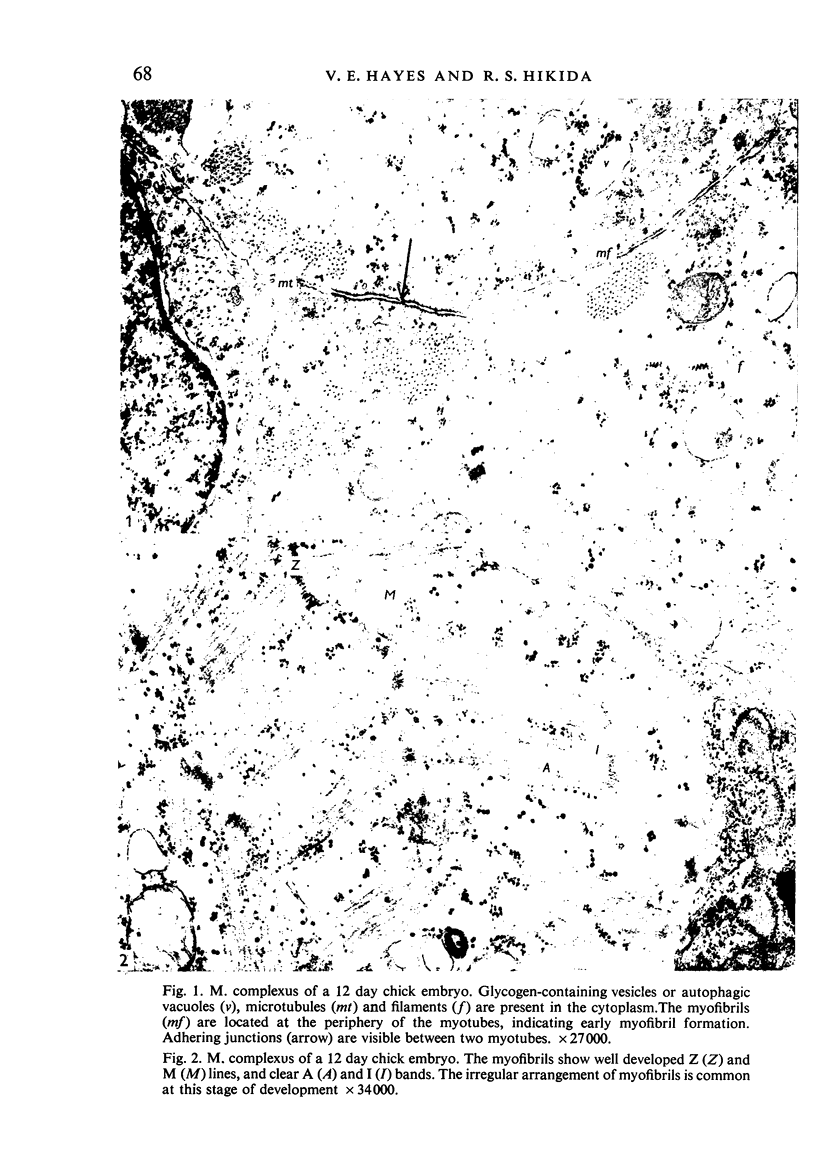
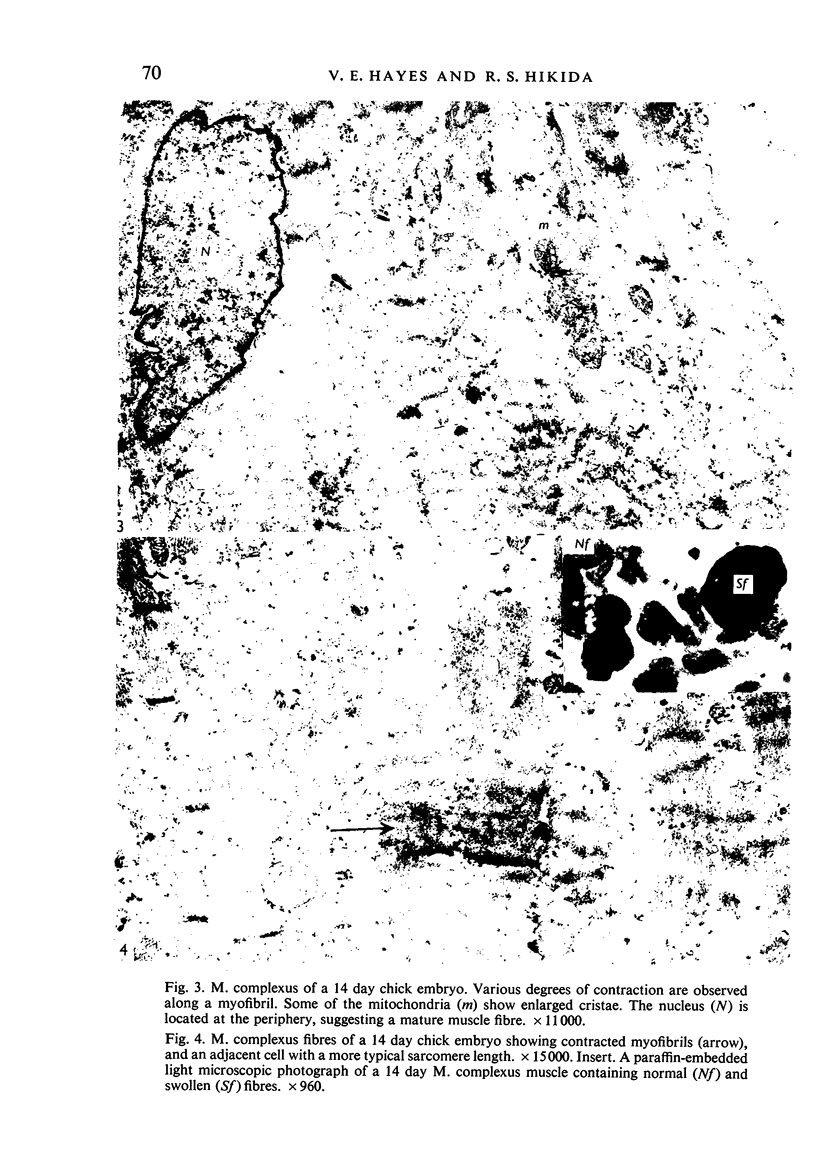
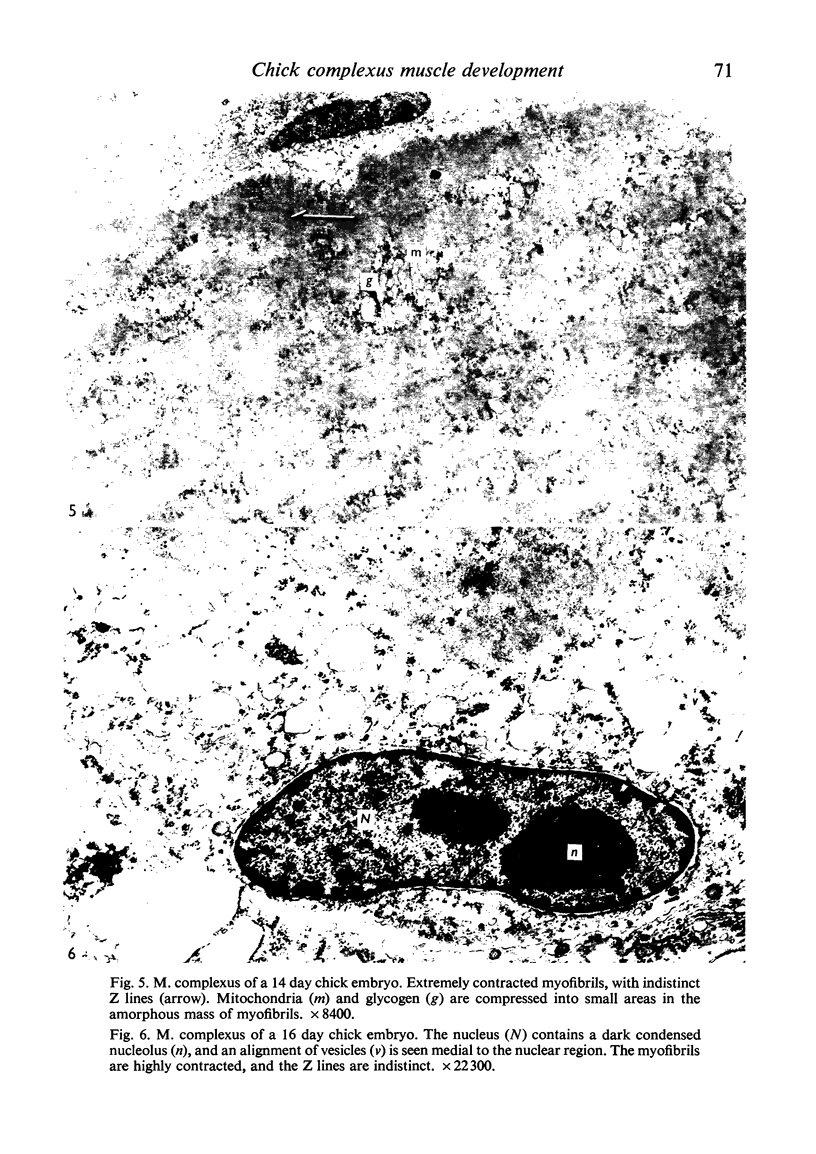
Abstract
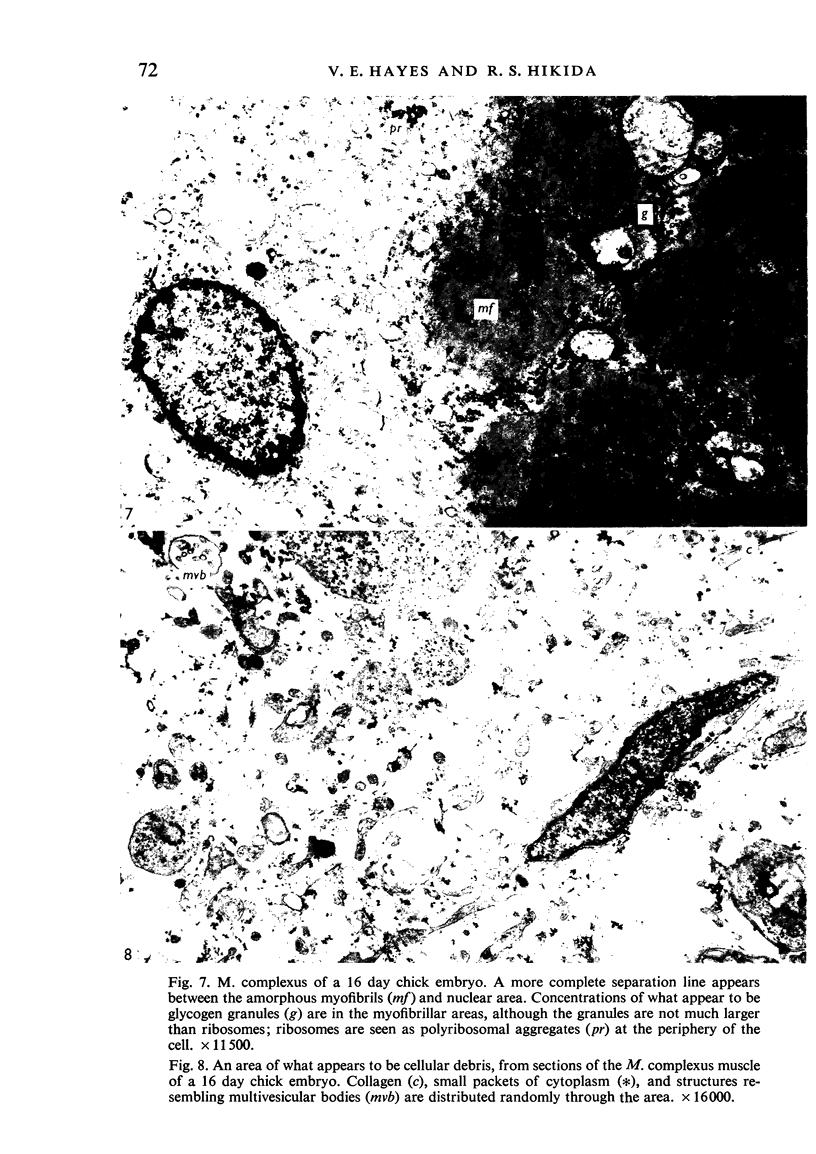
Ultrastructural study of the M. complexus muscle of the chick embryo has demonstrated two populations of fibres: the more common is the normal myotube or muscle fibre, which was observed in various stages of development; the other shows myofibrillar contractions which, at their greatest degree, produce the appearance of an amorphous mass of myofilaments. The contracted fibre had rounded and swollen mitochondria, and vacuoles (autophagic) containing glycogen, and it exhibited a cleavage of the cell which isolated the nuclear region from the main body of the fibre. When the fibres were less contracted they resembled degenerative mammalian fast-twitch-oxidative-glycolytic fibres after immobilization. The more normal fibre