Abstract
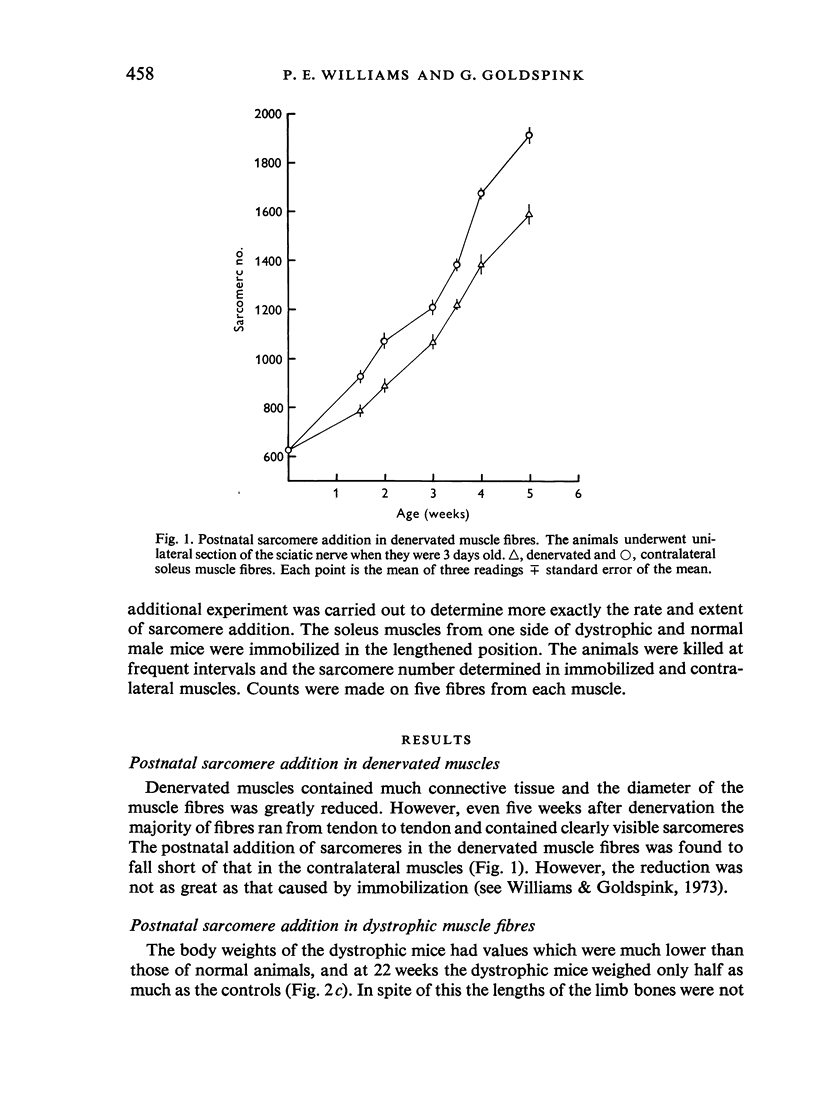
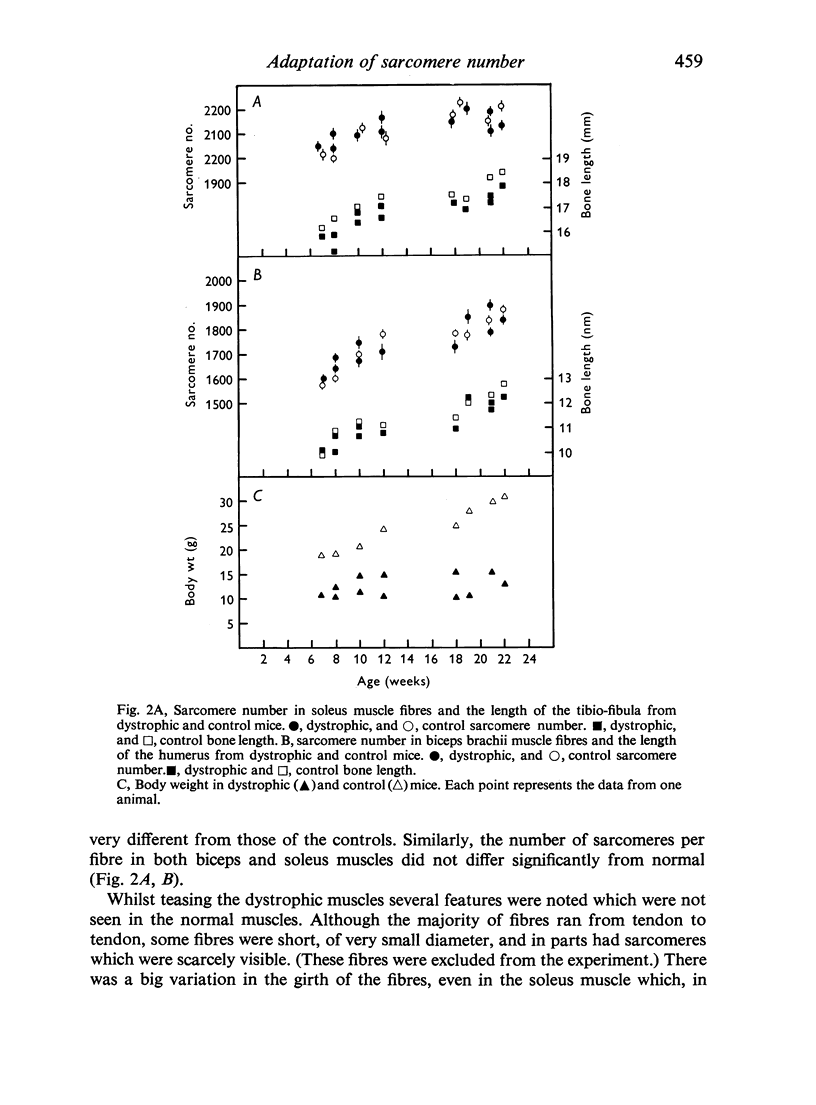
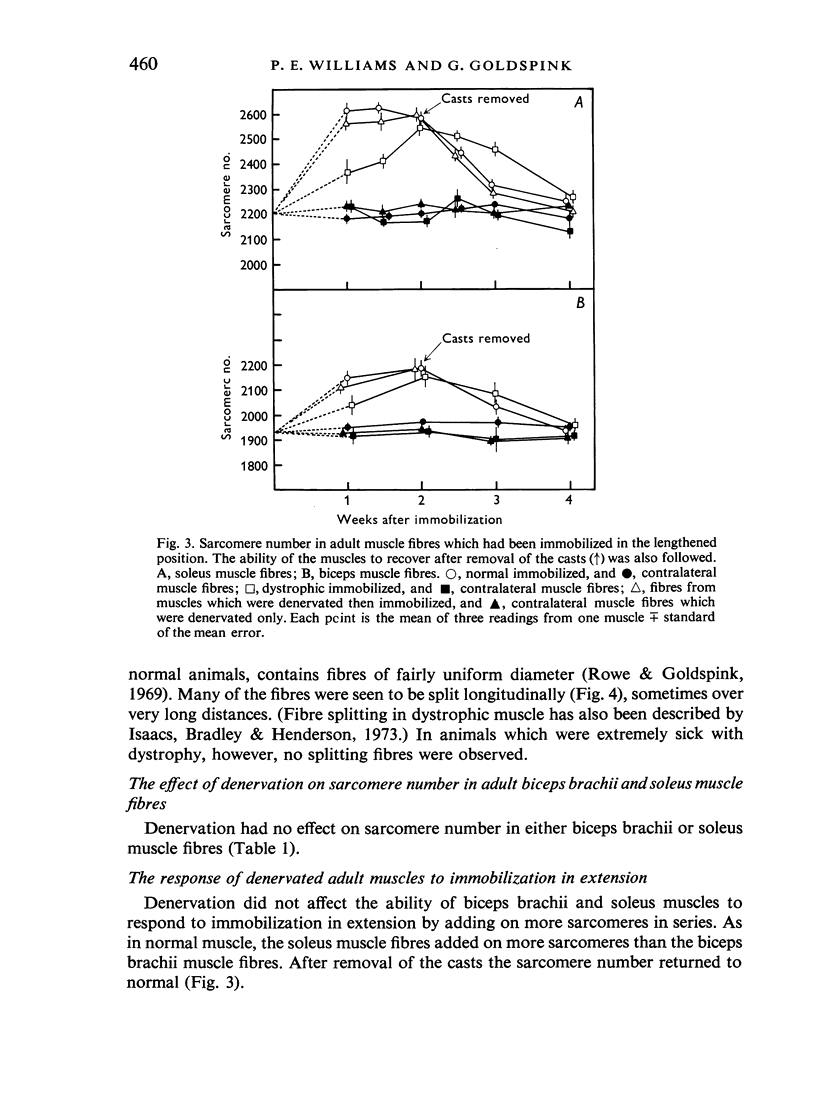
In young animals the elongation of the limb bones increases the functional lengths of the muscles. In adult animals the functional length of a muscle can be increased by immobilizing it in the lengthened position. In both cases the muscle adapts by adding on more sarcomeres in series. The role of the nerve supply in this adaptation has been investigated using denervated muscles and muscles from dystrophic animals where there is thought to be an abnormality of the nerve supply. Postnatal sarcomere addition in denervated muscles falls short of that of controls. Although this might mean that the nerve supply is necessary for normal addition of sarcomeres, it is just as likely that there is a change in gait resulting from denervation, which affects the sarcomere number. Sarcomere number in fully grown mice is not affected by denervation, nor is the ability of the muscle to adapt to immobilization in the lengthened position. This is true for fast-twitch as well as slow-twitch muscles. In dystrophic muscles postnatal sarcomere addition is normal, although the presence of a few short fibres in the muscle may mean that some muscle fibres cannot adapt to an