety: mortality; Acceptability: dropouts | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "the patients were randomly assigned to treatment with either amobarbital, oxazepam or Melperone" |
| Allocation concealment? | Unclear risk | "the patients were randomly assigned to treatment with either amobarbital, oxazepam or Melperone" |
| Blinding? subjective outcomes | Low risk | "patients were treated with amobarbital, oxazepam and Meplerone in a double blind design". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "patients were treated with amobarbital, oxazepam and Meplerone in a double blind design". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "four patients were excluded before the study was finished from the Melperone group because of epileptic fits" |
Burroughs 1985a.
| Methods | Randomised controlled trial | |
| Participants | No. = 44 Inclusion criteria: history of alcohol drinking in excess of 80 g/day for five or more years; history of previous alcohol withdrawal. Patient with minimal withdrawal syndrome Exclusion criteria: participants who had taken psychotropic drugs within 48h of hospital administration |
|
| Interventions | Group A (12) Chlormethiazole, Group B (11) placebo, Group C (10) chlordiazepoxide, Group D (11) bromocriptine | |
| Outcomes | Efficacy: global improvement, Changes in Gross scale and Borg scale; Safety: adverse events, severe life‐treating adverse events, mortality; Acceptability: dropouts , dropouts due to adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "the patients in each group were randomised to treatment using a pre‐fixed code devised by the hospital pharmacy from a random number table, with a blocked design to ensure roughly equal numbers in the different treatment groups" |
| Allocation concealment? | Low risk | "the patients in each group were randomised to treatment using a pre‐fixed code devised by the hospital pharmacy from a random number table, with a blocked design to ensure roughly equal numbers in the different treatment groups" |
| Blinding? subjective outcomes | Low risk | "the patients in each group were randomised to treatment in double blind fashion using a pre‐fixed code devised by the hospital pharmacy" "The drugs were masked in the same size capsule and were pre‐packaged into daily dosage containers". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "the patients in each group were randomised to treatment in double blind fashion using a pre‐fixed code devised by the hospital pharmacy" "The drugs were masked in the same size capsule and were pre‐packaged into daily dosage containers". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "three patients withdrawn from the study: Two in the minor withdrawal group from the placebo group. One in the major withdrawal group taking chlordiazepoxide". " Analysis of difference between groups was based on intent to treat regardless of subsequent withdrawal from the study" |
Burroughs 1985b.
| Methods | Randomised controlled trial | |
| Participants | No. = 27 Inclusion criteria: history of alcohol drinking in excess of 80 g/day for five or more years; history of previous alcohol withdrawal. Patients with intense withdrawal syndrome. Exclusion criteria: participants who had taken psychotropic drugs within 48h of hospital administration |
|
| Interventions | Group A (8) Chlormethiazole, Group B (10) chlordiazepoxide, Group C (9) bromocriptine | |
| Outcomes | Efficacy: global improvement, Changes in Gross scale and Borg scale; Safety: adverse events, severe life‐treating adverse events, mortality; Acceptability: dropouts, dropouts due to adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "the patients in each group were randomised to treatment using a pre‐fixed code devised by the hospital pharmacy from a random number table, with a blocked design to ensure roughly equal numbers in the different treatment groups" |
| Allocation concealment? | Low risk | "the patients in each group were randomised to treatment using a pre‐fixed code devised by the hospital pharmacy from a random number table, with a blocked design to ensure roughly equal numbers in the different treatment groups" |
| Blinding? subjective outcomes | Low risk | "the patients in each group were randomised to treatment in double blind fashion using a pre‐fixed code devised by the hospital pharmacy" "The drugs were masked in the same size capsule and were pre‐packaged into daily dosage containers". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "the patients in each group were randomised to treatment in double blind fashion using a pre‐fixed code devised by the hospital pharmacy" "The drugs were masked in the same size capsule and were pre‐packaged into daily dosage containers". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "three patients withdrawn from the study: Two in the minor withdrawal group from the placebo group. One in the major withdrawal group taking chlordiazepoxide". " Analysis of difference between groups was based on intent to treat regardless of subsequent withdrawal from the study" |
Chance 1991.
| Methods | Randomized controlled trial | |
| Participants | No. = 55. Inclusion criteria: patients with a complaint of seizure due exclusively to alcohol withdrawal Exclusion criteria: patients with seizure unrelated to alcohol withdrawal; detectable phenytoin levels (> 2.5 ìg/ml) or allergies or contraindications to hydantoin derivatives; women due the inability to detect early pregnancy and the teratogenic risk of phenytoin. |
|
| Interventions | Group A (27) phenytoin 50 mg/ml; Group B (27) 0.9% normal saline | |
| Outcomes | Efficacy: Seizure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Patients were randomly assigned to phenytoin or normal saline. The drugs solutions were in identical vials prepared by the pharmacy in an order determined by random‐number tables and sent to the ED labelled only by test solution numbers" |
| Allocation concealment? | Low risk | "Patients were randomly assigned to phenytoin or normal saline. The drugs solutions were in identical vials prepared by the pharmacy in an order determined by random‐number tables and sent to the ED labelled only by test solution numbers" |
| Blinding? subjective outcomes | Low risk | "double blind study". "The drugs solutions were in identical vials prepared by the pharmacy". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment. |
| Blinding? Objective outcomes | Low risk | "double blind study". "The drugs solutions were in identical vials prepared by the pharmacy". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment. |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Choi 2005.
| Methods | Randomized controlled trial | |
| Participants | No: 52. . Gender: male:93%; mean age: 46 Inclusion criteria: meet DSM‐IV criteria for alcohol dependence |
|
| Interventions | Group A (25) Topiramate fixed, single dose, 50mg; Group B (27): lorazepam 4mg | |
| Outcomes | Efficacy: Withdrawal symptoms (CIWA‐Ar scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Fifty‐two patients after providing written informed consent are randomised to either lorazepam (N=27) or Topiramate (N=25) groups" |
| Allocation concealment? | Unclear risk | "Fifty‐two patients after providing written informed consent are randomised to either lorazepam (N=27) or Topiramate (N=25) groups" |
| Blinding? subjective outcomes | Unclear risk | no information about blindness |
| Blinding? Objective outcomes | Low risk | no information about blindness COMMENT: objective outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Croissant 2009.
| Methods | Randomized controlled trial | |
| Participants | No: 56; Gender: male 82,25%; mean age: 45 Inclusion criteria: patients with diagnosis of alcohol dependence according to both ICD‐10 and DSM‐IV, 18 ‐ 65 years of age , who knew enough German to fill in the questionnaires provided and who declared their commitment to the goal of total abstinence. Exclusion criteria: patients with positive screening results for opiates, amphetamine and cocaine ; severe somatic, psychiatric, or terminal diseases ; pregnancy, lactation period, suicidal tendencies, and legal or illegal drug addiction (except nicotine dependence and infrequent THC consumption) within the previous six months. . Patients taking anti‐psychotic drugs or antidepressants, AEDs or benzodiazepines, and patients for whom OXC, Tiaprid or CLO were contraindicated |
|
| Interventions | Group A (27) Oxcarbazepine + Tiapride Group B (29): Chlormethiazole | |
| Outcomes | Efficacy: Ciwa‐Ar score;delirium, seizures Safety: adverse events, severe adverse events; Acceptability: drop out | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "We used a table of randomisation to assign subjects to the OXC / TIA or to the CLO‐group. Randomization was effected block by block by five patients, i.e. every five patients the treatment regime changed from OXC / TIA to CLO and vice versa." |
| Allocation concealment? | High risk | Information on allocation concealment not reported COMMENT: the concealment is judged inadequate given the type of block randomisation used |
| Blinding? subjective outcomes | High risk | Open label |
| Blinding? Objective outcomes | Low risk | Open label COMMENT: objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | analysis on an intention‐to‐treat basis (ITT), including all patients who received at least one dose of medication. |
Dencker 1978.
| Methods | Randomized controlled trial | |
| Participants | No. = 63; Gender: 59 males/1 female; Age (mean): Chlormethiazole, 37 years, Piracetam, 39 years; Inclusion criteria: In‐patients, with diagnosis of chronic alcoholism and a previous history of at least 10 years alcohol abuse; had been drinking for at least 7 days; in an acute alcohol withdrawal state. Exclusion criteria: history of heavy drug addiction |
|
| Interventions | Group A (29) Chlormethiazole; Group B (34) Piracetam 1600 mg three times daily | |
| Outcomes | Efficacy: Comprehensive Psychiatric Rating Scale (CPRS); Adjective Check‐List (ACL); Estimation Scale (DES); Memory Test; laboratory examination; physical, including neurological examination; Safety: Clinical Side‐Effect Scale (CSE) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "patients were randomly allocated to either Piracetam or Chlormerhiazole" |
| Allocation concealment? | Unclear risk | "patients were randomly allocated to either Piracetam or Chlormerhiazole" |
| Blinding? subjective outcomes | Low risk | study described as "double blind". Blinding of outcome assessor: "Al the rating were performed by two psychologists who did not communicate with the nursing staff" |
| Blinding? Objective outcomes | Low risk | study described as "double blind". Blinding of outcome assessor: "Al the rating were performed by two psychologists who did not communicate with the nursing staff" |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "three patients dropped out from trial". One from the Piracetam group, one from the Chlormethiazole group and the third od not stated from which group. Reason fro drop out given |
Elsing 1996.
| Methods | Randomized controlled trial | |
| Participants | No. = 21; Gender: 18 males/3 females; mean age: GHB, 43.5; Clomethiazole, 42.2 Inclusion criteria: Patients with severe alcohol withdrawal syndrome. |
|
| Interventions | Group A (10) Chlomethiazole 250 mg every 4 hours as a mixture ; Group B (11) GHB initially 30 mg/kg BW followed by 15 mg/kg BW i.v. | |
| Outcomes | Efficacy: Tremor; sweating; nausea; restlessness; | |
| Notes | Meeting Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "randomised prospective study" |
| Allocation concealment? | Unclear risk | "randomised prospective study" |
| Blinding? subjective outcomes | High risk | Blindness: not mentioned. COMMENT: we judged that the study was not blinded because the frequency of drug administration was different between the two groups |
| Blinding? Objective outcomes | Low risk | Blindness: not mentioned COMMENT: objective outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Elsing 2009.
| Methods | Randomised controle trail | |
| Participants | No: 26; Gender: 22 male, 4 female. Age: mean 45 Inclusion criteria: Alcohol addicted patients admitted to the intermediate and intensive care unit of the medical department either because of severe withdrawal syndrome or because of additional concomitant life threatening diseases Exclusion criteria: not reported |
|
| Interventions | Group a: (12) Chlormethiazole; Group B:(14) Gamma‐Hydroxybutyric acid (GHB) | |
| Outcomes | Efficacy: Ciwa‐Ar score; Safety: side effects, severe side effects Acceptability:. drop out; drop out due to side effect. Other: mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients were randomised to receive either GHB (initially 30 mg/kg BWfollowed by 15 mg/kg BW i. v.) or CLO (250 mg every 4 hours as a liquid)" |
| Allocation concealment? | Unclear risk | "Patients were randomised to receive either GHB (initially 30 mg/kg BWfollowed by 15 mg/kg BW i. v.) or CLO (250 mg every 4 hours as a liquid)" |
| Blinding? subjective outcomes | High risk | Open label |
| Blinding? Objective outcomes | Low risk | Open label COMMENT: objective outcome unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 1 patient dropped out. Reason given |
Flygering 1984.
| Methods | Randomized controlled trial | |
| Participants | No. = 72; Age = 19‐62; Gender = 65 males/7 females Inclusion criteria: Patients requiring hospitalisation for alcohol withdrawal symptoms Exclusion Criteria: Concomitant abuse of drugs; full‐blown or incipient delirium tremens; concomitant treatment with antiepileptics, barbital or other psychoactive drugs |
|
| Interventions | Group A (37) carbamazepine; Group B (35) barbital The daily dose individual | |
| Outcomes | Efficacy: Alcohol withdrawal symptoms; visual analogue scale expressing the patient's subjective feeling; consumption of trial medication and duration of treatment; cure rate during treatment; Safety: adverse drug reactions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients were randomly allocated to treatment" |
| Allocation concealment? | Unclear risk | "Patients were randomly allocated to treatment" |
| Blinding? subjective outcomes | Low risk | a double blind technique were used". Tablets had identical appearance". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | a double blind technique were used". Tablets had identical appearance". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 6 patients dropped out from treatment from each group. Reason fro drop out given. |
Gann 2004.
| Methods | Randomized controlled trial | |
| Participants | No.= 20 Gender: not reported; mean age: 41. Inclusion criteria: meet diagnosis of alcohol dependence according to DSM‐IV criteria Exclusion criteria: psychotic features; clinically significant cognitive impairment; abuse of other substances other than alcohol; significant medical problem |
|
| Interventions | Group A (11) Chlormethiazole capsule of 384mg; 3 cps on day one, 4 cps on day two, 3 cps on day three, 2 cps on day four, 1 cps on day five; Group B (9): placebo | |
| Outcomes | Efficacy: Withdrawal symptoms (CIWA‐Ar scale); Sleep (polysomnography, total sleep time, sleep efficiency, latency to stage 2, latency to REM, number of wake period, PSQI); | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "patients were randomised to group" |
| Allocation concealment? | Low risk | the randomisation list was prepared by the pharmacy. Investigators were unaware of the randomisation code" |
| Blinding? subjective outcomes | Low risk | double blind placebo controlled study |
| Blinding? Objective outcomes | Low risk | double blind placebo controlled study |
| Incomplete outcome data addressed? All outcomes except withdrawal | High risk | two patients (15%) in the experimental group and four in the placebo group (31%) excluded before the first night because the withdrawal symptoms reached an extent when medication was considered necessary |
Glatt 1966.
| Methods | Randomized controlled trial | |
| Participants | No. = 97; Gender=53 males/44 females Inclusion criteria: Alcoholics admitted to the St. Bernard's Hospital in varying states of alcoholic intoxication. |
|
| Interventions | Group A (49) Chlormethiazole; Group B (48) placebo | |
| Outcomes | Efficacy: Individual psychiatric symptoms; prevalence of sedation; over‐all effect of treatment assessed by the doctor and by the patient | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "The test tablets for each patients were provided with a serial number and distributed within the series by mean of random numbers" |
| Allocation concealment? | Unclear risk | "The test tablets for each patients were provided with a serial number and distributed within the series by mean of random numbers" |
| Blinding? subjective outcomes | Low risk | "The investigation was carried out as a double blind trial.". To render the placebo indistinguishable in taste from the active tablet, 10 mg of Chlormetiazole was added to the coating of the placebo tablet, an amount that is too small to have any clinical effect". Blindness of outcome assessor: "the outcomes were assessed by a doctor; at the end of the trial he was asked whether he thought the patients had been receiving the active tablet or the placebo" |
| Blinding? Objective outcomes | Low risk | "The investigation was carried out as a double blind trial.". To render the placebo indistinguishable in taste from the active tablet, 10 mg of Chlormetiazole was added to the coating of the placebo tablet, an amount that is too small to have any clinical effect". Blindness of outcome assessor: "the outcomes were assessed by a doctor; at the end of the trial he was asked whether he thought the patients had been receiving the active tablet or the placebo" |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "three patients did not complete the trial, two from the Chlormethiazole and one from the placebo group" |
Golbert 1967.
| Methods | Randomised controlled trial | |
| Participants | N=49, Gender: 100% male; age range: 31‐71 years Inclusion criteria: patients admitted to the Veterans Administration Hospital, and in whom alcohol withdrawal syndromes subsequently developed. 47 were classified as in the "tremulous state" and 2 patients were classified as in "acute hallucination" |
|
| Interventions | Group A(12) paraldehyde and chloral hydrate, Group B (12) chlordiazepoxide, Group C (13) promazine. Group D (12) alcohol. | |
| Outcomes | Efficacy: delirium, global improvement; Safety: mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | "Random selection was achieved by assigning each patients medication by rotation" |
| Allocation concealment? | High risk | "Random selection was achieved by assigning each patients medication by rotation" |
| Blinding? subjective outcomes | High risk | Blinding: Not mentioned. COMMENT:We judged that the study was not blind because the route of administration of treatment was different (alcohol administered orally vs drug administered intramuscularly) |
| Blinding? Objective outcomes | Low risk | Blinding: Not mentioned. COMMENT: objective outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no drop out from the study |
Kaim 1972.
