Abstract
Plant phytochemicals play an immense role in the synthesis of metal nanoparticles, and the quality of phytochemicals used in the synthesis depends on the extraction method, solvent, temperature, etc. An extensive number of studies were carried out on the extraction of phytochemicals using aqueous extract during the synthesis of nanomaterials. Compared to distilled water, methanol can extract diverse phytochemicals at lower temperatures, and very few studies were done on this. Therefore, the present study aims to synthesize silver nanoparticles (AgNPs) using the methanolic extract of Plumeria rubra (PR) leaves. The successful synthesis of AgNPs was achieved by reducing Ag⁺ ions using PR extract. The phytochemistry of PR extract was observed using qualitative screening, Fourier transform infrared spectroscopy (FTIR), and gas chromatography with flame ionisation detection (GC-FID). The observed surface plasmon resonance using UV-visible spectroscopy confirmed the formation of PR-AgNPs, and the surface functionalization identified with FTIR correlated with the phytochemistry of the PR extract. X-ray diffraction and high-resolution transmission electron microscopy (HRTEM) methods revealed the amorphous nature and particle size distribution. Field emission scanning electron microscopy (FESEM) shows that PR-AgNPs particles have irregular shapes, some of which are embedded on sheet-like structures. The successful inhibition of V. parahaemolyticus was observed at lower concentrations among other tested organisms, and it also reduced 2,2’-diphenyl-1-picryl hydrazyl (DPPH). The hemocompatibility and in vitro cytotoxicity assays showed that PR-AgNPs had moderate activity. This study successfully synthesized AgNPs using PR extract, and its biological activities in vitro were also found to be effective.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-14353-w.
Keywords: Antimicrobial resistance, AgNPs, Phytochemicals, Plumeria rubra.
Subject terms: Biological techniques, Nanoscience and technology
Introduction
Nanotechnology has introduced the development of novel materials as a frontier for diverse applications in scientific fields. Nanotechnology plays a significant role in synthesizing particles within the Nano size range of approximately 1 to 100 nm1. The large surface area-to-volume ratio, optical properties, and quantum confinement effects of nanoparticles attracted researchers to utilize them in broad scientific fields2,3 such as biomedical, engineering, pharmaceuticals, environmental pollution remediation, energy, and material sciences4. The materials at the nanoscale range offer benefits in the biomedical field due to their antimicrobial, anticancer, and anti-parasitic properties and are used in medical implants, imaging, and pharmaceutical preparations5,6.
Commercial products widely use silver nanoparticles (AgNPs), among other metallic nanoparticles, because of their antimicrobial and other versatile applications including food packaging, bio fertilizers, cosmetics, and medicines7,8. AgNPs prominently have antibacterial properties via various mechanisms, such as inducing oxidative stress in the bacterial cell wall, disrupting internal ion balance, inhibiting growth, and affecting chemical transportation across the cell wall. Generally, multicellular organisms remain unharmed by AgNPs until the concentrations are high9. Biological approaches for synthesizing AgNPs are regarded as beneficial due to their environmentally friendly nature and low cost relative to physical and chemical methods10. Recently, the synthesis of AgNPs has been achieved using green chemistry principles, which involve the utilization of naturally occurring reducing agents such as sugars, biodegradable polymers, plant secondary metabolites, and microorganisms in a sustainable way11.
The plant phytochemicals are effective in reducing oxidative stress, which is associated with various human ailments like diabetes, cancer, cardiovascular and neural disorders, etc. Fabrication of these phytochemicals on the surface of AgNPs enhances their effectivity and efficacy compared to other natural reducing agents used in the synthesis of nanomaterials12,13.
During AgNP green synthesis, plant phytochemicals reduce the Ag⁺ ions to Ag⁰ and act as capping agents, ultimately stabilizes the nanoparticles14,15. The phytochemicals are typically extracted using water as a solvent during nanoparticle synthesis. The limitations of this process are that it requires high temperature, breakdown of thermo-labile phytocompounds, low extraction efficiency, and limited solubility of semi-polar and non-polar compounds16. Whereas, methanol, a high-polar organic solvent, is able to extract a diverse number of phytochemicals at lower temperatures, which include non-polar, semi-polar, and polar phytocompounds17. Unlike water, methanol can penetrate the cell walls of plants and extract a large number of phytocompounds, and the obtained extract can be concentrated at lower temperatures18.
Several extraction procedures are used for the extraction of phytochemicals, and the quality and quantity of phytochemicals depend upon the type of solvent, temperature, and type of extraction method. The extraction methods include maceration, decoction, percolation, and Soxhlet extraction; among these, the Soxhlet extraction method is more efficient for extracting phytochemicals because it continuously cycles solvent through the plant sample. Previous studies have reported the use of methanolic extract in the synthesis of AgNPs, but they primarily focused on maceration, decoction, and percolation methods7,19–25.
The studies that were carried out previously are limited, and more studies are required for acquiring efficient and homogeneous nanoparticles using phytochemicals obtained from methanolic extracts of plant materials. After a thorough literature survey, there is no study reported on the synthesis of AgNPs using Plumeria rubra methanolic extract prepared using Soxhlet extraction.
The genus Plumeria of the Apocynaceae family consists of a diverse number of plant species commonly found in tropical and subtropical regions of the world. P. obtusa L., P. alba L., and P. rubra L. are the most abundantly found Plumeria species in the Indian subcontinent region. Previous studies have been carried out on the synthesis of AgNPs using various Plumeria species, but no work has been carried out on P. rubra26. P. rubra typically grows in parks, gardens, landscapes, and even temples, earning it the nickname “temple tree” or “graveyard flowers”26. P. rubra, a deciduous tree reaching a height of about 7–8 m, displays flowers of various colors, including red, white, pink, yellow, and tricolor. The leaves of P. rubra are thin and grayish-green, possessing a smooth and shiny appearance. Its fruits are brown color legumes, measuring 15–25 cm in length and a diameter about 1.5–2 cm. The medicinal properties of P. rubra plant was traditionally used in Ayurveda, for treating malarial infections and acting as an antiseptic and stimulant27. P. rubra leaves possess significant medicinal value, serving as a rubefacient, treating conditions like leprosy, inflammation, and cholera, and alleviating cough and cold symptoms. People have traditionally used P. rubra to treat diarrhea and typhoid28.
There are several studies have reported the synthesis of AgNPs using various green extracts, whereas the use of Plumeria rubra methanolic leaf extract for AgNP synthesis obtained through Soxhlet extraction method is unexplored. This method ensures recovery of large number of phytochemicals which are essential for reduction and stabilization of nanoparticles. The previous studies explore very fewer biomedical applications which are limited to understand the properties of green AgNPs. Therefore, the present study evaluated the in vitro antioxidant, antimicrobial, haemocompatibility and cytotoxicity activities of AgNPs synthesized using Plumeria rubra methanolic leaf extract.
