Abstract
Background
Influenza is a global public health and economic burden. Its seasonality patterns differ considerably between geographic regions, but the factors underlying these differences are not well characterized.
Methods
The data on influenza were obtained from 2019 to 2022 in Huaian. A descriptive study was used to describe the epidemiological characteristics.The DLNM(distributed lag nonlinear model) model was established to further analyze the relationship between influenza cases, meteorological factors and pollutants. In addition, the attribution risk analysis and the interaction analysis further explored the interaction between the attributable risk and meteorological factors of influenza in terms of meteorological factors.
Results
A total of 9205 cases of influenza were reported in Huaian City from 2019 to 2022, Jiangsu province, of which 4938 cases were males and 4267 cases were females.The DLNM results showed an inverted U-shaped relationship between PM2.5(Fine Particulate Matter) and temperature and influenza.The low concentration of PM2.5 and O3(Ozone) showed decreased risks, and the maximum effect values appeared on the 8th day (RR(Relative Ris) = 0.35,95 %CI(Confidence Interval): 0.25–0.49) and the 2nd day (RR = 0.63,95 %CI: 0.52–0.77). At the high concentration, the cumulative RR values of PM2.5 and O3 reached their maximum on the 8th day (RR = 1.93,95 %CI: 1.47–2.54) and the 9th day (RR = 2.58,95 %CI: 1.63–4.09). The attribution analysis based on DLNM showed that the AF(attributable fraction) value of influenza attributable to the high concentration of PM2.5 exposure was 15.90 %, equivalent to 1456 cases. AF of the high concentration of O3 was 8.12 % (743 cases). The AF of low temperature effect was 30.91 % (2830 cases). The interaction analysis showed that high temperature reduced the influence of PM2.5 on the onset of influenza, showing an antagonistic effect (RR = 0.31, 95 %CI: 0.15–0.65), IRR(interaction relative risk) and RERI(interaction relative risk) were 0.17 (95 %CI: 0.08–0.37) and −1.62 (95 %CI: 2.65∼-0.68), respectively.
Conclusion
The results show that low temperature significantly increases the risk of influenza. At the low concentration of PM2.5, the risk of influenza increases with increasing concentration but decreases at the high concentrations. At the high concentration of O3, the risk of influenza increases rapidly. 15.90 % of influenza cases may be attributed to the high concentration of PM2.5, equivalent to 1456 cases; temperature-induced cases mainly come from the low-temperature effect, with an AF value of 30.91 %, equivalent to 2830 cases. In addition, high temperature can effectively mitigate the impact of PM2.5 on influenza incidence, and outdoor exposure time should be minimized in low temperature and high PM2.5 weather.
Keywords: Influenza, Meteorological factors, Ambient air pollutants, Interactive effects
1. Introduction
Influenza is an acute respiratory disease caused by influenza viruses (divided into four types: A, B, C, and D) (Labella & Merel, 2013). The WHO(World Health Organization) reports that influenza is prevalent worldwide, with an estimated annual incidence of 5 %–10 % in adults and 20 %–30 % in children. Every year, influenza epidemics will lead to about 3 million to 50 million severe cases and about 290,000 to 650,000 deaths. In China, influenza is a class C legal infectious disease, and an average of 88,100 people die from influenza-related diseases every year. It is obvious that influenza viruses are highly infectious and can lead to severe morbidity, and the burden of influenza cannot be ignored. Therefore, a quantitative assessment of the environmental drivers of the influenza virus will provide a useful reference for optimizing public health prevention and control measures.
In recent years, climate change, air pollution and health hazards have been areas of great concern globally. WHO estimated that in 2019, environmental (outdoor) air pollution caused 4.2 million premature deaths worldwide. That mortality rate is due to exposure to PM (Particulate Matter), which can lead to cardiovascular and respiratory diseases as well as cancer. More and more studies have shown that climate change and air pollution are closely related to influenza epidemics/pandemics. Yang et al. (Yang, Yang, et al., 2023) pointed out that most air pollutants increase the risk of ILI (influenza-like illness). Xu et al. (Xu et al., 2013) found that PM10 and average temperature have a significant interaction with influenza in children. Yu et al. (Yu et al., 2013) found that cold temperatures, sunshine time and precipitation are the main causes of influenza in winter or spring in China. Emukule et al. (Emukule et al., 2016) found that in Kenya, the association between influenza and temperature and rainfall is not significant. Wang et al. (Wang et al., 2022) pointed out that low ambient temperature, low relative humidity and high pressure would increase the risk of influenza, while the interaction of low relative humidity with low ambient temperature and high pressure would aggravate the incidence of influenza. Seah et al. (Seah et al., 2023) observed a positive correlation between SO2(Sulfur Dioxide) exposure and the risk of subsequent infection with these two influenzas, ambient temperature is associated with a decline in reports of influenza A, and rainfall is positively correlated with a subsequent increase in reports of influenza A. However, since the pronounced seasonal geographic patterns and meteorological factors that affect the influenza outbreaks are inconsistent, the effect of meteorological factors on influenza can not be effectively concluded.
Based on the above discussion, this paper will take Huaian city, Jiangsu province as an example to study the impact of air pollutants and meteorological factors on the spread of influenza. Huaian city is located between 32°44′33°N-34°06′33°N and 118°12′33°E−119°36′30°E, in the transition zone between the warm temperate zone in the south and the subtropical zone in the north, consisting of four districts, namely Qingjiangpu, Huaiyin, Huaian and Hongze, and three counties, namely Lianshui, Xuyi and Jinhu. By the end of 2021, the permanent population of Huaian city was 45.622 million. At present, there is little research on the meteorological impact of influenza in Huaian city. In this study, the DLNM(distributed lag nonlinear model) is used to explore the expose-lag relationship between influenza and meteorological factors. The attribution risk analysis and the interaction analysis further discuss the interaction effects of influenza-attributable risk and meteorological factors. These analyses are intended to provide strategies for future influenza prevention and control.
2. Method
2.1. Data source
The influenza case data was obtained from the Huaian CDC(Center for Disease Control and Prevention) legally reported infectious disease database from January 1, 2019 to December 31, 2022. The China Meteorological Data Sharing Service system of the China Meteorological Administration was used to acquire daily meteorological data (Temperature (°C), Humidity (%), Windspeed (m/s), Air pressure(hPa)) over the study period 2019–2022 (http://data.cma.cn). The AQI PM2.5(Fine Particulate Matter) (μg/m3), PM10(Coarse Particulate Matter) (μg/m3), SO2(Sulfur Dioxide)(μg/m3), NO2(Nitrogen Dioxide)(μg/m3), O3(Ozone)(μg/m3), CO(Carbon Monoxide)(mg/m3) were collected from the China Meteorological Science data sharing Service system (http://hz.hjhj-e.com/home).
2.2. Statistical analysis
2.2.1. Descriptive statistics
Descriptive statistics were performed for continuous variables and expressed as mean and SD(Standard Deviation), the quantile was also calculated to present the distribution of data. Due to the data not subject to normal distribution, the association between each air pollutant and meteorological factors was estimated using the Spearman correlation.