| Methods | Randomised controlled trial | |
| Participants | No. = 202, Gender: 100% male Inclusion criteria: patients with a clearly established history of alcoholism and manifested all three of the cardinal symptoms of delirium tremens ‐ disorientation, tremor, and hallucinations ‐ during the episode that led to their hospitalisation. Exclusion criteria: frank schizophrenic reaction; obvious chronic brain syndrome; serious medical or surgical conditions; diabetes mellitus; or a diagnosis of epilepsy |
|
| Interventions | Group A (46) chlordiazepoxide, Group B (14) placebo, Group C (55) paraldehyde, Group D (41) pentobarbital, Group E (46) perphenazine, | |
| Outcomes | Efficacy: seizures; Nurse Rating Scale; Physicians Symptom Recor d;symptoms checklist; global rating; Safety: mortality; Acceptability: dropouts, dropouts due to adverse events; Other: Treatment Booklet | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "The random assignment of patients to treatments was controlled by a study code number;; patients were assigned consecutive numbers. Prepackaged box of study medication corresponding to the patient's code number." COMMENT: it is not clear how the code numbers were generated |
| Allocation concealment? | Low risk | "The random assignment of patients to treatments was controlled by a study code number;; patients were assigned consecutive numbers. Prepackaged box of study medication corresponding to the patient's code number." |
| Blinding? subjective outcomes | High risk | "The parenteral form of chlordiazepoxide is straw‐coloured and required to be mixed with an intramuscular diluent; The IM form of prephenazine is a clear fluid supplied in 2cc. ampoules: IM sodium pentobarbital is colourless fluid, supplied in 5cc ampules.To maintain a partial double blind all IM medication was prepared with matching placebo and each patients was supplied with two injections of equivalent amount, one of which was placebo" "Oral medication other than paraldehyde were in capsule of identical appearance" .Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, COMMENT: the authors described the study as partial double blind; we judged that physicians could easily understand the type of treatment given |
| Blinding? Objective outcomes | Low risk | "The parenteral form of chlordiazepoxide is straw‐coloured and required to be mixed with an intramuscular diluent; The IM form of perphenazine is a clear fluid supplied in 2cc. ampoules: IM sodium pentobarbital is colourless fluid, supplied in 5cc ampules.To maintain a partial double blind all IM medication was prepared with matching placebo and each patients was supplied with two injections of equivalent amount, one of which was placebo" "Oral medication other than paraldehyde were in capsule of identical appearance" COMMENT: objective outcomes unlikely to be influenced by lack of physician's blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "There were 21 early terminators. 13 for administrative reason and they were not included in the analysis, 8 for treatment failure and they were included in the analysis" |
Kalyoncu 1996.
| Methods | Randomised controlled trial | |
| Participants | No. = 83, Gender: 100% male; age range: 18‐65 years | |
| Interventions | Group A (34) diazepam; max. 80 mg/day for 7 days, Group B (33) carbamazepine; max. 800mg/day for 7 days. | |
| Outcomes | Efficacy: delirium, MMSE; CIWA score; SCL‐90‐R; Beck depression inventory; global pathology assessment; Safety: mortality; Acceptability: dropouts | |
| Notes | Meeting Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "patients were randomly assigned to either carbamazepine or diazepam" |
| Allocation concealment? | Unclear risk | "patients were randomly assigned to either carbamazepine or diazepam" |
| Blinding? subjective outcomes | Unclear risk | blinding not mentioned COMMENT: there are no sufficient information about treatments to judge if the study could be blinded |
| Blinding? Objective outcomes | Unclear risk | blinding not mentioned COMMENT: objective outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 16 patients dropped out from studies. reason given |
Koethe 2007.
| Methods | RCT | |
| Participants | No.= 50 Gender: 80 % male; mean age: 46. Inclusion criteria: meet diagnosis of alcohol dependence according to DSM‐IV criteria; age between 18 and 60 years; symptoms of alcohol addiction persisting for a period of six months; willing to be hospitalised for alcohol withdrawal; having a history of severe AWS during former detoxification treatments; mentally competent to give written informed consent. Esxclusion criteria: patients showing severe alcohol withdrawal symptoms requiring the administration of CLO to avoid withdrawal seizures; using illicit drugs, except nicotine; history of drug abuse within the past year; psychiatric disorders or mental impairment limiting the ability to comply with study; acute illness of the respiratory system or reduced breath function or with a known hypersensitivity to CBZ, OXC, or CLO; history of a laboratory abnormality consistent with,or other evidence of a severe medical or neurological disease or malignancy; showing signs of renal or liver insufficiency; hyponatraemia; breath alcohol concentration more than 1.5%; positive urine drug screening at baseline. pregnancy; breast feeding |
|
| Interventions |
Group A (24) Oxcarbazepine on titration dosage starting with 600 mg the first day. Reaching the final dose of 900 mg OXC at day 2, the medication was applied over 2 days and tapered off the following 2 days (days 4 and 5).; Group B (26) placebo CLO was permitted (192 mg CLO per capsule, up to 18 capsules a day), and administered to the subjects in a symptom‐triggered manner according to the SAB score |
|
| Outcomes | Efficacy:Amount of CLO capsules taken to limit the alcohol withdrawal symptoms; intensity of withdrawal syndrome (AES); anxiety (STAI); depression (BDI); sleep (PSQI); Safety: adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "After admission, subjects were randomly assigned to the 2 treatment groups" |
| Allocation concealment? | Unclear risk | "After admission, subjects were randomly assigned to the 2 treatment groups" |
| Blinding? subjective outcomes | Low risk | double blind placebo controlled trial Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | double blind placebo controlled trial Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 3 patients were excluded from the studies from each group; reason given |
Koppi 1987.
| Methods | Randomized controlled trial | |
| Participants | No. = 20; Gender: 19 males/1 female; Age: 21‐65. Inclusion criteria: Patients with chronic alcoholism presenting mild to moderate withdrawal symptoms (DSM III criteria), Exclusion criteria: other forms of addiction; psychoses; dementias; known epilepsy; severe medical or neurological illnesses; pregnancy. |
|
| Interventions | Group A (10) meprobamate mean 2400 mg/24h; Group B (10) Caroverine mean 120 mg/24h | |
| Outcomes | Efficacy: Intensity of clouded consciousness in organic brain syndromes (SKT)‐; Webster rating scale; nurse's observation scale for inpatient evaluation (NOSIE); severity of illness (NGI) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "On a random number basis patients were either assigned to Caroverine or meprobamate" |
| Allocation concealment? | Unclear risk | "On a random number basis patients were either assigned to Caroverine or meprobamate" |
| Blinding? subjective outcomes | Low risk | "double blind". "identical capsules were provided". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "double blind". "identical capsules were provided". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | only one patient dropped out from treatment |
Kramp 1978.
| Methods | Randomised controlled trial | |
| Participants | No. = 91, Gender: 89% male; age range: 21‐62 years Inclusion criteria: delirium tremens; history of alcohol abuse; actual condition was related to alcohol abuse; severity of the symptoms permitted admission and treatment according to the general routine of the department; intense gross tremor of the extremities and intense perspiration; duration of the symptoms should be at least some hours. Exclusion criteria: intake of psychoactive drugs during the last 24 hours before treatment; alcohol in the blood at the time of treatment; acute event in chronic alcoholic hallucinosis |
|
| Interventions | Group A (44) diazepam 20 mg i.m. plus placebo p.os; Group B (47) barbital 500 mg p.os plus placebo i.m. | |
| Outcomes | Efficacy: seizures, global improvement, doctor's assessment of efficacy; Safety: mortality; Acceptability: dropouts; Other: Physical status; mental condition | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "The patients were allocated double blind to treatment" COMMENT: it was not state if the study was randomised |
| Allocation concealment? | Unclear risk | "The patients were allocated double blind to treatment" |
| Blinding? subjective outcomes | Low risk | "The patients were allocated double blind to treatment with either barbital (by oral route ) or diazepam (by intramuscular route). Patients received tablets as well as injections when medication was given(active tables plus placebo injection or active injection plus placebo tablet) ". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "The patients were allocated double blind to treatment with either barbital (by oral route ) or diazepam (by intramuscular route). Patients received tablets as well as injections when medication was given(active tables plus placebo injection or active injection plus placebo tablet) ". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "18 patients were excluded for reasons unrelated to te treatment, 10 in the diazepam group and 8 in the barbital group". Reason fro exclusion reported |
Krupitsky 2007.
| Methods | Randomised controlled trial | |
| Participants | No.= 127 Gender: 100 % male; mean age: 43. Inclusion criteria: meet DSM‐IV criteria for alcohol dependence; history of most recent alcohol consumption between 8 and 48 hours before study entry; clinically significant alcohol withdrawal symptoms on the basis of the CIWA‐Ar. Exclusion criteria : use of psychoactive or anticonvulsant medications other than those prescribed in the study; opiate dependence; need of urgent treatment for other symptoms; to be at high risk for untoward side effects from study medications |
|
| Interventions | Group A (26) Topiramate 25mg every 6 hours for a total of 100 mg/d, Group B (26) Memantine 10 mg every 8 hours for a total of 30 mg/d, Group C (25) lamotrigine 25 mg every 6 hours for a total daily dose of 100 mg/d, Group D (25) diazepam 10 mg every 8 hours for a total daily dose of 30 mg/d, Group E (25) placebo, | |
| Outcomes | Efficacy: Withdrawal symptom (CIVA‐Ar observer and self rated); Dysphoric mood (MADRS scale); Safety: adverse events; Acceptability: dropouts | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Block randomisation was used with block sizes of 15, 20, and 25 randomly varied |
| Allocation concealment? | Low risk | patients randomly chose from blocks of 15, 20, or 25 envelopes, each envelope containing 1 treatment assignment. Assessors were unaware of block sizes and were not involved with any aspect of the randomisation. Patient treatment assignment was kept under lock and key throughout the study |
| Blinding? subjective outcomes | Low risk | placebo‐controlled randomised single‐blinded Blinding of outcome assessor: "raters blind to the treatment assignment administered an alcohol withdrawal severity scale ". Patients blinded to treatment completed a self reported withdrawal symptoms checklist "Because study medications were not encapsulated, there was a potential for subjects to learn their group assignment by studying their medications if they were aware of distinctive markings associated with each study medication. We suspect that the blind was largely intact in this patient group based on informal clinical interactions with patients, although the integrity of the blind was not formally assessed. To promote the integrity of the blind, no medication could be identified by its administration schedule |
| Blinding? Objective outcomes | Low risk | placebo‐controlled randomised single‐blinded Blinding of outcome assessor: "raters blind to the treatment assignment" |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "All analyses were performed on an intent‐to‐treat basis" 133 patients recruited, data presented for 127 patients. No information about the 5 patients not included; the number is small, so it is inlikely that this could bias the results. |
Lambie 1980.
| Methods | Randomized controlled trial | |
| Participants | No. = 48; Gender = 44 males/4 females; Age = 22‐60 (mean 46) years Inclusion criteria: Alcohol dependents showing symptoms of withdrawal; had been drinking within the last 48 hours. |
|
| Interventions | Group A (22): sodium valproate 400 mg eight hourly; Group B (27) control. | |
| Outcomes | Efficacy: Symptoms of withdrawal; degree of severity of symptoms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Pateints were randomly allocated to one of the two groups" |
| Allocation concealment? | Unclear risk | "Pateints were randomly allocated to one of the two groups" |
| Blinding? subjective outcomes | High risk | "Patients were unaware of which group they were as other drugs described below were also given" COMMENT: physicians were not blind to treatment; The control group received tranquillizer and/or Chlormethiazole, but it is not specified if the drugs were identical in appearance |
| Blinding? Objective outcomes | Low risk | "Patients were unawere of which group they were as other drugs described below were also given" COMMENT: The outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no drop out from the study |
Lapierre 1983.
| Methods | Randomised controlled trial | |
| Participants | No. = 40, Gender: 83% male; age range: 24‐60 years Inclusion criteria: patients admitted to hospital for moderate to acute symptoms of alcohol withdrawal; history of alcohol abuse of at least 5 years; current episode of heavy drinking of at least 10 days duration. Exclusion criteria: history or positive urine drug screen for other substances' addiction; acute infections; head or major bony injures; medical conditions involving the cardiovascular; endocrine; pulmonary; and nervous system; gross and severe physical deterioration secondary to excessive alcohol intake resulting in severe renal, hepatic, nutritional, hematological and electrolytic disturbances; and schizophrenic illness |
|
| Interventions | Group A (20) Chlormethiazole, Group B (20) chlordiazepoxide | |
| Outcomes | Efficacy: Changes in AWS; TSA; SSA; Safety: adverse events, severe life‐treating adverse events, mortality; Acceptability: dropouts, dropouts due to adverse events; Other: psychophysiological assessment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | "Treatment were randomly administered sequentially as the patients were included in the study" |
| Allocation concealment? | Unclear risk | "Treatment were randomly administered sequentially as the patients were included in the study" |
| Blinding? subjective outcomes | Low risk | "Double blind was assured by the double dummy technique in which the patient received equal number of either of the two active drugs and the placebo of the other". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "Double blind was assured by the double dummy technique in which the patient received equal number of either of the two active drugs and the placebo of the other". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | No withdrawn from the study |
Longo 2002.
| Methods | Randomised controlled trial | |
| Participants | No. = 16, Gender: 50% male; age: 18‐65 Inclusion criteria: DSM‐IV criteria for alcohol dependence; desire to quit drinking; acceptable health; reliable and compliant; CIWA‐Ar score > 8 and < 20 on entry. Exclusion criteria: history of seizures or delirium tremens; significant medical co‐morbidity; abuse or dependence on drugs other than alcohol, cannabis, nicotine or caffeine; DSM‐IV diagnosis of an Axis I psychiatric disorder for which pharmacotherapy was required; pregnancy or lack of birth control; treatment within the month prior to screening and during the six week period with medications which might influence drinking outcomes; no fixed domicile or collateral informant |
|
| Interventions | Group A (5) Depakote 5‐day detoxification, Group B (5) Depakote plus 6‐week maintenance, Group C (7) chlordiazepoxide | |
| Outcomes | Efficacy: changes in CIWA‐Ar scores, ADS; TLFB, drinking diary; treatment utilization; CGI; ASI; VCS; OCDS; VSS; SIP; Laboratory values; Safety: adverse events, mortality; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | "Patients were sequentially randomised into one of three group" |
| Allocation concealment? | Unclear risk | "Patients were randomised to receive a standard benzodiazepine detoxification or Depakote detox plus maintenance" |
| Blinding? subjective outcomes | Low risk | "CIWA‐Ar raters were unaware of subjects cohort group assignment" COMMENT: we judged that the study was not blinded because the treatment differed in duration and way and frequency of drug administration. |
| Blinding? Objective outcomes | Low risk | COMMENT: the outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Lucht 2003.
| Methods | Randomised controlled trial | |
| Participants | No. = 127, Gender: 93% male; mean age = 43.1 years Inclusion criteria: patients with alcohol dependence (ICD‐10) admitted for alcohol detoxification therapy; alcohol withdrawal syndrome; age > or = 18 years; direct admission. Exclusion criteria: delirium on admission; contraindications or severe side effects against the study medications; other drug/substance dependence; pregnancy and lactation; psychosis; severe physical diseases; more than 5 single doses of study medications 2 weeks prior to study |
|
| Interventions | Group A (31) Chlormethiazole (max. 3840 mg/day) for 9 days, Group B (28) carbamazepine (max. 1200 mg/day) for 9 days, Group C (34) diazepam (max. 80 mg/day) for 9 days, | |
| Outcomes | Efficacy: seizure; delirium; CIWA‐Ar score; global improvement, VAS; SCL‐90‐R; Safety: adverse events, mortality; Acceptability: dropouts | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | "Assignment took place in blocks of 10 in order of admission ( first 10 patients into group A, next 10 patients into group B, etc) |
| Allocation concealment? | High risk | "Assignment took place in blocks of 10 in order of admission ( first 10 patients into group A, next 10 patients into group B, etc) |
| Blinding? subjective outcomes | High risk | "patients, physicians and nurses were not blind to the study medication" |
| Blinding? Objective outcomes | Low risk | "patients, physicians and nurses were not blind to the study medication" COMMENT: lack of blinding unlikely to influence outcomes |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Madden 1969.
| Methods | Randomised controlled trial | |
| Participants | No. = 100; Gender: 73 males/27 females Inclusion criteria: alcoholics in treatment of dependency on alcohol and drugs showing alcohol withdrawal features on the day of admission; |
|
| Interventions | Group A: Chlormethiazole; Group B: trifluoperazine | |
| Outcomes | Efficacy: Overall degree of improvement on each of the initial three full days of treatment compared with the patients' state on the day of admission; daily assessment of the presence or absence of abstinence features | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients were randomly allocated to one or other preparation" |
| Allocation concealment? | Unclear risk | "Patients were randomly allocated to one or other preparation" |
| Blinding? subjective outcomes | Unclear risk | "identical capsules were used" COMMENT: it is not clear if personnel and outcome assessor were blind to treatment |
| Blinding? Objective outcomes | Low risk | "identical capsules were used" COMMENT: outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Malcolm 1989.
