Abstract
Making eye contact is the most powerful mode of establishing a communicative link between humans. During their first year of life, infants learn rapidly that the looking behaviors of others conveys significant information. Two experiments were carried out to demonstrate special sensitivity to direct eye contact from birth. The first experiment tested the ability of 2- to 5-day-old newborns to discriminate between direct and averted gaze. In the second experiment, we measured 4-month-old infants' brain electric activity to assess neural processing of faces when accompanied by direct (as opposed to averted) eye gaze. The results show that, from birth, human infants prefer to look at faces that engage them in mutual gaze and that, from an early age, healthy babies show enhanced neural processing of direct gaze. The exceptionally early sensitivity to mutual gaze demonstrated in these studies is arguably the major foundation for the later development of social skills.
The perception of faces, and the understanding that faces can reflect internal states of social partners, are vital skills for the typical development of humans. Of particular importance is processing information about eyes and eye-gaze direction. Although the perception of averted gaze can elicit an automatic shift of attention in the same direction (1), allowing the establishment of “joint attention” (2), mutual gaze (eye contact) provides the main mode of establishing a communicative context between humans (3–5). A number of lines of evidence suggest that specific neural mechanisms are engaged when human adults (6) or other primates (7) detect the direction of gaze in another's face. In addition, it is known that, from at least 4 months of age, human infants will shift their spatial attention toward the direction of a gaze shift when viewing a face (8, 9), and it is commonly agreed that such skills are vital for subsequent social development (10). However, considerable controversy remains with regard to whether the perception of eye gaze is a perceptual skill acquired through experience (11), or caused by innate mechanisms. This controversy is also relevant to the proposal that deficits in eye-gaze perception may be symptomatic of, or even contribute to, autism (12). Individuals with autism have difficulties with many forms of social communication, and their gaze processing is impaired at various levels, such as eye contact, gaze following, joint attention, and understanding gaze within a mentalistic framework (13–15).
It has been shown that human newborns have a visual preference for face-like stimuli (16), prefer faces with eyes opened (17), and tend to imitate certain facial gestures (18). Preferential attention to perceived faces with direct gaze would provide the most compelling evidence to date that human newborns are born prepared to detect socially relevant information. This was investigated in experiment 1. In experiment 2, we attempt to gain converging evidence for the differential processing of direct gaze in infants by recording event-related potentials (ERPs) from the scalp as infants view faces.