2.2.2. Distributed lag nonlinear model
The DLNM is commonly used to explore the association between air pollution or meteorological factors and influenza. This methodology describes associations by revealing potentially nonlinear and delayed effects for a time series and the shape of the relationship along both the space of the predictor and the lag dimension of its occurrence (Gasparrini et al., 2010). The DLNM model can simultaneously capture the nonlinear and lag effects of environmental factors on health outcomes, which is impossible to be achieved by traditional linear or single-dimensional models. The relationship between the occurrence of influenza and meteorological factors (e.g., temperature) and air pollutants (e.g., PM2.5, ozone) is often not simple linear or immediate. This complex expose-response relationship and time lag effects need to be modeled in two dimensions by the cross-basis function of the DLNM to more accurately quantify the risk. In addition, the flexibility of the DLNM allows the constructed model to adapt to the nonlinear morphology of different environmental factors. Studies have shown that influenza is associated with temperature, PM2.5, ozone and other pollution factors (Lau et al., 2021; Park, Son, Ryu, & et al, 2020; Wang et al., 2023), and our correlation analysis also proves this point (see Table 2), so we select temperature, PM2.5 and ozone for the DLNM analysis. We model meteorological factors and pollution factors separately in order to capture the risk effects of individual exposure factors, and then take into account the effects of co-exposure of meteorological factors and pollution factors on the risk of influenza in the interaction effect analysis. The DLNM with quasi-Poisson distribution is used to assess the association between daily meteorological factors and influenza cases as follows:
| (1) |
and then the model between daily pollution and influenza cases as follows:
| (2) |
where is the daily number of influenza at day t, the intercept of the model, denotes the cross basis function of PM2.5 or O3 to describe the non-linear delayed effect, and the lag will be determined later according to the optimal AIC(Akaike information criterion). are the effects of covariates in the model and a natural cubic spline with degree df. is an indicator of the day of week and holiday effect and the corresponding regression coefficient. is used to control the long-term trend and seasonality in the model. The AIC is used to choose the optimal df(degrees of freedom) and lag (Gasparrini et al., 2010).
Table 2.
Spearman correlation between influenza cases and environmental parameters in Huaian, China during 2019–2022.
| Temperature | Humidity | Windspeed | Air pressure | AQI | PM2.5 | PM10 | SO2 | NO2 | O3 | CO | Influenza | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | 1 | |||||||||||
| Humidity | 0.3a | 1 | ||||||||||
| Windspeed | −0.06a | −0.16a | 1 | |||||||||
| Air pressure | −0.89a | −0.35a | −0.03 | 1 | ||||||||
| AQI | −0.04 | −0.35a | −0.13a | 0.02 | 1 | |||||||
| PM2.5 | −0.52a | −0.26a | −0.14a | 0.46a | 0.69a | 1 | ||||||
| PM10 | −0.49a | −0.49a | −0.05a | 0.44a | 0.75a | 0.89a | 1 | |||||
| SO2 | −0.21a | −0.62a | −0.07a | 0.21a | 0.48a | 0.45a | 0.59a | 1 | ||||
| NO2 | −0.52a | −0.38a | −0.27a | 0.52a | 0.49a | 0.73a | 0.77a | 0.62a | 1 | |||
| O3 | 0.63a | −0.2a | −0.08a | −0.54a | 0.4a | −0.11a | −0.02 | 0.18a | −0.16a | 1 | ||
| CO | −0.28a | −0.08a | −0.12a | 0.18a | 0.61a | 0.73a | 0.67a | 0.46a | 0.56a | −0.05 | 1 | |
| Influenza | −0.16a | −0.02 | 0 | 0.14a | 0.08a | 0.08a | 0.09a | 0.15a | 0.04 | −0.07a | 0.26a | 1 |
P < 0.05.
The lag day analysis examines the delayed effects of environmental exposures (e.g., pollutants, temperature) on influenza cases by quantifying associations at specific time intervals (lag days) after exposure. Lag0 denotes the effect of exposure (e.g., PM2.5∼, temperature) on influenza cases on the same day. Lagi denotes the effect observed ith day after exposure.
The relative risk (RR) is an indicator used in epidemiology to measure the strength of the association between exposure factors and disease occurrence. It represents the ratio of the risk of developing disease in the exposed group to the risk of developing disease in the unexposed group as follows:
The AN(Attributable number) of influenza cases caused by pollution or temperature and corresponding AF(Attributable fractions) of influenza cases are calculated by using previously published methodologies to determine the burden of influenza cases attributable to PM as follows (Gasparrini & Leone, 2014):
| (3) |
Here and denote the fractions and number of influenza cases that may be attributed to the excessive pollution or temperature exposures on day t, and indicates the coefficient of the association pollution or temperature and influenza, is the difference in pollution or temperature exposures between the measured value and the reference value on day t, the count of influenza cases on day t. The sum is divided by the total sum of influenza cases to calculate the overall AF.
In addition, the IRR (interaction relative risk) and RERI (relative excess risk due to interaction) were calculated based on the multiplicative and additive model of interaction theory as follows:
| (4) |
where R01 represents the relative risk when meteorological factor = 0 and atmospheric pollutant factor = 1, R10 represents the relative risk when meteorological factor = 1 and atmospheric pollutant factor = 0, and R11 represents the relative risk when both are exposed. No interaction is observed when IRR = 1 or RERI = 0, synergistic interaction is observed when IRR>1 or RERI>0, and antagonistic interaction is observed when IRR<1 or RERI<0. Moreover, bivariate response surface models are used to visually explore the potential interactive effects between meteorological factors and pollution on the incidence of influenza.
The bivariate response surface analysis applies non-parametric functions to introduce air temperature and air pollutants into the model as bivariate continuous equations and draws 3D graphs of air temperature, air pollutants and outcome variables to more intuitively reflect the changes among the three. The specific formula for model construction is shown as
where is the expected value of influenza onset, te the tensor product smoothing function, and temp the average daily temperature.
Finally, we replace the degrees of freedom for the PM2.5, O3 or temperature (change df from 3 to 7) and lagged variable (lagged time from 0 to 10) in our results to check the robustness of the result.
2.3. Methodology of seasonal model and wavelet coherence analysis
The wavelet analysis is commonly utilized to analyze time series that contain non-stationary power at many different frequencies. It is based on the wavelet transform which breaks the signal into a sum of scaled and translated mother wavelets. The wavelet analysis is based on a wavelet transform defined as
where ∗ denotes the complex conjugate form and represents a family of functions derived from a single function called the mother wavelet. The signal is decomposed in these functions which can be expressed in terms of two parameters, one for time position , and the other for the scale of wavelets s.
In the present study, we use the Morlet mother wavelet:
To quantify the time series synchronization between the influenza incidences and the different meteorological factors and ambient air pollutants, we compute the wavelet coherence given by
The angle brackets indicate smoothing in both time and frequency, and are the wavelet transform of the series and , respectively. is the cross-wavelet transform. The value of range between 0 and 1, where 1 represents a perfect linear relationship between time series and . In addition to this analysis, it is possible to compute the phase difference associated to the two signals, which gives information about series synchronization (i.e., in phase or out of phase). The Morlet wavelet is complex, so the phase difference can be computed in terms of the real () and the imaginary () part, as shown in:
All the analyses above are performed by using the software R (V.4.1.2) with the “dlnm”, “splines”, “interaction R”, “epiR” packages. A two-sided P value of less than 0.05 is considered statistically significant.