Result and discussion
Phytochemistry of Plumeria rubra methanolic extract
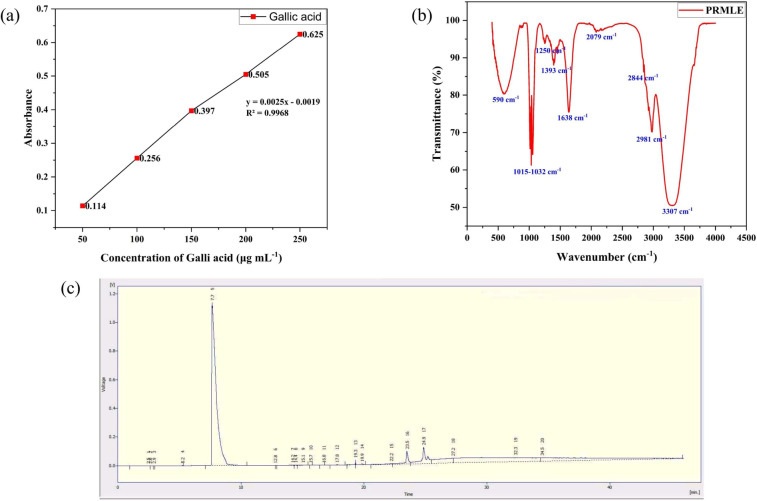
The reducing, stabilizing capacities and owing to its easiness, the plant phytochemicals made a distinct attention in the field of metal/metal oxide NP synthesis and received a considerable interest in the last few decades29. In the present study, the phytochemistry of methanolic extract of Plumeria rubra leaves was evaluated using qualitative phytochemical screening, total polyphenol content (TPC), and gas chromatography with flame ionisation detection (GC-FID) methods. The qualitative phytochemical investigation found the presence of alkaloids, cardiac glycosides, terpenoids, and flavonoids (Table 1), and the estimated total polyphenol content (TPC) was expressed as 135 mg of GAE g⁻¹ of dry extract wt, which is calculated using the linear regression curve of gallic acid shown in Fig. 1(a). The ubiquitous nature of polyphenols can be used useful in NP synthesis which improves their efficacy in relevant field of application30.
Table 1.
Qualitative phytochemical screening of Plumeria rubra methanolic leaf extract.
| S.No | Phytochemical Screening Test | Observation |
|---|---|---|
| 1 | Test for alkaloids (Mayer’s test) | Positive |
| 2 | Test for tannins (Ferric chloride test) | Negative |
| 3 | Test for saponins (Frothing’s test) | Negative |
| 4 | Test for steroids (Liebermann-Burchard reaction) | Negative |
| 5 | Test for flavonoids (Shinoda test) | Positive |
| 6 | Test for cardiac glycosides (Keller – kiliani test) | Positive |
| 7 | Test for phlobatannins (HCl test) | Negative |
| 8 | Test for anthraquinones (Borntager test) | Negative |
| 9 | Test for terpenoids (Salkowski test) | Positive |
| 10 | Test for carbohydrates (Molisch test) | Negative |
Fig. 1.
Phytochemistry of Plumeria rubra leaf methanolic extract; (a) Gallic acid standard curve (b) FTIR spectrum of methanolic extract and (c) GC-FID analysis of methanolic extract.
The FTIR analysis of the methanolic extract from Plumeria rubra leaves showed (Fig. 1(b)) a major transmittance peak at 3307 cm⁻¹, along with moderate and minor intensity peaks at 2844 cm⁻¹, 2981 cm⁻¹, 1638 cm⁻¹, 1015–1250 cm⁻¹, and 590 cm⁻¹, which indicate the stretching of functional groups such as hydroxyl (O-H), aliphatic (C-H), conjugated C = C, and carbonyl (C-O). These groups actively participate in the synthesis of AgNPs, and the peak at 590 cm⁻¹ is due to residual traces of metals.
The methanolic extract of Plumeria rubra leaves was also subjected to GC-FID analysis (Table 2; Fig. 1(c)) and revealed the presence of various phytochemicals such as phenolics and their derivatives (methyl salicylate and ferulic acid), terpenoid derivatives (β-sitosterol, stigmasterol, and squalene), cardiac glycosides (cardenolide), and other saturated and unsaturated fatty acids. The phenolics, terpenoids, and glycosides act as reducing agents by donating electrons to the metal ions and facilitate subsequent formation of nanoparticles31,32. The earlier studies also found that saturated and unsaturated fatty acids act as stabilizers and play a role in the growth of nanoparticles33,34. Thus, the GC-FID results of the present study revealed phytochemicals that play a significant role in the synthesis of metal nanoparticles.
Table 2.
Phytochemical constituents of Plumeria rubra methanolic leaf extract identified using GC-FID.
| S.No | Retention time (RT) | Identified compound | Approximate area (~%) | Observed molecular weight (m/z) (g mol−1) |
|---|---|---|---|---|
| 1 | 7.7 | Methyl salicylate | 30–35% | 152.15 |
| 2 | 12.8 | Myristic acid | 2% | 228.37 |
| 3 | 13.2 | Palmitic acid methyl ester | 3% | 270.5 |
| 4 | 14.2 | Oleic acid methyl ester | 2% | 296.5 |
| 5 | 15.1 | Stearic acid | 1.5% | 284.5 |
| 6 | 16.8 | Ferulic acid | 1% | 194.18 |
| 7 | 19.3 | β – Sitosterol | 2.5% | 414.7 |
| 8 | 22.2 | α- hydroxyl linoleic acid | 3% | 296.4 |
| 9 | 23.5 | Linoleic acid methyl ester | 4% | 294.5 |
| 10 | 24.9 | Stigmasterol | 5% | 412.7 |
| 11 | 27.2 | Arachidic acid | 1% | 312.5 |
| 12 | 32.3 | Cardenolide | 1% | 342.5 |
| 13 | 34.5 | Squalene | 2% | 410.79 |
Bio-reduction of AgNPs
The bio-reduction of AgNPs was carried out using methanolic extract of Plumeria rubra leaves. The addition of re-dissolved PR extract to 5 mM of AgNO3 solution resulted in instantaneous transformation of yellowish green to dark black which indicates the successful reduction of AgNPs. The color of the colloidal solution is depends on SPR absorption, size and shape of the AgNPs35. The exact mechanism of AgNPs synthesis yet to understand but the studies reported that synthesis occurs in two consecutive stages: First the reduction of metal ions by plant phytochemicals and second agglomeration of colloidal nanoparticles. This ultimately result in formation of agglomeric clusters22. Several analytical techniques were used to confirm the physiochemical properties (optical and morphological) of synthesized PR-AgNPs.