| Methods | Randomised controlled trial | |
| Participants | No. = 66, Gender: 100% male; age range: 18‐65 years Inclusion criteria: met DSM‐III criteria for alcohol dependency, MMSE >25; CIWA = or > 20. Exclusion criteria: history of daily use of CNS active drugs, including prescription, nonprescription, and illicit agents; 5 or more days of illicit drug abuse (other than alcohol) in the 30 days before admission; allergic or adverse reactions to oxazepam or carbamazepine; manic‐depressive illness, schizophrenia, or dementia; history of hepatic encephalopathy, jaundice, ascites, diabetes, renal disease, neurologic disease (excluding peripheral neuropathy), or leukopenia; liver function transaminase levels (SGOT, LDH, SGPT) 2.5 times higher than normal; total WBC <4000/mm3; platelet count <100,000/mm3; participating in any drug |
|
| Interventions | Group A (32) carbamazepine 800 mg/day for 7 days, Group B (34) oxazepam 120 mg/day for 7 days | |
| Outcomes | Efficacy: changes in CIWA‐Ar scores, physiological measures; neurological measures; self‐report measures; standard psychological testing ( SCL‐90‐R, [Beck depression inventory, State‐Trait anxiety inventory, Wechsler Memory scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Subjetcs were blindly assigned to a group who received carbamazepine or to a group who received oxazepam" |
| Allocation concealment? | Unclear risk | "Subjetcs were blindly assigned to a group who received carbamazepine or to a group who received oxazepam" |
| Blinding? subjective outcomes | Low risk | study described ad "double blind". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment. |
| Blinding? Objective outcomes | Low risk | study described ad "double blind". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment. |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 20 subjects dropped out from the study. Reason for drop out give, No difference in number of subjects dropped out between groups |
Malcolm 2002.
| Methods | Randomised controlled trial | |
| Participants | No. = 136, Gender: 75% male Inclusion criteria: met DSM‐IV criteria for alcohol dependence and alcohol withdrawal; blood alcohol level = or <0.1 g/dl; residence within 50 miles of the study site; MMSE = or >26; admission score on CIWA‐Ar = or >10. Exclusion criteria: all substance abuse syndromes other than alcohol, nicotine or cannabis; major Axis I psychiatric disorder; use of medication in the preceding 30 days that could alter the withdrawal process; history of head injury or other neurologic illness including idiopathic epilepsy; medical instability; electroencephalogram abnormalities; grossly abnormal laboratory values |
|
| Interventions | Group A (61) carbamazepine 600‐800 mg on day 1 tapering to 200 mg on day 5, Group B (75) lorazepam 6‐8 mg on day 1 tapering to 2 mg on day 5. | |
| Outcomes | Efficacy: changes in CIWA‐Ar scores; ADS; daily drinking log; Zung Anxiety scale; Beck depression inventory; ability to return to work; sleep quality measures; Safety: mortality | |
| Notes | Malcolm2 2002 is the same RCT assessing different AW outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Subjects randomisation was based on on a computer generated schedule administered by a research pharmacist not involved in data collection" |
| Allocation concealment? | Low risk | "Subjects randomisation was based on on a computer generated schedule administered by a research pharmacist not involved in data collection" |
| Blinding? subjective outcomes | Low risk | Study describes as double blind". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment. |
| Blinding? Objective outcomes | Low risk | Study describes as double blind". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment. |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "Retention rate did not differ between the groups". Reported flow chart of treatment retention |
Malcolm 2007.
| Methods | Randomised controlled trial | |
| Participants | No.= 101 Gender: 75 % male; mean age: 41. Inclusion criteria: meet DSM‐IV1 criteria for alcohol dependence and for current alcohol withdrawal syndrome; medically stable and not likely to require hospitalisation for medical complications within 10 days of entry into the study; score of 10 or higher (CIWA‐Ar); negative urine drug screens for benzodiazepines, other sedative‐hypnotics, opiates, and amphetamine. Exclusion criteria: history of taking medications known to ameliorate or intensify the AWS ; diagnosis of any other substance‐dependence syndrome other than alcohol , except cannabis and cocaine; history of idiopathic epilepsy, schizophrenia, bipolar disorder, or dementia; liver function tests 4 times higher than the upper range of normal; history of hepatic encephalopathy, jaundice, ascites, insulin‐dependent diabetes mellitus, or renal insufficiency . |
|
| Interventions | Group A (20) Gabapentin, mid‐range dose 300 mg 3 times a day for 3 days and 300 mg twice daily for the last day, Group B (21) lorazepam 2 mg 3 times a day for the first 3 days and 2 mg twice daily on the last day of treatment | |
| Outcomes | Efficacy: Insomnia measured by CIWA‐AR scale; Quality of sleep measured by Beck Depression Inventory (BDI); Sleepiness measured by the Eporth Sleepiness Scale; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Subjects were randomised using a stratified permuted block method in which individuals with particular characteristics were assigned to medication groups with constraint that the assignment was balanced within successive blocks of subjects. We used a relatively small block size of q=4in order to promote a high degree of balance. Stratifications were based on the intersection of previous alcohol treatment history (0‐1 or more than 1 past treated withdrawals) and sex (male or female). |
| Allocation concealment? | Unclear risk | not specified if providers including subjects were aware of the criteria used to allocate patients to groups |
| Blinding? subjective outcomes | Low risk | "double blind study" "Medications prepared for the project were identical capsules prepared and distributed by the Alcohol Research Center Shared Scientific Core pharmacists under supervision by the University Research Pharmacy Office" |
| Blinding? Objective outcomes | Low risk | "double blind study" "Medications prepared for the project were identical capsules prepared and distributed by the Alcohol Research Center Shared Scientific Core pharmacists under supervision by the University Research Pharmacy Office" |
| Incomplete outcome data addressed? All outcomes except withdrawal | Unclear risk | reported the number of subjects excluded or who dropped out for treatment but not divided for group assignment |
Manhem 1985.
| Methods | Randomized controlled trial | |
| Participants | N= 67; Age: 32‐60 Inclusion criteria: alcohol dependents (DSM III) with acute alcohol withdrawal syndrome requiring drug therapy; Exclusion criteria: history, medical examination or laboratory evidence suggesting severe neurologic, psychiatric, hepatic or cardio‐vascular illness. patients using prescription or illicit drugs |
|
| Interventions | Group A (10) Chlormethiazole 4 x 500 mg; Group B (10) clonidine 4 x 150 ìg | |
| Outcomes | Efficacy: Withdrawal Assessment Scale; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? subjective outcomes | Unclear risk | Unclear |
| Blinding? Objective outcomes | Unclear risk | Unclear |
| Incomplete outcome data addressed? All outcomes except withdrawal | Unclear risk | Unclear |
Mariani 2006.
| Methods | Randomised controlled trial | |
| Participants | No.= 27 Gender: 70 % male; mean age: 44. Inclusion criteria: met DSM‐IV1 criteria for alcohol dependence; age between 18 and 60 years; score of ten or higher as measured by the CIWA‐Ar); willingness to sign informed consent. Exclusion criteria: any major DSM‐IV Axis I non‐substance‐related psychiatric disorder; any known allergy to gabapentin or phenobarbital; pregnancy; documented AIDS (HIV+ status was not an exclusion criterion); severe unstable medical illness; alcohol withdrawal delirium; use of opioids (methadone maintenance was not an exclusion criterion) or sedative‐hypnotic agents (eg, benzodiazepines or barbiturates). |
|
| Interventions |
Group A (14) gabapentin Day 1 total of 2400 mg in the first 24 hours Day 2‐4. 600 mg Group B (13) Phenobarbital Day 1‐3 60mg , day 4. 30mg |
|
| Outcomes | Efficacy: Withdrawal symptom (CIWA‐Ar); mood (POMS); craving (ACS); irritability (BDHI);anxiety (Hamilton?A);dysphoria (RDS); sleep (SPS, hours of sleep) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "patients were randomised to receive one of the two treatments" |
| Allocation concealment? | High risk | "The principal investigator conducted all screening interviews and administered all rating scales. The study was open‐label—all participants, research staff, and clinical staff were aware of the identity of the treatment medication. |
| Blinding? subjective outcomes | High risk | "The study was open‐label—all participants, research staff, and clinical staff were aware of the identity of the treatment medication." |
| Blinding? Objective outcomes | Low risk | "The study was open‐label—all participants, research staff, and clinical staff were aware of the identity of the treatment medication". COMMENT: objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | The proportion of completers did not differ between treatment groups: gabapentin, 71%; phenobarbital, 62%( p : 0.70). reason for drop out given |
McGrath 1975.
| Methods | Randomised controlled trial | |
| Participants | No: 100, Gender: not reported, age range: not reported Inclusion criteria: patients with acute withdrawals phase of alcoholism. No further detailde given Exclusion criteria: not reported |
|
| Interventions |
Group A: Chlormethiazole (50) group B: Chlordiazepoxide (50) |
|
| Outcomes |
Efficacy: delirium Acceptaibility: drop out |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "One hundred consecutive admissions to a specialized unit for the treatment of alcoholism in a psychiatric hospital were allotted code numbers and in accordance with a list of randomised numbers" |
| Allocation concealment? | Low risk | "The true nature of the medication was known only to the hospital pharmacist who packaged them for each patient according to number. It was agreed that the code could be broken only if a patient's condition deteriorated to the extent that knowledge of the medication he was receiving was essential." |
| Blinding? subjective outcomes | Low risk | "As the two preparations being tested were very different in presentation, one being a tablet and the other a capsule with Arachis oil, it was decided that both tablet and capsule would be given to each patient. Patients received either active capsules and placebo tablets, or vice versa." |
| Blinding? Objective outcomes | Low risk | "As the two preparations being tested were very different in presentation, one being a tablet and the other a capsule with Arachis oil, it was decided that both tablet and capsule would be given to each patient. Patients received either active capsules and placebo tablets, or vice versa." |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 14 patients dropped out from Chlordiazepoxide group and 7 from Chlormethiazole because feelings of tension and restlessness or to breaking of the code due to the development of delirium tremens |
Murphy 1983.
| Methods | Randomized controlled trial | |
| Participants | No. = 68; Gender: 51 males/17 females; Age: 21‐65 years Inclusion criteria: addicted to alcohol with withdrawal symptoms Exclusion criteria: alcoholic hallucinatory syndrome or delirium; psychosis of any kind or past psychosis not related to alcohol abuse; clinical or documented evidence of brain damage including the Wernicke‐Korsakoff syndrome; intercurrent physical illness necessitating intervention; any evidence of epilepsy not related to alcohol abuse; ingestion of psychotropic drugs less than 24 h before admission. |
|
| Interventions | Group A (29) Chlormethiazole; Group B (30) Tiapride; Group C (9) placebo | |
| Outcomes | Efficacy: Depression (Wakefield Inventory of Zung's Self‐Rated Depressive Scale); Severity of Alcohol Dependence Questionnaire; withdrawal symptoms (Severity Assessment Scale of Gross); craving for alcohol; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients were randomly assigned to one of the treatment group after stratification for age, sex and blood alcohol concentration" |
| Allocation concealment? | Unclear risk | "Patients were randomly assigned to one of the treatment group after stratification for age, sex and blood alcohol concentration" |
| Blinding? subjective outcomes | Low risk | "The study is a double blind, blindness being achieved by the use of double dummy technique". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "The study is a double blind, blindness being achieved by the use of double dummy technique". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 11 patients dropped out of the trial. Rason for drop out given. Number of drop out balanced among group (5 from Tiapride, 4 from Chlormethiazole, 2 from placebo) |
Myrick 2000.
| Methods | Randomized controlled trial | |
| Participants | No. = 11; Gender = Both males and females. Age = 18‐65 Inclusion criteria: Alcohol dependents (DSM II R) requiring alcohol detoxification; (CIWA‐Ar) scale of 6 or higher. Exclusion criteria: serious medical conditions; major psychiatric disorders; liver function tests 3 times greater than normal. |
|
| Interventions | Group A (6) divalproex 500mg 3 times a day for 4 days)+Lorazepam 2mg; Group B (5) lorazepam 2mg | |
| Outcomes | Efficacy: CIWA‐Ar score; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Subjects were placed randomly on Depakote group. The CIWA‐ar only group is the comparison group". |
| Allocation concealment? | Unclear risk | "Subjects were placed randomly on Depakote group. The CIWA‐ar only group is the comparison group". |
| Blinding? subjective outcomes | Low risk | "Open label clinical trial". Blindness of outcome assessor: "CIWA‐Ar raters were unaware of treatment assignment" |
| Blinding? Objective outcomes | Low risk | "Open label clinical trial". COMMENT: objective outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the trial |
Nimmerichter 2002.
| Methods | Randomized controlled trial | |
| Participants | No. = 98; Gender = 89 males/9 females; Age = 31.7‐50.7 Inclusion criteria: Patients meet at least 3 DSM‐IV and ICD‐10 criteria for the diagnosis of alcohol dependence; severity of withdrawal syndrome = or > 20 points on the CIWA‐Ar,; elevated GGT and/or MCV and/or relative carbohydrate‐deficient transferrin levels. Exclusion criteria: patients already detoxified or dependent on substances other than alcohol; illnesses such as decompensated liver cirrhosis, acute pancreatitis, reduced respiratory function, acute pulmonary or bronchial disease, myocardial infarction during the preceding 6 months and cardiac arrhythmia; dementia, schizophrenia, polysubstance misuse or dependence or use of benzodiazepines and hypno‐sedatives; suspected sleep apnoea syndrome; allergy to either of the study drugs |
|
| Interventions | Group A (33) Chlormethiazole 1000 mg daily; Group B (33) GHB 50 mg GHB/kg body wt; Group C (32) GHB 100 mg GHB/kg body wt | |
| Outcomes | Efficacy: Withdrawal syndrome (CIWA‐Ar); clinical and biological parameters; Lubeck Craving‐Risk‐Relapse questionnaire (LCRR‐1); additional study medication; Acceptabilty: side effects; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Participants were randomised to one of the three treatments by means of a computerized randomisation schedule" |
| Allocation concealment? | Unclear risk | "Participants were randomised to one of the three treatments by means of a computerized randomisation schedule" |
| Blinding? subjective outcomes | Low risk | Double blind, double dummy. Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | Double blind, double dummy. Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "11 patients discontinued the study prematurely". Reason for drop out given. Analysis performed on the intention to treat basis |
Radouco‐Thomas 1989.
| Methods | Randomised controlled trial | |
| Participants | No.= 67; Gender: 100% male; age range: 32‐60 years Inclusion criteria: patients admitted to the detoxification unit of Quebec Hospital; met the DSM‐III criteria for alcohol dependence; presented acute alcohol withdrawal syndrome requiring drug therapy. Exclusion criteria: history, medical examination or laboratory evidence suggesting severe neurologic, psychiatric, hepatic or cardiovascular illness and the use of prescription or illicit drugs (polydrug addiction |
|
| Interventions | Group A (30) oral phenobarbital, Group B (30) oral chlordiazepoxide. Additional medication doses were given according to the clinician judgment | |
| Outcomes | Efficacy: seizures, CIWA‐Ar score, REG; vital signs and sleep evaluation; DSST, PPT; HSCL‐35, Zung Self Rating Anxiety Scale, Zerssen bipolar mood test‐Z; changes in blood alcohol levels; Safety: adverse events, severe life‐treating adverse events, mortality; Acceptability: dropouts | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "After enrolment, patients were assigned to receive the next in a sequentially numbered supply of medication. The sequence was previously determined by the hospital pharmacist from a table of random numbers with a blocked design to ensure a roughly equal numbers in the two treatment group and in the different season of the year" |
| Allocation concealment? | Low risk | "After enrolment, patients were assigned to receive the next in a sequentially numbered supply of medication. The sequence was previously determined by the hospital pharmacist from a table of random numbers with a blocked design to ensure a roughly equal numbers in the two treatment group and in the different season of the year" |
| Blinding? subjective outcomes | Low risk | Double blind. "Double dummy administration procedure". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | Double blind. "Double dummy administration procedure". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 7 patients drop out from the study. Reason for drop out given. No differences in the number of drop out between groups |
Rathlev 1994.
| Methods | Randomized controlled trial | |
| Participants | No. = 100; Gender = 96 males/4 females
Age (mean) = placebo, 41.5 years; phenytoin, 42.4 years. Inclusion criteria: Patients with a witnessed generalized seizure in the setting of decreasing alcohol intake, and had no other known or suspected cause of seizures. Exclusion criteria: known or potential causes of seizure by head CT scan, EEG, lumbar puncture, or laboratory studies; onset of seizures before age 25; focal seizures or status epilepticus; positive phenytoin level; focal neurologic examination; known ingestion of drugs that potentially cause seizures; treatment with benzodiazepines for alcohol withdrawal symptoms; significant head trauma; pregnancy; phenytoin allergy. |
|
| Interventions | Group A (49) phenytoin 15 mg/kg at a rate equivalent to 50 mg/min over 20 minutes by iv pump; Group B (51) placebo | |
| Outcomes | Efficacy: seizure; Acceptability: completion of a six‐hour observation period after infusion. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Patients were randomised to receive either phenytoin or saline placebo according to a table of random number" |
| Allocation concealment? | Unclear risk | "Patients were randomised to receive either phenytoin or saline placebo according to a table of random number" |
| Blinding? subjective outcomes | Low risk | Double blind study. "The drugs ware prepared in identical ampoules and were administered in a blinded fashion". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | Double blind study. "The drugs ware prepared in identical ampoules and were administered in a blinded fashion". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Reoux 2001.