2.4. Sensitivity analysis
Given temporal overlap with the COVID-19 pandemic, non-pharmaceutical interventions (NPIs) such as mask mandates and travel restrictions may have introduced confounding effects. To address this, we conducted sensitivity analyses using exclusively pre-pandemic (2019) data.
3. Results
3.1. Descriptive analysis
Table 1 present the descriptive results of meteorological factors, pollutants and cases of influenza. During the time of 2019–2022, there were 9205 influenza cases reported in Huaian City, Jiangsu Province, including 4938 cases (53.64 %) in males and 4267 cases (46.36 %) in females, with 6962 cases (75.63 %) occurring children under the age of 15. The daily mean values of temperature, relative humidity, wind speed, air pressure, AQI(Air Quality Index), PM2.5, PM10, SO2, NO2, O3, CO and a number of cases were 15.73 °C, 74.77 %, 2.09 m/s, 1015.16hpa, 78.25, 39.76 μg/m3, 67.18 μg/m3, 7.43 μg/m3, 25.63 μg/m3, 101.72 μg/m3, 0.64 mg/m3 and 3.63, respectively. The correlation among meteorological factors, air pollutants and influenza is shown in Table 2, indicating that the daily cases of influenza were negatively correlated with temperature and O3, but positively correlated with air pressure, AQI, PM2.5, PM10, SO2 and CO. In addition, Fig. S1 shows the time series of influenza cases with meteorological factors and pollutants from 2019 to 2022.
Table 1.
Basic information about the onset of influenza, meteorological factors, and pollutants in Huaian, China from 2019 to 2022.
| Group | Mean | SD(Total) | Min-Max | P5 | P25 | P50 | P75 | P95 |
|---|---|---|---|---|---|---|---|---|
| Influenza | ||||||||
| Cases(count/day) | 6.3 | 15.47(9205) | 0–118 | 0 | 0 | 0 | 4 | 36 |
| Man(count/day) | 3.38 | 8.33(4938) | 0–68 | 0 | 0 | 0 | 2 | 20 |
| Woman(count/day) | 2.92 | 7.48(4267) | 0–60 | 0 | 0 | 0 | 2 | 17 |
| 0∼15years(count/day) | 4.77 | 12.17(6962) | 0–108 | 0 | 0 | 0 | 3 | 27 |
| >15years(count/day) | 1.54 | 4.73(2243) | 0–49 | 0 | 0 | 0 | 1 | 10 |
| Meteorological variable | ||||||||
| Temperature (◦C) | 15.73 | 9.36 | −9.1–32.6 | 1.3 | 7.6 | 15.5 | 24.3 | 29.1 |
| Humidity (%) | 74.77 | 13.14 | 33–100 | 52 | 66 | 76 | 85 | 94 |
| Windspeed (m/s) | 2.09 | 0.98 | 0.2–6.7 | 0.8 | 1.4 | 1.9 | 2.6 | 3.9 |
| Air pressure(hPa) | 1015.16 | 9.82 | 985–1041 | 1000 | 1007 | 1016 | 1023 | 1031 |
| Pollution | ||||||||
| AQI(Air Quality Index) | 78.25 | 36.02 | 15–434 | 36 | 53 | 71 | 96 | 144 |
| PM2.5(Fine Particulate Matter)(μg/m3) | 39.76 | 27.8 | 5–210 | 12 | 20 | 32 | 51 | 93 |
| PM10(Coarse Particulate Matter)(μg/m3) | 67.18 | 43.37 | 6–534 | 22 | 38 | 56 | 86 | 142 |
| SO2(Sulfur Dioxide)(μg/m3) | 7.43 | 3.07 | 3–33 | 4 | 5 | 7 | 9 | 13 |
| NO2(Nitrogen Dioxide)(μg/m3) | 25.63 | 12.98 | 4–80 | 10 | 16 | 22 | 33 | 52 |
| O3(Ozone)(μg/m3) | 101.72 | 41.51 | 8–266 | 47 | 70 | 97 | 127 | 181 |
| CO(Carbon Monoxide)(mg/m3) | 0.64 | 0.22 | 0.2-1.8 | 0.3 | 0.5 | 0.6 | 0.8 | 1 |
3.2. The lagged effects of environmental factors on influenza
The DLNM model information can be found in Tables S1 and S2. Fig. 1(A)–(C) shows the response curves for the cumulative lagged effects of PM2.5, O3 and temperature on influenza at extreme levels, respectively. Using the median of each environmental factor as a reference, the risk of PM2.5 and influenza exhibited an inverted 'U' shape, with a dangerous effect at 68 μg/m3 and further increasing along with higher PM2.5 concentrations until 126 μg/m3, and then decreasing with increased concentrations. A J-shaped correlation between O3 and influenza was observed, with the risk of influenza rising rapidly after O3 concentrations above 113 μg/m3. The effect of temperature on influenza appeared from −3 °C and progressively increased, peaking at 4 °C and then decreasing, showing an inverted 'U' shape.
Fig. 1.
The overall cumulative exposure response relationships (A–C) and cumulative lagged effects at low levels (D–F) and high levels (G–I) for meteorological factors and air pollutants temperature.
We replace the degrees of freedom for the environmental and time variables in our results and find minor changes in the overall effect, which implies that our main findings are robust (See Fig. S2).
(D)-(F) and (G)–(I) in Fig. 1 show the cumulative effects of low and high levels of environmental factors, respectively. Among them, both the low concentrations of PM2.5 and O3 exhibited decreased risk, significant at lags 1–10 days, and 0–10 days, with maximum values occurring on day 8 (RR(Relative Risk) = 0.35,95 %CI:0.25–0.49), and day 2 (RR = 0.63,95 %CI:0.52–0.77), respectively. Whereas the high concentrations of PM2.5 and O3 showed significant hazard effects during lags 0–10 days, and 1–10 days, with maximum values on lag days 8 and 9, respectively, and the cumulative RR values were (RR = 1.93,95 %CI:1.47–2.54) and (RR = 2.58,95 %CI:1.63–4.09). For temperature, none of the effects were significant for different extreme levels (See Table 3).
Table 3.