increase in the functional length of the muscle accompanying bone growth. Adult dystrophic muscle is capable of adapting to immobilization in the lengthened position. However, although the total number of additional sarcomeres is the same as in normal immobilized muscle, they are added on at a slower rate. The experiments show that although denervated and dystrophic muscle fibres are in a state of atrophy they are still capable of adding on sarcomeres in series when the functional length of the muscle is increased. It would appear that the mechanism which enables the muscle to respond in this way to an increased functional length does not involve the nerve supply. This work was supported by a grant from the National Fund for Research into Crippling Diseases.
Full text
PDF


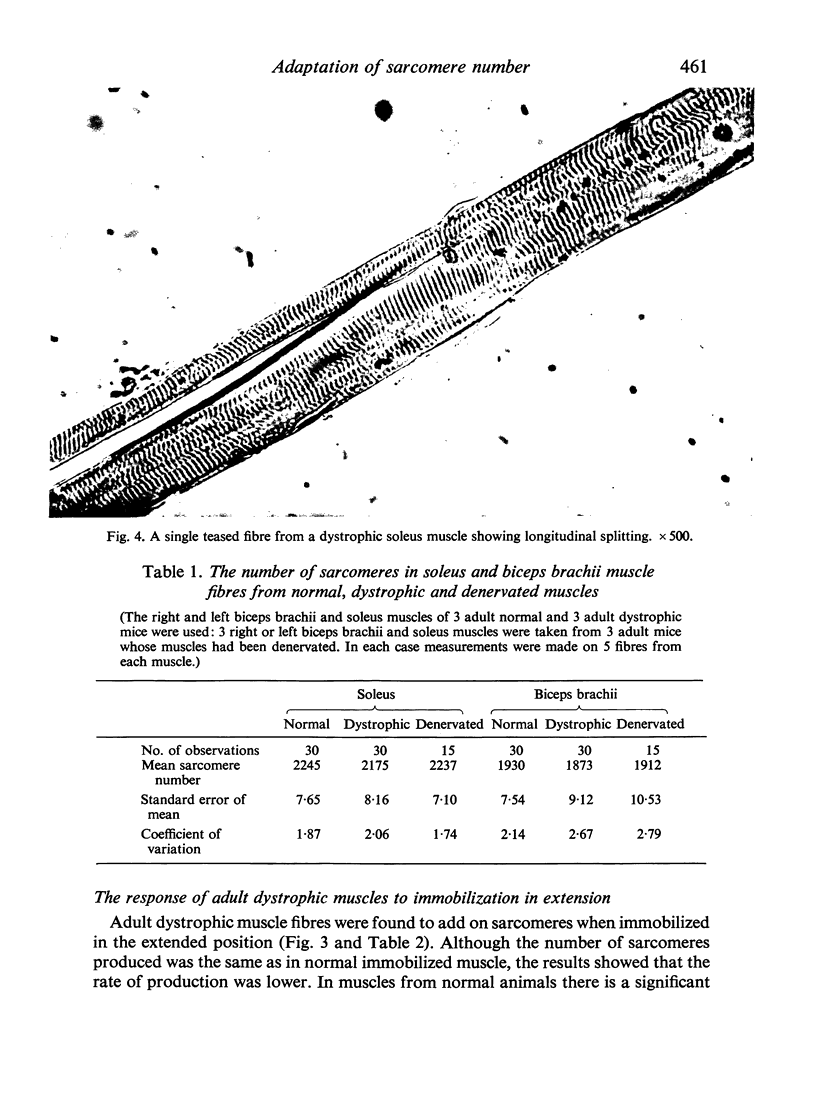
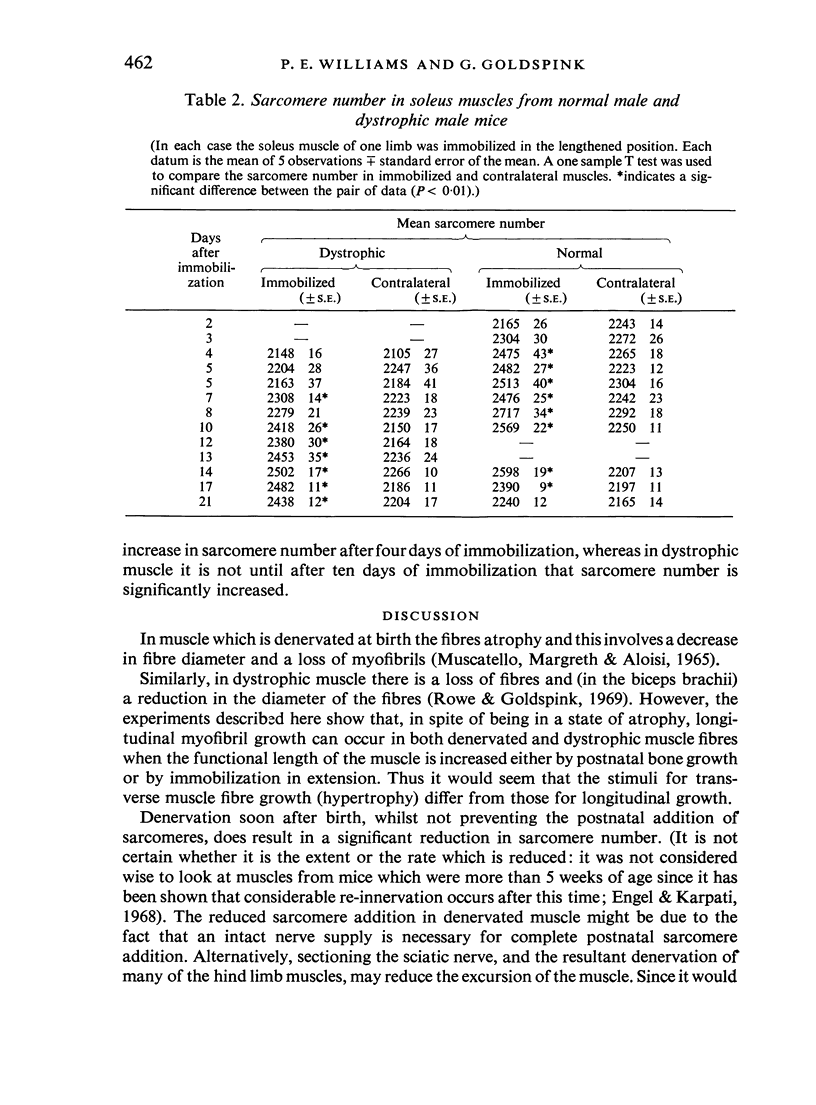



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDER A. B., CRAWFORD G. N., EDWARDS R. G. The effect of denervation on the longitudinal growth of a voluntary muscle. Proc R Soc Lond B Biol Sci. 1960 Mar 1;151:551–562. doi: 10.1098/rspb.1960.0016. [DOI] [PubMed] [Google Scholar]