| Methods | Randomized controlled trial | |
| Participants | No. = 36; Gender = 35 males/1 female; Age = 40.8‐58 Inclusion criteria: volunteers seeking treatment for alcohol dependence and withdrawal; met DSM‐IV criteria for alcohol dependence; experiencing at least moderate acute alcohol withdrawal (score of 10 or greater on the CIWA‐Ar scale) Exclusion Criteria: 6 or more days of illicit drug use, other than cannabis, in the 30 days before admission; allergic or adverse reaction to prior use of oxazepam or valproate; current use of an anticonvulsant medication or benzodiazepine; pregnancy; medical condition potentially risky |
|
| Interventions | Group A (18) divalproex sodium 500 mg 3 times a day; Group B (18) inactive placebo | |
| Outcomes | Efficacy: Total milligrams of oxazepam required to control AWS symptoms; withdrawal symptoms (CIWA‐Ar); changes in SCL‐90 scores; Acceptability: side‐effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Subjects were randomly assigned to either a placebo or divalproex sodium condition." |
| Allocation concealment? | Unclear risk | "Subjects were randomly assigned to either a placebo or divalproex sodium condition." |
| Blinding? subjective outcomes | Low risk | Study described as double blind. Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | Study described as double blind. Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "7 subjects (3 in the divalproex, 4 in the placebo) dropped out before the remission of the acute withdrawal symptoms and were not included in the analysis" |
Ritola 1981.
| Methods | Randomized controlled trial | |
| Participants | No. = 68; Gender = 100% males; Age =37.4‐41.6. Inclusion criteria: patients considered sufficiently severe as to require hospitalisation. Exclusion Criteria: clear abnormalities in cardiac, hepatic or renal functions; using psychoactive agents other than the trial preparations |
|
| Interventions | Group A (34): carbamazepine 200 mg; Group B: (34) Chlormethiazole 300 mg | |
| Outcomes | Efficacy: Withdrawal symptoms; patient's subjective feeling; overall evaluation of the treatment (patient, nurse, physician); Acceptability:side effects; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "The patients were randomly allocated to two treatments" |
| Allocation concealment? | Unclear risk | "The patients were randomly allocated to two treatments" |
| Blinding? subjective outcomes | Low risk | "The double blind and double dummy technique was achieved by using placebo capsules and placebo tablets which were given toghether with the arrpopiate active drugs". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "The double blind and double dummy technique was achieved by using placebo capsules and placebo tablets which were given toghether with the arrpopiate active drugs". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 5 patients (44 from Chlormethiazole and 1 from carbamazepine) dropped out from the study. Reason for drop out given |
Robinson 1989.
| Methods | Randomized controlled trial | |
| Participants | No. = 32; Gender: 26 males/6 females
Age (mean): Clonidine, 50.8±9.3 years; Chlormethiazole, 44.8 ± 8.4 years. Inclusion criteria: patients requiring drug treatment for the suppression of withdrawal symptoms. Exclusion Criteria: no significant symptoms of alcohol withdrawal (WSRscore < 3); standing systolic blood pressure < 100 mmHg; major withdrawal symptoms (WSR score > 20); history of previous withdrawal seizures; multidrug dependency problem; taking drugs affecting sympathetic nervous activity in the week prior to admission |
|
| Interventions | Group A (16): Chlormethiazole; Group B: (16) clonidine | |
| Outcomes | Efficacy: alcohol withdrawal symptoms (WSR scale); | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Medication was administered in a randomised double blind manner". |
| Allocation concealment? | Unclear risk | "Medication was administered in a randomised double blind manner". |
| Blinding? subjective outcomes | Low risk | "Medication was administered in a randomised double blind manner".. Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | "Medication was administered in a randomised double blind manner".. Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | High risk | "The study was stopped when 25% of the patients had been withdrawn from the trial. All 16 patients who received chlormethiazole completed the trial uneventfully. However there was a significant number (8) of withdrawals for the clonidine group (P:0.002) |
Rosenthal 1998.
| Methods | Randomized controlled trial | |
| Participants | No. = 42; Gender: 36 males/6 females; Mean age: Valproate group, 43.5±6.7 years; Phenobarbital group, 41.5 ± 10.7 years.
Inclusion criteria: alcohol dependents in withdrawal (DSM‐IV criteria); willingness to sign informed consent, age between 18 and 55 years; and criteria for alcohol dependence in withdrawal. Exclusion criteria: any major DSM‐IV Axis I disorder; documented liver disease; known allergy to valproate; pregnancy; documented AIDS (HIV+ not an exclusion criterion); coexisting use of cocaine or illicit opioids (methadone maintenance not an exclusion criterion); abuse of a half‐life benzodiazepine |
|
| Interventions | Group A phenobarbital; Group B valproate | |
| Outcomes | Efficacy: withdrawal symptoms and signs: Modified Selective Severity Assessment (MSSA); modified Abstinence Symptoms Questionnaire (ASQ);Profile of Mood States (POMS‐SF); Buss‐Durkee Hostility Index (BDHI). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients were randomised on day 1 to receive either phenobarbital or valproate" |
| Allocation concealment? | Unclear risk | "Patients were randomised on day 1 to receive either phenobarbital or valproate" |
| Blinding? subjective outcomes | High risk | "The study was open label" |
| Blinding? Objective outcomes | Low risk | "The study was open label" COMMENT:outcomes are unlikely to be influenced by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 5 patients dropped out from study, 4 from valproate and one from control. Reason fro drop out given |
Rothstein 1973.
| Methods | Randomized controlled trial | |
| Participants | No. = 200 Inclusion criteria: have a history of chronic alcoholism; have been drinking heavily every day for at least five days immediately preceding his admission; give written, informed consent Exclusion criteria: previous long‐term diphenylhydantoin therapy for convulsive disorders (other than withdrawal seizures); seizures during the two weeks just before admission |
|
| Interventions | Group A (100) diphenylhydantoin 2 x 200 mg orally; Group B chlordiazepoxide + thiamine(100) | |
| Outcomes | Efficacy: Seizures | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "The 200 patients were randomly assigned to either a diphenylhydantoin group or a control group using the following procedure:at the start of the study we obtained a random number list of 200 number balanced after each 20 to assure equal odd and even numbers.Upon admission each new patient eligible was assigned the nest available number in the random number list; cases with odd numbers received diphenylhydantoin and cases with even numbers served as control. " |
| Allocation concealment? | Unclear risk | "The 200 patients were randomly assigned to either a diphenylhydantoin group or a control group using the following procedure:at the start of the study we obtained a random number list of 200 number balanced after each 20 to assure equal odd and even numbers.Upon admission each new patient eligible was assigned the nest available number in the random number list; cases with odd numbers received diphenylhydantoin and cases with even numbers served as control. " |
| Blinding? subjective outcomes | High risk | blinding not mentioned COMMENT: we judged that study was not blind because the diphenylhydantoin group received this drug as an adjunct to the drugs received by the control group |
| Blinding? Objective outcomes | Low risk | blinding not mentioned COMMENT: outcome unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Sampliner 1974.
| Methods | Randomized controlled trial | |
| Participants | No. =157. Inclusion criteria: history of seizures in adulthood; no medications in the two weeks before admission; steady, heavy alcoholic intake for the preceding four weeks. |
|
| Interventions | Group A (70) diphenylhydantoin 300 mg/day; Group B (66) placebo | |
| Outcomes | Efficacy: seizures | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Patients were assigned randomly to diphenylhydantoin or placebo according to a computer generated set of numbers" |
| Allocation concealment? | Unclear risk | "Patients were assigned randomly to diphenylhydantoin or placebo according to a computer generated set of numbers" |
| Blinding? subjective outcomes | Unclear risk | "Diphenylhydantoin or placebo were blindly administered." " Diphenylhydantoin and placebo were placed in gelatine capsules" COMMENT: it is not clear if also personnel and outcome assessors were blind to treatment assignment |
| Blinding? Objective outcomes | Low risk | "Diphenylhydantoin or placebo were blindly administered." " Diphenylhydantoin and placebo were placed in gelatine capsules" COMMENT: it is not clear if also personnel and outcome assessors were blind to treatment assignment but outcomes are unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 8 patients from diphenylhydantoin group and 13 from the placebo group were excluded from the study. Reason for exclusion given |
Santo 1985.
| Methods | Randomized clinical trial | |
| Participants | No. = 38; Gender: All males
Age: Group 1: 44 years, Group 2: 43 years. Inclusion criteria: Patients with alcohol withdrawal syndrome hospitalised for reasons other than alcoholism Exclusion criteria: severe delirium tremens. |
|
| Interventions | Group A (15) Tetrabamate 300 mg; Group B (17) Tiapride 100 mg. | |
| Outcomes | Efficay: Number of patients with withdrawal symptoms at day 14; duration of symptoms per group | |
| Notes | Study in Spanish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | a tables of random numbers was used |
| Allocation concealment? | Unclear risk | a tables of random numbers was used |
| Blinding? subjective outcomes | Low risk | study described as double blind Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | study described as double blind Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 3 patients from each group were excluded from the study. Reason for exclusion given |
Schik 2005.
| Methods | Randomized clinical trial | |
| Participants | No.= 29 Gender: 83 % male; mean age: 41. Inclusion criteria: voluntary admitted to hospital with a alcohol withdrawal syndrome according to ICD‐10 Exclusion criteria: polydrug abuse; delirium tremens upon admission; severe hepatic or haematological complications; hyponatraemia; abnormal ECG; known epileptic seizures; history of head injury; neuroleptic or antiepileptic medication as well as MAO‐inhibitor intake |
|
| Interventions |
Group A (14 ) carbamazepine, 600 mg on days 1 ‐ 3, 300 mg on day 4 and a last dose of 100 mg on day 5. Group B (15) oxcarbazepine , 900 mg on days 1 ‐ 3, 450 mg on day 4 and a final dose of 150 mg on day 5 |
|
| Outcomes | Efficacy: Withdrawal symptom (CIWA‐Ar); Acceptability: subjective perception of side affect and symptoms (VAS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "14 patients were assigned randomly to each of the two treatment groups". |
| Allocation concealment? | Unclear risk | "14 patients were assigned randomly to each of the two treatment groups." |
| Blinding? subjective outcomes | High risk | single blind "The type of medication was not disclosed to the patient, but the physician who measured withdrawal symptoms of the particular patient was aware of the treatment regimen." |
| Blinding? Objective outcomes | Low risk | "The type of medication was not disclosed to the patient, but the physician who measured withdrawal symptoms of the particular patient was aware of the treatment regimen." COMMENT: obljective outcomes are unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Seifert 2004.
| Methods | Randomized controlled trial | |
| Participants | No. =159; Gender = 150 males/9 females; Age = 18‐83 Inclusion criteria: met the DSM‐III‐R criteria for alcohol dependence. Exclusion criteria: less than 18 years of age; chronic obstructive disease; poor pulmonary function or pneumonia; bradycardia; systolic blood pressure of less than 95 mmHg; second‐ or third‐degree atrioventricular node block; history of current use or abuse of benzodiazepines, barbiturates, clonidine, or beta‐adrenergic receptor blockers. |
|
| Interventions | Group A (21) carbamazepine; Group B (16) Chlormethiazole | |
| Outcomes | Efficacy; Memory test (Benton); Zahlen‐Verbindungs test ;Beck Depression Inventory; Anxiety Sensitivity Index; Verbal Lern‐ und Merkfahigkeitstest; Mehrfach‐Wortschatz‐Intelligenz test (MWT‐B); European Addiction Severity Index (EuropASI); Clinical Institute Withdrawal Scale (CIWA‐A); | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | study described as randomised |
| Allocation concealment? | Unclear risk | study described as randomised |
| Blinding? subjective outcomes | High risk | study described as single blind COMMENT: personnel and outcome assessor not blinded |
| Blinding? Objective outcomes | Low risk | study described as single blind COMMENT: oucomes unlikely to be biased by lack of blindness |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Spies 1996.
| Methods | Randomised controlled trial | |
| Participants | No. = 159; Gender: 94% male; age range: 18‐83 years Inclusion criteria: multiple‐injured alcohol‐dependent patients who met the DSM‐III‐R criteria for alcohol dependence. Exclusion criteria: age < 18 years; chronic obstructive lung disease and poor pulmonary function or pneumonia; bradycardia (heart rate < 45/min); systolic blood pressure < 95 mmHg; second‐ or third‐degree atrioventricular node block; history of current use or abuse of benzodiazepines, barbiturates, clonidine, or beta‐adrenergic receptor blockers |
|
| Interventions | Group A (50) Clomethiazole/haloperidol, Group B (55) flunitrazepam/haloperidol, Group C (54) flunitrazepam/clonidine. | |
| Outcomes | Efficacy: changes in CIWA‐Ar scores, Duration of controlled or assisted mechanical ventilation; Safety: mortality; Acceptability: dropouts, dropouts due to adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patient was randomised to receive one of the three treatment" |
| Allocation concealment? | Unclear risk | "Patient was randomised to receive one of the three treatment" |
| Blinding? subjective outcomes | Unclear risk | Study reported as blinded. "The investigator who documented the alcoholism related history was unaware of the treatment and of the complication during the ICU stay" COMMENT: it is not clear if the patients and outcome assessor were blinded. |
| Blinding? Objective outcomes | Low risk | Study reported as blinded. "The investigator who documented the alcoholism related history was unaware of the treatment and of the complication during the ICU stay" COMMENT: outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 6 patients from the flunitrazepam/clonidine group, 10 patients from the Chlormethiazole/haloperidol group, 5 patients in the flunitrazepam/haloperidol group were excluded from the studies for medical reasons described in the study |
Stanhope 1989.
| Methods | Randomized controlled trial | |
| Participants | No. = 304; Gender = 256 males; 48 females
age = 40.5 years (mean age for carbamazepine); 39.9 years (mean age for placebo) Inclusion criteria Patients presenting for detoxification from alcohol Exclusion criteria: allergy to carbamazepine or Chlormethiazole reported before entry; known use of anticonvulsant before entry; previous repeated early self‐discharge; two admissions by Langton Centre terminated under 3 days duration; a previous admission already included in the study; alcohol not the principal drug of dependence. |
|
| Interventions | Group A (157) carbamazepine; Group B (147) placebo | |
| Outcomes | Efficacy: Daily withdrawal scores; seizures;Acceptability: Mode of discharge (completed detoxifications); Length of stay;Safety: adverse events; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | "Alternate allocation was used rather than random allocation for logistic reason. The initial case was allocated randomly; and on five subsequent occasions, when the code was inadvertently broken, a fresh random start was made" |
| Allocation concealment? | High risk | "Alternate allocation was used rather than random allocation for logistic reason. The initial case was allocated randomly; and on five subsequent occasions, when the code was inadvertently broken, a fresh random start was made" |
| Blinding? subjective outcomes | Unclear risk | "Identical tablets were supplied by Ciba Geigy" COMMENT: it is not clear if personnel and outcome assessor were blind |
| Blinding? Objective outcomes | Low risk | "Identical tablets were supplied by Ciba Geigy" COMMENT: outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | 7 patients withdrawn from the study. Reason for withdrawn given. Not specified form which group patients withdrawn, but numbers are small |
Stuppaeck 1992.
| Methods | Randomised controlled trial | |
| Participants | No. = 60; Gender: 82% male Inclusion criteria: DSM‐III criteria for alcohol dependence; CIWA‐Ar score > 20 on admission; be able to sign informed consent. Exclusion criteria: age <18 or >65 years; severe somatic illness; polysubstance dependence; pre‐treatment with psychotropic drugs; full‐blown alcohol delirium |
|
| Interventions | Group A (29) carbamazepine, Group B (29) oxazepam Additional B‐polyvitamin compound was given orally. | |
| Outcomes | Efficacy: seizures, delirium, changes in CIWA‐Ar scores; self‐rating adjective checklist; CGI scale; Safety: adverse events, mortality; Acceptability: dropouts, dropouts due to adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients were randomly allocated to a double blind study comparing CBZ abs oxazepam" |
| Allocation concealment? | Unclear risk | "Patients were randomly allocated to a double blind study comparing CBZ abs oxazepam" |
| Blinding? subjective outcomes | Low risk | Study defined as double blind. "Oxazepam and CBZ were administered in identical capsules". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Blinding? Objective outcomes | Low risk | Study defined as double blind. "Oxazepam and CBZ were administered in identical capsules". Blinding of outcomes assessor: it was not stated if the outcome assessor was blind, but because the outcomes were recorded during the treatment we judged that they were assessed by the blind personnel who gave the treatment |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "4 patients from each group dropped out from treatment." Reason for drop out given |
Stuppaeck 1998.