Cumulative lag effect of meteorological factors and air pollutants on influenza at extremely low level vs. high level.
| Lag days | low level |
high level |
||||
|---|---|---|---|---|---|---|
| PM2.5 | O3 | Temperature | PM2.5 | O3 | Temperature | |
| lag0 | 0.87(0.74,1.03) | 0.7(0.59,0.83) | 0.89(0.61,1.3) | 1.29(1.13,1.46) | 1.19(0.97,1.46) | 0.8(0.42,1.52) |
| lag1 | 0.70(0.58,0.86) | 0.63(0.52,0.77) | 0.82(0.57,1.18) | 1.63(1.41,1.88) | 1.58(1.24,2.03) | 1.01(0.55,1.85) |
| lag2 | 0.64(0.51,0.8) | 0.64(0.52,0.8) | 0.88(0.59,1.3) | 1.67(1.43,1.94) | 1.74(1.32,2.29) | 0.85(0.46,1.56) |
| lag3 | 0.6(0.47,0.77) | 0.67(0.52,0.86) | 0.97(0.63,1.49) | 1.59(1.34,1.88) | 1.75(1.29,2.38) | 0.63(0.34,1.16) |
| lag4 | 0.54(0.41,0.71) | 0.67(0.51,0.89) | 1(0.62,1.62) | 1.58(1.31,1.92) | 1.82(1.3,2.55) | 0.55(0.28,1.06) |
| lag5 | 0.47(0.36,0.63) | 0.67(0.5,0.9) | 0.99(0.6,1.65) | 1.67(1.35,2.07) | 1.97(1.38,2.81) | 0.55(0.28,1.1) |
| lag6 | 0.41(0.31,0.56) | 0.67(0.48,0.92) | 0.98(0.58,1.68) | 1.79(1.42,2.26) | 2.16(1.48,3.16) | 0.59(0.29,1.22) |
| lag7 | 0.37(0.27,0.51) | 0.68(0.48,0.97) | 1.01(0.57,1.8) | 1.89(1.47,2.45) | 2.35(1.56,3.55) | 0.62(0.28,1.36) |
| lag8 | 0.35(0.25,0.49) | 0.74(0.5,1.07) | 1.12(0.61,2.05) | 1.93(1.47,2.54) | 2.51(1.62,3.89) | 0.6(0.26,1.38) |
| lag9 | 0.35(0.25,0.49) | 0.84(0.56,1.25) | 1.34(0.7,2.56) | 1.87(1.4,2.5) | 2.58(1.63,4.09) | 0.51(0.21,1.23) |
| lag10 | 0.37(0.25,0.53) | 1.02(0.66,1.58) | 1.78(0.87,3.63) | 1.7(1.24,2.33) | 2.55(1.55,4.2) | 0.38(0.15,0.98) |
3.3. Attribution risk analysis of environmental factors on influenza
Based on the DLNM model constructed above, this study conducts the attribution analysis on the impact of PM2.5, O3 and temperature on influenza, and calculates the total effect, low-level and high-level AF values from the backward and forward perspectives respectively, taking the median of the data as a reference(See Table 4 for details).
Table 4.
AF(attributable fractions) value of meteorological factors and pollutants on influenza.
| PM2.5 |
Ozone |
Temperature |
||||
|---|---|---|---|---|---|---|
| B-AF(%) | F-AF(%) | B-AF(%) | F-AF(%) | B-AF(%) | F-AF(%) | |
| Overall | 0.02(-12.89,9.96) | −6.10(-22.63,4.99) | 15.95(-5.49,30.85) | 8.29(-17.57,24.65) | −4.96(-94.96,35.16) | −7.77(-100.06,33.65) |
| Low | −16.24(-27.94,-7.11) | −20.60(-35.96,-9.48) | 8.28(-12.14,22.50) | 2.65(-23.17,18.58) | 30.91(1.12,47.13) | 30.21(0.14,46.41) |
| High | 15.90(4.43,24.54) | 14.50(3.30,22.28) | 8.12(2.38,12.89) | 5.63(-0.15,10.10) | −35.91(-123.74,0.62) | −37.98(-128.98,-0.48) |
∗B-AF: F-AF.
From the backward-looking perspective, the AF value for the total effect was 0.02 %. The AF value for the low concentrations of PM2.5 was −16.24 %, which corresponded to a reduction of 1487 cases.
In contrast, the AF value for the high concentrations of PM2.5 was 15.90 %, which represented an increase of 1456 cases. Obviously, the occurrence of influenza is more attributable to the high concentrations of PM2.5 exposure, while the low concentrations of PM2.5 exposure counterbalance this effect.
For the AF value of the backward viewing angle, the AF value of the total effect was 15.95 %. Among them, 8.28 % (758 cases) were attributed to the low O3 concentration, while 8.12 % (743 cases) were attributed to the high O3 concentration. Both the low and high concentrations of ozone may be the attribution source of influenza cases.
The AF for temperature was −4.96 %, with 30.91 % for low temperature (2830 cases) and −35.91 % for high-temperature effect (−3287 cases). It can be seen that the cases attributed to temperature are mainly from the hypothermia effect.
3.4. Interaction analysis of environmental factors on influenza
Fig. 2 shows a significant effect of interaction between temperature, PM2.5 and O3 on influenza. The risk of influenza morbidity was smaller under the influence of high temperatures with the high PM2.5 concentrations as well as high temperatures with the high O3 concentrations. As can be seen from Table 5, The interaction effects of different environmental factors on influenza were further explored by dividing meteorological factors and pollutants into binary variables based on median values for the stratified analysis. The results demonstrated that the risk of influenza morbidity was reduced at high temperatures with the high PM2.5 concentrations (RR = 0.31, 95 % CI: 0.15–0.65) with the interaction exhibiting an antagonistic effect, the IRR and RERI were 0.17 (95 % CI: 0.08–0.37) and −1.62 (95 % CI: 2.56 ∼ −0.68). Females (RR = 0.33, 95 % CI: 0.16–0.69) and people over 15 years old (RR = 0.29, 95 % CI: 0.13–0.63) presented the same results. Although high temperatures with the high O3 concentrations also reduced the risk of influenza, the interaction between IRR and RERI were only consistent in males (IRR = 0.17, 95 % CI: 0.08–0.37, RERI = -1.82, 95 % CI: 2.92–0.72) and the 0-15-years (IRR = 0.15, 95 % CI: 0.05–0.49, RERI = -1.17, 95 % CI: 2.02–0.32).
Fig. 2.
Three-dimensional map of the interaction effect of temperature, PM2.5(Fine Particulate Matter) and O3(Ozone) with temperature on influenza in Huaian, China during 2019–2022. (A) Interaction effect of PM2.5 with temperature. (B) Interaction effect of O3 with temperature.
Table 5.