Experiment 1: Newborns' Preference for Mutual Gaze
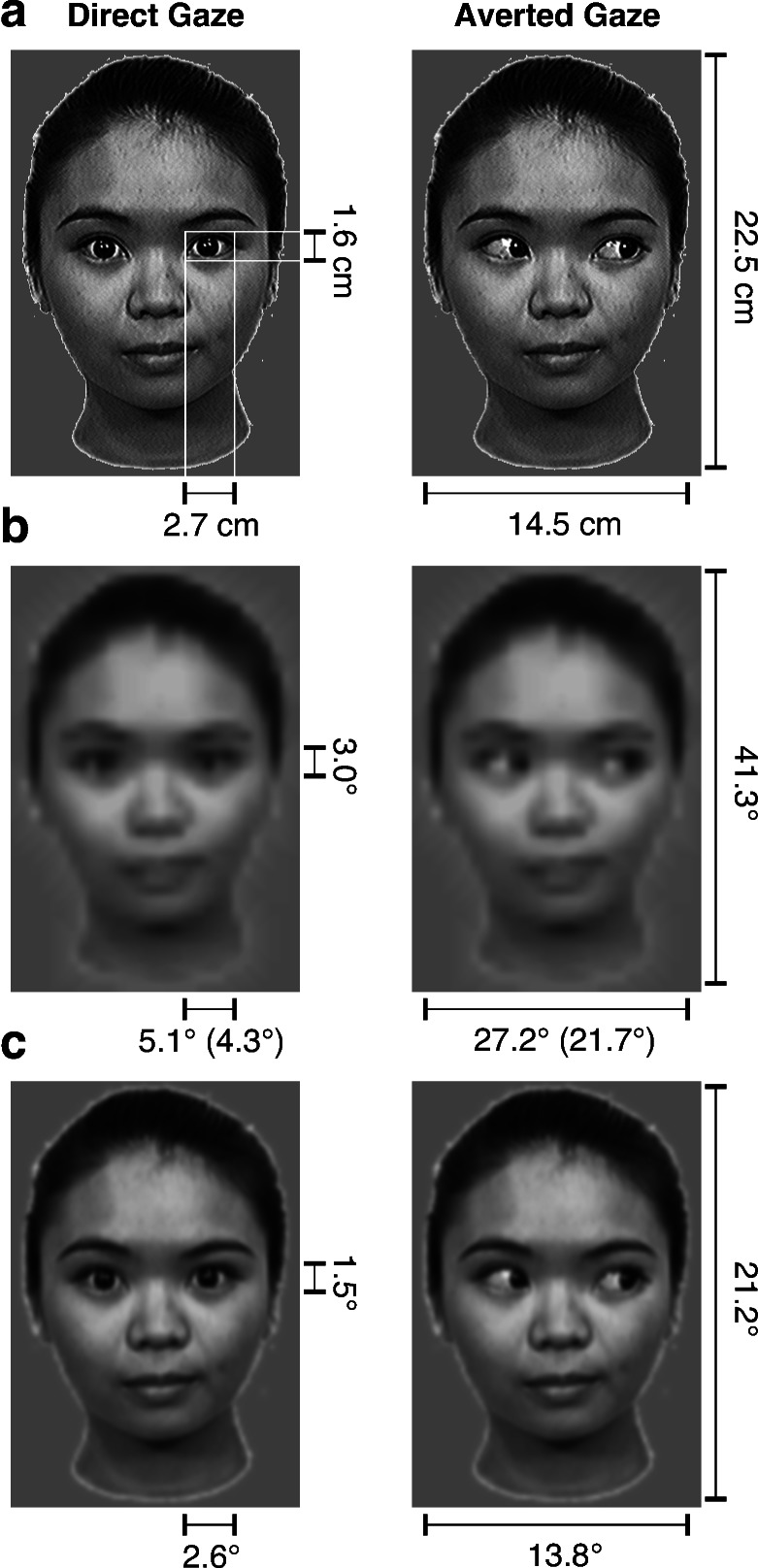
Seventeen healthy human newborn infants, all within the first 5 days of life (between 24 and 120 h postnatal age, mean 72 h), were presented with paired photographic face stimuli (see Fig. 1 a and b). All of the newborns (7 males and 10 females) were free of any known neurological or ocular abnormality and met the screening criteria of normal delivery, a birth-weight between 2,600 and 4,000 g, and an Apgar score of at least 8 at 5 min after birth. They were tested in the Pediatric Clinic of the University of Padua. The infants sat on the experimenter's lap, 30 cm from a translucent screen. The newborns eye level was aligned to the center of the screen at the same height as the eyes of the stimulus face. A video camera focused on the infants' face, allowing the experimenter to monitor their eye movements. The infants were shown two pictures of the same face, one on the right and one on the left of the center of the screen. One of the pair had direct gaze, whereas in the other face the eyes were averted randomly to the right or left. The inner edges of the images were 8.5 cm from the center. Once the newborn was seated in front of the screen, a flickering (300-ms on/off cycle) red light-emitting diode (LED) attracted his/her attention to the center. When she/he fixated it, the LED was turned off and a pair of faces were presented side by side. Two presentation sequences were used in which the position of the two stimuli alternated between the two trials. Half of the infants saw gaze averted to the right and half to the left. The analysis revealed no effects of stimulus order, averted gaze direction, or interactions between these factors. The stimuli remained on the screen as long as the infants fixated one of them. When they shifted their gaze from the display for more than 10 s, the experimenter turned off the stimuli and the center LED turned on. Videotapes of the babies' eye movements throughout the trial were analyzed by two coders who were blind as to which stimulus was presented on each side. The coders recorded, separately for each stimulus and each trial, the number of orienting responses and the total fixation time.
Figure 1.
Experimental stimuli. (a) Stimuli in both experiments were color photographic images of female faces directing their gaze straight-on to the viewers (Direct Gaze) or averted to one side (Averted Gaze). (b) Low-pass filtered versions of the stimuli illustrate the estimated resolution of the images in the visual system according to newborns' average visual acuity (19). Measures in the figure indicate viewing angles of faces and eyes when fixated or when in the periphery (in brackets). (c) These pictures illustrate the estimated resolution of the images according to 4-month-old infants' average visual acuity (19).