| Methods | Randomized controlled trial | |
| Participants | No. = 38. Inclusion criteria: Inpatients met DSM‐IV criteria for alcohol dependence and a minimum CIWA ‐A score of 18. |
|
| Interventions | Group A (19) vigabatrin 2000 mg/day from day1‐3, then placebo from day4‐7; Group B (19) oxazepam 120 mg/day from day1‐3 and 90 mg/day from day 4‐ | |
| Outcomes | Efficacy: Modified German version of the Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA‐A); Clinical Global Impression Scale (CGI); Visual Analogue Scale (VAS); Fischer's Unwanted Symptoms Check List (FUSCL) | |
| Notes | Meeting abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | unclear |
| Allocation concealment? | Unclear risk | unclear |
| Blinding? subjective outcomes | Low risk | double blind |
| Blinding? Objective outcomes | Low risk | double blind |
| Incomplete outcome data addressed? All outcomes except withdrawal | Unclear risk | unclear |
Teijeiro 1975.
| Methods | Randomized Controlled Trial | |
| Participants | No. = 55 | |
| Interventions | Group A (30) atrium 300 mg; Group B (25) Heminiurine | |
| Outcomes | Efficacy: Tolerance; Tremor; Anxiety | |
| Notes | Study in French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | B Unclear |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? subjective outcomes | Unclear risk | Unclear |
| Blinding? Objective outcomes | Unclear risk | Unclear |
| Incomplete outcome data addressed? All outcomes except withdrawal | Unclear risk | Unclear |
Thompson 1975.
| Methods | Randomized controlled trial | |
| Participants | No. = 34; Gender = 26 males/8 females
Age = 22‐58 Inclusion criteria: Patients admitted consecutively to the Osler Medical Service with advanced delirium tremens |
|
| Interventions | Group A (17) paraldehyde 10 ml rectally every 30 minutes; Group B (17) diazepam 10 mg intravenously initially and then mg intravenously every 5 minutes | |
| Outcomes | Efficacy: Time for induction of a calm state; duration of delirium ; dose of diazepam and paraldehyde given after initial calming until patient was entirely recovered from delirium tremens; Safety: adverse events . | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "17 patients have delirium tremens not complicated by other serious illnesses; 17 patients had delirium tremens complicated by serious ilness. The two groups were allocated to therapy by means of separate series of sealed envelopes ordered from random number tables" |
| Allocation concealment? | Low risk | "17 patients have delirium tremens not complicated by other serious illnesses; 17 patients had delirium tremens complicated by serious ilness. The two groups were allocated to therapy by means of separate series of sealed envelopes ordered from random number tables" |
| Blinding? subjective outcomes | High risk | blindness not mentioned COMMENT: the study was judged not blind because the way of administration were different for two treatments |
| Blinding? Objective outcomes | Low risk | blindness not mentioned COMMENT: outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | no withdrawn from the study |
Tubridy 1988.
| Methods | Randomised controlled trial | |
| Participants | No. = 102; Age range: 21‐65 years Inclusion criteria: alcohol dependent participants admitted to St John of God Hospital, Dublin. Exclusion criteria: patients sensitive to benzodiazepines or Chlormethiazole; dependent on or abused substances other than alcohol; psychotic or suffered from another mental disorder; serious physical illness; pregnant or lactating; taking other psychotropic drugs; history of withdrawal seizures |
|
| Interventions | Group A (49) Chlormethiazole, Group B (51) alprazolam All patients received daily intravenous injections of vitamin B complex and ascorbic acid on days 1‐5 | |
| Outcomes | Efficacy: seizures, global improvement, doctor's assessment of efficacy, patient's assessment of efficacy, Patient's general physical condition; presence of symptoms of alcohol withdrawal throughout the trial; HARS; doctor's and patient's global ratings of well‐being; changes in blood tests measures; Safety: adverse events, severe life‐treating adverse events, mortality; Acceptability: dropouts, dropouts due to adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Patients were randomised to one or the two treatment group by consecutive assignment of patients identification numbers that had previously been distributed between the two treatments by a computer generated list of random numbers" |
| Allocation concealment? | Unclear risk | "Patients were randomised to one or the two treatment group by consecutive assignment of patients identification numbers that had previously been distributed between the two treatments by a computer generated list of random numbers" |
| Blinding? subjective outcomes | Unclear risk | blindness not mentioned |
| Blinding? Objective outcomes | Low risk | blindness not mentioned COMMENT: outcomes unlikely to be biased by lack of blinding |
| Incomplete outcome data addressed? All outcomes except withdrawal | Low risk | "Data from two patients in each group were lost" |
ACL: Adjective Check List; ACS: Alcohol Craving Scale; ADS: Alcohol Dependence Scale; ALT: Alanine Aminotransferase Transaminase; ASI: Addiction Severity Index; ASQ: Abstinence Symptoms Questionnaire; AST: Aspartate Aminotransferase Transaminase; AWS: alcohol withdrawal symptoms; BAC: Blood Alcohol Concentration; BDHI: Buss‐Durkee Hostility Inventory BDI: Beck Depression Inventory; CGI: Clinical Global Impression; CIWA‐Ar: Rivised Clinical Institude Withdrawal Assessment for Alcohol; CLO: Chlormethiazole; CNS: Central Nervous System CPRS: Comprehensive Psychiatric Rating Scale; CSE: Clinical Side‐Effect Scale; DES: Daily Estimation Scale; DOTES: Dosage Record and Treatment Emergent Symptom Scale; DSM III: Diagnostic and Statistical Manual for Mental Disorders, third edition; DSM IV: Diagnostic and Statistical Manual for Mental Disorders, fourth edition; DSST: Digit Symbol Substitution Test; EuropASI: European Addiction Severity Index; FUSCL: Fischer's Unwanted Symptoms Check List; GGT: Gamma Glutamyl Transferase HARS: Hamilton Anxiety Rating Scale; HSCL: Hopkins Symptoms Check List; ICD‐10: Internationa Classification of Diseases; LCRR‐1: Lubeck Craving‐Risk‐Relapse questionnaire; MADRS: MontgomeryÅsberg Depression Rating Scale; MAO Inhibitors: Monoamine Oxidase inhibitors; MAWS: Mainz Alcohol Withdrawal Scale; MMSE: Mini‐Mental State Examination; MSSA: Modified Selective Severity Assessment; MWT‐B: Mehrfach‐Wortschatz‐Intelligenz test; NGI: Nurse's Global Impression; NOSIE: nurse's observation scale for inpatient evaluation; OCDS: Obsessive Compulsive Drinking Scale; PPT: Purdue Pegboard Test; PSQI: Pittsburgh Sleep Quality Index; RCT: randomised controlled trial; RDS: Reward Deficiency Syndrome REG: Rada‐Extensive Grid; SCL‐90‐R: Symptom Checklist 90 revised SGOT: Serum Glutamate Oxaloacetate Transaminase; SGPT: Serum Glutamic Pyruvic Transaminase; SIP: Short Index of Problems; SKT: Syndrom‐kurztest; SPS: Sleep Promoting Substance SSA: Selected Severity Assessment; STAI: State Anxiety Inventory; TLFB: Time Line Follow Back; TSA: Total Severity Assessment; VAS: visual analogue scale; VCS: Visual Craving Scale; VSS: Visual Success Scale; WHO: World Health Organization WSR: Withdrawal Symptom Rating;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Addolorato 2002 | Type of intervention not in the inclusion criteria |
| Adinoff 1994 | Type of intervention not in the inclusion criteria |
| Aliyev 2008 | Type of intervention not in the inclusion criteria |
| Ansoms 2000 | Type of study design not in the inclusion criteria |
| Anton 2009 | Outcome measures presented in a way not suitable for meta‐analysis |
| Athen 1976 | Type of intervention not in the inclusion criteria |
| Athen 1977 | Duplicate publication |
| Athen 1986 | Study design not in the inclusion criteria |
| Balldin 1992 | Study design not in the inclusion criteria |
| Baltieri 2003 | Type of intervention not in the inclusion criteria |
| Banki 1983 | Study design not in the inclusion criteria |
| Block 1974 | Type of outcome measures not in the inclusion criteria |
| Bonnet 2009 | Study design not in the inclusion criteria |
| Borg 1980 | Study design not in the inclusion criteria |
| Brady 2002 | Type of outcome measures not in the inclusion criteria |
| Bullock 1989 | Type of intervention not in the inclusion criteria |
| Caspari 1992 | Study design not in the inclusion criteria |
| Cazzato 1982 | Study design not in the inclusion criteria |
| Chick 2000 | Type of intervention not in the inclusion criteria |
| Cushman 1985 | Type of intervention not in the inclusion criteria |
| Delamaire 1986 | Type of outcome measures not in the inclusion criteria |
| Demmel 2000 | Type of outcome measures not in the inclusion criteria |
| Dobrydnjov 2004 | Type of intervention not in the inclusion criteria |
| Franz 2001 | Study design not in the inclusion criteria |
| Frecker 1982 | Study design not in the inclusion criteria |
| Gallant 1989 | Study design not in the inclusion criteria |
| Gallant 1992 | Study design not in the inclusion criteria |
| Glatt 1965 | Duplicate publication |
| Gordon 2006 | Type of intervention not in the inclusion criteria |
| Gottesleben 1995 | Type of intervention not in the inclusion criteria |
| Griffith 1986 | Type of outcome measures not in the inclusion criteria |
| Gual 2001 | Type of intervention not in the inclusion criteria |
| Gual 2002 | Type of intervention not in the inclusion criteria |
| Guarino 1989 | Type of outcome measures not in the inclusion criteria |
| Hague 1976 | Type of intervention not in the inclusion criteria |
| Harfst 1967 | Study design not in the inclusion criteria |
| Heil 1990 | Type of outcome measures not in the inclusion criteria |
| Hillbom 1989 | Type of outcome measures not in the inclusion criteria |
| Hutchison 2001 | Type of intervention not in the inclusion criteria |
| Ilyuchina 1995 | Study design not in the inclusion criteria |
| Janiri 1996 | Type of intervention not in the inclusion criteria |
| Johnson 2003 | Type of outcome measures not in the inclusion criteria |
| Johnson 2004 | Type of outcome measures not in the inclusion criteria |
| Kaim 1974 | Study design not in the inclusion criteria |
| Kaplan 1972 | Study design not in the inclusion criteria |
| Karam‐Hage 2003 | Type of outcome measures not in the inclusion criteria |
| Karst 2002 | Type of intervention not in the inclusion criteria |
| Keaney 2001 | Type of intervention not in the inclusion criteria |
| Kiefer 2003 | Type of intervention not in the inclusion criteria |
| Kramp 1979 | Type of outcome measures not in the inclusion criteria |
| Kranzler 1996 | Type of outcome measures not in the inclusion criteria |
| Kraus 1985 | Type of intervention not in the inclusion criteria |
| Lange‐Ass 2003 | Study design not in the inclusion criteria |
| Le Bon 2003 | Type of intervention not in the inclusion criteria |
| Le Fauve 2004 | Type of intervention not in the inclusion criteria |
| Leivonen 1966 | Study design not in the inclusion criteria |
| Loo 1986 | Type of outcome measures not in the inclusion criteria |
| Malcolm 1992 | Type of intervention not in the inclusion criteria |
| Martinotti 2007 | Type of intervention not in the inclusion criteria |
| Mendels 1985 | Type of intervention not in the inclusion criteria |
| Miller 1988 | Type of outcome measures not in the inclusion criteria |
| Montejo 2007 | Study design not in the inclusion criteria |
| Mueller 1997 | Type of intervention not in the inclusion criteria |
| Mukherjee 1983 | Type of intervention not in the inclusion criteria |
| Muller 1969 | Study design not in the inclusion criteria |
| Myrick 2009 | Outcome measures presented in a way not suitable for meta‐analysis |
| Naranjo 2000 | Type of intervention not in the inclusion criteria |
| Newman 1995 | Study design not in the inclusion criteria |
| Pettinati 2000 | Type of intervention not in the inclusion criteria |
| Ponce 2005 | Study design not in the inclusion criteria |
| Rodgers 1999 | Study design not in the inclusion criteria |
| Rubio 2002 | Type of intervention not in the inclusion criteria |
| Rychlik 2001 | Type of outcome measures not in the inclusion criteria |
| Sass 1996 | Type of intervention not in the inclusion criteria |
| Schmidt 1994 | Type of outcome measures not in the inclusion criteria |
| Schulte 1987 | Study design not in the inclusion criteria |
| Schwarz 1982 | Type of outcome measures not in the inclusion criteria |
| See 2002 | Duplicate publication |
| Shaw 1994 | Type of intervention not in the inclusion criteria |
| Sillanpaa 1979 | Duplicate publication |
| Smith 1987 | Type of intervention not in the inclusion criteria |
| Snel 1983 | Type of intervention not in the inclusion criteria |
| Sobcyzk 1980 | Study design not in the inclusion criteria |
| Solomon 1983 | Type of intervention not in the inclusion criteria |
| Spies 1995 | Type of outcome measures not in the inclusion criteria |
| Sternebring 1983 | Study design not in the inclusion criteria |
| Stojek 1987 | Study design not in the inclusion criteria |
| Streeton 2001 | Study design not in the inclusion criteria |
| Swift 2003 | Study design not in the inclusion criteria |
| Tacke 1975 | Type of intervention not in the inclusion criteria |
| Teijeiro 1976 | Type of outcome measures not in the inclusion criteria |
| Tempesta 2000 | Type of intervention not in the inclusion criteria |
| Thomas 1964 | Study design not in the inclusion criteria |
| Tress 1977 | Study design not in the inclusion criteria |
| Wadstein 1986 | Duplicate publication |
| Wegner 1965 | Study design not in the inclusion criteria |
| Welbel 1982 | Study design not in the inclusion criteria |
| Whitworth 1996 | Type of intervention not in the inclusion criteria |
| Wiesbeck 1999 | Type of intervention not in the inclusion criteria |
AW: alcohol withdrawal CCT: controlled clinical trial RCT: randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Gottesleben 1994.
| Methods | RCT |
| Participants | 28 patients during drug therapy for alcoholic pre delirium |
| Interventions | slow‐release carbamazepine (Timonil registered trade mark 150 retard) versus Chlomethiazole |
| Outcomes | duration and severity of withdrawal symptoms. |
| Notes | we are trying to find the study |
Hart 1964.
| Methods | it is impossible from title to know the study design |
| Participants | 175 of alcohol withdrawal |
| Interventions | promazine and paraldehyde |
| Outcomes | it is impossible from title to know which outcomes were considered |
| Notes | we are trying to find the study |
Trevisan 2008.
| Methods | RCT |
| Participants | Fifty‐seven male veterans presenting for alcohol detoxification |
| Interventions | valproic acid, gabapentin, or placebo for a total of 4 weeks. |
| Outcomes | symptoms of physiologic withdrawal during the first 5 days of treatment and doses of benzodiazepine required; |
| Notes | we are trying to find the study |
Contributions of authors
Vecchi performed the literature searches; Mitrova organised papers collection; Minozzi and Amato reviewed the papers, abstracted data from the papers for meta‐analysis. Amato and Minozzi wrote abstract, introduction, discussion and conclusions sections. Minozzi wrote methods , assessed methodological quality of included studies and wrote results sections. Davoli supervised to all the process and all authors provided comments to the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
EDAP Project (Evidence for Drugs and Alcohol Policy) sponsored by the European Community‐ Directorate Public Health (Grant Agreement SPC.2002454), Not specified.