Interactive analysis between temperature and pollutants on influenza cases in Huaian, China during 2019–2022.
| Variable | Subgroup |
Individual Effects |
Interactive Terms |
Multiplicative Interaction |
Additive Interaction |
|
|---|---|---|---|---|---|---|
| Temperature(15.5 °C) | Pollutant | IRR (interaction relative risk) | RERI (relative excess risk due to interaction) | |||
| PM2.5 (32 μg/m3) | Total | 0.91(0.62,1.35) | 2.02(1.42,2.88)∗ | 0.31(0.15,0.65)∗ | 0.17(0.08,0.37)∗ | −1.62(-2.56,-0.68) ∗ |
| Man | 0.24(0.22,0.26)∗ | 0.62(0.58,0.66)∗ | 0.46(0.43,0.48)∗ | 0.32(0.29,0.36) ∗ | 0.6(0.55,0.65) ∗ | |
| Woman | 0.97(0.65,1.44) | 2.10(1.46,3.01)∗ | 0.33(0.16,0.69)∗ | 0.16(0.08,0.35) ∗ | −1.73(-2.73,-0.73) ∗ | |
| 0∼15years | 0.27(0.24,0.31)∗ | 0.66(0.61,0.72)∗ | 0.47(0.44,0.5)∗ | 0.38(0.32,0.44) ∗ | 0.54(0.47,0.61) ∗ | |
| >15years | 0.85(0.57,1.28) | 1.95(1.35,2.80)∗ | 0.29(0.13,0.63)∗ | 0.18(0.08,0.4) ∗ | −1.51(-2.43,-0.58) ∗ | |
| O3 (97 μg/m3) | Total | 0.2(0.18,0.24)∗ | 0.57(0.52,0.62)∗ | 0.44(0.41,0.47)∗ | 0.26(0.22,0.32) ∗ | 0.67(0.6,0.73) ∗ |
| Man | 1.08(0.71,1.63) | 2.13(1.45,3.13)∗ | 0.38(0.18,0.8)∗ | 0.17(0.08,0.37) ∗ | −1.82(-2.92,-0.72) ∗ | |
| Woman | 0.27(0.24,0.30)∗ | 0.66(0.62,0.71)∗ | 0.53(0.5,0.56)∗ | 0.33(0.29,0.38) ∗ | 0.6(0.54,0.66) ∗ | |
| 0∼15years | 0.53(0.33,0.85)∗ | 1.78(1.21,2.64)∗ | 0.14(0.05,0.44)∗ | 0.15(0.05,0.49) ∗ | −1.17(-2.02,-0.32) ∗ | |
| >15years | 0.17(0.14,0.21)∗ | 0.50(0.44,0.57)∗ | 0.27(0.24,0.3)∗ | 0.32(0.24,0.41) ∗ | 0.60(0.52,0.68) ∗ | |
3.5. Results of seasonal model and wavelet coherence analysis
Wavelet transform and wavelet transform coherence provide information about whether two time series are correlated or covariant at a particular time and frequency table.
The wavelet coefficient contour map can reflect the variation law of the occurrence period and amplitude of influenza over time. According to this law, people can roughly infer the occurrence time and intensity of influenza outbreaks. According to the daily influenza data in Huaian from 2019 to 2022, in Fig. 3, we give the schematic diagram of the wavelet power spectrum of daily influenza data, PM2.5, PM10, SO2, NO2, O3, CO, average temperature, humidity, wind speed, pressure and rainfall in Huaian from 2019 to 2022. In Fig. 3(A), the wavelet power spectrum of influenza shows that there is a fixed cycle pattern for influenza over the two years of 2019–2020, with periods ranging from about 30 days to 300 days. The wavelet power spectra of meteorological factors and pollutants in Fig. 3(B–L) show that there are periodic patterns of more than 250 days in the 400-1000 days (2020–2021) period.
Fig. 3.
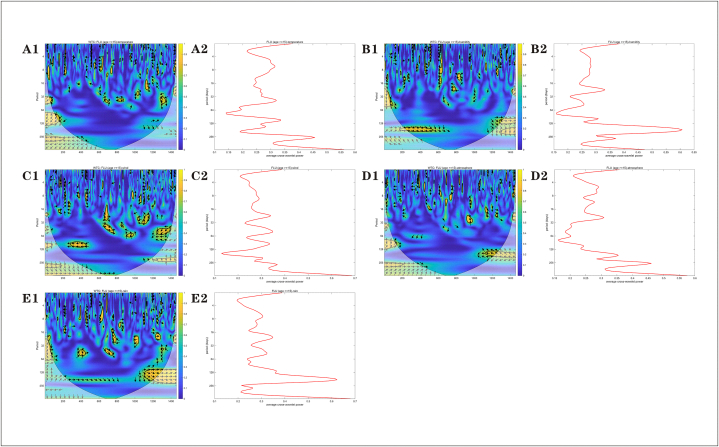
Wavelet power spectrum. The black solid line shows the regions of power significant at the 5 % level computed based on 1000 Monte-Carlo simulations. The corn shadow (gray area) indicated the region with edge effects. The power value of the test of seasonality were coded from dark blue for low power to dark yellow for high power. (A) influenza (B) PM2.5(Fine Particulate Matter) (C) PM10(Coarse Particulate Matter) (D) SO2(Sulfur Dioxide) (E) NO2(Nitrogen Dioxide) (F) O3(Ozone) (G) CO(Carbon Monoxide) (H) temperature (I) humidity (J) wind speed (K) atmosphere (L) rain.
Fig. 4, Fig. 5 are the wavelet consistency correlation power spectrum and its average power spectrum between the influenza outbreak and 11 factors, including PM2.5, PM10, SO2, NO2, O3, CO, average temperature, humidity, wind speed, pressure and rainfall in Huaian from 2019 to 2022. In addition, a significant positive correlation was found between humidity and rain and influenza over a period of 1200 to 1400 days (2022). Combined with 95 % confidence intervals, the correlations between influenza and O3, humidity, and rainfall are more significant.
Fig. 4.
Wavelet consistency correlation power spectrum(A1-F1) and wavelet average power spectrum(A2-F2). The power value of the test of seasonality were coded from dark blue for low power to dark yellow for high power. Arrows indicate the relevant direction. (A) PM2.5 (B) PM10 (C) SO2 (D) NO2 (E) O3 (F) CO.
Fig. 5.
Wavelet consistency correlation power spectrum(A1-E1) and wavelet average power spectrum(A2-E2). The power value of the test of seasonality were coded from dark blue for low power to dark yellow for high power. Arrows indicate the relevant direction. (A) temperature (B) humidity (C) wind (D) atmosphere (E) rain.
3.6. Results of sensitivity analysis
The sensitivity analysis is shown in Fig. S3. The sensitivity analysis is shown in Fig. S3. The relationship between PM2.5 and the incidence of influenza shows an inverted U-shaped curve. Higher levels of PM2.5 can increase the risk of influenza outbreaks. When the concentration of O3 exceeds 200 μ/m3, the number of influenza cases significantly increases. For the two types of air pollutants, the results of the sensitivity analysis are consistent with our overall results. The relationship between temperature and the response to influenza an N-shaped curve. At temperatures below 27 °C, temperatures are all risk factors for the onset of influenza. At the low temperatures and at 25 °C, there is the greatest risk.
4. Discussions
Influenza is an acute infectious disease caused by the influenza virus, and its occurrence and transmission are also affected by the pathogen itself, the social environment and the meteorological environment (Lu et al., 2021). At the same time, seasonal driving factors are an important feature of influenza, which often limits the spread of the virus to a certain period of time (Mummert et al., 2013). Many studies have shown that meteorological factors and environmental pollutants are related to the seasonality of influenza, but the research conclusions are inconsistent in different regions and different climates (Ali et al., 2018; Chen et al., 2017a). Therefore, this study collected data on 9205 influenza cases in Huaian City, Jiangsu Province from 2019 to 2022. The DLNM analysis showed that PM2.5, temperature and O3 concentration all contributed to the incidence of influenza. At the low concentrations of PM2.5, the risk of influenza increased significantly with the increase of concentration; at the high concentrations of PM2.5, this effect was significantly slowed down. O3 showed a significantly increasing risk at the high concentrations. Within a certain range of temperature, the risk of influenza increased with the decrease in temperature. Subsequent interaction analysis showed that high temperatures can slow down the promotion effect of PM2.5 on influenza.