Surface plasmon resonance (SPR) and surface functional group of PR-AgNPs
The bio-reduction of AgNPs by the phytochemicals of Plumeria rubra methanolic leaf extract was monitored by measuring SPR with UV-Visible spectroscopy from wavelength region of 200–800 nm and PR-AgNPs exhibited a characteristic SPR at λmax = 432 nm (Supplementary data 1 (Fig. S1)). The tauc’s plot of PR-AgNPs were represented in Fig. 2(a) (Direct band gap) and Fig. 2(b) (Indirect band gap). The SPR or also known as Localized SPR (LSPR) a characteristic optical property of metallic NPs and AgNPs typically exhibit SPR between 400 and 470 nm36. The SPR effect arises from the interaction between incident photons (light) and nanomaterial surface electrons, which is facilitatedby the precise wavelength and frequency of the photons. This interaction induces plasmonic effects and excites the electrons37. Similar studies reported with SPR peaks at 430 nm (Diospyros paniculata root methanolic extract)24 and 436 nm (Aegle marmelos fruit methanolic extract)7. In contrast to this AgNPs synthesized using C. maritima seed methanolic extract showed a slight shift in SPR at 369 nm25.
Fig. 2.
Optical Properties of PR-AgNPs (a) UV-Tauc’s plot (Direct) (b) UV-Tauc’s plot (Indirect) (c) FTIR transmittance spectrum (d) X-ray diffractogram.
As shown in Fig. 2(c) FTIR analysis revealed surface functional groups of PR-AgNPs from 500 to 4500 cm−1 region. The major vibrational signals at 1374 cm−1 and 1588 cm−1 corresponds to O-H bending (alcohol) and N-O stretching (nitro compound) respectively. Other vibrational signals were found at 3205, 3790 cm−1 (O-H stretching (alcohol)), 2920 cm−1 (Asymmetric C-H stretching (Alkane)), 2358 cm−1 (-C = N stretching), 1762 cm−1 (C = O stretching (carbonyl group)), 1047 cm−1 (CO-O-CO stretching (Anhydride)), 822 cm−1 (C-H bending) and a metal oxide bond at 588 cm−1. This revealed that the phytochemicals of PR-methanolic leaf extract were participated in reduction and capping of PR-AgNPs. Many of these functional groups of FTIR are belongs to the phytochemicals in the PR extract.
The phytochemicals of the methanolic extract of PR leaves analysed using qualitative screening, FTIR and GC-FID provided the basis for a hypothetical mechanism of PR-AgNP formation and schematic representation shown in Fig. 3. The alkaloids, flavonoids, terpenoids, and cardiac glycosides in the extract play a critical role in reducing silver ions (Ag⁺) to metallic silver (Ag⁰) through electron transfer. The O-H bending and stretching signals indicate the abundance and reducing activity of phenolic compounds. Similarly, carbonyl groups (C = O) of terpenoids and glycosides also participate in the reduction process. The asymmetric C-H stretching and C = N stretching of alkaloids play a role in stabilizing PR-AgNPs. The appearance of a metal-oxide bond at 588 cm−1 indicates the interaction between functional groups and silver ions, ensuring stabilization. Thus, these functional groups are efficient in reduction of silver ions and stabilization of PR-AgNPs, preventing agglomeration and stabilization.
Fig. 3.
Schematic representation of PR-AgNP synthesis. (a) PR methanolic extract and its phytochemistry (b) Addition of PR methanolic extract to AgNO3 solution (c) Bio-reduction of Ag + ions (d) Reduced silver and polymerization (e) Surface functionalized PR-AgNPs.
Phase analysis
Figure 2(d) represents the X-ray diffractogram of PR-AgNPs, confirming the face-centered cubic (fcc) amorphous nature of PR-AgNPs in line with the standard reference JCPDS data with card No. 01-087-0597. The hkl values of (111), (200), (220), (311), and (222) at 2θ angles of 38.11°, 44.27°, 64.42°, 77.47°, and 81.53°, respectively, confirmed the synthesis of PR-AgNPs. The spectrographic analysis showed the traces of Ca, Fe, and Cu, and the melting point was 1233.6 K. The occurrence of unindexed peaks in the diffractogram may be due to either crystal diffraction planes of AgNPs surface silver oxide or phytochemicals from plant extract acting as capping agents38. The crystallinity percentage, crystallite size, d-spacing (in Å), and FWHM for each diffraction peak are calculated and given in the supplementary data 1 (Supplementary Table 1). The calculated average crystallite size using Scherrer equation was 16.44 nm (Eq. 1). In contrast to this, previous studies reported average crystallite sizes of 15.9339 and 181.36 nm7.
 |
1 |
D = Crystallite size.
K = Shape factor (0.9).
λ = X-ray Wavelength.
β = Full width at half maximum (FWHM) of the diffraction peak.
θ = Bragg’s angle.
Morphology and elemental composition
The morphological characteristics, elemental distribution and composition of the synthesized PR-AgNPs were evaluated through FESEM and EDX analysis, as depicted in Fig. 4 (a–c). The FESEM micrograph (Fig. 4(a-b)) represents the morphology of irregular shapes of AgNPs, and aggregated on sheet like structures. The morphological pattern is may be due to the role of phytochemicals in capping, nucleation, and growth processes. The elemental mapping (Fig. 4(c)) confirms the uniform distribution of Ag throughout the sample, and the EDX spectrum (Fig. 4(d)) exhibits a prominent peak at 3 keV, characteristic of the plasmon resonance of silver, along with additional minor peaks associated with silver and other elements (as a part of plant material). These results collectively confirm the influence of phytochemicals in PR-methanolic extracts on the morphological characteristic elemental distribution and composition of PR-AgNPs. There are reports showing less agglomeration and more spherical morphology when using methanolic plant extracts to synthesize AgNPs7,39,40.
Fig. 4.
Morphology and Elemental Composition of PR-AgNPs (a&b) FESEM micrograph (c) Elemental mapping and (d) EDAX spectrum.