population was identical with that of the pectoralis muscle, which was used as a control. These results suggest that the contracted fibres are degenerating, which agrees with conclusions by earlier investigators using light miscroscopy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fischman D. A. The synthesis and assembly of myofibrils in embryonic muscle. Curr Top Dev Biol. 1970;5:235–280. doi: 10.1016/s0070-2153(08)60057-5. [DOI] [PubMed] [Google Scholar]
- Gori Z. Proliferations of the sarcoplasmic reticulum and the T system in denervated muscle fibers. Virchows Arch B Cell Pathol. 1972;11(2):147–160. doi: 10.1007/BF02889394. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Oppenheim R. Prehatching motility and hatching behavior in the chick. J Exp Zool. 1967 Nov;166(2):171–203. doi: 10.1002/jez.1401660203. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Formation of elaborate networks of T-system tubules in cultured skeletal muscle with special reference to the T-system formation. J Cell Biol. 1968 Jul;38(1):51–66. doi: 10.1083/jcb.38.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton B. H., Konigsberg I. R. A fine-structural analysis of the fusion of myogenic cells. J Cell Biol. 1972 May;53(2):348–364. doi: 10.1083/jcb.53.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatello U., Margreth A., Aloisi M. On the differential response of sarcoplasm and myoplasm to denervation in frog muscle. J Cell Biol. 1965 Oct;27(1):1–24. doi: 10.1083/jcb.27.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino C., Franzini-Armstrong C. Recent contributions of electron microscopy to the study of normal and pathological muscle. Int Rev Exp Pathol. 1969;7:139–226. [PubMed] [Google Scholar]
- Ramachandran S., Klicka J., Ungar F. Biochemical changes in the musculus complexus of the chick (Gallus domesticus). Comp Biochem Physiol. 1969 Aug 15;30(4):631–640. doi: 10.1016/0010-406x(69)92140-9. [DOI] [PubMed] [Google Scholar]
- Rigdon R. H., Ferguson T. M., Trammel J. L., Couch J. R., German H. L. Necrosis in the "pipping" muscle of the chick. Poult Sci. 1968 May;47(3):873–877. doi: 10.3382/ps.0470873. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. Contracture knots in normal and diseased muscle fibres. Brain. 1973 Sep;96(3):637–640. doi: 10.1093/brain/96.3.637. [DOI] [PubMed] [Google Scholar]
- Tomanek R. J., Lund D. D. Degeneration of different types of skeletal muscle fibres. I. Denervation. J Anat. 1973 Dec;116(Pt 3):395–407. [PMC free article] [PubMed] [Google Scholar]
- Tomanek R. J., Lund D. D. Degeneration of different types of skeletal muscle fibres. II. Immobilization. J Anat. 1974 Dec;118(Pt 3):531–541. [PMC free article] [PubMed] [Google Scholar]