Exposure to particulate air pollution may increase the risk of human diseases, especially cardiovascular and respiratory diseases (Dominici et al., 2006; Feng et al., 2016). And Wang et al. (Wang et al., 2023) showed a nonlinear relationship between PM2.5 and influenza incidence by analyzing influenza cases in Shijiazhuang, when the concentration of PM2.5 was the highest, the influenza incidence risk was the highest. The study of air pollution in Beijing (Feng et al., 2016) also found a strong positive correlation between PM2.5 and influenza incidence. This is consistent with our findings, as a kind of particulate matter, PM2.5 can mediate inflammatory responses through NLRP(NOD-like receptor pyrin domain-containing) and other signaling pathways (Jia et al., 2021). At the same time, the metal components contained in particulate matter may react with the human body to activate inflammatory pathways (Woodby et al., 2021). This inflammatory state disrupts the integrity of the airway mucosal barrier and weakens the innate antiviral immune response, making it easier for influenza viruses to breach host defenses. The results of Tao et al. (Tao et al., 2020) show that PM2.5 may activate AHR(Aryl Hydrocarbon Receptor) signaling to weaken innate resistance to influenza infection, and change macrophage inflammatory response by inhibiting the expression of IFN-β(Interferon-beta) during influenza infection, thus increasing the susceptibility of the body to influenza virus. Moreover, PM2.5 as a viral vector may prolong the survival time of viruses in the air, and this mechanism is particularly significant in winter under low temperature and high humidity conditions. Notably, the effect on the risk of influenza was attenuated when the PM2.5 concentration is too high. This may be due to the fact that in the high concentrations of PM2.5, the harsh environment visible to the naked eye drives people to wear protective measures such as masks, thereby reducing the actual exposure to PM2.5.
Besides PM2.5, as one of the air pollutants, Ozone also contributes to the incidence and spread of influenza. Wang et al. (Wang et al., 2023) pointed out that in the 7-day lag model of influenza in Shijiazhuang, when the O3 concentration was 310 μg/m3, the RR of influenza was 2.28 (95 %CI: 1.19–4.38). O3 affects influenza susceptibility through oxidative stress and immune regulation. Kesic et al. (Kesic et al., 2012) found that high O3 exposure can damage tight junction proteins in airway epithelial cells and promote the protease-antiprotease imbalance, forming pathological changes similar to chronic obstructive pulmonary disease, creating physical conditions for virus invasion. O3 can inhibit the function of CD8+ T cells and reduce the secretion of interferon-γ, which is especially prominent in the elderly. In the study of influenza transmissibility in China by Yang et al. (Yang, Zhang, et al., 2023),Yang, Zhang, et al., 2023n U-shaped relationship was found between ozone concentration and influenza RR, that is, exposure to both low concentration and extremely high concentration of ambient O3 increased the risk of influenza. However, a study on the effect of ozone on influenza in Hong Kong showed that exposure to environmental ozone is associated with reduced influenza transmissibility, possibly due to the virus-killing properties of ozone (Ali et al., 2018; Tseng, 2006). One possible reason is that low concentrations of O3 may activate the type 2 immune response by inducing IL-33(Interleukin 33), which shows the paradoxical phenomenon of enhancing antiviral immunity (Bonilla et al., 2012).
In addition, our analysis revealed an inverted U-shaped relationship between temperature and influenza incidence: incidence decreased at both temperature extremes, while elevated risk occurred at low-to-moderate temperatures. The virus is more stable in a low temperature and dry environment, and people mostly gather indoors, which increases the chance of transmission. But too high or too low temperature can inhibit the spread and infection of the virus through different ways. Extremely low temperatures may inhibit viral activity, whereas high temperatures generally lead to reduced outdoor activities, decreased congregation of individuals, and improved indoor ventilation, all of which contribute to a lower risk of infection. So far, there have been many research results on the relationship between temperature and influenza consistent with our findings. Most studies have shown that low temperature has an important impact on the spread of influenza virus (Tamerius et al., 2019). Zhang et al. (Zhang et al., 2022) confirmed that the cumulative relative risk of low temperature (5.1 °C) was 2.13 (95 %CI: 1.41–3.22), and 60 % of the incidence of influenza can be attributed to sensitive temperature. Among them, children under 5 years old and adolescents aged 6–17 have higher sensitivity. The influencing mechanism may be due to the higher transmission efficiency of influenza in low-temperature environments, and the transmission and survival ability of the influenza virus decreases with the increase of temperature between 5 and 20 °C (Lowen & Steel, 2014). At the same time, in low temperature and large temperature difference environments, human immunity is reduced, which also leads to the easier invasion of influenza virus into the human body (Park, Son, Ryu, & et al, 2020). In addition, low temperatures are mostly in winter, and more indoor gathering environment also leads to the easier spread of influenza. The antagonism of high temperature on PM2.5 affecting influenza may be due to the enhancement of human immunity, enhance enzyme activity, and further strengthen defense and immunity against infection (Appenheimer & Evans, 2018).
This study reveals a significant antagonistic interaction between high temperatures and high PM2.5 on influenza risk in Huaian, China (RR = 0.31, 95 % CI: 0.15–0.65), contrasting with prior reports of synergistic heat-pollution effects on respiratory diseases (Li et al., 2024; Zhang et al., 2021). Although most literature reports exacerbated health risks under co-exposure conditions (e.g., Zhang et al. demonstrated that temperature amplifies air pollution effects on influenza incidence (Zhang et al., 2021). Li et al. found high PM2.5 concentrations intensify temperature impacts (Li et al., 2024).), our results reveal a threshold-dependent reversal: at PM2.5 levels ≥32 μg/m3, elevated temperatures attenuate PM2.5-associated influenza transmission. This aligns with Chen et al.'s finding that PM2.5-related influenza risks exhibit higher RR during cold days than warm periods, Temperature and PM2.5 exhibit a significant antagonistic effect (Chen et al., 2017b). The antagonistic interaction between elevated PM2.5 and high temperatures may operate through two primary mechanisms: First, concurrent exposure to high temperatures and PM2.5 induces inflammatory response saturation in the respiratory system, manifesting as diminishing marginal effects. Additionally, high temperatures accelerate moisture evaporation from PM2.5 surfaces, compromising the humidity-dependent microenvironment essential for viral survival. This process destabilizes viral-particle binding integrity, thereby reducing the efficacy of PM2.5 as a transmission vector. Therefore, public health strategies must prioritize mitigating synergistic influenza risks during northern China's cold-dry winters, where stagnant atmospheric conditions exacerbate PM2.5 pollution—demanding strategic reallocation of prevention resources toward high-risk periods and regions.