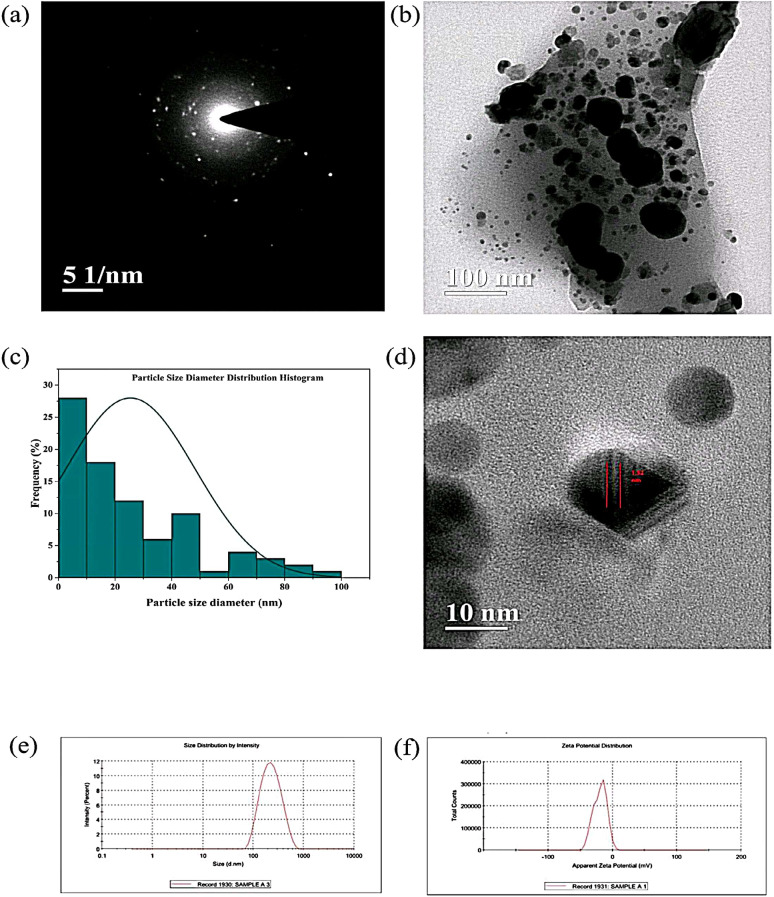
SAED pattern and size distribution
The selected area electron diffraction (SAED) pattern of PR-AgNPs, as shown in Fig. 5(a), indicates their amorphous nature, correlated with the X-ray diffractogram. The HRTEM micrograph (Fig. 5(b)) reveals the less agglomerated nanoparticles, and particle size diameter distribution of PR-AgNPs was performed using Image J software. The diameters of the particles range from 0 to 100 nm (Fig. 5(c)), with an average diameter of 25.42 nm and the calculated maximum fine fringes of PR-AgNPs are 1.32 nm (Fig. 5(d)).
Fig. 5.
SAED Pattern, Size distribution and DLS of PR-AgNPs (a) SAED pattern (b) HRTEM micrograph (c) Size diameter histogram (d) Fine fringes (e) Hydrodynamic size and (f) Zeta potential.
Dynamic light scattering
Dynamic light scattering (DLS) analysis was employed to evaluate the hydrodynamic size and zeta potential of PR-AgNPs. The Z-average hydrodynamic size was determined to be 196.6 nm, with a polydispersity index (PDI) of 0.187, indicating moderate size uniformity (Fig. 5(e)). The PDI value ranges from 0 to 1 represents homogeneous distribution of particles with slight to moderate aggregation41. Zeta potential analysis (Fig. 5(f)) revealed a value of −19.3 ± 11.7 mV, reflecting the surface charge and stability of the nanoparticles in suspension. A tendency of ZP towards zero suggests particle agglomeration; however, particles exhibiting a negative zeta potential generally demonstrate greater stability and lower agglomeration in solutions42. The high conductivity of 4.06 mS suggests the presence of ionic species, likely influenced by the phytochemical capping agents. These results show the successful synthesis of stable PR-AgNPs with controlled size and surface characteristics, suitable for diverse applications.
In vitro biological activities
The in vitro biological activities of synthesized PR-AgNPs were subjected to antimicrobial, antioxidant, inhibition of heat-induced RBC haemolysis, and cytotoxicity assays. The photographs of experiments are shown in supplementary data 1 (Fig. S2-S4).
Antimicrobial activity of PR-AgNPs
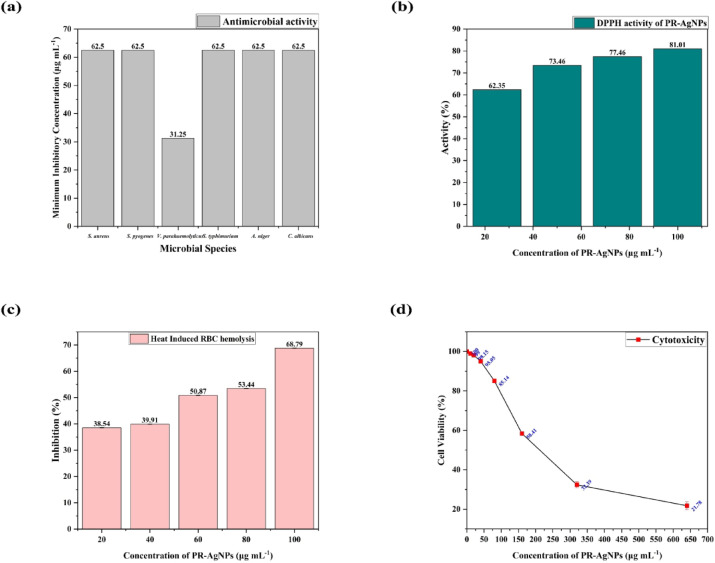
The antimicrobial activity of PR-AgNPs on 6 tested microbial species was measured in terms of MIC (µg mL- 1). The PR-AgNPs showed an almost similar range of MIC (62.5 ± 0 µg mL- 1) on all tested species except for V. parahaemolyticus (31.2 ± 0 µg mL- 1) depicted in Fig. 6 (a). The activity of PR-AgNPs depends on various factors such as synthesis method, shape, size, and surface functionalization43. AgNPs synthesized using a variety of green reducing agents exhibit different mechanisms of action on microbes. To date, no study has reported a precise antimicrobial mechanism of action for AgNPs and using various analytical techniques such as SEM, LC MS, NMR, etc., researchers reported possible antimicrobial mechanisms of nanoparticles44. In a study, AgNPs synthesized using Plumeria rubra flower methanolic extract added with polyvinyl alcohol revealed MIC values ranging from 15.6 to 250 µg mL⁻¹45 and the present study showed a moderate MIC value, but only methanolic extract was used without any stabilizing agents. In another similar study, aqueous extract of Plumeria alba leaves used to synthesize AgNPs revealed a maximum zone of inhibition of 16 mm at 100 µg mL⁻¹46. There are several studies, which effectively showed antimicrobial activity of AgNPs but it’s in vivo efficacy and adversity must be determined with future studies.
Fig. 6.
In vitro biological activities of PR-AgNPs (a) Antimicrobial activity (b) DPPH activity (c) inhibition of RBC hemolysis and (d) cytotoxicity.