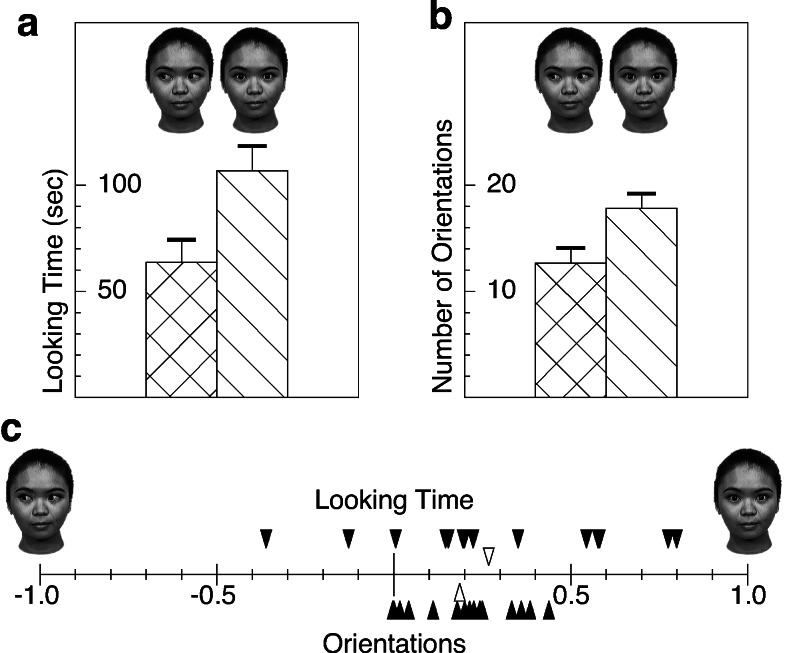
Newborns looked significantly longer at the direct gaze (mean 106.8 s) than at the averted gaze (mean 63.7 s) (parametric t test on log-transformed data: t16 = 3.211, P < 0.01; Wilcoxon test: z = 2.580, P < 0.01) (see Fig. 2a). In addition, they oriented significantly more frequently to the direct gaze face (mean 17.8) than to the other (mean 12.7) (t test: t16 = 5.290, P < 0.0001; Wilcoxon test: z = 3.334, P < 0.001) (see Fig. 2b). All of the newborns looked more times toward, and all but two looked longer at, the face with direct gaze (see Fig. 2c). Preference scores for direct gaze (d) over averted gaze (a) were calculated as (d − a)/(d + a) separately for the looking time and orientation measure. Preference scores significantly differed from zero for both measures (t16 = 3.326, P < 0.005 and t16 = 5.303, P < 0.0001, respectively).
Figure 2.
Results of the preferential looking study with newborns. (a) Mean looking times (and SE) spent at the two stimulus types. Newborns spent significantly more time looking at the face with mutual gaze than looking at the face with averted gaze. (b) Mean number of orientations toward each type of stimulus. (c) Filled triangles indicate reference scores for the direct gaze over the averted gaze for each individual newborn. Open triangles indicate average preference scores.
Experiment 2: Enhanced Neural Processing of Faces in 4-Month-Old Infants
To test 4-month-old infants' sensitivity to gaze direction, we presented them with the same faces that we used with newborns while we recorded their ERPs. ERPs have been shown to be sensitive to small differences in transient brain activation caused by processing of visual stimuli in infants (20) and they provide the most feasible neuroimaging method to study the brain development of healthy babies. We hypothesized that an early preference for eye contact would facilitate the processing of faces with direct gaze.
Nine male and six female full-term, healthy infants, aged between 127 and 146 days (mean age 135 days) participated in the study. (An additional nine infants were excluded from the analyses because of too few trials completed and/or excessive movement artifacts). The infants sat on their parent's lap 60 cm from a 40 cm × 29 cm computer monitor within an acoustically and electrically shielded and dimly lit room. A video camera mounted below the monitor and centered on the infant's face allowed us to record his/her gaze. Their attention was drawn to the middle of screen by a dynamic color cartoon. When they fixated it, the stimulus froze for 800-1200 ms before a face replaced it for 1,000 ms. Faces with direct or averted gaze were presented in random order and with equal probability for as long as the babies were willing to look at them (see Fig. 1 a and c). Infants who were included in the final sample typically completed 40–150 trials before the session was concluded. The brain electric activity was recorded by using a Geodesic Sensor Net consisting of 62 silver-silver chloride electrodes evenly distributed across the scalp and the vertex lead serving as reference (21). The electrical potential was amplified with 0.1- to 100-Hz bandpass, digitized at a 250-Hz sampling rate, and stored on computer disk for the off-line analysis. The infants' visual behavior was coded from videotape to exclude trials where they did not fixate the screen during stimulation. Participants who were included in the final sample contributed at least 15 trials per condition to their ERPs (mean number of trials: 30.8 for direct gaze and 32.8 for averted gaze). Averaged ERPs were calculated time-locked to stimulus presentation onset, and baseline-corrected to the average amplitude of the 100-ms interval preceding stimulus onset. ERPs were re-referenced to the average potential over the scalp and filtered by a digital elliptical low-pass filter at 35 Hz.