Declarations of interest
None.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Agricola 1982 {published data only}
- Agricola R, Mazzarino M, Urani R, Gallo V, Grossi E. Treatment of acute alcohol withdrawal syndrome with carbamazepine: a double‐blind comparison with tiapride. Journal of Internal Medical Research 1982;10(3):160‐5. [DOI] [PubMed] [Google Scholar]
Alldredge 1989 {published data only}
- Alldredge BK, Lowenstein DH, Simon RP. Placebo‐controlled trial of intravenous diphenylhydantoin for short‐term treatment of alcohol withdrawal seizures. American Journal of Medicine 1989;87(6):645‐8. [DOI] [PubMed] [Google Scholar]
Balldin 1986 {published data only}
- Balldin J, Bokstrom K. Treatment of alcohol abstinence symptoms with the alpha 2‐agonist clonidine. Acta Psychiatrica Scandinavica Supplementum 1986;327:131‐43. [PubMed] [Google Scholar]
Bjorkqvist 1976 {published data only}
- Bjorkqvist SE, Isohanni M, Makela R, Malinen L. Ambulant treatment of alcohol withdrawal symptoms with carbamazepine: a formal Multicentre double‐blind comparison with placebo. Acta Psychiatrica Scandinavica 1976;53(5):333‐42. [DOI] [PubMed] [Google Scholar]
Blanchard 1985 {published data only}
- Blanchard C. Atrium 300 and Alcohol Withdrawal: Double‐Blind Placebo‐Controlled Study in 38 hospitalised patients [Atrium 300 et Sevrage Alcoolique. Etude en Double Aveugle Versus Placebo chez 38 malades hospitalises]. Psychological Medicine (Paris) 1985;17(1):159‐63. [Google Scholar]
Bonnet 2003 {published data only}
- Bonnet U, Banger M, Leweke FM, Specka M, Muller BW, Hashemi T, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two‐centre trial. Journal of Clinical Psychopharmacology 2003;23(5):514‐9. [DOI] [PubMed] [Google Scholar]
- Bonnet U, Speeka M, Leweke FM, Nyhuis P, Banger M. Gabapentin's acute effect on mood profile ‐ a controlled study on patients with alcohol withdrawal. Progress in Neuro‐Pharmacology & Biological Psychiatry 2007;31:434‐438. [DOI] [PubMed] [Google Scholar]
Borg 1986 {published data only}
- Borg S, Kvande H, Valverius P. Clinical conditions and central dopamine metabolism in alcoholics during acute withdrawal under treatment with different pharmacological agents. Psychopharmacology (Berlin) 1986;88(1):12‐7. [DOI] [PubMed] [Google Scholar]
Burroughs 1985a {published data only}
- Burroughs AK, Morgan MY, Sherlock S. Double‐blind controlled trial of bromocriptine, chlordiazepoxide and Chlormethiazole for alcohol withdrawal symptoms. Alcohol and Alcoholism 1985;20(3):263‐71. [PubMed] [Google Scholar]
Burroughs 1985b {published data only}
- Burroughs AK, Morgan MY, Sherlock S. Double‐blind controlled trial of bromocriptine, chlordiazepoxide and Chlormethiazole for alcohol withdrawal symptoms. Alcohol and Alcoholism 1985;20(3):263‐71. [PubMed] [Google Scholar]
Chance 1991 {published data only}
- Chance JF. Emergency department treatment of alcohol withdrawal seizures with phenytoin. Annals of Emergency Medicine 1991;20(5):520‐2. [DOI] [PubMed] [Google Scholar]
Choi 2005 {published data only}
- Choi EA, Ki SW, Kim SE, Kim JW, Park JK. The efficacy and safety of topiramate in the treatment of alcohol withdrawal. Journal of Korean Neuropsychiatric Association 2005;44:328‐33. [Google Scholar]
Croissant 2009 {published data only}
- Croissant B, Loeber S, Diehl A, Nakovics H, Wagner F, Kiefer F, et al. Oxcarbazepine in combination with Tiaprid in inpatient alcohol‐withdrawal‐‐a RCT. Pharmacopsychiatry 2009;42:175 – 81. [DOI] [PubMed] [Google Scholar]
Dencker 1978 {published data only}
- Dencker SJ, Wilhelmson G, Carlsson E, Bereen FJ. Piracetam and Chlormethiazole in acute alcohol withdrawal: a controlled clinical trial. Journal of Internal Medical Research 1978;6(5):395‐400. [DOI] [PubMed] [Google Scholar]
Elsing 1996 {published data only}
- Elsing C, Schimanski U, Stremmel W. Randomized controlled trial for the treatment of alcohol withdrawal syndrome: gamma‐hydroxybutyric acid abstract. European Journal of Clinical Investigation 1996;26 S:A17. [Google Scholar]
Elsing 2009 {published data only}
- Elsing C, Stremmel W, Grenda U, Herrmann T. Gamma‐hydroxybutyric acid versus clomethiazole for the treatment of alcohol withdrawal syndrome in a medical intensive care unit: an open, single‐center randomized study. The American Journal of Drug and Alcohol Abuse 2009;35:189‐192. [DOI] [PubMed] [Google Scholar]
Flygering 1984 {published data only}
- Flygenring J, Hansen J, Holst B, Petersen E, Sorensen A. Treatment of alcohol withdrawal symptoms in hospitalised patients. A randomised, double‐blind comparison of carbamazepine (Tegretol) and barbital (Diemal). Acta Psychiatrica Scandinavica 1984;69(5):398‐408. [DOI] [PubMed] [Google Scholar]
Gann 2004 {published data only}
- Gann H, Feige B, Cloot O, Wasen H, Zinzgraf D, Hohagen F, et al. Polysomnography during withdrawal with Chlormethiazole or placebo in alcohol dependent patients‐‐a double‐blind and randomised study. Pharmacopsychiatry 2004;37:228‐35. [PUBMED: 15359376] [DOI] [PubMed] [Google Scholar]
Glatt 1966 {published data only}
- Glatt MM, George HR, Frisch EP. Evaluation of Chlormethiazole in treatment for alcohol withdrawal syndrome. Results of a controlled trial. Acta Psychiatrica Scandinavica Supplementum 1966;192:121‐37. [DOI] [PubMed] [Google Scholar]
Golbert 1967 {published data only}
- Golbert TM, Sanz CJ, Rose HD, Leitschuh TH. Comparative evaluation of treatments of alcohol withdrawal syndromes. Journal of the American Medical Association 1967;201(2):99‐102. [PubMed] [Google Scholar]
Kaim 1972 {published data only}
- Kaim SC, Klett CJ. Treatment of delirium tremens: a comparative evaluation of four drugs. Quarterly Journal of Studies on Alcohol 1972;33:1065‐72. [PubMed] [Google Scholar]
Kalyoncu 1996 {published data only}
- Kalyoncu O, Beyazyurek M, Kuru L, Solukcu R, Yazman U. Double‐blind comparative trial with carbamazepine vs diazepam treatment of alcohol withdrawal. European Neuropsychopharmacology 1996;6 Suppl 3:1‐2. [Google Scholar]
Koethe 2007 {published data only}
- Koethe D, Juelicher A, Nolden BM, Braunwarth WD, Klosterkotter J, Niklewski G, et al. Oxcarbazepine‐‐efficacy and tolerability during treatment of alcohol withdrawal: a double‐blind, randomised, placebo‐controlled multicenter pilot study. Alcoholism, Clinical and Experimental Research 2007;31(7):1188‐94. [DOI] [PubMed] [Google Scholar]
Koppi 1987 {published data only}
- Koppi S, Eberhardt G, Haller R, Konig P. Calcium‐channel‐blocking agent in the treatment of acute alcohol withdrawal‐‐Caroverine versus meprobamate in a randomised double‐blind study. Neuropsychobiology 1987;17(1‐2):49‐52. [DOI] [PubMed] [Google Scholar]
Kramp 1978 {published data only}
- Kramp P, Rafaelsen OJ. Delirium tremens: a double‐blind comparison of diazepam and barbital treatment. Acta Psychiatrica Scandinavica 1978;58(2):174‐90. [DOI] [PubMed] [Google Scholar]
Krupitsky 2007 {published data only}
- Krupitsky EM, Rudenko AA, Burakov AA, Slavina TY, Grinenko AA, Pittman B, et al. Antiglutamatergic strategies for ethanol detoxification: comparison with placebo and diazepam. Alcoholism, Clinical and Experimental Research 2007;31(4):604‐11. [DOI] [PubMed] [Google Scholar]
Lambie 1980 {published data only}
- Lambie DG, Johnson RH, Vijayasenan ME, Whiteside EA. Sodium valproate in the treatment of the alcohol withdrawal syndrome. Australian and New Zeland Journal of Psychiatry 1980;14(3):213‐5. [DOI] [PubMed] [Google Scholar]
Lapierre 1983 {published data only}
- Lapierre YD, Bulmer DR, Oyewumi LK, Mauguin ML, Knott VJ. Comparison of chlormethiazole (Heminevrin) and chlordiazepoxide (Librium) in the treatment of acute alcohol withdrawal. Neuropsychobiology 1983;10(2‐3):127‐30. [DOI] [PubMed] [Google Scholar]
Longo 2002 {published data only}
- Longo LP, Campbell T, Hubatch S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. Journal of Addictive Diseases 2002;21(2):55‐64. [DOI] [PubMed] [Google Scholar]
Lucht 2003 {published data only}
- Lucht M, Kuehn KU, Armbruster J, Abraham G, Gaensicke M, Barnow S, et al. Alcohol withdrawal treatment in intoxicated vs non‐intoxicated patients: a controlled open‐label study with tiapride/carbamazepine, chlormethiazole and diazepam. Alcohol and Alcoholism 2003;38(2):168‐75. [DOI] [PubMed] [Google Scholar]
Madden 1969 {published data only}
- Madden JS, Jones D, Frisch EP. Chlormethiazole and trifluoperazine in alcohol withdrawal. British Journal of Psychiatry 1969;115(527):1191‐2. [DOI] [PubMed] [Google Scholar]
Malcolm 1989 {published data only}
- Malcolm R, Ballenger JC, Sturgis ET, Anton R. Double‐blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawal. American Journal of Psychiatry 1989;146(5):617‐21. [DOI] [PubMed] [Google Scholar]
Malcolm 2002 {published data only}
- Malcolm R, Myrick H, Roberts J, Wang W, Anton RF, Ballenger JC. The effects of carbamazepine and lorazepam on single versus multiple previous alcohol withdrawals in an outpatient randomised trial. Journal of General Internal Medicine 2002;17(5):349‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malcolm 2007 {published data only}
- Malcolm R, Myrick LH, Veatch LM, Boyle E, Randall PK. Self‐reported sleep, sleepiness, and repeated alcohol withdrawals: a randomised, double blind, controlled comparison of lorazepam vs gabapentin. Journal of Clinical Sleep Medicine 2007;3(1):24‐32. [PubMed] [Google Scholar]
Manhem 1985 {published data only}
- Manhem P, Nilsson LH, Moberg AL, Wadstein J, Hokfelt B. Alcohol withdrawal: effects of clonidine treatment on sympathetic activity, the renin‐aldosterone system, and clinical symptoms. Alcoholism, Clinical and Experimental Research 1985;9(3):238‐43. [DOI] [PubMed] [Google Scholar]
Mariani 2006 {published data only}
- Mariani JJ, Rosenthal RN, Tross S, Singh P, Anand OP. A randomised, open‐label, controlled trial of gabapentin and phenobarbital in the treatment of alcohol withdrawal. American Journal on Addictions 2006;15(1):76‐84. [DOI] [PubMed] [Google Scholar]
McGrath 1975 {published data only}
- McGrath SD. A controlled trial of Chlormethiazole and chlordiazepoxide in the treatment of the acute withdrawal phase of alcoholism. British Journal of Addiction to Alcohol and Other Drugs 1975;70 Suppl 1:81‐90. [DOI] [PubMed] [Google Scholar]
Murphy 1983 {published data only}
- Murphy DJ, Shaw GK, Clarke I. Tiapride and chlormethiazole in alcohol withdrawal: a double‐blind trial. Alcohol and Alcoholism 1983;18(3):227‐37. [Google Scholar]
Myrick 2000 {published data only}
- Myrick H, Brady KT, Malcolm R. Divalproex in the treatment of alcohol withdrawal. American Journal of Drug and Alcohol Abuse 2000;26(1):155‐60. [DOI] [PubMed] [Google Scholar]
Nimmerichter 2002 {published data only}
- Nimmerrichter AA, Walter H, Gutierrez‐Lobos KE, Lesch OM. Double‐blind controlled trial of gamma‐hydroxybutyrate and Chlormethiazole in the treatment of alcohol withdrawal. Alcohol and Alcoholism 2002;37(1):67‐73. [DOI] [PubMed] [Google Scholar]
Radouco‐Thomas 1989 {published data only}
- Radouco‐Thomas S, Garcin F, Guay D, Marquis PA, Chabot F, Huot J, et al. Double blind study on the efficacy and safety of Tetrabamate and chlordiazepoxide in the treatment of the acute alcohol withdrawal syndrome. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 1989;13(1‐2):55‐75. [DOI] [PubMed] [Google Scholar]
Rathlev 1994 {published data only}
- Rathlev NK, D'Onofrio G, Fish SS, Harrison PM, Bernstein E, Hossack RW, et al. The lack of efficacy of phenytoin in the prevention of recurrent alcohol‐related seizures. Annals of Emergency Medicine 1994;23(3):513‐8. [DOI] [PubMed] [Google Scholar]
Reoux 2001 {published data only}
- Reoux JP, Saxon AJ, Malte CA, Baer JS, Sloan KL. Divalproex sodium in alcohol withdrawal: a randomised double‐blind placebo‐controlled clinical trial. Alcoholism, Clinical and Experimental Research 2001;25(9):1324‐9. [PubMed] [Google Scholar]
Ritola 1981 {published data only}
- Ritola E, Malinen L. A double‐blind comparison of carbamazepine and chlormethiazole in the treatment of alcohol withdrawal syndrome. Acta Psychiatrica Scandinavica 1981;64(3):254‐9. [DOI] [PubMed] [Google Scholar]
Robinson 1989 {published data only}
- Robinson BJ, Robinson GM, Maling TJ, Johnson RH. Is clonidine useful in the treatment of alcohol withdrawal?. Alcoholism, Clinical and Experimental Research 1989;13(1):95‐8. [DOI] [PubMed] [Google Scholar]
Rosenthal 1998 {published data only}
- Rosenthal RN, Perkel C, Singh P, Anand O, Miner CR. A pilot open randomised trial of valproate and phenobarbital in the treatment of acute alcohol withdrawal. American Journal on Addictions 1998;7(3):189‐97. [DOI] [PubMed] [Google Scholar]
Rothstein 1973 {published data only}
- Rothstein E. Prevention of alcohol withdrawal seizures: the roles of diphenylhydantoin and chlordiazepoxide. American Journal of Psychiatry 1973;130(12):1381‐2. [DOI] [PubMed] [Google Scholar]
Sampliner 1974 {published data only}
- Sampliner R, Iber FL. Diphenylhydantoin control of alcohol withdrawal seizures. Results of a controlled study. Journal of the American Medical Association 1974;230(10):1430‐2. [PubMed] [Google Scholar]
Santo 1985 {published data only}
- Santo Domingo Carrasco J, Bravo Ortiz MF, Barroso Canizares A, Caballero Martin L. Double‐blind study of the efficacy of Tetrabamate and Tiapride in the treatment of alcohol deprivation syndrome [Estudio a doble ciego de la eficacia del tetrabamato y el tiapride en el tratamiento del sindrome de deprivacion alcoholica]. Medicina Clinica (Barcelona) 1985;85(13):533‐6. [PubMed] [Google Scholar]
Schik 2005 {published data only}
- Schik G, Wedegaertner FR, Liersch J, Hoy L, Emrich HM, Schneider U. Oxcarbazepine versus carbamazepine in the treatment of alcohol withdrawal. Addiction Biology 2005;10(3):283‐88. [DOI] [PubMed] [Google Scholar]
Seifert 2004 {published data only}
- Seifert J, Peters E, Jahn K, Metzner C, Ohlmeier M, Wildt B, et al. Treatment of alcohol withdrawal: chlormethiazole vs. carbamazepine and the effect on memory performance‐a pilot study. Addiction Biology 2004;9(1):43‐51. [DOI] [PubMed] [Google Scholar]
Spies 1996 {published data only}
- Spies CD, Dubisz N, Neumann T, Blum S, Muller C, Rommelspacher H, et al. Therapy of alcohol withdrawal syndrome in intensive care unit patients following trauma: results of a prospective, randomized trial. Critical Care Medicine 1996;24(3):414‐22. [DOI] [PubMed] [Google Scholar]
Stanhope 1989 {published data only}
- Stanhope JM. The use of carbamazepine in chormethiazole‐modified withdrawal from alcohol. Australian Drug and Alcohol Review 1989;8:5‐8. [Google Scholar]
Stuppaeck 1992 {published data only}
- Stuppaeck CH, Pycha R, Miller C, Whitworth AB, Oberbauer H, Fleischhacker WW. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double‐blind study. Alcohol and Alcoholism 1992;27(2):153‐8. [PubMed] [Google Scholar]
Stuppaeck 1998 {published data only}
- Stuppaeck C, Whitworth A, Deisenhammer E, Honeder M, Kurz M, Telser S. Vigabatrin in the treatment of alcohol withdrawal syndrome : a double‐blind , randomized study. XXIst Collegium Internationale Neuro‐psychopharmacologicum, Glasgow, Scotland.12th‐16th July, 1998 1998:PW13010.