- BAJUSZ E. "RED" SKELETAL MUSCLE FIBERS: RELATIVE INDEPENDENCE OF NEURAL CONTROL. Science. 1964 Aug 28;145(3635):938–939. doi: 10.1126/science.145.3635.938. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R. Morphology and histochemistry of the soleus muscle from normal and exercised rats. Am J Anat. 1970 Jan;127(1):81–87. doi: 10.1002/aja.1001270107. [DOI] [PubMed] [Google Scholar]
- Engel W. K., Brooke M. H., Nelson P. G. Histochemical studies of denervated or tenotomized cat muscle: illustrating difficulties in relating experimental animal conditions to human neuromuscular diseases. Ann N Y Acad Sci. 1966 Sep 9;138(1):160–185. doi: 10.1111/j.1749-6632.1966.tb41164.x. [DOI] [PubMed] [Google Scholar]
- Engel W. K., Karpati G. Impaired skeletal muscle maturation following neonatal neurectomy. Dev Biol. 1968 Jun;17(6):713–723. doi: 10.1016/0012-1606(68)90015-8. [DOI] [PubMed] [Google Scholar]
- Gallup B., Dubowitz V. Letter: Failure of "dystrophic" neurones to support functional regeneration of normal or dystrophic muscle in culture. Nature. 1973 Jun 1;243(5405):287–289. doi: 10.1038/243287a0. [DOI] [PubMed] [Google Scholar]
- Goldspink G., Tabary C., Tabary J. C., Tardieu C., Tardieu G. Effect of denervation on the adaptation of sarcomere number and muscle extensibility to the functional length of the muscle. J Physiol. 1974 Feb;236(3):733–742. doi: 10.1113/jphysiol.1974.sp010463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Craggs E. C. The longitudinal division of fibres in overloaded rat skeletal muscle. J Anat. 1970 Nov;107(Pt 3):459–470. [PMC free article] [PubMed] [Google Scholar]