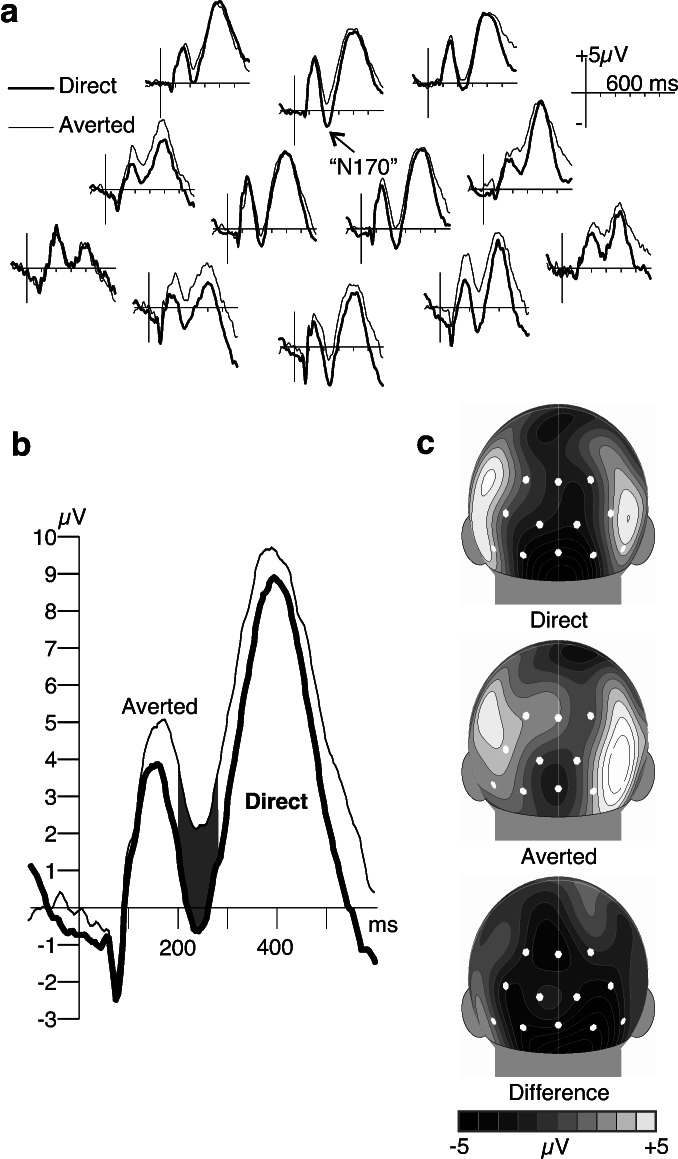
The ERPs over the occipital cortex showed the deflections (P1–N1–P2) that are expected to occur in response to onsets of large visual stimuli (Fig. 3a). In our analyses we focused on an ERP component known to be sensitive to faces (N170) in adults (23) and recently identified in 6-month-old infants (24). The putative “infant N170” shares some functional properties with the adult component and is the first negative going deflection after the P1 over posterior sides. As in previous studies with infants (24), the “infant N170” component peaked around 240 ms after stimulus. Its amplitude was higher in response to direct gaze than to averted gaze (see Fig. 3b). The 12 occipital electrodes were collapsed into three lateral groups (left, medial, right) and a two-way ANOVA (laterality vs. stimulus type) was calculated on the average ERP amplitude within the 200- to 280-ms latency range. This analysis revealed a significant main effect of stimulus type (F1,14 = 7.748, P < 0.02). Twelve of 15 infants showed a more negative “infant N170” (P < 0.02; binomial test) to the direct gaze. There was also an effect of laterality (F2,28 = 3.538, P < 0.05) with the “infant N170” amplitude being more negative over the medial than over the lateral leads. Post hoc Scheffé tests revealed medial–lateral contrasts but no differences between the left and right hemispheres. The surface voltage maps also indicated medial occipital distribution of the “infant N170” (see Fig. 3c) and its enhancement because of perceiving direct gaze.