Teijeiro 1975 {published data only}
- Teijeiro J. A double‐blind comparative study of atrium 300 and Heminiurine in chronic alcoholics [Etude comparee en double‐aveugle de l'atrium 300 et de l'hemineurine chez des alcooliques chroniques]. Medicine et Hygiene (Geneve) 1975;33(1164):1141‐2. [PubMed] [Google Scholar]
Thompson 1975 {published data only}
- Thompson WL, Johnson AD, Maddrey WL. Diazepam and paraldehyde for treatment of severe delirium tremens. A controlled trial. Annals of Internal Medicine 1975;82(2):175‐80. [DOI] [PubMed] [Google Scholar]
Tubridy 1988 {published data only}
- Tubridy P. Alprazolam versus Chlormethiazole in acute alcohol withdrawal. British Journal of Addiction 1988;83(5):581‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Addolorato 2002 {published data only}
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double‐blind randomised controlled study. Alcohol and Alcoholism 2002;37(5):504‐8. [DOI] [PubMed] [Google Scholar]
Adinoff 1994 {published data only}
- Adinoff B. Double‐blind study of alprazolam, diazepam, clonidine, and placebo in the alcohol withdrawal syndrome: preliminary findings. Alcoholism, Clinical and Experimental Research 1994;18(4):873‐8. [DOI] [PubMed] [Google Scholar]
Aliyev 2008 {published data only}
- Aliyev ZN, Aliyev NA. Valproate treatment of acute alcohol hallucinosis: a double‐blind, placebo‐controlled study. Alcohol and Alcoholism 2008;43(4):456‐9. [DOI] [PubMed] [Google Scholar]
Ansoms 2000 {published data only}
- Ansoms C, Deckers F, Lehert P, Pelc I, Potgieter A. An open study with Acamprosate in Belgium and Luxemburg: results on socio‐ demographics, supportive treatment and outcome. European Addiction Research 2000;6(3):132‐40. [DOI] [PubMed] [Google Scholar]
Anton 2009 {published data only}
- Anton RF, Myrick H, Baros AM, Latham PK, Randall PK, Wright TM, et al. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. Journal of Clinical Psychopharmacology 2009;29(4):334‐42. [DOI] [PubMed] [Google Scholar]
Athen 1976 {published data only}
- Athen D, Bender W, Meyendorf R. Criteria of the efficacy of therapeutic measures in alcoholic delirium. Study on the effectiveness of aprotinin in alcoholic delirium [Beurteilung der Wirksamkeit therapeutischer Massnahmen beim Alkoholdelir. Untersuchung uber die Wirksamkeit von Aprotinin beim Alkoholdelir]. Psychiatria Clinica 1976;9(3‐4):183‐98. [PubMed] [Google Scholar]
Athen 1977 {published data only}
- Athen D, Hippius H, Meyendorf R, Riemer C, Steiner C. Comparison of the effectiveness of neuroleptics and Chlormethiazole in the treatment of alcoholic delirium (author's translation) [Ein Vergleich der Wirksamkeit von Neuroleptika und Chlormethiazol bei der Behandlung des Alkoholdelirs]. Nervenarzt (Berlin) 1977;48(10):528‐32. [PubMed] [Google Scholar]
Athen 1986 {published data only}
- Athen D. Comparative investigation of Chlormethiazole and neuroleptic agents in the treatment of alcoholic delirium. Acta Psychiatrica Scandinavica Supplementum 1986;329:167‐70. [DOI] [PubMed] [Google Scholar]
Balldin 1992 {published data only}
- Balldin J, Berggren U, Engel J, Lindstedt G, Sundkler A, Walinder J. Alpha‐2‐adrenoceptor sensitivity in early alcohol withdrawal. Biological Psychiatry 1992;31(7):712‐9. [DOI] [PubMed] [Google Scholar]
Baltieri 2003 {published data only}
- Baltieri DA, Andrade AG. Efficacy of Acamprosate in the treatment of alcohol‐dependent outpatients. Revista Brasileira de Psiquiatria 2003;25(3):156‐9. [DOI] [PubMed] [Google Scholar]
Banki 1983 {published data only}
- Banki CM. Comparative study with Grandaxin and diazepam in alcohol withdrawal syndrome and geronto psychiatric diseases. Therapia Hungarica 1983;31(3):120‐5. [PubMed] [Google Scholar]
Block 1974 {published data only}
- Block C, Borendal‐Jansson B, Carlsson C. A double‐blind crossover comparison between Chlothiapine and diazepam in the treatment of mental symptoms in chronic alcoholics. International Journal of Clinical Pharmacology 1974;9(4):321‐5. [PubMed] [Google Scholar]
Bonnet 2009 {published data only}
- Bonnet U, Hamzavi‐Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol & Alcoholism 2010;45:143‐5. [DOI] [PubMed] [Google Scholar]
Borg 1980 {published data only}
- Borg V. Clinical trial of Tegretol and Anafranil/Sinequan in alcohol abstinence symptoms [Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke]. Tidsskrift for den Norske Laegeforening 1980;100(5):277‐8. [PubMed] [Google Scholar]
Brady 2002 {published data only}
- Brady KT, Myrick H, Henderson S, Coffey SF. The use of divalproex in alcohol relapse prevention: a pilot study. Drug and Alcohol Dependence 2002;67(3):323‐30. [DOI] [PubMed] [Google Scholar]
Bullock 1989 {published data only}
- Bullock ML, Culliton PD, Olander RT. Controlled trial of acupuncture for severe recidivist alcoholism. The Lancet 1989;1(8652):1435‐9. [DOI] [PubMed] [Google Scholar]
Caspari 1992 {published data only}
- Caspari D, Wappler M, Bellaire W. Treatment of delirium tremens‐‐a comparison between Chlormethiazole and clorazepate with reference to effectiveness and rate of side effects [Zur Behandlung des Delirium tremens‐‐ein Vergleich zwischen Clomethiazol und Clorazepat hinsichtlich Effektivitat und Nebenwirkungsrate]. Psychiatrische Praxis 1992;19(1):23‐7. [PubMed] [Google Scholar]
Cazzato 1982 {published data only}
- Cazzato G, Gioseffi M, Torre P, Coppola N. Prevention and therapy of delirium tremens with tiapride and chlordesmethyldiazepam [Prevenzione e terapia del delirium tremens con tiapride e clordemetildiazepam]. Rivista di Neurologia 1982;52(6):331‐42. [PubMed] [Google Scholar]
Chick 2000 {published data only}
- Chick J, Howlett H, Morgan MY, Ritson B. United Kingdom Multicentre Acamprosate Study (UKMAS): a 6‐month prospective study of Acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol and Alcoholism 2000;35(2):176‐87. [DOI] [PubMed] [Google Scholar]
Cushman 1985 {published data only}
- Cushman P Jr, Lerner W, Cramer M. Adrenergic agonist therapy in alcohol withdrawal states in man. Psychopharmacology Bulletin 1985;21(3):651‐6. [PubMed] [Google Scholar]
Delamaire 1986 {published data only}
- Delamaire D, Carpentier MC, Eudier F. Trial of Tiapride in patients discharged after hospitalisation for alcohol withdrawal. A randomised, double‐blind trial in fifty‐one patients with alcohol related liver disease. La semaine des hopitaux: organe fonde par l' Association d' enseignement medical des hopitaux de Paris 1986;62(39):3109‐14. [Google Scholar]
Demmel 2000 {published data only}
- Demmel R, Rist F, Olbrich R. Autonomic reactivity to mental stressors after single administration of lorazepam in male alcoholics and healthy controls. Alcohol and Alcoholism 2000;35(6):617‐24. [DOI] [PubMed] [Google Scholar]
Dobrydnjov 2004 {published data only}
- Dobrydnjov I, Axelsson K, Berggren L, Samarutel J, Holmstrom B. Intrathecal and oral clonidine as prophylaxis for postoperative alcohol withdrawal syndrome: a randomised double‐blinded study. Anesthesia and Analgesia 2004;98(3):738‐44. [DOI] [PubMed] [Google Scholar]
Franz 2001 {published data only}
- Franz M, Dlabal H, Kunz S, Ulferts J, Gruppe H, Gallhofer B. Treatment of alcohol withdrawal: tiapride and carbamazepine versus chlormethiazole. A pilot study. European Archives of Psychiatry and Clinical Neuroscience 2001;251(4):185‐92. [DOI] [PubMed] [Google Scholar]
Frecker 1982 {published data only}
- Frecker RC, Shaw JM, Zilm DH, Jacob MS, Sellers EM, Degani N. Nonpharmacological supportive care compared to Chlormethiazole infusion in the management of severe acute alcohol withdrawal. Journal of Clinical Psychopharmacology 1982;2(4):277‐80. [PubMed] [Google Scholar]
Gallant 1989 {published data only}
- Gallant DM. Improvements in treatment of alcohol withdrawal syndromes. Alcoholism, Clinical and Experimental Research 1989;13(5):721‐2. [DOI] [PubMed] [Google Scholar]
Gallant 1992 {published data only}
- Gallant DM. One more look at carbamazepine in the treatment of alcohol withdrawal. Alcoholism, Clinical and Experimental Research 1992;16(6):1174‐5. [DOI] [PubMed] [Google Scholar]
Glatt 1965 {published data only}
- Glatt MM, George HR, Frisch EP. Controlled trial of Chlormethiazole in treatment of the alcoholic withdrawal phase. British Medical Journal 1965;2:401‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2006 {published data only}
- Gordon SM, Sterling R, Siatkowski C, Raively K, Weinstein S, Hill PC. Inpatient desire to drink as a predictor of relapse to alcohol use following treatment. The American Journal on Addictions 2006;15(3):242‐5. [DOI] [PubMed] [Google Scholar]
Gottesleben 1995 {published data only}
- Gottesleben A, Willemsen D, Wolf C. Slow‐release carbamazepine for therapy of alcoholic predelirium‐ results of a double‐blind clinical trial using timonol 150 retard [Retardiertes Carbamazepin in der Therapie des alkoholischen Pradelirs]. Aktuelle Neurologie 1995;22:60‐5. [Google Scholar]
Griffith 1986 {published data only}
- Griffith JD, Jasinski DR, Casten GP, McKinney GR. Investigation of the abuse liability of buspirone in alcohol‐dependent patients. American Journal of Medicine 1986;80(3B):30‐5. [DOI] [PubMed] [Google Scholar]
Gual 2001 {published data only}
- Gual A, Lehert P. Acamprosate during and after acute alcohol withdrawal: a double‐blind placebo‐controlled study in Spain. Alcohol and Alcoholism 2001;36(5):413‐8. [DOI] [PubMed] [Google Scholar]
Gual 2002 {published data only}
- Gual A, Monras M, Ortega L. Efficacy of tiapride in the maintenance of abstinence in weaned alcoholics. Results of a double‐blind trial against placebo. [Eficacia de tiapride en el mantenimiento de la abstinencia en alcoholicos desintoxicados. Resultados de un ensayo clinico a doble ciego frente a placebo]. Adicciones 2002;14(3):321‐6. [Google Scholar]
Guarino 1989 {published data only}
- Guarino JJ, Roache JD, Kirk WT, Griffiths RR. Comparison of the behavioral effects and abuse liability of ethanol and pentobarbital in recreational sedative abusers. NIDA Research Monograph 1989;95:453‐4. [PubMed] [Google Scholar]
Hague 1976 {published data only}
- Hague WH, Wilson LG, Dudley DL, Cannon DS. Post‐detoxification drug treatment of anxiety and depression in alcohol addicts. Journal of Nervous and Mental Disease 1976;162(5):354‐9. [DOI] [PubMed] [Google Scholar]
Harfst 1967 {published data only}
- Harfst MJ, Greene JG, Lassae FG. Controlled trial comparing amobarbital and Chlormethiazole in alcohol withdrawal symptoms. Quarterly Journal of Studies on Alcohol 1967;28(4):641‐8. [PubMed] [Google Scholar]
Heil 1990 {published data only}
- Heil T, Martens D, Eyrich K. Alcohol withdrawal syndrome in the postoperative phase‐‐therapy or prevention? [Das Alkoholentzugssyndrom in der postoperativen Phase‐‐Therapie oder Prophylaxe?]. Langenbecks Archiv fur Chirurgie. Supplement II, Verhandlungen der Deutschen Gesellschaft fur Chirurgie. Deutsche Gesellschaft fur Chirurgie. Kongress 1990;Supplement II:1137‐40. [PubMed] [Google Scholar]
Hillbom 1989 {published data only}
- Hillbom M, Tokola R, Kuusela V, Karkkainen P, Kalli‐Lemma L, Pilke A, et al. Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol 1989;6(3):223‐6. [DOI] [PubMed] [Google Scholar]
Hutchison 2001 {published data only}
- Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D, Almeida A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology (Berlin) 2001;155(1):27‐34. [DOI] [PubMed] [Google Scholar]
Ilyuchina 1995 {published data only}
- Ilyuchina VA, Nikitina LI. Clinical physiological study of the therapeutic effects of phenytoin in acute alcohol withdrawal and the asthenic‐autonomic syndrome in patients with chronic alcoholism. Alcohol 1995;12(6):511‐7. [DOI] [PubMed] [Google Scholar]
Janiri 1996 {published data only}
- Janiri L, Gobbi G, Mannelli P, Pozzi G, Serretti A, Tempesta E. Effects of fluoxetine at antidepressant doses on short‐term outcome of detoxified alcoholics. International Clinical Psychopharmacology 1996;11(2):109‐17. [PubMed] [Google Scholar]
Johnson 2003 {published data only}
- Johnson BA, Ait‐Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. The Lancet 2003;361(9370):1677‐85. [DOI] [PubMed] [Google Scholar]
Johnson 2004 {published data only}
- Johnson BA, Ait‐Daoud N, Akhtar FZ, Ma JZ. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol‐dependent individuals: a randomized controlled trial. Archives of General Psychiatry 2004;61(9):905‐12. [DOI] [PubMed] [Google Scholar]
Kaim 1974 {published data only}
- Kaim SC. Prevention of delirium tremens: use of phenothiazines versus drugs cross‐dependent with alcohol. Advances in Biochemical Psychopharmacology 1974;9(0):685‐90. [PubMed] [Google Scholar]
Kaplan 1972 {published data only}
- Kaplan R, Blume S, Rosenberg S, Pitrelli J, Turner WJ. Phenytoin, Metronidazole and multivitamins in the treatment of alcoholism. Quarterly Journal of Studies on Alcohol 1972;33(1):94‐104. [PubMed] [Google Scholar]
Karam‐Hage 2003 {published data only}
- Karam‐Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry and Clinical Neurosciences 2003;57(5):542‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karst 2002 {published data only}
- Karst M, Passie T, Friedrich S, Wiese B, Schneider U. Acupuncture in the treatment of alcohol withdrawal symptoms: a randomised, placebo‐controlled inpatient study. Addiction Biology 2002;7(4):415‐9. [DOI] [PubMed] [Google Scholar]
Keaney 2001 {published data only}
- Keaney F, Strang J, Gossop M, Marshall EJ, Farrell M, Welch S, et al. A double‐blind randomised placebo‐controlled trial of Lofexidine in alcohol withdrawal: Lofexidine is not a useful adjunct to chlordiazepoxide. Alcohol and Alcoholism 2001;36(5):426‐30. [DOI] [PubMed] [Google Scholar]
Kiefer 2003 {published data only}
- Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, et al. Comparing and combining naltrexone and Acamprosate in relapse prevention of alcoholism: a double‐blind, placebo‐controlled study. Archives of General Psychiatry 2003;60(1):92‐9. [DOI] [PubMed] [Google Scholar]
Kramp 1979 {published data only}
- Kramp P, Ronsted P, Hansen T. Barbital and diazepam plasma levels during treatment of delirium tremens. Acta Psychiatrica Scandinavica 1979;59(3):263‐75. [DOI] [PubMed] [Google Scholar]
Kranzler 1996 {published data only}
- Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive‐behavioral therapy in type B alcoholics. Alcoholism, Clinical and Experimental Research 1996;20(9):1534‐41. [DOI] [PubMed] [Google Scholar]
Kraus 1985 {published data only}
- Kraus ML, Gottlieb LD, Horwitz RI, Anscher M. Randomized clinical trial of atenolol in patients with alcohol withdrawal. The New England Journal of Medicine 1985;313(15):905‐9. [DOI] [PubMed] [Google Scholar]
Lange‐Ass 2003 {published data only}
- Lange‐Asschenfeldt C, Muller MJ, Szegedi A, Anghelescu I, Klawe C, Wetzel H. Symptom‐triggered versus standard Chlormethiazole treatment of inpatient alcohol withdrawal: clinical implications from a chart analysis. European Addiction Research 2003;9(1):1‐7. [DOI] [PubMed] [Google Scholar]
Le Bon 2003 {published data only}
- Bon O, Murphy JR, Staner L, Hoffmann G, Kormoss N, Kentos M, et al. Double‐blind, placebo‐controlled study of the efficacy of trazodone in alcohol post‐withdrawal syndrome: polysomnographic and clinical evaluations. Journal of Clinical Psychopharmacology 2003;23(4):377‐83. [DOI] [PubMed] [Google Scholar]
Le Fauve 2004 {published data only}
- Fauve CE, Litten RZ, Randall CL, Moak DH, Salloum IM, Green AI. Pharmacological treatment of alcohol abuse/dependence with psychiatric comorbidity. Alcoholism, Clinical and Experimental Research 2004;28(2):302‐12. [DOI] [PubMed] [Google Scholar]
Leivonen 1966 {published data only}
- Leivonen P, Stenij P, Thesleff CJ. Experience with three drugs in ambulatory treatment of alcohol patients. Acta Psychiatrica Scandinavica Supplementum 1966;192:177‐81. [DOI] [PubMed] [Google Scholar]