In vitro antioxidant activity
The in vitro antioxidant activity of PR-AgNPs was evaluated using the DPPH assay, where the concentration-dependent free radical scavenging was observed, ranging from 62.35 to 81.01% at concentrations of 25 to 100 µg mL⁻¹ (Fig. 6(b)). The calculated IC50 value of PR-AgNPs for DPPH is 10.85 µg mL⁻¹, whereas standard ascorbic acid showed an IC50 value of 2.46 µg mL⁻¹. At 517 nm, DPPH usually has a deep purple colour. When antioxidant molecules donate an electron or transfer an H atom, it creates a reduced hydrazine form. This changes the colour from deep purple to yellow to colourless, depending on efficacy of the antioxidant molecules47. Phenolic compounds of plants are effective H donors, which in turn are considered efficient antioxidant molecules. Surface functionalization of phenolic compounds in nanoparticles may enhance their free radical scavenging efficacy48. The AgNPs synthesized using methanolic extracts of Caesalpinia pulcherrima stem49 and Reynoutria japonica root50 showed DPPH activity with IC50 values of 664 µg mL⁻¹ and 19.25 µg mL⁻¹, respectively.
Inhibition of heat-induced RBC hemolysis
The inhibition of heat-induced RBC hemolysis of PR-AgNPs was tested in in vitro conditions using chicken RBC and a percentage of inhibitions (%) ranging from 38.54 to 68.79% (Fig. 6(c)) at concentrations of 20 to 100 µg mL⁻¹. The calculated IC50 value of PR-AgNPs is 31.30 µg mL⁻¹, and standard aspirin showed an IC50 value of 12.74 µg mL⁻¹. This assay is commonly known as hemocompatibility, and the induced thermal stress on RBC cells causes destabilization of cell membranes, elevation of osmotic fragility, and alterations in cell rigidity through oxidative stress51. The adhesion of NPs to the RBC may protect the membrane integrity and stabilize the cellular structure through scavenging free radicals52. The AgNPs synthesized using Mentha spicata L. extract revealed hemocompatibility of less than 2%53, while the bAgNPs synthesized using Syzygium cymosum leaf extract showed no significant hemocompatibility54.
In vitro cytotoxicity
The in vitro cytotoxicity of PR-AgNPs on SH-SY5Y cell lines was evaluated at concentrations ranging from 10 to 640 µg mL⁻¹, showing concentration-dependent reduction in cell viability from 98.86 to 23.79% (Fig. 6(d)). The calculated IC50 value, representing the concentration at which 50% of the cells were inhibited, was determined to be 72.54 µg mL⁻¹. Several mechanisms, including oxidative stress induction, plasma membrane disruption, denaturation, and cellular component damage, can trigger the cytotoxicity of PR-AgNPs. The cytotoxic potentiality is dependent on several factors such as the type of metal/metal oxide, molarity of precursor molecules, surface functionalization, and synthesis method55. AgNPs synthesized using Citrus unshiu fruit peel extract showed effective cytotoxicity on HEK293 (37.73 µg mL⁻¹) and A549 (56.37 µg mL⁻¹) cell lines, which are in almost similar to the present study56.
Limitations
Besides the advantages and applications of nanomaterials synthesized using green precursors or derivatives, there are associated disadvantages. The quality and quantity of plant phytochemicals are geographically variable, so that plants collected at different geographical locations may show variable properties. Reproducibility and large-scale production of these nanomaterials are always challenging.
Conclusion
The bio-reduction of AgNPs was successfully achieved using methanolic extract of Plumeria rubra leaves. The preliminary phytochemical screening, FTIR and GC-FID methods of methanolic extract found the presence of alkaloids, cardiac glycosides, terpenoids, flavonoids, and fatty acid molecules which play a crucial role in the synthesis of metal nanoparticles. The formation of PR-AgNPs was confirmed preliminarily by evaluating SPR using UV-Vis spectroscopy at 432 nm, and FTIR analysis revealed surface functionalization with O-H, C = O, C-H, and N-O functional groups which are correlated with the phytochemistry of PR extract. This revealed the role of various phytochemicals in synthesis of nanoparticles. The PR-AgNPs exhibited irregularity in shape, and most of the particles are in embedded on sheet like structure with particle diameter size ranging from 1 to 100 nm. The antimicrobial activity was tested against 6 pathogenic organisms, and on V. parahaemolyticus, AgNPs showed inhibition at a concentration of 31.25 µg mL⁻¹. The PR-AgNPs exhibited IC50 values of 10.86, 31.30, and 72.54 µg mL⁻¹ for DPPH, hemocompatibility, and cytotoxicity assays. Thus, the efficacy of nanoparticles for specific applications depends upon their surface functionalization. Future studies could explore the synthesis of AgNPs using various concentrations of plant extract to reduce agglomeration and refine the size of the NPs. The present study limited antibacterial activity only using a single assay, but the mechanism of action of antimicrobial activity could be analysed using mass spectrophotometry and electron microscopy methods. The reproducibility of the present study remains a significant concern due to the agglomeration and non-homogeneous size distribution.
Methods
Processing of leaves and Preparation of methanolic extract
The freshly collected leaves of Plumeria rubra (obtained from Adikavi Nannaya University) were identified by Dr. A. Matta Reddy, Associate Professor, School of Life and Health Sciences, Adikavi Nannaya University, and further, their morphological characteristics were confirmed using the reports of Bihani, T., 2021, and Malaspina Paola et al., 202426,57. These leaves are washed with double distilled water, and dried at room temperature and made into fine powder using a conventional blender. The methanolic extract was prepared as follows: The Soxhlet was loaded with 25 g of plant powder in a thimble, and 300 mL of methanol solvent was added to the round-bottom flask, and the equipment was run for 6–7 cycles. The reaction mixture was collected in a round-bottomed flask and filtered through muslin cloth, followed by Whatman No.1 filter paper. The resulting reaction mixture was further concentrated at reduced pressure using a rotary evaporator, and the final yield was stored at 4 °C.
Phytochemical screening and total polyphenol content (TPC) Estimation
The phytochemicals of PR-Methanolic extract were observed using conformational tests such as alkaloids (Mayers and Wagner’s), tannins, saponins, steroids, flavonoids, cardiac glycosides, phlobatannins, anthraquinones, terpenoids, and carbohydrates. The Folin-Ciocalteu (FC) method was used to estimate the amount of TPC58. The detailed procedures of phytochemical screening and TPC are given in supplementary data 1. The phytochemistry of methanolic extract further evaluated using FTIR (400–4,000 cm−1 (Bruker Alpha II)) and GC-FID (Shimadzu GC-2010 plus) techniques.