Figure 3.
ERPs recorded to faces with direct and averted gaze in 4-month-old infants. (a) All but one of the electrodes over the occipital cortex recorded an enhanced N170 response (peaking at 240 ms after stimulus) to the faces with direct gaze compared with the faces with averted gaze. (b) ERPs to the two kinds of stimuli averaged across all occipital electrode sites. (c) Spherical spline interpolations (22) for the surface distributions of the average amplitude at 240-ms latency show an enhanced medial occipital response to mutual gaze compared with averted gaze. White spots mark the electrode locations used in the statistical analyses.
The amplitude of the P1 response (mean peak latency 136 ms) was also analyzed to make sure that the differences on the N170 was not a carryover effect. A two-way ANOVA (laterality vs. stimulus type) on the average ERP amplitude within the 100-to 180-ms latency range revealed no significant effect of stimulus (F1,14 = 0.99, not significant). There was an effect of laterality (F2,28 = 3.756, P < 0.05), with the P1 amplitude being more positive over the medial than over the lateral leads. No differences between left and right hemisphere were observed. Point-by-point t tests on the average ERPs across the occipital electrodes revealed significant stimulus effects only within the poststimulus 176- to 304-ms interval, indicating that the effect was restricted to the “infant N170” latency range.
Discussion
The results of experiment 1 demonstrated attention to direct eye gaze from birth. Note that the psychophysically very small difference between the direct and averted gaze stimuli (see Fig. 1b) makes it unlikely that the preference at birth arises from nonspecific visual processes, such as those favoring certain spatial frequencies (25). Instead, the preference is probably a result of a fast and approximate analysis of the visual input, dedicated to find socially relevant stimuli for further processing. Experiment 2 provides converging evidence indicating enhanced face processing in the infant brain when viewing faces with direct gaze. Although it is possible that ERP differences could arise from the slight asymmetry introduced by the eyes being averted, the fact that the significant effects only occurred after early stages of visual processing make this interpretation unlikely.
There are at least two candidate mechanisms that may underlie newborns preference for direct eye gaze. Baron-Cohen (26) proposed that there is a specific mechanism, termed the “Eye Direction Detector (EDD),” that (i) detects the presence of eyes, and (ii) represents their direction and behavior. The first of these functions was argued by Baron-Cohen to be obligatory and innate, whereas the second emerges later. A second possibility is that the more frequent orienting to the direct gaze in newborns is mediated by the same mechanism that underlies their tendency to orient to faces in general. Specifically, Johnson and Morton (26) hypothesized that subcortical circuits supported a primitive representation of high-contrast elements relating to the location of the eyes and mouth. A face with direct gaze would better fit the spatial relation of elements in this template than one with gaze averted, suggesting that the functional role of this putative mechanism is more general than previously supposed.
Although both of the above theories are broadly consistent with our findings, we currently favor the Johnson and Morton account for two reasons. First, because there are eyes present in both the direct and averted gaze faces in our experiments, a mechanism that simply detects the presence of absence of eyes should be equally activated by both stimuli. Second, in 4-month-old infants, the presence of direct gaze facilitated the neural processes that are associated with the earliest steps of face encoding (23, 28). The issue of whether there are changes in the neural substrate of eye gaze perception over the first few months of life will require further research. Nevertheless, our data are consistent with the view that preferential attention to direct gaze in newborns results in facilitation of face processing when accompanied by direct gaze by 4 months of age. Although this interpretation of our data are consistent with neuroimaging evidence from adults (29), it does not provide support for arguments involving an innate neural module for eye-gaze detection that is dissociable from general face processing.
Additional evidence for the special status of direct gaze in early infancy comes from studies showing that 4-month-old infants' attention is only directed by the perception of averted gaze when it is preceded by a period of mutual gaze (T.F., M.H.J., E. Mansfield, C. Lai, and F.S., unpublished data). Further, 3-month-old infants smile less when an adult averts her gaze and show recovery of smiling when eye contact is resumed (4). The significance of mutual gaze in the development of human relationships has also been shown in many other studies, revealing its function to provide information, to regulate adult-infant interaction, to exercise social control, and to facilitate task goals (3, 30).
Interpretation of eye-gaze signals as referential communicative acts is probably a human-specific adaptation (31) that is essential for developing a rich understanding of others' mental states, often called a “theory of mind” (32). An early sensitivity for such signals, as demonstrated in these studies, facilitates this development.