- Hamburgh M., Peterson E., Bornstein M. B., Kirk C. Capacity of foetal spinal cord obtained from dystrophic mice (dy2J) to promote muscle regeneration. Nature. 1975 Jul 17;256(5514):219–220. doi: 10.1038/256219a0. [DOI] [PubMed] [Google Scholar]
- Harris J. B. The resting membrane potential of fibres of fast and slow twitch muscles in normal and dystrophic mice. J Neurol Sci. 1971 Jan;12(1):45–52. doi: 10.1016/0022-510x(71)90250-4. [DOI] [PubMed] [Google Scholar]
- Isaacs E. R., Bradley W. G., Henderson G. Longitudinal fibre splitting in muscular dystrophy: a serial cinematographic study. J Neurol Neurosurg Psychiatry. 1973 Oct;36(5):813–819. doi: 10.1136/jnnp.36.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComas A. J., Sica R. E., Upton A. R., Petito F. Trophic functions of the neuron. IV. Clinical disorders of trophic functions muscular dystrophy? Sick motoneurons and muscle disease. Ann N Y Acad Sci. 1974 Mar 22;228(0):261–279. doi: 10.1111/j.1749-6632.1974.tb20514.x. [DOI] [PubMed] [Google Scholar]
- Muscatello U., Margreth A., Aloisi M. On the differential response of sarcoplasm and myoplasm to denervation in frog muscle. J Cell Biol. 1965 Oct;27(1):1–24. doi: 10.1083/jcb.27.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R. Expression of the dystrophia muscularis (dy) recessive gene in mice. Nature. 1974 Oct 18;251(5476):621–622. doi: 10.1038/251621a0. [DOI] [PubMed] [Google Scholar]
- Peterson A. C. Chimaera mouse study shows absence of disease in genetically dystrophic muscle. Nature. 1974 Apr 12;248(449):561–564. doi: 10.1038/248561a0. [DOI] [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Muscle fibre growth in five different muscles in both sexes of mice. II. Dystrophic mice. J Anat. 1969 May;104(Pt 3):531–538. [PMC free article] [PubMed] [Google Scholar]
- Tabary J. C., Tabary C., Tardieu C., Tardieu G., Goldspink G. Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. J Physiol. 1972 Jul;224(1):231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEST W. T., MURPHY E. D. Histopathology of hereditary, progressive muscular dystrophy in inbred strain 129 mice. Anat Rec. 1960 Jul;137:279–295. doi: 10.1002/ar.1091370306. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. Longitudinal growth of striated muscle fibres. J Cell Sci. 1971 Nov;9(3):751–767. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973 Oct;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]