Phyto-mediated synthesis of silver nanoparticles (AgNPs)
The concentrated PR methanolic extract was re-dissolved in distilled water (1 mg mL−1) and ultra-sonication was carried out for 30 min to ensure complete dissolving. For the synthesis of AgNPs, 5mM of silver nitrate (AgNO3) was dissolved in 90 mL of distilled water and kept on magnetic stirrer in dark at 70 °C. The sonicated PR extract was slowly released into AgNO3 solution with a speed of 1 min mL−1 and color of the colloidal solution was turned from pale yellow to dark brown, which thereby indicates the formation of AgNPs and the reaction was continued for next 24 h. The colloidal solution was centrifuged and the supernatant was discarded and the pellet was washed thrice with 70% ethyl alcohol and dried at 70 °C using hot air oven. The final PR-AgNPs were stored at 4 °C. The photographs of synthesis process are shown in supplementary data Fig. S5.
Evaluation of optical and morphological properties of PR-AgNPs
The optical properties of PR-AgNPs were evaluated using following analytical techniques: surface plasmon resonance (SPR) of by UV-Vis spectrophotometer from 200 to 800 nm wavelength region (UV-Shimadzu 2,600), surface functionalization by FTIR from 400 to 4,000 cm−1 (Bruker Alpha II), phase analysis by X-ray Diffractometer from 10–90o at 2θ (BRUKER). The morphological and size analysis was carried out using field emission scanning electron microscopy (FESEM) (JEOL), high resolution transmission electron microscopy (HRTEM) (Thermo Scientific) and Zeta sizer (MALVERN)59,60.
Antimicrobial activity
The antimicrobial properties of PR-AgNPs were determined by evaluating Minimum Inhibitory Concentration (MIC) using broth micro dilution method on two gram positive bacteria (Staphylococcus aureus MTCC 87 and Streptococcus pyogenes MTCC 442), two gram negative bacteria (Vibrio parahaemolyticus MTCC 451 and Salmonella enterica typhimurium MTCC 98) and two fungal species (Aspergillus niger MTCC 282 and Candida albicans MTCC 183). The freeze-dried cultures were inoculated into Luria Bertani (LB) broth, incubated overnight at 37 °C and the final optical density of each culture was adjusted to 105 CFU mL−1 prior to the experiment. This assay was performed using two-fold dilution method in 96 well micro titer plates and PR-AgNPs (1,000–3.9 µg mL−1) were added to the LB broth. Each microbial broth with 0.1% TTC solution was added to the wells with PR-AgNPs and incubated at 37 °C for 24 h. A positive and negative controls were maintained. After incubation the wells with no bacterial growth at minimum concentration of PR-AgNPs was calculated.
In vitro antioxidant property
The antioxidant property of PR-AgNPs was evaluated using DPPH (2,2-diphenyl-1-picryl-hydrazyl) free radical scavenging activity. In brief, 3 mM of DPPH solution was prepared freshly and absorbance (0.600–0.650) adjusted at 517 nm, stored in dark until use. Different concentrations of PR-AgNPs (25–100 µg mL−1) in methanol were sonicated and added to the DPPH reagent. These solutions were incubated in dark for 1 h and absorbance was read at 517 nm. Ascorbic acid was used as standard and % of DPPH scavenging activity was calculated using Eq. (2) and Inhibitory Concentration 50 (IC50) using Eq. (3).
| 2 |
| 3 |
Inhibition of heat induced RBC hemolysis
The inhibition of heat induced RBC hemolysis was carried out using hemocompatibility assay on chicken RBC. The blood sample was collected directly from commercial chicken markets without any involvement of authors of the present study. The collected heparinized blood was centrifuged to remove the serum and re-dissolved in 10% PBS (1:10 v/v). Different concentrations of PR-AgNPs (20–100 µg mL−1) were added to the PBS (pH 7.4) and PBS dissolved blood sample. The samples were incubated at 37 °C for 5 min and subsequently transferred to water bath at 56 °C for 10 min of incubation. The reaction mixtures were cooled down to room temperature and centrifuged at 2,500 rpm for 3 min and the absorbance of supernatant was read at 540 nm. The inhibition (%) and the IC50 values were calculated according to the Eqs. 2&3.
In vitro cytotoxicity
The MTT assay was performed on SH-SY5Y cells using a standard protocol. Cells were revived from cryopreservation, washed with 1X PBS, and suspended in media after centrifugation. Cell counting was conducted using a hemocytometer, and cells were seeded at a density of 10,000 cells per well in a 96-well plate. The media was removed after 24 h and cells were washed with PBS. The PR-AgNPs of various concentrations (10–640 µg mL−1) dissolved in media, followed by incubation for another 24 h. After the incubation, the cells were washed with PBS, and 10 µg of MTT solution (5 mg mL−1 in PBS) was added to each well and incubation carried out for next 4 h. Then, the media was removed, followed by addition of 120 µL of DMSO to dissolve formazan crystals. The optical density of each well at each concentration was measured using an ELISA plate reader at 570 and 590 nm to evaluate cell viability. The cell viability (%) and the IC50 values were calculated according to the Eqs. 2&3.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to the School of Life and Health Sciences, Adikavi Nannaya University, Rajamahendravaram, Andhra Pradesh, India and Sathyabama University, Chennai for providing necessary research facilities and outsouring services.
Author contributions
AM & AMR: Design of the work & Methodology; PVN, DK, KR, & IJNP: Drafting & Revision; LPP, GSS, BSK, SF: Interpretation of data; DR, BM, PBK: Acquisition & Analysis.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1. Kirubakaran, D. et al. Green synthesis of zinc oxide nanoparticles using acmella caulirhiza leaf extract: characterization and assessment of antibacterial, antioxidant, Anti-inflammatory and hemolytic properties. Biomed. Mater. Devices. 10.1007/s44174-025-00283-8 (2025). [Google Scholar]
- 2.Yugay, Y. A. et al. Biosynthesis of functional silver nanoparticles using callus and hairy root cultures of aristolochia manshuriensis. J Funct. Biomater14, (2023). [DOI] [PMC free article] [PubMed]
- 3.Chandra, D. K., Reis, R. L., Kundu, S. C., Kumar, A. & Mahapatra, C. Nanomaterials-Based hybrid Bioink platforms in advancing 3D Bioprinting technologies for regenerative medicine. ACS Biomater. Sci. Eng.10.1021/acsbiomaterials.4c00166 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar, M. et al. Synthesis of chitosan/pva/copper oxide nanocomposite using anacardium occidentale extract and evaluating its antioxidant, antibacterial, anti-inflammatory and cytotoxic activities. Sci. Rep.15, 3931 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma, A., Sagar, A., Rana, J. & Rani, R. Green synthesis of silver nanoparticles and its antibacterial activity using fungus talaromyces purpureogenus isolated from taxus baccata Linn. Micro Nano Syst. Lett10, (2022).