Acknowledgments
We thank Bruce Hood for the pictures used to generate the stimuli; Leslie Tucker, Eileen Mansfield, Ágnes Volein, and Luisa Zulian for their assistance in recording and coding data, Stefano Massaccesi for software; and Beatrice dalla Barba for collaboration. This work was supported by United Kingdom Medical Research Council Program Grant G9715587, Birkbeck College, and Università degli studi di Padova.
Abbreviation
- ERP
event-related potentials
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Vis Cogn. 1999;6:509–540. [Google Scholar]
- 2.Butterworth G, Jarrett N. Br J Dev Psychol. 1991;9:55–72. [Google Scholar]
- 3.Kleinke C L. Psychol Bull. 1986;100:78–100. [PubMed] [Google Scholar]
- 4.Hains S M J, Muir D W. Child Dev. 1996;67:1940–1951. [PubMed] [Google Scholar]
- 5.Symons L A, Hains S M J, Muir D W. Inf Behav Dev. 1998;21:531–536. [Google Scholar]
- 6.Kampe K K W, Frith C D, Dolan R J, Frith U. Nature (London) 2001;413:589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H, Kohshima S. Nature (London) 1997;387:767–768. doi: 10.1038/42842. [DOI] [PubMed] [Google Scholar]
- 8.Hood B M, Willen J D, Driver J. Psychol Sci. 1998;9:53–56. [Google Scholar]
- 9.Farroni T, Johnson M H, Brockbank M, Simion F. Vis Cogn. 2000;7:705–718. [Google Scholar]
- 10.Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- 11.Vecera S P, Johnson M H. Vis Cogn. 1995;2:101–129. [Google Scholar]
- 12.Klin A, Sparrow S S, de Bildt A, Cicchetti D V, Cohen D J, Volkmar F R. J Autism Dev Disord. 1999;29:499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- 13.Leekam S, Baron-Cohen S, Perrett D I, Milders M, Brown S. Br J Dev Psychol. 1997;15:77–95. [Google Scholar]
- 14.Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. Dev Psychol. 1997;33:781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- 15.Baron-Cohen S, Cambell R, Karmiloff-Smith A, Grant J, Walker J. Br J Dev Psychol. 1995;13:379–398. [Google Scholar]
- 16.Johnson M H, Dziurawiec S, Ellis H, Morton J. Cognition. 1992;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 17.Batki A, Baron-Cohen S, Wheelwright S, Connellan J, Ahluwalia J. Inf Behav Dev. 2000;23:223–229. [Google Scholar]
- 18.Meltzoff A N, Moore M K. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- 19.Salomao S R, Ventura D F. Invest Ophthalmol Visual Sci. 1995;36:657–670. [PubMed] [Google Scholar]
- 20.Csibra G, Davis G, Spratling M W, Johnson M H. Science. 2000;290:1582–1585. doi: 10.1126/science.290.5496.1582. [DOI] [PubMed] [Google Scholar]
- 21.Tucker D. Electroencephalogr Clin Neurophysiol. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- 22.Perrin F, Pernier J, Bertrand O, Echallier J F. Electroencephalogr Clin Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- 23.Bentin S, Allison T, Puce A, Perez E, McCarthy G. J Cognit Neurosci. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Haan M, Pascalis O, Johnson M H. J Cognit Neurosci. 2002;14:199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- 25.Banks M S, Salapatek P. J Exp Child Psychol. 1981;31:1–45. doi: 10.1016/0022-0965(81)90002-3. [DOI] [PubMed] [Google Scholar]
- 26.Baron-Cohen S. Cah Psych Cogn. 1994;13:513–552. [Google Scholar]
- 27.Johnson M H, Morton J. Biology and Cognitive Development: The Case of Face Recognition. Oxford: Blackwell; 1991. [Google Scholar]
- 28.Eimer E. Clin Neurophysiol. 2000;111:694–705. doi: 10.1016/s1388-2457(99)00285-0. [DOI] [PubMed] [Google Scholar]
- 29.George N, Driver J, Dolan R J. Neuroimage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- 30.Blass E M, Camp C A. Dev Psychol. 2001;37:762–774. doi: 10.1037//0012-1649.37.6.762. [DOI] [PubMed] [Google Scholar]
- 31.Povinelli D J, Giambrone S. Child Dev. 2001;72:691–695. doi: 10.1111/1467-8624.00307. [DOI] [PubMed] [Google Scholar]
- 32.Frith C D, Frith U. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]