Loo 1986 {published data only}
- Loo H, Guelfi JD, Malka R, Brion S, Cottin M, Gailledreau J, et al. [Anxiolytic efficacy and tolerance of Tetrabamate in anxious patients abusing alcohol. Multicenter double‐blind versus placebo study]. L' encephale 1986;12(5):291‐7. [PubMed] [Google Scholar]
Malcolm 1992 {published data only}
- Malcolm R, Anton RF, Randall CL, Johnston A, Brady K, Thevos A. A placebo‐controlled trial of buspirone in anxious inpatient alcoholics. Alcoholism, Clinical and Experimental Research 1992;16(6):1007‐13. [DOI] [PubMed] [Google Scholar]
Martinotti 2007 {published data only}
- Martinotti G, Romanelli R, Nicola M, Reina D, Mazza M, Janiri L. Oxcarbazepine at high dosages for the treatment of alcohol dependence. The American Journal on Addictions 2007;16(3):247‐8. [DOI] [PubMed] [Google Scholar]
Mendels 1985 {published data only}
- Mendels J, Wasserman TW, Michals TJ, Fine EW. Halazepam in the management of acute alcohol withdrawal syndrome. Journal of Clinical Psychiatry 1985;46(5):172‐4. [PubMed] [Google Scholar]
Miller 1988 {published data only}
- Miller TP, Taylor JL, Tinklenberg JR. A comparison of assessment techniques measuring the effects of methylphenidate, secobarbital, diazepam and diphenhydramine in abstinent alcoholics. Neuropsychobiology 1988;19(2):90‐6. [DOI] [PubMed] [Google Scholar]
Montejo 2007 {published data only}
- Montejo JZ, Galligo FC. Treatment of the alcohol withdrawal syndrome and other drugs. Formacion Medica Continuada En Atencion Primaria 2007;14(1):40‐6. [Google Scholar]
Mueller 1997 {published data only}
- Mueller TI, Stout RL, Rudden S, Brown RA, Gordon A, Solomon DA, et al. A double‐blind, placebo‐controlled pilot study of carbamazepine for the treatment of alcohol dependence. Alcoholism, Clinical and Experimental Research 1997;21(1):86‐92. [PubMed] [Google Scholar]
Mukherjee 1983 {published data only}
- Mukherjee PK. A comparison of the efficacy and tolerability of clobazam and chlordiazepoxide in the treatment of acute withdrawal from alcohol in patients with primary alcoholism. Journal of International Medical Research 1983;11(4):205‐11. [DOI] [PubMed] [Google Scholar]
Muller 1969 {published data only}
- Muller DJ. A comparison of three approaches to alcohol‐withdrawal states. Southern Medical Journal 1969;62(4):495‐6. [DOI] [PubMed] [Google Scholar]
Myrick 2009 {published data only}
- Myrick H, Malcolm R, Randall PK, Boyle E, Anton RF, Becker HC, et al. A double‐blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcoholism: Clinical and Experimental Research 2009;33(9):1582‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naranjo 2000 {published data only}
- Naranjo CA, Knoke DM, Bremner KE. Variations in response to citalopram in men and women with alcohol dependence. Journal of Psychiatry & Neuroscience: JPN 2000;25(3):269‐75. [PMC free article] [PubMed] [Google Scholar]
Newman 1995 {published data only}
- Newman JP, Terris DJ, Moore M. Trends in the management of alcohol withdrawal syndrome. The Laryngoscope 1995;105(1):1‐7. [DOI] [PubMed] [Google Scholar]
Pettinati 2000 {published data only}
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcoholism, Clinical and Experimental Research 2000;24(7):1041‐9. [PubMed] [Google Scholar]
Ponce 2005 {published data only}
- Ponce G, Rodriguez‐Jimenez R, Ortiz H, Rubio G, Jimenez‐Arriero MA, Palomo T. Oxcarbazepine in the prevention of epileptic syndromes in alcohol detoxification. Revista de Neurologia 2005;40(10):577‐80. [PubMed] [Google Scholar]
Rodgers 1999 {published data only}
- Rodgers JE, Crouch MA. Phenobarbital for alcohol withdrawal syndrome. American Journal of Health‐System Pharmacy: AJHP 1999;56(2):175‐8. [DOI] [PubMed] [Google Scholar]
Rubio 2002 {published data only}
- Rubio G, Ponce G, Ortiz S, Oliva J, Manzanares J, Jimenez‐Arriero M, et al. Comparison between gabapentin and Acamprosate in the treatment of alcohol dependence patients. European Neuropsychopharmacology 2002;12 Suppl 3:S397. [Google Scholar]
Rychlik 2001 {published data only}
- Rychlik R, Paschen B, Kirchhoff D, Daniel D, Pfeil T, Kilburg A. Adjuvant drug treatment of alcoholism with Acamprosate: between sectorial budgets and disease management. Deutsche Medizinische Wochenschrift 2001;126(33):899‐904. [DOI] [PubMed] [Google Scholar]
Sass 1996 {published data only}
- Sass H, Soyka M, Mann K, Zieglgansberger W. Relapse prevention by Acamprosate. Results from a placebo‐controlled study on alcohol dependence. Archives of General Psychiatry 1996;53(8):673‐80. [DOI] [PubMed] [Google Scholar]
Schmidt 1994 {published data only}
- Schmidt LG, Dufeu P, Kuhn S, Rommelspacher H. Relapse prevention in alcoholics with an anti craving drug treatment: first results of the Berlin Study. Pharmacopsychiatry 1994;27 Suppl 1:21‐3. [DOI] [PubMed] [Google Scholar]
Schulte 1987 {published data only}
- Schulte RM. Therapy of pre‐delirium alcohol withdrawal syndrome. Results of a comparative study of carbamazepine and Chlormethiazole. Fortschritte der Medizin 1987;105(25):493‐6. [PubMed] [Google Scholar]
Schwarz 1982 {published data only}
- Schwarz E, Kielholz P, Hobi V, Goldberg L, Hofstetter M, Ladewig D. Changes in EEG, blood levels, mood scales and performance scores during long term treatment with diazepam, phenobarbital or placebo in patients. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 1982;6(3):249‐63. [DOI] [PubMed] [Google Scholar]
See 2002 {published data only}
- See S. Carbamazepine effective for alcohol withdrawal. The Journal of Family Practise 2002;51(9):778. [PubMed] [Google Scholar]
Shaw 1994 {published data only}
- Shaw GK, Waller S, Majumdar SK, Alberts JL, Latham CJ, Dunn G. Tiapride in the prevention of relapse in recently detoxified alcoholics. British Journal of Psychiatry 1994;165(4):515‐23. [DOI] [PubMed] [Google Scholar]
Sillanpaa 1979 {published data only}
- Sillanpaa M, Sonck T. Finnish experiences with carbamazepine (Tegretol) in the treatment of acute withdrawal symptoms in alcoholics. Journal of International Medical Research 1979;7(3):168‐73. [DOI] [PubMed] [Google Scholar]
Smith 1987 {published data only}
- Smith DB, Mattson RH, Cramer JA, Collins JF, Novelly RA, Craft B. Results of a nationwide Veterans Administration Cooperative Study comparing the efficacy and toxicity of carbamazepine, phenobarbital, phenytoin, and primidone. Epilepsia 1987;28 Suppl 3:S50‐8. [DOI] [PubMed] [Google Scholar]
Snel 1983 {published data only}
- Snel H, Lehmann E, Velikonja M. Piracetam in the treatment of alcohol‐induced delirium [Piracetam in der Behandlung des alkoholbedingten Delirs]. MMW. Munchener Medizinische Wochenschrift 1983;125(42):947‐9. [PubMed] [Google Scholar]
Sobcyzk 1980 {published data only}
- Sobcyzk P, Jena K, Klir T. Comparative evaluation of the effectiveness of various methods of treating delirium tremens [Porownawcza ocena skutecznosci niektorych metod leczenia majaczenia dezennego]. Psychiatria Polska 1980;14(5):481‐6. [PubMed] [Google Scholar]
Solomon 1983 {published data only}
- Solomon J, Rouck LA, Koepke HH. Double‐blind comparison of lorazepam and chlordiazepoxide in the treatment of the acute alcohol abstinence syndrome. Clinical Therapeutics 1983;6(1):52‐8. [PubMed] [Google Scholar]
Spies 1995 {published data only}
- Spies CD, Dubisz N, Funk W, Blum S, Muller C, Rommelspacher H, et al. Prophylaxis of alcohol withdrawal syndrome in alcohol‐dependent patients admitted to the intensive care unit after tumour resection. British Journal of Anaesthesia 1995;75(6):734‐9. [DOI] [PubMed] [Google Scholar]
Sternebring 1983 {published data only}
- Sternebring B, Holm R, Wadstein J. Reduction in early alcohol abstinence fits by administration of carbamazepine syrup instead of tablets. European Journal Clinical Pharmacology 1983;24(5):611‐3. [DOI] [PubMed] [Google Scholar]
Stojek 1987 {published data only}
- Stojek A, Bilikiewicz A, Lerch A. Carbamazepine and physostigmine eyedrops in the treatment of early alcohol withdrawal and alcohol‐related hypertension. Psychiatria Polska 1987;21(5):369‐75. [PubMed] [Google Scholar]
Streeton 2001 {published data only}
- Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta‐analysis of randomised controlled trials. Alcohol and Alcoholism 2001;36(6):544‐52. [DOI] [PubMed] [Google Scholar]
Swift 2003 {published data only}
- Swift RM. Topiramate for the treatment of alcohol dependence: initiating abstinence. The Lancet 2003;361(9370):1666‐7. [DOI] [PubMed] [Google Scholar]
Tacke 1975 {published data only}
- Tacke B, Kempf H. Double‐blind study of Aprotininum (Trasylol) in the management of alcoholic delirium [Doppelblindstudie uber Aprotinium (Trasylol) in der Behandlung des Alkoholdelirs]. International Pharmacopsychiatry 1975;10(1):37‐41. [PubMed] [Google Scholar]
Teijeiro 1976 {published data only}
- Teijeiro J. A comparative double‐blind study of atrium 300 and Hemineurine in chronic alcoholics. Schweizerische Rundschau fur Medizin Praxis 1976;65(14):420‐2. [PubMed] [Google Scholar]
Tempesta 2000 {published data only}
- Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: a placebo‐controlled study. Alcohol and Alcoholism 2000;35(2):202‐9. [DOI] [PubMed] [Google Scholar]
Thomas 1964 {published data only}
- Thomas DW, Freedman DX. Treatment of the Alcohol Withdrawal Syndrome. Comparison of Promazine and Paraldehyde. Journal of the American Medical Association 1964;188:316‐8. [DOI] [PubMed] [Google Scholar]
Tress 1977 {published data only}
- Tress W. New type of treatment for alcohol withdrawal syndrome [Ein neuartiges therapie verfahren des alkoholischen entzugs syndroms]. TherapieWoche 1977;27(51):9304‐12. [Google Scholar]
Wadstein 1986 {published data only}
- Wadstein J, Manhem P, Nilsson LH, Moberg AL, Hokfelt B. Clonidine versus Chlormethiazole in alcohol withdrawal. Acta Psychiatrica Scandinavica Supplementum 1986;327:144‐8. [PubMed] [Google Scholar]
Wegner 1965 {published data only}
- Wegner ME, Fink DW. Chlordiazepoxide (librium) compared with meprobamate and promazine (prozine) for the withdrawal symptoms of acute alcoholism. Wisconsin Medical Journal 1965;64(11):436‐40. [PubMed] [Google Scholar]
Welbel 1982 {published data only}
- Welbel L, Zareba J, Kowalewska I. Clomethiazole (Chlormethiazole) in the treatment of delirium tremens [Klometiazol (chlorometiazol) w leczeniu majaczenia alkoholowego]. Psychiatria Polska 1982;16(1‐2):19‐24. [PubMed] [Google Scholar]
Whitworth 1996 {published data only}
- Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, et al. Comparison of Acamprosate and placebo in long‐term treatment of alcohol dependence. The Lancet 1996;347(9013):1438‐42. [DOI] [PubMed] [Google Scholar]
Wiesbeck 1999 {published data only}
- Wiesbeck GA, Weijers HG, Chick J, Naranjo CA, Boening J. Ritanserin in relapse prevention in abstinent alcoholics: results from a placebo‐controlled double‐blind international multicenter trial. Ritanserin in Alcoholism Work Group. Alcoholism, Clinical and Experimental Research 1999;23(2):230‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gottesleben 1994 {published data only}
- Gottesleben A, Wolf C, Willemsen D. Slow‐release carbamazepine for therapy of alcoholic pre delirium: Results of a double blind clinical trial using Timonil 150 retard. European Journal of Clinical Research 1994;5:51‐61. [Google Scholar]
Hart 1964 {published data only}
- Hart WT. A comparison of promazine and paraldehyde in 175 cases of alcohol withdrawal. Psychiatric Quarterly 1964;38:619‐26. [Google Scholar]
Trevisan 2008 {published data only}
- Trevisan L.A, Ralevski E, Keegan K, Oville A, Vuppalapati D, Gonzalez G, et al Addictive Disorders and their Treatment 2008 7:3 (119‐128). Alcohol detoxification and relapse prevention using valproic acid versus gabapentin in alcohol‐dependent patients. Addictive Disorders and their Treatment 2008;7(3):119‐128. [Google Scholar]
Additional references
Amato 2010
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD005063] [DOI] [PubMed] [Google Scholar]
De Witte 2003
- Witte P, Pinto E, Ansseau M, Verbanck P. Alcohol and withdrawal: from animal research to clinical issues. Neuroscience and Biobehavioral Reviews 2003;27(3):189‐97. [DOI] [PubMed] [Google Scholar]
Dodd 2000
- Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate‐mediated transmission, alcohol, and alcoholism. Neurochemistry International 2000;37(5‐6):509‐33. [DOI] [PubMed] [Google Scholar]
DSM‐IV‐R
- AA VV. Diagnostic and Statistical Manual of Mental Disorders., Fourth Edition‐revised. American Psychiatric Association , 1995. [Google Scholar]
Fox 2003
- Fox C, Cook C, Loughlin P, Mangal R. Acamprosate for alcohol dependence. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004332] [DOI] [Google Scholar]
Gillman 2007
- Gillman MA, Lichtigfeld F, Young T. Psychotropic analgesic nitrous oxide for alcoholic withdrawal states. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005190.pub2] [DOI] [PubMed] [Google Scholar]
Gilman 1996
- Gilman S, Koeppe RA, Adams K, Johnson‐Greene D, Junck L, Kluin KJ, et al. Positron emission tomographic studies of cerebral benzodiazepine‐receptor binding in chronic alcoholics. Annals of Neurology 1996;40(2):163‐71. [DOI] [PubMed] [Google Scholar]
Hasin 1990
- Hasin DS, Grant B, Endicott J. The natural history of alcohol abuse: implications for definitions of alcohol use disorders. The American Journal of Psychiatry 1990;147(11):1537‐41. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Kohl 1998
- Kohl RR, Katner JS, Chernet E, McBride WJ. Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopharmacology 1998;139(1‐2):79‐85. [DOI] [PubMed] [Google Scholar]
Koob 1997
- Koob GF, LeMoal M. Drug abuse: hedonic homeostatic dysregulation. Science 1997;278(5335):52‐8. [DOI] [PubMed] [Google Scholar]
Leggio 2008
- Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non‐benzodiazepine GABAergicmedications. Progress in Neuro‐psychopharmacology & Biological Psychiatry 2008;32(5):1106‐17. [DOI] [PubMed] [Google Scholar]
Leone 2010
- Leone MA, Vigna‐Taglianti F, Avanzi G, Brambilla R, Faggiano F. Gamma‐hydroxybutyrate (GHB) for treatment of alcohol withdrawal and prevention of relapses. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD006266] [DOI] [PubMed] [Google Scholar]
Mayo‐Smith 1997
- Mayo‐Smith MF. Pharmacological management of alcohol withdrawal: a meta‐analysis and evidence‐based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. Journal of the American Medical Association 1997;278(2):144‐51. [DOI] [PubMed] [Google Scholar]
Nutt 1999
- Nutt D. Alcohol and the brain. Pharmacological insights for psychiatrists. The British Journal of Psychiatry 1999;175:114‐9. [DOI] [PubMed] [Google Scholar]
Petty 1993
- Petty F, Fulton M, Moeller FG, Kramer G, Wilson L, Fraser K, et al. Plasma gamma‐aminobutyric acid (GABA) is low in alcoholics. Psychopharmacology Bulletin 1993;29(2):277‐81. [PubMed] [Google Scholar]
Polycarpou 2005
- Polycarpou A, Papanikolau P, Ioannidis JP, Contopoulos Ioannidis D. Anticonvulsants for alcohol withdrawal. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005064.pub2] [DOI] [PubMed] [Google Scholar]
Slawecki 1999
- Slawecki CJ, Somes C, Ehlers CL. Effects of chronic ethanol exposure on neurophysiological responses to corticotropin‐releasing factor and neuropeptide Y. Alcohol and Alcoholism 1999;34(3):289‐99. [DOI] [PubMed] [Google Scholar]
Tejani 2010
- Tejani AM, Chan AHill Wah, Kuo IF, Li J. Magnesium for alcohol withdrawal. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD008358] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 1995
- Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis of human alcoholism. The American Journal of Psychiatry 1995;152(3):332‐40. [DOI] [PubMed] [Google Scholar]