While the inverted U-shaped PM2.5 relationship and J-shaped O3 curve initially appear paradoxical, they likely arise from a complex interplay of behavioral adjustments, biological saturation, model limitations, and interaction effects. Future studies should incorporate individual-level exposure data, longer lag periods, and mechanistic models (e.g., in vitro viral stability assays) to disentangle these dynamics. Public health strategies should emphasize pollution mitigation while recognizing that extreme levels may trigger unintended behavioral or biological responses.
The existing consensus shows that seasonal influenza outbreaks are caused by a variety of factors, and their environmental factors mainly depend on environmental conditions, air quality, temperature, humidity, etc. In order to analyze the specific quantitative correlation between the influenza outbreak in Huaian from 2019 to 2022 and which factors, many target factors such as air quality index AQI, PM2.5, PM10, SO2, NO2, O3, CO, average temperature, humidity, wind speed, pressure and rainfall are selected for analysis. Wavelet is a powerful tool commonly used for the non-stationary time series analysis and the spatial pattern analysis. The wavelet coherence analysis, which separates noise from meaningful seasonal trends, such as weekly weather changes, by decomposing the time series into different frequency components, reveals temporal correlations between influenza incidence and factors such as humidity and rainfall. The significant positive correlation between humidity, rainfall, and influenza observed in our study highlights the role of these meteorological factors as seasonal triggers of disease in different regions. In temperate regions such as Huai 'an, the low humidity (<40 %) during cooler months increased viral droplet survival and airborne transmission, while rainfall events may have caused indoor crowding and amplified contact-based transmission. Rather than being random noise, these temporal correlations reflect systematic lagged interactions between environmental conditions and influenza dynamics. The quantification of this lag relationship can prompt local public health departments to use humidity and rainfall trends as early warning signals to predict influenza epidemics in advance, so that proactive measures such as targeted vaccination or hospital resource allocation can be taken.
The study period overlaps with the COVID-19 pandemic, which emerged in late 2019 and intensified globally in 2020. Public health interventions such as mask mandates, social distancing, and lockdowns likely reduced influenza transmission during 2020–2022, as observed in global reports of diminished influenza activity (Chen et al., 2024). This suppression could lead to underestimation of typical influenza incidence and alter the observed relationships between environmental factors and cases. For instance, reduced human mobility during lockdowns may have temporarily lowered ambient pollutant levels (e.g., PM2.5 and NO2), potentially confounding the exposure-risk associations (Ali et al., 2022). Conversely, heightened health awareness and diagnostic focus on COVID-19 might have affected influenza case reporting, either through underdiagnosis (due to overwhelmed healthcare systems) or improved surveillance (via parallel testing protocols) (Schaffner et al., 2021). These dynamics highlight the need to interpret findings within the context of pandemic-influenced behavioral and environmental changes.
However, this study has some limitations. First, the meteorological data and pollutant data used in this study may differ from the actual exposure levels of individuals, which may lead to a deviation between the study results and the actual situation. Second, this study, using daily incidence numbers, and the small sample size in the number of incidence days, may limit certain statistical power and the influenza data did not include specific subtypes. Besides, the effects of vaccination coverage, socioeconomic status, population density and other factors on the association between meteorological factors and pollutants on influenza infection were not considered in this study, which may lead to biased results. Finally, this study spans a period intersecting with the COVID-19 pandemic, which introduced unprecedented behavioral and environmental shifts. The pandemic's public health measures (e.g., masking, reduced mobility) may have artificially suppressed influenza transmission and altered pollutant patterns, limiting the generalizability of results to non-pandemic conditions. Additionally, healthcare prioritization of COVID-19 could have impacted influenza case reporting consistency. Future studies should account for pandemic-related confounders or focus on pre/post-pandemic comparisons to disentangle these effects.
Comparative analysis between primary and sensitivity models confirmed robust exposure-response relationships for both O3 and PM2.5 with influenza incidence. Among our primary findings, there is an inverted U-shaped relationship between temperature and influenza. However, the results in the sensitivity analysis differ significantly from the primary findings. A study conducted before the COVID-19 pandemic indicated that the risk of influenza increased significantly when the temperature was between 0 and 5 °C. This is consistent with our research findings (Park, Son, Ryu, Choi, et al., 2020). The observed discrepancies between sensitivity and primary analyses may stem from limited statistical power due to sample size constraints, potentially introducing estimation bias. Although our results do guarantee a certain degree of robustness, there are still some limitations in our study, such as using the data covering the COVID-19 pandemic and the relatively small sample size. In the future work, we will need larger amounts of data to verify our results.
5. Conclusion
Our results show that low temperature significantly increases the risk of influenza. For air pollutants such as PM2.5 and ozone, the risk of influenza is also increased. At the low concentrations of PM2.5, the risk of influenza increases with increasing concentration but decreases at the high concentrations. At the high concentrations of O3, the risk of influenza increases rapidly. 15.90 % of influenza cases may be attributed to the high concentrations of PM2.5, equivalent to 1456 cases; temperature-induced cases mainly come from the low-temperature effect, with an AF value of 30.91 %, equivalent to 2830 cases. In addition, high temperatures can effectively mitigate the impact of PM2.5 on influenza incidence, and outdoor exposure time should be minimized in low temperatures and high PM2.5 weather. These analyses will provide scientific guidance for policymakers and health agencies to take action to reduce the incidence of influenza.
CRediT authorship contribution statement
Xiaomeng Wang: Writing – original draft, Software, Methodology, Formal analysis, Conceptualization. Jianli Hu: Resources, Methodology, Investigation, Formal analysis, Data curation. Zhiming Wang: Software, Methodology, Formal analysis. Yongli Cai: Writing – review & editing, Project administration, Funding acquisition. Daihai He: Writing – review & editing, Validation, Funding acquisition.
Declaration of Competing interest
Daihai He is an associate editor of the journal Infectious Disease Modelling, he was not involved in the editorial review or decision to publish this article. All authors declare no competing interests.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No. 12071173 and 12171192), the Scientific Research Project of Jiangsu Provincial Health Commission (No. DX202302) and Huaian Key Laboratory for Infectious Diseases Control and Prevention, China (HAP201704).
Handling Editor: Dr Yiming Shao
Footnotes
Peer review under the responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.idm.2025.07.010.
Contributor Information
Yongli Cai, Email: yonglicai@hytc.edu.cn.