- 6.Saqib, S. et al. Postharvest disease Inhibition in fruit by synthesis and characterization of Chitosan iron oxide nanoparticles. Biocatal. Agric. Biotechnol.28, 101729 (2020). [Google Scholar]
- 7.Devi, M. et al. Green synthesis of silver nanoparticles using methanolic fruit extract of Aegle Marmelos and their antimicrobial potential against human bacterial pathogens. J. Tradit Complement. Med.10, 158–165 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saqib, S. et al. Bimetallic Assembled Silver Nanoparticles Impregnated in Aspergillus fumigatus Extract Damage the Bacterial Membrane Surface and Release Cellular Contents. Coatings vol. 12 at (2022). 10.3390/coatings12101505
- 9.Pangi, V. N., Marukurti, A., Reddy, A. M. & Medapalli, S. R. Synthesis of biogenic silver nanoparticles (bAgNPs) using leaf extract of mirabilis Jalapa and evaluation of Anti-vibriocidal, Anti-oxidant properties and cytotoxicity. Bionanoscience13, 376–392 (2023). [Google Scholar]
- 10.Saif, S., Tahir, A. & Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials6, 1–26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ying, S. et al. Green synthesis of nanoparticles: current developments and limitations. Environ. Technol. Innov.26, 102336 (2022). [Google Scholar]
- 12.Kirubakaran, D. et al. Biogenic synthesis of copper nanoparticle using impatiens chinensis L: insights into antimicrobial, antioxidant and anticancer activity. J. Mol. Struct.1317, 138991 (2024). [Google Scholar]
- 13.Kirubakaran, D. et al. Bio-fabrication of zinc oxide nanoparticles using strobilanthes cordifolia: characterization and evaluation of antioxidant, anti-cholinergic, anti-inflammatory and wound healing activities. ChemistrySelect9, e202302792 (2024).
- 14.Ankegowda, V. M. et al. Phyto-Mediated synthesis of silver nanoparticles using terminalia chebula fruit extract and evaluation of its cytotoxic and antimicrobial potential. Molecules25, (2020). [DOI] [PMC free article] [PubMed]
- 15.Kirubakaran, D. et al. A comprehensive review on the green synthesis of nanoparticles: advancements in biomedical and environmental applications. Biomed. Mater. Devices. 10.1007/s44174-025-00295-4 (2025). [Google Scholar]
- 16.Azmir, J. et al. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng.117, 426–436 (2013). [Google Scholar]
- 17.Altemimi, A. et al. Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (Basel Switzerland)6, (2017). [DOI] [PMC free article] [PubMed]
- 18.Ramabulana, T., Ndlovu, M., Mosa, R. A., Sonopo, M. S. & Selepe, M. A. Phytochemical profiling and isolation of bioactive compounds from Leucosidea sericea (Rosaceae). ACS Omega. 7, 11964–11972 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Nemrawi, N., Hameedat, F. & El-Elimat, T. Green synthesis of silver nanoparticles using Bellevalia flexuosa leaves extract. Sci Pharm90, (2022).
- 20.Phukan, K., Devi, R. & Chowdhury, D. Green synthesis of gold Nano-bioconjugates from onion Peel extract and evaluation of their antioxidant, Anti-inflammatory, and cytotoxic studies. ACS Omega. 6, 17811–17823 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flieger, J. et al. Green synthesis of silver nanoparticles using natural extracts with proven antioxidant activity. Molecules26, (2021). [DOI] [PMC free article] [PubMed]
- 22.Ullah, I. et al. Peganum Harmala L. extract-based gold (Au) and silver (Ag) nanoparticles (NPs): green synthesis, characterization, and assessment of antibacterial and antifungal properties. Food Sci. Nutr. 1–14. 10.1002/fsn3.4112 (2024). [DOI] [PMC free article] [PubMed]
- 23.Singh, R. Current research in green and sustainable chemistry green synthesis of silver nanoparticles using methanol extract of Ipomoea carnea jacq. To combat multidrug resistance bacterial pathogens. Curr. Res. Green. Sustain. Chem.4, 100152 (2021). [Google Scholar]
- 24.Rao, N. H. et al. Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater. Sci. Eng. C. 62, 553–557 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Elazab, N. T., Baka, Z. A. M., Saleh, H. H. & El-Zahed, M. M. Green synthesis of silver nanoparticles using cakile maritima seed extract: molecular, antifungal and physiological studies. Physiol. Mol. Plant. Pathol.129, 102183 (2024). [Google Scholar]
- 26.Bihani, T. Plumeria rubra L.– A review on its ethnopharmacological, morphological, phytochemical, Pharmacological and toxicological studies. J. Ethnopharmacol.264, 113291 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Kaur, J., Sanghavi, A. D., Chopra, A., Lobo, R. & Saha, S. Antimicrobial and cytotoxicity properties of plumeria Alba flower extract against oral and periodontal pathogens: A comparative in vitro study. J. Indian Soc. Periodontol. 26, 334–341 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar Sharma, S., Ali*, M., Kumar, N. & Sultana, S. Rasool mir, S. Chemical constituents from the stem barks of plumeria rubra L. Res. J. Pharmacogn. 5, 69–78 (2018). [Google Scholar]
- 29.Ovais, M., Khalil, A. T., Ayaz, M. & Ahmad, I. Chapter 7 - Metal oxide nanoparticles and plants. in Phytonanotechnology (eds. Thajuddin, N. & Mathew, S.) 123–141 (Elsevier, 2020). 10.1016/B978-0-12-822348-2.00007-3
- 30.Aguirre, A. & Borneo, R. Chapter 4 - Improving Bioavailability of Polyphenols Using Nanodelivery Systems Based on Food Polymers. in Polyphenols in Plants (Second Edition) (ed. Watson, R. R.) 59–65Academic Press, (2019). 10.1016/B978-0-12-813768-0.00004-9
- 31.Hosseingholian, A., Gohari, S. D., Feirahi, F., Moammeri, F. & Mesbahian, G. Materials today sustainability recent advances in green synthesized nanoparticles: from production to application. Mater. Today Sustain.24, 100500 (2023). [Google Scholar]
- 32.Kasthuri, K. & K. I., V. S. A. Green synthesis of metal nanoparticles from three medicinal plants: a review of environmental and health applications. Discov Catal.10.1007/s44344-025-00007-6 (2025). [Google Scholar]
- 33.Yue, Y. & Yokota, Y. iScience Ll biosynthesis of highly branched gold nanoparticles through structural engineering of fatty acids. ISCIENCE26, 105864 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matei, A., Stoian, M., Crăciun, G. & Țucureanu, V. Chemical Synthesis and Characterization of Fatty Acid-Capped ZnO Nanoparticles. Journal of Composites Science vol. 8 at (2024). 10.3390/jcs8100429
- 35.Dipole, D. & Method, A. A. Study of the surface plasmon resonance of silver nanoparticles by the A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: effect of shape, size, structure, and assembly. (2010). 10.1007/s11468-009-9120-4
- 36.Alzoubi, F. Y., Ahmad, A. A., Aljarrah, I. A. & Migdadi, A. B. Al-Bataineh, Q. M. Localize surface plasmon resonance of silver nanoparticles using Mie theory. J. Mater. Sci. Mater. Electron.34, 1–10 (2023). [Google Scholar]
- 37.Abaid, R. et al. Biosynthesizing Cassia fistula Extract-Mediated silver nanoparticles for MCF – 7 cell lines Anti-Cancer assay. (2023). 10.1021/acsomega.3c02225 [DOI] [PMC free article] [PubMed]
- 38.Alamier, W. M. et al. Green synthesis of silver nanoparticles using acacia ehrenbergiana plant cortex extract for efficient removal of Rhodamine B cationic dye from wastewater and the evaluation of antimicrobial activity. ACS Omega. 8, 18901–18914 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh, R. & Navneet Green synthesis of silver nanoparticles using methanol extract of Ipomoea carnea jacq. To combat multidrug resistance bacterial pathogens. Curr. Res. Green. Sustain. Chem.4, 100152 (2021). [Google Scholar]
- 40.Pavić, V. et al. Antibacterial efficacy and characterization of silver nanoparticles synthesized via methanolic extract of Fomes fomentarius L. Fr. Molecules29, (2024). [DOI] [PMC free article] [PubMed]
- 41.Pangi, V. N., Marukurti, A., Reddy, A. M. & Medapalli, S. R. Synthesis of biogenic silver nanoparticles (bAgNPs) using leaf extract of mirabilis Jalapa and evaluation of Anti-vibriocidal, Anti-oxidant properties and cytotoxicity. Bionanoscience10.1007/s12668-023-01060-x (2023). [Google Scholar]
- 42.Marukurti, A., Reddy, A. M. & Praveena, V. D. Sustainable Biogenic Synthesis of Silver Nanoparticles (AgNPs) Using Aegle Marmelos and Evaluation of their Photocatalytic, Antimicrobial and Cytotoxicity Properties (Atlantis Press International BV, 2025). 10.2991/978-94-6463-648-2
- 43.Hijazi, B. U., Faraj, M., Mhanna, R. & El-Dakdouki, M. H. Biosynthesis of silver nanoparticles as a reliable alternative for the catalytic degradation of organic dyes and antibacterial applications. Curr. Res. Green. Sustain. Chem.8, 100408 (2024). [Google Scholar]
- 44.Ananda, T., Modi, A., Managuli, V. & Mukhopadhyay, C. Antimicrobial property of silver nanoparticles: effects of concentration and temperature on bacterial isolates. J. Pure Appl. Microbiol.17, 1118–1127 (2023). [Google Scholar]
- 45.Elwakil, B. H., Elsabrouty, M. H., Eskandrani, A., Paudel, K. R. & Moneer, E. A. Multifunctional plumeria rubra nanoparticles: antimicrobial, antiviral, antiparasitic and anti-inflammatory activities. Heliyon11, e42044 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudrappa, M. et al. Plumeria alba-Mediated green synthesis of silver nanoparticles exhibits antimicrobial effect and Anti-Oncogenic activity against glioblastoma U118 MG cancer cell line. Nanomater (Basel Switzerland) 12, (2022). [DOI] [PMC free article] [PubMed]
- 47.Baliyan, S. et al. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of ficus religiosa. Molecules27, (2022). [DOI] [PMC free article] [PubMed]
- 48.Gulcin, İ. & Alwasel, S. H. DPPH Radical Scavenging Assay. Processes11, (2023).
- 49.Moteriya, P. & Chanda, S. Biosynthesis of silver nanoparticles formation from caesalpinia pulcherrima stem metabolites and their broad spectrum biological activities. J. Genet. Eng. Biotechnol.16, 105–113 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khuda, F. et al. Assessment of antioxidant and cytotoxic potential of silver nanoparticles synthesized from root extract of reynoutria Japonica Houtt. Arab J. Chem15, (2022).
- 51.Yedgar, S., Barshtein, G. & Gural, A. Hemolytic activity of nanoparticles as a marker of their hemocompatibility. Micromachines13, 1–15 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehrizi, T. Z. Hemocompatibility and hemolytic effects of functionalized nanoparticles on red blood cells: A recent review study. Nano16, 2130007 (2021). [Google Scholar]
- 53.Khalid, Z. et al. Biosynthesis, structural characterization of silver nanoparticles synthesized using an eco-friendly method with mentha spicata L. extract and their antimicrobial activity and toxicological risk assessment. Results Chem.7, 101487 (2024). [Google Scholar]
- 54.Mahmud, K. M. et al. Investigation of antimicrobial activity and biocompatibility of biogenic silver nanoparticles synthesized using syzigyum cymosum extract. ACS Omega. 7, 27216–27229 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Awashra, M. & Młynarz, P. The toxicity of nanoparticles and their interaction with cells: an in vitro metabolomic perspective. Nanoscale Adv.5, 2674–2723 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mafhala, L. et al. Antibacterial and cytotoxicity activity of green synthesized silver nanoparticles using aqueous extract of Naartjie (Citrus unshiu) fruit peels. Emerg. Contam.10, 100348 (2024). [Google Scholar]
- 57.Malaspina, P. et al. Beyond the scent: new evidence about micromorphological, phytochemical and biological features of plumeria rubra ‘tonda palermitana’ (Apocynaceae). Plants (Basel Switzerland)13, (2024). [DOI] [PMC free article] [PubMed]
- 58.Mishra, P., Yadav, K. S., Gautam, G., COMPARATIVE & QUALITATIVE AND QUANTITATIVE PHYTOCHEMICAL ANALYSIS OF CALOTROPIS GIGANTEA AND CALOTROPIS PROCERA ROOTS. 8, 179–184 (2018).
- 59.Raman, G. et al. Synthesis of silver nanoparticles@carbon Dot nanocomposites using Aegle Marmelos and euphorbia hirta: anti vibriocidal and antioxidant properties. Results Chem.13, 101964 (2025). [Google Scholar]
- 60.Marukurti, A. et al. Atlantis Press,. Sustainable Biogenic Synthesis of Silver Nanoparticles (AgNPs) using Aegle marmelos and Evaluation of their Photocatalytic, Antimicrobial and Cytotoxicity Properties BT - Proceedings of the International Conference on Bio-Based Environment for Sustainable Territory (ICBEST 2024). in 178–194 (2025). 10.2991/978-94-6463-648-2_15
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.