Daihai He, Email: daihai.he@polyu.edu.hk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ali S.T., Lau Y.C., Shan S., et al. Prediction of upcoming global infection burden of influenza seasons after relaxation of public health and social measures during the COVID-19 pandemic: a modelling study[J] Lancet Global Health. 2022;10(11):e1612–e1622. doi: 10.1016/S2214-109X(22)00358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S.T., Wu P., Cauchemez S., et al. Ambient ozone and influenza transmissibility in hong kong[J] European Respiratory Journal. 2018;51(5) doi: 10.1183/13993003.00369-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenheimer M.M., Evans S.S. Temperature and adaptive immunity[J] Handbook of Clinical Neurology. 2018;156:397–415. doi: 10.1016/B978-0-444-63912-7.00024-2. [DOI] [PubMed] [Google Scholar]
- Bonilla W.V., Frohlich A., Senn K., et al. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses[J] Science. 2012;335(6071):984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- Chen Z., Tsui J.L., Gutierrez B., et al. COVID-19 pandemic interventions reshaped the global dispersal of seasonal influenza viruses[J] Science. 2024;386(6722) doi: 10.1126/science.adq3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Zhang W., Li S., et al. The impact of ambient fine particles on influenza transmission and the modification effects of temperature in China: A multi-city study[J] Environment International. 2017;98:82–88. doi: 10.1016/j.envint.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Zhang W., Li S., et al. The impact of ambient fine particles on influenza transmission and the modification effects of temperature in China: A multi-city study[J] Environment International. 2017;98:82–88. doi: 10.1016/j.envint.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F., Peng R.D., Bell M.L., et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases[J] JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emukule G.O., Mott J.A., Spreeuwenberg P., et al. Influenza activity in Kenya, 2007-2013: Timing, association with climatic factors, and implications for vaccination campaigns[J] Influenza Other Respir Viruses. 2016;10(5):375–385. doi: 10.1111/irv.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Li J., Sun W., et al. Impact of ambient fine particulate matter (PM2.5) exposure on the risk of influenza-like-illness: A time-series analysis in beijing, china[J] Environmental Health. 2016;15:17. doi: 10.1186/s12940-016-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A., Armstrong B., Kenward M.G. Distributed lag non-linear models[J] Statistics in Medicine. 2010;29(21):2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A., Leone M. Attributable risk from distributed lag models[J] BMC Medical Research Methodology. 2014;14:55. doi: 10.1186/1471-2288-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Liu Y., Guo D., et al. PM2.5-induced pulmonary inflammation via activating of the NLRP3/caspase-1 signaling pathway[J] Environmental Toxicology. 2021;36(3):298–307. doi: 10.1002/tox.23035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesic M.J., Meyer M., Bauer R., et al. Exposure to ozone modulates human airway protease/antiprotease balance contributing to increased influenza A infection[J] PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labella A.M., Merel S.E. Influenza[J] Medical Clinics of North America. 2013;97(4):621–645. doi: 10.1016/j.mcna.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Lau S.Y., Cheng W., Yu Z., et al. Independent association between meteorological factors, PM2.5, and seasonal influenza activity in hangzhou, Zhejiang Province, china[J] Influenza Other Respir Viruses. 2021;15(4):513–520. doi: 10.1111/irv.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wang X., Wu Y., et al. Temperature variability and influenza incidence in China: Effect modification by ambient fine particulate matter[J] Journal of Hazardous Materials. 2024;480 doi: 10.1016/j.jhazmat.2024.136114. [DOI] [PubMed] [Google Scholar]
- Lowen A.C., Steel J. Roles of humidity and temperature in shaping influenza seasonality[J] Journal of Virology. 2014;88(14):7692–7695. doi: 10.1128/JVI.03544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Yang Z., Karawita A.C., et al. Limited evidence for the role of environmental factors in the unusual peak of influenza in Brisbane during the 2018-2019 Australian summer[J] The Science of the Total Environment. 2021;776 doi: 10.1016/j.scitotenv.2021.145967. [DOI] [PubMed] [Google Scholar]
- Mummert A., Weiss H., Long L.P., et al. A perspective on multiple waves of influenza pandemics[J] PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E., Son W.S., Ryu Y., Choi S.B., Kwon O., Ahn I. Effects of temperature, humidity, and diurnal temperature range on influenza incidence in a temperate region[J] Influenza Other Respir Viruses. 2020;14(1):11–18. doi: 10.1111/irv.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.E., Son W.S., Ryu Y., et al. Effects of temperature, humidity, and diurnal temperature range on influenza incidence in a temperate region[J] Influenza Other Respir Viruses. 2020;14(1):11–18. doi: 10.1111/irv.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Gabbay R.A., Taylor A.J. Protecting vulnerable patients from influenza during the COVID-19 pandemic: An urgent call to action for health care professionals[J] Infectious Diseases in Clinical Practice. 2021;29(4):e202–e203. doi: 10.1097/IPC.0000000000001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seah A., Loo L.H., Jamali N., et al. The influence of air quality and meteorological variations on influenza A and B virus infections in a paediatric population in singapore[J] Environmental Research. 2023;216(Pt 1) doi: 10.1016/j.envres.2022.114453. [DOI] [PubMed] [Google Scholar]
- Tamerius J., Uejio C., Koss J. Seasonal characteristics of influenza vary regionally across US[J] PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0212511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R.J., Cao W.J., Li M.H., et al. PM2.5 compromises antiviral immunity in influenza infection by inhibiting activation of NLRP3 inflammasome and expression of interferon-beta[J] Molecular Immunology. 2020;125:178–186. doi: 10.1016/j.molimm.2020.07.001. [DOI] [PubMed] [Google Scholar]
- Tseng C.L.C. Ozone for inactivation of aerosolized bacteriophages[J] 2006;9(40):683–689. [Google Scholar]
- Wang X., Cai J., Liu X., et al. Impact of PM(2.5) and ozone on incidence of influenza in shijiazhuang, China: A time-series study[J] Environmental Science and Pollution Research International. 2023;30(4):10426–10443. doi: 10.1007/s11356-022-22814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhang L., Lei R., et al. Effects and interaction of meteorological parameters on influenza incidence during 2010-2019 in lanzhou, china[J] Frontiers in Public Health. 2022;10 doi: 10.3389/fpubh.2022.833710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodby B., Arnold M.M., Valacchi G. SARS-CoV-2 infection, COVID-19 pathogenesis, and exposure to air pollution: What is the connection?[J] Annals of the New York Academy of Sciences. 2021;1486(1):15–38. doi: 10.1111/nyas.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Hu W., Williams G., et al. Air pollution, temperature and pediatric influenza in Brisbane, australia[J] Environment International. 2013;59:384–388. doi: 10.1016/j.envint.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Yang J., Yang Z., Qi L., et al. Influence of air pollution on influenza-like illness in China: a nationwide time-series analysis[J] EBioMedicine. 2023;87 doi: 10.1016/j.ebiom.2022.104421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhang T., Yang L., et al. Association between ozone and influenza transmissibility in china[J] BMC Infectious Diseases. 2023;23(1):763. doi: 10.1186/s12879-023-08769-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Alonso W.J., Feng L., et al. Characterization of regional influenza seasonality patterns in China and implications for vaccination strategies: Spatio-temporal modeling of surveillance data[J] PLoS Medicine. 2013;10(11) doi: 10.1371/journal.pmed.1001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Meng Y., Song H., et al. The modification effect of temperature on the relationship between air pollutants and daily incidence of influenza in ningbo, China [J] Respiratory Research. 2021;22(1):153. doi: 10.1186/s12931-021-01744-6. Published 2021 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Peng Z., Meng Y., et al. Temperature and influenza transmission: Risk assessment and attributable burden estimation among 30 cities in china[J] Environmental Research. 2022;215(Pt 1) doi: 10.1016/j.envres.2022.114343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.